Can Essential Oils Provide an Alternative Adjuvant Therapy for COVID-19 Infections and Pain Management at the Same Time?
Abstract
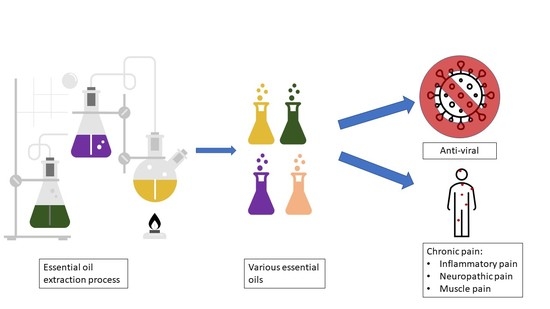
1. Introduction
2. In Silico Effectiveness of Essential Oil Constituents
3. The Pathway from In Silico to In Vivo
4. Essential Oils in Pain Management and Opioid Dependence
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryman, D. Aromatherapy: The Encyclopaedia of Plants and Oils and How They Help You; Piatkus Books: London, UK, 1991; ISBN 9780749911560. [Google Scholar]
- Silveira, D.; Prieto-Garcia, J.M.; Boylan, F.; Estrada, O.; Fonseca-Bazzo, Y.M.; Jamal, C.M.; Magalhães, P.O.; Pereira, E.O.; Tomczyk, M.; Heinrich, M. COVID-19: Is There Evidence for the Use of Herbal Medicines as Adjuvant Symptomatic Therapy? Front. Pharmacol. 2020, 11, 1479. [Google Scholar] [CrossRef] [PubMed]
- Grigore, A.; Cord, D.; Tanase, C.; Albulescu, R. Herbal medicine, a reliable support in COVID therapy. J. Immunoass. Immunochem. 2020, 41, 976–999. [Google Scholar] [CrossRef] [PubMed]
- Setzer, W.N. Essential oils and anxiolytic aromatherapy. Nat. Prod. Commun. 2009, 4, 1934578X0900400928. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kimura, T.; Hayashi, T. Aromatic effects of a Japanese citrus fruit-yuzu (Citrus junos Sieb. ex Tanaka)-on psychoemotional states and autonomic nervous system activity during the menstrual cycle: A single-blind randomized controlled crossover study. Biopsychosoc. Med. 2016, 10, 11. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Sheafer, H.; Tepper, D. The Effectiveness of Aromatherapy in Reducing Pain: A Systematic Review and Meta-Analysis. Pain Res. Treat. 2016, 2016, 8158693. [Google Scholar] [CrossRef]
- Farrar, A.J.; Farrar, F.C. Clinical Aromatherapy. Nurs. Clin. N. Am. 2020, 55, 489–504. [Google Scholar] [CrossRef]
- Damiescu, R.; Banerjee, M.; Lee, D.Y.W.; Paul, N.W.; Efferth, T. Health(care) in the Crisis: Reflections in Science and Society on Opioid Addiction. Int. J. Environ. Res. Public Health 2021, 18, 341. [Google Scholar] [CrossRef] [PubMed]
- Darke, S.; Larney, S.; Farrell, M. Yes, people can die from opiate withdrawal. Addiction 2017, 112, 199–200. [Google Scholar] [CrossRef]
- Kosten, T.R.; Baxter, L.E. Review article: Effective management of opioid withdrawal symptoms: A gateway to opioid dependence treatment. Am. J. Addict. 2019, 28, 55–62. [Google Scholar] [CrossRef]
- Srivastava, A.B.; Mariani, J.J.; Levin, F.R. New directions in the treatment of opioid withdrawal. Lancet 2020, 395, 1938–1948. [Google Scholar] [CrossRef]
- Valussi, M.; Antonelli, M.; Donelli, D.; Firenzuoli, F. Appropriate use of essential oils and their components in the management of upper respiratory tract symptoms in patients with COVID-19. J. Herb. Med. 2021, 28, 100451. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Hires, C.; Keenan, L.; Dunne, E. Aromatherapy blend of thyme, orange, clove bud, and frankincense boosts energy levels in post-COVID-19 female patients: A randomized, double-blinded, placebo controlled clinical trial. Complement. Ther. Med. 2022, 67, 102823. [Google Scholar] [CrossRef] [PubMed]
