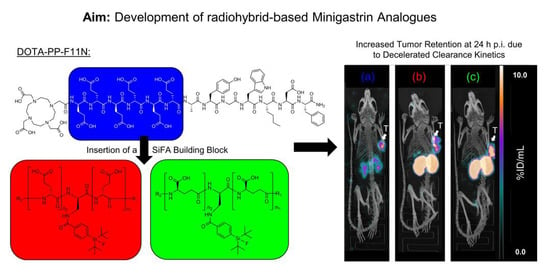
Introduction of a SiFA Moiety into the D-Glutamate Chain of DOTA-PP-F11N Results in Radiohybrid-Based CCK-2R-Targeted Compounds with Improved Pharmacokinetics In Vivo
Abstract
1. Introduction
2. Results
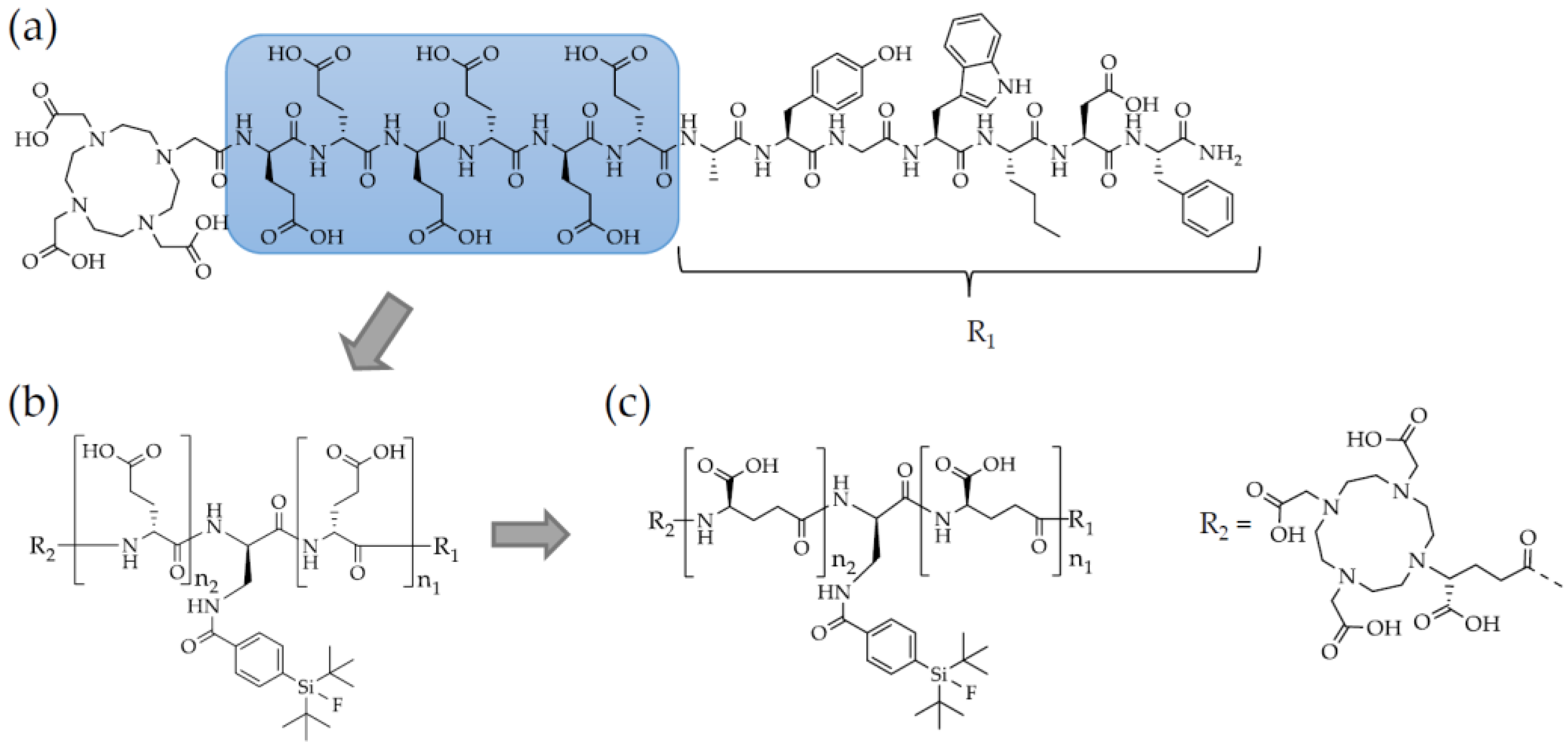
2.1. Synthesis and Radiolabelling
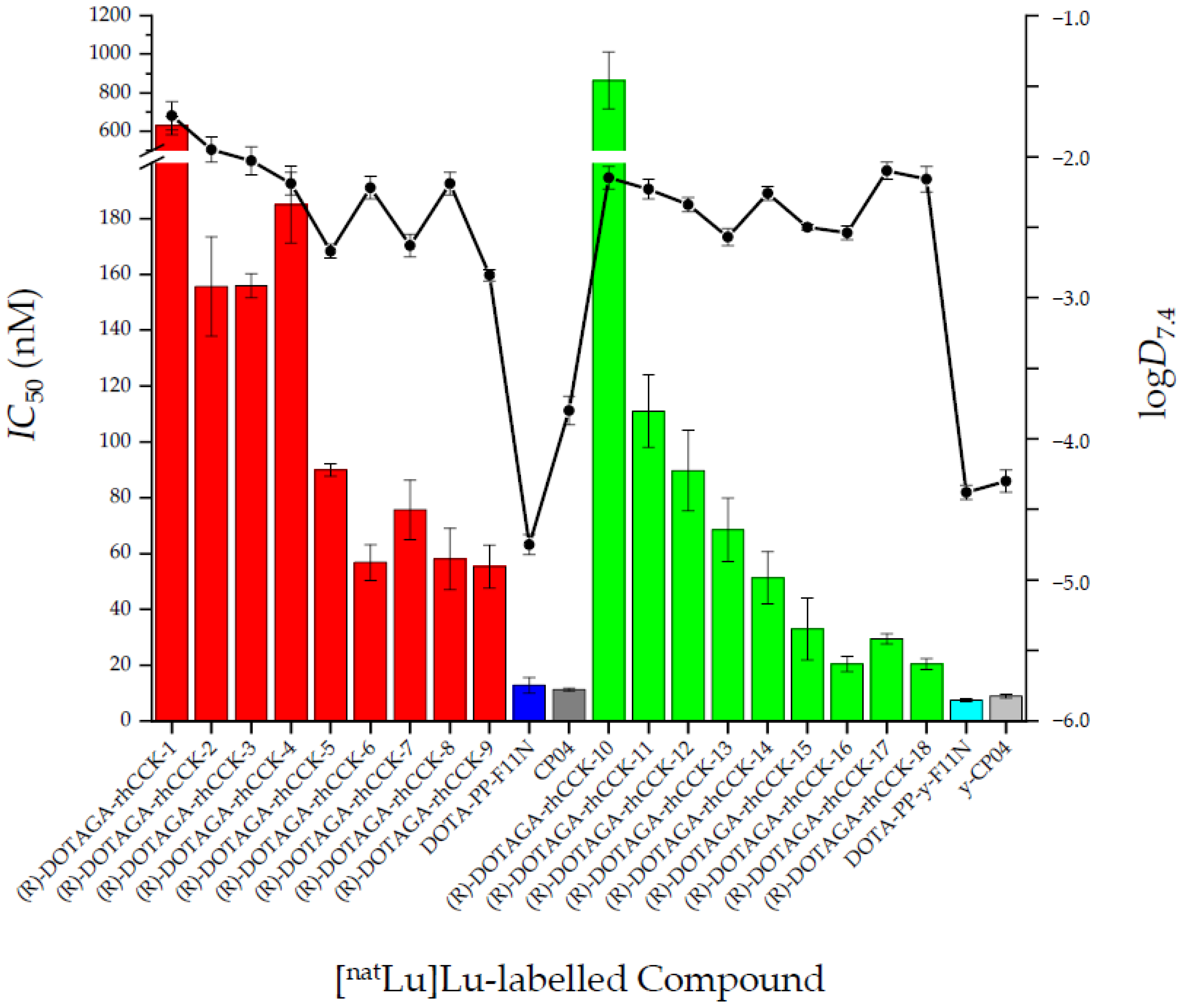
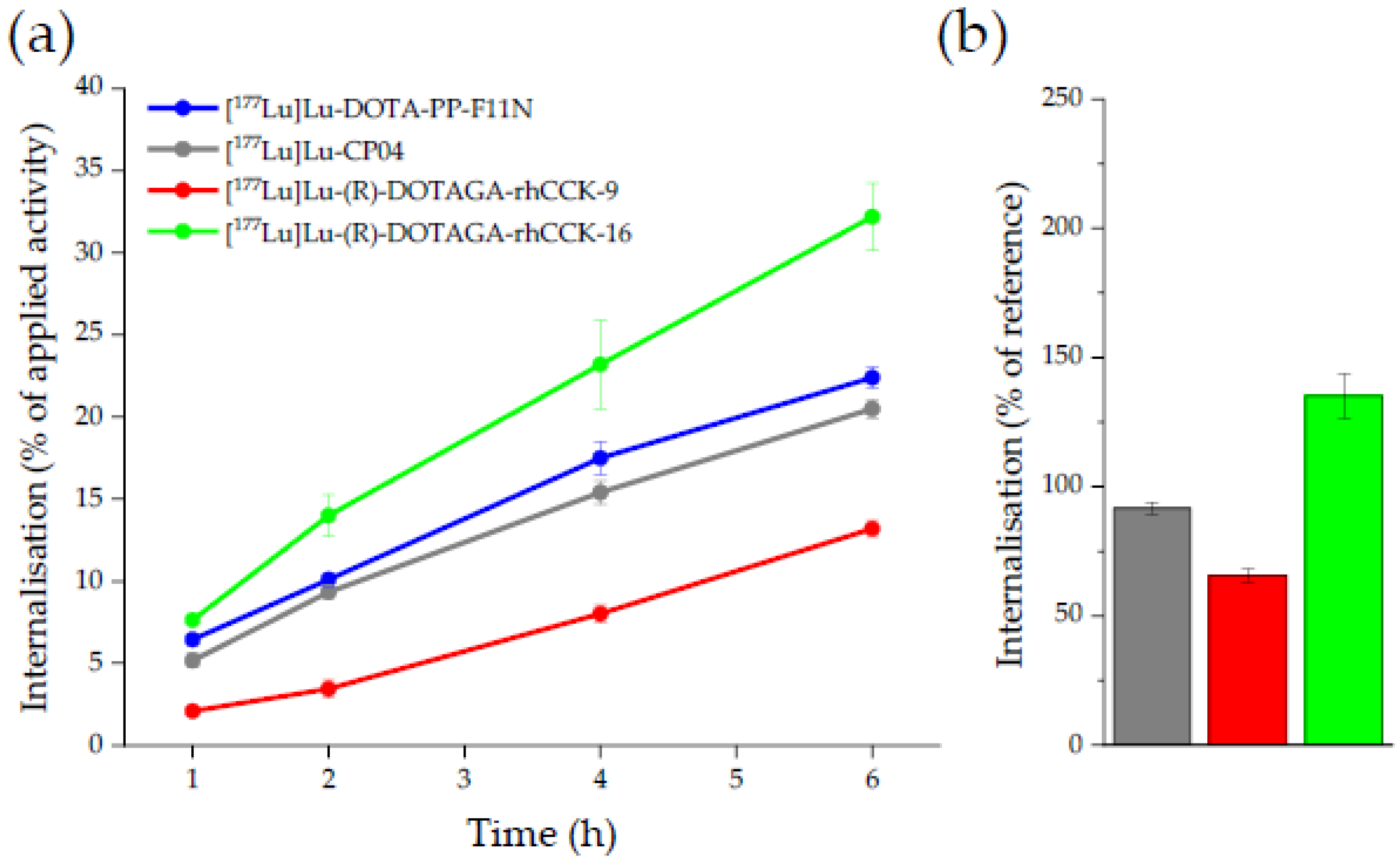
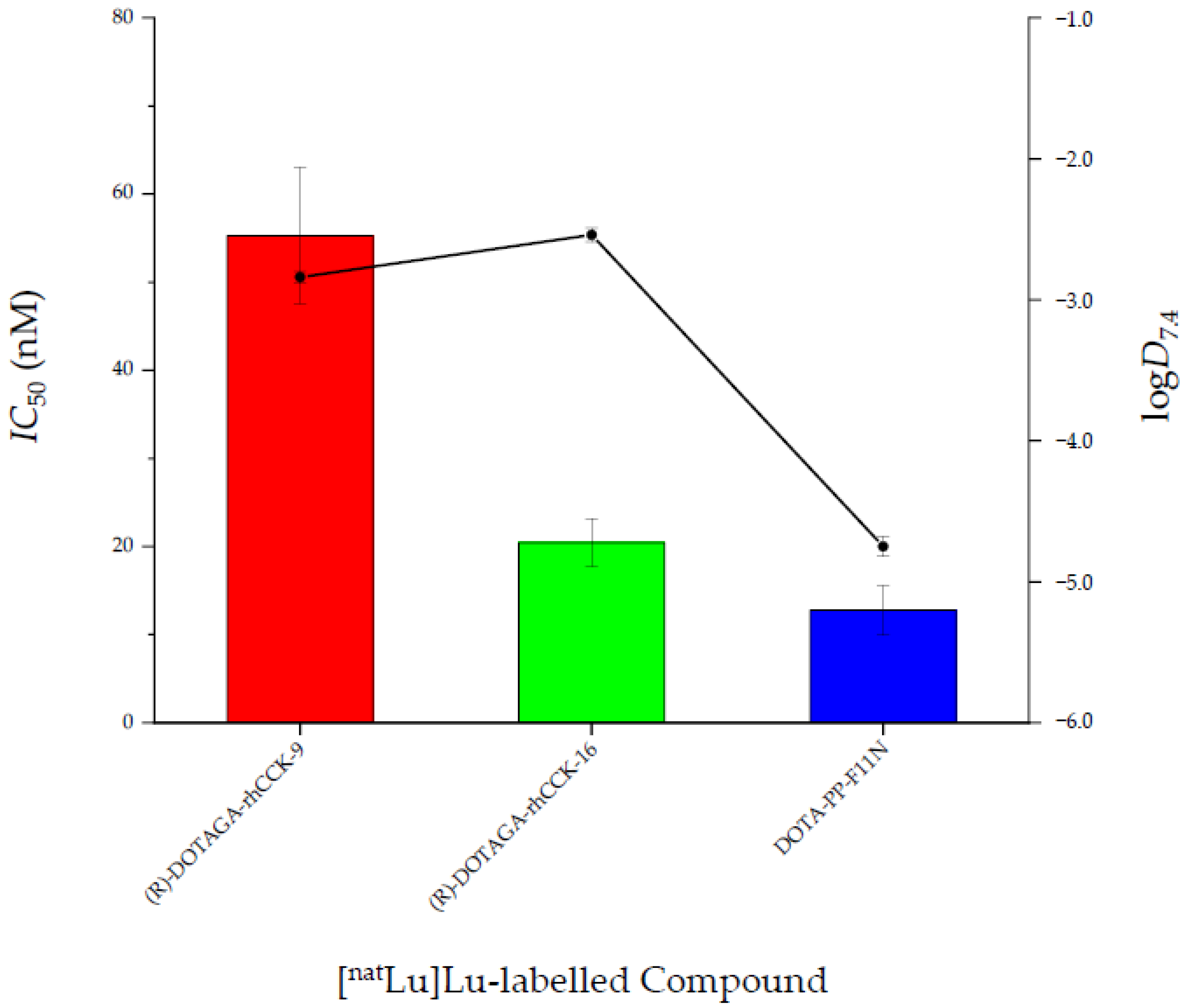
2.2. In Vitro Characterisation
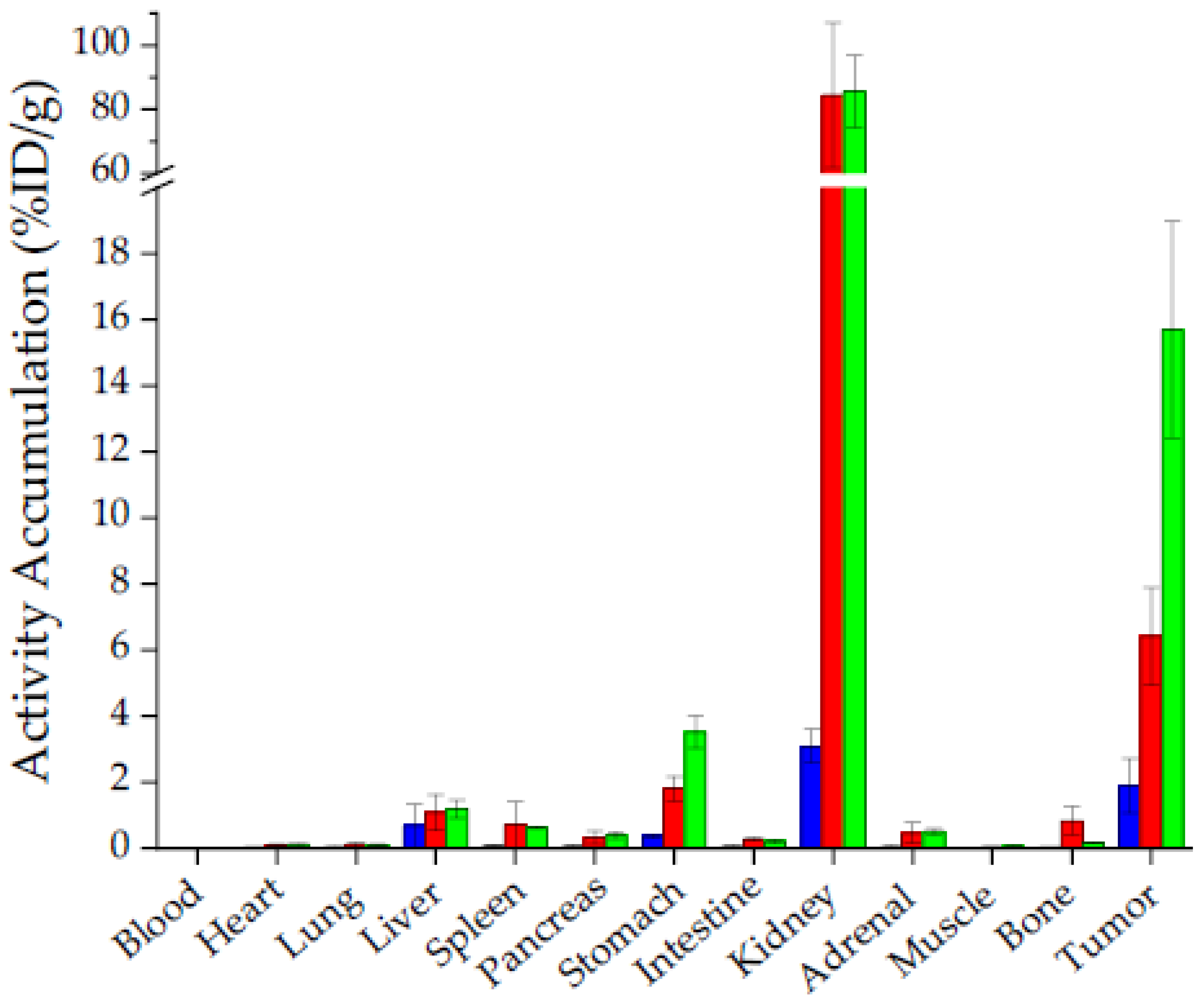
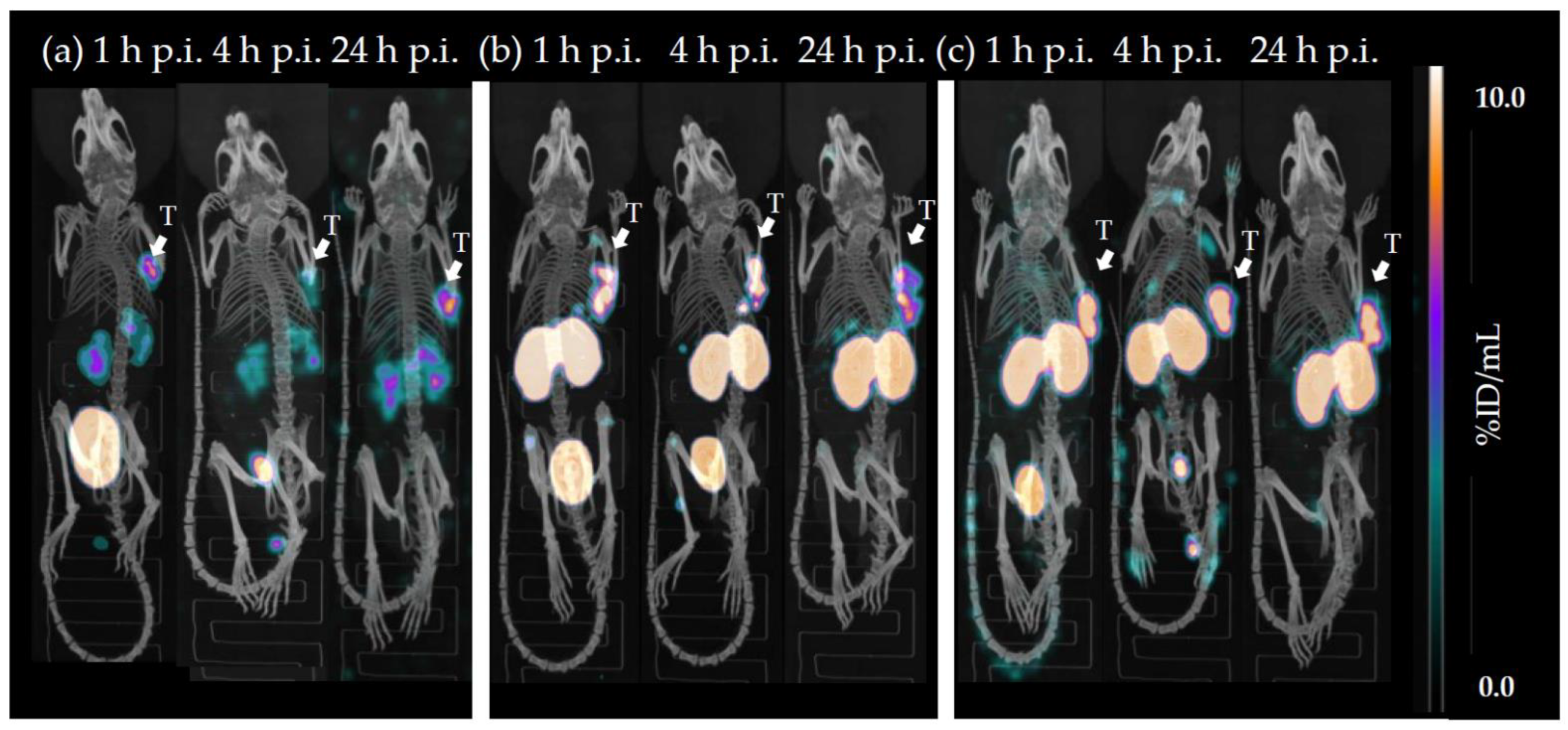
2.3. In Vivo Characterisation
3. Discussion
4. Materials and Methods
4.1. Chemical Synthesis and Labelling Procedures
4.2. In Vitro Experiments
4.3. In Vivo Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stamatakos, M.; Paraskeva, P.; Stefanaki, C.; Katsaronis, P.; Lazaris, A.; Safioleas, K.; Kontzoglou, K. Medullary thyroid carcinoma: The third most common thyroid cancer reviewed. Oncol. Lett. 2011, 2, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A., Jr.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Hadoux, J.; Schlumberger, M. Chemotherapy and tyrosine-kinase inhibitors for medullary thyroid cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 335–347. [Google Scholar] [CrossRef]
