3.1. Chemistry
All chemicals and solvents were purchased from different chemical suppliers and were used without further purification unless stated otherwise. Dichloromethane (DCM) was distilled under nitrogen immediately before use. The drying agent used for DCM was calcium hydride. Reactions were monitored by thin layer chromatography (TLC) carried out on aluminum sheets precoated with silica gel 60 F254 (Merck KGaA, Darmstadt, Germany). Compounds were visualized under UV light (254 nm). Column chromatography was performed on Merck silica gel 60 (40–63 µm) as a stationary phase. Melting points were measured in open capillary tubes in a Thomas HooverTM apparatus (Thomas Scientific, Seattle, WA, United States) and are uncorrected. IR spectra were determined as KBr pellets on a ShimadzuTM model 470 spectrophotometer (Shimadzu Co., Kyoto, Japan) and are expressed in cm−1. The 1H and 13C NMR spectra were recorded on a Bruker AvanceTM 300 (300 MHz/75.5 MHz) (Bruker Bioscience, Billerica, MA, United States) or a JEOL EclipseTM 270 (270 MHz/67.9 MHz) (JEOL Ltd., Tokyo, Japan) spectrometer using CDCl3 as the solvent, and are reported in ppm downfield from the residual CHCl3 (δ 7.25 ppm for 1H-NMR and 77.0 ppm for 13C-NMR). Signal multiplicity is given as singlet (s), doublet (d), double doublet (dd), multiplet (m), quartet (q), where coupling constant (J) values were estimated in Hertz. A Perkin ElmerTM 2400 CHN elemental analyzer (Perkin Elmer, Inc., Waltham, MA, United States) was used to obtain the elemental analyses, and the results were within ±0.4% of the predicted values. Exact molecular masses were determined on a Finnigan TSQ Quantum Ultra (IET. Ltd., Mundelein, IL, USA) spectrometer equipped with an electrospray ion source.
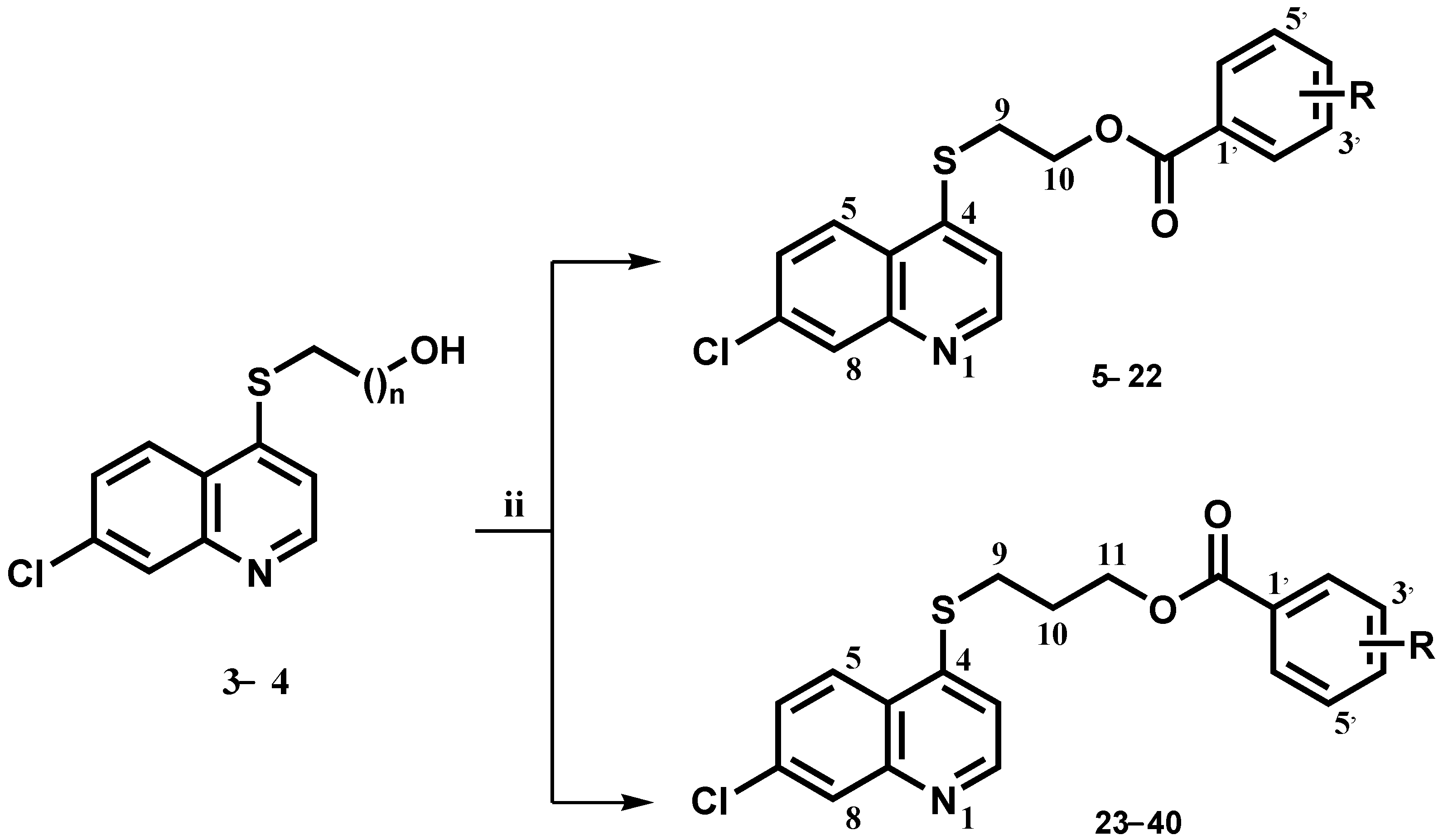
3.1.1. General Procedure for the Synthesis of Compounds 3,4
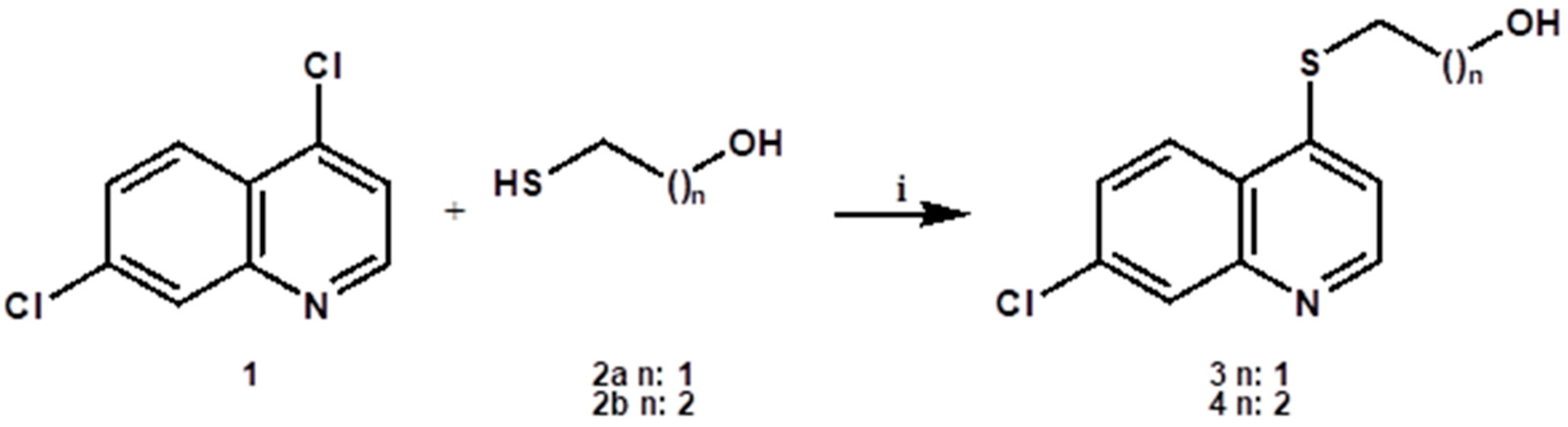
To a solution of 4,7-Dichloroquinoline 1 (5.0 g 25 mmol) in dry ethanol (100 mL) was added dropwise mercapto alcohol 2a or 2b (30 mmol) and triethylamine (5.3 mL, 37.5 mmol). The mixture was stirred at reflux temperature (80 °C) for 5 days, under a N2 atmosphere and then allowed to cool down to room temperature. The solvent was evaporated under reduced pressure. To the resulting solid was added ethyl acetate (150 mL), and the organic layer was subsequently washed with water (100 mL), with 10% sodium bicarbonate (2 × 20 mL) and a saturated NaCl solution (50 mL). Anhydrous sodium sulfate was finally added to the organic layer, which was then filtered and evaporated under reduced pressure. The compounds were then purified by column chromatography.
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethanol (
3). Column chromathography DCM:EtOAc:MeOH (7:2:1). White solid, yield: 52%; m.p. 106–107 °C, (161 °C) [
27]; IR (KBr) cm
−1: 3270, 2985, 1593;
1H NMR (CDCl
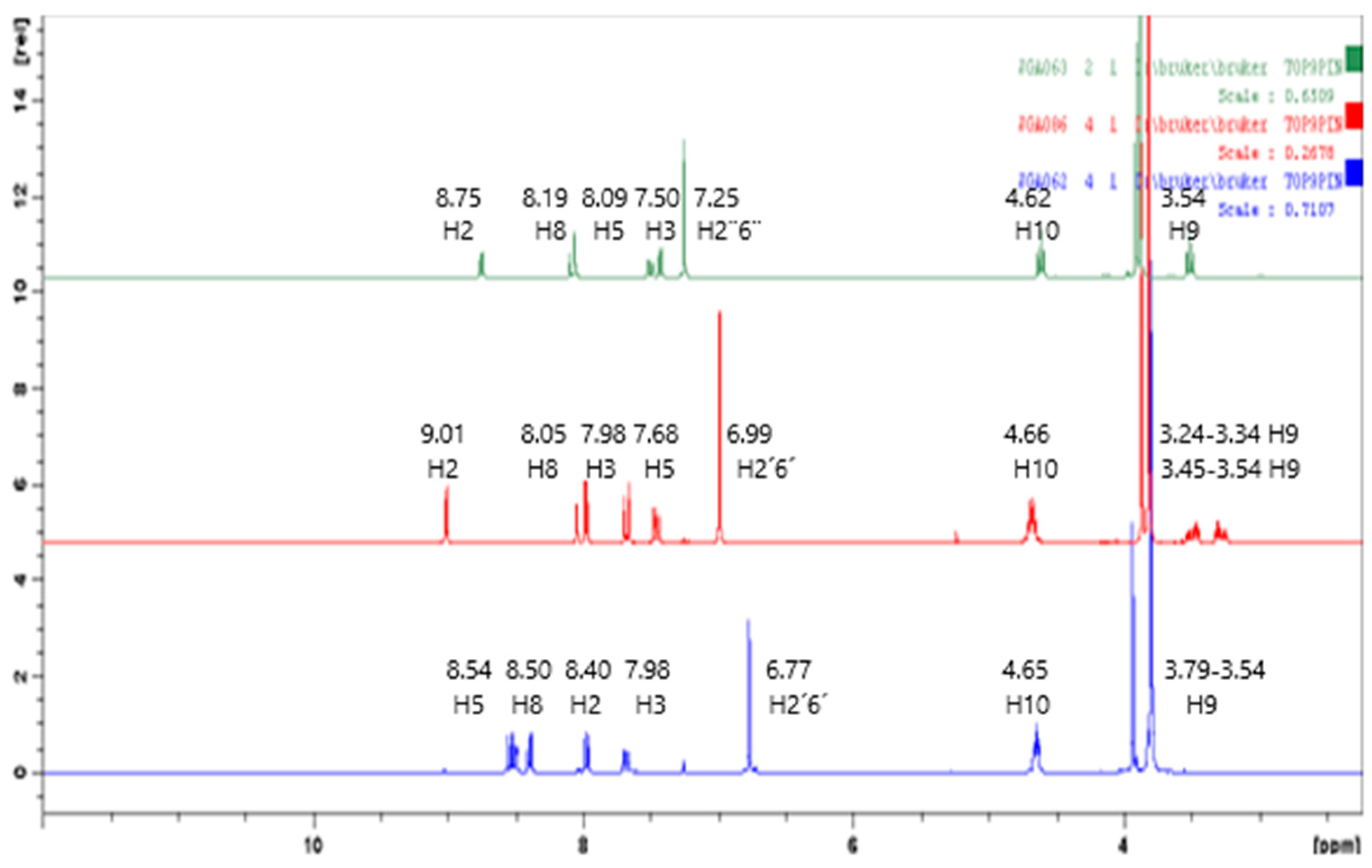
3, 300 MHz) δ ppm: 3.33 (t, 2H, H9, J = 6.1 Hz), 3.99 (t, 2H, H10, J = 6.1 Hz), 7.21 (d, 1H, H3, J = 4.9 Hz), 7.48 (dd, 1H, H6, J = 2.0, 9.0 Hz), 8.04–8.07 (m, 2H, H5,8), 8.65 (d, 1H, H2, J = 4.8 Hz);
13C NMR (CDCl
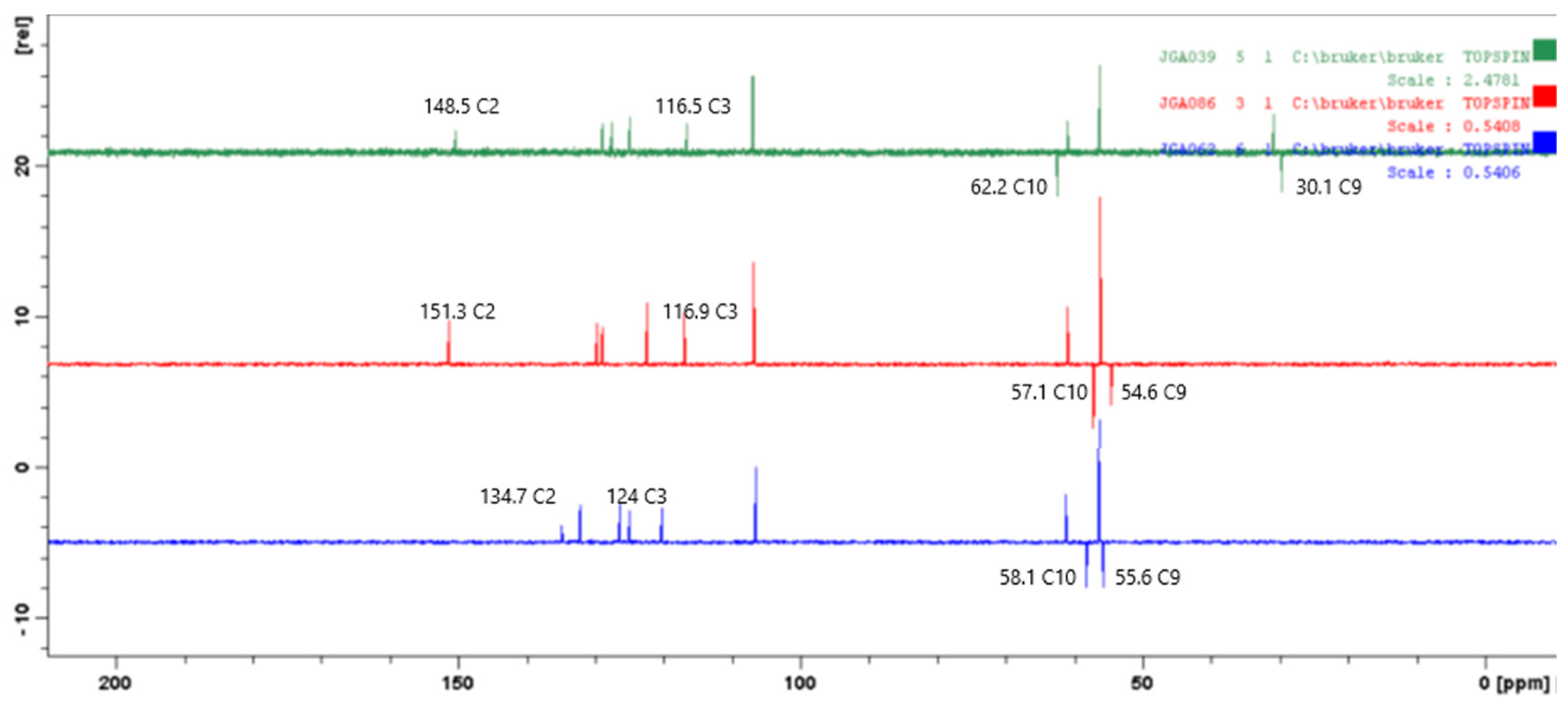
3, 75 MHz) δ ppm: 34.1 (C9), 60.2 (C10), 116.4 (C3), 124.9 (C5), 125,1, 127.4 (C8), 128.8 (C6), 135.8, 147.1, 147.9, 150.1 (C2). Anal. calcd. for: C
11H
10ClNOS: C 55.11, H 4.20, N 5.84; Found: C 55.10, H 4.23, N 5.97. MS:
m/
z 240.02 (M+H
+. 11%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propan-1-ol (4). Column chromathography DCM:EtOAc:MeOH (7:2.5:0.5). Yellow solid, yield: 71%; m.p. 125–127 °C; IR (KBr) cm−1: 3450, 2850, 1598; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.01–2.09 (m, 2H, H10), 2.48 (brs, 1H, OH), 3.23 (t, 2H, H9, J = 7.2 Hz), 3.86 (t, 2H, H11, J = 5.9 Hz), 7.15 (d, 1H, H3, J = 4.9 Hz), 7.46 (dd, 1H, H6, J = 2.2, 8.9 Hz), 8.01 (d, 1H, H5, J = 8.9 Hz), 8.03 (d, 1H, H8, J = 2.3 Hz), 8.63 (d, 1H, H2, J = 4.9 Hz). 13C NMR (CDCl3 75 MHz) δ ppm: 27.7 (C10), 31.1 (C9), 60.9 (C11), 116.0 (C3), 125.1, 127.4, 128.8, 135.8, 147.9, 148.3, 150.2. Anal. calcd. for: C12H12ClNOS: C 56.80, H 4.77, N 5.52; Found: C 56.77, H 4.79, N 5.75. MS: m/z 254.04 (M+H+. 13%).
3.1.2. General Procedure for the Synthesis of Compounds 5–40
A solution of the selected benzoic acid derivative (1.2 mmol) in dry DCM (15 mL) was treated with EDCI (1.5 mmol) and DMAP (0.4 mmol). The mixture was shaken at −10 °C for 30 min. The respective intermediates, 3 or 4 (0.65 mmol), dissolved in dry DCM (1 mL), were slowly added, and the resulting mixture was left stirring for 24 h at room rt, under a N2 atmosphere. Next, water was added and the aqueous fraction was extracted with DCM (2 × 10 mL). The organic layer was washed with 10% sodium bicarbonate (2 × 10 mL), a saturated NaCl solution (3 × 10 mL), and finally dried over Na2SO4, filtered, and evaporated under reduced pressure to give the crude product. The compounds were then purified by recrystallization or column chromatography.
