Unravelling Potential Health-Beneficial Properties of Corema album Phenolic Compounds: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
3. Results
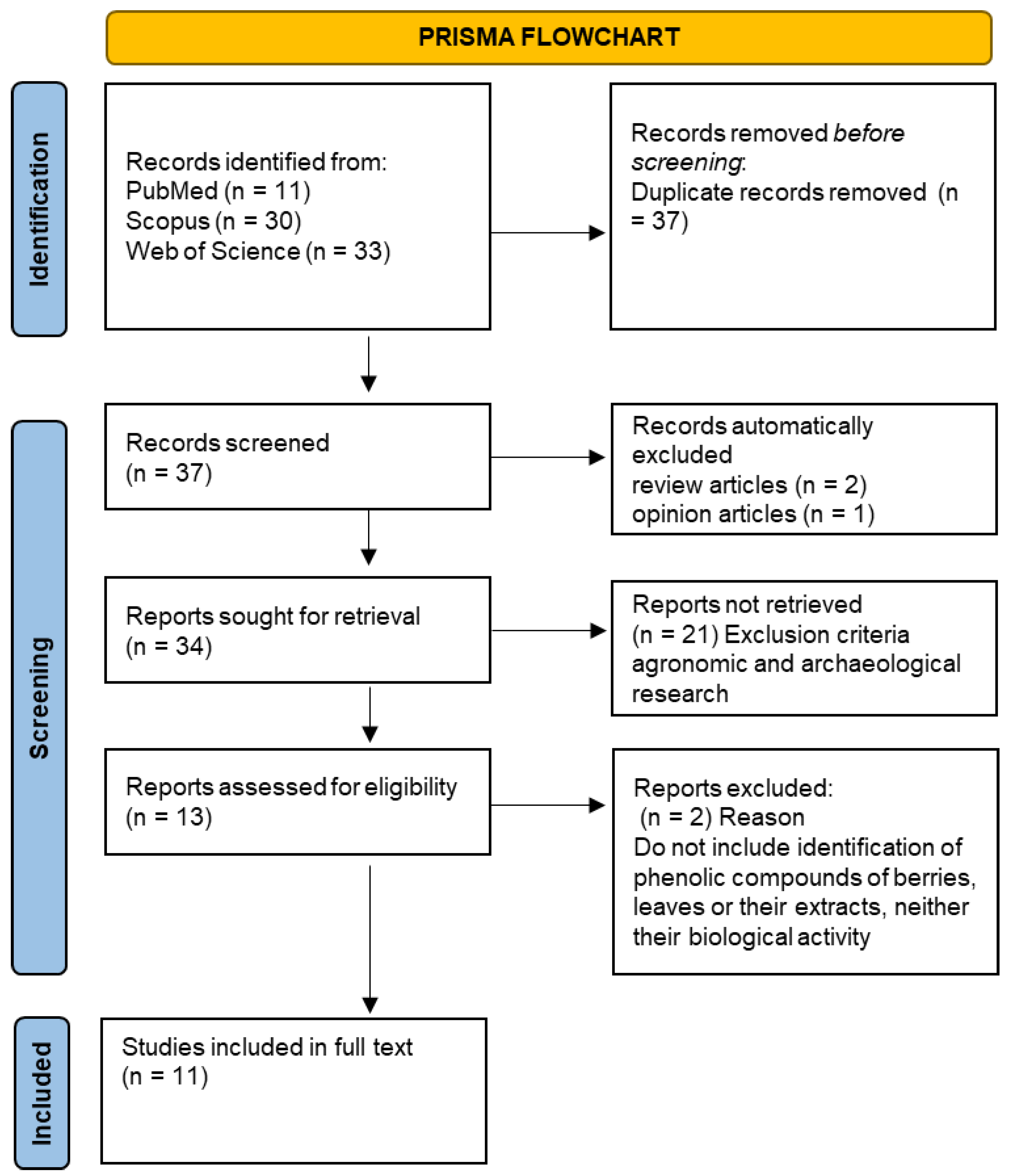
3.1. Literature Search Process
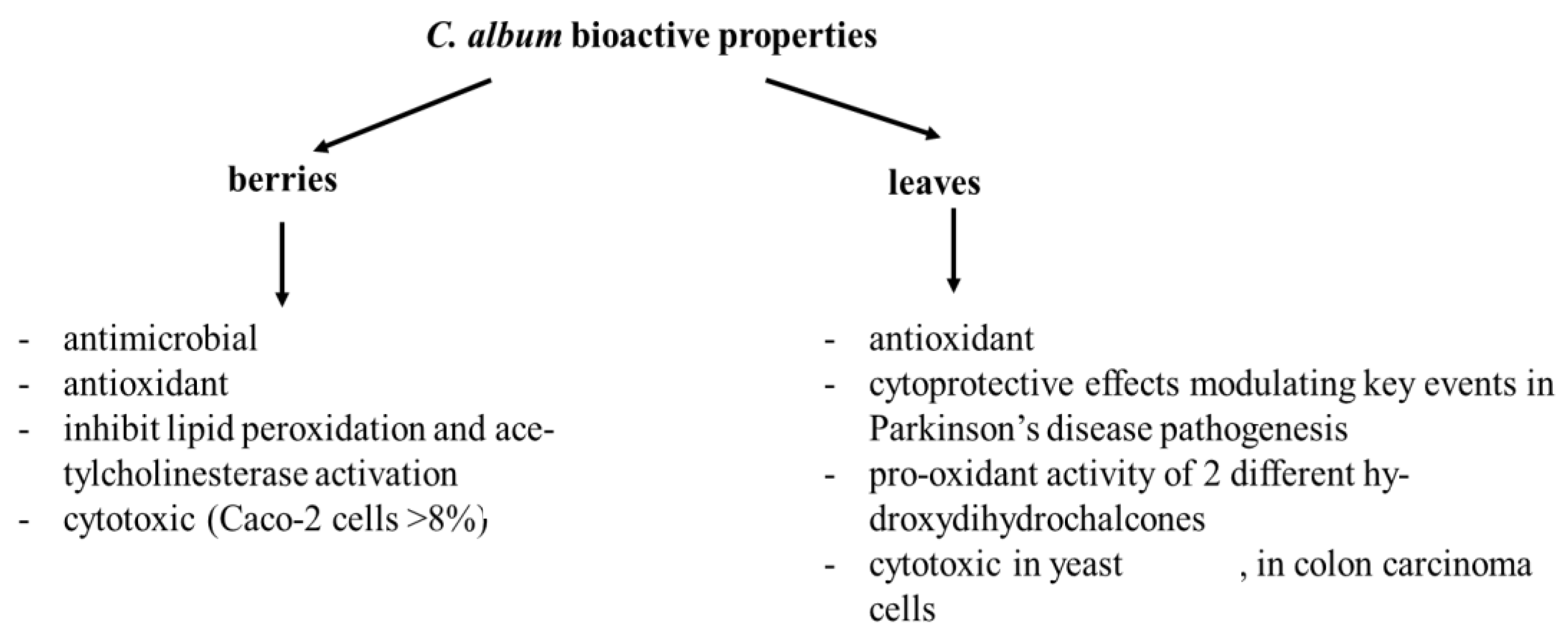
3.2. Phenolic Compounds in Berries and Leaves from C. album
4. Discussion
4.1. Suppression of NF-kB Transcription Factor Activation
4.2. Modulation of Inflammatory Mediators/Enzymes
4.3. Induction of Apoptosis
4.4. Modulation of Mitogen Activated Protein Kinase
4.5. Cell Cycle Arrest
4.6. Zeduction of Oxidative Stress
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.C.; Gonçalves, F.; Guiné, R. Contribution for the physical-chemical characterization of Portuguese Crowberry (Corema album). Int. J. Food Sci. Nutr. 2017, 2, 9–14. [Google Scholar]
- Martine, C.T.; Lubertazzi, D.; Dubrul, A. The biology of Corema conradii: Natural history, reproduction, and observations of a post-fire seedling recruitment. Northeast. Nat. 2005, 12, 267–286. [Google Scholar] [CrossRef]
- Guitián, P.; Medrano, M.; Rodríguez, M. Reproductive biology of Corema album (L.) D. Don (Empetraceae) in the northwest Iberian Peninsula. Acta Bot. Gall. 1997, 144, 119–128. [Google Scholar] [CrossRef][Green Version]
- Zunzunegui, M.; Barradas, M.D.; Clavijo, A.; Cansino, L.A.; Lhout, F.A.; Novo, F.G. Ecophysiology, growth timing and reproductive effort of three sexual foms of Corema album (Empetraceae). Plant Ecol. 2006, 183, 35–46. [Google Scholar] [CrossRef]
- de Oliveira, P.B.; Dale, A. Corema album (L.) D. Don, the white crowberry—A new crop. J. Berry Res. 2012, 2, 123–133. [Google Scholar] [CrossRef]
- León-González, A.J.; Mateos, R.; Ramos, S.; Martín, M.Á.; Sarriá, B.; Martín-Cordero, C.; López-Lázaro, M.; Bravo, L.; Goya, L. Chemo-protective activity and characterization of phenolic extracts from Corema album. Food Res. Int. 2012, 49, 728–738. [Google Scholar] [CrossRef]
- López-Dóriga, I.L. The archaeobotany and ethnobotany of Portuguese or white crowberry (Corema album (L.) D. Don). Ethnobiol. Lett. 2018, 9, 19–32. [Google Scholar] [CrossRef]
- Gras, A.; Garnatje, T.; Ibáñez, N.; López-Pujol, J.; Nualart, N.; Vallès, J. Medicinal plant uses and names from the herbarium of Francesc Bolòs (1773-1844). J. Ethnopharmacol 2017, 204, 142–168. [Google Scholar] [CrossRef]
- Martin, D.; Marques, J.; Amado, A.M.; Barroca, M.J.; Moreira da Silva, A.; Batista de Carvalho, L.A.E.; Marques, M.P.M. Shedding light into the health-beneficial properties of Corema album—A vibrational spectroscopy study. J. Raman Spectrosc. 2020, 51, 313–322. [Google Scholar] [CrossRef]
- Marques, J.; Martin, D.; Amado, A.M.; Lysenko, V.; Osório, N.; Batista de Carvalho, L.A.E.; Marques, M.P.M.; Barroca, M.J.; Moreira da Silva, A. Novel Insights into Corema album Berries: Vibrational Profile and Biological Activity. Plants 2021, 10, 1761. [Google Scholar] [CrossRef] [PubMed]
- León-González, A.J.; Truchado, P.; Tomás-Barberán, F.A.; López-Lázaro, M.; Barradas, M.C.D.; Martín-Cordero, C. Phenolic acids, flavonols and anthocyanins in Corema album (L.) D. Don berries. J. Food Compos. Anal. 2013, 29, 58–63. [Google Scholar] [CrossRef]
- Andrade, S.C.; Guiné, R.P.F.; Gonçalves, F.J.A. Evaluation of phenolic compounds, antioxidant activity and bioaccessibility in white crowberry (Corema album). J. Food Meas. Charact. 2017, 11, 1936–1946. [Google Scholar] [CrossRef]
- Brito, C.; Bertotti, T.; Primitivo, M.J.; Neves, M.; Pires, C.L.; Cruz, P.F.; Martins, P.A.T.; Rodrigues, A.C.; Moreno, M.J.; Brito, R.M.M.; et al. Corema album spp: Edible wild crowberries with a high content in minerals and organic acids. Food Chem. 2021, 345, 128732. [Google Scholar] [CrossRef] [PubMed]