- Hazard, J.B. The C cells (parafollicular cells) of the thyroid gland and medullary thyroid carcinoma. A review. Am. J. Pathol. 1977, 88, 213–250. [Google Scholar]
- Resteghini, C.; Cavalieri, S.; Galbiati, D.; Granata, R.; Alfieri, S.; Bergamini, C.; Bossi, P.; Licitra, L.; Locati, L.D. Management of tyrosine kinase inhibitors (TKI) side effects in differentiated and medullary thyroid cancer patients. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Waser, B. Unexpected high incidence of cholecystokinin-B/gastrin receptors in human medullary thyroid carcinomas. Int. J. Cancer 1996, 67, 644–647. [Google Scholar] [CrossRef]
- Behr, T.M.; Jenner, N.; Béhé, M.; Angerstein, C.; Gratz, S.; Raue, F.; Becker, W. Radiolabeled Peptides for Targeting Cholecystokinin-B/Gastrin Receptor-Expressing Tumors. J. Nucl. Med. 1999, 40, 1029–1044. [Google Scholar]
- Behe, M.; Becker, W.; Gotthardt, M.; Angerstein, C.; Behr, T.M. Improved kinetic stability of DTPA-dGlu as compared with conventional monofunctional DTPA in chelating indium and yttrium: Preclinical and initial clinical evaluation of radiometal labelled minigastrin derivatives. