Effect of Plasmonic Gold Nanoprisms on Biofilm Formation and Heat Shock Proteins Expression in Human Pathogenic Bacteria
Abstract
:1. Introduction
2. Results
2.1. AuNPs Characterization
2.2. Antibacterial Property of AuNPs
2.3. Biofilm Formation
2.4. Biofilm Inhibition
2.5. Effect of AuNPs on GroES and GroEL Expression
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Gold Nanoparticles
4.2.1. Synthesis
4.2.2. Characterization
4.3. Antibacterial Activity of Au NPs: Minimum Inhibitory and Minimum Bactericidal Concentration
4.4. Biofilm Formation on Polystyrene
4.5. Biofilm Inhibition
4.6. Western Blot for GroEL and GroES Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katas, H.; Lim, C.; Nor Azlan, A.; Buang, F.; Mh Busra, M. Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from lignosus rhinocerotis and chitosan. Saudi. Pharm. J. 2019, 27, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Rossolini, G.; Arena, F.; Pecile, P.; Pollini, S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef]
- Li, X.; Robinson, S.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.; Sahar, A.; Riley, M.; Rotello, V. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS. Nano. 2014, 8, 10682–10686. [Google Scholar] [CrossRef]
- Lenchenko, E.; Blumenkrants, D.; Sachivkina, N.; Shadrova, N.; Ibragimova, A. Morphological and adhesive properties of Klebsiella pneumoniae biofilms. Vet. World. 2020, 13, 197–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, R.; Leid, J.; Calhoun, J.; Costerton, J.; Shirtliff, M. Osteomyelitis and the role of biofilms in chronic infection. FEMS. Immunol. Med. Microbiol. 2008, 52, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Simões, M.; Bennett, R.N.; Rosa, E.A.S. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep. 2009, 26, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Vinod Kumar, K.; Lall, C.; Vimal Raj, R.; Vedhagiri, K.; Kartick, C.; Surya, P.; Natarajaseenivasan, K.; Vijayachari, P. Overexpression of heat shock groel stress protein in Leptospiral biofilm. Microb. Pathog. 2017, 102, 8–11. [Google Scholar] [CrossRef]
- Stamm, L.; Gherardini, F.; Parrish, E.; Moomaw, C. Heat shock response of spirochetes. Infect. Immun. 1991, 59, 1572–1575. [Google Scholar] [CrossRef] [Green Version]
- Susin, M.; Baldini, R.; Gueiros-Filho, F.; Gomes, S. GroES/GroEL and Dnak/Dnaj have distinct roles in stress responses and during cell cycle progression in Caulobacter crescentus. J. Bacteriol. 2006, 188, 8044–8053. [Google Scholar] [CrossRef] [Green Version]
- Maleki, F.; Khosravi, A.; Nasser, A.; Taghinejad, H.; Azizian, M. Bacterial heat shock protein activity. J. Clin. Diagn. Res. 2016, 10, BE01–BE03. [Google Scholar] [CrossRef]