- Macedo, D.; Tavares, L.; McDougall, G.J.; Vicente Miranda, H.; Stewart, D.; Ferreira, R.B.; Tenreiro, S.; Outeiro, T.F.; Santos, C.N. (Poly)phenols protect from α-synuclein toxicity by reducing oxidative stress and promoting autophagy. Hum. Mol. Genet. 2015, 24, 1717–1732. [Google Scholar] [CrossRef] [PubMed]
- Jardim, C.; Macedo, D.; Figueira, I.; Dobson, G.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Menezes, R.; Santos, C.N. (Poly)phenol metabolites from Arbutus unedo leaves protect yeast from oxidative injury by activation of antioxidant and protein clearance pathways. J. Funct. Foods 2017, 32, 333–346. [Google Scholar] [CrossRef]
- León-González, A.J.; López-Lázaro, M.; Espartero, J.L.; Martín-Cordero, C. Cytotoxic Activity of Dihydrochalcones Isolated from Corema Album Leaves against HT-29 Colon Cancer Cells. Nat. Prod. Commun. 2013, 8, 1934578X1300800. [Google Scholar] [CrossRef]
- Leon-Gonzalez, A.J.; Manson, M.M.; Lopez-Lazaro, M.; Navarro, I.; Martin-Cordero, C. Induction of apoptosis and cell cycle arrest in human colon carcinoma cells by corema album leaves. Nat. Prod. Commun. 2014, 9, 1934578X1400900117. [Google Scholar] [CrossRef]
- Wong, E. The role of chalcones and flavanones in flavonoid biosynthesis. Phytochemistry 1968, 7, 1751–1758. [Google Scholar] [CrossRef]
- Sánchez-Picó, Á.; León-González, A.J.; Martín-Cordero, C.; Daga, R.R. Screening for natural anticancer agents using a fission yeast bioassay. Phytochem. Lett. 2014, 8, 184–189. [Google Scholar] [CrossRef]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef]
- Jacinto, J.; Giovanetti, M.; Oliveira, P.B.; Valdiviesso, T.; Máguas, C.; Alegria, C. Quality attributes of cultivated white crowberries (Corema album (L.) D. Don) from a multi-origin clonal field. Euphytica 2021, 217, 40. [Google Scholar] [CrossRef]
- Fresco, P.; Borges, F.; Marques, M.P.; Diniz, C. The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr. Pharm. Des. 2010, 16, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ha, S.J.; Lee, H.J.; Kim, M.J.; Kim, J.H.; Kim, Y.T.; Song, K.M.; Kim, Y.J.; Kim, H.K.; Jung, S.K. Protective effect of Tremella fuciformis Berk extract on LPS-induced acute inflammation via inhibition of the NF-κB and MAPK pathways. Food Funct. 2016, 7, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zheng, T.; Liang, Y.; Duan, L.; Zhang, Y.; Wang, L.-J.; He, G.; Xiao, H. P-Coumaric Acid Protects Human Lens Epithelial Cells Against Oxidative Stress-Induced Apoptosis by MAPK signaling. Oxidative Med. Cell. Longev. 2018, 2018, 8549052. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liang, Q.H.; Xiong, X.G.; Wang, Y.; Zhang, Z.H.; Sun, M.J.; Lu, X.; Wu, D. Anti-Inflammatory Effects of p-Coumaric Acid, a Natural Compound of Oldenlandia diffusa, on Arthritis Model Rats. Evid. Based Complement. Altern. Med. 2018, 2018, 5198594. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.R.; Ramachandran, S.; Pugalendi, K.V.; Menon, V.P. Ferulic acid inhibits UV-B–induced oxidative stress in human lymphocytes. Nutr. Res. 2007, 27, 559–564. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, Z.; Li, J.; Shi, Y.; Jin, N.; Zou, W.; Gao, Q.; Wang, W.; Liu, F. Ferulic acid inhibits bovine endometrial epithelial cells against LPS-induced inflammation via suppressing NK-κB and MAPK pathway. Res. Vet. Sci. 2019, 126, 164–169. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Qi, J.; Liu, R.; Zhang, H.; He, L. Ferulic acid inhibits H2O2-induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NADPH oxidase and NF-κB pathway. Int. Immunopharmacol. 2015, 28, 1018–1025. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, J.; Wang, L.; Li, B.; Guo, J.; Guan, X.; Han, Q.; Zhang, H. Caffeic acid reduces cutaneous tumor necrosis factor alpha (TNF-alpha), IL-6 and IL-1beta levels and ameliorates skin edema in acute and chronic model of cutaneous inflammation in mice. Biol. Pharm. Bull. 2014, 37, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.R.; Karthikeyan, A.; Karthikeyan, S.; Reddy, B.V.J.M.; Biochemistry, C. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-kappaB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Sheng, Y.; Lu, B.; Ji, L. The therapeutic detoxification of chlorogenic acid against acetaminophen-induced liver injury by ameliorating hepatic inflammation. Chem. Biol. Interact. 2015, 238, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.Y.; Yang, H.R.; Zhang, J.; Li, D.; Lin, J.; Wang, L.; Xu, X. The studies of chlorogenic Acid antitumor mechanism by gene chip detection: The immune pathway gene expression. J. Anal. Methods Chem. 2013, 2013, 617243. [Google Scholar] [CrossRef] [PubMed]
- Búfalo, M.C.; Ferreira, I.; Costa, G.; Francisco, V.; Liberal, J.; Cruz, M.T.; Lopes, M.C.; Batista, M.T.; Sforcin, J.M. Propolis and its constituent caffeic acid suppress LPS-stimulated pro-inflammatory response by blocking NF-κB and MAPK activation in macrophages. J. Ethnopharmacol. 2013, 149, 84–92. [Google Scholar] [CrossRef]
- Yun, N.; Kang, J.W.; Lee, S.M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255. [Google Scholar] [CrossRef]
- Gao, X.H.; Zhang, S.D.; Wang, L.T.; Yu, L.; Zhao, X.L.; Ni, H.Y.; Wang, Y.Q.; Wang, J.D.; Shan, C.H.; Fu, Y.J. Anti-Inflammatory Effects of Neochlorogenic Acid Extract from Mulberry Leaf (Morus alba L.) Against LPS-Stimulated Inflammatory Response through Mediating the AMPK/Nrf2 Signaling Pathway in A549 Cells. Molecules 2020, 25, 1385. [Google Scholar] [CrossRef]
- Kim, M.; Choi, S.Y.; Lee, P.; Hur, J. Neochlorogenic Acid Inhibits Lipopolysaccharide-Induced Activation and Pro-inflammatory Responses in BV2 Microglial Cells. Neurochem. Res. 2015, 40, 1792–1798. [Google Scholar] [CrossRef]
- Chang, C.H.; Chang, Y.T.; Tseng, T.H.; Wang, C.J. Mulberry leaf extract inhibit hepatocellular carcinoma cell proliferation via depressing IL-6 and TNF-alpha derived from adipocyte. J. Food Drug Anal. 2018, 26, 1024–1032. [Google Scholar] [CrossRef]
- Park, S.Y.; Jin, M.L.; Yi, E.H.; Kim, Y.; Park, G. Neochlorogenic acid inhibits against LPS-activated inflammatory responses through up-regulation of Nrf2/HO-1 and involving AMPK pathway. Environ. Toxicol. Pharmacol. 2018, 62, 1–10. [Google Scholar] [CrossRef]
- Colombo, M.; Figueiró, F.; de Fraga Dias, A.; Teixeira, H.F.; Battastini, A.M.O.; Koester, L.S. Kaempferol-loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int. J. Pharm. 2018, 543, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zuo, J.; Tan, C.; Xian, S.; Luo, C.; Chen, S.; Yu, L.; Luo, Y. Kaempferol, a Flavonoid Compound from Gynura Medica Induced Apoptosis and Growth Inhibition in Mcf-7 Breast Cancer Cell. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Bao, J.; Wei, Y.; Chen, Y.; Mao, X.; Li, J.; Yang, Z.; Xue, Y. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Cui, W.; Yang, X.; Tong, B. Kaempferol inhibits the growth and metastasis of cholangiocarcinoma in vitro and in vivo. Acta Biochim. Et Biophys. Sin. 2016, 48, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Lee, Y.H.; Sharma, A.R.; Park, J.B.; Jagga, S.; Sharma, G.; Lee, S.S.; Nam, J.S. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. Korean J. Physiol. Pharm. 2017, 21, 205–213. [Google Scholar] [CrossRef]