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl-4-methoxybenzoate (5). Column chromathography DCM:EtOAc (8:2). White solid, yield: 92%; m.p. 116–118 °C; IR (KBr) cm−1: 3030, 2971, 1699, 1242; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.48 (t, 2H, H9, J = 6.9 Hz), 3.85 (s, 3H, OMe), 4.58 (t, 2H, H10, J = 6.7 Hz), 6.89 (d, 2H, H3′,5′, J = 8.9 Hz), 7.41 (d, 1H, H3, J = 4.7 Hz), 7.50 (dd, 1H, H6, J = 2.3, 8.9 Hz), 7.95 (d, 2H, H2′,6′, J = 8.9 Hz), 8.06 (d, 1H, H8, J = 2.3 Hz), 8.10 (d, 1H, H5, J = 8.9 Hz), 8.72 (d, 1H, H2, J = 4.7 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 30.0 (C9), 55.6 (OMe), 62.2 (C10), 113.7 (C3′ or 5′), 113.8 (C3′ or 5′), 116.7 (C3), 121.9, 125.1 (C5), 127.6 (C6), 128.8 (C8), 131.8 (C2′ or 6′), 132.2 (C2′ or 6′), 136.0, 147.0, 148.0, 150.4 (C2), 163.8, 166.2 (C11). Anal. calcd. for: C19H16ClNO3S: C 61.04, H 4.31, N 3.75; Found: C 60.98, H 4.33, N 3.87. MS: m/z 374.06 (M+H+. 22%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl 2,3-dimethoxybenzoate (6). Column chromathography DCM:EtOAc (8:2). White solid, yield: 91%; m.p. 108–109 °C; IR (KBr) cm−1: 2941, 1700, 1258; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.52 (t, 2H, H9, J = 6.7 Hz), 3.88 (s, 3H, OMe), 3.90 (s, 3H, OMe), 4.61 (t, 2H, H10, J = 6.7 Hz), 7.07 (d, 1H, H5′, J = 4.5 Hz), 7.25–7.28 (m, 2H, H3, 4′), 7.44 (d, 1H, H6′, J = 4.7 Hz), 7.52 (d, 1H, H6, J = 8.9 Hz), 8.08 (d, 1H, H5, J = 8.9 Hz), 8.15 (brs, 1H, H8), 8.72 (d, 1H, H2, J = 4.9 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 29.8 (C10), 56.1 (OMe), 61.5 (OMe), 62.3 (C11),116.2 (C3 or 3′), 116.6 (C3 or 3′), 122.2 (C4′), 123.9 (C5′), 125.0 (C5), 125.3, 127.4 (C6), 128.9 (C8), 135.7, 146.4, 148.1, 149.3, 150.4 (C2), 153.6, 165.8 (C11). Anal. calcd. for: C20H18ClNO4S: C 59.48, H 4.49, N 3.47; Found: C 59.48, H 4.47, N 3.72. MS: m/z 404.07 (M+H+. 21%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl 2,4-dimethoxybenzoate (7). White solid, yield: 86% crystallized from ethanol; m.p. 107–108 °C; IR (KBr) cm−1: 2944, 1678, 1273; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.53 (t, 2H, H9, J = 6.9 Hz), 3.85 (s, 3H, OMe), 3.88 (s, 3H, OMe), 4.57 (t, 2H, H10, J = 6.9 Hz), 6.43–6.47 (m, 2H, H3′,5′), 7.54–7.57 (m, 2H, H3, 6), 7.79 (dd, 1H, H6′, J = 1.7, 7.7 Hz), 8.11 (d, 1H, H5, J = 9.2 Hz), 8.25 (d, 1H, H8, J = 1.9 Hz), 8.71 (d, 1H, H2, J = 5.2 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 30.1 (C9), 55.6 (OMe), 56.1 (OMe), 61.7 (C10), 99.1 (C5′), 104.9 (C3′), 111.5, 116.6 (C3), 125.1 (C5), 127.4 (C8), 128.2 (C6), 133.9 (C6′), 137.1, 139.5, 148.2 (C2), 161.9, 164.9, 165.1 (C12). Anal. calcd. for: C20H18ClNO4S: C 59.48, H 4.49, N 3.47; Found: C 59.50, H 4.49, N 3.67. MS: m/z 404.07 (M+H+. 13%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl-2,5-dimethoxybenzoate (8). Column chromathography DCM:EtOAc (8:2). White solid, yield: 99%; m.p. 98–100 °C; IR (KBr) cm−1: 3048, 2925, 1700, 1240; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.56 (t, 2H, H9, J = 6.9 Hz), 3.76 (s, 3H, OMe), 3.84 (s, 3H, OMe), 4.61 (t, 2H, H10, J = 6.9 Hz), 6.92 (d, 1H, H3′, J = 9.2 Hz), 7.05 (dd, 1H, H4′, J = 3.2, 9.2 Hz), 7.28 (d, 1H, H3, J = 4.4 Hz), 7.57–7.60 (m, 2H, H6,6′), 8.12 (d, 1H, H5, J = 9.1 Hz), 8.33 (brs, 1H, H8), 8.72 (d, 1H, H2, J = 4.5 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 30.2 (C9), 56.0 (OMe), 56.8 (OMe), 62.1 (C10), 114.1 (C3′), 116.3 (C6 or 6′), 116.5 (C3), 120.0 (C4′), 125.0, 125.1 (C5), 126.2 (C8), 128.8 (C6 or 6′), 138.0, 146.9 (C2), 153.2, 153.9, 165.4 C11). Anal. calcd. for: C20H18ClNO4S: C 59.48, H 4.49, N 3.47; Found: C 59.49, H 4.48, N 3.61. MS: m/z 404.07 (M+H+. 21%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl 2,6-dimethoxybenzoate (9). White solid, yield: 76% crystallized from ethanol; m.p. 116–118 °C; IR (KBr) cm−1: 3084, 2960, 1728, 1251; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.52 (t, 2H, H9, J = 6.9 Hz), 3.78 (s, 3H, OMe), 3.84 (s, 3H, OMe), 4.61 (t, 2H, H10, J = 6.9 Hz), 6.55 (d, 2H, H3′,5′, J = 8.4 Hz), 7.29 (d, 1H, H4′, J = 8.4 Hz), 7.52–7.56 (m, 2H, H3,6), 8.09 (d, 1H, H5, J = 8.9 Hz), 8.23 (m, 1H, H8), 8.70 (d, 1H, H2). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 28.8 (C9), 56.1 (OMe), 62.4 (C10), 104.2 (C3′, 5′), 112.4, 116.3 (C3), 125.1 (C5), 127.0 (C8), 128.4 (C6), 131.6 (C4′), 137.5, 147.7 (C2), 157.6, 166.2 (C11). Anal. calcd. for: C20H18ClNO4S: C 59.48, H 4.49, N 3.47; Found: C 59.46, H 4.53, N 3.65. MS: m/z 404.07 (M+H+. 17%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl 3,4-dimethoxybenzoate (10). White solid, yield: 79% crystallized from ethanol; m.p. 109–110 °C; IR (KBr) cm−1: 2987, 1699, 1240; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.58 (t, 2H, H9, J = 6.9 Hz), 3.90 (s, 3H, OMe), 3.93 (s, 3H, OMe), 4.62 (t, 2H, H10, J = 6.9 Hz), 6.86 (d, 1H, H5′, J = 8.7 Hz), 7.49 (d, 1H, H2′, J = 1.9 Hz), 7.59–7.62 (m, 3H, H3,6,6′), 8.12 (d, 1H, H5, J = 9.2 Hz), 8.38 (brs, 1H, H8), 8.74 (d, 1H, H2, J = 5.4 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 30.4 (C9), 56.2 (OMe), 61.7 (C10), 110.6 (C5′), 112.4 (C2′), 114.7, 115.8 (C3), 121.7, 123.9 (C6′), 124.9, 125.1 (C5), 129.4 (C8), 138.9, 142.0, 145.3 (C2), 149.0, 153.9, 166.1 (C11). Anal. calcd. for: C20H18ClNO4S: C 59.48, H 4.49, N 3.47; Found: C 59.48, H 4.52, N 3.56. MS: m/z 404.07 (M+H+. 26%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl 3,5-dimethoxybenzoate (11). White solid, yield: 84% crystallized from ethanol; m.p. 100–102 °C; IR (KBr) cm−1: 2993, 1718, 1240; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.56 (t, 2H, H9, J = 6.9 Hz), 3.80 (s, 6H, OMe), 4.63 (t, 2H, H10, J = 6.7 Hz), 6.64 (t, 1H, H4′, J = 2.2 Hz), 7.12 (d, 2H, H2′, 6′, J = 2.2 Hz), 7.41 (d, 1H, H3, J = 4.9 Hz), 7.49 (dd, 1H, H6, J = 2.2, 9.1 Hz), 8.06 (d, 1H, H5, J = 8.9 Hz), 8.07 (d, 1H, H8, J = 1.9 Hz), 8.73 (d, 1H, H2, J = 5.2 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 30.1 (C9), 55.6 (OMe), 62.6 (C10), 106.1 (C4′), 107.5 (C2′,6′), 116.9 (C3), 125.1 (C5 or 8), 125.3, 127.7 (C6), 128.8 (C5 or 8), 131.4, 136.2, 147.0, 147.8, 150.0 (C2), 160.9, 166.1 (C11). Anal. calcd. for: C20H18ClNO4S: C 59.48, H 4.49, N 3.47; Found: C 59.47, H 4.50, N 3.68. MS: m/z 404.07 (M+H+. 35%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl 2,3,4-trimethoxybenzoate (12). White solid, yield: 80% crystallized from ethanol; m.p. 94–96 °C; IR (KBr) cm−1: 2980, 1699, 1245; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.55 (t, 2H, H9, J = 6.7 Hz), 3.85 (s, 3H, OMe), 3.90 (s, 3H, OMe), 3.93 (s, 3H, OMe), 4.60 (t, 2H, H10, J = 6.7 Hz), 6.66 (d, 1H, H5′, J = 8.9 Hz), 7.52–7.56 (m, 3H, H3,6,6′), 8.09 (d, 1H, H5, J = 8.9), 8.26–8.31 (m, 1H, H8), 8.72 (d, 1H, H2, J = 5.2 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 30.3 (C9), 56.2 (OMe), 61.0 (OMe), 61.8 (OMe), 61.9 (C10), 107.1 (C5′), 116.3 (C3), 117.0, 125.1 (C5), 126.9 (C6), 127.0 (C8), 128.5 (C6′), 137.6, 143.4, 144.7, 147.6 (C2), 155.0, 157.8, 165.1 (C11). Anal. calcd. for: C21H20ClNO5S: C 58.13, H 4.65, N 3.23; Found: C 58.13, H 4.67, N 3.40. MS: m/z 434.08 (M+H+. 75%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl 2,4,5-trimethoxybenzoate (13). Column chromathography DCM:EtOAc (8:2). White solid, yield: 80%; m.p. 134–135 °C; IR (KBr) cm−1: 2930, 1666, 1204; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.51 (t, 2H, H9, J = 7.1 Hz), 3.84 (s, 3H, OMe), 3.91 (s, 3H, OMe), 3.95 (s, 3H, OMe), 4.59 (t, 2H, H10, J = 7.0 Hz), 6.53 (s, 1H, H3′), 7.38 (s, 1H, H6′), 7.46 (d, 1H, H3, J = 4.9 Hz), 7.50 (dd, 1H, H6, J = 2.1, 8.9 Hz), 8.06 (d, 1H, H8, J = 2.3 Hz), 8.09 (d, 1H, H5, J = 9.2 Hz), 8.75 (d, 1H, H2, J = 4.9 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 29.7 (C9), 56.1 (OMe), 56.5 (OMe), 57.0 (OMe), 62.0 (C10), 97.5 (C3′), 109.6, 114.5 (C6′), 116.6 (C3), 125.0 (C5), 125.1, 127.4 (C6), 129.0 (C8), 135.7, 142.6, 146.6, 148.1, 150.5 (C2), 154.2, 156.2, 165.2 (C11). Anal. calcd. for: C21H20ClNO5S: C 58.13, H 4.65, N 3.23; Found: C 58.15, H 4.64, N 3.27. MS: m/z 434.08 (M+H+. 83%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl 3,4,5-trimethoxybenzoate (14). Column chromathography DCM:EtOAc (9:1). White solid, yield: 89%; m.p. 139–140 °C; IR (KBr) cm−1: 2892, 1700, 1209; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.54 (d, 2H, H9, J = 6.9 Hz), 3.87 (s, 6H, OMe), 3.90 (s, 3H, OMe), 4.62 (t, 2H, H10, J = 6.9 Hz), 7.25 (s, 2H, H2′,6′), 7.50 (d, 1H, H3, J = 4.9 Hz), 7.54 (dd, 1H, H6, J = 1.9, 9.2 Hz), 8.09 (d, 1H, H5, J = 8.9 Hz), 8.19 (d, 1H, H8, J = 1.9 Hz), 8.75 (d, 1H, H2, J = 5.2 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 30.1 (C9), 56.4 (OMe × 2), 61.0 (OMe), 62.2 (C10), 107.4 (C2′,6′), 116.5 (C3), 124.3, 125.1 (C5), 127.5 (C6), 128.3 (C8), 137.1, 143.3, 148.4 (C2), 148.5, 153.2, 166.0 (C11). Anal. calcd. for: C21H20ClNO5S: C 58.13, H 4.65, N 3.23; Found: C 58.13, H 4.65, N 3.34. MS: m/z 434.11 (M+H+. 100%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl 2-chlorobenzoate (15). White solid, yield: 75% crystallized from ethanol; m.p. 108 °C; IR (KBr) cm−1: 2944, 1692, 1213; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.55 (d, 2H, H9, J = 6.7 Hz), 4.65 (t, 2H, H10, J = 6.7 Hz), 7.27 (d, 1H, H3, J = 5.9 Hz), 7.29–7.32 (m, 1H, H5′), 7.40–7.50 (m, 2H, H3′,4′), 7.55 (dd, 1H, H6, J = 2.0, 8.9 Hz), 7.77 (d, 1H, H6′, J = 7.2 Hz), 8.11 (d, 1H, H5, J = 9.2 Hz), 8.20 (m, 1H, H8), 8.73 (s, 1H, H2). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 30.1 (C9), 62.7 (C10), 116.6 (C3), 125.1 (C5), 126.7 (C5′), 127.8 (C8), 128.2 (C6), 129.4, 131.3 (C3′ or 4′), 131.5 (C6′), 133.1 (C3′ or 4′), 134.0, 137.0, 146.0, 148.7 (C2), 165.3 (C11). Anal. calcd. for: C18H13Cl2NO2S: C 57.15, H 3.46, N 3.70; Found: C 57.17, H 3.47, N 3.83. MS: m/z 378.10 (M+H+. 52%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl 3-chlorobenzoate (16). White solid, yield: 79% crystallized from ethanol; m.p. 94–96 °C; IR (KBr) cm−1: 2937, 1730, 1246; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.55 (d, 2H, H9, J = 6.7 Hz), 4.65 (t, 2H, H10, J = 6.7 Hz), 7.37 (t, 1H, H5′, J = 7.9 Hz), 7.49 (d, 1H, H3, J = 5.2 Hz), 7.53–7.55 (m, 1H, H4′), 7.58 (d, 1H, H6, J = 1.9 Hz), 7.86 (d, 1H, H6′, J = 7.6 Hz), 7.95 (t, 1H, H2′, J = 1.7 Hz), 8.11 (d, 1H, H5, J = 9.2 Hz), 8.25 (d, 1H, H8, J = 1.5 Hz), 8.74 (d, 1H, H2, J = 5.2 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 30.3 (C9), 62.5 (C10), 116.5 (C3), 125.1 (C5), 126.9 (C8), 127.8 (C6′), 128.6 (C6), 129.8 (C2′), 129.9 (C5′), 131.2, 133.5 (C4′), 134.8, 147.8 (C2), 165.1 (C11). Anal. calcd. for: C18H13Cl2NO2S: C 57.15, H 3.46, N 3.70; Found: C 57.15, H 3.48, N 3.79. MS: m/z 378.09 (M+H+. 61%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl 4-methoxy-3-nitrobenzoate (17). Column chromathography DCM:EtOAc (8:2). White solid, yield: 93%; m.p. 171–173 °C; IR (KBr) cm−1: 3030, 2963, 1717, 1519, 1233; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.51 (t, 2H, H9, J = 6.8 Hz), 4.03 (s, 3H, OMe), 4.64 (t, 2H, H10, J = 6.8 Hz), 7.11 (d, 1H, H5′, J = 8.9 Hz), 7.39 (d, 1H, H3, J = 4.8 Hz), 7.50 (dd, 1H, H6′, J = 2.2, 8.9 Hz), 8.05 (d, 1H, H2′, J = 2.1 Hz), 8.09 (d, 1H, H5, J = 9.0 Hz), 8.13 (dd, 1H, H6, J = 2.2, 8.8 Hz), 8.45 (d, 1H, H8, J = 2.1 Hz), 8.76 (d, 1H, H2, J = 4.8 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 30.0 (C9), 57.0 (OMe), 62.9 (C10), 113.3 (C5′), 116.9 (C3), 121.9, 125.1 (C5), 125.2, 127.4 (C6 or 2′), 127.6 (C6 or 2′), 129.1 (C8), 135.4 (C6′), 135.9, 146.2, 148.2, 150.4 (C2), 156.5, 164.2 (C11). Anal. calcd. for: C19H15ClN2O5S: C 54.48, H 3.61, N 6.69; Found: C 54.50, H 3.60, N 6.81. MS: m/z 419.07 (M+H+. 36%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl 5-methyl-2-nitrobenzoate (18). Column chromathography DCM:EtOAc (8:2). White solid, yield: 79%; m.p. 93–95 °C; IR (KBr) cm−1: 3076, 2977, 1731, 1524, 1200; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.46 (s, 3H, CH3), 3.49 (t, 2H, H9, J = 7.1 Hz), 4.61 (t, 2H, H10, J = 6.9 Hz), 7.37 (d, 1H, H3, J = 4.8 Hz), 7.39–7.43 (m, 2H, H4′,6′), 7.50 (dd, 1H, H6, J = 2.2, 8.9 Hz), 7.89 (d, 1H, H3′, J = 8.3 Hz), 8.05 (d, 1H, H8, J = 2.1 Hz), 8.08 (d, 1H, H5, J = 9.0 Hz), 8.74 (d, 1H, H2, J = 4.8 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.4 (CH3), 29.1 (C9), 63.6 (C10), 116.8 (C3), 124.3 (C3′), 125.1 (C5), 125.2, 127.5 (C6), 127.7, 129.0 (C8), 130.0 (C6′), 132.2 (C4′), 135.9, 145.0, 145.5, 146.1, 148.2, 150.5 (C2), 165.8 (C11). Anal. calcd. for: C19H15ClN2O4S: C 56.65, H 3.75, N 6.95; Found: C 56.67, H 3.74, N 7.19. MS: m/z 403.15 (M+H+. 78%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl 3,5-dimethylbenzoate (19). Column chromathography DCM:EtOAc (9:1). White solid, yield: 91%; m.p. 102–104 °C; IR (KBr) cm−1: 3024, 2972, 1729, 1204; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.34 (s, 6H, Me), 3.49 (t, 2H, H9, J = 6.8 Hz), 4.60 (t, 2H, H10, J = 6.8 Hz), 7.19 (brs, 1H, H3), 7.42 (d, 1H, H4′, J = 4.7 Hz), 7.49 (d, 1H, H6, J = 8.9 Hz), 7.58 (brs, 2H, H2′,6′), 8.05–8.09 (m, 2H, H5,8), 8.74 (d, 1H, H2, J = 4.8 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.3 (2 × CH3), 30.0 (C9), 62.5 (C10), 116.9 (C3), 125.2 (C5), 125.3, 127,4 (C2′,6′), 127.5 (C6), 129.1 (C8), 129.4, 135.1 (C4′), 135.9, 138.3, 146.6, 148.3, 150.5 (C2), 166.8 (C11). C20H18ClNO2S: C 64.59, H 4.88, N 3.77; Found: C 64.60, H 4.89, N 3.89. MS: m/z 372.12. (M+H+. 47%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl 4-(trifluoromethyl)benzoate (20). Column chromathography DCM:EtOAc (8:2). White solid, yield: 75%; m.p. 134–136 °C; IR (KBr) cm−1: 3066, 2981, 1711, 1282; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.51 (t, 2H, H9, J = 6.8 Hz), 4.65 (t, 2H, H10, J = 6,8 Hz), 7.37 (d, 1H, H3, J = 4.8 Hz), 7.48 (dd, 1H, H6, J = 2.3, 8.9 Hz), 7.69 (d, 2H, H3′,5′, J = 8.2 Hz), 8.04–8.09 (m, 4H, H5,8,2′,6′), 8.74 (d, 1H, H2, J = 4.8 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 30.0 (C9), 63.0 (C10), 116.9 (C3), 125.1 (C5), 125.2 (C6), 125.6 (C3′,5′), 127.6 (C8), 129.1 (C2′,6′), 130.2, 132.8, 134.7, 135.1, 136.0, 146.2, 148.2, 150.5 (C2), 165.2 (C11). C19H13ClF3NO2S: C 55.41, H 3.18, N 3.40; Found: C 55.43, H 3.20, N 3.61. MS: m/z 412.16. (M+H+. 56%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl-4-tert-butylbenzoate (21). Column chromathography DCM:EtOAc (9.5:0.5). White solid, yield: 91%; m.p. 120–122 °C; IR (KBr) cm−1: 3037, 2963, 1713, 1268; 1H NMR (CDCl3, 270 MHz) δ ppm: 1.32 (s, 9H, Me), 3.49 (t, 2H, H9, J = 6.9 Hz), 4.60 (t, 2H, H10, J = 6.9 Hz), 7.40–7.45 (m, 3H, H3,3′,5′), 7.49 (dd, 1H, H6, J = 2.2, 8.9Hz), 7.90 (dt, 2H, H2′,6′, J = 1.7, 6.9 Hz), 8.04 (d, 1H, H8, J = 2.0 Hz), 8.07 (d, 1H, H5, J = 9.2 Hz), 8.75 (d, 1H, H2, J = 4.9 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 30.3 (C9), 31.1 (3 × CH3), 35.2, 62.3 (C10), 117.1 (C3), 125.2, 125.3 (C5), 125.5 (C3′,5′), 126.8, 127.5 (C6), 129.0 (C8), 129.6 (C2′,6′), 135.9, 146.6, 148.2, 150.4 (C2), 157.2, 166.4 (C11). C22H22ClNO2S: C 66.07, H 5.54, N 3.50; Found: C 66.05, H 5.56, N 3.74. MS: m/z 400.13. (M+H+. 33%).