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Laverman, P.; Joosten, L.; Eek, A.; Roosenburg, S.; Peitl, P.K.; Maina, T.; Macke, H.; Aloj, L.; von Guggenberg, E.; Sosabowski, J.K.; et al. Comparative biodistribution of 12 (1)(1)(1)In-labelled gastrin/CCK2 receptor-targeting peptides. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1410–1416. [Google Scholar] [CrossRef]
- Sauter, A.W.; Mansi, R.; Hassiepen, U.; Muller, L.; Panigada, T.; Wiehr, S.; Wild, A.M.; Geistlich, S.; Béhé, M.; Rottenburger, C.; et al. Targeting of the Cholecystokinin-2 Receptor with the Minigastrin Analog (177)Lu-DOTA-PP-F11N: Does the Use of Protease Inhibitors Further Improve In Vivo Distribution? J. Nucl. Med. 2019, 60, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Schibli, R. Mini-Gastrin Analogue, in Particular for Use in CCK2 Receptor Positive Tumour Diagnosis and/or Treatment. U.S. Patent 10,130,724, 20 November 2018. [Google Scholar]
- Rottenburger, C.; Nicolas, G.P.; McDougall, L.; Kaul, F.; Cachovan, M.; Vija, A.H.; Schibli, R.; Geistlich, S.; Schumann, A.; Rau, T.; et al. Cholecystokinin 2 Receptor Agonist (177)Lu-PP-F11N for Radionuclide Therapy of Medullary Thyroid Carcinoma: Results of the Lumed Phase 0a Study. J. Nucl. Med. 2020, 61, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, A.; Di Carlo, D.; Schmidt, A.; Beck, R.; Eiber, M.; Schwaiger, M.; Wester, H.