- Lv, L.; Liu, C.; Chen, C.; Yu, X.; Chen, G.; Shi, Y.; Qin, F.; Ou, J.; Qiu, K.; Li, G. Quercetin and doxorubicin co-encapsulated biotin receptor-targeting nanoparticles for minimizing drug resistance in breast cancer. Oncotarget 2016, 7, 32184–32199. [Google Scholar] [CrossRef]
- Maurya, A.K.; Vinayak, M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol. Biol. Rep. 2015, 42, 1419–1429. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, S.; Shin, Y.S.; Cho, M.; Kang, H.; Cho, H. Anti-Cancer Effect of Quercetin in Xenograft Models with EBV-Associated Human Gastric Carcinoma. Molecules 2016, 21, 1286. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Kim, S.J.; Kim, J.H. Quercetin-3-O-glucoside suppresses pancreatic cancer cell migration induced by tumor-deteriorated growth factors in vitro. Oncol. Rep. 2016, 35, 2473–2479. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, Y.; Mao, M.; Lv, J.; Zhang, L.; Li, S.; Li, X.; Zheng, X. Antioxidant mechanism of Rutin on hypoxia-induced pulmonary arterial cell proliferation. Molecules 2014, 19, 19036–19049. [Google Scholar] [CrossRef] [PubMed]
- Koval’Skii, I.V.; Krasnyuk, I.I.; Krasnyuk, I.I.; Nikulina, O.I.; Belyatskaya, A.V.; Kharitonov, Y.Y.; Feldman, N.B.; Lutsenko, S.V. Mechanisms of Rutin Pharmacological Action (Review). Pharm. Chem. J. 2014, 48, 73–76. [Google Scholar] [CrossRef]
- Kostić, D.A.; Dimitrijević, D.S.; Stojanović, G.S.; Palić, I.R.; Đorđević, A.S.; Ickovski, J.D. Xanthine Oxidase: Isolation, Assays of Activity, and Inhibition. J. Chem. 2015, 2015, 294858. [Google Scholar] [CrossRef]
- Saleh, A.; ElFayoumi, H.M.; Youns, M.; Barakat, W. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. Naunyn Schmiedebergs Arch. Pharm. 2019, 392, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, A.Y.; Ye, X.; Li, B.; Rojanasakul, Y.; Rankin, G.O.; Chen, Y.C. Myricetin inhibits proliferation of cisplatin-resistant cancer cells through a p53-dependent apoptotic pathway. Int. J. Oncol. 2015, 47, 1494–1502. [Google Scholar] [CrossRef]
- Zang, W.; Wang, T.; Wang, Y.; Li, M.; Xuan, X.; Ma, Y.; Du, Y.; Liu, K.; Dong, Z.; Zhao, G. Myricetin exerts anti-proliferative, anti-invasive, and pro-apoptotic effects on esophageal carcinoma EC9706 and KYSE30 cells via RSK2. Tumour. Biol. 2014, 35, 12583–12592. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Yu, X.; Xu, S.; Li, D.; Zheng, Q.; Yin, Y. Myricetin Suppresses the Propagation of Hepatocellular Carcinoma via Down-Regulating Expression of YAP. Cells 2019, 8, 358. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, M.; Wang, L.; Yu, S. Anti-tumor effects and associated molecular mechanisms of myricetin. Biomed. Pharmacother. 2019, 120, 109506. [Google Scholar] [CrossRef]
- Urias-Lugo, D.A.; Heredia, J.B.; Muy-Rangel, M.D.; Valdez-Torres, J.B.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Anthocyanins and Phenolic Acids of Hybrid and Native Blue Maize (Zea mays L.) Extracts and Their Antiproliferative Activity in Mammary (MCF7), Liver (HepG2), Colon (Caco2 and HT29) and Prostate (PC3) Cancer Cells. Plant Foods Hum. Nutr. 2015, 70, 193–199. [Google Scholar] [CrossRef]
- Pyo, M.Y.; Yoon, S.J.; Yu, Y.; Park, S.; Jin, M. Cyanidin-3-glucoside suppresses Th2 cytokines and GATA-3 transcription factor in EL-4 T cells. Biosci. Biotechnol. Biochem. 2014, 78, 1037–1043. [Google Scholar] [CrossRef]
- Zeng, L.; Gao, J.; Zhang, R. Study on anti-tumor effect of cyanidin-3-glucoside on ovarian cancer. Zhongguo Zhong Yao Za Zhi 2012, 37, 1651–1654. [Google Scholar] [PubMed]
- Guo, H.; Liu, G.; Zhong, R.; Wang, Y.; Wang, D.; Xia, M. Cyanidin-3-O-beta-glucoside regulates fatty acid metabolism via an AMP-activated protein kinase-dependent signaling pathway in human HepG2 cells. Lipids Health Dis. 2012, 11, 10. [Google Scholar] [CrossRef]