- Gomes, S.L.; Simaõ, R.C.G. Stress response: Heat. In Encyclopedia of Microbiology; Schaechter, M., Ed.; Elsevier Academic Press: Oxford, UK, 2009; pp. 464–474. [Google Scholar]
- Yafout, M.; Ousaid, A.; Khayati, Y.; El Otmani, I. Gold nanoparticles as a drug delivery system for standard chemotherapeutics: A new lead for targeted pharmacological cancer treatments. Sci. Afr. 2021, 11, e00685. [Google Scholar] [CrossRef]
- Thambiraj, S.; Hema, S.; Ravi Shankaran, D. Functionalized gold nanoparticles for drug delivery applications. Mater. Today Proc. 2018, 5, 16763–16773. [Google Scholar] [CrossRef]
- Kong, F.; Zhang, J.; Li, R.; Wang, Z.; Wang, W.; Wang, W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Kandi, V.; Kandi, S. Antimicrobial properties of nanomolecules: Potential candidates as antibiotics in the era of multi-drug resistance. Epidemiol. Health 2015, 37, e2015020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhibber, S.; Nag, D.; Bansal, S. Inhibiting biofilm formation by Klebsiella pneumoniae B5055 using an iron antagonizing molecule and a bacteriophage. BMC Microbiol. 2013, 13, 174. [Google Scholar] [CrossRef] [Green Version]
- Mezni, A.; Dammak, T.; Fkiri, A.; Mlayah, A.; Abid, Y.; Smiri, L.S. Photochemistry at the surface of gold nanoprisms from surface-enhanced raman scattering blinking. J. Phys. Chem. C 2014, 118, 17956–17967. [Google Scholar] [CrossRef]
- Zawrah, M.F.; AbdEl-Moez, S.I. Antimicrobial activities of gold nanoparticles against major foodborne pathogens. Life Sci. 2011, 8, 37–44. [Google Scholar]
- Dibrov, P.; Dzioba, J.; Gosink, K.; Häse, C. Chemiosmotic mechanism of antimicrobial activity of ag + in Vibrio cholerae. Antimicrob. Agents Chemother. 2002, 46, 2668–2670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penders, J.; Stolzoff, M.; Hickey, D.; Andersson, M.; Webster, T. Shape-dependent antibacterial effects of non-cytotoxic gold nanoparticles. Int. J. Nanomed. 2017, 12, 2457–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Chen, P.; Chen, S.; Yuan, Z.; Yu, C.; Ren, B.; Zhang, K. In situ study of the antibacterial activity and mechanism of action of silver nanoparticles by surface-enhanced Raman spectroscopy. Anal. Chem. 2013, 85, 5436–5443. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. 2007, 18, 225103. [Google Scholar] [CrossRef]
- Sohm, B.; Immel, F.; Bauda, P.; Pagnout, C. Insight into the primary mode of action of tio2 nanoparticles on Escherichia coli in the dark. Proteomics. 2014, 15, 98–113. [Google Scholar] [CrossRef]
