Water as Green Solvent: Methods of Solubilisation and Extraction of Natural Products—Past, Present and Future Solutions
Abstract
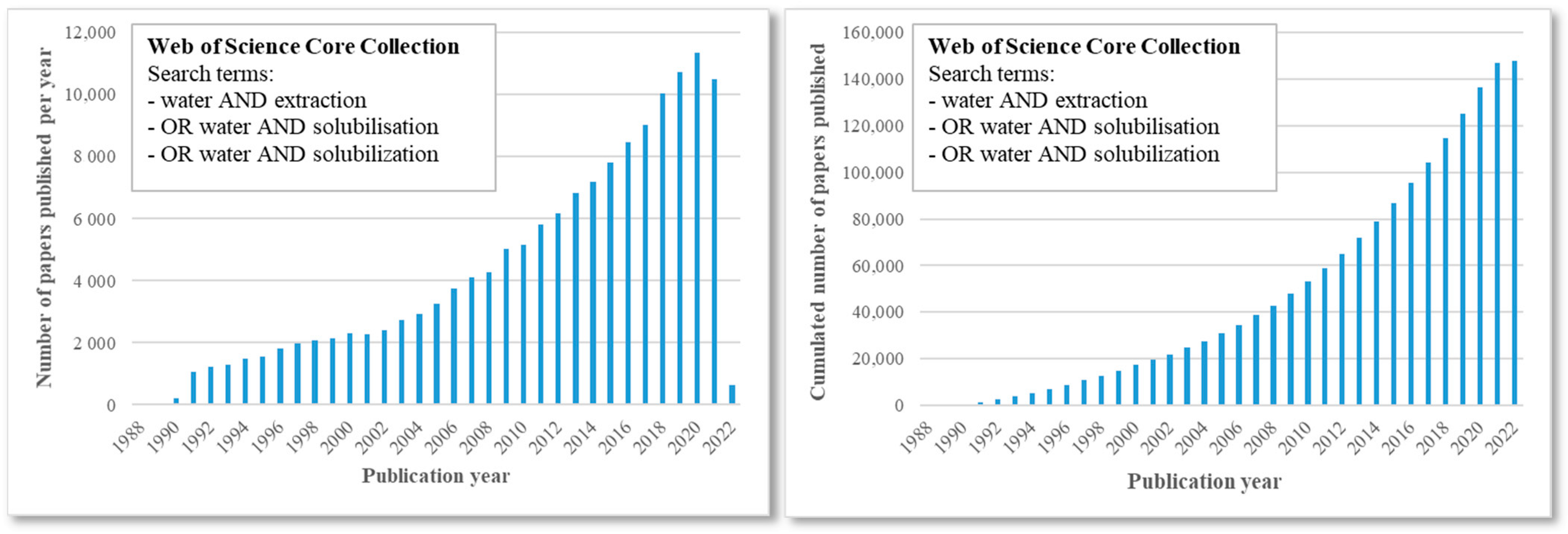
1. Introduction
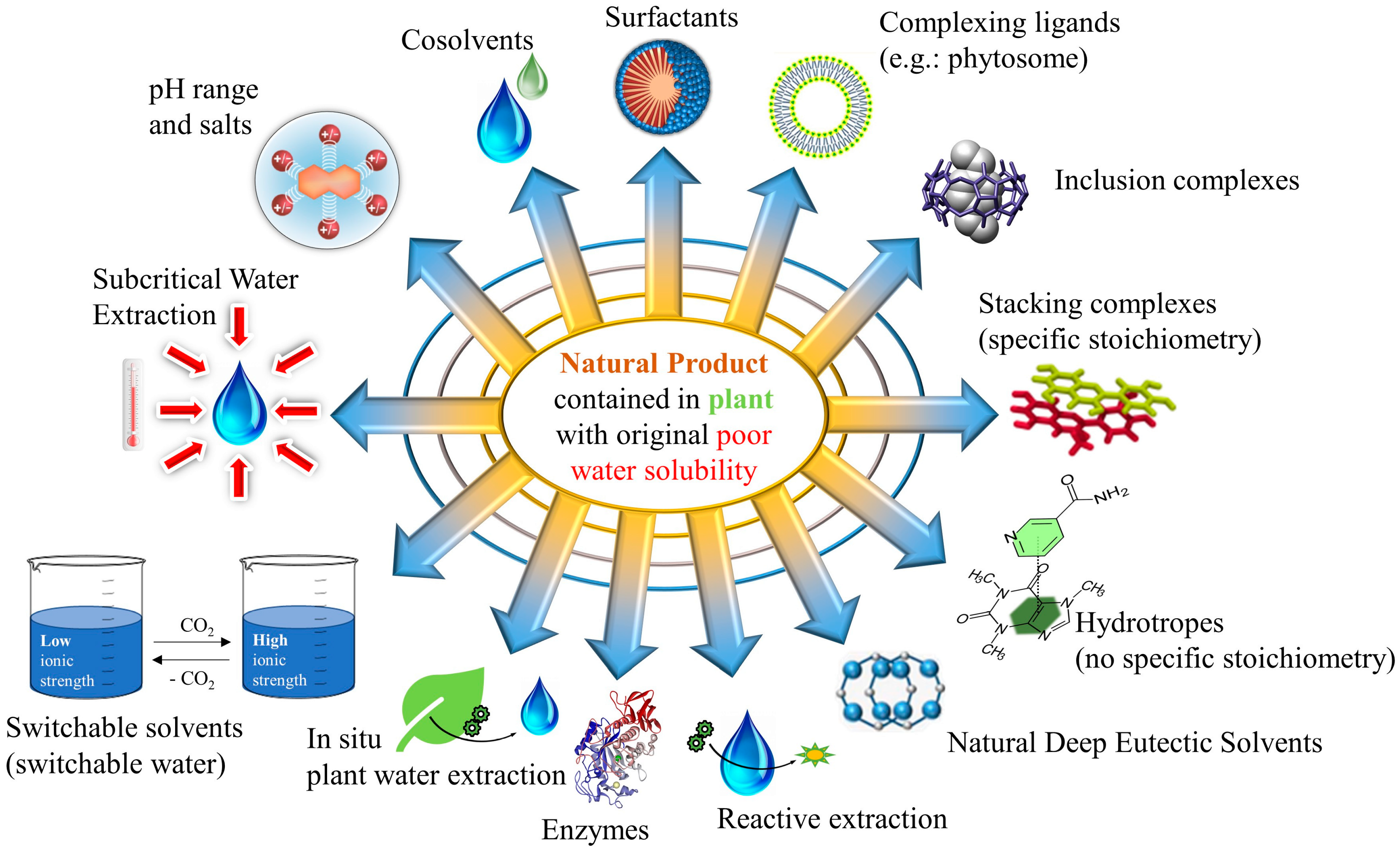
2. Relevant Methods to Enhance the Solvent Potential of Water
2.1. Method Overview
2.1.1. pH Range and Salts
2.1.2. Cosolvents
2.1.3. Surfactants
2.1.4. Complexing Ligands
2.1.5. Inclusion Complexes
2.1.6. Stacking Complexes
2.1.7. Hydrotropes
2.1.8. NADES
2.1.9. Reactive Extraction
2.1.10. Enzymes
2.1.11. ISPWE
2.1.12. Switchable Solvents
2.1.13. SWE
2.2. Successful Cases of Green Extraction Using Each Method
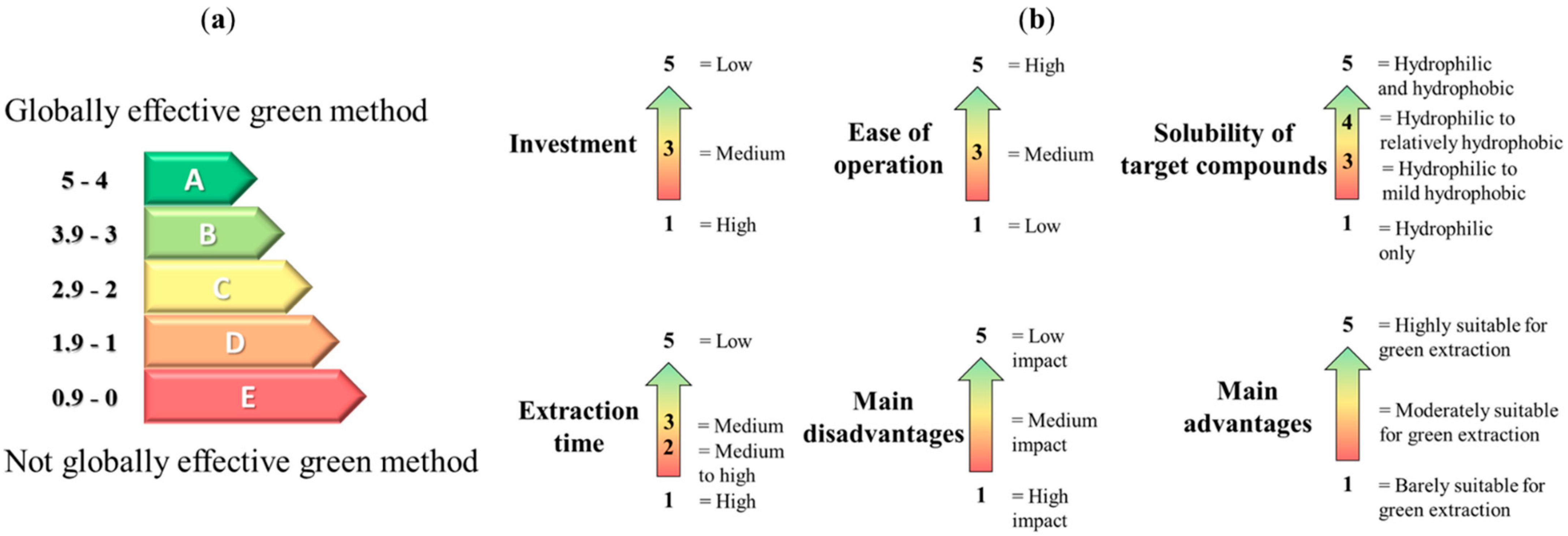
2.3. Method Analysis, Comparison and Rating
3. Future of Water-Based Extraction: Combined Methods at 2 or 3 Levels
4. Contribution of These Methods in Terms of Sustainability: Consolidating the SDGs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Westall, F.; Brack, A. The Importance of Water for Life. Space Sci. Rev. 2018, 214, 50. [Google Scholar] [CrossRef]
- Zhou, F.; Hearne, Z.; Li, C.-J. Water—The Greenest Solvent Overall. Curr. Opin. Green Sustain. Chem. 2019, 18, 118–123. [Google Scholar] [CrossRef]
- Hartonen, K.; Riekkola, M.-L. Water as the First Choice Green Solvent. In The Application of Green Solvents in Separation Processes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 19–55. ISBN 978-0-12-805297-6. [Google Scholar]
- Sadatshojaei, E.; Wood, D.A. Water, the Most Accessible Eco-Friendly Solvent, and Extraction and Separation Agent. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 283–292. ISBN 978-0-12-821884-6. [Google Scholar]
- Water as the Green Solvent in Organic Synthesis. In Materials Research Foundations; Materials Research Forum LLC: Millersville, PA, USA, 2019; Volume 54, pp. 182–201. ISBN 978-1-64490-031-4.