- Mahdood, B.; Imani, B.; Khazaei, S. Effects of Inhalation Aromatherapy with Rosa damascena (Damask Rose) on the State Anxiety and Sleep Quality of Operating Room Personnel During the COVID-19 Pandemic: A Randomized Controlled Trial. J. Perianesthesia Nurs. 2022, 37, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Ren, C.J.; Fielding, G.A.; Pitti, A.; Kasumi, T.; Wajda, M.; Lebovits, A.; Bekker, A. Treatment with lavender aromatherapy in the post-anesthesia care unit reduces opioid requirements of morbidly obese patients undergoing laparoscopic adjustable gastric banding. Obes. Surg. 2007, 17, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Dhany, A.L.; Mitchell, T.; Foy, C. Aromatherapy and massage intrapartum service impact on use of analgesia and anesthesia in women in labor: A retrospective case note analysis. J. Altern. Complement. Med. 2012, 18, 932–938. [Google Scholar] [CrossRef]
- Salamati, A.; Mashouf, S.; Sahbaei, F.; Mojab, F. Effects of inhalation of lavender essential oil on Open-Heart surgery pain. Iran. J. Pharm. Res. 2014, 13, 1257. [Google Scholar]
- Oberleitner, L.M.; Beitel, M.; Schottenfeld, R.S.; Kerns, R.D.; Doucette, C.; Napoleone, R.; Liong, C.; Barry, D.T. Drug Counselors’ Attitudes Toward Nonpharmacologic Treatments for Chronic Pain. J. Addict. Med. 2016, 10, 34. [Google Scholar] [CrossRef]
- Rombolà, L.; Amantea, D.; Russo, R.; Adornetto, A.; Berliocchi, L.; Tridico, L.; Corasaniti, M.; Sakurada, S.; Sakurada, T.; Bagetta, G.; et al. Rational Basis for the Use of Bergamot Essential Oil in Complementary Medicine to Treat Chronic Pain. Mini-Rev. Med. Chem. 2016, 16, 721–728. [Google Scholar] [CrossRef]
- Lee, G.; Park, J.; Kim, M.S.; Seol, G.H.; Min, S.S. Analgesic effects of eucalyptus essential oil in mice. Korean J. Pain 2019, 32, 79–86. [Google Scholar] [CrossRef]
- Donatello, N.N.; Emer, A.A.; Salm, D.C.; Ludtke, D.D.; Bordignon, S.A.S.R.; Ferreira, J.K.; Salgado, A.S.I.; Venzke, D.; Bretanha, L.C.; Micke, G.A.; et al. Lavandula angustifolia essential oil inhalation reduces mechanical hyperalgesia in a model of inflammatory and neuropathic pain: The involvement of opioid and cannabinoid receptors. J. Neuroimmunol. 2020, 340, 577145. [Google Scholar] [CrossRef]
- Santos, A.; Percy, M.; Rabinowitsch, D. Evaluating the Aromatherapy Recommendation for Pain in the Holistic Nurses’ Pain Relief Tools for Patients and Self-Care. J. Holist. Nurs. 2022, 40, 99–107. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.K.R.; Figueiredo, P.L.B.; Byler, K.G.; Setzer, W.N. Essential Oils as Antiviral Agents, Potential of Essential Oils to Treat SARS-CoV-2 Infection: An In-Silico Investigation. Int. J. Mol. Sci. 2020, 21, 3426. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Nagarajan, S.K.; Ramesh, V.; Palaniyandi, V.; Selvam, S.P.; Madhavan, T. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J. Mol. Struct. 2020, 1221, 128823. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; Laird, K.; Wilson, P.B. Structure-activity modelling of essential oils, their components, and key molecular parameters and descriptors. Mol. Cell. Probes 2018, 38, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, I.; Hassani, F.; Bekkel Brikci, S.; Ghalem, S. In silico study the inhibition of angiotensin converting enzyme 2 receptor of COVID-19 by Ammoides verticillata components harvested from Western Algeria. J. Biomol. Struct. Dyn. 2020, 39, 3263–3276. [Google Scholar] [CrossRef]