2-[(7-Chloroquinolin-4-yl)sulfanyl]ethyl-2-methoxybenzoate (22). Column chromathography DCM:EtOAc (8:2). White solid, yield: 87%; m.p. 98–99 °C; IR (KBr) cm−1: 3020, 2980, 1715, 1220; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.48 (t, 2H, H9, J = 6.9 Hz), 3.89 (s, 3H, OMe), 4.59 (t, 2H, H10, J = 6.9 Hz), 6.92–6.98 (m, 2H, H3′,5′), 7.41 (d, 1H, H3, J = 4.8 Hz), 7.45–7.50 (m, 2H, H6,4′), 7.76 (dd, 1H, H6′, J = 1.4, 7.7 Hz), 8.04–8.08 (m, 2H, H5,8), 8.72 (d, 1H, H2, J = 4.8 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 29.8 (C9), 56.1 (OMe), 62.3 (C10), 112.1, 116.7, 119.2, 120.2, 125.1, 125.1, 127.4, 129.1, 131.9, 134.2, 135.8, 146.6, 148.2, 150.5 (C2), 159.6, 165.8 (C11). Anal. calcd. for: C19H16ClNO3S: C 61.04, H 4.31, N 3.75; Found: C 61.05, H 4.35, N 3.91. MS: m/z 374.10. (M+H+. 29%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-4-methoxybenzoate (23). Column chromathography DCM:EtOAc (8:2). White solid, yield: 96%; m.p. 106–108 °C; IR (KBr) cm−1: 3010, 2926, 1701, 1259; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.22–2.31 (m, 2H, H10), 3.27 (t, 2H, H9, J = 7.2 Hz), 3.87 (s, 3H, OMe), 4.48 (t, 2H, H11, J = 6.0 Hz), 6.93 (d, 2H, H3′, 5′, J = 9.0 Hz), 7.20 (d, 1H, H3, J = 4.9 Hz), 7.49 (dd, 1H, H6′, J = 2.1, 9.0 Hz), 8.01 (d, 2H, H2′, 6′, J = 9.0 Hz), 8.05 (d, 1H, H8, J = 2.4 Hz), 8.06 (d, 1H, H5, J = 8.8 Hz), 8.68 (d, 1H, H2, J = 4.8 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 28.0 (C10), 28.1 (C9), 55.6 (OMe), 63.0 (C11), 113.9 (C3′,5′), 116.3 (C3), 122.5, 125.2 (C5 or 8), 127.5 (C6), 129.1 (C8 or 5), 131.8 (C2′,6′), 135.9, 147.5, 148.2, 150.4 (C2), 163.7, 166.3 (C12). Anal. calcd. for: C20H18ClNO3S: C 61.93, H 4.68, N 3.61; Found: C 61.96, H 4.71, N 3.72. MS: m/z 388.10. (M+H+. 100%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-2,3-dimethoxybenzoate (24). Column chromathography DCM:EtOAc (8:2). White solid, yield: 91%; m.p. 78–80 °C; IR (KBr) cm−1: 3031, 2912, 1728, 1254; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.24–2.30 (m, 2H, H10), 3.29 (t, 2H, H9, J = 7.3 Hz), 3.89 (s, 3H, OMe), 3.90 (s, 3H, OMe), 4.50 (t, 2H, H11, J = 5.9 Hz), 7.08–7.10 (m, 2H, H5′,4′), 7.21 (d, 1H, H3, J = 4.9 Hz), 7.32 (dd, 1H, H6′, J = 2.7, 6.8 Hz), 7.49 (dd, 1H, H6, J = 2.1, 9.0 Hz), 8.05 (d, 1H, H8, J = 2.5 Hz), 8.06 (d, 1H, H5, J = 8.8 Hz), 8.68 (d, 1H, H2, J = 4.8 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 27.9 (C9,10), 56.2 (OMe), 61.7 (OMe), 63.3 (C11), 116.1 (C3), 116.3 (C4′), 122.3 (C5′), 124.1 (C6′), 125.1 (C5), 125.2, 126.0, 127.4 (C6), 129.1 (C8), 135.8, 147.4, 148.2, 149.2, 150.4 (C2), 153.7, 166.4 (C12). Anal. calcd. for: C21H20ClNO4S: C 60.35, H 4.82, N 3.35; Found: C 60.39, H 4.81, N 3.47. MS: m/z 418.08. (M+H+. 37%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-2,4-dimethoxybenzoate (25). Column chromathography DCM:EtOAc (8:2). White solid, yield: 89%; m.p. 74–76 °C; IR (KBr) cm−1: 3040, 2944, 1688, 1280; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.23–2.30 (m, 2H, H10), 3.33 (t, 2H, H9, J = 7.4 Hz), 3.85 (s, 3H, OMe), 3.86 (s, 3H, OMe), 4.44 (t, 2H, H11, J = 5.9 Hz), 6.48–6.51 (m, 2H, H3′,5′), 7.32 (d, 1H, H3, J = 4.9 Hz), 7.54 (dd, 1H, H6, J = 1.9, 8.9 Hz), 7.84 (d, 1H, H6′, J = 9.2 Hz), 8.08 (d, 1H, H5, J = 8.9 Hz), 8.26 (d, 1H, H8, J = 1.9 Hz), 8.65 (d, 1H, H2, J = 4.9 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 27.8 (C10), 28.4 (C9), 55.6 (OMe), 56.1 (OMe), 62.3 (C11), 99.2 (C3′ or 5′), 105.0 (C3′ or 5′), 111.9, 115.0 (C3), 125.1 (C5), 126.5 (C8), 129.2 (C6), 133.9 (C6′), 146.8 (C2), 161.5, 164.7, 165.6 (C12). Anal. calcd. for: C21H20ClNO4S: C 60.35, H 4.82, N 3.35; Found: C 60.33, H 4.84, N 3.51. MS: m/z 418.11. (M+H+. 49%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-2,5-dimethoxybenzoate (26). Column chromathography DCM:EtOAc (8:2). White solid, yield: 96%; m.p. 97–98 °C; IR (KBr) cm−1: 2971, 1696, 1286; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.20–2.29 (m, 2H, H10), 3.29 (t, 2H, H9, J = 7.3 Hz), 3.79 (s, 3H, OMe), 3.84 (s, 3H, OMe), 4.48 (t, 2H, H11, J = 5.9 Hz), 6.93 (d, 1H, H3′, J = 9.1 Hz), 7.05 (dd, 1H, H4′, J = 3.2, 9.1 Hz), 7.23 (d, 1H, H3, J = 4.9 Hz), 7.35 (d, 1H, H6′, J = 3.2 Hz), 7.49 (dd, 1H, H6, J = 2.2, 8.9 Hz), 8.05–8.07 (m, 2H, H5,8), 8.68 (d, 1H, H2, J = 4.9 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 27.9 (C9,10), 56.1 (OMe), 56.8 (OMe), 63.3 (C11), 113.9 (C3′), 116.2 (C3 or 6′), 116.4 (C3 or 6′), 119.6 (C4′), 120.5, 125.1, 125.2 (C5), 127.4 (C8), 129.1 (C6), 135.8, 147.6 (C2), 148.2, 150.4, 153.2, 153.6, 166.2 (C12). Anal. calcd. for: C21H20ClNO4S: C 60.35, H 4.82, N 3.35; Found: C 60.35, H 4.83, N 3.57. MS: m/z 418.08. (M+H+. 61%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-3,5-dimethoxybenzoate (27). White solid, yield: 95% crystallized from ethanol; m.p. 148 °C; IR (KBr) cm−1: 2944, 1712, 1248; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.26–2.31 (m, 2H, H10), 3.30 (t, 2H, H9, J = 7.2 Hz), 3.82 (s, 6H, OMe), 4.49 (t, 2H, H11, J = 5.9 Hz), 6.65 (t, 1H, H4′, J = 2.2 Hz), 7.17 (d, 2H, H2′,6′, J = 2.2 Hz), 7.27 (d, 1H, H3, J = 4.9 Hz), 7.54 (dd, 1H, H6, J = 1.9, 8.6 Hz), 8.08 (d, 1H, H5, J = 8.6 Hz), 8.22 (brs, 1H, H8), 8.68 (d, 1H, H2, J = 4.9 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 27.8 (C10), 28.4 (C9), 55.7 (2 × OMe), 63.2 (C11), 105.6 (C4′), 107.5 (C2′,6′), 115.6 (C3), 124.9 (C5), 125.1 (C8), 128.6 (C6), 131.8, 137.8, 147.0 (C2), 152.4, 157.9, 160.9, 166.2 (C12). Anal. calcd. for: C21H20ClNO4S: C 60.35, H 4.82, N 3.35; Found: C 60.39, H 4.79, N 3.60. MS: m/z 418.12. (M+H+. 27%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-2,3,4-trimethoxybenzoate (28). White solid, yield: 85% crystallized from ethanol; m.p. 84 °C; IR (KBr) cm−1: 2963, 1692, 1216; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.20–2.30 (m, 2H, H10), 3.30 (t, 2H, H9, J = 7.2 Hz), 3.87 (s, 3H, OMe), 3.90 (s, 3H, OMe), 3.92 (s, 3H, OMe), 4.46 (t, 2H, H11, J = 5.9 Hz), 6.70 (d, 1H, H5′, J = 8.9 Hz), 7.28 (d, 1H, H3, J = 4.5 Hz), 7.52 (dd, 1H, H6, J = 1.9, 9.2 Hz), 7.59 (d, 1H, H6′, J = 8.9 Hz), 8.07 (d, 1H, H5, J = 9.2 Hz), 8.17 (d, 1H, H8, J = 1.9 Hz), 8.67 (d, 1H, H2, J = 4.5 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 27.9 (C10), 28.2 (C9), 56.2 (OMe), 61.0 (OMe), 61.8 (OMe), 62.9 (C11), 107.2 (C5′), 116.0 (C3), 117.7, 125.1 (C5), 126.9 (C6′), 127.7 (C8), 128.0 (C6), 136.9, 143.2, 146.2, 148.6 (C2), 150.1, 154.8, 157.5, 165.5 (C12). Anal. calcd. for: C22H22ClNO5S: C 58.99, H 4.95, N 3.13; Found: C 59.01, H 4.96, N 3.32. MS: m/z 448.11. (M+H+. 100%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-2,4,5-trimethoxybenzoate (29). Column chromathography DCM:EtOAc (8:2). White solid, yield: 58%; m.p. 118–120 °C; IR (KBr) cm−1: 3028, 2905, 1714, 1229; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.18–2.27 (m, 2H, H10), 3.27 (t, 2H, H9, J = 7.3 Hz), 3.84 (s, 3H, OMe), 3.86 (s, 3H, OMe), 3.92 (s, 3H, OMe), 4.45 (t, 2H, H11, J = 6.0 Hz), 6.51 (s, 1H, H3′), 7.20 (d, 1H, H3, J = 4.9 Hz), 7.40 (s, 1H, H6′), 7.46 (dd, 1H, H6, J = 2.3, 8.8 Hz), 8.01 (d, 1H, H8, J = 2.3 Hz), 8.03 (d, 1H, H5, J = 8.8 Hz), 8.64 (d, 1H, H2, J = 4.9 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 27.9 (C9,10), 56.1 (OMe), 56.6 (OMe), 57.0 (OMe), 62.9 (C11), 97.7 (C3′), 110.4, 114.7 (C6′), 116.1 (C3), 125.1 (C5), 125.1, 127.3 (C6), 129.0 (C8), 135.8, 142.7, 147.6, 148.1, 150.3 (C2), 153.9, 155.8, 165.8 (C12). Anal. calcd. for: C22H22ClNO5S: C 58.99, H 4.95, N 3.13; Found: C 58.96, H 4.95, N 3.29. MS: m/z 448.13. (M+H+. 100%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-3,4,5-trimethoxybenzoate (30). Column chromathography DCM:EtOAc (9:1). White solid, yield: 97%; m.p. 98–100 °C; IR (KBr) cm−1: 3048, 2925, 1704, 1220; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.22–2.32 (m, 2H, H10), 3.24 (t, 2H, H9, J = 7.2 Hz), 3.88 (s, 6H, 2 × OMe), 3.89 (s, 3H, OMe), 4.49 (t, 2H, H11, J = 6.2 Hz), 7.17 (d, 1H, H3, J = 4.8 Hz), 7.28 (s, 2H, H2′, 6′), 7.47 (dd, 1H, H6, J = 2.1, 9.0 Hz), 8.03 (d, 1H, H8, J = 2.1 Hz), 8.04 (d, 1H, H5, J = 9.0 Hz), 8.67 (d, 1H, H2, J = 4.8 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 27.9 (C10), 28.1 (C9), 56.4 (2 × OMe), 61.1 (OMe), 63.5 (C11), 107.1 (C2′,6′), 116.3 (C3), 125.0 (C5), 125.1, 125.2, 127.5 (C6), 129.1 (C8), 135.9, 142.7, 147.4, 148.2, 150.3 (C2), 153.1, 166.2 (C12). Anal. calcd. for: C22H22ClNO5S: C 58.99, H 4.95, N 3.13; Found: C 58.98, H 4.96, N 3.41. MS: m/z 448.09. (M+H+. 100%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-2-methoxybenzoate (31). Column chromathography DCM:EtOAc (8:2). White solid, yield: 76%; m.p. 95–97 °C; IR (KBr) cm−1: 2950, 1714, 1225; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.25–2.32 (m, 2H, H10), 3.36 (t, 2H, H9, J = 7.2 Hz), 3.88 (s, 3H, OMe), 4.48 (t, 2H, H11, J = 5.9 Hz), 6.97–7.00 (m, 2H, H3′,5′), 7.36 (d, 1H, H3, J = 5.2 Hz), 7.45–7.49 (m, 1H, H4′), 7.57 (dd, 1H, H6, J = 1.9, 8.9 Hz), 7.79 (dd, 1H, H6′, J = 1.7, 7.9 Hz), 8.10 (d, 1H, H5, J = 8.9 Hz), 8.35 (d, 1H, H8, J = 1.9 Hz), 8.65 (d, 1H, H2, J = 5.2 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 27.8 (C10), 28.3 (C9), 56.1 (OMe), 62.8 (C11), 112.3 (C3′ or 5′), 115.3 (C3), 120.0 (C3′ or 5′), 120.4, 122.9, 124.8, 125.1 (C5), 125.9 (C8), 128.8 (C6), 131.7 (C6′), 133.9 (C4′), 138.4, 146.5 (C2), 159.3, 166.2 (C12). Anal. calcd. for: C20H18ClNO3S: C 61.93, H 4.68, N 3.61; Found: C 61.95, H 4.68, N 3.69. MS: m/z 388.10. (M+H+. 