Recent Developments in Electrochemical Sensors for the Detection of Antibiotic-Resistant Bacteria
Abstract
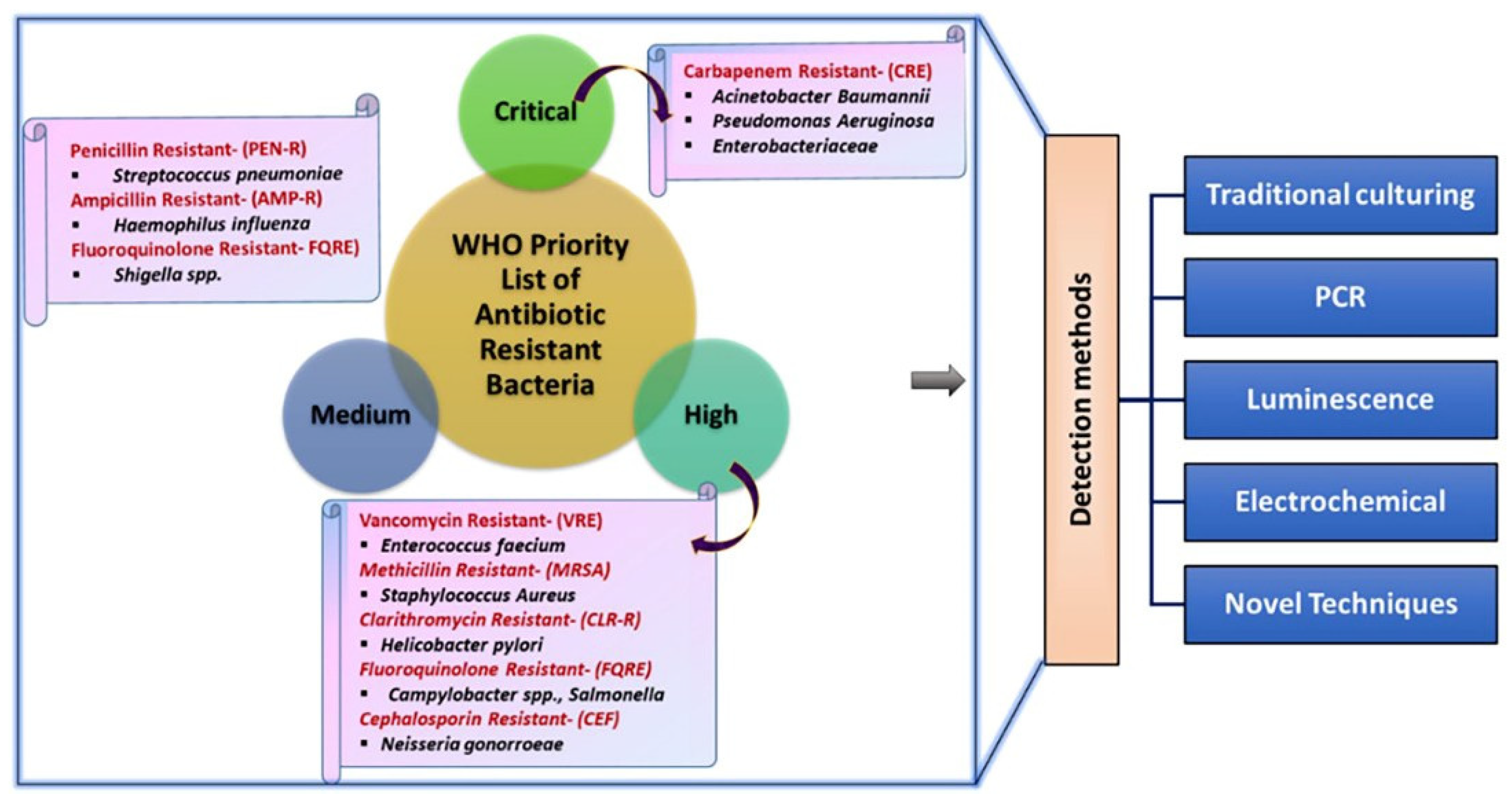
1. Introduction
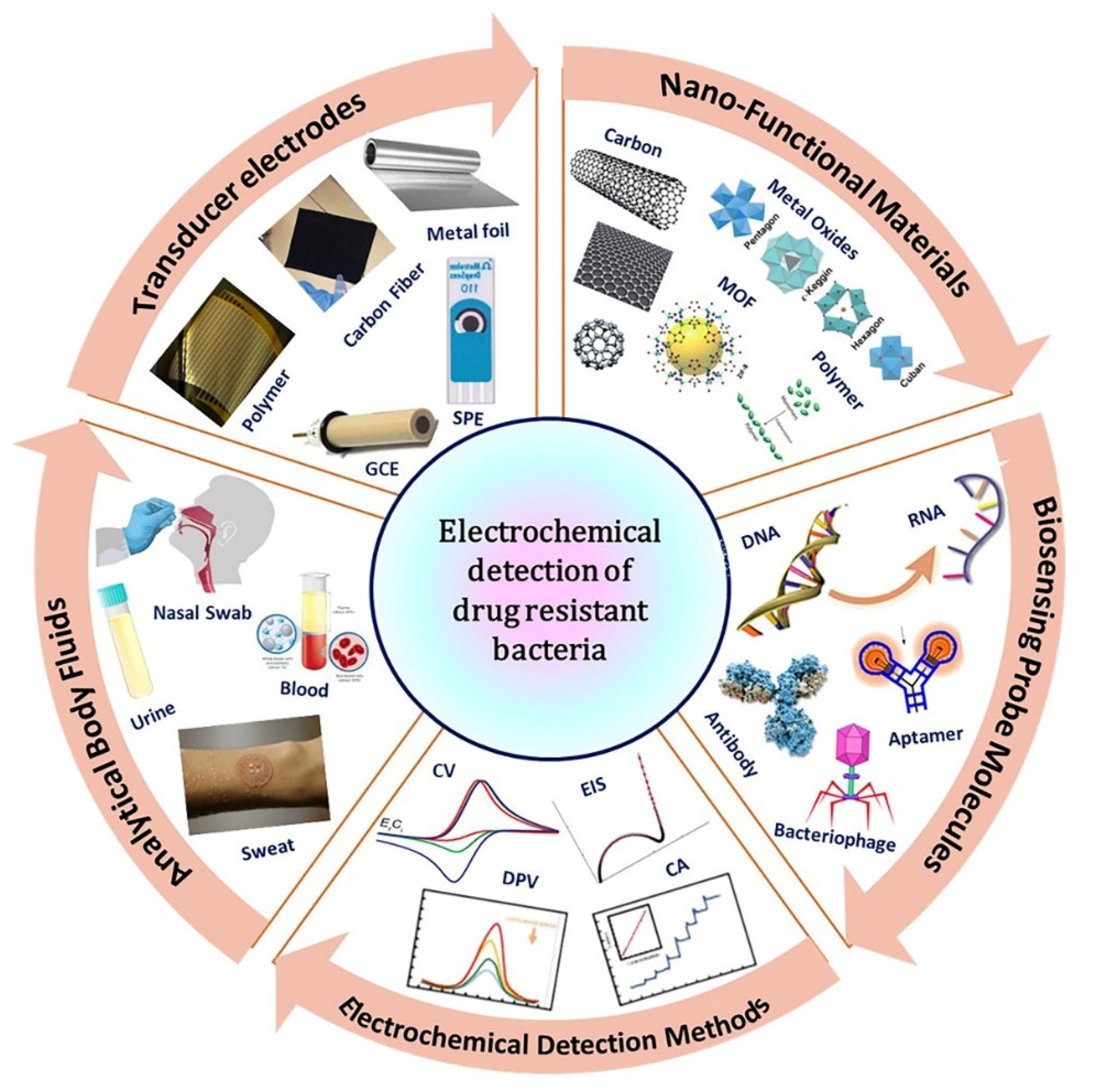
2. The Basic Principle of Electrochemical Sensors for The Detection of Antibiotic-Resistant Bacteria
2.1. Recognition Elements
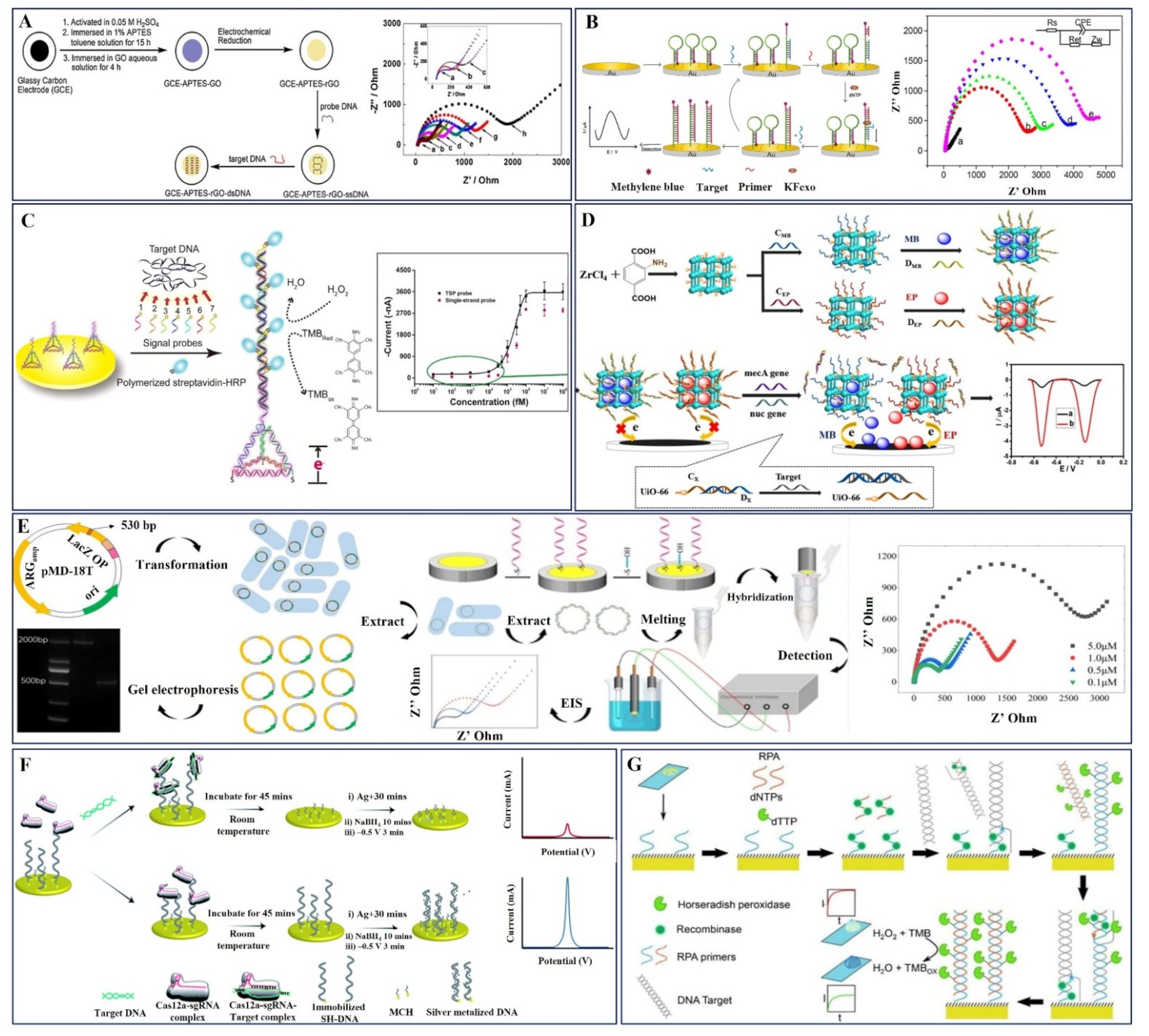
2.2. DNA-Based Electrochemical Sensors
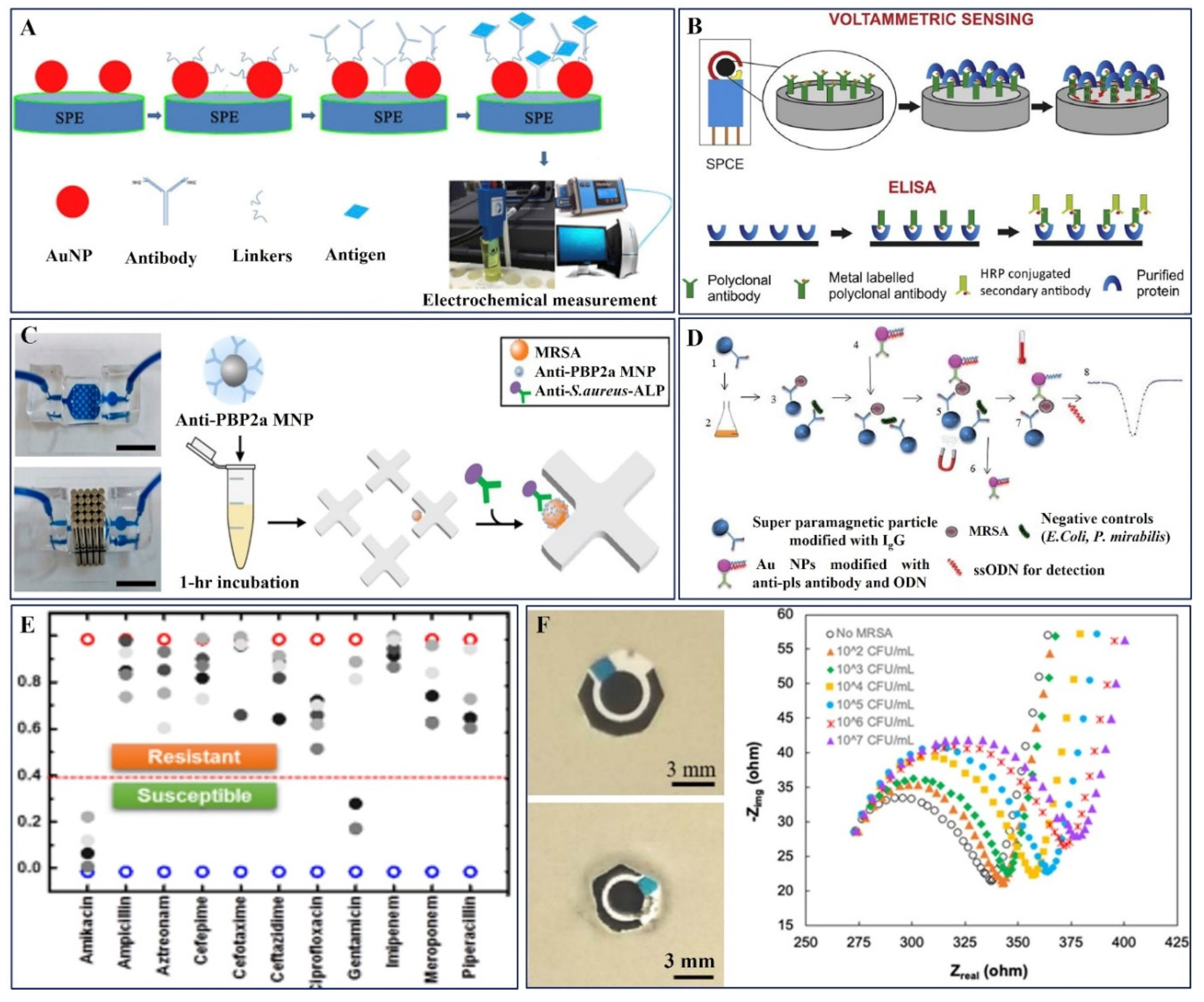
2.3. Antibody-Based Electrochemical Sensors
2.4. Aptamer-Based Electrochemical Sensors
2.5. Bacteriophages-Based Electrochemical Sensors
3. Electrochemical Sensors for Resistant Bacterial Detection
3.1. Nanofunctional Materials-Based Sensor Platform
3.1.1. AST-DNA Probe-Based Electrochemical Sensors
3.1.2. AST-Antibody Probe-Based Electrochemical Sensors
3.1.3. AST-Aptamer Probe-Based Electrochemical Sensors
3.1.4. AST-Bacteriophages Probe-Based Electrochemical Sensors
3.2. Bimodal Action of Nanomaterials-Electrochemical Sensor and Antibiotics
| S. No | Working Electrode | Antibiotic | Target Bacteria | Probe | Electro Chemical Method | Detection Range | LOD | Interference | Body Fluid | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
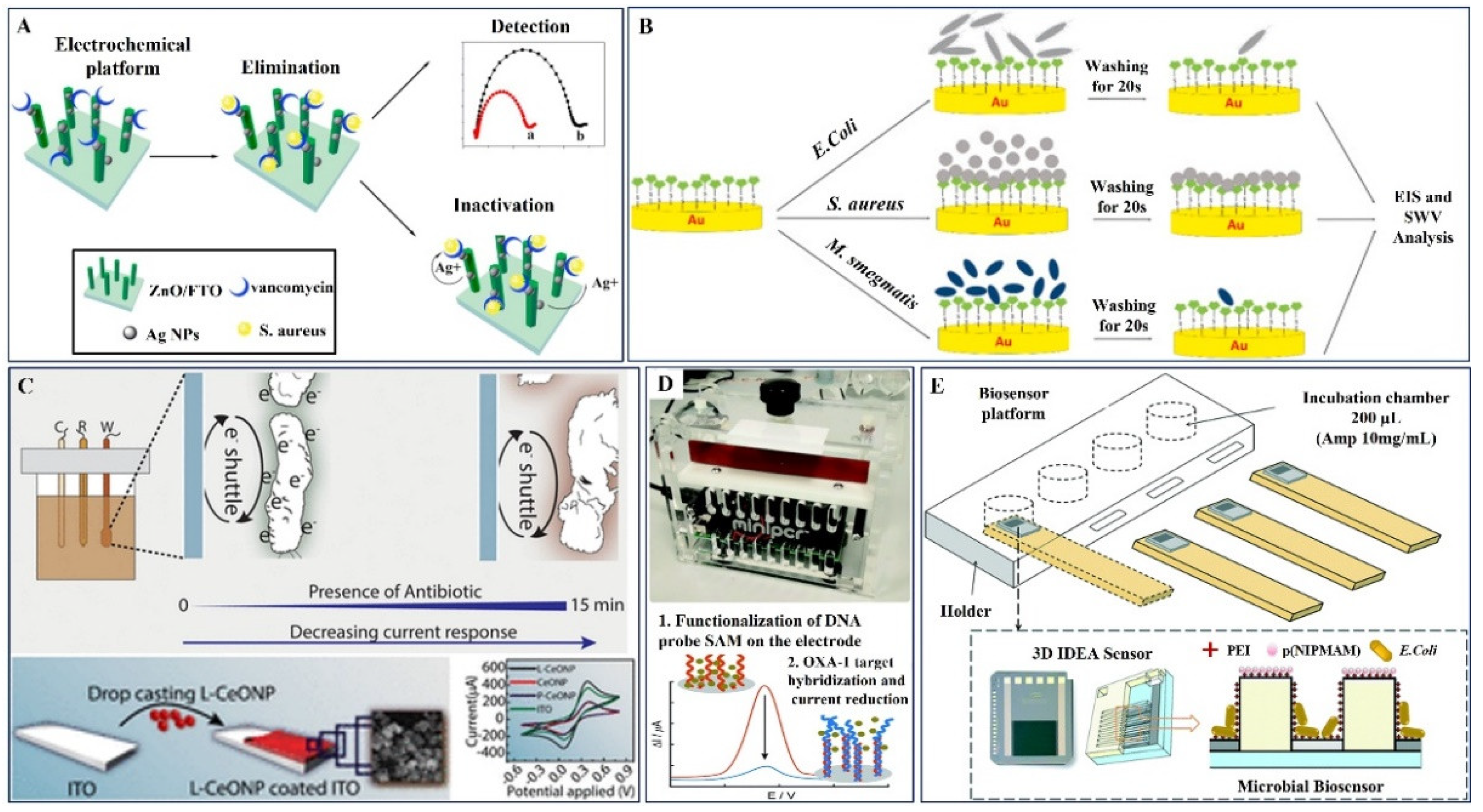
| 1. | AgNPs/3D-ZnO Check | Vancomycin | S. aureus | Van | EIS | 1000–2000 CFU/mL | 330 CFU/mL | E. coli | -- | [97] |
| 2. | Thiolated vancomycin/SPGE | Vancomycin | S. aureus | HS-Van | EIS | 101 to 108 CFU/mL | <39 CFU/mL | -- | -- | [98] |
| 3. | L-lysine coated CeO/ITO (EAST) | Ciprofloxacin, Cefixime, Amoxycillin | E. coli and B. Sutbilis | -- | CV | 0.001×106–10 ×106 CFU/mL for E. coli and 250 ×1012–280 ×1012 CFU/mL for B. Sutbilis | -- | -- | -- | [100] |
| 4. | Polycrystalline gold electrode | Oxacillin | OXA-1 DNA | Complementary OXA-1 DNA | DPV | -- | DNA from the TetA gene | -- | [101] | |
| 5. | E. coli Bacteria/PEI/p(NIPMAM/PDMS microgel (3D-IDEA) | Ampicillin | E. coli | PEI/p(NIPMAM/PDMS microgel | EIS | 2–8 mg/L | 2 mg/L | ampicillin resistant and non-resistant E. coli | -- | [102] |
| 6. | Agarose gel modified Au electrode | Amoxicillin, Oxacillin | S. aureus, MRSA | -- | EIS and DPV | 8 μg/mL and 50 μg/mL | -- | -- | -- | [103] |
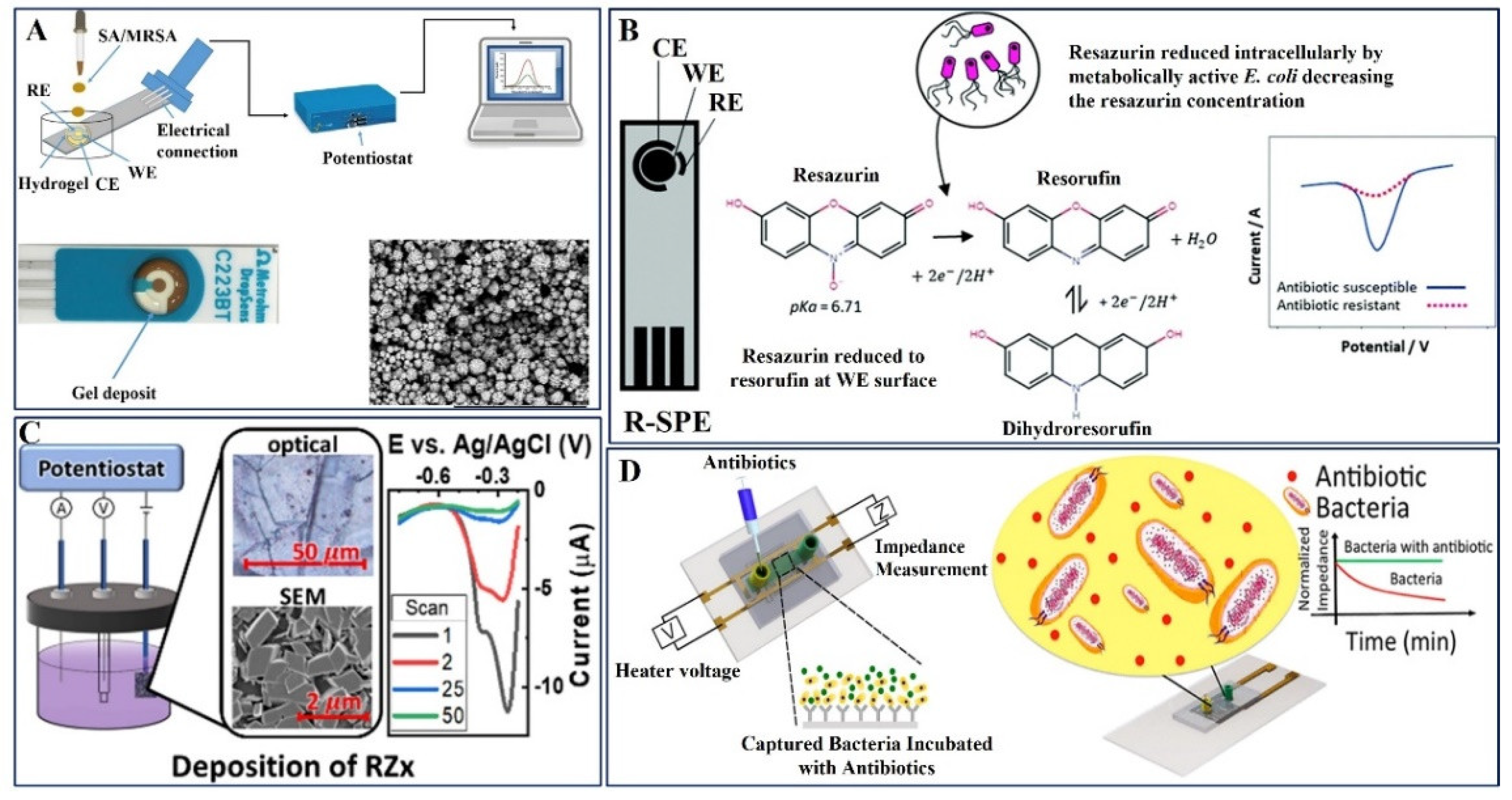
| 7. | Resazurin-modified graphite SPE | Gentamicin | E. coli | Resazurin | DPV | 0–1000 μM | 15.6 μM | -- | Artificial Urine | [104] |
| 8. | Nafion coated RZx on graphite sheets | Ampicillin, Kanamycin | E. coli | -- | DPV | 0.001–10 μM | 16 μg/mL | -- | Whole blood, Milk | [105] |
| 9. | Silver interdigitated carbon working electrode | Ampicillin, ciprofloxacin and Erythromycin, Daptomycin, Gentamicin, Methicillin | E. coli and MRSA | Label free | Normalized EIS | 0.1 μM–100 μM | 0.1 μM | single-base, double-base, and three-base mismatch DNA | Whole blood, Human urine | [106] |
| 10. | Au electrode | Rifampicin | Mycobacterium tuberculosis | Solid-phase isothermal primer | SWV | 6 μM–140 μM | 6 μM | A mixture of the four dNFcTP | -- | [107] |
| 11. | SDNA1-SbT@SiO2NSs complex and Sb2S3/ZnS/ITO | Penicillin | bla-CTX-M-1 and bla-TEM | DNA | Photo electrochemistry | 1 nM to 10 μM | 1 nM | Acidic pH for bla-CTX-M-1 and alkaline pH for bla-TEM | Plasmid | [108] |
| 12. | Au electrode/glass substrate | Gentamicin | E. coli, S. aureus | Aptamer | Capacitance | 0–50 μg/mL | - | Aptamer of A. baumannii and E. faecalis | -- | [109] |
| 13. | GCE | Ofloxacin, Penicillin, Cefepime | E. coli | DPV | 1 × 105 CFU–5 × 107 CFU/mL | 10 CFU/mL | -- | -- | [110] | |
| 14. | Miniature incubation chamber WE | Ampicillin, Ciprofloxacin | E. coli, Klebsiella nueumoniae | DPV | 1–1000 CFU/mL | 1 CFU/mL | -- | Human Urine | [111] | |
| 15. | Pt/Ti/Glass | Ampicillin, Kanamycin, tetracycline | E. coli, Klebsiella nueumoniae | DPV | 0–0.9 mM | 0.12 mM | -- | -- | [112] | |
| 16. | SPE | Methicillin | mecA DNA from MRSA | mec A1 and mec A2 Primer | CV | 3 × 104–3 × 106 CFU/mL | - | NTC negative control | Nasal swab | [113] |