- Catella, C.; Camero, M.; Lucente, M.S.; Fracchiolla, G.; Sblano, S.; Tempesta, M.; Martella, V.; Buonavoglia, C.; Lanave, G. Virucidal and antiviral effects of Thymus vulgaris essential oil on feline coronavirus. Res. Vet. Sci. 2021, 137, 44–47. [Google Scholar] [CrossRef]
- Rouf, R.; Uddin, S.J.; Sarker, D.K.; Islam, M.T.; Ali, E.S.; Shilpi, J.A.; Nahar, L.; Tiralongo, E.; Sarker, S.D. Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 2020, 104, 219–234. [Google Scholar] [CrossRef]
- Thuy, B.T.P.; My, T.T.A.; Hai, N.T.T.; Hieu, L.T.; Hoa, T.T.; Thi Phuong Loan, H.; Triet, N.T.; Anh, T.T.V.; Quy, P.T.; Van Tat, P.; et al. Investigation into SARS-CoV-2 Resistance of Compounds in Garlic Essential Oil. ACS Omega 2020, 5, 8312–8320. [Google Scholar] [CrossRef]
- My, T.T.A.; Loan, H.T.P.; Hai, N.T.T.; Hieu, L.T.; Hoa, T.T.; Thuy, B.T.P.; Quang, D.T.; Triet, N.T.; Anh, T.T.V.; Dieu, N.T.X.; et al. Evaluation of the Inhibitory Activities of COVID-19 of Melaleuca cajuputi Oil Using Docking Simulation. ChemistrySelect 2020, 5, 6312–6320. [Google Scholar] [CrossRef]
- Amparo, T.R.; Seibert, J.B.; Silveira, B.M.; Costa, F.S.F.; Almeida, T.C.; Braga, S.F.P.; da Silva, G.N.; dos Santos, O.D.H.; de Souza, G.H.B. Brazilian essential oils as source for the discovery of new anti-COVID-19 drug: A review guided by in silico study. Phytochem. Rev. 2021, 20, 1013–1032. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.N.; de Sá, É.R.A.; Bezerra, R.D.S.; Souza, J.L.; Lima, F.D.C.A. Constituents of buriti oil (Mauritia flexuosa L.) like inhibitors of the SARS-Coronavirus main peptidase: An investigation by docking and molecular dynamics. J. Biomol. Struct. Dyn. 2020, 39, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, C.; Pieraccini, G.; Santilli, C.; Tani, C.; Bottoni, M.; Schiff, S.; Fico, G.; Papini, A.; Falsini, S. Anatomical Investigation and GC/MS Analysis of ‘Coco de Mer’, Lodoicea maldivica (Arecaceae). Chem. Biodivers. 2020, 17, e2000707. [Google Scholar] [CrossRef] [PubMed]
- Ulasli, M.; Gurses, S.A.; Bayraktar, R.; Yumrutas, O.; Oztuzcu, S.; Igci, M.; Igci, Y.Z.; Cakmak, E.A.; Arslan, A. The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family. Mol. Biol. Rep. 2014, 41, 1703–1711. [Google Scholar] [CrossRef]
- Islam, M.N.; Hossain, K.S.; Sarker, P.P.; Ferdous, J.; Hannan, M.A.; Rahman, M.M.; Chu, D.; Uddin, M.J. Revisiting pharmacological potentials of Nigella sativa seed: A promising option for COVID-19 prevention and cure. Phyther. Res. 2020, 35, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Koshak, A.E.; Koshak, E.A.; Mobeireek, A.F.; Badawi, M.A.; Wali, S.O.; Malibary, H.M.; Atwah, A.F.; Alhamdan, M.M.; Almalki, R.A.; Madani, T.A. Nigella sativa for the treatment of COVID-19: An open-label randomized controlled clinical trial. Complement. Ther. Med. 2021, 61, 102769. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical Analysis andin vitro Antiviral Activities of the Essential Oils of Seven Lebanon Species. Chem. Biodivers. 2008, 5, 461–470. [Google Scholar] [CrossRef]