- Smitha, S.L.; Gopchandran, K.G. Surface Enhanced Raman scattering, antibacterial and antifungal active triangular gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 102, 114–119. [Google Scholar] [CrossRef]
- Joo, H.-S.; Otto, M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem. Biol. 2012, 19, 1503–1513. [Google Scholar] [CrossRef] [Green Version]
- Paharik, A.E.; Horswill, A.R. The Staphylococcal Bioflm: Adhesins, Regulation, and Host Response. Microbiol. Spectr. 2016, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Pandit, S.; Beshay, M.; Mokkapati, V.R.S.S.; Garnaes, J.; Olsson, M.E.; Sultan, A.; Mackevica, A.; Mateiu, R.V.; Lütken, H.; et al. Anti-biofilm effects of gold and silver nanoparticles synthesized by the rhodiola rosea rhizome extracts. Artif. Cells. Nanomed. Biotechnol. 2018, 46, S886–S899. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Khan, A.K.; Anwar, A.; Ali, S.A.; Shah, M.R. Biofilm inhibitory effect of chlorhexidine conjugated gold nanoparticles against Klebsiella pneumoniae. Microb. Pathog. 2016, 98, 50–56. [Google Scholar] [CrossRef]
- Bohne, J.; Sokolovic, Z.; Goebel, W. Transcriptional regulation of PRFA and PRFA-regulated virulence genes in listeria monocytogenes. Mol. Microbiol. 1994, 11, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Makumire, S.; Revaprasadu, N.; Shonhai, A. Dnak protein alleviates toxicity induced by citrate-coated gold nanoparticles in Escherichia coli. PLoS ONE 2015, 10, e0121243. [Google Scholar]
- Ben Abdallah, F.; Ellafi, A.; Lagha, R.; Bakhrouf, A.; Namane, A.; Rousselle, J.-C.; Lenormand, P.; Kallel, H. Identification of outer membrane proteins of Vibrio parahaemolyticus and Vibrio alginolyticus altered in response to γ-irradiation or long-term starvation. Res. Microbiol. 2010, 161, 869–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kustos, I.; Kocsis, B.; Kilár, F. Bacterial outer membrane protein analysis by electrophoresis and microchip technology. Expert Rev. Proteomics 2007, 4, 91–106. [Google Scholar] [CrossRef]
- Bagamboula, C.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. J. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- El-Deeb, B.; Elhariry, H.; Mostafa, N.Y. Antimicrobial Activity of Silver and Gold Nanoparticles Biosynthesized Using Ginger Extract. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1085. [Google Scholar]
- Gulluce, M.; Sahin, F.; Sokmen, M. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. Food. Chem. 2007, 103, 1449–1456. [Google Scholar] [CrossRef]