4. Summary and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burnham, C.A.D.; Leeds, J.; Nordmann, P.; O’Grady, J.; Patel, J. Diagnosing Antimicrobial Resistance. Nat. Rev. Microbiol. 2017, 15, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Brennan-Krohn, T.; Smith, K.P.; Kirby, J.E. The Poisoned Well: Enhancing the Predictive Value of Antimicrobial Susceptibility Testing in the Era of Multidrug Resistance. J. Clin. Microbiol. 2017, 55, 2304–2308. [Google Scholar] [CrossRef] [PubMed]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Alzahrani, A.J.; Tobaiqy, M.; Alresasi, A.M.; Bu-Shehab, I.; Al-Hadary, I.; Alhmeed, N.; Alismail, M.; et al. Antimicrobial Susceptibility of Gram-Positive and Gram-Negative Bacteria: A 5-Year Retrospective Analysis at a Multi-Hospital Healthcare System in Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 43. [Google Scholar] [CrossRef]
- Zhang, Q.; Plummer, P.J. Mechanisms of Antibiotic Resistance in Campylobacter. Campylobacter 2014, 263–276. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, S.; Shrestha, P.; Adhikari, B. Antimicrobial Use in Food Animals and Human Health: Time to Implement ‘One Health’ Approach. Antimicrob. Resist. Infect. Control 2020, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Kaprou, G.D.; Bergšpica, I.; Alexa, E.A.; Alvarez-Ordóñez, A.; Prieto, M. Rapid Methods for Antimicrobial Resistance Diagnostics. Antibiotics 2021, 10, 209. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, S. Electrochemical Sensors for Antibiotic Susceptibility Testing: Strategies and Applications. Chemosensors 2022, 10, 53. [Google Scholar] [CrossRef]
- Jean, S.S.; Harnod, D.; Hsueh, P.R. Global Threat of Carbapenem-Resistant Gram-Negative Bacteria. Front. Cell. Infect. Microbiol. 2022, 12, 823684. [Google Scholar] [CrossRef]
- Jubeh, B.; Breijyeh, Z.; Karaman, R. Resistance of Gram-Positive Bacteria to Current Antibacterial Agents and Overcoming Approaches. Molecules 2020, 25, 2888. [Google Scholar] [CrossRef]
- Vasala, A.; Hytönen, V.P.; Laitinen, O.H. Modern Tools for Rapid Diagnostics of Antimicrobial Resistance. Front. Cell. Infect. Microbiol. 2020, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef]
- Song, D.; Lei, Y. Mini-Review: Recent Advances in Imaging-Based Rapid Antibiotic Susceptibility Testing. Sens. Actuators Rep. 2021, 3, 100053. [Google Scholar] [CrossRef]
- Sekyere, J.O.; Asante, J. Emerging Mechanisms of Antimicrobial Resistance in Bacteria and Fungi: Advances in the Era of Genomics. Future Microbiol. 2018, 13, 241–262. [Google Scholar] [CrossRef]
- Charnock, C.; Samuelsen, Ø.; Nordlie, A.L.; Hjeltnes, B. Use of a Commercially Available Microarray to Characterize Antibiotic-Resistant Clinical Isolates of Klebsiella Pneumoniae. Curr. Microbiol. 2018, 75, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, K.-M. Bond-Dissociation Energies of Cations—Pushing the limits to quantum state resolution. Data Mass Spectrom. Rev. 2011, 30, 221–235. [Google Scholar] [CrossRef]
- Leonard, H.; Colodner, R.; Halachmi, S.; Segal, E. Recent Advances in the Race to Design a Rapid Diagnostic Test for Antimicrobial Resistance. ACS Sens. 2018, 3, 2202–2217. [Google Scholar] [CrossRef]
- Davenport, M.; Mach, K.E.; Shortliffe, L.M.D.; Banaei, N.; Wang, T.H.; Liao, J.C. New and Developing Diagnostic Technologies for Urinary Tract Infections. Nat. Rev. Urol. 2017, 14, 298–310. [Google Scholar] [CrossRef]
- Narayana Iyengar, S.; Dietvorst, J.; Ferrer-Vilanova, A.; Guirado, G.; Muñoz-Berbel, X.; Russom, A. Toward Rapid Detection of Viable Bacteria in Whole Blood for Early Sepsis Diagnostics and Susceptibility Testing. ACS Sens. 2021, 6, 3357–3366. [Google Scholar] [CrossRef]
- Schuetz, A.N. Antimicrobial Resistance and Susceptibility Testing of Anaerobic Bacteria. Clin. Infect. Dis. 2014, 59, 698–705. [Google Scholar] [CrossRef]
- Van Belkum, A.; Bachmann, T.T.; Lüdke, G.; Lisby, J.G.; Kahlmeter, G.; Mohess, A.; Becker, K.; Hays, J.P.; Woodford, N.; Mitsakakis, K.; et al. Developmental Roadmap for Antimicrobial Susceptibility Testing Systems. Nat. Rev. Microbiol. 2019, 17, 51–62. [Google Scholar] [CrossRef]