-J. Radiohybrid Ligands: A Novel Tracer Concept Exemplified by 18F- or 68Ga-Labeled rhPSMA Inhibitors. J. Nucl. Med. 2020, 61, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, A.; Kunert, J.P.; Fischer, S.; Felber, V.; Beck, R.; Rose, F.; D’Alessandria, C.; Weber, W.; Wester, H.J. Synthesis and Preclinical Evaluation of (177)Lu-Labeled Radiohybrid PSMA Ligands for Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2022, 63, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Kronke, M.; Wurzer, A.; Ulbrich, L.; Jooss, L.; Maurer, T.; Horn, T.; Schiller, K.; Langbein, T.; Buschner, G.; et al. (18)F-rhPSMA-7 positron emission tomography for the detection of biochemical recurrence of prostate cancer following radical prostatectomy. J. Nucl. Med. 2020, 61, 696–701. [Google Scholar] [CrossRef]
- Kronke, M.; Wurzer, A.; Schwamborn, K.; Ulbrich, L.; Jooss, L.; Maurer, T.; Horn, T.; Rauscher, I.; Haller, B.; Herz, M.; et al. Histologically-confirmed diagnostic efficacy of (18)F-rhPSMA-7 positron emission tomography for N-staging of patients with primary high risk prostate cancer. J. Nucl. Med. 2020, 61, 710–715. [Google Scholar] [CrossRef]