- Rastogi, H.; Jana, S. Evaluation of Physicochemical Properties and Intestinal Permeability of Six Dietary Polyphenols in Human Intestinal Colon Adenocarcinoma Caco-2 Cells. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Krewson, C.F.; Naghski, J. Some Physical Properties of Rutin. J. Am. Pharm. Assoc. Sci. Ed. 1952, 41, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.O. (Bill) Solubility and Solubilization in Aqueous Media by Samuel, H. Yalkowsky (University of Arizona). Oxford University Press: New York. 1999. Xvi + 464 pp. $165. ISBN 0-8412-3576-7. J. Am. Chem. Soc. 2000, 122, 9882. [Google Scholar] [CrossRef]
- Mercer, S.M.; Jessop, P.G. “Switchable Water”: Aqueous Solutions of Switchable Ionic Strength. ChemSusChem 2010, 3, 467–470. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Jacotet-Navarro, M.; Laguerre, M.; Fabiano-Tixier, A.-S.; Tenon, M.; Feuillère, N.; Bily, A.; Chemat, F. What Is the Best Ethanol-Water Ratio for the Extraction of Antioxidants from Rosemary? Impact of the Solvent on Yield, Composition, and Activity of the Extracts. Electrophoresis 2018, 39, 1946–1956. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-Assisted Extractions of Polyphenols–A Comprehensive Review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Chemat, F.; Vorobiev, E. (Eds.) Green Food Processing Techniques, 1st ed.; Elsevier: Cambridge, UK, 2019; ISBN 978-0-12-815353-6. [Google Scholar]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- León-Carmona, J.R.; Galano, A.; Alvarez-Idaboy, J.R. Deprotonation Routes of Anthocyanidins in Aqueous Solution, PK a Values, and Speciation under Physiological Conditions. RSC Adv. 2016, 6, 53421–53429. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Kunz, W. Specific Ion Effects in Colloidal and Biological Systems. Curr. Opin. Colloid Interface Sci. 2010, 15, 34–39. [Google Scholar] [CrossRef]
- Hyde, A.M.; Zultanski, S.L.; Waldman, J.H.; Zhong, Y.-L.; Shevlin, M.; Peng, F. General Principles and Strategies for Salting-Out Informed by the Hofmeister Series. Org. Process Res. Dev. 2017, 21, 1355–1370. [Google Scholar] [CrossRef]
- Liu, L.; Yu, X.; Zhao, Z.; Xu, L.; Zhang, R. Efficient Salt-Aided Aqueous Extraction of Bitter Almond Oil: Efficient Salt-Aided Aqueous Extraction of Bitter Almond Oil. J. Sci. Food Agric. 2017, 97, 3814–3821. [Google Scholar] [CrossRef]
- Hatti-Kaul, R. Aqueous Two-Phase Systems. Mol. Biotechnol. 2001, 19, 9. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Zhang, J.; Zhan, J.; Wu, C.; Yu, W.; Fan, W.; Tang, J.; Zhang, Q.; Zhang, J. Sustainable Bioactive Salts Fully Composed of Natural Products for Enhanced Pharmaceutical Applicability. ACS Sustain. Chem. Eng. 2022, 10, 10369–10382. [Google Scholar] [CrossRef]
- Jeffries, W. The dielectric constant of mixtures of ethyl alcohol and water from −5 TO 40°. J. Am. Chem. Soc. 1931, 53, 3292–3301. [Google Scholar] [CrossRef]
- Li, A.; Andren, A.W.; Yalkowsky, S.H. Choosing a Cosolvent: Solubilization of Naphthalene and Cosolvent Property. Environ. Toxicol. Chem. 1996, 15, 2233–2239. [Google Scholar] [CrossRef]
- Prat, D.; Pardigon, O.; Flemming, H.-W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; et al. Sanofi’s Solvent Selection Guide: A Step Toward More Sustainable Processes. Org. Process Res. Dev. 2013, 17, 1517–1525. [Google Scholar] [CrossRef]
- Paleologos, E.K.; Giokas, D.L.; Karayannis, M.I. Micelle-Mediated Separation and Cloud-Point Extraction. TrAC Trends Anal. Chem. 2005, 24, 426–436. [Google Scholar] [CrossRef]
- Petigny, L.; Özel, M.Z.; Périno, S.; Wajsman, J.; Chemat, F. Water as Green Solvent for Extraction of Natural Products. In Green Extraction of Natural Products; Chemat, F., Strube, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 237–264. ISBN 978-3-527-67682-8. [Google Scholar]
- Bi, W.; Tian, M.; Row, K.H. Extraction and Concentration of Tanshinones in Salvia Miltiorrhiza Bunge by Task-Specific Non-Ionic Surfactant Assistance. Food Chem. 2011, 126, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, S.H.; Lee, C.T.; Chmelka, B.F.; Israelachvili, J.N. General Hydrophobic Interaction Potential for Surfactant/Lipid Bilayers from Direct Force Measurements between Light-Modulated Bilayers. Proc. Natl. Acad. Sci. USA 2011, 108, 15699–15704. [Google Scholar] [CrossRef] [PubMed]
- Abo Enin, H.A. Self-Nanoemulsifying Drug-Delivery System for Improved Oral Bioavailability of Rosuvastatin Using Natural Oil Antihyperlipdemic. Drug Dev. Ind. Pharm. 2015, 41, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Ameur, S.; Haddou, B.; Derriche, Z.; Canselier, J.P.; Gourdon, C. Cloud Point Extraction of Δ9-Tetrahydrocannabinol from Cannabis Resin. Anal. Bioanal. Chem. 2013, 405, 3117–3123. [Google Scholar] [CrossRef][Green Version]