- Oulkheir, S.; Aghrouch, M.; El Mourabit, F.; Dalha, F.; Graich, H.; Amouch, F.; Ouzaid, K.; Moukale, A.; Chadli, S. Antibacterial Activity of Essential Oils Extracts from Cinnamon, Thyme, Clove and Geranium Against a Gram Negative and Gram-Positive Pathogenic Bacteria. J. Dis. Med. Plants 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Jadhav, S.; Shah, R.; Bhave, M.; Palombo, E.A. Inhibitory activity of yarrow essential oil on Listeria planktonic cells and biofilms. J. Food Control 2013, 29, 125–130. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, N.; Ayed, A.; Ammar, B.; Zrigui, R.; Nicaud, J.-M.; Kallel, H. Development of a cultivation process for the enhancement of human interferon alpha 2b production in the oleaginous yeast, Yarrowia Lipolytica. Microb. Cell. Factories 2011, 10, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
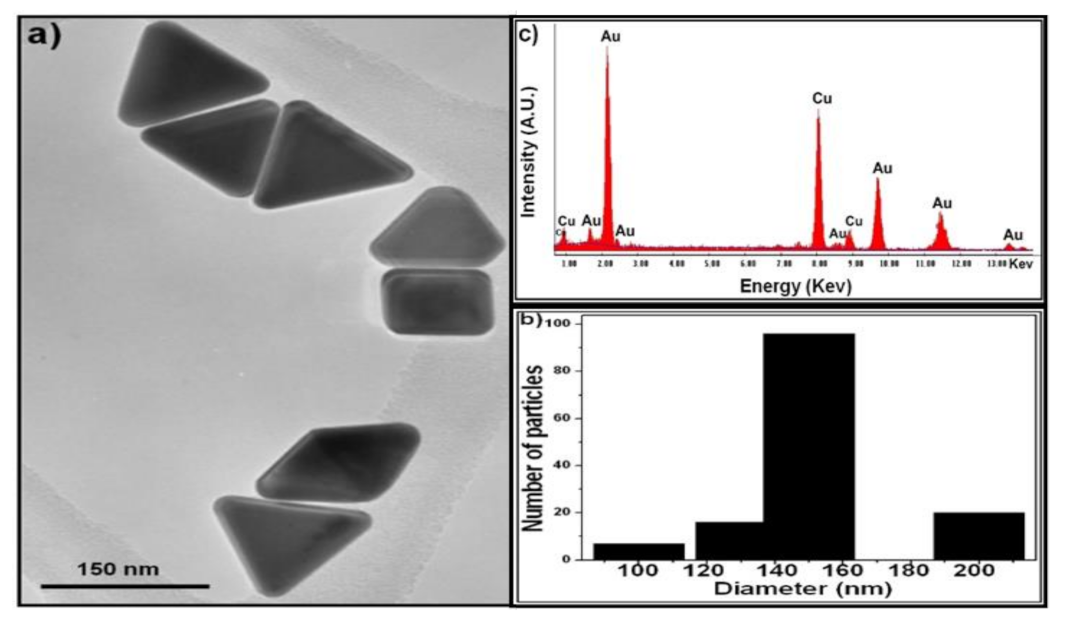

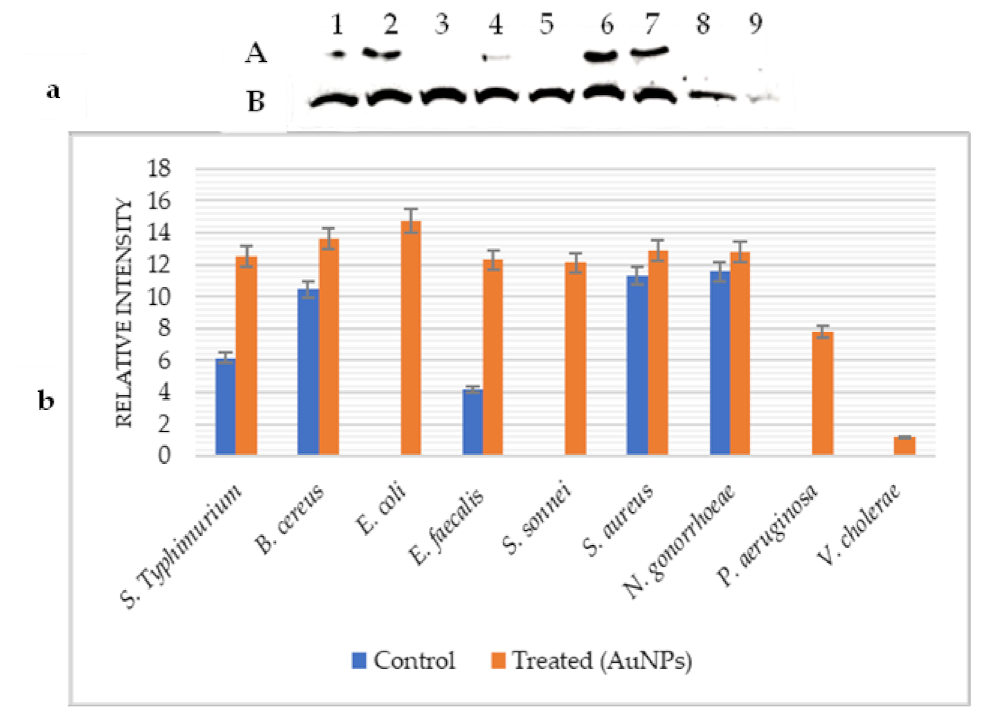
| Strains | Control OD570 ± SD | Biofilm Phenotype | AuNPs OD570 ± SD | Biofilm Phenotype | Inhibition (%) |
|---|---|---|---|---|---|
| S. Typhimurium | 0.37 ± 0.026 | low-grade positive | 0.065 ± 0.073 | negative | 82.43 |
| B. cereus | 0.046 ± 0.003 | negative | - | - | - |
| E. coli | 0.119 ± 0.042 | low-grade positive | 0.12 ± 0.062 | low-grade positive | 0 |
| E. faecalis | 0.599 ± 0.067 | low-grade positive | 0.206 ± 0.032 | low-grade positive | 65.60 |
| S. sonnei | 0.142 ± 0.022 | low-grade positive | 0.131 ± 0.046 | low-grade positive | 0 |
| S. aureus | 3.22 ± 0.088 | highly positive | 0.6 ± 0.058 | low-grade positive | 81.36 |
| N. gonorrhoeae | 0.361 ± 0.014 | low-grade positive | 0.215 ± 0.023 | low-grade positive | 40.44 |
| P. aeruginosa | 0.409 ± 0.076 | low-grade positive | 0.084 ± 0.016 | negative | 79.46 |
| V. cholerae | 0.032 ± 0.002 | negative | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagha, R.; Abdallah, F.B.; Mezni, A.; Alzahrani, O.M. Effect of Plasmonic Gold Nanoprisms on Biofilm Formation and Heat Shock Proteins Expression in Human Pathogenic Bacteria. Pharmaceuticals 2021, 14, 1335. https://doi.org/10.3390/ph14121335
Lagha R, Abdallah FB, Mezni A, Alzahrani OM. Effect of Plasmonic Gold Nanoprisms on Biofilm Formation and Heat Shock Proteins Expression in Human Pathogenic Bacteria. Pharmaceuticals. 2021; 14(12):1335. https://doi.org/10.3390/ph14121335
Chicago/Turabian StyleLagha, Rihab, Fethi Ben Abdallah, Amine Mezni, and Othman M. Alzahrani. 2021. "Effect of Plasmonic Gold Nanoprisms on Biofilm Formation and Heat Shock Proteins Expression in Human Pathogenic Bacteria" Pharmaceuticals 14, no. 12: 1335. https://doi.org/10.3390/ph14121335
APA StyleLagha, R., Abdallah, F. B., Mezni, A., & Alzahrani, O. M. (2021). Effect of Plasmonic Gold Nanoprisms on Biofilm Formation and Heat Shock Proteins Expression in Human Pathogenic Bacteria. Pharmaceuticals, 14(12), 1335. https://doi.org/10.3390/ph14121335