- Oh, S.W.; Wurzer, A.; Teoh, E.J.; Oh, S.; Langbein, T.; Kronke, M.; Herz, M.; Kropf, S.; Wester, H.J.; Weber, W.A.; et al. Quantitative and Qualitative Analyses of Biodistribution and PET Image Quality of Novel Radiohybrid PSMA, (18)F-rhPSMA-7, in Patients with Prostate Cancer. J. Nucl. Med. 2020, 61, 702–709. [Google Scholar] [CrossRef]
- Kroenke, M.; Mirzoyan, L.; Horn, T.; Peeken, J.C.; Wurzer, A.; Wester, H.J.; Makowski, M.; Weber, W.A.; Eiber, M.; Rauscher, I. Matched-Pair Comparison of (68)Ga-PSMA-11 and (18)F-rhPSMA-7 PET/CT in Patients with Primary and Biochemical Recurrence of Prostate Cancer: Frequency of Non-Tumor-Related Uptake and Tumor Positivity. J. Nucl. Med. 2021, 62, 1082–1088. [Google Scholar] [CrossRef]
- Guenther, T.; Deiser, S.; Felber, V.; Beck, R.; Wester, H.J. Substitution of L-Tryptophan by a-Methyl-L-Tryptophan in 177Lu-RM2 Results in 177Lu-AMTG, a High-Affinity Gastrin-Releasing Peptide Receptor Ligand with Improved In Vivo Stability. J. Nucl. Med. 2022, 63, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Uprimny, C.; von Guggenberg, E.; Svirydenka, A.; Mikolajczak, R.; Hubalewska-Dydejczyk, A.; Virgolini, I.J. Comparison of PET/CT imaging with [(18)F]FDOPA and cholecystokinin-2 receptor targeting [(68)Ga]Ga-DOTA-MGS5 in a patient with advanced medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 48, 935–936. [Google Scholar] [CrossRef]