- Ni, T.; Yang, W.; Xing, Y. Protective effects of delphinidin against H2O2-induced oxidative injuries in human retinal pigment epithelial cells. Biosci. Rep. 2019, 39, BSR20190689. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Park, Y.-J.; Song, M.-G.; Kim, D.R.; Zada, S.; Kim, D.-H. Cytoprotective Effects of Delphinidin for Human Chondrocytes against Oxidative Stress through Activation of Autophagy. Antioxidants 2020, 9, 83. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Adhami, V.M.; Esnault, S.; Sechi, M.; Siddiqui, I.A.; Satyshur, K.A.; Syed, D.N.; Dodwad, S.M.; Chaves-Rodriquez, M.I.; Longley, B.J.; et al. Dual Inhibition of PI3K/Akt and mTOR by the Dietary Antioxidant, Delphinidin, Ameliorates Psoriatic Features In Vitro and in an Imiquimod-Induced Psoriasis-Like Disease in Mice. Antioxid. Redox Signal. 2017, 26, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Khanna, D. Green tea catechins: Defensive role in cardiovascular disorders. Chin. J. Nat. Med. 2013, 11, 345–353. [Google Scholar] [CrossRef]
- Suzuki, J.; Ogawa, M.; Futamatsu, H.; Kosuge, H.; Sagesaka, Y.M.; Isobe, M. Tea catechins improve left ventricular dysfunction, suppress myocardial inflammation and fibrosis, and alter cytokine expression in rat autoimmune myocarditis. Eur. J. Heart Fail. 2007, 9, 152–159. [Google Scholar] [CrossRef]
- Cheng, A.W.; Tan, X.; Sun, J.Y.; Gu, C.M.; Liu, C.; Guo, X. Catechin attenuates TNF-alpha induced inflammatory response via AMPK-SIRT1 pathway in 3T3-L1 adipocytes. PLoS ONE 2019, 14, e0217090. [Google Scholar] [CrossRef]
- Frei, B.; Higdon, J.V. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Jun, M.; Jeong, W.S. Procyanidins from wild grape (Vitis amurensis) seeds regulate ARE-mediated enzyme expression via Nrf2 coupled with p38 and PI3K/Akt pathway in HepG2 cells. Int. J. Mol. Sci. 2012, 13, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Truong, V.L.; Ko, S.Y.; Nguyen, X.N.; Ingkasupart, P.; Jun, M.; Shin, J.Y.; Jeong, W.S. Antioxidant and Hepatoprotective Effects of Procyanidins from Wild Grape (Vitis amurensis) Seeds in Ethanol-Induced Cells and Rats. Int. J. Mol. Sci. 2016, 17, 758. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Tang, Q.; Huang, H.; Hao, W.; Wei, X. Grape-seed proanthocyanidins inhibit the lipopolysaccharide-induced inflammatory mediator expression in RAW264.7 macrophages by suppressing MAPK and NF-kappab signal pathways. Env. Toxicol. Pharm. 2016, 41, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages. Nutr. Res. Pract. 2016, 10, 251. [Google Scholar] [CrossRef]
- Cho, B.O.; Yin, H.H.; Park, S.H.; Byun, E.B.; Ha, H.Y.; Jang, S.I. Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of NF-κB and STAT1 activation and Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated RAW264.7 macrophages. Biosci. Biotechnol. Biochem. 2016, 80, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-H.; Zhu, J.-X.; Feng, H.; Ni, J.; Zhang, N.; Chen, S.; Liu, H.-J.; Yang, Z.; Deng, W.; Tang, Q.-Z. Myricetin Possesses Potential Protective Effects on Diabetic Cardiomyopathy through Inhibiting IκBα/NFκB and Enhancing Nrf2/HO-1. Oxidative Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, O.; Nass, J.; Saeed, M.E.; Schuler, B.; Efferth, T. Kaempferol Is an Anti-Inflammatory Compound with Activity towards NF-κB Pathway Proteins. Anticancer Res. 2015, 35, 2645–2650. [Google Scholar] [PubMed]