100%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-2-chlorobenzoate (32). White solid; yield: 81% crystallized from ethanol; m.p. 100–102 °C; IR (KBr) cm−1: 2950, 1720, 1230; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.22–2.31 (m, 2H, H10), 3.29 (t, 2H, H9, J = 7.2 Hz), 4.52 (t, 2H, H11, J = 5.9 Hz), 7.21 (d, 1H, H3, J = 4.9 Hz), 7.29–7.35 (m, 1H, H5′), 7.43–7.46 (m, 2H, H3′,4′), 7.49 (dd, 1H, H6, J = 1.9, 9.2 Hz), 7.80–7.83 (m, 1H, H6′), 8.05 (d, 1H, H5, J = 9.2 Hz), 8.09 (d, 1H, H8, J = 1.9 Hz), 8.68 (brs, 1H, H2). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 27.8 (C10), 28.2 (C9), 63.8 (C11), 116.3 (C3), 125.1 (C5), 126.7, 127.6, 128.6, 130.2, 131.2, 131.5, 132.8, 133.7, 136.1, 147.4, 148.1, 149.7 (C2), 165.7 (C12). Anal. calcd. for: C19H15Cl2NO2S: C 58.17, H 3.85, N 3.57; Found: C 58.16, H 3.87, N 3.73. MS: m/z 392.02. (M+H+. 75%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-3-chlorobenzoate (33). White solid, yield: 80% crystallized from ethanol; m.p. 96–98 °C; IR (KBr) cm−1: 2920, 1700, 1210; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.22–2.32 (m, 2H, H10), 3.26 (t, 2H, H9, J = 7.2 Hz), 4.50 (t, 2H, H11, J = 5.9 Hz), 7.20 (d, 1H, H3, J = 4.9 Hz), 7.38 (t, 1H, H5′, J = 7.9 Hz), 7.49 (dd, 1H, H6, J = 1.9, 9.2 Hz), 7.52–7.56 (m, 1H, H4′), 7.89–7.93 (m, 1H, H6′), 8.00 (t, 1H, H2′, J = 1.7 Hz), 8.05 (d, 1H, H5, J = 9.2 Hz), 8.08 (d, 1H, H8, J = 1.9 Hz), 8.69 (brs, 1H, H2). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 27.8 (C10), 28.2 (C9), 63.6 (C11), 116.3 (C3), 125.1 (C5), 127.7 (C6), 127.8 (C6′), 128.6 (C8), 129.7 (C2′), 129.8 (C5′), 131.8, 133.3 (C4′), 134.8, 136.2, 143.5, 147.5, 148.1, 149.7 (C2), 165.2 (C12). Anal. calcd. for: C19H15Cl2NO2S: C 58.17, H 3.85, N 3.57; Found: C 58.21, H 3.83, N 3.82. MS: m/z 392.04. (M+H+. 63%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-2,4-dichlorobenzoate (34). White solid, yield: 76% crystallized from ethanol; m.p. 88–89 °C; IR (KBr) cm−1: 3060, 2930, 1690, 1210; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.23–2.33 (m, 2H, H10), 3.30 (t, 2H, H9, J = 7.2 Hz), 4.52 (t, 2H, H11, J = 5.9 Hz), 7.25 (d, 1H, H3, J = 4.2 Hz), 7.31 (dd, 1H, H5′, J = 1.9, 8.4 Hz), 7.48 (d, 1H, H3′, J = 1.9 Hz), 7.53 (dd, 1H, H6, J = 1.9, 9.2 Hz), 7.80 (d, 1H, H6′, J = 8.4 Hz), 8.07 (d, 1H, H5, J = 9.2 Hz), 8.19 (d, 1H, H8, J = 1.9 Hz), 8.69 (d, 1H, H2, J = 4.2 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 27.6 (C10), 28.2 (C9), 63.9 (C11), 116.0 (C3), 125.1 (C5 or 8),127.2 (C5′), 127.6 (C6 or 3′), 128.1 (C8 or 5), 128.2, 131.2 (C3′ or 6), 132.6 (C6′), 134.8, 137.1, 138.8, 148.3 (C2), 164.8 (C12). Anal. calcd. for: C19H14Cl3NO2S: C 53.48, H 3.31, N 3.28; Found: C 53.51, H 3.31, N 3.45. MS: m/z 426.94. (M+H+. 80%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-4-methoxy-3-nitrobenzoate (35). Column chromathography DCM:EtOAc (9:1). White solid, yield: 97%; m.p. 138–140 °C; IR (KBr) cm−1: 3020, 2958, 1715, 1507, 1247; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.24–2.33 (m, 2H, H10), 3.26 (t, 2H, H9, J = 7.1 Hz), 4.03 (s, 3H, OMe), 4.52 (t, 2H, H11, J = 6.1 Hz), 7.13 (d, 1H, H5′, J = 8.8 Hz), 7.19 (d, 1H, H3, J = 4.8 Hz), 7.49 (dd, 1H, H6, J = 2.4, 8.8 Hz), 8.04 (d, 1H, H8, J = 2.4 Hz), 8.06 (d, 1H, H5, J = 8.8 Hz), 8.20 (dd, 1H, H6′, J = 2.2, 8.8 Hz), 8.50 (d, 1H, H2′, J = 2.2 Hz), 8.70 (d, 1H, H2, J = 4.8 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 27.7 (C10), 28.1 (C9), 57.0 (OMe), 63.9 (C11), 113.4 (C5′), 116.4 (C3), 122.4, 125.1 (C5), 125.2, 127.3 (C2′ or 8), 127.5 (C2′ or 8), 129.1 (C6), 135.5 (C6′), 135.9, 139.5, 147.2, 148.2, 150.4 (C2), 156.4, 164.4 (C12). Anal. calcd. for: C20H17ClN2O5S: C 55.49, H 3.96, N 6.47; Found: C 55.47, H 4.01, N 6.72. MS: m/z 433.09. (M+H+. 46%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-5-methyl-2-nitrobenzoate (36). Column chromathography DCM:EtOAc (9:1). White solid, yield: 67%; m.p. 91–92 °C; IR (KBr) cm−1: 3062, 2971, 1731, 1505, 1205; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.12–2.21 (m, 2H, H10), 2.39 (s, 3H, CH3), 3.15 (t, 2H, H9, J = 7.2 Hz), 4.46 (t, 2H, H11, J = 5.9 Hz), 7.13 (d, 1H, H3, J = 4.9 Hz), 7.33 (dd, 1H, H4′, J = 1.0, 8.3 Hz), 7.39 (dd, 1H, H6, J = 2.2, 8.94 Hz), 7.44 (d, 1H, H6′, J = 1.0 Hz), 7.79 (d, 1H, H3′, J = 8.3 Hz), 7.94–7.97 (m, 2H, H5,8), 8.62 (d, 1H, H2, J = 4.9 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.3 (CH3), 27.3 (C10), 27.6 (C9), 64.6 (C11), 116.1 (C3), 124.1 (C3′), 124.9, 125.0 (C5), 127.1 (C6), 127.7, 128.8 (C8), 130.1 (C6′), 132.1 (C4′), 135.5, 144.7, 145.7, 147.2, 147.9, 150.3 (C2), 165.7 (C12). Anal. calcd. for: C20H17ClN2O4S: C 57.62, H 4.11, N 6.72; Found: C 57.63, H 4.13, N 6.87. MS: m/z 417.10. (M+H+. 56%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-3,5-dimethylbenzoate (37). Column chromathography DCM:EtOAc (9:1). White solid, yield: 92%; m.p. 136–138 °C; IR (KBr) cm−1: 3029, 2772, 1706, 1224; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.22–2.32 (m, 2H, H10), 2.37 (s, 6H, 2CH3), 3.27 (t, 2H, H9, J = 7.2 Hz), 4.49 (t, 2H, H11, J = 6.0 Hz), 7.20–7.26 (m, 2H, H3,4′), 7.49 (dd, 1H, H6, J = 2.1, 9.1 Hz), 7.66 (brs, 2H, H2′,6′), 8.06 (brs, 1H, H8), 8.07 (d, 1H, H5, J = 9.0 Hz), 8.68 (d, 1H, H2, J = 4.8 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.3 (2 × CH3), 27.9 (C10), 28.0 (C9), 63.2 (C11), 116.3 (C3), 125.1 (C5), 125.2, 127.4 (C2′,5′), 127.5 (C6), 129.1 (C8), 129.9, 135.0 (C3′), 135.9, 138.3, 147.5, 148.2, 150.4 (C2), 166.9 (C11). Anal. calcd. for: C21H20ClNO2S: C 65.36, H 5.22, N 3.63; Found: C 65.39, H 5.24, N 3.79. MS: m/z 386.11. (M+H+. 100%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-4-(trifluoromethyl)benzoate (38). Column chromathography DCM:EtOAc (8:2). White solid, yield: 90%; m.p. 122–124 °C; IR (KBr) cm−1: 3049, 2985, 1733, 1236; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.25–2.34 (m, 2H, H10), 3.27 (t, 2H, H9, J = 7.1 Hz), 4.55 (t, 2H, H11, J = 6.1 Hz), 7.19 (d, 1H, H3, J = 4.8 Hz), 7.49 (dd, 1H, H6, J = 2.3, 9.0 Hz), 7.72 (d, 2H, H3′,5′, J = 8.2 Hz), 8.05 (d, 1H, H8, J = 2.3 Hz), 8.06 (d, 1H, H5, J = 8.9 Hz), 8.16 (d, 2H, H2′,6′, J = 8.2 Hz), 8.70 (d, 1H, H2, J = 4.8 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 27.7 (C10), 28.0 (C9), 63.9 (C11), 116.4 (C3), 125.1 (C5), 125.2, 125.6 (J = 14.8 Hz), 127.5 (C8), 129.1 (C2′ or 6′), 130.1 (C2′ or 6′), 132.2 (J = 4.4 Hz), 133.3, 134.8 (J = 131.4 Hz), 136.0, 147.2, 148.3, 150.4 (C2), 165.4 (C12). Anal. calcd. for: C20H15ClF3NO2S: C 56.41, H 3.55, N 3.29; Found: C 56.45, H 3.54, N 3.41. MS: m/z 426.07. (M+H+. 48%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-4-tert-butylbenzoate (39). Column chromathography DCM:EtOAc (8:2). White solid, yield: 80%; m.p. 88–90 °C; IR (KBr) cm−1: 3039, 2959, 1702, 1204; 1H NMR (CDCl3, 270 MHz) δ ppm: 1.34 (s, 9H, 3 × CH3), 2.22–2.28 (m, 2H, H10), 3.26 (t, 2H, H9, J = 7.4 Hz), 4.49 (t, 2H, H11, J = 5.9 Hz), 7.20 (d, 1H, H3, J = 4.9 Hz), 7.45–7.50 (m, 3H, H 6,3′,5′), 7.98 (d, 2H, H2′,6′, J = 8.2 Hz), 8.06 (d, 1H, H5, J = 8.9 Hz), 8.06 (d, 1H, H8, J = 1.7 Hz), 8.68 (d, 1H, H2, J = 4.9 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 28.0 (C10), 28.2 (C9), 31.2 (3 × CH3), 35.2, 63.0 (C11), 116.5 (C3), 125.1 (C6), 125.2, 125.5 (C3′,5′), 127.3, 127.4 (C5), 129.0 (C8), 129.5 (C2′,6′), 135.9, 147.4, 148.2, 150.2 (C2), 157.0, 166.5 (C12). Anal. calcd. for: C23H24ClNO2S: C 66.73, H 5.84, N 3.38; Found: C 66.79, H 5.87, N 3.56. MS: m/z 414.12. (M+H+. 53%).
3-[(7-Chloroquinolin-4-yl)sulfanyl]propyl-2-fluorobenzoate (40). White solid, yield: 82% crystallized from ethanol; m.p. 112–113 °C; IR (KBr) cm−1: 3084, 2930, 1700, 1225; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.21–2.31 (m, 2H, H10), 3.30 (t, 2H, H9, J = 7.16 Hz), 4.50 (t, 2H, H11, J = 5.9 Hz), 7.11–7.21 (m, 2H, H3′,5′), 7.24–7.27 (d, 1H, H3, J = 4.2), 7.49 (dd, 1H, H6, J = 1.9, 9.2 Hz), 7.52–7.55 (m, 1H, H4′), 7.94 (td, 1H, H6′, J = 1.7, 7.4 Hz), 8.05 (d, 1H, H5, J = 9.2 Hz), 8.11 (d, 1H, H8, J = 1.9 Hz), 8.67 (d, 1H, H2, J = 4.2 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 27.8 (C10), 28.0 (C9), 63.5 (C11), 116.2 (C3), 116.9 (C5′), 117.3, 118.7, 124.2 (C3′ J = 16.5 Hz), 125.1 (C5), 127.8 (C6), 128.2 (C8), 132.3 (C6′), 134.8 (C4′ J = 8.8 Hz), 136.5, 147.0, 149.1, 149.2 (C2), 160.1, 164.5 (C12). Anal. calcd. for: C19H15ClFNO2S: C 60.72, H 4.02, N 3.73; Found: C 60.73, H 4.02, N 3.90. MS: m/z 376.05. (M+H+. 87%).
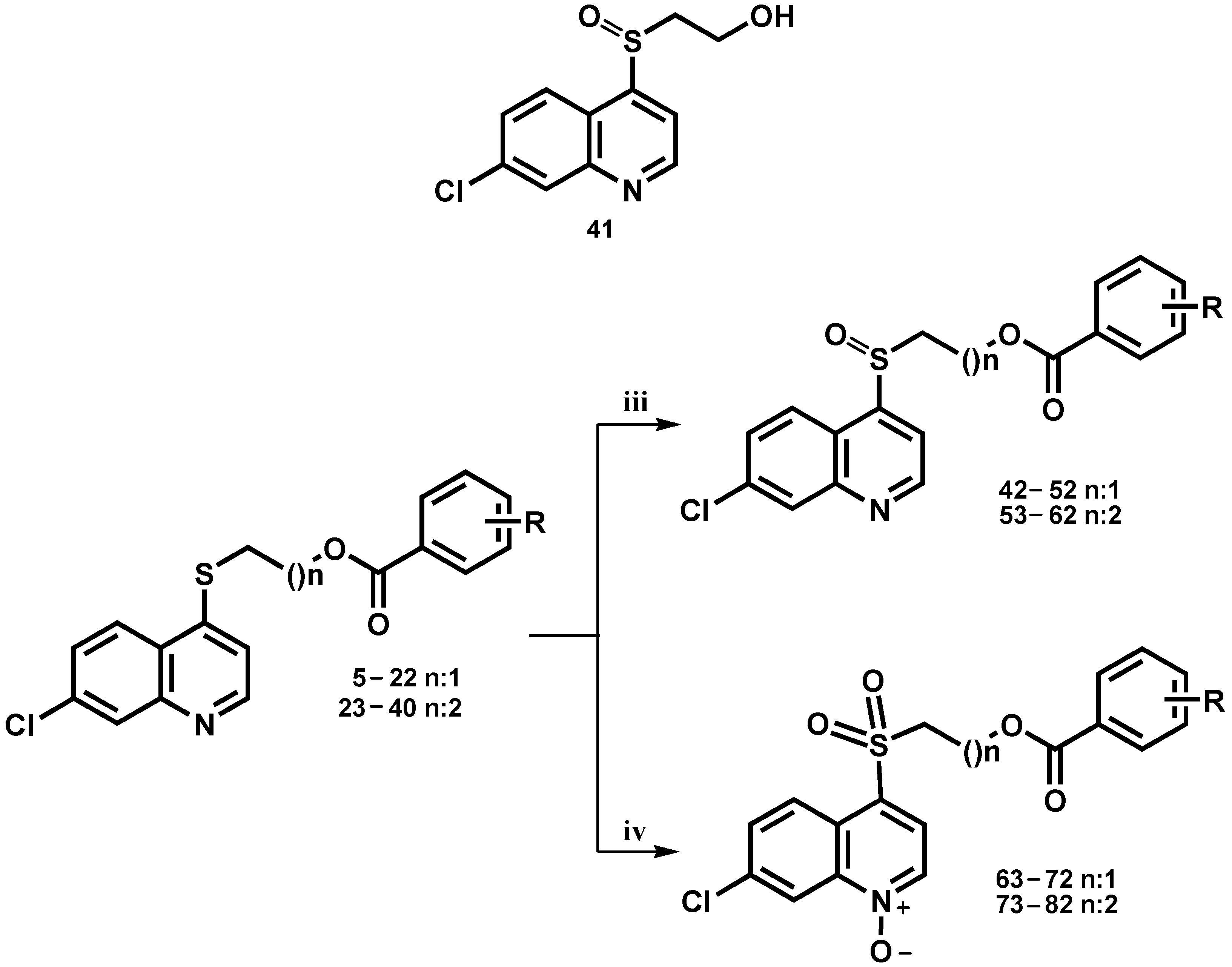
3.1.3. General Procedure for the Synthesis of Compounds 41–62
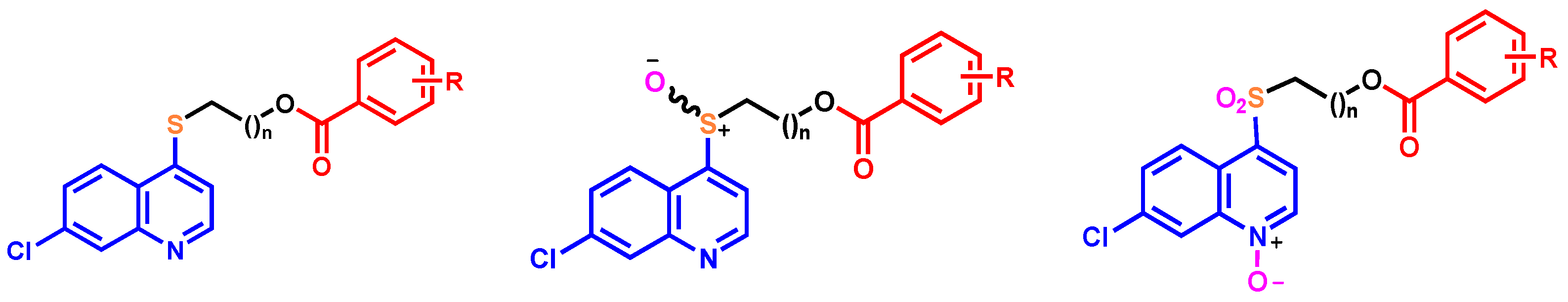
A solution of 3 and the corresponding sulfanyl benzoate derivative 5, 6, 8, 13, 14, 17–25, 29, 30, 37, and 38 (0.25 mmol) in dry DCM (5 mL) was treated with m-CPBA (1.2 mmol). The mixture was shaken at room temperature (rt) for 10 min, under a N2 atmosphere. The organic layer was washed with an aqueous mixture of saturated sodium bicarbonate and saturated sodium bisulfite (1:1) (3 × 10 mL), water (2 × 20 mL), a saturated NaCl solution (2 × 10 mL), and was finally dried over Na2SO4, filtered, and evaporated under reduced pressure to give the crude product. The compounds were then purified by column chromatography.