- Lu, M.; Qiu, Q.; Luo, X.; Liu, X.; Sun, J.; Wang, C.; Lin, X.; Deng, Y.; Song, Y. Phyto-Phospholipid Complexes (Phytosomes): A Novel Strategy to Improve the Bioavailability of Active Constituents. Asian J. Pharm. Sci. 2019, 14, 265–274. [Google Scholar] [CrossRef]
- Arenas-Lago, D.; Rodríguez-Seijo, A.; Andrade Couce, L.; Vega, F.A. A Multianalytical Approach for the Assessment of Toxic Element Distribution in Soils from Mine and Quarry Areas. In Assessment, Restoration and Reclamation of Mining Influenced Soils; Elsevier: Amsterdam, The Netherlands, 2017; pp. 33–62. ISBN 978-0-12-809588-1. [Google Scholar]
- Diarra, I.; Kotra, K.K.; Prasad, S. Assessment of Biodegradable Chelating Agents in the Phytoextraction of Heavy Metals from Multi–Metal Contaminated Soil. Chemosphere 2020, 273, 128483. [Google Scholar] [CrossRef]
- Huang, W.-C.; Li, B.; Qi, X.; Mao, X. New Type of Green Extractant for Oil Production: Citric Acid/Citric Acid Sodium Extraction System. Food Chem. 2020, 310, 125815. [Google Scholar] [CrossRef]
- Hu, H.; Luo, F.; Wang, M.; Fu, Z.; Shu, X. New Method for Extracting and Purifying Dihydromyricetin from Ampelopsis Grossedentata. ACS Omega 2020, 5, 13955–13962. [Google Scholar] [CrossRef]
- Quercefit®. Available online: https://www.indena.com/products/see-through-science/quercefit/ (accessed on 10 November 2022).
- Di Pierro, F.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A.; et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int. J. Gen. Med. 2021, 14, 2359–2366. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fourmentin, M.; Morin-Crini, N. Principales applications des complexes d’inclusion cyclodextrine/substrat. Innov. Technol. 2019. [Google Scholar] [CrossRef]
- Yang, L.-J.; Xia, S.; Ma, S.-X.; Zhou, S.-Y.; Zhao, X.-Q.; Wang, S.-H.; Li, M.-Y.; Yang, X.-D. Host–Guest System of Hesperetin and β-Cyclodextrin or Its Derivatives: Preparation, Characterization, Inclusion Mode, Solubilization and Stability. Mater. Sci. Eng. C 2016, 59, 1016–1024. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 Years of Cyclodextrin Discovery for Health, Food, Agriculture, and the Industry: A Review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Cyclolab. Available online: https://cyclolab.hu/ (accessed on 10 November 2022).
- Baron, M. Towards a Greener Pharmacy by More Eco Design. Waste Biomass Valorization 2012, 3, 395–407. [Google Scholar] [CrossRef]
- Zhuang, W.-R.; Wang, Y.; Cui, P.-F.; Xing, L.; Lee, J.; Kim, D.; Jiang, H.-L.; Oh, Y.-K. Applications of π-π Stacking Interactions in the Design of Drug-Delivery Systems. J. Controll. Release 2019, 294, 311–326. [Google Scholar] [CrossRef]
- Liu, X.; Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Cell Wall Polysaccharides and Polyphenols: Effect of Molecular Internal Structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Rasool, A.A.; Hussain, A.A.; Dittert, L.W. Solubility Enhancement of Some Water-Insoluble Drugs in the Presence of Nicotinamide and Related Compounds. J. Pharm. Sci. 1991, 80, 387–393. [Google Scholar] [CrossRef]
- Mazaud, A.; Lebeuf, R.; Laguerre, M.; Nardello-Rataj, V. Hydrotropic Extraction of Carnosic Acid from Rosemary with Short-Chain Alkyl Polyethylene Glycol Ethers. ACS Sustain. Chem. Eng. 2020, 8, 15268–15277. [Google Scholar] [CrossRef]
- Desai, M.A.; Parikh, J. Hydrotropic Extraction of Citral from Cymbopogon Flexuosus(Steud.) Wats. Ind. Eng. Chem. Res. 2012, 51, 3750–3757. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, S.; Papp, M.; Park, K.; Pinal, R. Hydrotropic Solubilization of Poorly Water-Soluble Drugs. J. Pharm. Sci. 2010, 99, 3953–3965. [Google Scholar] [CrossRef] [PubMed]
- González, C.G.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Application of Natural Deep Eutectic Solvents for the “Green” Extraction of Vanillin from Vanilla Pods. Flavour Fragr. J. 2018, 33, 91–96. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents–Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Chemat; Abert Vian; Ravi; Khadhraoui; Hilali; Perino; Tixier Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [CrossRef] [PubMed]
- Lavaud, A.; Laguerre, M.; Birtic, S.; Fabiano, T.A.S.; Roller, M.; Chemat, F.; Bily, A.C. Solvant Eutectique D’extraction, Procédé D’extraction Par Eutectigenèse Utilisant Ledit Solvant, et Extrait Issu Dudit Procédé D’extraction. French Patent FR3034625A1, 14 October 2016. [Google Scholar]
- Green Fractionation|Givaudan. Available online: https://www.givaudan.com/fragrance-beauty/active-beauty/innovative-technologies/green-fractionation (accessed on 10 November 2022).