- Schnitzler, P.; Reichling, J. Wirksamkeit von Pflanzenprodukten gegen Herpesinfektionen. HNO 2011, 59, 1176–1184. [Google Scholar] [CrossRef]
- Wilkin, P.J.; Al-Yozbaki, M.; George, A.; Gupta, G.K.; Wilson, C.M. The Undiscovered Potential of Essential Oils for Treating SARS-CoV-2 (COVID-19). Curr. Pharm. Des. 2020, 26, 5261–5277. [Google Scholar] [CrossRef]
- Melegari, G.; Iseppi, R.; Mariani, M.; Giuliani, E.; Caciagli, V.; Bertellini, E.; Messi, P.; Barbieri, A. Keyboard Contamination in Intensive Care Unit: Is Cleaning Enough? Prospective Research of In Situ Effectiveness of a Tea Tree Oil (KTEO) Film. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1323, pp. 91–102. [Google Scholar]
- Senthil Kumar, K.J.; Vani, M.G.; Wang, C.S.; Chen, C.C.; Chen, Y.C.; Lu, L.P.; Huang, C.H.; Lai, C.S.; Wang, S.Y. Geranium and lemon essential oils and their active compounds downregulate angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 spike receptor-binding domain, in epithelial cells. Plants 2020, 9, 770. [Google Scholar] [CrossRef]
- Yadalam, P.K.; Varatharajan, K.; Rajapandian, K.; Chopra, P.; Arumuganainar, D.; Nagarathnam, T.; Sohn, H.; Madhavan, T. Antiviral Essential Oil Components Against SARS-CoV-2 in Pre-procedural Mouth Rinses for Dental Settings During COVID-19: A Computational Study. Front. Chem. 2021, 9, 642026. [Google Scholar] [CrossRef] [PubMed]
- Stathis, C.; Victoria, N.; Loomis, K.; Nguyen, S.A.; Eggers, M.; Septimus, E.; Safdar, N. Review of the use of nasal and oral antiseptics during a global pandemic. Future Microbiol. 2021, 16, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Clarkson, J.E.; Goulao, B.; Glenny, A.-M.; McBain, A.J.; Schilder, A.G.M.; Webster, K.E.; Worthington, H.V. Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them. Cochrane Database Syst. Rev. 2020, 9, CD013627. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Oliveira, G.F.; Melo, M.S.; Cavalcanti, S.C.H.; Antoniolli, A.R.; Bonjardim, L.R.; Silva, F.A.; Santos, J.P.A.; Rocha, R.F.; Moreira, J.C.F.; et al. Bioassay-guided Evaluation of Antioxidant and Antinociceptive Activities of Carvacrol. Basic Clin. Pharmacol. Toxicol. 2010, 107, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante Melo, F.H.; Rios, E.R.V.; Rocha, N.F.M.; Citó, M.d.C.d.O.; Fernandes, M.L.; de Sousa, D.P.; de Vasconcelos, S.M.M.; de Sousa, F.C.F. Antinociceptive activity of carvacrol (5-isopropyl-2-methylphenol) in mice. J. Pharm. Pharmacol. 2012, 64, 1722–1729. [Google Scholar] [CrossRef]