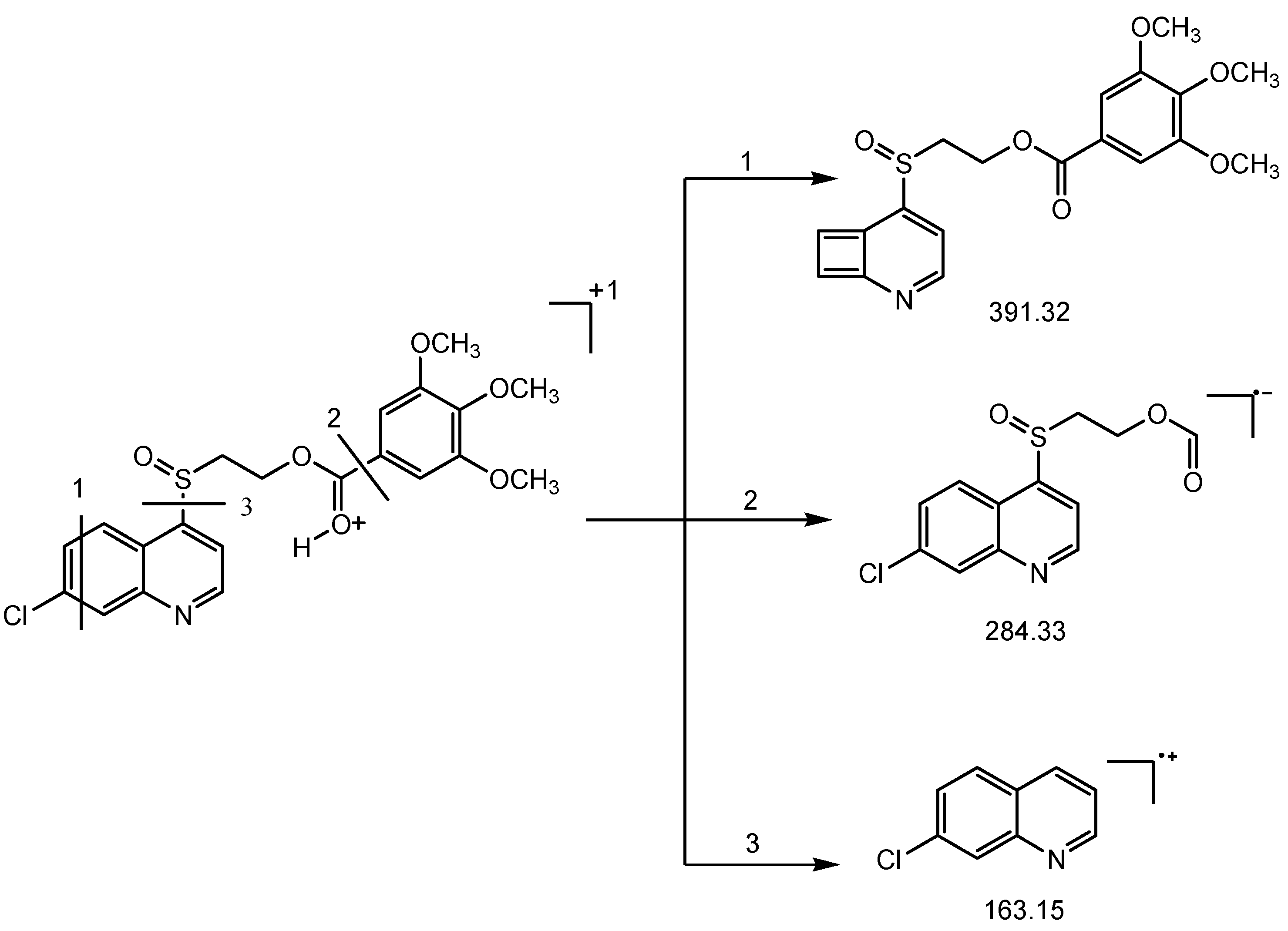
2-[(7-Chloroquinolin-4-yl)sulfinyl]ethanol (41). Column chromathography Cyclohexane:Acetone (7:3). White solid, yield: 51%; m.p. 144–146 °C; IR (KBr) cm−1: 3073, 2982, 1023; 1H NMR (DMSO-d6, 300 MHz) δ ppm: 2.97–3.04 (m, 1H, H9a), 3.26–3.34 (m, 1H, H9b), 3.75–3.83 (m, 1H, H10a), 3.89–3.99 (m, 1H, H10b), 7.87 (dd, 1H, H6, J = 2.2, 8.9 Hz), 8.03 (d, 1H, H3, J = 4.3 Hz), 8.06 (d, 1H, H5, J = 8.9 Hz), 8.30 (d, 1H, H8, J = 2.2 Hz), 9.21 (d, 1H, H2, J = 4.3 Hz). 13C NMR (DMSO-d6, 75 MHz) δ ppm: 54.1 (C9), 59.2 (C10), 117.0 (C3), 121.5, 124.2 (C5), 128.5 (C6), 128.6 (C8), 135.0, 147.7, 151.8 (C2), 152.4. Anal. calcd. for: C11H10ClNO2S: C 51.66, H 3.94, N 5.48; Found: C 51.63, H 3.95, N 5.62. MS: m/z 256.05. (M+H+. 78%).
2-[(7-Chloroquinolin-4-yl)sulfinyl]ethyl-4-methoxybenzoate (42). Column chromathography DCM:EtOAc (9.5:0.5). Cream solid, yield: 63%; m.p. 126–128 °C; IR (KBr) cm−1: 3052, 2929, 1706, 1248, 1020; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.23–3.31 (m, 1H, H9a), 3.47–3.56 (m, 1H, H9b), 3.84 (s, 3H, OMe), 4.67–4.72 (m, 2H, H10), 6.84 (d, 2H, H3′,5′, J = 8.8 Hz), 7.51 (dd, 1H, H6, J = 2.0, 8.9 Hz), 7.66 (d, 2H, H2′,6′, J = 8.8 Hz), 7.74 (d, 1H, H5, J = 8.9 Hz), 8.01 (d, 1H, H3, J = 4.4 Hz), 8.12 (d, 1H, H8, J = 1.9 Hz), 9.04 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 54.9 (C9), 55.6 (OMe), 56.6 (C10), 113.8 (C3′,5′), 117.0 (C3), 121.3, 121.7, 122.6 (C5), 129.1 (C6), 129.8 (C8), 131.6 (C2′,6′), 136.4, 148.4, 150.8, 151.4 (C2), 163.8, 165.6 (C11). Anal. calcd. for: C19H16ClNO4S: C 58.54, H 4.14, N 3.59; Found: C 58.55, H 4.17, N 3.70. MS: m/z 390.07. (M+H+. 71%).
2-[(7-Chloroquinolin-4-yl)sulfinyl]ethyl-2,3-dimethoxybenzoate (43). Column chromathography DCM:EtOAc (5:5). Cream solid, yield: 93%; m.p. 110 °C; IR (KBr) cm−1: 3087, 2939, 1721, 1228, 1031; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.16–3.23 (m, 1H, H9a), 3.46–3.55 (m, 1H, H9b), 3.86 (s, 3H, OMe), 3.87 (s, 3H, OMe), 4.70–4.75 (m, 2H, H10), 7.03–7.10 (m, 3H, H4′,5′,6′), 7.52 (dd, 1H, H6, J = 2.1, 8.9 Hz), 7.78 (d, 1H, H5, J = 8.9 Hz), 8.01 (d, 1H, H3, J = 4.4 Hz), 8.13 (d, 1H, H8, J = 2.1 Hz), 9.05 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 55.5 (C9), 56.1 (OMe), 57.4 (C10), 61.6 (OMe), 116.4, 116.8 (C3), 121.7, 122.1 (C5′), 122.7 (C5), 124.0 (C6′), 124.9, 129.1 (C6), 129.7 (C8), 136.4, 148.4, 149.3, 151.0, 151.4 (C2), 153.6, 165.5 (C11). Anal. calcd. for: C20H18ClNO5S: C 57.21, H 4.32, N 3.34; Found: C 57.16, H 4.32, N 3.59. MS: m/z 420.07. (M+H+. 61%).
2-[(7-Chloroquinolin-4-yl)sulfinyl]ethyl-2,5-dimethoxybenzoate (44). Column chromathography DCM:EtOAc (8:2). Cream solid, yield: 85%; m.p. 112–113 °C; IR (KBr) cm−1: 3056, 2960, 1724, 1204, 1038; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.20–3.28 (m, 1H, H9a), 3.44–3.53 (m, 1H, H9b), 3.75 (s, 3H, OMe), 3.79 (s, 3H, OMe), 4.67–4.70 (m, 2H, H10), 6.86 (d, 1H, H3′, J = 9.0 Hz), 7.02 (dd, 1H, H4′, J = 3.2, 9.0 Hz), 7.09 (d, 1H, H6′, J = 3.2 Hz), 7.49 (dd, 1H, H6, J = 2.1, 8.9 Hz), 7.75 (d, 1H, H5, J = 8.9 Hz), 8.00 (d, 1H, H3, J = 4.4 Hz), 8.08 (d, 1H, H8, J = 2.1 Hz), 9.02 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 55.3 (C9), 55.9 (OMe), 56.6 (OMe), 57.1 (C10), 113.7 (C3′), 116.2, 116.8 (C3), 119.0, 120.0 (C4′), 121.7, 122.6 (C5), 129.0 (C6), 129.7 (C8), 136.3, 148.3, 150.9, 151.3 (C2), 152.9, 153.7, 165.2 (C11). Anal. calcd. for: C20H18ClNO5S: C 57.21, H 4.32, N 3.34; Found: C 57.22, H 4.31, N 3.43. MS: m/z 420.05. (M+H+. 77%).
2-[(7-Chloroquinolin-4-yl)sulfinyl]ethyl-2,4,5-trimethoxybenzoate (45). Column chromathography DCM:EtOAc (8:2). White solid, yield: 91%; m.p. 151–153 °C; IR (KBr) cm−1: 3071, 2940, 1711, 1204, 1022; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.22–3.30 (m, 1H, H9a), 3.44–3.53 (m, 1H, H9b), 3.82 (s, 3H, OMe), 3.85 (s, 3H, OMe), 3.94 (s, 3H, OMe), 4.67 (t, 2H, H10, J = 4.9 Hz), 6.48 (s, 1H, H3′), 7.20 (s, 1H, H6′), 7.48 (dd, 1H, H6, J = 2.1, 8.9 Hz), 7.76 (d, 1H, H5, J = 8.9 Hz), 8.02 (d, 1H, H3, J = 4.4 Hz), 8.10 (d, 1H, H8, J = 2.1 Hz), 9.04 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 55.5 (C9), 56.2 (OMe), 56.5 (OMe), 56.8 (OMe), 56.9 (C10), 97.4 (C3′), 109.2, 114.4 (C6′), 116.9 (C3), 121.7, 122.7 (C5), 129.0 (C6), 129.7 (C8), 136.3, 142.6, 148.4, 151.0, 151.4 (C2), 154.3, 156.1, 164.9 (C12). Anal. calcd. for: C21H20ClNO6S: C 56.06, H 4.48, N 3.11; Found: C 56.04, H 4.50, N 3.27. MS: m/z 450.11. (M+H+. 83%).
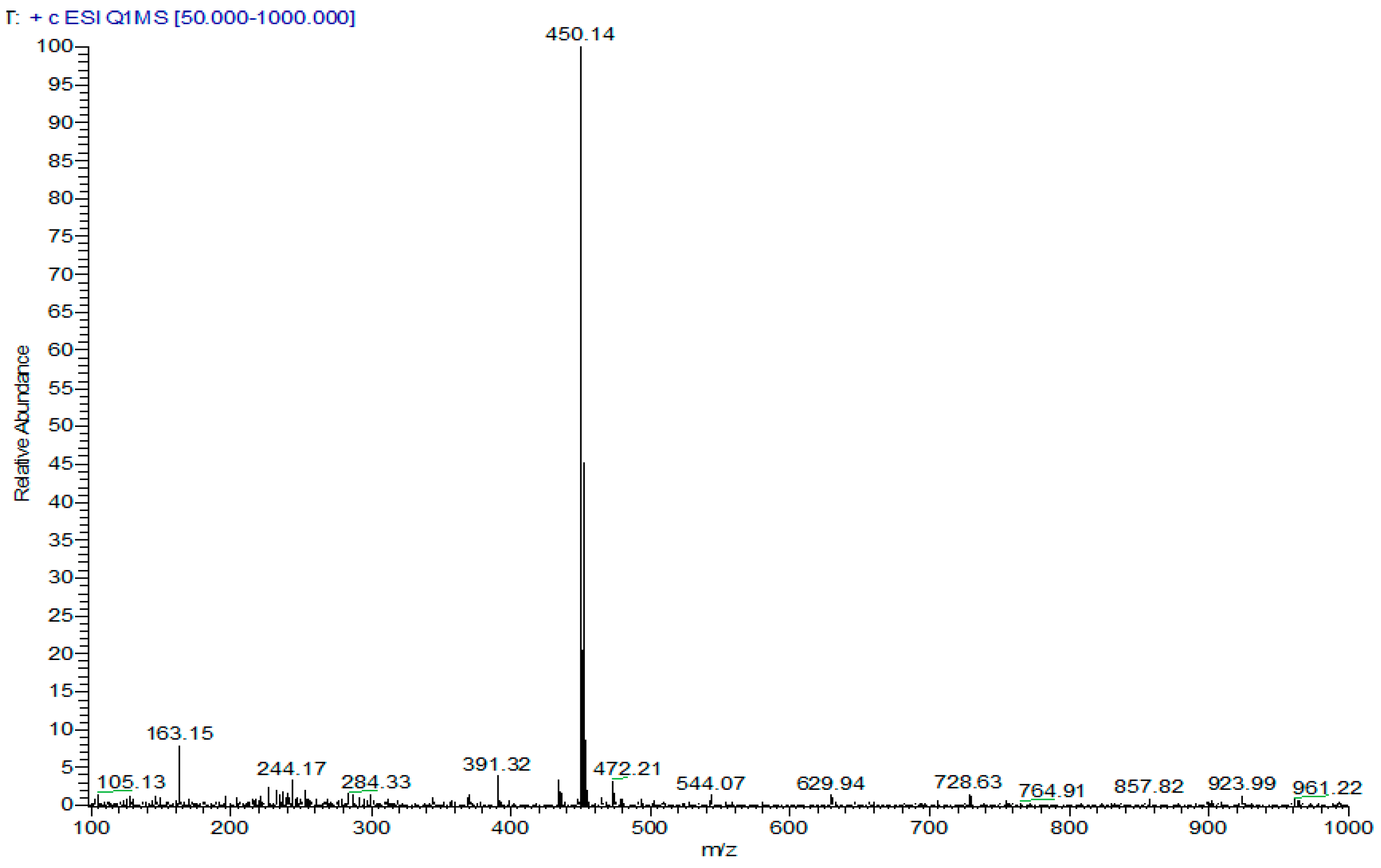
2-[(7-Chloroquinolin-4-yl)sulfinyl]ethyl-3,4,5-trimethoxybenzoate (46). Column chromathography DCM:EtOAc (8:2). Pink solid, yield: 83%; m.p. 144–146 °C; IR (KBr) cm−1: 3076, 2960, 1706, 1222, 1044; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.24–3.32 (m, 1H, H9a), 3.45–3.54 (m, 1H, H9b), 3.87 (s, 6H, 2 × OMe), 3.87 (s, 3H, OMe), 4.66–4.71 (m, 2H, H10), 6.99 (s, 2H, H2′,6′), 7.46 (dd, 1H, H6, J = 2.1, 8.9 Hz), 7.68 (d, 1H, H5, J = 8.9 Hz), 7.98 (d, 1H, H3, J = 4.4 Hz), 8.05 (d, 1H, H8, J = 2.1 Hz), 9.01 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 54.6 (C9), 56.2 (2 × OMe), 57.1 (C10), 60.9 (OMe), 106.8 (C2′,6′), 116.9 (C3), 121.5, 122.4 (C5), 123.8, 128.9 (C6), 129.7 (C8), 136.3, 142.7, 148.3, 150.6, 151.3 (C2), 152.9, 165.5 (C11). Anal. calcd. for: C21H20ClNO6S: C 56.06, H 4.48, N 3.11; Found: C 56.05, H 4.47, N 3.23. MS: m/z 450.14. (M+H+. 100%).
2-[(7-Chloroquinolin-4-yl)sulfinyl]ethyl-2-methoxybenzoate (47). Column chromathography DCM:EtOAc (9:1). Pink solid, yield: 78%; m.p. 105–107 °C; IR (KBr) cm−1: 3079, 2965, 1719, 1240, 1043; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.22–3.29 (m, 1H, H9a), 3.46–3.55 (m, 1H, H9b), 3.85 (s, 3H, OMe), 4.68–4.70 (m, 2H, H10), 6.89–6.96 (m, 2H, H3′,5′), 7.45–7.51 (m, 3H, H4′,6′,6), 7.76 (d, 1H, H5, J = 8.9 Hz), 8.02 (d, 1H, H3, J = 4.2 Hz), 8.11 (d, 1H, H8, J = 2.0 Hz), 9.03 (d, 1H, H2, J = 4.2 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 55.2 (C9), 56.0 (OMe), 56.9 (C10), 112.1, 116.9 (C3), 118.6, 120.2, 121.7, 122.7 (C5), 129.0 (C6), 129.7 (C8), 131.7, 134.3, 136.3, 148.4, 151.0, 151.4 (C2), 159.4, 165.3 (C12). Anal. calcd. for: C19H16ClNO4S: C 58.53, H 4.14, N 3.59; Found: C 58.55, H 4.17, N 3.72. MS: m/z 390.08. (M+H+. 100%).
2-[(7-Chloroquinolin-4-yl)sulfinyl]ethyl-4-methoxy-3-nitrobenzoate (48). Column chromathography DCM:EtOAc (9:1). Pink solid, yield: 88%; m.p. 148–150 °C; IR (KBr) cm−1: 3035, 2981, 1721, 1527, 1238, 1052; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.27–3.35 (m, 1H, H9a), 3.49–3.57 (m, 1H, H9b), 4.01 (s, 3H, OMe), 4.70–4.75 (m, 2H, H10), 7.06 (d, 1H, H5′, J = 8.9 Hz), 7.56 (dd, 1H, H6, J = 2.1, 8.9 Hz), 7.75 (d, 1H, H5, J = 8.9 Hz), 7.85 (dd, 1H, H6′, J = 2.2, 8.9 Hz), 7.99 (d, 1H, H3, J = 4.4 Hz), 8.08 (d, 1H, H8, J = 2.1 Hz), 8.13 (d, 1H, H2′, J = 2.2 Hz), 9.02 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 54.1 (C9), 57.0 (OMe), 57.3 (C10), 113.3 (C5′), 117.0 (C3), 121.2, 121.5, 122.5 (C5), 127.1 (C2′), 129.2 (C6), 129.7 (C8), 135.2 (C6′), 136.5, 139.1, 148.3, 150.5, 151.4 (C2), 156.5, 163.7 (C12). Anal. calcd. for: C19H15ClN2O6S: C 52.48, H 3.48, N 6.44; Found: C 52.49, H 3.48, N 6.62. MS: m/z 435.07. (M+H+. 80%).