- Gatuline® Link n Lift|Anti-Wrinkle Active for the Eye-Contour. Available online: https://www.gattefosse.com/personal-care-actives/gatuline-link-n-lift (accessed on 10 November 2022).
- Di Mauro, A.; Fallico, B.; Passerini, A.; Rapisarda, P.; Maccarone, E. Recovery of Hesperidin from Orange Peel by Concentration of Extracts on Styrene−Divinylbenzene Resin. J. Agric. Food Chem. 1999, 47, 4391–4397. [Google Scholar] [CrossRef]
- Klein, R.; Kellermeier, M.; Drechsler, M.; Touraud, D.; Kunz, W. Solubilisation of Stearic Acid by the Organic Base Choline Hydroxide. Colloids Surf. Physicochem. Eng. Asp. 2009, 338, 129–134. [Google Scholar] [CrossRef]
- Labavitch, J.M.; Powell, A.L.T.; Greve, L.C.; Blanco-Ulate, B.; Cantu, D.; Vicente, A.R. Cell Wall Metabolism: The Yin and Yang of Fruit Postharvest Biology. Acta Hortic. 2015, 1079, 27–40. [Google Scholar] [CrossRef]
- Domínguez González, H.; Muñoz González, M.J. (Eds.) Water Extraction of Bioactive Compounds: From Plants to Drug Development; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-809380-1. [Google Scholar]
- Goyal, M.R.; Joy, P.P.; Suleria, H. (Eds.) Plant Secondary Metabolites for Human Health: Extraction of Bioactive Compounds. In Innovations in Plant Science for Better Health: From Soil to Fork, 1st ed.; Apple Academic Press: Palm Bay, FL, USA, 2019; ISBN 978-0-429-42532-5. [Google Scholar]
- Périno-Issartier, S.; Zill-e-Huma; Abert-Vian, M.; Chemat, F. Solvent Free Microwave-Assisted Extraction of Antioxidants from Sea Buckthorn (Hippophae Rhamnoides) Food By-Products. Food Bioprocess Technol. 2011, 4, 1020–1028. [Google Scholar] [CrossRef]
- Vorobiev, E.; Lebovka, N. Processing of Foods and Biomass Feedstocks by Pulsed Electric Energy; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-40916-6. [Google Scholar]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Vanderveen, J.R.; Burra, S.; Geng, J.; Goyon, A.; Jardine, A.; Shin, H.E.; Andrea, T.; Dyson, P.J.; Jessop, P.G. Characterizing the Effects of a “Switchable Water” Additive on the Aqueous Solubility of Small Molecules. Chemphyschem 2018, 19, 2093–2100. [Google Scholar] [CrossRef]
- Jessop, P.G.; Mercer, S.M.; Heldebrant, D.J. CO2-Triggered Switchable Solvents, Surfactants, and Other Materials. Energy Environ. Sci. 2012, 5, 7240. [Google Scholar] [CrossRef]
- Pan, H.; Nie, S.; Kou, P.; Wang, L.; Wang, Z.; liu, Z.; Zhao, C.; Wang, X.; Fu, Y. An Enhanced Extraction and Enrichment Phytochemicals from Rosa Roxburghii Tratt Leaves with Ultrasound-Assist CO2-Based Switchable-Solvent and Extraction Mechanism Study. J. Mol. Liq. 2021, 337, 116591. [Google Scholar] [CrossRef]
- Thiruvenkadam, S.; Izhar, S.; Yoshida, H.; Danquah, M.K.; Harun, R. Process Application of Subcritical Water Extraction (SWE) for Algal Bio-Products and Biofuels Production. Appl. Energy 2015, 154, 815–828. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Marina, M.L.; Plaza, M. Water as Green Extraction Solvent: Principles and Reasons for Its Use. Curr. Opin. Green Sustain. Chem. 2017, 5, 31–36. [Google Scholar] [CrossRef]
- Phenobio Technology Protects Natural Resources-Lubrizol. Available online: https://www.lubrizol.com/Personal-Care/Blog/2021/09/Phenobio-technology-protects-natural-resources (accessed on 10 November 2022).
- Sensient Technologies Acquires Mazza Innovation. Available online: https://investor.sensient.com/news/news-details/2018/Sensient-Technologies-Acquires-Mazza-Innovation/default.aspx (accessed on 10 November 2022).
- Sensient Natural Extracts. Natural Sources, Pure Extracts. Available online: https://www.sensientnaturalextracts.com/technology/ (accessed on 10 November 2022).