- Sun, Z.; Li, Q.; Hou, R.; Sun, H.; Tang, Q.; Wang, H.; Hao, Z.; Kang, S.; Xu, T.; Wu, S. Kaempferol-3-O-glucorhamnoside inhibits inflammatory responses via MAPK and NF-kappaB pathways in vitro and in vivo. Toxicol. Appl. Pharm. 2019, 364, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Sikder, K.; Bhattacharjee, S.; Majumdar, S.B.; Ghosh, S.; Majumdar, S.; Dey, S. Quercetin alleviates inflammation after short-term treatment in high-fat-fed mice. Food Funct. 2013, 4, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.J.; Kim, C.S.; Kang, J.H.; Park, J.Y.; Choe, S.Y.; Hong, S.M.; Yoo, H.; Park, T.; Yu, R. Quercetin suppresses MIP-1alpha-induced adipose inflammation by downregulating its receptors CCR1/CCR5 and inhibiting inflammatory signaling. J. Med. Food 2014, 17, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar] [CrossRef]
- Chang, Y.C.; Tsai, M.H.; Sheu, W.H.; Hsieh, S.C.; Chiang, A.N. The therapeutic potential and mechanisms of action of quercetin in relation to lipopolysaccharide-induced sepsis in vitro and in vivo. PLoS ONE 2013, 8, e80744. [Google Scholar] [CrossRef] [PubMed]
- Napimoga, M.H.; Clemente-Napimoga, J.T.; Macedo, C.G.; Freitas, F.F.; Stipp, R.N.; Pinho-Ribeiro, F.A.; Casagrande, R.; Verri, W.A., Jr. Quercetin inhibits inflammatory bone resorption in a mouse periodontitis model. J. Nat. Prod. 2013, 76, 2316–2321. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef]
- Zhang, W.; Li, B.; Guo, Y.; Bai, Y.; Wang, T.; Fu, K.; Sun, G. Rhamnetin attenuates cognitive deficit and inhibits hippocampal inflammatory response and oxidative stress in rats with traumatic brain injury. Cent. Eur. J. Immunol. 2015, 40, 35–41. [Google Scholar] [CrossRef]
- Hirai, S.; Kim, Y.I.; Goto, T.; Kang, M.S.; Yoshimura, M.; Obata, A.; Yu, R.; Kawada, T. Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages. Life Sci. 2007, 81, 1272–1279. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- Rasul, A.; Millimouno, F.M.; Ali Eltayb, W.; Ali, M.; Li, J.; Li, X. Pinocembrin: A novel natural compound with versatile pharmacological and biological activities. BioMed Res. Int. 2013, 2013, 379850. [Google Scholar] [CrossRef]
- Gagliotti Vigil de Mello, S.V.; Frode, T.S. In Vitro and In Vivo Experimental Model-based Approaches for Investigating Anti-inflammatory Properties of Coumarins. Curr. Med. Chem. 2018, 25, 1446–1476. [Google Scholar] [CrossRef]
- Shin, S.Y.; Yoon, H.; Ahn, S.; Kim, D.W.; Bae, D.H.; Koh, D.; Lee, Y.H.; Lim, Y. Structural properties of polyphenols causing cell cycle arrest at G1 phase in HCT116 human colorectal cancer cell lines. Int. J. Mol. Sci. 2013, 14, 16970–16985. [Google Scholar] [CrossRef]
- Feng, R.; Lu, Y.; Bowman, L.L.; Qian, Y.; Castranova, V.; Ding, M. Inhibition of activator protein-1, NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J. Biol. Chem. 2005, 280, 27888–27895. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Angeles Martín, M.; Goya, L.; Bravo, L.; Ramos, S. Time-course regulation of survival pathways by epicatechin on HepG2 cells. J. Nutr. Biochem. 2009, 20, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.A.; Acharya, J.D.; Mehendale, N.K.; Kamat, S.S.; Ghaskadbi, S.S. Pterostilbene reverses palmitic acid mediated insulin resistance in HepG2 cells by reducing oxidative stress and triglyceride accumulation. Free Radic. Res. 2019, 53, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, G.; Morales Escobar, L.; Braca, A.; De Tommasi, N. Antioxidant chalcone glycosides and flavanones from Maclura (Chlorophora) tinctoria. J. Nat. Prod. 2003, 66, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
| Group | Sub-Group | Compound | General Structure | Ref. |
|---|---|---|---|---|
| PHENOLIC ACIDS | Benzoic acid | 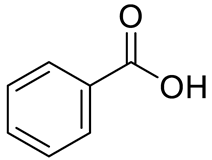 | [12] | |
| Salicilic acid | 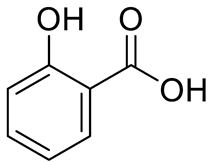 | [12] | ||
| Tannic acid | 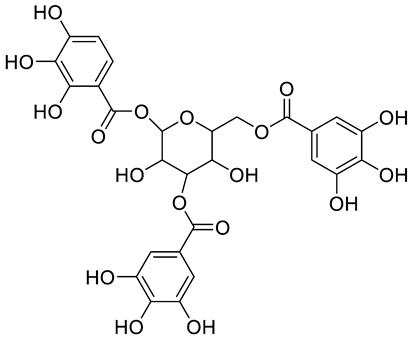 | [13] | ||
