In Silico Screening and In Vivo Evaluation of Potential CACNA2D1 Antagonists as Intraocular Pressure-Reducing Agents in Glaucoma Therapy
Abstract
:1. Introduction
2. Results and Discussions
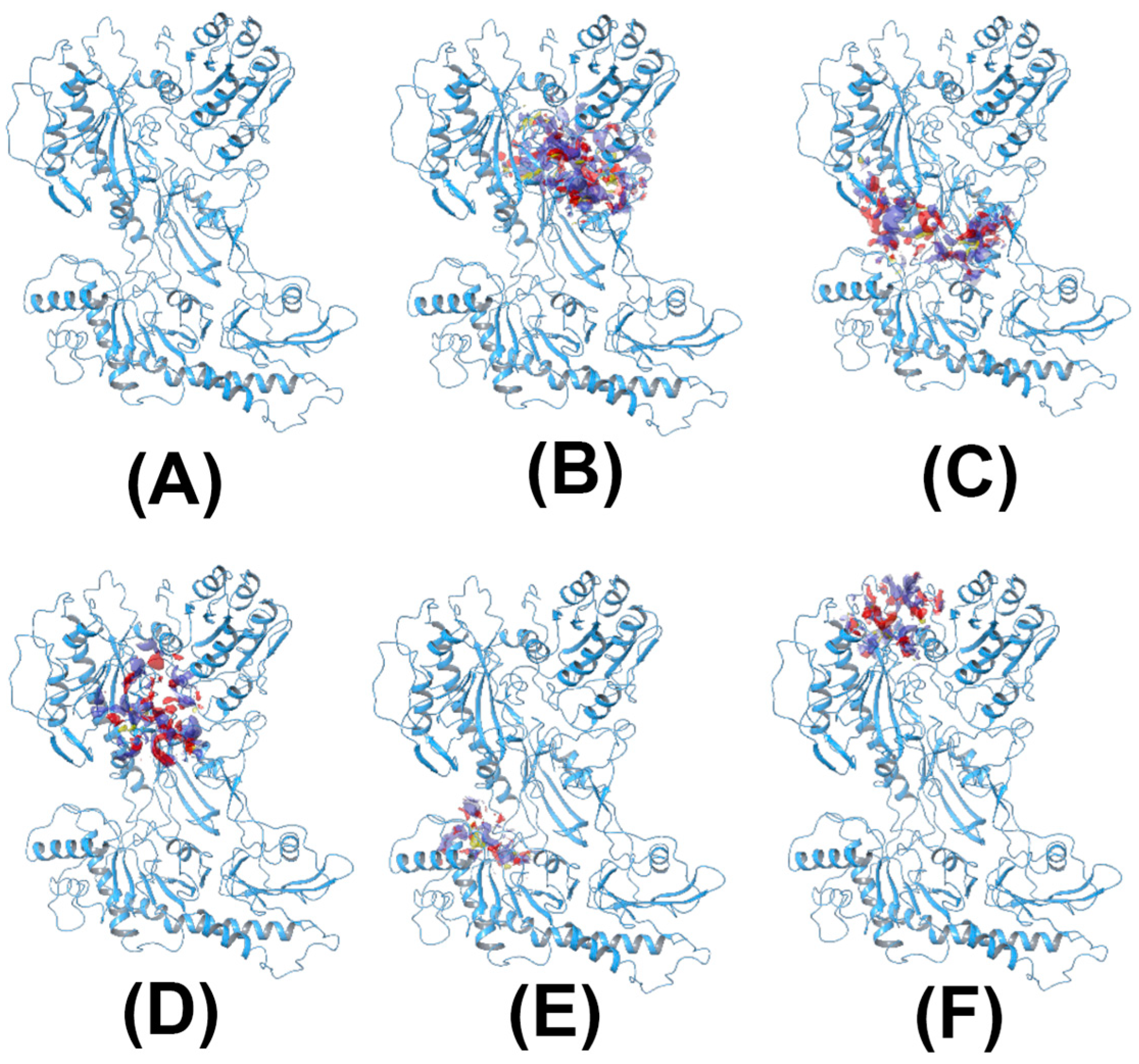
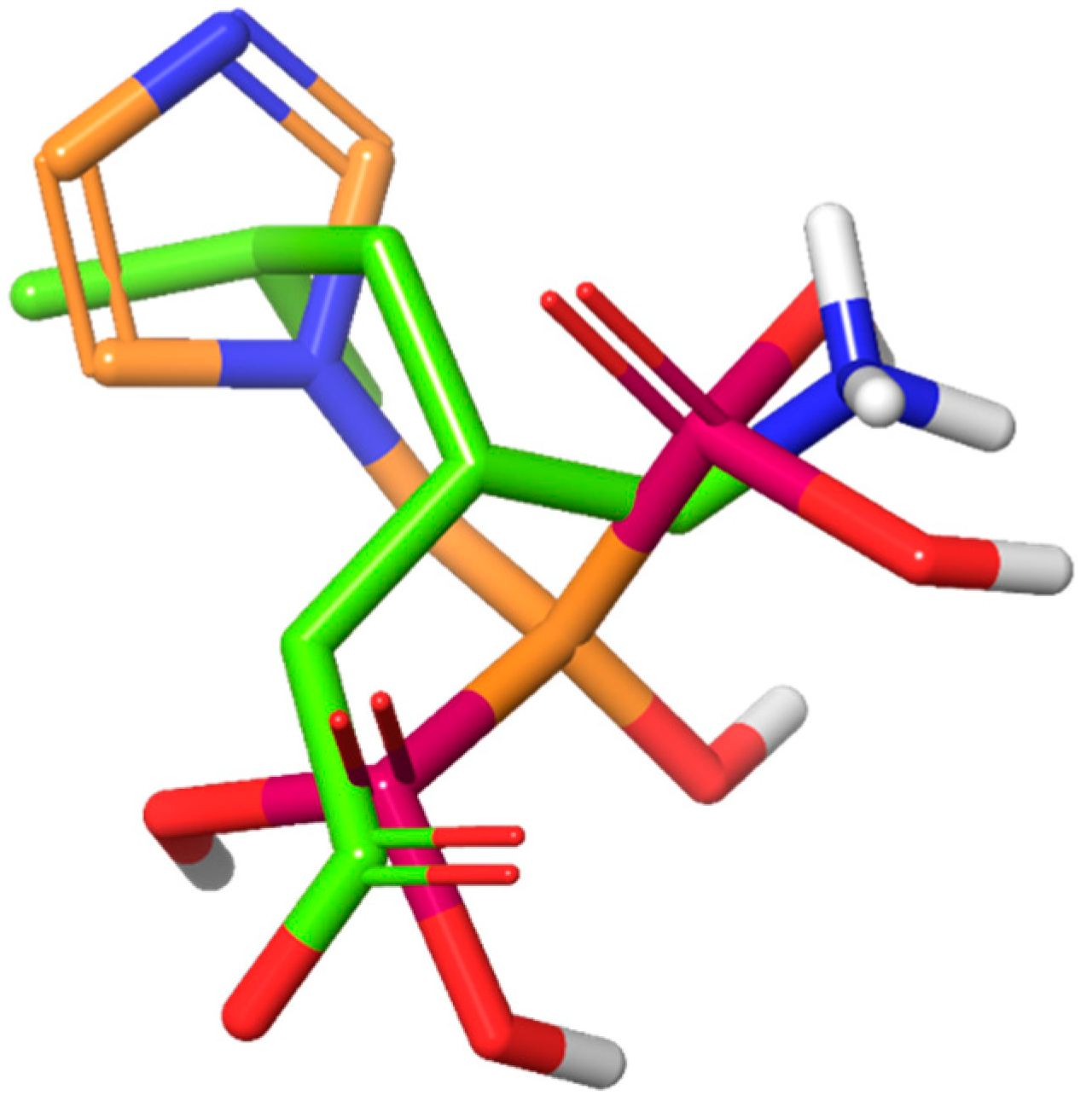
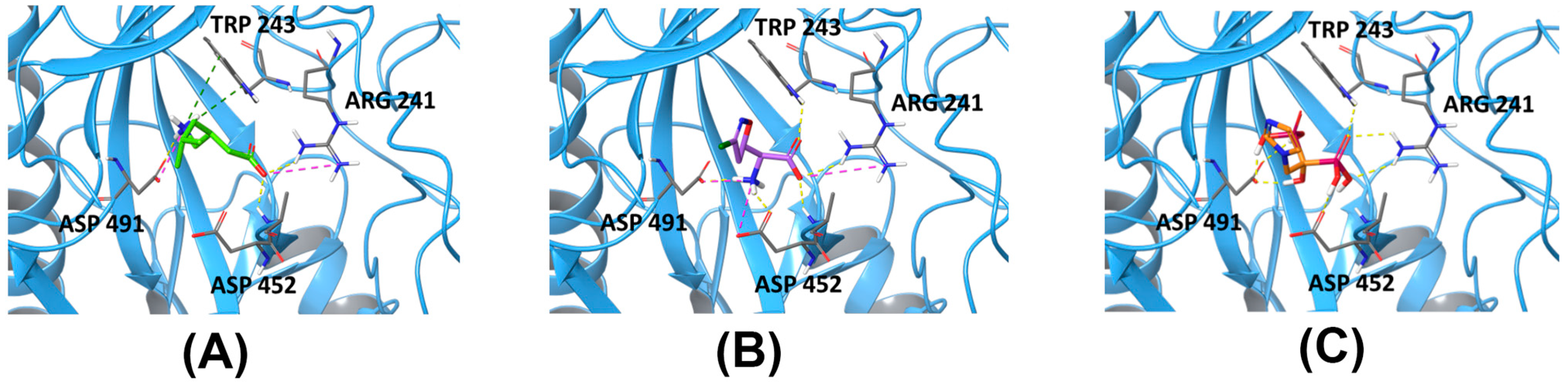
2.1. Homology Model of CACNA2D1 and In Silico Screening
2.2. Preparation of Viscous Eye Drops Containing 0.6% w/v of Different Drug Molecules
2.3. pH Measurement of Different Eye Drops
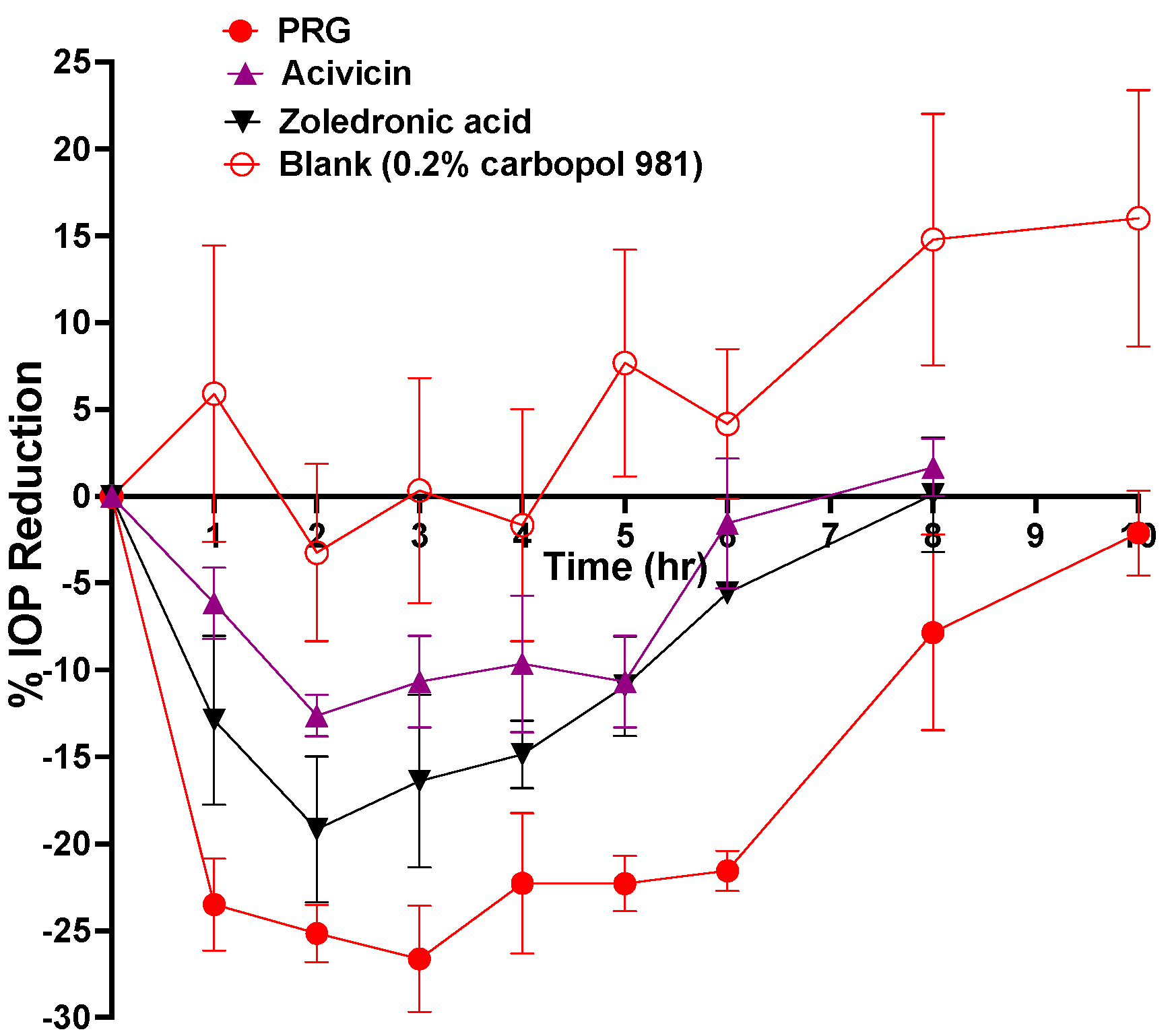
2.4. In Vivo IOP-Lowering Efficacy Evaluation of Different Eye Drops after a Single Dose Application