- Kubiliene, L.; Jekabsone, A.; Zilius, M.; Trumbeckaite, S.; Simanaviciute, D.; Gerbutaviciene, R.; Majiene, D. Comparison of Aqueous, Polyethylene Glycol-Aqueous and Ethanolic Propolis Extracts: Antioxidant and Mitochondria Modulating Properties. BMC Complement. Altern. Med. 2018, 18, 165. [Google Scholar] [CrossRef]
- Mantegna, S.; Binello, A.; Boffa, L.; Giorgis, M.; Cena, C.; Cravotto, G. A One-Pot Ultrasound-Assisted Water Extraction/Cyclodextrin Encapsulation of Resveratrol from Polygonum Cuspidatum. Food Chem. 2012, 130, 746–750. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, H.; Wang, S.; Li, T.; Huang, X.; Yan, M.; Cao, X.; Xu, B.; Wang, P.; Lei, H. A New Strategy Based on Acid-Alkali Complexation for Rapidly and Accurately Fishing Phytochemicals in Sennae Folium. Chin. Herb. Med. 2020, 12, 188–194. [Google Scholar] [CrossRef]
- Sharma, R.A.; Gaikar, V.G. Hydrotropic Extraction of Reserpine from Rauwolfia Vomitoria Roots. Sep. Sci. Technol. 2012, 47, 827–833. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.; Hu, H.; Wan, J.; Li, P. Natural Deep Eutectic Solvents for Simultaneous Extraction of Multi-Bioactive Components from Jinqi Jiangtang Preparations. Pharmaceutics 2019, 11, 18. [Google Scholar] [CrossRef]
- Meini, M.-R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of Phenolic Antioxidants from Syrah Grape Pomace through the Optimization of an Enzymatic Extraction Process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef]
- Périno, S.; Pierson, J.T.; Ruiz, K.; Cravotto, G.; Chemat, F. Laboratory to Pilot Scale: Microwave Extraction for Polyphenols Lettuce. Food Chem. 2016, 204, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chun, B. Extraction of Bioactive Compounds from Pseuderanthemum Palatiferum (Nees) Radlk. Using Subcritical Water and Conventional Solvents: A Comparison Study. J. Food Sci. 2019, 84, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Sed, G.; Cicci, A.; Jessop, P.G.; Bravi, M. A Novel Switchable-Hydrophilicity, Natural Deep Eutectic Solvent (NaDES)-Based System for Bio-Safe Biorefinery. RSC Adv. 2018, 8, 37092–37097. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, X.; Geng, Q.; Li, M. Combination of Span 20 and PH-Assisted Walnut Oil Extraction during Aqueous Extraction Process. LWT 2018, 91, 477–483. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; First Publ. New as Paperback; Oxford University Press: Oxford, UK, 2000; ISBN 978-0-19-850698-0. [Google Scholar]
- Anastas, P.T.; Zimmerman, J.B. Peer Reviewed: Design through the 12 Principles of Green Engineering. Environ. Sci. Technol. 2003, 37, 94A–101A. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]


| Method | Matrix | Target NP(s) | Experimental Conditions | Results (Compared to the Control) | Refs. |
|---|---|---|---|---|---|
pH and salts | Bitter almond (Prunus amygdalus L.) kernel powder | Monounsaturated and polyunsaturated fatty acids contained in the oil (e.g., oleic and linolenic acids)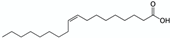 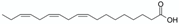 | 1 h stirred maceration at 84 °C with sodium bicarbonate (0.4 M) in water Matrix/Solvent = 1/5 w/w 30 min centrifugation at 2368× g Control: same treatment with pure water | 91% oil extraction yield with sodium bicarbonate (>55% with control) Reduced creamy phase Less toxic compounds extracted (hydrocyanic acid) | [19] |
Cosolvents | Propolis powder | Phenolic compounds (e.g., vanillin) | 5 h maceration at room temperature with 20% propylene glycol in water Matrix/Solvent = 1/10 w/V Control: same treatment with pure water | 20% propylene glycol in water can double the extraction of total phenolic compounds from propolis and triple the antioxidant activity | [72] |
Surfactants | Cannabis (Cannabis sativa L.) resin | Δ9-tetrahydrocannabinol (THC)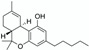 | CPE with non-ionic surfactant Dowfax 20B102 (ethylene oxide-propylene oxide copolymer-based) 4 h maceration at 45 °C Matrix/Solvent = 1/2000 w/V Controls: same treatment with pure hexane or pure methanol | Dowfax is approximately 4 times more efficient than hexane and methanol controls (62% THC extraction versus 14 and 17%, respectively) Possibility to enhance this extraction yield by combining this method with the use of a salt: 81% THC recovery with the addition of 1% Na2SO4 | [30] |
Complexing ligands | Chinese vine tea (Ampelopsis grossedentata) leaf powder  | Dihydromyricetin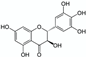 | Reverse method to extract NP from a matrix: use of the complexing properties of the NP itself (chelation extraction). 2 h maceration at 90 °C (pH = 2, HCl 2M) with zinc salt (complexation of ionic zinc by dihydromyricetin). Reverse extraction of dihydromyricetin with disodium EDTA (stronger binding affinity with zinc than dihydromyricetin). Dihydromyricetin released in high quantities in the extract and EDTA-zinc complexes easily removed via filtration. Matrix/Solvent = 1/20 w/V | 1 2% dihydromyricetin recovery yield and 94% purity (versus 8 and 91%, respectively obtained with the reference method of repeated crystallisation [54]) | [35] |