- Nijhuis, R.H.T.; Guerendiain, D.; Claas, E.C.J.; Templeton, K.E. Comparison of EPlex Respiratory Pathogen Panel with Laboratory-Developed Real-Time PCR Assays for Detection of Respiratory Pathogens. J. Clin. Microbiol. 2017, 55, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Mahbubur Rahman, M.; Li, X.B.; Lopa, N.S.; Ahn, S.J.; Lee, J.J. Electrochemical DNA Hybridization Sensors Based on Conducting Polymers. Sensors 2015, 15, 3801–3829. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.; Hu, C.; Wang, Y.; Wang, L.; Peng, F.; Geng, P.F.; Guan, M. Recent Advancements in Microfluidic Chip Biosensor Detection of Foodborne Pathogenic Bacteria: A Review. Anal. Bioanal. Chem. 2022, 414, 2883–2902. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.O.; Cetin, A.E.; Azimzadeh, M.; Topkaya, S.N. Pathogen Detection with Electrochemical Biosensors: Advantages, Challenges and Future Perspectives. J. Electroanal. Chem. 2021, 882, 114989. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical Biosensors for Pathogen Detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Sheikhzadeh, E.; Beni, V.; Zourob, M. Nanomaterial Application in Bio/Sensors for the Detection of Infectious Diseases. Talanta 2021, 230, 122026. [Google Scholar] [CrossRef]
- Muniandy, S.; Teh, S.J.; Thong, K.L.; Thiha, A.; Dinshaw, I.J.; Lai, C.W.; Ibrahim, F.; Leo, B.F. Carbon Nanomaterial-Based Electrochemical Biosensors for Foodborne Bacterial Detection. Crit. Rev. Anal. Chem. 2019, 49, 510–533. [Google Scholar] [CrossRef]
- Trotter, M.; Borst, N.; Thewes, R.; von Stetten, F. Review: Electrochemical DNA Sensing—Principles, Commercial Systems, and Applications. Biosens. Bioelectron. 2020, 154, 112069. [Google Scholar] [CrossRef]
- Lee, V.B.C.; Mohd-Naim, N.F.; Tamiya, E.; Ahmed, M.U. Trends in Paper-Based Electrochemical Biosensors: From Design to Application. Anal. Sci. 2018, 34, 7–18. [Google Scholar] [CrossRef]
- Karbelkar, A.a.; Furst, A.L. Electrochemical Diagnostics for Bacterial Infectious Diseases. ACS Infect. Dis. 2020, 6, 1567–1571. [Google Scholar] [CrossRef]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Zhou, Y.G. Nano-Impact Electrochemistry: Analysis of Single Bioentities. TrAC—Trends Anal. Chem. 2020, 123, 115768. [Google Scholar] [CrossRef]
- Curulli, A. Nanomaterials in Electrochemical Sensing Area: Applications and Challenges in Food Analysis. Molecules 2020, 25, 5759. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Bezaatpour, A.; Jafari, H.; Boukherroub, R.; Szunerits, S. Electrochemical Methodologies for the Detection of Pathogens. ACS Sens. 2018, 3, 1069–1086. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A Review of the Identification and Detection of Heavy Metal Ions in the Environment by Voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef]
- Liu, X.; Huang, D.; Lai, C.; Zeng, G.; Qin, L.; Zhang, C.; Yi, H.; Li, B.; Deng, R.; Liu, S.; et al. Recent Advances in Sensors for Tetracycline Antibiotics and Their Applications. TrAC—Trends Anal. Chem. 2018, 109, 260–274. [Google Scholar] [CrossRef]
- Aleixo, H.; Okumura, L.L.; Gurgel, A.; Silva, A.F.S.; Diniz, J.A. Multiwalled Carbon Nanotube/Ionic Liquid Paste Electrode for Voltammetric Determination of Sulfachlorpyridazine. Anal. Methods 2019, 11, 1743–1750. [Google Scholar] [CrossRef]
- Gondim, C.S.; Durán, G.M.; Contento, A.M.; Ríos, Á. Development and Validation of an Electrochemical Screening Methodology for Sulfonamide Residue Control in Milk Samples Using a Graphene Quantum Dots@Nafion Modified Glassy Carbon Electrode. Food Anal Methods 2018, 11, 1711–1721. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (Eis): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- McEachern, F.; Harvey, E.; Merle, G. Emerging Technologies for the Electrochemical Detection of Bacteria. Biotechnol. J. 2020, 15, e2000140. [Google Scholar] [CrossRef] [PubMed]