- Melo, F.H.C.; Venâncio, E.T.; De Sousa, D.P.; De França Fonteles, M.M.; De Vasconcelos, S.M.M.; Viana, G.S.B.; De Sousa, F.C.F. Anxiolytic-like effect of Carvacrol (5-isopropyl-2-methylphenol) in mice: Involvement with GABAergic transmission. Fundam. Clin. Pharmacol. 2009, 24, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Naeem, K.; Tariq Al Kury, L.; Nasar, F.; Alattar, A.; Alshaman, R.; Shah, F.A.; Khan, A.; Li, S. Natural Dietary Supplement, Carvacrol, Alleviates LPS-Induced Oxidative Stress, Neurodegeneration, and Depressive-Like Behaviors via the Nrf2/HO-1 Pathway. J. Inflamm. Res. 2021, 14, 1313–1329. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Schrott, L.M.; Snelling, S.; Ndi, J.; Arnold, T.; Korneeva, N.L. Chronic oxycodone induces integrated stress response in rat brain. BMC Neurosci. 2015, 16, 58. [Google Scholar] [CrossRef]
- Fan, R.; Schrott, L.M.; Arnold, T.; Snelling, S.; Rao, M.; Graham, D.; Cornelius, A.; Korneeva, N.L. Chronic oxycodone induces axonal degeneration in rat brain. BMC Neurosci. 2018, 19, 15. [Google Scholar] [CrossRef]
- Motaghinejad, M.; Karimian, S.M.; Motaghinejad, O.; Shabab, B.; Asadighaleni, M.; Fatima, S. The effect of various morphine weaning regimens on the sequelae of opioid tolerance involving physical dependency, anxiety and hippocampus cell neurodegeneration in rats. Fundam. Clin. Pharmacol. 2015, 29, 299–309. [Google Scholar] [CrossRef]
- Nesterkina, M.; Kravchenko, I. Synthesis and Pharmacological Properties of Novel Esters Based on Monocyclic Terpenes and GABA. Pharmaceuticals 2016, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Salmalian, H.; Saghebi, R.; Moghadamnia, A.A.; Bijani, A.; Faramarzi, M.; Nasiri Amiri, F.; Bakouei, F.; Behmanesh, F.; Bekhradi, R. Comparative effect of thymus vulgaris and ibuprofen on primary dysmenorrhea: A triple-blind clinical study. Casp. J. Intern. Med. 2014, 5, 82–88. [Google Scholar]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Azimullah, S.; Meeran, M.; Ansari, S.; Ojha, S. Neuroprotective Effects of Thymol, a Dietary Monoterpene Against Dopaminergic Neurodegeneration in Rotenone-Induced Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 1538. [Google Scholar] [CrossRef]
- Asadbegi, M.; Komaki, A.; Salehi, I.; Yaghmaei, P.; Ebrahim-Habibi, A.; Shahidi, S.; Sarihi, A.; Soleimani Asl, S.; Golipoor, Z. Effects of thymol on amyloid-β-induced impairments in hippocampal synaptic plasticity in rats fed a high-fat diet. Brain Res. Bull. 2018, 137, 338–350. [Google Scholar] [CrossRef]
- FangFang; Li, H.; Qin, T.; Li, M.; Ma, S. Thymol improves high-fat diet-induced cognitive deficits in mice via ameliorating brain insulin resistance and upregulating NRF2/HO-1 pathway. Metab. Brain Dis. 2017, 32, 385–393. [Google Scholar] [CrossRef]
- da Fonsêca, D.V.; da Silva Maia Bezerra Filho, C.; Lima, T.C.; de Almeida, R.N.; de Sousa, D.P. Anticonvulsant Essential Oils and Their Relationship with Oxidative Stress in Epilepsy. Biomolecules 2019, 9, 835. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phyther. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Duncker, S.C.; Philippe, D.; Martin-Paschoud, C.; Moser, M.; Mercenier, A.; Nutten, S. Nigella sativa (Black Cumin) seed extract alleviates symptoms of allergic diarrhea in mice, involving opioid receptors. PLoS ONE 2012, 7, e39841. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Ghannadi, A.; Jafarabadi, H. Black cumin seed essential oil, as a potent analgesic and antiinflammatory drug. Phyther. Res. 2004, 18, 195–199. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.-F.M.; Matsumoto, K.; Watanabe, H. Antinociceptive effects of Nigella sativa oil and its major component, thymoquinone, in mice. Eur. J. Pharmacol. 2000, 400, 89–97. [Google Scholar] [CrossRef]