Inclusion complexes | Giant knotweed (Polygonum cuspidatum) root powder | Resveratrol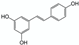 | 1 h ultrasound-assisted extraction with 1.5 wt% β-CD in water Matrix/Solvent = 1/50 w/V Conventional solvent: same treatment with pure methanol Control: same treatment with water | 125 times more resveratrol extracted with 1.5 wt% β-CD than with the control. As efficient as pure methanol. Similar antioxidant activities between methanolic and β-CD aqueous extracts | [73] |
Stacking complexes | Sennae folium (dried Cassia angustifolia Vahl or Cassia acutifolia Delile leaf) powder | Sennoside A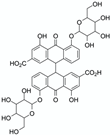 | 30 min ultrasound-assisted extraction 0.1% NaHCO3 Matrix/Solvent = 1/16.67 w/V Precipitation extraction with berberine (added in excess) Filtration, washing and drying Controls: organic bases such as sulphanilamide or ammonium thiosulfate | More efficient selective sennoside extraction with berberine than with controls. Specific binding molar ratio: 2:1 (berberine:sennoside A) | [74] |
Hydrotropes 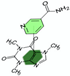 | Poison Devil’s pepper (Rauwolfia vomitoria) root bark powder | Reserpine 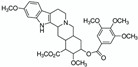 | 40 min stirred maceration at room temperature with hydrotrope sodium cumene sulfonate dissolved in water (2 M). Matrix/Solvent = 1/10 w/V Control: same treatment with pure methanol Reference (assumed total extraction): 48 h reflux extraction (Soxhlet) with chloroform | Hydrotrope extraction as efficient as the reference Soxhlet extraction with chloroform, 72 times shorter. Also more than 3 times greater than methanol. | [75] |
NADES  | Powder from traditional Chinese medicine herbal preparation JinQi Jiangtang (consisting of Coptis chinensis, Astragalus membranaceus, and Lonicera japonica)  | Phenolic compounds and alkaloids (e.g., chlorogenic acid and groenlandicine)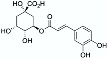  | 1 h ultrasound-assisted extraction with aqueous NADES (50% water content) composed of choline chloride:Levulinic acid 1:2 (mol:mol) Matrix/Solvent = 1/125 w/V Controls: same treatment with 70% methanol and with water | Aqueous NADES more efficient than both controls in extracting chlorogenic acid and groenlandicine | [76] |
Reactive extraction | Italian blood orange (Citrus sinensis) peel (industrial by-product)  | Hesperidin 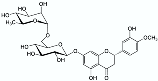 | 1 h stirred maceration at 60 °C after pH adjustment using calcium hydroxide (pH = 12), followed by neutralisation with hydrochloric acid (pH = 6) to modify hesperidin structure before resin purification. Matrix/Solvent = 1/5 w/V | 40% extraction yield, 93% purity | [56] |
Enzymes  | Syrah grape (Vitis vinifera ‘Syrah’) pomace  | Phenolic compounds (e.g., p-coumaric acid)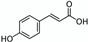 | 1 h stirred maceration (orbital shaker, 125 rpm) at 45 °C, pH = 5 (acetate buffer at 50 mM) using cellulase and tannase alone and in combination. Control: same treatment with water | Individually, cellulase and tannase greatly enhanced extraction yields of gallic acid, p-coumaric acid, and total phenolic compounds (from 2 to 8 times) compared to control. Combination of both enzymes categories is beneficial. | [77] |
ISPWE | Lettuce (Lettuce sativa) | Phenolic compounds (e.g., quercetin) 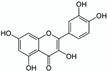 | 20 min SFME at 250–300 W (lab and pilot scale, 4 and 150 L reactors respectively) Conventional extraction: 5 min ultra-homogenisation (4000 rpm) at room temperature with 80% ethanol (Matrix/Solvent = 1/10 w/V) | Quercetin and luteolin at least 5 times more concentrated in SFME extracts (lab and pilot scales) compared to conventional extracts. | [78] |
Switchable solvents 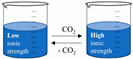 | Pure compounds in water (solubilisation tests) | Various NPs (e.g., benzoic acid and capsaicin)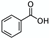 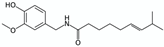 | Switchable water obtained with N,N,N’,N’-tetramethylbutane-1,4-diamine (1:5 base:water V:V ≈ 0.9 M) with or without CO2 (1 atm of air or CO2) Control: pure water (1 atm of air) | Capsaicin and benzoic acid far more soluble in switchable water than in control (877 and 73 times respectively) | [64] |
SWE | Pseuderanthemum palatiferum (Nees) Radlk. leaf powder  | Phenolic compounds, flavonoids and saponins. | 15 min SWE at 130 to 190 °C, 80 bar Matrix/Solvent = 1/70 w/V Conventional solvent and extraction procedures: 19 h stirred maceration with methanol at 25 °C (Matrix/Solvent = 1/100 w/V) 7 h Soxhlet reflux with 70% ethanol (Matrix/Solvent = 1/100 w/V) 30 min stirred maceration with hot water at 80 °C (Matrix/Solvent = 1/25 w/V) | SWE most efficient and fastest method SWE extracts far richer in NPs, exhibit 2 to 20 times more antioxidant activity, as well as more antimicrobial power (inhibition zone) compared to conventional extracts | [79] |