- Hörmann, A.A.; Klingler, M.; Rangger, C.; Mair, C.; Decristoforo, C.; Uprimny, C.; Virgolini, I.J.; von Guggenberg, E. Radiopharmaceutical Formulation and Preclinical Testing of 68Ga-Labeled DOTA-MGS5 for the Regulatory Approval of a First Exploratory Clinical Trial. Pharmaceuticals 2021, 14, 575. [Google Scholar] [CrossRef] [PubMed]
- 177Lu-PP-F11N for Receptor Targeted Therapy and Imaging of Metastatic Thyroid Cancer. Available online: https://ClinicalTrials.gov/show/NCT02088645 (accessed on 23 July 2022).
- Radiolabelled CCK-2/Gastrin Receptor Analogue for Personalized Theranostic Strategy in Advanced MTC. Available online: https://ClinicalTrials.gov/show/NCT03246659 (accessed on 23 July 2022).
- Bernard-Gauthier, V.; Wangler, C.; Schirrmacher, E.; Kostikov, A.; Jurkschat, K.; Wangler, B.; Schirrmacher, R. (1)(8)F-labeled silicon-based fluoride acceptors: Potential opportunities for novel positron emitting radiopharmaceuticals. Biomed. Res. Int. 2014, 2014, 454503. [Google Scholar] [CrossRef] [PubMed]