2-[(7-Chloroquinolin-4-yl)sulfinyl]ethyl-5-methyl-2-nitrobenzoate (49). Column chromathography DCM:EtOAc (9:1). Pink solid, yield: 92%; m.p. 81–83 °C; IR (KBr) cm−1: 3067, 2969, 1731, 1526, 1200, 1065; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.45 (s, 3H, CH3), 3.07–3.14 (m, 1H, H9a), 3.43–3.53 (m, 1H, H9b), 4.74–4.79 (m, 2H, H10), 7.30 (brs, 1H, H6′), 7.39–7.42 (m, 1H, H4′), 7.58 (dd, 1H, H6, J = 2.1, 8.9 Hz), 7.82 (d, 1H, H3′, J = 8.3 Hz), 7.85 (d, 1H, H5, J = 8.9 Hz), 7.98 (d, 1H, H3, J = 4.4 Hz), 8.09 (d, 1H, H8, J = 2.1 Hz), 9.03 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.4 (CH3), 54.5 (C9), 58.1 (C10), 116.7 (C3), 121.6, 123.0 (C5), 124.2, 127.1, 129.2 (C6), 129.5 (C8), 130.1, 132.3, 136.5, 145.0, 148.3, 151.1, 151.3 (C2), 165.2 (C11). Anal. calcd. for: C19H15ClN2O5S: C 54.48, H 3.61, N 6.69; Found: C 54.45, H 3.59, N 6.83. MS: m/z 419.10. (M+H+. 100%).
2-[(7-Chloroquinolin-4-yl)sulfinyl]ethyl-3,5-dimethylbenzoate (50). Column chromathography DCM:EtOAc (8:2). White solid, yield: 83%; m.p. 106–108 °C; IR (KBr) cm−1: 3032, 2971, 1707, 1221, 1041; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.32 (s, 6H, CH3), 3.28–3.36 (m, 1H, H9a), 3.50–3.59 (m, 1H, H9b), 4.66–4.80 (m, 2H, H10), 7.17 (brs, 1H, H4′), 7.30 (brs, 2H, H2′,6′), 7.52 (dd, 1H, H6, J = 2.0, 8.9 Hz), 7.75 (d, 1H, H5, J = 8.9 Hz), 8.03 (d, 1H, H3, J = 4.4 Hz), 8.11 (d, 1H, H8, J = 1.9 Hz), 9.05 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.2 (2 × CH3), 54.6 (C9), 56.6 (C10), 117.1 (C3), 121.7, 122.6 (C5), 127.2 (C2′,6′), 128.8 (C6), 129.1, 129.8 (C8), 135.3 (C4′), 136.4, 138.2, 148.4, 150.8, 151.3 (C2), 166.2 (C11). Anal. calcd. for: C20H18ClNO3S: C 61.93, H 4.68, N 3.61; Found: C 61.93, H 4.69, N 3.77. MS: m/z 388.12. (M+H+. 100%).
2-[(7-Chloroquinolin-4-yl)sulfinyl]ethyl-4-(trifluoromethyl)benzoate (51). Column chromathography DCM:EtOAc (8:2). White solid, yield: 78%; m.p. 148–150 °C; IR (KBr) cm−1: 3070, 2970, 1727, 1220, 1036; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.27–3.36 (m, 1H, H9a), 3.50–3.60 (m, 1H, H9b), 4.78 (t, 2H, H10, J = 4.9 Hz), 7,56 (dd, 1H, H6, J = 1.9, 8.9 Hz), 7,66 (d, 2H, H, 3′,5′ J = 8.7 Hz), 7.74 (d, 1H, H5, J = 8,9 Hz), 7.84 (d, 2H, H2′,6′, J = 8.7 Hz), 8.05 (d, 1H, H3, J = 4.4 Hz), 8.13 (d, 1H, H8, J = 1.9 Hz), 9.08 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 67.9 MHz) δ ppm: 54.4 (C9), 57.5 (C10), 117.1 (C3), 121.7, 122.4 (C5), 125.5 (C3′ or 5′), 125.6 (C3′ or 5′), 129.2 (C6), 129.9 (C8,2′,6′), 132.3, 134.8, 135.3, 136.6, 148.5, 150.7, 151.3 (C2), 164.7 (C11). Anal. calcd. for: C19H13ClF3NO3S: C 53.34, H 3.06, N 3.27; Found: C 53.37, H 3.08, N 3.45. MS: m/z 428.11. (M+H+. 100%).
2-[(7-Chloroquinolin-4-yl)sulfinyl]ethyl-4-tert-butylbenzoate (52). Column chromathography DCM:EtOAc (7:3). White solid, yield: 82%; m.p. 106 °C; IR (KBr) cm−1: 3081, 2975, 1726, 1267, 1015; 1H NMR (CDCl3, 300 MHz) δ ppm: 1.35 (s, 9H, CH3), 3.26–3.34 (m, 1H, H9a), 3.49–3.58 (m, 1H, H9b), 4.73 (t, 2H, H10, J = 5.0 Hz), 7.41 (d, 2H, H3′,5′, J = 8.4 Hz), 7.53 (dd, 1H, H6, J = 2.1, 8.9 Hz), 7.66 (d, 2H, H2′,6′, J = 8.4 Hz), 7.75 (d, 1H, H5, J = 8.9 Hz), 8.05 (d, 1H, H3, J = 4.4 Hz), 8.12 (d, 1H, H8, J = 2.1 Hz), 9.08 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 31.2 (CH3), 35.3 (C12), 55.1 (C9), 56.8 (C10), 117.1 (C3), 121.8, 122.6 (C5), 125.6 (C3′,5′), 126.2, 129.1 (C6 or 8), 129.5 (C2′,6′), 129.9 (C6 or 8), 136.5, 148.5, 150.8, 151.5 (C2), 157.4, 166.0 (C11). Anal. calcd. for: C22H22ClNO3S: C 63.53, H 5.33, N 3.37; Found: C 63.52, H 5.34, N 3.49. MS: m/z 416.15. (M+H+. 100%).
3-[(7-Chloroquinolin-4-yl)sulfinyl]propyl-4-methoxybenzoate (53). Column chromathography DCM:EtOAc (5:5). Cream solid, yield: 63%; m.p. 126–128 °C; IR (KBr) cm−1: 3078, 2962, 1717, 1257, 1016; 1H NMR (CDCl3, 300 MHz) δ ppm: 1.97–2.10 (m, 1H, H10a), 2.30–2.48 (m, 1H, H10b), 2.88–3.04 (m, 1H, H9a), 3.19–3.29 (m, 1H, H9b), 3.83 (s, 3H, OMe), 4.31–4.47 (m, 2H, H11), 6.86 (d, 2H, H3′,5′, J = 8.9 Hz), 7.46 (dd, 1H, H6, J = 2.1, 8.9 Hz), 7.74 (d, 1H, H5, J = 8.9 Hz), 7.83 (d, 2H, H2′,6′, J = 8.9 Hz), 7.98 (d, 1H, H3, J = 4.4 Hz), 8.17 (d, 1H, H8, J = 2.1 Hz), 9.08 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.8 (C10), 52.2 (C9), 55.5 (OMe), 62.5 (C11), 113.8 (C3′,5′), 117.1 (C3), 121.7, 122.0, 122.8 (C5), 128.9 (C6), 129.8 (C8), 131.6 (C2′,6′), 136.4, 148.5, 150.9, 151.3 (C2), 163.7, 166.0 (C12). Anal. calcd. for: C20H18ClNO4S: C 59.48, H 4.49, N 3.47; Found: C 59.45, H 4.49, N 3.53. MS: m/z 404.14. (M+H+. 100%).
3-[(7-Chloroquinolin-4-yl)sulfinyl]propyl-2,3-dimethoxybenzoate (54). Column chromathography DCM:EtOAc (9:1). Cream solid, yield: 94%; m.p. 111–112 °C; IR (KBr) cm−1: 3070, 2922, 1701, 1259, 1051; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.00–2.14 (m, 1H, H10a), 2.33–2.47 (m, 1H, H10b), 2.90–3.00 (m, 1H, H9a), 3.24–3.34 (m, 1H, H9b), 3.77 (s, 3H, OMe), 3.88 (s, 3H, OMe), 4.37–4.54 (m, 2H, H11), 7.05 (d, 2H, H4′,5′, J = 4.8 Hz), 7.19 (t, 1H, H6′, J = 4.9 Hz), 7.49 (dd, 1H, H6, J = 1.4, 8.9 Hz), 7.77 (d, 1H, H5, J = 8.9 Hz), 8.00 (d, 1H, H3, J = 4.4 Hz), 8.20 (brs 1H, H8), 9.10 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.9 (C10), 52.2 (C9), 56.2 (OMe), 61.5 (OMe), 62.9 (C11), 116.1, 117.1 (C3), 121.8, 122.1 (C5), 123.0, 124.0, 125.7, 129.1 (C6), 129.8 (C8), 136.5, 148.6, 149.1, 151.0, 151.3 (C2), 153.7, 166.2 (C12). Anal. calcd. for: C21H20ClNO5S: C 58.13, H 4.65, N 3.23; Found: C 58.10, H 4.67, N 3.29. MS: m/z 434.12. (M+H+. 100%).
3-[(7-Chloroquinolin-4-yl)sulfinyl]propyl-,5-dimethoxybenzoate (55). Column chromathography DCM:EtOAc (9:1). Clear oil, yield: 73%; IR (NaCl) cm−1: 3054, 2947, 1699, 1217, 1047; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.00–2.14 (m, 1H, H10a), 2.31–2.45 (m, 1H, H10b), 2.89–2.98 (m, 1H, H9a), 3.23–3.32 (m, 1H, H9b), 3.68 (s, 3H, OMe), 3.75 (s, 3H, OMe), 4.35–4.52 (m, 2H, H11), 6.86 (d, 1H, H3′, J = 9.1 Hz), 7.00 (dd, 1H, H4′, J = 3.2, 9.1 Hz), 7.22 (d, 1H, H6′, J = 3.2 Hz), 7.47 (dd, 1H, H6, J = 2.1, 8.9 Hz), 7.77 (d, 1H, H5, J = 8.9 Hz), 7.99 (d, 1H, H3, J = 4.4 Hz), 8.18 (d, 1H, H8, J = 2.1 Hz), 9.09 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 22.0 (C10), 52.5 (C9), 55.9 (OMe), 56.5 (OMe), 62.7 (C11), 113.7 (C3′), 116.4, 117.0 (C3), 119.6, 120.0, 121.7, 122.9 (C5), 129.0 (C6), 129.7 (C8), 136.4, 148.5, 151.1, 151.3 (C2), 153.1, 153.5, 165.8 (C12). Anal. calcd. for: C21H20ClNO5S: C 58.13, H 4.65, N 3.23; Found: C 58.17, H 4.69, N 3.42. MS: m/z 434.10. (M+H+. 89%).
3-[(7-Chloroquinolin-4-yl)sulfinyl]propyl-2,4,5-trimethoxybenzoate (56). Column chromathography DCM:EtOAc (8:2). Yellow oil, yield: 79%; IR (NaCl) cm−1: 3071, 2940, 1711, 1204, 1022; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.01–2.08 (m, 1H, H10a), 2.33–2.42 (m, 1H, H10b), 2.89–2.98 (m, 1H, H9a), 3.22–3.30 (m, 1H, H9b), 3.70 (s, 3H, OMe), 3.80 (s, 3H, OMe), 3.91 (s, 3H, OMe), 4.35–4.56 (m, 2H, H11), 6.45 (s, 1H, H3′), 7.31 (s, 1H, H6′), 7.45 (dd, 1H, H6, J = 1.5, 8.9 Hz), 7.76 (d, 1H, H5, J = 8.9 Hz), 8.00 (d, 1H, H3, J = 4.2 Hz), 8.18 (d, 1H, H8, J = 1.5 Hz), 9.09 (d, 1H, H2, J = 4.2 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 22.1 (C10), 52.8 (C9), 56.1 (OMe), 56.7 (OMe), 56.9 (OMe), 62.4 (C11), 98.0 (C3′), 110.4, 115.0 (C6′), 117.0 (C3), 121.8, 122.9 (C5), 128.9 (C6), 129.8 (C8), 136.4, 142.9, 148.6, 151.3 (C2), 151.4, 154.2, 155.8, 165.5 (C12). Anal. calcd. for: C22H22ClNO6S: C 56.96, H 4.78, N 3.02; Found: C 57.01, H 4.78, N 2.97. MS: m/z 464.13. (M+H+. 100%).
3-[(7-Chloroquinolin-4-yl)sulfinyl]propyl-3,4,5-trimethoxybenzoate (57). Column chromathography DCM:EtOAc (9:1). Cream solid, yield: 75%; m.p. 148 °C; IR (KBr) cm−1: 3016, 2993, 1613, 1238, 1021; 1H NMR (CDCl3, 300 MHz) δ ppm: 1.98–2.11 (m, 1H, H10a), 2.33–2.46 (m, 1H, H10b), 2.86–2.95 (m, 1H, H9a), 3.16–3.26 (m, 1H, H9b), 3.82 (s, 3H, OMe), 3.86 (s, 6H, OMe), 4.32–4.50 (m, 2H, H11), 7.17 (brs, 2H, H2′,6′), 7.47 (dd, 1H, H6, J = 1.9, 8.9 Hz), 7.74 (d, 1H, H5, J = 8.9 Hz), 7.97 (d, 1H, H3, J = 4.4 Hz), 8.15 (d, 1H, H8, J = 1.9 Hz), 9.07 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.8 (C10), 52.2 (C9), 56.3 (OMe), 61.0 (2 × OMe), 63.1 (C11), 106.9 (C2′,6′), 117.0 (C3), 121.6, 122.7 (C5), 124.5, 128.9 (C6), 129.8 (C8), 136.4, 142.6, 148.5, 150.9, 151.3 (C2), 153.0, 165.9 (C12). Anal. calcd. for: C22H22ClNO6S: C 56.96, H 4.78, N 3.02; Found: C 56.93, H 4.77, N 3.19. MS: m/z 464.09. (M+H+. 100%).
3-[(7-Chloroquinolin-4-yl)sulfinyl]propyl-2-methoxybenzoate (58). Column chromathography DCM:EtOAc (8:2). Brown oil, yield: 78%; IR (NaCl) cm−1: 3044, 2963, 1735, 1237, 1056; 1H NMR (CDCl3, 300 MHz) δ ppm: 1.97–2.12 (m, 1H, H10a), 2.40–2.47 (m, 1H, H10b), 2.88–3.05 (m, 1H, H9a), 3.21–3.31 (m, 1H, H9b), 3.72 (s, 3H, OMe), 4.32–4.49 (m, 2H, H11), 6.89–6.94 (m, 2H, H3′ or 4′ or 5′ or 6′), 7.41–7.47 (m, 2H, H3′ or 4′ or 5′ or 6′), 7.65 (dd, 1H, H6, J = 1.9, 8.9 Hz), 7.75 (d, 1H, H5, J = 8.9 Hz), 7.98 (d, 1H, H3, J = 4.4 Hz), 8.17 (d, 1H, H8, J = 1.9 Hz), 9.07 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.9 (C10), 52.5 (C9), 55.8 (OMe), 62.5 (C11), 112.1 (C3′ or 5′), 117.0 (C3), 119.5, 120.2 (C3′ or 5′), 121.7, 122.9 (C5), 128.9 (C6), 129.8 (C8), 131.7 (C6′), 133.9 (C4′), 136.4, 148.5, 151.1, 151.3 (C2), 159.2, 166.0 (C12). Anal. calcd. for: C20H18ClNO4S: C 59.48, H 4.49, N 3.47; Found: C 59.52, H 4.50, N 3.58. MS: m/z 404.13. (M+H+. 100%).
3-[(7-Chloroquinolin-4-yl)sulfinyl]propyl-4-methoxy-3-nitrobenzoate (59). Column chromathography DCM:EtOAc (9:1). Cream solid, yield: 80%; m.p. 124 °C; IR (KBr) cm−1: 3077, 2948, 1707, 1532, 1232, 1072; 1H NMR (CDCl3, 300 MHz) δ ppm: 1.97–2.11 (m, 1H, H10a), 2.30–2.44 (m, 1H, H10b), 2.88–2.98 (m, 1H, H9a), 3.20–3.29 (m, 1H, H9b), 3.99 (s, 3H, OMe), 4.34–4.48 (m, 2H, H11), 7.08 (d, 1H, H5′, J = 8.9 Hz), 7.52 (dd, 1H, H6, J = 2.1, 8.9 Hz), 7.76 (d, 1H, H5, J = 8.9 Hz), 7.98 (d, 1H, H3, J = 4.4 Hz), 8.03 (dd, 1H, H6′, J = 2.2, 8.8 Hz), 8.14 (d, 1H, H8, J = 2.0 Hz), 8.32 (d, 1H, H2′, J = 2.1 Hz), 9.08 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.6 (C10), 51.6 (C9), 57.0 (OMe), 63.4 (C11), 113.3 (C5′), 117.2 (C3), 121.6, 121.9, 122.7 (C5), 127.2 (C2′), 129.0 (C6), 129.8 (C8), 135.3 (C6′), 136.4, 139.3, 148.5, 150.7, 151.3 (C2), 156.3, 164.1 (C12). Anal. calcd. for: C20H17ClN2O6S: C 53.51, H 3.82, N 6.24; Found: C 53.51, H 3.79, N 6.37. MS: m/z 449.11. (M+H+. 98%).
3-[(7-Chloroquinolin-4-yl)sulfinyl]propyl-5-methyl-2-nitrobenzoate (60). Column chromathography DCM:EtOAc (9:1). Cream solid, yield: 69%; m.p. 116 °C; IR (KBr) cm−1: 3039, 2987, 1736, 1521, 1200, 1063; 1H NMR (CDCl3, 300 MHz) δ ppm: 1.95–2.08 (m, 1H, H10a), 2.29–2.40 (m, 1H, H10b), 2.42 (s, 3H, CH3), 2.83–2.92 (m, 1H, H9a), 3.17–3.27 (m, 1H, H9b), 4.31–4.39 (m, 1H, H11a), 4.47–4.54 (m, 1H, H11b), 7.36–7.38 (m, 2H, H4′,5), 7.54 (dd, 1H, H6, J = 2.1, 8.9 Hz), 7.81 (d, 2H, H3′,6′, J = 8.9 Hz), 7.97 (d, 1H, H3, J = 4.1 Hz), 8.15 (d, 1H, H8, J = 2.1 Hz), 9.07 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.4 (CH3), 21.6 (C10), 51.8 (C9), 64.4 (C11), 117.0 (C3), 121.7, 123.1 (C5), 124.1 (C3′), 127.7, 129.0 (C6), 129.6 (C8), 130.1 (C6′), 132.2 (C4′), 136.4, 144.9, 145.4, 148.5, 150.9, 151.2 (C2), 165.7 (C12). Anal. calcd. for: C20H17ClN2O5S: C 55.49, H 3.96, N 6.47; Found: C 55.47, H 3.97, N 6.61. MS: m/z 433.10. (M+H+. 96%).