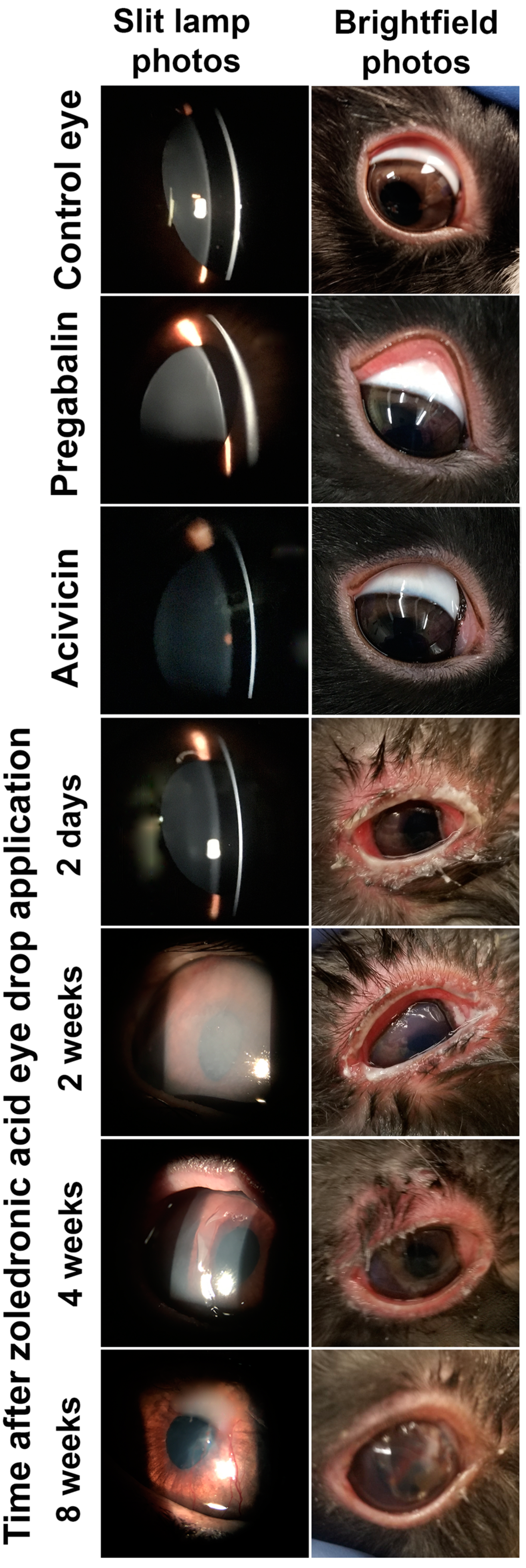
2.5. In Vivo Safety Evaluation of Different Eye Drops after a Single Dose Application
3. Materials and Methods
3.1. Materials
3.2. Animals
3.3. Methods
3.3.1. Homology Model of CACNA2D1
3.3.2. Virtual Screening
3.3.3. Preparation of Viscous Eye Drops Containing 0.6% w/v of Different Drug Molecules
3.3.4. pH Measurement of Different Eye Drops
3.3.5. In Vivo Evaluation of Different Eye Drops after a Single-Dose Application
- 1.
- Efficacy evaluation
- 2.
- Safety evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quigley, H.A. Number of people with glaucoma worldwide. Br. J. Ophthalmol. 