- Rashid, J.I.A.; Yusof, N.A. The Strategies of DNA Immobilization and Hybridization Detection Mechanism in the Construction of Electrochemical DNA Sensor: A Review. Sens. Biosens. Res. 2017, 16, 19–31. [Google Scholar] [CrossRef]
- Drummond, T.G.; Hill, M.G.; Barton, J.K. Electrochemical DNA Sensors. Nat. Biotechnol. 2003, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Pellitero, M.A.; Shaver, A.; Arroyo-Currás, N. Critical Review—Approaches for the Electrochemical Interrogation of DNA-Based Sensors: A Critical Review. J. Electrochem. Soc. 2020, 167, 037529. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, D.; Qiu, W.; Gao, F.; Jiang, S.; Wang, Q. An Ultrasensitive DNA Biosensor Based on Covalent Immobilization of Probe DNA on Fern Leaf-like α-Fe2O3 and Chitosan Hybrid Film Using Terephthalaldehyde as Arm-Linker. Biosens. Bioelectron. 2015, 72, 175–181. [Google Scholar] [CrossRef]
- Ulianas, A.; Heng, L.Y.; Hanifah, S.A.; Ling, T.L. An Electrochemical DNA Microbiosensor Based on Succinimide-Modified Acrylic Microspheres. Sensors 2012, 12, 5445–5460. [Google Scholar] [CrossRef]
- Schroeder, H.W.; Cavacini, L. Structure and Function of Immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The Role of Electrochemical Immunosensors in clinical analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef]
- Lim, S.A.; Ahmed, M.U. Electrochemical Immunosensors and Their Recent Nanomaterial-Based Signal Amplification Strategies: A Review. RSC Adv. 2016, 6, 24995–25014. [Google Scholar] [CrossRef]
- Samuel, V.R.; Rao, K.J. A Review on Label Free Biosensors. Biosens. Bioelectron. X 2022, 11, 100216. [Google Scholar] [CrossRef]
- Ghindilis, A.L.; Atanasov, P.; Wilkins, E. Enzyme-Catalyzed Direct Electron Transfer: Fundamentals and Analytical Applications. Electroanalysis 1997, 9, 661–674. [Google Scholar] [CrossRef]
- Chikkaveeraiah, B.V.; Bhirde, A.A.; Morgan, N.Y.; Eden, H.S.; Chen, X. Electrochemical Immunosensors for Detection of Cancer Protein Biomarkers. ACS Nano 2012, 6, 6546–6561. [Google Scholar] [CrossRef]
- Wilson, M.S. Electrochemical Immunosensors for the Simultaneous Detection of Two Tumor Markers. Anal. Chem. 2005, 77, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lin, Y. Nanomaterial Labels in Electrochemical Immunosensors and Immunoassays. Talanta 2007, 74, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Tombelli, S.; Minunni, M.; Mascini, M. Analytical Applications of Aptamers. Biosens. Bioelectron. 2005, 20, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Li, W.; Li, J. Applications of Aptasensors in Clinical Diagnostics. Sensors 2012, 12, 1181–1193. [Google Scholar] [CrossRef]
- Kinsella, M.; Monk, C. The emerging field of RNA nanotechnology. Nat. Nanotechnol. 2010, 5, 833–842. [Google Scholar] [CrossRef]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. SELEX-A (r)Evolutionary Method to Generate High-Affinity Nucleic Acid Ligands. Biomol. Eng. 2007, 24, 381–403. [Google Scholar] [CrossRef]
- Ku, T.H.; Zhang, T.; Luo, H.; Yen, T.M.; Chen, P.W.; Han, Y.; Lo, Y.H. Nucleic Acid Aptamers: An Emerging Tool for Biotechnology and Biomedical Sensing. Sensors 2015, 15, 16281–16313. [Google Scholar] [CrossRef]
- Malik, P.; Gupta, R.; Malik, V.; Ameta, R.K. Emerging Nanomaterials for Improved Biosensing. Meas. Sens. 2021, 16, 100050. [Google Scholar] [CrossRef]
- Verheust, C.; Pauwels, K.; Mahillon, J.; Helinski, D.R.; Herman, P. Contained Use of Bacteriophages: Risk Assessment and Biosafety Recommendations. Appl. Biosaf. 2010, 15, 32–44. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Qiu, T.; Gao, M.; Wang, X. Bacteriophages: Underestimated Vehicles of Antibiotic Resistance Genes in the Soil. Front. Microbiol. 2022, 13, 936267. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Arshad, F.; Eissa, S.; Safavieh, M.; Alattas, S.G.; Ahmed, M.U.; Zourob, M. Recent Developments towards Portable Point-of-Care Diagnostic Devices for Pathogen Detection. Sens. Diagn. 2022, 1, 87–105. [Google Scholar] [CrossRef]