- Sangi, S.; Ahmed, S.P.; Channa, M.A.; Ashfaq, M.; Mastoi, S.M. A new and novel treatment of opioid dependence: Nigella sativa 500 mg. J. Ayub Med. Coll. Abbottabad 2008, 20, 118–124. [Google Scholar] [PubMed]
- Abdel-Zaher, A.O.; Mostafa, M.G.; Farghly, H.M.; Hamdy, M.M.; Omran, G.A.; Al-Shaibani, N.K.M. Inhibition of brain oxidative stress and inducible nitric oxide synthase expression by thymoquinone attenuates the development of morphine tolerance and dependence in mice. Eur. J. Pharmacol. 2013, 702, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Zaher, A.O.; Abdel-Rahman, M.S.; ELwasei, F.M. Blockade of Nitric Oxide Overproduction and Oxidative Stress by Nigella sativa Oil Attenuates Morphine-Induced Tolerance and Dependence in Mice. Neurochem. Res. 2010, 35, 1557–1565. [Google Scholar] [CrossRef]
- Abdel-Zaher, A.O.; Abdel-Rahman, M.S.; ELwasei, F.M. Protective effect of Nigella sativa oil against tramadol-induced tolerance and dependence in mice: Role of nitric oxide and oxidative stress. Neurotoxicology 2011, 32, 725–733. [Google Scholar] [CrossRef]
- Elkhateeb, A.; El Khishin, I.; Megahed, O.; Mazen, F. Effect of Nigella sativa Linn oil on tramadol-induced hepato- and nephrotoxicity in adult male albino rats. Toxicol. Reports 2015, 2, 512–519. [Google Scholar] [CrossRef]
- Adnan, L.H.M.; Mohamad, N.; Mat, K.C.; Abu Bakar, N.H.; Hashim, S.N.; Shariff, M.H.M.; Mansor, M.I. Attenuation of morphine-induced camp overshoot by thymoquinone in opioid receptor expressing cells (U87 MG) mediated by chronic morphine treatment. J. Eng. Appl. Sci. 2018, 13, 8906–8911. [Google Scholar] [CrossRef]
- Omar, N. Nigella sativa oil alleviates ultrastructural alterations induced by tramadol in rat motor cerebral cortex. J. Microsc. Ultrastruct. 2016, 4, 76–84. [Google Scholar] [CrossRef]
- Ullah, I.; Badshah, H.; Naseer, M.I.; Lee, H.Y.; Kim, M.O. Thymoquinone and Vitamin C Attenuates Pentylenetetrazole-Induced Seizures Via Activation of GABAB1 Receptor in Adult Rats Cortex and Hippocampus. Neuromol. Med. 2015, 17, 35–46. [Google Scholar] [CrossRef]
- Meral, I.; Esrefoglu, M.; Dar, K.; Ustunova, S.; Aydin, M.; Demirtas, M.; Arifoglu, Y. Effects of Nigella sativa on apoptosis and GABA A receptor density in cerebral cortical and hippocampal neurons in pentylenetetrazol induced kindling in rats. Biotech. Histochem. 2016, 91, 493–500. [Google Scholar] [CrossRef]
- Gilani, A.H.; Aziz, N.; Khurram, I.M.; Chaudhary, K.S.; Iqbal, A. Bronchodilator, spasmolytic and calcium antagonist activities of Nigella sativa seeds (Kalonji): A traditional herbal product with multiple medicinal uses. J. Pak. Med. Assoc. 2001, 51, 115. [Google Scholar] [PubMed]
- Adnan, L.H.M.; Abu Bakar, N.H.; Mohamad, N. Opioid dependence and substitution therapy: Thymoquinone as potential novel supplement therapy for better outcome for methadone maintenance therapy substitution therapy. Iran. J. Basic Med. Sci. 2014, 17, 926. [Google Scholar] [CrossRef] [PubMed]