| Method | pH and Salts | Cosolvents | Surfactants | Complexing Ligands | Inclusion Complexes | Stacking Complexes | Hydrotropes | NADES | Reactive Extraction | Enzymes | ISPWE | Switchable Solvent | SWE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| System description | Addition of salts to increase solubility | Addition of solvent(s) to tune water polarity, proticity and viscosity | Addition of surfactants to create micelles | Addition of a complexing agent to capture the target compound | Addition of an inclusion agent to host the target compound | Addition of a stacking agent to increase solubility | Addition of organic or natural agent to increase solubility | Addition of natural, small organic molecules at specific molar ratio | Addition of salts to extract and simultaneously transform the target compound | Addition of enzymes in water under specific conditions to denature the matrix | Physical treatments to extract plant metabolites using its own water content | Addition of organic bases and CO2 to switch water behaviour | Water at a high temperature and pressure to keep it in liquid state |
| Investment | Low | Low | Low to medium | Low | Medium | Medium to high | Low to medium | Low | Low | Medium | High | Low to medium | High |
| Ease of operation | High | High | Medium | Medium | High | Medium | High | Medium | Low to medium | Low | Medium to high | Medium | Medium |
| Hydropathy of target NPs | Hydrophilic and lipophilic | Hydrophilic to mildly lipophilic | Hydrophilic to mildly lipophilic | Hydrophilic and lipophilic (with phytosomes) | Hydrophilic to relatively lipophilic | Hydrophilic and lipophilic | Hydrophilic to mildly lipophilic | Hydrophilic to relatively lipophilic | Hydrophilic and lipophilic | Hydrophilic and lipophilic | Hydrophilic to mild lipophilic | Hydrophilic and lipophilic | Hydrophilic to mildly lipophilic |
| Extraction time | Medium | Medium to high | Medium | Medium to high | Medium | Medium | Low | Medium | Medium to high | High | Low | Medium to high | Medium |
| Main disadvantages | Very specific (precise conditions necessary) | Limited concentration authorised in food products, obligation to remove it | Surfactant removal | Lack of data in plant extraction | Difficult to combine with other methods | Lack of data in plant extraction | High concentration of hydrotropes needed | Patented use | Lack of data in plant extraction | Enzyme price, precise conditions necessary | Not particularly tuneable or easy to implement | Still needs toxic organic agents (albeit in small quantities) | Not suitable for thermosensitive molecules, high pressure |
| Main advantages | Useful method mainly if used in combination with others, intensification techniques | Cosolvents could be part of next steps in formulation | Simultaneous extraction of polar and apolar molecules | Extremely target-specific, potential drug delivery system (enhanced stability), could be part of next steps in formulation | Extremely target-specific, potential drug delivery system (enhanced stability), could be part of next steps in formulation | Extremely target-specific, could be part of next steps in formulation | Hydrotropes could be part of next steps in formulation | Biomimetic (natural, GRAS)tuneable quantity of water added, enables intensification | Highly efficient while using very low-cost agents | Matrix pretreatment | No solvent needed and short treatment | Ease of recovery of both product and extractant and specifically designed to facilitate industrial implementation | Tuneable solvent power |
 | |||||||||||||
| Method | pH and Salts | Cosolvents | Surfactants | Complexing Ligands | Inclusion Complexes | Stacking Complexes | Hydrotropes | NADES | Reactive Extraction | Enzymes | ISPWE | Switchable Solvent | SWE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Investment | 5 | 5 | 4 | 5 | 3 | 2 | 4 | 5 | 5 | 3 | 1 | 4 | 1 |
| Ease of operation | 5 | 5 | 3 | 3 | 5 | 3 | 5 | 3 | 2 | 1 | 4 | 3 | 3 |
| Solubility of the target compounds | 5 | 3 | 3 | 5 | 4 | 5 | 4 | 4 | 5 | 5 | 3 | 5 | 3 |
| Extraction time | 3 | 2 | 3 | 2 | 3 | 3 | 5 | 3 | 2 | 1 | 5 | 2 | 3 |
| Main disadvantages | 2 | 2 | 2 | 3 | 3 | 3 | 2 | 4 | 3 | 2 | 2 | 2 | 2 |
| Main advantages | 2 | 3 | 4 | 4 | 5 | 3 | 4 | 5 | 5 | 4 | 5 | 5 | 4 |
| Average score | 3.7 | 3.3 | 3.2 | 3.7 | 3.8 | 3.2 | 4.0 | 4.0 | 3.7 | 2.7 | 3.3 | 3.5 | 2.7 |
| Equivalent letter | B | B | B | B | B | B | A | A | B | C | B | B | C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajoie, L.; Fabiano-Tixier, A.-S.; Chemat, F. Water as Green Solvent: Methods of Solubilisation and Extraction of Natural Products—Past, Present and Future Solutions. Pharmaceuticals 2022, 15, 1507. https://doi.org/10.3390/ph15121507
Lajoie L, Fabiano-Tixier A-S, Chemat F. Water as Green Solvent: Methods of Solubilisation and Extraction of Natural Products—Past, Present and Future Solutions. Pharmaceuticals. 2022; 15(12):1507. https://doi.org/10.3390/ph15121507
Chicago/Turabian StyleLajoie, Léo, Anne-Sylvie Fabiano-Tixier, and Farid Chemat. 2022. "Water as Green Solvent: Methods of Solubilisation and Extraction of Natural Products—Past, Present and Future Solutions" Pharmaceuticals 15, no. 12: 1507. https://doi.org/10.3390/ph15121507
APA StyleLajoie, L., Fabiano-Tixier, A.-S., & Chemat, F. (2022). Water as Green Solvent: Methods of Solubilisation and Extraction of Natural Products—Past, Present and Future Solutions. Pharmaceuticals, 15(12), 1507. https://doi.org/10.3390/ph15121507