- Singh, A.; Poshtiban, S.; Evoy, S. Recent Advances in Bacteriophage Based Biosensors for Food-Borne Pathogen Detection. Sensors 2013, 13, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Quesada-González, D.; Merkoçi, A. Nanomaterial-Based Devices for Point-of-Care Diagnostic Applications. Chem. Soc. Rev. 2018, 47, 4697–4709. [Google Scholar] [CrossRef]
- Da Silva, E.T.S.G.; Souto, D.E.P.; Barragan, J.T.C.; de F.Giarola, J.; de Moraes, A.C.M.; Kubota, L.T. Electrochemical Biosensors in Point-of-Care Devices: Recent Advances and Future Trends. ChemElectroChem 2017, 4, 778–794. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Guo, Y. Recent Trends in Nanomaterial-Based Biosensors for Point-of-Care Testing. Front. Chem. 2020, 8, 586702. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Qian, K. Nanomaterial-Based Electrochemical Sensors: Mechanism, Preparation, and Application in Biomedicine. Adv. Nanobiomed. Res. 2021, 1, 2000104. [Google Scholar] [CrossRef]
- He, Z.; Liu, C.; Li, Z.; Chu, Z.; Chen, X.; Chen, X.; Guo, Y. Advances in the Use of Nanomaterials for Nucleic Acid Detection in Point-of-Care Testing Devices: A Review. Front. Bioeng. Biotechnol. 2022, 10, 1020444. [Google Scholar] [CrossRef]
- Porto, L.S.; Silva, D.N.; de Oliveira, A.E.F.; Pereira, A.C.; Borges, K.B. Carbon Nanomaterials: Synthesis and Applications to Development of Electrochemical Sensors in Determination of Drugs and Compounds of Clinical Interest. Rev. Anal. Chem. 2020, 38, 1–16. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review—Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Kirchner, E.M.; Hirsch, T. Recent Developments in Carbon-Based Two-Dimensional Materials: Synthesis and Modification Aspects for Electrochemical Sensors. Microchim. Acta 2020, 187, 441. [Google Scholar] [CrossRef] [PubMed]
- Şerban, I.; Enesca, A. Metal Oxides-Based Semiconductors for Biosensors Applications. Front. Chem. 2020, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Löffler, S.; Antypas, H.; Choong, F.X.; Peter, K.; Richter-Dahlfors, A. Conjugated Oligo- and Polymers for Bacterial Sensing. Front. Chem. 2019, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.C.; Pang, J.; Biswas, S.; Wang, K.; Wang, C.; Xia, X.H. Ultrasensitive Detection of Bacteria Using a 2D MOF Nanozyme-Amplified Electrochemical Detector. Anal. Chem. 2021, 93, 8544–8552. [Google Scholar] [CrossRef]
- Gupta, A.; Bhardwaj, S.K.; Sharma, A.L.; Kim, K.H.; Deep, A. Development of an Advanced Electrochemical Biosensing Platform for E. Coli Using Hybrid Metal-Organic Framework/Polyaniline Composite. Environ. Res. 2019, 171, 395–402. [Google Scholar] [CrossRef]
- Gill, A.A.S.; Singh, S.; Thapliyal, N.; Karpoormath, R. Nanomaterial-Based Optical and Electrochemical Techniques for Detection of Methicillin-Resistant Staphylococcus Aureus: A Review. Microchim. Acta 2019, 186, 114. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Chen, P.; Zhou, X.; Yang, Y.; Wu, S.; Niu, L.; Han, Y.; Wang, L.; Chen, P.; et al. Label-Free, Electrochemical Detection of Methicillin-Resistant Staphylococcus Aureus DNA with Reduced Graphene Oxide-Modified Electrodes. Biosens. Bioelectron. 2011, 26, 3881–3886. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Z.; Li, Y.; Xie, G. Amplified Electrochemical Detection of MecA Gene in Methicillin-Resistant Staphylococcus Aureus Based on Target Recycling Amplification and Isothermal Strand-Displacement Polymerization Reaction. Sens. Actuators B Chem. 2015, 221, 148–154. [Google Scholar] [CrossRef]
- Liu, M.; Xiang, H.; Hua, E.; Wang, L.; Jing, X.; Cao, X.; Sheng, S.; Xie, G. Ultrasensitive Electrochemical Biosensor for the Detection of the MecA Gene Sequence in Methicillin Resistant Strains of Staphylococcus Aureus Employing Gold Nanoparticles. Anal. Lett. 2014, 47, 579–591. [Google Scholar] [CrossRef]