3-[(7-Chloroquinolin-4-yl)sulfinyl]propyl-3,5-dimethylbenzoate (61). Column chromathography DCM:EtOAc (1:1). Cream solid, yield: 75%; m.p. 105–107 °C; IR (KBr) cm−1: 3070, 2989, 1715, 1218, 1037; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.00–2.14 (m, 1H, H10a), 2.34–2.46 (m, 1H, H10b), 2.31 (s, 6H, CH3), 2.89–2.98 (m, 1H, H9a), 3.21–3.31 (m, 1H, H9b), 4.34–4.42 (m, 1H, H11a), 4.43–4.51 (m, 1H, H11b), 7.17 (brs, 1H, H4′), 7.45 (dd, 1H, H6, J = 2.1, 8.9 Hz), 7.52 (brs, 2H, H2′,6′), 7.74 (d, 1H, H5, J = 8.9 Hz), 7.99 (d, 1H, H3, J = 4.4 Hz), 8.17 (d, 1H, H8, J = 2.1 Hz), 9.09 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.2 (2 × CH3), 21.9 (C10), 52.3 (C9), 62.6 (C11), 117.1 (C3), 121.7, 121.8, 122.8 (C5), 127.3 (C2′,6′), 128.9 (C6), 129.5, 129.8 (C8), 135.0 (C4′), 136.4, 138.2, 148.5, 150.9, 151.4 (C2), 166.6 (C12). Anal. calcd. for: C21H20ClNO3S: C 62.76, H 5.02, N 3.49; Found: C 62.77, H 4.98, N 3.65. MS: m/z 402.15. (M+H+. 100%).
3-[(7-Chloroquinolin-4-yl)sulfinyl]propyl-4-(trifluoromethyl)benzoate (62). Column chromathographyc DCM:EtOAc (8:2). White solid, yield: 68%; m.p. 86–87 °C; IR (KBr) cm−1: 2958, 1730, 1280, 1020; 1H NMR (CDCl3, 300 MHz) δ ppm: 1.99–2.13 (m, 1H, H10a), 2.33–2.47 (m, 1H, H10b), 2.89–2.99 (m, 1H, H9a), 3.20–3.30 (m, 1H, H9b), 4.37–4.52 (m, 2H, H11), 7.50 (dd, 1H, H6, J = 2.1, 8.9 Hz), 7.65 (d, 2H, H3′,5′, J = 8.7 Hz), 7.75 (d, 1H, H5, J = 8.9 Hz), 7.98–8.01 (m, 3H, H3,2′,6′), 8.17 (d, 1H, H8, J = 2.1 Hz), 9.09 (d, 1H, H2, J = 4.4 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.6 (C10), 51.7 (C9), 63.5 (C11), 117.6 (C3), 121.7, 122.7 (C5), 125.6 (q, J = 14.7 Hz), 129.0 (C6), 129.9 (C8), 130.0 (C2′ or 6′), 130.0 (C2′ or 6′), 132.8 (d, J = 4.5 Hz), 134.8 (q, J = 129.9 Hz), 136.5, 148.5, 150.7, 151.3 (C2), 165.1 (C12). Anal. calcd. for: C20H15ClF3NO3S: C 54.37, H 4.32, N 3.17; Found: C 54.39, H 4.32, N 3.34. MS: m/z 442.08. (M+H+. 75%).
3.1.4. General Procedure for the Synthesis of Compounds 63–82
A solution of the corresponding sulfanyl benzoate derivative (0.25 mmol) in dry DCM (5 mL) was treated with m-CPBA (2.5 mmol). The mixture was shaken at room temperature (rt) between 8–15 h, under a N2 atmosphere. The organic layer was washed with an aqueous mixture of saturated sodium bicarbonate and saturated sodium bisulfite (1:1) (3 × 10 mL), water (2 × 20 mL), a saturated NaCl aqueous solution (2 × 10 mL), and was finally dried over Na2SO4, filtered, and evaporated under reduced pressure to give the crude product. The compounds were then purified by column chromatography.
2-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]ethyl-4-methoxybenzoate (63). Column chromathography DCM:EtOAc (9:1). White solid, yield: 61%; m.p. 142–144 °C; IR (KBr) cm−1: 3042, 2988, 1709, 1298, 1243, 1137, 1056; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.80 (t, 2H, H9, J = 5.5 Hz), 3.86 (brs, 3H, OMe), 4.63 (t, 2H, H10, J = 5.3 Hz), 6.76 (d, 2H, H2′,6′, J = 8.9 Hz), 7.31 (d, 2H, H3′,5′, J = 8.9 Hz), 7.69 (dd, 1H, H6, J = 2.2, 9.1 Hz), 7.98 (d, 1H, H3, J = 6.5 Hz), 8.39 (d, 1H, H2, J = 6.5 Hz), 8.52 (d, 1H, H8, J = 2.2 Hz), 8.56 (d, 1H, H5, J = 9.1 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 55.7 (OMe), 56.0 (C9), 57.9 (C10), 113.8 (C3′,5′), 120.3 (C8), 120.4, 124.4, 124.8 (C3), 126.3 (C5), 130.5 (C2′,6′), 131.0 (C6), 132.1, 134.9 (C2), 138.0, 143.0, 164.0, 165.1 (C11). Anal. calcd. for: C19H16ClNO6S: C 54.10, H 3.82, N 3.32; Found: C 54.12, H 3.85, N 3.47. MS: m/z 422.06. (M+H+. 100%).
2-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]ethyl-2,3-dimethoxybenzoate (64). Column chromathography DCM:EtOAc (9:1). White solid, yield: 51%; m.p. 110 °C; IR (KBr) cm−1: 2986, 1725, 1291, 1265, 1145, 1078; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.68 (brs, 3H, OMe), 3.75 (t, 2H, H9, J = 5.6 Hz), 3.85 (brs, 3H, OMe), 4.61 (t, 2H, H10, J = 5.4 Hz), 6.57 (dd, 1H, H4′, J = 1.5, 7.8 Hz), 6.88 (t, 1H, H5′, J = 7.9 Hz), 7.00 (dd, 1H, H6′, J = 1.4, 8.2 Hz), 7.66 (dd, 1H, H6, J = 2.2, 9.1 Hz), 7.92 (d, 1H, H3, J = 6.5 Hz), 8.34 (d, 1H, H2, J = 6.5 Hz), 8.51 (d, 1H, H8, J = 2.2 Hz), 8.54 (d, 1H, H5, J = 9.1 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 55.8 (C9), 56.1 (OMe), 57.9 (C10), 61.4 (OMe), 116.5 (C4′), 120.1 (C8), 121.1 (C5′), 123.7 (C6′), 123.8, 124.3, 124.8 (C3), 126.2 (C5), 130.4, 131.9 (C6), 134.8 (C2), 137.8, 142.8, 149.1, 153.5, 164.5 (C11). Anal. calcd. for: C20H18ClNO7S: C 53.16, H 4.02, N 3.10; Found: C 53.16, H 4.03, N 3.27. MS: m/z 452.07. (M+H+. 100%).
2-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]ethyl-2,5-dimethoxybenzoate (65). Column chromathography DCM:EtOAc (9.5:0.5). Yellow solid, yield: 72%; m.p. 133–135 °C; IR (KBr) cm−1: 3087, 2991, 1728, 1239, 1227, 1145, 1052; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.70 (s, 3H, OMe), 3.75 (s, 3H, OMe), 3.79 (t, 2H, H9, J = 5.3 Hz), 4.64 (t, 2H, H10, J = 5.1 Hz), 6.73 (d, 1H, H6′, J = 2.9 Hz), 6.81 (d, 1H, H3′, J = 9.1 Hz), 7.01 (dd, 1H, H4′, J = 2.9, 9.0 Hz), 7.67 (dd, 1H, H6, J = 1.6, 9.1 Hz), 7.95 (d, 1H, H3, J = 6.5 Hz), 8.32 (d, 1H, H2, J = 6.5 Hz), 8.46 (d, 1H, H8, J = 1.5 Hz), 8.56 (d, 1H, H5, J = 9.1 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 55.9 (OMe), 55.9 (C9), 56.3 (OMe), 58.1 (C10), 113.4 (C3′), 116.1 (C6′), 117.7, 119.9 (C4′), 120.1 (C8), 124.4 (C3), 124.8, 126.3 (C5), 130.6, 131.9 (C6), 134.7 (C2), 137.8, 142.8, 152.8, 153.5, 164.4 (C11). Anal. calcd. for: C20H18ClNO7S: C 53.16, H 4.02, N 3.10; Found: C 53.22, H 4.07, N 3.19. MS: m/z 452.05. (M+H+. 100%).
2-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]ethyl-2,4,5-trimethoxybenzoate (66). Column chromathography DCM:EtOAc (9.5:0.5). Yellow solid, yield: 71%; m.p. 148–150 °C; IR (KBr) cm−1: 3080, 2998, 1726, 1295, 1243, 1160, 1025; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.67 (s, 3H, OMe), 3.74 (s, 3H, OMe), 3.77 (t, 2H, H9, J = 5.3 Hz), 3.94 (s, 3H, OMe), 4.58 (t, 2H, H10, J = 5.1 Hz), 6.35 (s, 1H, H3′), 6.86 (s, 1H, H6′), 7.62 (dd, 1H, H6, J = 2.0, 9.1 Hz), 7.92 (d, 1H, H3, J = 6.5 Hz), 8.32 (d, 1H, H2, J = 6.5 Hz), 8.43 (d, 1H, H8, J = 1.9 Hz), 8.52 (d, 1H, H5, J = 9.1 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 55.9 (C9), 56.2 (OMe), 56.3 (2 × OMe), 57.7 (C10), 96.9 (C3′), 107.9, 113.7 (C6′), 119.9 (C8), 124.3, 124.7 (C3), 126.2 (C5), 130.5, 131.7 (C6), 134.6 (C2), 137.6, 142.4, 142.7, 154.3, 155.6, 164.3 (C11). Anal. calcd. for: C21H20ClNO8S: C 52.34, H 4.18, N 2.91; Found: C 52.36, H 4.17, N 3.12. MS: m/z 482.06. (M+H+. 100%).
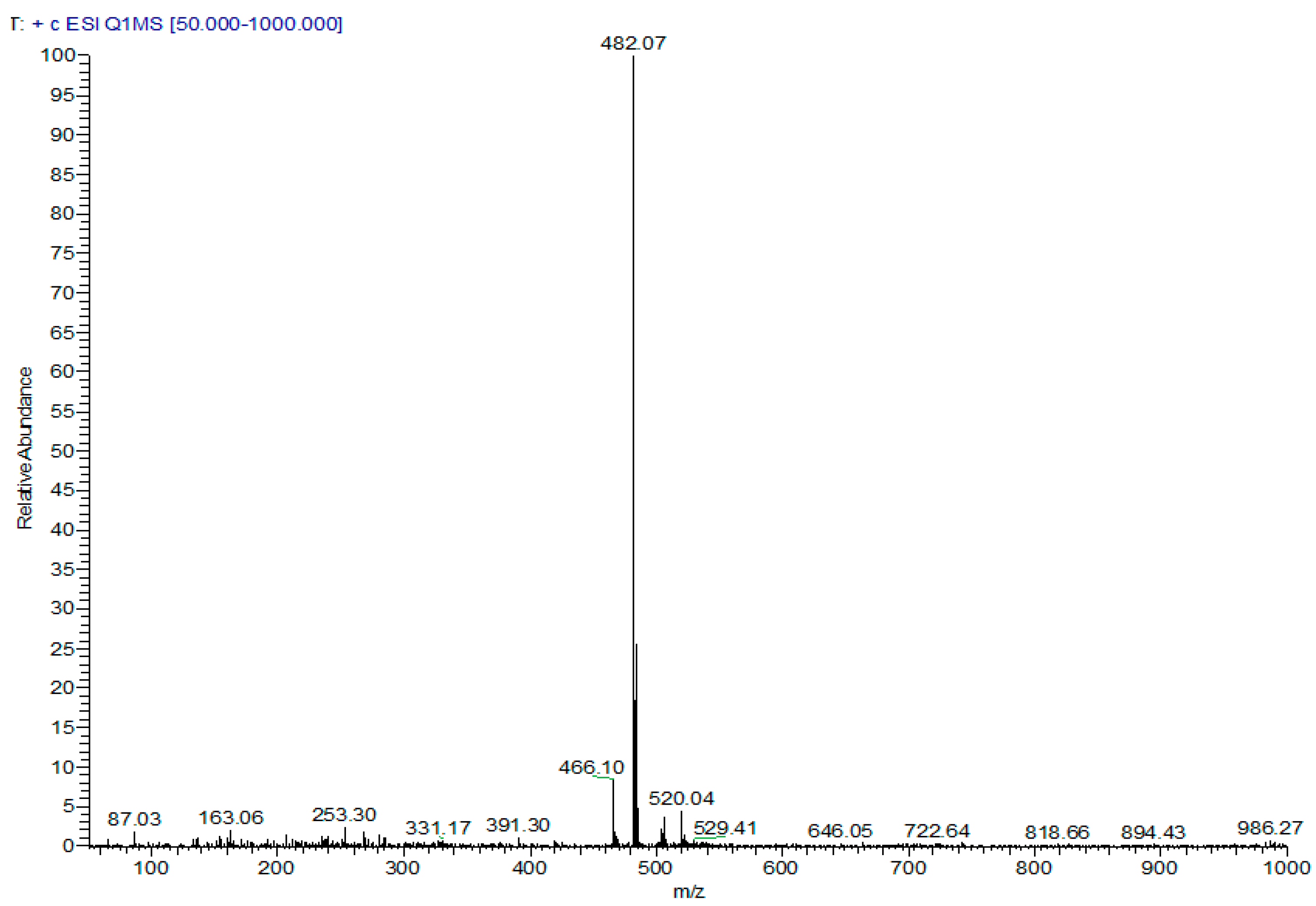
2-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]ethyl-3,4,5-trimethoxybenzoate (67). Column chromathography DCM:EtOAc (9.5:0.5). Yellow solid, yield: 51%; m.p. 168 °C; IR (KBr) cm−1: 3017, 2993, 1723, 1294, 1234, 1143, 1036; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.79–3.83 (m, 8H, 2 × OMe, H9), 3.94 (s, 3H, OMe), 4.65 (t, 2H, H10, J = 5.4 Hz), 6.77 (s, 2H, H2′,6′), 7.69 (dd, 1H, H6, J = 2.2, 9.1 Hz), 7.98 (d, 1H, H3, J = 6.5 Hz), 8.40 (d, 1H, H2, J = 6.5 Hz), 8.50 (d, 1H, H8, J = 2.2 Hz), 8.54 (d, 1H, H5, J = 9.1 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 55.6 (C9), 56.3 (OMe), 58.1 (C10), 61.0 (OMe), 106.4 (C2′,6′), 120.1 (C8), 123.2, 124.3, 124.8 (C3), 126.2 (C5), 130.0, 132.1 (C6), 134.7 (C2), 138.0, 143.0, 153.0, 165.3 (C11). Anal. calcd. for: C21H20ClNO8S: C 52.34, H 4.18, N 2.91; Found: C 52.31, H 4.21, N 3.23. MS: m/z 482.07. (M+H+. 100%).
2-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]ethyl-2-methoxybenzoate (68). Column chromathography DCM:EtOAc (9.5:0.5). Solid light orange, yield: 72%; m.p. 106–108 °C; IR (KBr) cm−1: 3015, 2995, 1731, 1297, 1235, 1142, 1043; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.73 (s, 3H, OMe), 3.78 (t, 2H, H9, J = 5.5 Hz), 4.62 (t, 2H, H10, J = 5.3 Hz), 6.79 (t, 1H, H3′, J = 7.9 Hz), 6.86 (d, 1H, H4′ or 5′, J = 8.4 Hz), 7.09 (dd, 1H, H4′ or 5′, J = 1.7, 7.8 Hz), 7.44 (td, 1H, H6′, J = 1.8, 7.9 Hz), 7.65 (dd, 1H, H6, J = 2.2, 9.1 Hz), 7.94 (d, 1H, H3, J = 6.5 Hz), 8.31 (d, 1H, H2, J = 6.5 Hz), 8.43 (d, 1H, H8, J = 2.2 Hz), 8.55 (d, 1H, H5, J = 9.1 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 55.8 (OMe), 55.9 (C9), 57.9 (C10), 112.0 (C3′), 117.2, 120.0, 124.4, 124.8 (C3), 126.3 (C5), 130.6 (C6′), 131.1, 131.9 (C6), 134.6 (C4′), 134.7 (C2), 137.8, 142.7, 159.2, 164.4 (C11). Anal. calcd. for: C19H16ClNO6S: C 54.10, H 3.82, N 3.32; Found: C 54.10, H 3.83, N 3.49. MS: m/z 422.08. (M+H+. 100%).
2-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]ethyl-4-methoxy-3-nitrobenzoate (69). Column chromathography DCM:EtOAc (8:2). Solid light yellow, yield: 53%; m.p. 205–206 °C; IR (KBr) cm−1: 3035, 2921, 1724, 1530, 1300, 1238, 1142, 1080; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.80 (t, 2H, H9, J = 5.6 Hz), 4.06 (s, 3H, OMe), 4.71 (t, 2H, H10, J = 5.4 Hz), 7.04 (d, 1H, H5′, J = 8.9 Hz), 7.65 (dd, 1H, H6′, J = 2.2, 8.8 Hz), 7.75 (dd, 1H, H6, J = 2.3, 9.1 Hz), 8.02 (d, 1H, H3, J = 6.5 Hz), 8.06 (d, 1H, H2′, J = 2.2 Hz), 8.45 (d, 1H, H2, J = 6.5 Hz), 8.56 (d, 1H, H8, J = 2.3 Hz), 8.59 (d, 1H, H5, J = 9.1 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 55.6 (C9), 57.2 (OMe), 58.5 (C10), 113.5 (C5′), 119.6, 120.2 (C8), 120.6, 124.3, 124.9 (C3), 126.3 (C5), 127.0 (C2′), 128.8, 132.4 (C2), 134.7 (C2′),134.9, 138.4, 143.1, 156.9, 163.4 (C11). Anal. calcd. for: C19H15ClN2O8S: C 48.88, H 3.24, N 6.00; Found: C 48.89, H 3.27, N 5.89. MS: m/z 467.05. (M+H+. 100%).
2-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]ethyl-5-methyl-2-nitrobenzoate (70). Column chromathography DCM:EtOAc (9.5:0.5). Yellow solid, yield: 48%; m.p. 148–150 °C; IR (KBr) cm−1: 2983, 1743, 1523, 1341, 1297, 1139; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.45 (s, 3H, CH3), 3.71 (t, 2H, H9, J = 5.6 Hz), 4.68 (t, 2H, H10, J = 5.4 Hz), 7.06 (s, 1H, H6′), 7.39 (d, 1H, H4′, J = 8.3 Hz), 7.69 (dd, 1H, H6, J = 2.0, 9.1 Hz), 7.75 (d, 1H, H3′, J = 8.3 Hz), 7.94 (d, 1H, H3, J = 6.5 Hz), 8.38 (d, 1H, H2, J = 6.5 Hz), 8.52 (d, 1H, H8, J = 2.0 Hz), 8.58 (d, 1H, H5, J = 9.1 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.5 (CH3), 55.5 (C9), 59.0 (C10), 120.0 (C8), 124.3, 124.4, 124.9, 126.4, 126.5, 129.5, 130.3, 132.1, 132.6, 134.8, 138.1, 142.7, 145.0, 145.4, 164.8 (C11). Anal. calcd. for: C19H15ClN2O7S: C 50.62, H 3.35, N 6.21; Found: C 50.65, H 3.34, N 6.47. MS: m/z 451.03. (M+H+. 100%).