| Hydroxibenzoic acids | p-hydroxybenzoic acid (R=R1=R2=H) and derivatives Vanillic acid (R=R1=H; R2=OCH3) Protocatechuic acid (R=R1=H; R2=OH) Syringic aid (R=H; R1=R2= OCH3) Gallic acid (R=H; R1=R2=OH) |  | [7,10,11,12,14] | |
| Hydroxicinnamic acids | t-Cinnamic acid |  | [12] | |
| p-coumaric acid (R=R1=R2=H) Sinapic acid (R=H; R1=R2=OCH3) Ferulic acid (R=R2=H; R1=OCH3) and derivatives Caffeic acid and derivatives (R=R2=H; R1=OH/O-Hexose; R2=H) | 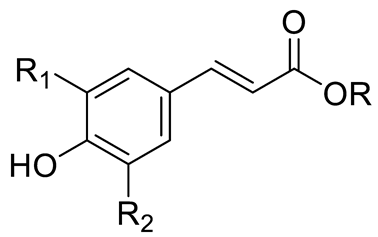 | [7,12,14] | ||
| Chlorogenic acid (R=R1=R2=H; R3=Caffeic acid) Neochlorogenic acid (R=R1=R2= H; R3= Caffeic acid) Cryptochlorogenic acid (R=R2=R4=H; R3= Caffeic acid) | 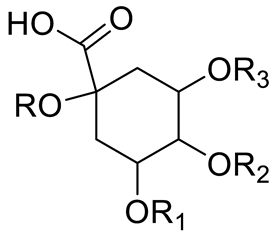 | [7,14,15,16] | ||
| FLAVONOIDS | Flavonols | Kaempherol (R1=R2=R4=OH; R3=R5=R6=H) and derivatives: - i.e., Kaempherol 3-O-galactoside (R6=galactose) - i.e., Kaempherol 3-O-glucoside (R6=glucose) | 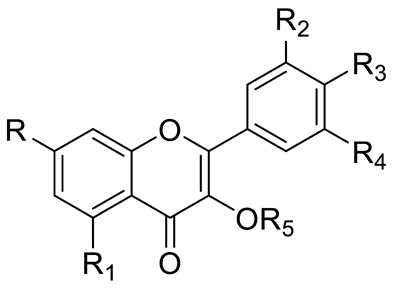 | [7,14,15,16] |
| Quercetin (R=R1=R2=R3=OH; R4=R5=H) and derivatives: - i.e., Quercetin 3-O-glucoside (R5=glucose) - i.e., Quercetin 3-O-arabinoside (R5=arabinose) - i.e., Quercetin 3-O-galactoside (R5=galactose) - i.e., Quercetin rhamnoside (R5=rhamnose) Rutin (R= R1= R2=R3=OH; R4=H; R5=glucopyranose) | [7,15,16] | |||
| Myricetin (R=R1=R2=R3=OH; R4=H; R5=OH) and derivatives: - i.e., Myricetin 3-O-glucoside (R=R1=R2=R3=OH; R4=H; R5=O-glucose) | [7,15,16] | |||
| Catechin (R=R1=R2=R3=R4=R5=OH) and derivatives | [15] | |||
| Procyanidin (R=H, n=1) and derivatives: - i.e., Procyanidin Dimer type A (R=H, n=2) | 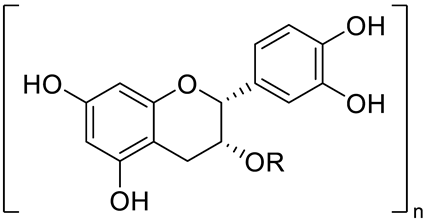 | [15] | ||
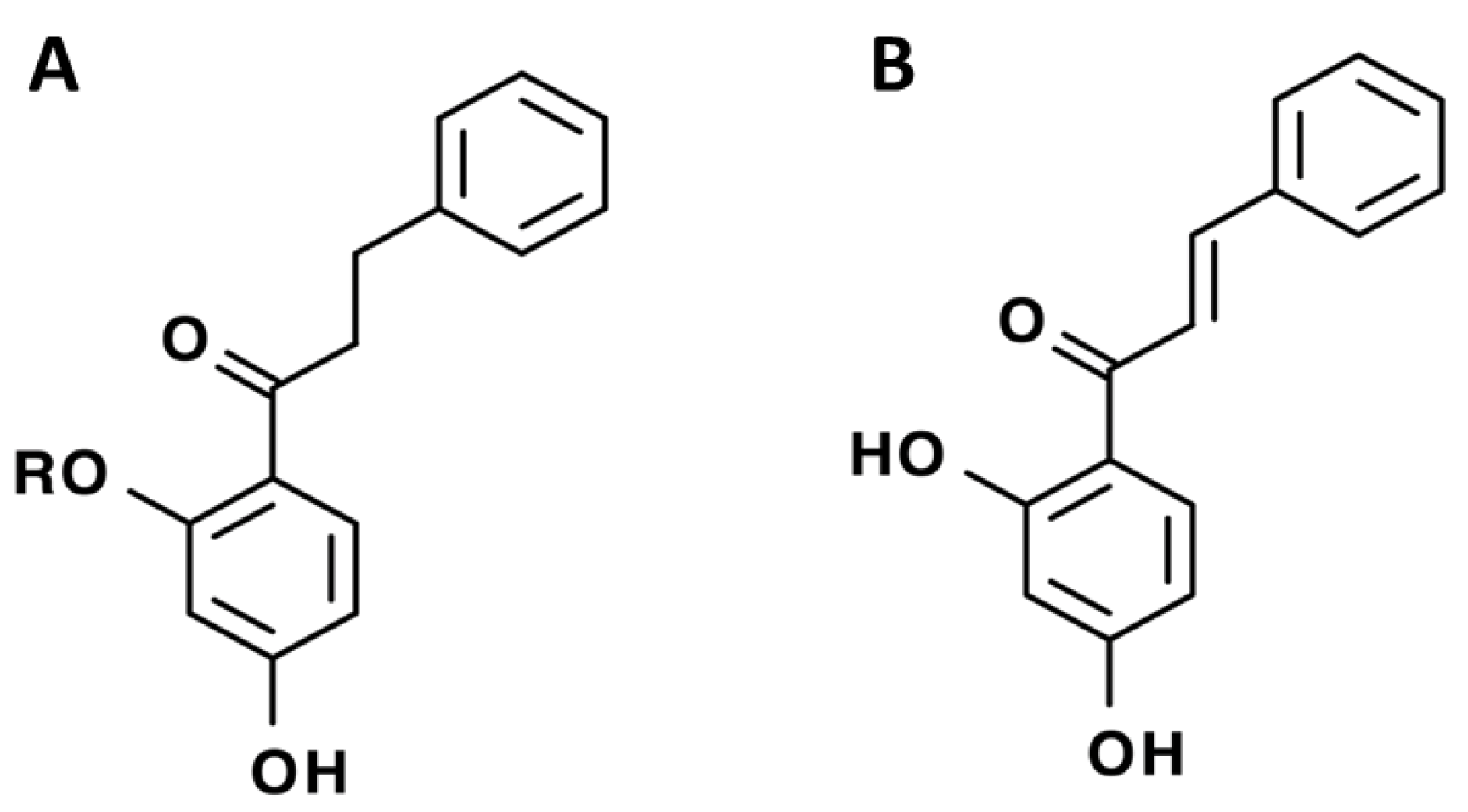
| Flavanones | pinocembrin | 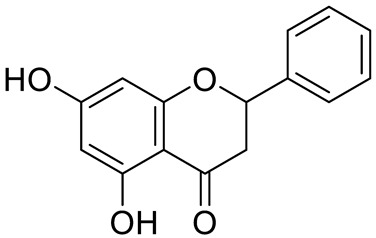 | [7] | |
| 6-geranylnaringenin | 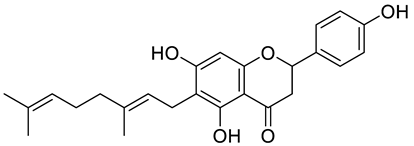 | [7] | ||
| Anthocyanins | Cyanidin (R1=R2=OH; R3=R4=R5=R6=H) and derivatives - i.e., Cyanidin 3-O-glucoside (R6=glucose) - i.e., Cyanidin 3-O-arabinoside (R6=arabinose) |  | [7] | |
| Delphinidin (R1=R2=R3=R4=R5=OH; R6=H) and derivatives: - i.e., Delphinidin 3-O-glucoside (R6= glucose) | [7] | |||
| STILBENES | Resveratrol (R1=R2=H) and derivatives: - i.e., Pterostilbene (R1=R2=CH3) - i.e., Stilbene Hexoside (R2=Hexose) | 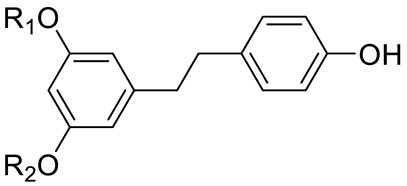 | [7,15] |
| Group | Sub-Group | Compound | General Structure | Ref. |
|---|---|---|---|---|
| PHENOLIC ACIDS | Hydroxycinnamic acids | Coumaric acid (R=R1=R2=H) and derivatives: - i.e., Coumaroyl Glucose (R=Glucose) | 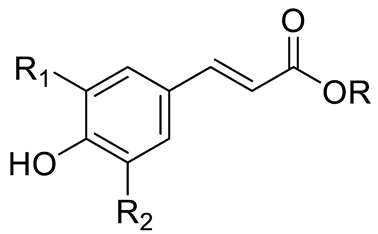 | [15,16] |
| FLAVONOIDS | Flavanols | Catechin (R=R1=R2=R3=R4=R5=OH) and derivatives: - i.e., Catechin 3-O-glucose (R3=Glucose) Epicatechin (R=R1=R2=R5=OH; R3=R4=H) | 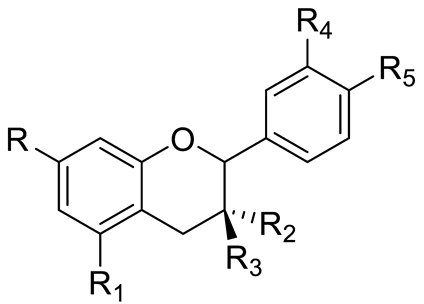 | [15,16] |
| Procyanidin (R=H, n=1) and derivatives: - i.e., Procyanidin Trimer (R=H, n=3) - i.e., Procyanidin Tretramer (R=H, n=4) - i.e., Procyanidin Galhate (R=Galhate, n=1) |  | [15,16] | ||
| Flavones or Flavonols | Myricetin (R=R1=R2=R3=R5=OH; R4=H) and derivatives: - i.e., Myricetin 3-O-galactoside (R5=O-galactose) - i.e., Myricetin 3-O-glucoside (R5=O-glucose) - i.e., Myricetin Xyloside (R5=O-xylose) - i.e., Myricetin Rhamnoside (R5=O-rhamnose) - i.e., Myricetin Methyl ether Hexoside | 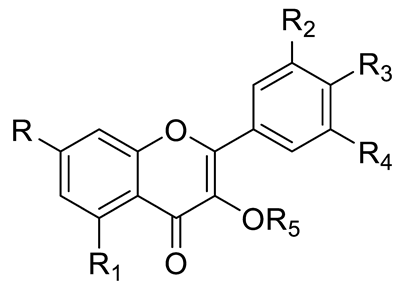 | [15,16] | |
| Kaempherol (R=R1=R3=OH; R2=R4=R5=H) and derivatives: - i.e., Kaempherol Hexoside (R5=Hexose) |  | [15] | ||
| Rhamnetin (R=OCH3; R1=R2=R3=OH; R4=R5=H) and derivatives: - i.e., Rhamnetin Hexoside (R5=Hexose) | [16] | |||
| Quercetin (R=R1=R2=R3=OH; R4=R5=H) and derivatives: - i.e., Quercitin-3-O-glucoside (R5=glucose) - i.e., Quercitin-3-O-galactoside (R5=galactose) - i.e., Quercetin Rhamnosyl Hexoside (R5=Rhamnosoyl Hexose) - i.e., Methyl-quercitin hexoside (R5=Hexose) Rutin (R= R1= R2=R3=OH; R4=H; R5=glucopyranose) | [15,16] | |||