- Xu, L.; Liang, W.; Wen, Y.; Wang, L.; Yang, X.; Ren, S.; Jia, N.; Zuo, X.; Liu, G. An Ultrasensitive Electrochemical Biosensor for the Detection of MecA Gene in Methicillin-Resistant Staphylococcus Aureus. Biosens. Bioelectron. 2018, 99, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Li, Z.; Luo, F.; Lu, Y.; Chu, Z.; Zhang, J.; Zhang, F.; Wang, Q.; He, P. Simultaneous Electrochemical Determination of Nuc and MecA Genes for Identification of Methicillin-Resistant Staphylococcus Aureus Using N-Doped Porous Carbon and DNA-Modified MOF. Microchim. Acta 2021, 188, 39. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Qu, A.; Li, M.; Tang, R.; Fu, L.; Liu, X.; Wang, P.; Wu, C. Electrochemical Sensor for Directional Recognition and Measurement of Antibiotic Resistance Genes in Water. Anal. Chem. 2022, 94, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Suea-Ngam, A.; Howes, P.D.; Demello, A.J. An Amplification-Free Ultra-Sensitive Electrochemical CRISPR/Cas Biosensor for Drug-Resistant Bacteria Detection. Chem. Sci. 2021, 12, 12733–12743. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, A.; Pratibha, P.; Marx, A.; Corrigan, D.K. Electrochemical Detection of Oxacillin Resistance Using Direct-Labeling Solid-Phase Isothermal Amplification. ACS Sens. 2021, 6, 3773–3780. [Google Scholar] [CrossRef]
- Watanabe, K.; Kuwata, N.; Sakamoto, H.; Amano, Y.; Satomura, T.; Suye, S.I. A Smart DNA Sensing System for Detecting Methicillin-Resistant Staphylococcus Aureus Using Modified Nanoparticle Probes. Biosens. Bioelectron. 2015, 67, 419–423. [Google Scholar] [CrossRef]
- Khue, V.Q.; Huy, T.Q.; Phan, V.N.; Tuan-Le, A.; Thanh Le, D.T.; Tonezzer, M.; Hong Hanh, N.T. Electrochemical Stability of Screen-Printed Electrodes Modified with Au Nanoparticles for Detection of Methicillin-Resistant Staphylococcus Aureus. Mater. Chem. Phys. 2020, 255, 123562. [Google Scholar] [CrossRef]
- Mandal, S.S.; Navratna, V.; Sharma, P.; Gopal, B.; Bhattacharyya, A.J. Titania Nanotube-Modified Screen Printed Carbon Electrodes Enhance the Sensitivity in the Electrochemical Detection of Proteins. Bioelectrochemistry 2014, 98, 46–52. [Google Scholar] [CrossRef]
- Nemr, C.R.; Smith, S.J.; Liu, W.; Mepham, A.H.; Mohamadi, R.M.; Labib, M.; Kelley, S.O. Nanoparticle-Mediated Capture and Electrochemical Detection of Methicillin-Resistant Staphylococcus Aureus. Anal. Chem. 2019, 91, 2847–2853. [Google Scholar] [CrossRef]
- Cihalova, K.; Hegerova, D.; Dostalova, S.; Jelinkova, P.; Krejcova, L.; Milosavljevic, V.; Krizkova, S.; Kopel, P.; Adam, V. Particle-Based Immunochemical Separation of Methicillin Resistant: Staphylococcus Aureus with Indirect Electrochemical Detection of Labeling Oligonucleotides. Anal. Methods 2016, 8, 5123–5128. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, S.M.; Oh, J.; Park, I.H.; Song, J.H.; Han, M.; Yong, D.; Lim, K.J.; Shin, J.S.; Yoo, K.H. Electrical Antimicrobial Susceptibility Testing Based on Aptamer-Functionalized Capacitance Sensor Array for Clinical Isolates. Sci. Rep. 2020, 10, 13709. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Zhou, Y.; Ramasamy, R.P. A Bacteriophage-Based Electrochemical Biosensor for Detection of Methicillin-Resistant Staphylococcus Aureus. J. Electrochem. Soc. 2021, 168, 057523. [Google Scholar] [CrossRef]
- Munir, M.U.; Ahmad, M.M. Nanomaterials Aiming to Tackle Antibiotic-Resistant Bacteria. Pharmaceutics 2022, 14, 582. [Google Scholar] [CrossRef]
- Singh, J.; Vishwakarma, K.; Ramawat, N.; Rai, P.; Singh, V.K.; Mishra, R.K.; Kumar, V.; Tripathi, D.K.; Sharma, S. Nanomaterials and Microbes’ Interactions: A Contemporary Overview. 3 Biotech 2019, 9, 68. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Zhang, D. A Novel Multifunctional Electrochemical Platform for Simultaneous Detection, Elimination, and Inactivation of Pathogenic Bacteria Based on the Vancomycin-Functionalised AgNPs/3D-ZnO Nanorod Arrays. Biosens. Bioelectron. 2017, 98, 248–253. [Google Scholar] [CrossRef]
- Norouz Dizaji, A.; Ali, Z.; Ghorbanpoor, H.; Ozturk, Y.; Akcakoca, I.; Avci, H.; Dogan Guzel, F. Electrochemical-Based “Antibiotsensor” for the Whole-Cell Detection of the Vancomycin-Susceptible Bacteria. Talanta 2021, 234, 122695. [Google Scholar] [CrossRef]