2-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]ethyl-,5-dimethylbenzoate (71). Column chromathography DCM:EtOAc (8:2). White solid, yield: 70%; m.p. 172–174 °C; IR (KBr) cm−1: 2931, 1720, 1275, 1158, 1025; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.27 (s, 6H, 2 × CH3), 3.83 (t, 2H, H9, J = 5.4 Hz), 4.66 (t, 2H, H10, J = 5.3 Hz), 6.97 (s, 2H, H2′,6′), 7.15 (s, 1H, H4′), 7.68 (dd, 1H, H6, J = 2.2, 9.12 Hz), 7.98 (d, 1H, H3, J = 6.5 Hz), 8.38 (d, 1H, H2, J = 6.5 Hz), 8.48 (d, 1H, H8, J = 2.2 Hz), 8.55 (d, 1H, H5, J = 9.1 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.2 (2 × CH3), 55.9 (C9), 58.2 (C10), 120.2 (C8), 124.3, 124.8 (C3), 126.2 (C5), 126.6 (C2′,6′), 128.0, 130.5, 132.1 (C6), 134.7, 135.5 (C2), 138.0, 138.5, 142.9, 165.8 (C11). Anal. calcd. for: C20H18ClNO5S: C 57.21, H 4.32, N 3.34; Found: C 57.18, H 4.35, N 3.51. MS: m/z 420.07. (M+H+. 100%).
2-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]ethyl-4-(trifluoromethyl)benzoate (72). Column chromathography DCM:EtOAc (8:2). White solid, yield: 73%; m.p. 115–117 °C; IR (KBr) cm−1: 3029, 2922, 1731, 1318, 1265, 1239, 1069; 1H NMR (CDCl3, 300 MHz) δ ppm: 3.79 (t, 2H, H9, J = 5.6 Hz), 4.72 (t, 2H, H10, J = 5.4 Hz), 7.59–7.66 (m, 4H, H2′,3′,5′,6′), 7.70 (dd, 1H, H6, J = 2.3, 9.1 Hz), 8.01 (d, 1H, H3, J = 6.5 Hz), 8.46 (d, 1H, H2, J = 6.5 Hz), 8.54 (d, 1H, H8, J = 2.2 Hz), 8.58 (d, 1H, H5, J = 9.1 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 55.7 (C9), 58.5 (C10), 120.3 (C8), 124.3, 124.9 (C3), 125.6 (q, J = 14.6 Hz), 126.2 (C5), 129.6 (C2′,6′), 129.9, 130.5, 131.6 (d, J = 4.4 Hz), 132.3 (C6), 134.9 (C2), 135.2 (q, J = 130.7 Hz), 138.3, 143.0, 151.1, 164.4 (C11). 19F NMR (CDCl3) δ ppm: −63.24. Anal. calcd. for: C19H13ClF3NO5S: C 49.63, H 2.85, N 3.05; Found: C 49.65, H 2.87, N 3.27. MS: m/z 460.12. (M+H+. 78%).
3-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]propyl-4-methoxybenzoate (73). Column chromathography DCM:EtOAc (8:2). White solid, yield: 64%; m.p. 135–137 °C; IR (KBr) cm−1: 3057, 2979, 1699, 1310, 1211, 1146, 1084; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.16–2.25 (m, 2H, H10), 3.43 (t, 2H, H9, J = 7.6 Hz), 3.85 (s, 3H, OMe), 4.31 (t, 2H, H11, J = 6.0 Hz), 6.85 (d, 2H, H3′,5′, J = 8.85 Hz), 7.68 (d, 1H, H6, J = 2.1, 9.1 Hz), 7.75 (d, 2H, H2′,6′, J = 8.9 Hz), 7.99 (d, 1H, H3, J = 6.5 Hz), 8.53 (d, 1H, H2, J = 6.5 Hz), 8.59 (d, 1H, H5, J = 9.1 Hz), 8.71 (d, 1H, H8, J = 2.1 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 22.7 (C10), 53.4 (C9), 55.6 (OMe), 61.7 (C11), 113.8 (C3′,5′), 120.4 (C8), 121.6, 124.4, 124.9 (C3), 126.4 (C5), 129.7, 131.5 (C2′,6′). 132.1 (C6), 134.9 (C2), 138.2, 143.0, 163.8, 165.8 (C12). Anal. calcd. for: C20H18ClNO6S: C 55.11, H 4.16, N 3.21; Found: C 55.10, H 4.16, N 3.41. MS: m/z 436.08. (M+H+. 100%).
3-[( N-Oxide 7-chloroquinolin-4-yl)sulfonyl]propyl-2,3-dimethoxybenzoate (74). Column chromathography DCM:EtOAc (9:1). Cream solid, yield: 75%; m.p. 106–108 °C; IR (KBr) cm−1: 3059, 2939, 1704, 1305, 1235, 1149, 1043; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.18–2.27 (m, 2H, H10), 3.45 (t, 2H, H9, J = 7.6 Hz), 3.77 (s, 3H, OMe), 3.87 (s, 3H, OMe), 4.36 (t, 2H, H11, J = 5.9 Hz), 7.02–7.05 (m, 2H, H4′,5′), 7.13 (dd, 1H, H6′, J = 3.4, 6.18 Hz), 7.69 (dd, 1H, H6, J = 2.16, 9.09 Hz), 7.97 (d, 1H, H3, J = 6.48 Hz), 8.51 (d, 1H, H2, J = 6.5 Hz), 8.60 (d, 1H, H5, J = 9.1 Hz), 8.71 (d, 1H, H8, J = 2.2 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 22.5 (C10), 53.3 (C9), 56.1 (OMe), 61.4 (OMe), 62.1 (C11), 116.1 (C4′), 120.2 (C8), 122.0 (C5′), 124.0 (C6′), 124.3, 124.7 (C3), 125.3, 126.6 (C5), 129.9, 132.1 (C6), 134.9 (C2), 138.2, 143.0, 149.0, 153.5, 165.9 (C12). Anal. calcd. for: C21H20ClNO7S: C 54.14, H 4.33, N 3.01; Found: C 54.12, H 4.32, N 3.27. MS: m/z 466.09. (M+H+. 100%).
3-[( N-Oxide 7-chloroquinolin-4-yl)sulfonyl]propyl-2,5-dimethoxybenzoate (75). Column chromathographyc DCM:EtOAc (9:1). Cream solid, yield: 65%; m.p. 127–128 °C; IR (KBr) cm−1: 3058, 2968, 1712, 1300, 1214, 1144, 1027; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.15–2.24 (m, 2H, H10), 3.46 (t, 2H, H9, J = 6.7 Hz), 3.75 (s, 6H, OMe), 4.33 (t, 2H, H11, J = 6.6 Hz), 6.86 (d, 1H, H3′, J = 9.1 Hz), 6.99 (dd, 1H, H4′, J = 3.2, 9.1 Hz), 7.15 (d, 1H, H6′, J = 3.2 Hz), 7.67 (dd, 1H, H6, J = 2.2, 9.1 Hz), 7.97 (d, 1H, H3, J = 6.5 Hz), 8.51 (d, 1H, H2, J = 6.5 Hz), 8.60 (d, 1H, H5, J = 9.1 Hz), 8.69 (d, 1H, H8, J = 2.2 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 22.6 (C10), 53.4 (C9), 55.9 (OMe), 56.6 (OMe), 62.0 (C11), 113.7, 116.3, 119.6, 120.3 (C8), 124.3 (C4′), 124.8 (C3), 126.5 (C5), 129.9, 132.0 (C6), 134.9 (C2), 138.2, 143.0, 153.0, 153.5, 165.6 (C12). Anal. calcd. for: C21H20ClNO7S: C 54.14, H 4.33, N 3.01; Found: C 54.17, H 4.28, N 3.19. MS: m/z 466.11. (M+H+. 100%).
3-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]propyl-2,4,5-trimethoxybenzoate (76). Column chromathography DCM:EtOAc (9:1). Yellow solid, yield: 61%; m.p. 148 °C; IR (KBr) cm−1: 3054, 2931, 1679, 1314, 1245, 1209, 1041; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.14–2.23 (m, 2H, H10), 3.46 (t, 2H, H9, J = 7.6 Hz), 3.77 (s, 3H, OMe), 3.81 (s, 3H, OMe), 3.92 (s, 3H, OMe), 4.29 (t, 2H, H11, J = 5.9 Hz), 6.44 (s, 1H, H3′), 7.22 (s, 1H, H6′), 7.65 (dd, 1H, H6, J = 2.2, 9.1 Hz), 7.97 (d, 1H, H3, J = 6.5 Hz), 8.51 (d, 1H, H2, J = 6.5 Hz), 8.59 (d, 1H, H5, J = 9.1 Hz), 8.68 (d, 1H, H8, J = 2.2 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 22.7 (C10), 53.5 (C9), 56.1 (OMe), 56.5 (OMe), 56.8 (OMe), 61.6 (C11), 97.4 (C3′), 109.5, 114.4 (C6′), 120.2 (C8), 124.3, 124.8 (C3), 126.5 (C5), 129.8, 131.9 (C6), 134.9 (C2), 138.1, 142.6, 143.0, 154.1, 155.7, 165.3 (C12). Anal. calcd. for: C22H22ClNO8S: C 53.28, H 4.47, N 2.82; Found: C 53.28, H 4.48, N 3.07. MS: m/z 496.10. (M+H+. 100%).
3-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]propyl-3,4,5-trimethoxybenzoate (77). Column chromathography DCM:EtOAc (9:1). White solid, yield: 64%; m.p. 187–188 °C; IR (KBr) cm−1: 3056, 2927, 1708, 1314, 1213, 1147, 1027; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.17–2.26 (m, 2H, H10), 3.40 (t, 2H, H9, J = 7.5 Hz), 3.83 (s, 6H, 2 × OMe), 3.87 (s, 3H, OMe), 4.34 (t, 2H, H11, J = 6.1 Hz), 7.10 (s, 2H, H2′,6′), 7.65 (dd, 1H, H6, J = 2.2, 9.1 Hz), 7.97 (d, 1H, H3, J = 6.5 Hz), 8.51 (d, 1H, H2, J = 6.5 Hz), 8.58 (d, 1H, H5, J = 9.1 Hz), 8.67 (d, 1H, H8, J = 2.2 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 22.6 (C10), 53.3 (C9), 56.3 (2 × OMe), 61.0 (OMe), 62.3 (C11), 106,8 (C2′,6′), 120.3 (C8), 124.2, 124.3, 124.9 (C3), 126.3 (C5), 129.6, 132.0 (C6), 134.9 (C2), 138.2, 142.6, 143.0, 153.0, 165.7 (C12). Anal. calcd. for: C22H22ClNO8S: C 53.28, H 4.47, N 2.82; Found: C 53.30, H 4.45, N 2.98. MS: m/z 496.09. (M+H+. 100%).
3-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]propyl-2-methoxybenzoate (78). Column chromathography DCM:EtOAc (9.5:0.5). Solid light orange, yield: 67%; m.p. 106–108 °C; IR (KBr) cm−1: 3053, 2933, 1718, 1305, 1212, 1145, 1020; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.13–2.22 (m, 2H, H10), 3.45 (t, 2H, H9, J = 7.7 Hz), 3.80 (s, 3H, OMe), 4.31 (t, 2H, H11, J = 5.9 Hz), 6.87–6.93 (m, 2H, H3′,5′), 7.41–7.47 (td, 1H, H4′, J = 1.8, 8.6 Hz), 7.58 (dd, 1H, H6′, J = 1.8, 7.6 Hz), 7.66 (dd, 1H, H6, J = 2.2, 9.1 Hz), 7.97 (d, 1H, H3, J = 6.5 Hz), 8.51 (d, 1H, H2, J = 6.5 Hz), 8.60 (d, 1H, H5, J = 9.1 Hz), 8.69 (d, 1H, H8, J = 2.2 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 22.6 (C10), 53.4 (C9), 55.9 (OMe), 61.8 (C11), 112.1 (C3′), 119.1, 120.2 (C5′ or 8), 120.3 (C5′ or 8), 124.3, 124.8 (C3), 126.5 (C5), 129.8, 131.5 (C6′), 131.9 (C6), 134.0 (C4′), 134.9 (C2), 138.1, 142.9, 159.2, 165.7 (C12). Anal. calcd. for: C20H18ClNO6S: C 55.11, H 4.16, N 3.21; Found: C 55.10, H 4.15, N 3.39. MS: m/z 436.07. (M+H+. 100%).
3-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]propyl-4-methoxy-3-nitrobenzoate (79). Column chromathographyc DCM:EtOAc (9:1). Solid light yellow, yield: 63%; m.p. 205–206 °C; IR (KBr) cm−1: 3038, 2927, 1706, 1530, 1364, 1239, 1144, 1079; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.23–2.32 (m, 2H, H10), 3.43 (t, 2H, H9, J = 7.5 Hz), 4.04 (s, 3H, OMe), 4.40 (t, 2H, H11, J = 6.2 Hz), 7.11 (d, 1H, H5′, J = 8.9 Hz), 7.75 (dd, 1H, H6, J = 2.2, 9.1 Hz), 8.02 (d, 1H, H3, J = 6.5 Hz), 8.06 (dd, 1H, H6′, J = 2.2, 8.8 Hz), 8.33 (d, 1H, H2′, J = 2.2 Hz), 8.55 (d, 1H, H2, J = 6.5 Hz), 8.64 (d, 1H, H5, J = 9.1 Hz), 8.75 (d, 1H, H8, J = 2.2 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 22.6 (C10), 53.3 (C9), 57.1 (OMe), 62.7 (C11), 113.4 (C5′), 120.5 (C8), 121.7, 124.4, 125.0 (C3), 126.4 (C5), 127.2 (C2′), 129.7, 132.3 (C6), 135.0 (C2), 135.4 (C6′), 138.4, 156.6, 164.1 (C12). Anal. calcd. for: C20H17ClN2O8S: C 49.95, H 3.56, N 5.83; Found: C 49.97, H 3.56, N 5.95. MS: m/z 481.06. (M+H+. 83%).
3-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]propyl-5-methyl-2-nitrobenzoate (80). Column chromathography DCM:EtOAc (9:1). White solid, yield: 60%; m.p. 182–184 °C; IR (KBr) cm−1: 3021, 2971, 1740, 1512, 1341, 1203, 1037; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.19–2.28 (m, 2H, H10), 2.46 (s, 3H, CH3), 3.40 (t, 2H, H9, J = 7.6 Hz), 4.42 (t, 2H, H11, J = 5.8 Hz), 7.39–7.41 (m, 2H, H 4′,6′), 7.76 (dd, 1H, H6, J = 2.3, 9.2 Hz), 7.80 (d, 1H, H3′, J = 8.8 Hz), 8.01 (d, 1H, H3, J = 6.5 Hz), 8.55 (d, 1H, H2, J = 6.5 Hz), 8.68 (d, 1H, H5, J = 9.1 Hz), 8.77 (d, 1H, H8, J = 2.2 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.5 (CH3), 22.2 (C10), 53.5 (C9), 63.7 (C11), 120.3 (C8), 124.2 (C3′), 124.5 (C3), 124.8, 126.8 (C5), 127.5, 130.1, 130.3 (C6′), 132.2 (C6), 132.4 (C4′), 134.9 (C2), 138.3, 143.1, 145.0, 145.6, 165.6 (C12). Anal. calcd. for: C20H17ClN2O7S: C 51.67, H 3.69, N 6.03; Found: C 51.69, H 3.66, N 6.23. MS: m/z 465.07. (M+H+. 97%).
3-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]propyl-3,5-dimethylbenzoate (81). Column chromathography DCM:EtOAc (9:1). White solid, yield: 66%; m.p. 188–190 °C; IR (KBr) cm−1: 3019, 2915, 1707, 1363, 1290, 1043; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.19–2.28 (m, 2H, H10), 2.32 (s, 6H, 2 × CH3), 3.44 (t, 2H, H9, J = 7.6 Hz), 4.34 (t, 2H, H11, J = 6.0 Hz), 7.18 (brs, 1H, H4′), 7.45 (s, 2H, H2′, 6′), 7.66 (dd, 1H, H6, J = 2.2, 9.1 Hz), 7.99 (d, 1H, H3, J = 6.5 Hz), 8.53 (d, 1H, H2, J = 6.5 Hz), 8.61 (d, 1H, H5, J = 9.1 Hz), 8.70 (d, 1H, H8, J = 2.2 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 21.3 (2 × CH3), 22.7 (C10), 53.4 (C9), 61.9 (C11), 120.4 (C8), 124.4, 124.9 (C3), 126.4 (C5), 127.2 (C2′,6′), 129.2, 129.7, 132.1 (C6), 134.9, 135.2 (C2), 138.2, 138.3, 143.1, 166.4 (C12). Anal. calcd. for: C21H20ClNO5S: C 58.13, H 4.65, N 3.23; Found: C 58.19, H 4.69, N 3.28. MS: m/z 434.12. (M+H+. 100%).
3-[(N-Oxide 7-chloroquinolin-4-yl)sulfonyl]propyl-4-(trifluoromethyl)benzoate (82). Column chromathography DCM:EtOAc (7:3). White solid, yield: 58%; m.p. 139–140 °C; IR (KBr) cm−1: 3021, 2910, 1718, 1320, 1283, 1151, 1022; 1H NMR (CDCl3, 300 MHz) δ ppm: 2.21–2.30 (m, 2H, H10), 3.43 (t, 2H, H9, J = 7.4 Hz), 4.41 (t, 2H, H11, J = 5.9 Hz), 7.64–7.71 (m, 3H, H3′,5′,6), 7.98 (d, 2H, H2′,6′, J = 8.4 Hz), 8.00 (d, 1H, H3, J = 6.9 Hz), 8.54 (d, 1H, H2, J = 6.9 Hz), 8.61 (d, 1H, H5, J = 9.1 Hz), 8.71 (d, 1H, H8, J = 1.2 Hz). 13C NMR (CDCl3, 75 MHz) δ ppm: 22.6 (C10), 53.3 (C9), 62.7 (C11), 120.4 (C8), 124.3, 125.0 (C3), 125.6 (q, J = 14.4 Hz), 126.4 (C5), 129.6, 130.0 (C2′,6′), 132.1 (C6), 132.6 (d, J = 3.8 Hz), 134.9 (q, J =126.1 Hz), 138.3, 143.1, 164.9 (C12). 19F NMR (CDCl3) δ ppm: −63.14. Anal. calcd. for: C20H15ClF3NO5S: C 50.69, H 3.19, N 2.96; Found: C 50.69, H 3.21, N 3.25. MS: m/z 474.07. (M+H+. 78%).
 , EDCI, DMAP, CH2Cl2, rt.
, EDCI, DMAP, CH2Cl2, rt.
 , EDCI, DMAP, CH2Cl2, rt.
, EDCI, DMAP, CH2Cl2, rt.