- Liapi, C.; Anifantis, G.; Chinou, I.; Kourounakis, A.P.; Theodosopoulos, S.; Galanopoulou, P. Antinociceptive properties of 1,8-cineole and β- pinene, from the essential oil of Eucalyptus camaldu lensis leaves, in rodents. Planta Med. 2007, 73, 1247–1254. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Nasir, H.M.; Ahmad, A.; Setapar, S.H.M.; Ahmad, H.; Noor, M.H.M.; Rafatullah, M.; Khatoon, A.; Kausar, M.A.; Ahmad, I.; et al. Enrichment of Eucalyptus oil nanoemulsion by micellar nanotechnology: Transdermal analgesic activity using hot plate test in rats’ assay. Sci. Rep. 2019, 9, 13678. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.S.; Kang, P.; Min, S.S.; Lee, J.M.; Kim, H.K.; Seol, G.H. Effect of eucalyptus oil inhalation on pain and inflammatory responses after total knee replacement: A randomized clinical trial. Evid.-Based Complement Altern. Med. 2013, 2013, 502727. [Google Scholar] [CrossRef] [PubMed]
- Sahraei, H.; Ghoshooni, H.; Hossein Salimi, S.; Mohseni Astani, A.; Shafaghi, B.; Falahi, M.; Kamalnegad, M. The effects of fruit essential oil of the Pimpinella anisum on acquisition and expression of morphine induced conditioned place preference in mice. J. Ethnopharmacol. 2002, 80, 43–47. [Google Scholar] [CrossRef]
- Darvishzadeh-Mahani, F.; Esmaeili-Mahani, S.; Komeili, G.; Sheibani, V.; Zare, L. Ginger (Zingiber officinale Roscoe) prevents the development of morphine analgesic tolerance and physical dependence in rats. J. Ethnopharmacol. 2012, 141, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Torkzadeh-Mahani, S.; Nasri, S.; Esmaeili-Mahani, S. Ginger (zingiber officinale roscoe) prevents morphine-induced addictive behaviors in conditioned place preference test in rats. Addict. Health 2014, 6, 65. [Google Scholar]
- Sepahvand, R.; Esmaeili-Mahani, S.; Arzi, A.; Rasoulian, B.; Abbasnejad, M. Ginger (Zingiber officinale Roscoe) elicits antinociceptive properties and potentiates morphine-induced analgesia in the rat radiant heat tail-flick test. J. Med. Food 2010, 13, 1397–1401. [Google Scholar] [CrossRef]
- Torkzadeh-Mahani, S.; Esmaeili-Mahani, S.; Nasri, S.; Darvishzadeh, F.; Naderi, R. Ginger Extract Reduces Chronic Morphine-Induced Neuroinflammation and Glial Activation in Nucleus Accumbens of Rats. Addict. Health 2019, 11, 66. [Google Scholar] [CrossRef]
- Yip, Y.B.; Tam, A.C.Y. An experimental study on the effectiveness of massage with aromatic ginger and orange essential oil for moderate-to-severe knee pain among the elderly in Hong Kong. Complement. Ther. Med. 2008, 16, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Riva, A.; Morazzoni, P.; Allegrini, P.; Faliva, M.A.; Naso, M.; Miccono, A.; Peroni, G.; Degli Agosti, I.; Perna, S. The effect and safety of highly standardized Ginger (Zingiber officinale) and Echinacea (Echinacea angustifolia) extract supplementation on inflammation and chronic pain in NSAIDs poor responders. A pilot study in subjects with knee arthrosis. Nat. Prod. Res. 2017, 31, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Grabnar, M.; Roach, M.J.; Abd-Elsayed, A.; Kim, C. Impact of Lavender on Pain and Anxiety Levels Associated with Spine Procedures. Ochsner J. 2021, 21, 358–363. [Google Scholar] [CrossRef]