- Ramalingam, B.; Parandhaman, T.; Das, S.K. Antibacterial Effects of Biosynthesized Silver Nanoparticles on Surface Ultrastructure and Nanomechanical Properties of Gram-Negative Bacteria Viz. Escherichia Coli and Pseudomonas Aeruginosa. ACS Appl. Mater. Interfaces 2016, 8, 4963–4976. [Google Scholar] [CrossRef]
- Rao, R.P.; Sharma, S.; Mehrotra, T.; Das, R.; Kumar, R.; Singh, R.; Roy, I.; Basu, T. Rapid Electrochemical Monitoring of Bacterial Respiration for Gram-Positive and Gram-Negative Microbes: Potential Application in Antimicrobial Susceptibility Testing. Anal. Chem. 2020, 92, 4266–4274. [Google Scholar] [CrossRef]
- Butterworth, A.; Corrigan, D.K.; Ward, A.C. Electrochemical Detection of Oxacillin Resistance with SimpleStat: A Low Cost Integrated Potentiostat and Sensor Platform. Anal. Methods 2019, 11, 1958–1965. [Google Scholar] [CrossRef]
- Brosel-Oliu, S.; Mergel, O.; Uria, N.; Abramova, N.; Van Rijn, P.; Bratov, A. 3D Impedimetric Sensors as a Tool for Monitoring Bacterial Response to Antibiotics. Lab Chip 2019, 19, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Hannah, S.; Addington, E.; Alcorn, D.; Shu, W.; Hoskisson, P.A.; Corrigan, D.K. Rapid Antibiotic Susceptibility Testing Using Low-Cost, Commercially Available Screen-Printed Electrodes. Biosens. Bioelectron. 2019, 145, 111696. [Google Scholar] [CrossRef] [PubMed]
- Crane, B.; Hughes, J.P.; Rowley Neale, S.J.; Rashid, M.; Linton, P.E.; Banks, C.E.; Shaw, K.J. Rapid Antibiotic Susceptibility Testing Using Resazurin Bulk Modified Screen-Printed Electrochemical Sensing Platforms. Analyst 2021, 146, 5574–5583. [Google Scholar] [CrossRef]
- Bolotsky, A.; Muralidharan, R.; Butler, D.; Root, K.; Murray, W.; Liu, Z.; Ebrahimi, A. Organic Redox-Active Crystalline Layers for Reagent-Free Electrochemical Antibiotic Susceptibility Testing (ORACLE-AST). Biosens. Bioelectron. 2021, 172, 112615. [Google Scholar] [CrossRef]
- Safavieh, M.; Pandya, H.J.; Venkataraman, M.; Thirumalaraju, P.; Kanakasabapathy, M.K.; Singh, A.; Prabhakar, D.; Chug, M.K.; Shafiee, H. Rapid Real-Time Antimicrobial Susceptibility Testing with Electrical Sensing on Plastic Microchips with Printed Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 12832–12840. [Google Scholar] [CrossRef]
- Ortiz, M.; Jauset-Rubio, M.; Skouridou, V.; Machado, D.; Viveiros, M.; Clark, T.G.; Simonova, A.; Kodr, D.; Hocek, M.; O’Sullivan, C.K. Electrochemical Detection of Single-Nucleotide Polymorphism Associated with Rifampicin Resistance in Mycobacterium Tuberculosis Using Solid-Phase Primer Elongation with Ferrocene-Linked Redox-Labeled Nucleotides. ACS Sens. 2021, 6, 4398–4407. [Google Scholar] [CrossRef]
- Li, X.; Lu, J.; Feng, L.; Zhang, L.; Gong, J. Smart PH-Regulated Switchable Nanoprobes for Photoelectrochemical Multiplex Detection of Antibiotic Resistance Genes. Anal. Chem. 2020, 92, 11476–11483. [Google Scholar] [CrossRef]
- Jo, N.; Kim, B.; Lee, S.M.; Oh, J.; Park, I.H.; Jin Lim, K.; Shin, J.S.; Yoo, K.H. Aptamer-Functionalized Capacitance Sensors for Real-Time Monitoring of Bacterial Growth and Antibiotic Susceptibility. Biosens. Bioelectron. 2018, 102, 164–170. [Google Scholar] [CrossRef]
- Li, C.; Sun, F. Graphene-Assisted Sensor for Rapid Detection of Antibiotic Resistance in Escherichia coli. Front. Chem. 2021, 9, 696906. [Google Scholar] [CrossRef]
- Besant, J.D.; Sargent, E.H.; Kelley, S.O. Rapid Electrochemical Phenotypic Profiling of Antibiotic-Resistant Bacteria. Lab Chip 2015, 15, 2799–2807. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Singh, D.; Mishra, K.P.; Kaur, G.; Dhull, N.; Tomar, M.; Gupta, V.; Kumar, B.; Ganju, L. Rapid Antibiotic Susceptibility Testing by Resazurin Using Thin Film Platinum as a Bio-Electrode. J. Microbiol. Methods 2019, 162, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Seki, M. PCR-Based Method for Rapid and Minimized Electrochemical Detection of MecA Gene of Methicillin-Resistant Staphylococcus Aureus and Methicillin-Resistant Staphylococcus Epidermis. Glob. J. Infect. Dis. Clin. Res. 2015, 2, 008–012. [Google Scholar] [CrossRef]