| Pinocembrin | 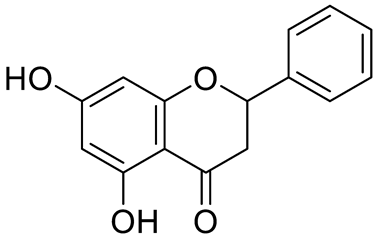 | [18] | ||
| STILBENES | Stilbenes and derivatives | 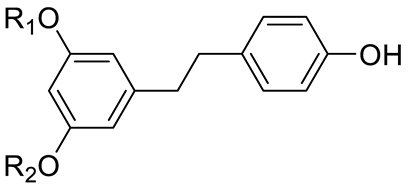 | [15,16] |
| Compound | Protective Mechanisms (s) | Experimental Model | Ref. |
|---|---|---|---|
| p-hydroxybenzoic acid |
| Mouse macrophages | [24] |
| p-coumaric acid |
| - Human epithelial cells - Animal models: rheumatoid arthritis rats | [25,26] |
| Ferulic acid |
| - Human lymphocytes - Bovine endometrial epithelial cells - Rat vascular smooth muscle cells | [27,28,29] |
| Caffeic acid and derivatives |
| - Human cancer cells fibrosarcoma - Animal model: albino mice (BALB/c) | [30,31] |
| Chlorogenic acid |
| - Mouse macrophages | [32,33,34,35,36] |
| Neochlorogenic acid |
| - Human cancer cells: lung - Mouse cells: macrophages, microglia, fibroblasts | [37,38,39,40] |
| Kaempherol and derivatives |
| - Human cancer cells: brain, breast, stomach, liver, QBC939 (human cholangiocarcinoma) - HCCC9810 (mice) and (human) | [41,42,43,44,45] |
| Quercetin and derivatives Rutin |
| - Human cancer cells: breast, liver - Human cancer cells: neuroblastoma - Animal models: Calf lung and muscle cells; Albino rats of Wistar strain. | [46,47,48,49,50] [51,52,53,54] |
| Myricetin and derivatives |
| - Human cancer cells: esophagus, ovary, and liver | [55,56,57,58] |
| Cyanidin and derivatives |
| - Human cancer cells: breast, liver, colon, prostate and ovarian. - Animal model: Murine thymoma | [59,60,61,62] |
| Delphinidin and derivatives |
| - Human cells (normal): eye, keratinocytes - Transformed cell line: human chondrocyte | [63,64,65] |
| Compound | Protective Mechanisms (s) | Experimental Model | Ref. |
|---|---|---|---|
| Catechin and derivatives Epicatechin |
| - Animal studies: mice and rats. - Animal model: experimental autoimmune myocarditis rats, mouse fibroblasts | [66,67,68,69] |
| Procyanidin and derivatives |
| - Human cancer cells: liver - Animal models: Rat liver, mouse macrophages | [70,71,72,73] |
| Myricetin and derivatives |
| - Animal model: mouse macrophage, diabetic cardiomyopathy mice | [74,75] |
| Kaempherol and derivatives |
| - Human cancer cells: leukemia Animal model: mouse macrophage | [76,77] |
| Quercetin and derivatives |
| - Animal model: HFD-induced inflammatory mice, mouse macrophages, male C57BL/6 mice, periodontitis mice | [78,79,80,81,82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerquido, A.S.; Vojtek, M.; Ribeiro-Oliveira, R.; Viegas, O.; Sousa, J.B.; Ferreira, I.M.P.L.V.O.; Diniz, C. Unravelling Potential Health-Beneficial Properties of Corema album Phenolic Compounds: A Systematic Review. Pharmaceuticals 2022, 15, 1231. https://doi.org/10.3390/ph15101231
Cerquido AS, Vojtek M, Ribeiro-Oliveira R, Viegas O, Sousa JB, Ferreira IMPLVO, Diniz C. Unravelling Potential Health-Beneficial Properties of Corema album Phenolic Compounds: A Systematic Review. Pharmaceuticals. 2022; 15(10):1231. https://doi.org/10.3390/ph15101231
Chicago/Turabian StyleCerquido, Ana Sofia, Martin Vojtek, Rita Ribeiro-Oliveira, Olga Viegas, Joana Beatriz Sousa, Isabel M. P. L. V. O. Ferreira, and Carmen Diniz. 2022. "Unravelling Potential Health-Beneficial Properties of Corema album Phenolic Compounds: A Systematic Review" Pharmaceuticals 15, no. 10: 1231. https://doi.org/10.3390/ph15101231
APA StyleCerquido, A. S., Vojtek, M., Ribeiro-Oliveira, R., Viegas, O., Sousa, J. B., Ferreira, I. M. P. L. V. O., & Diniz, C. (2022). Unravelling Potential Health-Beneficial Properties of Corema album Phenolic Compounds: A Systematic Review. Pharmaceuticals, 15(10), 1231. https://doi.org/10.3390/ph15101231