- Malaspina, S.; Taimen, P.; Kallajoki, M.; Oikonen, V.; Kuisma, A.; Ettala, O.; Mattila, K.; Boström, P.J.; Minn, H.; Kalliokoski, K.; et al. Uptake of (18)F-rhPSMA-7.3 in Positron Emission Tomography Imaging of Prostate Cancer: A Phase 1 Proof-of-Concept Study. Cancer Biother. Radiopharm. 2022, 37, 205–213. [Google Scholar] [CrossRef]
- Feuerecker, B.; Chantadisai, M.; Allmann, A.; Tauber, R.; Allmann, J.; Steinhelfer, L.; Rauscher, I.; Wurzer, A.; Wester, H.J.; Weber, W.A.; et al. Pre-therapeutic comparative dosimetry of (177)Lu-rhPSMA-7.3 and (177)Lu-PSMAI&T in patients with metastatic castration resistant prostate cancer (mCRPC). J. Nucl. Med. 2021, 63, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Yusufi, N.; Wurzer, A.; Herz, M.; D’Alessandria, C.; Feuerecker, B.; Weber, W.; Wester, H.J.; Nekolla, S.; Eiber, M. Comparative Preclinical Biodistribution, Dosimetry, and Endoradiotherapy in Metastatic Castration-Resistant Prostate Cancer Using (19)F/(177)Lu-rhPSMA-7.3 and (177)Lu-PSMA I&T. J. Nucl. Med. 2021, 62, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Imaging Study to Investigate the Safety and Diagnostic Performance of rhPSMA 7.3 (18F) in Newly Diagnosed Prostate Cancer (LIGHTHOUSE). Available online: https://ClinicalTrials.gov/show/NCT04186819 (accessed on 2 November 2022).