| S. No | Working Electrode | Antibiotic | Target Bacteria | Probe | Electro Chemical Method | Hybridization Time | Detection Range | LOD | Interference | Body Fluid | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | GCE-APTES-rGO-dsDNA | Methicillin | DNA from MRSA S. aureus | ssDNA | EIS | 30 min | 0.1 pM–1 μM | 0.1 pM | n-DNA | -- | [79] |
| 2. | mecA gene/MCH/hairpin probe/Au electrode | Methicillin | mecA DNA from MRSA | E-DNA | SWV | 2 h | 0–400 pM | 63 fM | one-base mismatched (T2), three-base mismatched (T3), and non-complementary(T4) DNAs | -- | [80] |
| 3. | mecA gene/Au/GCE | Methicillin | mecA DNA from MRSA | mecA gene | DPV | -- | 50–250 pM | 23 pM | one-base mismatch and complementary DNAs | -- | [81] |
| 4. | MSP-TSP/Au electrode | Methicillin | 130 nt synthetic ssDNA and gDNA | Multi-Signal Probes | EIS | 4 h | 100 nM–10 fM | 10 fM and 57 fM | Non-complementary E. coli gDNA | -- | [82] |
| 5. | UiO-66/BMZIF-derived NPCs | Methicillin | mecA and nuc gene DNA from MRSA | ssDNA | DPV | 1 h | 5–1 × 105 fM | 1.6 fM and 3.6 fM | One (T1), and three bases (T2) mismatched and non-complementary DNA (T3) | -- | [83] |
| 6. | MCH-sDNA-GE | Ampicillin | β-lactam gene | ssDNA-GE | EIS | 1 h | 3.1–480 pM | 3.1 pM | single, double, and three-base mismatch DNA | -- | [84] |
| 7. | E-Si-CRISPR | Methicillin | mecA DNA from MRSA | Aptamer gRNA | SWV | 45 min | 10 fM–0.1 nM | 3.5 fM and 10 fM | Colonies of E. coli, E. Faeclias, L. Monocytogens and S. epdermidis, AND MSSA | Lysate and Human serum | [85] |
| 8. | Screen printed Au SPGE | Oxacillin | DNA AMR gene sequence E. coli | Solid-phase RPA primers | Amperometry | 12 h | 319−20,830 CFU/mL | 319 CFU/mL | -- | -- | [86] |
| 9. | MNP/DNA1-Au/DNA-2 | Methicillin | mecA DNA from MRSA | Ferrocene-labeled probes | CV | -- | 10–166 pM | 10 pM | DNA from S. aureeus and E. coli | -- | [87] |
| 10. | Au/SPCE | Methicillin | Antigen | Monoclonal anti-MRSA antibody and Aptamer gRNA | CV, DPV | -- | 10–106 CFU/mL | 13 CFU/mL | E. coli O157:H7 | [88] | |
| 11. | TiO2-NTs | Methicillin | S. aureus | PBP2a Protein | CV | -- | 1–100 ng/μL | 1 ng/μL | Recombinant protein PTP10D | -- | [89] |
| 12. | Au electrode | Methicillin | PBP2a antibody | Monoclonal anti-MRSA antibody | DPV | -- | 3–105 CFU/mL | 3 CFU/mL | Nontarget strains MSSA, MSSE, and MRSE | Nasal swab | [90] |
| 13. | Au nanoparticles modified by anti-Pls | Methicillin | Antigen | MRSA-specific antibody | SWV | -- | 0.2–10 μM 4 × 107–2 × 104 CFU/mL | 2 × 104 CFU/mL | E. coli and P. mirabili ODN | -- | [91] |
| 14. | e-AST system on Au | 11 antibiotic drugs | E. coli U433 | 60 aptamers | Capacitance | -- | 0.5–128 mg/mL | -- | -- | [92] | |
| 15. | PEI-f-CNT | Methicillin | MRSA USA300 strain | SATA-8505, bacteriophage | EIS | -- | 102–107 CFU/mL | 1.23 × 102 CFU/mL in aqueous solution 1.29 × 102 CFU/mL in blood plasma | SATA-8505’s nonhost organisms as E. coli and P. putida | Blood plasma | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madhu, S.; Ramasamy, S.; Choi, J. Recent Developments in Electrochemical Sensors for the Detection of Antibiotic-Resistant Bacteria. Pharmaceuticals 2022, 15, 1488. https://doi.org/10.3390/ph15121488
Madhu S, Ramasamy S, Choi J. Recent Developments in Electrochemical Sensors for the Detection of Antibiotic-Resistant Bacteria. Pharmaceuticals. 2022; 15(12):1488. https://doi.org/10.3390/ph15121488
Chicago/Turabian StyleMadhu, Sekar, Sriramprabha Ramasamy, and Jungil Choi. 2022. "Recent Developments in Electrochemical Sensors for the Detection of Antibiotic-Resistant Bacteria" Pharmaceuticals 15, no. 12: 1488. https://doi.org/10.3390/ph15121488
APA StyleMadhu, S., Ramasamy, S., & Choi, J. (2022). Recent Developments in Electrochemical Sensors for the Detection of Antibiotic-Resistant Bacteria. Pharmaceuticals, 15(12), 1488. https://doi.org/10.3390/ph15121488