- Franklyne, J.S.; Gopinath, P.M.; Mukherjee, A.; Chandrasekaran, N. Nanoemulsions: The rising star of antiviral therapeutics and nanodelivery system—current status and prospects. Curr. Opin. Colloid Interface Sci. 2021, 54, 101458. [Google Scholar] [CrossRef] [PubMed]
- Van Der Watt, G.; Laugharne, J.; Janca, A. Complementary and alternative medicine in the treatment of anxiety and depression. Curr. Opin. Psychiatry 2008, 21, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.F.; Chung, K.F.; Ng, K.Y.; Yu, Y.M.; Zhang, S.P.; Ng, B.F.L.; Ziea, E.T.C. Prescription of Chinese Herbal Medicine in Pattern-Based Traditional Chinese Medicine Treatment for Depression: A Systematic Review. Evid.-Based Complement Altern. Med. 2015, 2015, 160189. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.S.; Seo, K.H.; Lee, S.H.; Jang, J.E.; Jung, Y.M.; Kim, M.J.; Yeon, J.Y. Massage with or without aromatherapy for symptom relief in people with cancer. Cochrane Database Syst. Rev. 2016, 2016, CD009873. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Lan, S.H.; Yen, Y.Y.; Hsieh, Y.P.; Lan, S.J. Aromatherapy intervention on anxiety and pain during first stage labour in nulliparous women: A systematic review and meta-analysis. J. Obstet. Gynaecol. 2020, 41, 21–31. [Google Scholar] [CrossRef]
| Essential Oils | Active Compounds | Preclinical Trials | Clinical Trials |
|---|---|---|---|
| Nigella sativa L. | thymoquinone, nigellone, -hederin, carvacrol, α- and β-pinene, thymol |
|
|
| Thymus vulgaris L. | thymol, carvacrol p-cymene, γ-terpinene, β-linalool |
|
|
| Eucalyptus globus Labill. | 1,8-cineol, α-pinene, jensenone |
|
|
| Zingiber officinale Roscoe | Gingerol, shogaols, paradols, zingerone |
|
|
| Melaleuca alternifolia | α-terpineol, terpinene-4-ol, terpinolene |
| |
| Lavandula angustifolia Mill. | linalool, linalyl acetate, 1,8-cineole, β -ocimene, 88 terpinen-4-ol, and camphor |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damiescu, R.; Lee, D.Y.W.; Efferth, T. Can Essential Oils Provide an Alternative Adjuvant Therapy for COVID-19 Infections and Pain Management at the Same Time? Pharmaceuticals 2022, 15, 1387. https://doi.org/10.3390/ph15111387
Damiescu R, Lee DYW, Efferth T. Can Essential Oils Provide an Alternative Adjuvant Therapy for COVID-19 Infections and Pain Management at the Same Time? Pharmaceuticals. 2022; 15(11):1387. https://doi.org/10.3390/ph15111387
Chicago/Turabian StyleDamiescu, Roxana, David Y. W. Lee, and Thomas Efferth. 2022. "Can Essential Oils Provide an Alternative Adjuvant Therapy for COVID-19 Infections and Pain Management at the Same Time?" Pharmaceuticals 15, no. 11: 1387. https://doi.org/10.3390/ph15111387
APA StyleDamiescu, R., Lee, D. Y. W., & Efferth, T. (2022). Can Essential Oils Provide an Alternative Adjuvant Therapy for COVID-19 Infections and Pain Management at the Same Time? Pharmaceuticals, 15(11), 1387. https://doi.org/10.3390/ph15111387