- Imaging Study to Investigate Safety and Diagnostic Performance of rhPSMA 7.3 (18F) PET Ligand in Suspected Prostate Cancer Recurrence (SPOTLIGHT). Available online: https://ClinicalTrials.gov/show/NCT04186845 (accessed on 2 November 2022).
- Anti-tumour Activity of (177Lu) rhPSMA-10.1 Injection. Available online: https://ClinicalTrials.gov/show/NCT05413850 (accessed on 2 November 2022).
- Assessing Radio-hybrid Prostate Specific Membrane Antigen (rhPSMA-7.3) (18F) in Healthy Volunteers and Subjects with Prostate Cancer. Available online: https://ClinicalTrials.gov/show/NCT03995888 (accessed on 2 November 2022).
- An Investigational Scan (rh PSMA 7.3 PET/MRI) for the Detection of Recurrent Disease and Aid in Radiotherapy Planning in Biochemically Recurrent Prostate Cancer. Available online: https://ClinicalTrials.gov/show/NCT04978675 (accessed on 2 November 2022).
- Kunert, J.P.; Fischer, S.; Wurzer, A.; Wester, H.J. Albumin-Mediated Size Exclusion Chromatography: The Apparent Molecular Weight of PSMA Radioligands as Novel Parameter to Estimate Their Blood Clearance Kinetics. Pharmaceuticals 2022, 15, 1161. [Google Scholar] [CrossRef] [PubMed]
- Klingler, M.; Summer, D.; Rangger, C.; Haubner, R.; Foster, J.; Sosabowski, J.; Decristoforo, C.; Virgolini, I.; von Guggenberg, E. DOTA-MGS5, a New Cholecystokinin-2 Receptor-Targeting Peptide Analog with an Optimized Targeting Profile for Theranostic Use. J. Nucl. Med. 2019, 60, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Schottelius, M.; Osl, T.; Poschenrieder, A.; Hoffmann, F.; Beykan, S.; Hänscheid, H.; Schirbel, A.; Buck, A.K.; Kropf, S.; Schwaiger, M.; et al. [(177)Lu]pentixather: Comprehensive Preclinical Characterization of a First CXCR4-directed Endoradiotherapeutic Agent. Theranostics 2017, 7, 2350–2362. [Google Scholar] [CrossRef] [PubMed]
- Dalm, S.U.; Nonnekens, J.; Doeswijk, G.N.; de Blois, E.; van Gent, D.C.; Konijnenberg, M.W.; de Jong, M. Comparison of the Therapeutic Response to Treatment with a 177Lu-Labeled Somatostatin Receptor Agonist and Antagonist in Preclinical Models. J. Nucl. Med. 2016, 57, 260–265. [Google Scholar] [CrossRef]
- Nicolas, G.P.; Mansi, R.; McDougall, L.; Kaufmann, J.; Bouterfa, H.; Wild, D.; Fani, M. Biodistribution, Pharmacokinetics, and Dosimetry of (177)Lu-, (90)Y-, and (111)In-Labeled Somatostatin Receptor Antagonist OPS201 in Comparison to the Agonist (177)Lu-DOTATATE: The Mass Effect. J. Nucl. Med. 2017, 58, 1435–1441. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holzleitner, N.; Günther, T.; Beck, R.; Lapa, C.; Wester, H.-J. Introduction of a SiFA Moiety into the D-Glutamate Chain of DOTA-PP-F11N Results in Radiohybrid-Based CCK-2R-Targeted Compounds with Improved Pharmacokinetics In Vivo. Pharmaceuticals 2022, 15, 1467. https://doi.org/10.3390/ph15121467
Holzleitner N, Günther T, Beck R, Lapa C, Wester H-J. Introduction of a SiFA Moiety into the D-Glutamate Chain of DOTA-PP-F11N Results in Radiohybrid-Based CCK-2R-Targeted Compounds with Improved Pharmacokinetics In Vivo. Pharmaceuticals. 2022; 15(12):1467. https://doi.org/10.3390/ph15121467
Chicago/Turabian StyleHolzleitner, Nadine, Thomas Günther, Roswitha Beck, Constantin Lapa, and Hans-Jürgen Wester. 2022. "Introduction of a SiFA Moiety into the D-Glutamate Chain of DOTA-PP-F11N Results in Radiohybrid-Based CCK-2R-Targeted Compounds with Improved Pharmacokinetics In Vivo" Pharmaceuticals 15, no. 12: 1467. https://doi.org/10.3390/ph15121467
APA StyleHolzleitner, N., Günther, T., Beck, R., Lapa, C., & Wester, H.-J. (2022). Introduction of a SiFA Moiety into the D-Glutamate Chain of DOTA-PP-F11N Results in Radiohybrid-Based CCK-2R-Targeted Compounds with Improved Pharmacokinetics In Vivo. Pharmaceuticals, 15(12), 1467. https://doi.org/10.3390/ph15121467