1996, 80, 389–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alm, A.; Grierson, I.; Shields, M.B. Side effects associated with prostaglandin analog therapy. Surv. Ophthalmol. 2008, 53, S93–S105. [Google Scholar] [CrossRef] [PubMed]
- Beidoe, G.; Mousa, S.A. Current primary open-angle glaucoma treatments and future directions. Clin. Ophthalmol. 2012, 6, 1699–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.; Lee, G.; Lefebvre, D.R.; Kronberg, B.; Loomis, S.; Brauner, S.C.; Turalba, A.; Rhee, D.J.; Freitag, S.K.; Pasquale, L.R. A cross-sectional survey of the association between bilateral topical prostaglandin analogue use and ocular adnexal features. PLoS ONE 2013, 8, e61638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tataru, C.P.; Purcarea, V.L. Antiglaucoma pharmacotherapy. J. Med. Life 2012, 5, 247–251. [Google Scholar] [PubMed]
- Vyzulta®. (Latanoprostene Bunod Ophthalmic Solution) [Package Insert]; Bausch & Lomb: Bridgewater, NJ, USA, 2017. [Google Scholar]
- Rhopressa®. (Netarsudil Ophthalmic Solution) [Package Insert]; Aerie Pharmaceuticals, Inc.: Irvine, CA, USA, 2017. [Google Scholar]
- Nijm, L.M.; De Benito-Llopis, L.; Rossi, G.C.; Vajaranant, T.S.; Coroneo, M.T. Understanding the dual dilemma of dry eye and glaucoma: An international review. Asia-Pacfic J. Ophthalmol. 2020, 9, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Chintalapudi, S.R.; Maria, D.; Wang, X.D.; Bailey, J.N.C.; Hysi, P.G.; Wiggs, J.L.; Williams, R.W.; Jablonski, M.M.; NEIGHBORHOOD Consortium; International Glaucoma Genetics Consortium. Systems genetics identifies a role for Cacna2d1 regulation in elevated intraocular pressure and glaucoma susceptibility. Nat. Commun. 2017, 8, 1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.M.; Maria, D.N.; Mishra, S.R.; Guragain, D.; Wang, X.; Jablonski, M.M. Once daily pregabalin eye drops for management of glaucoma. ACS Nano 2019, 13, 13728–13744. [Google Scholar] [CrossRef] [PubMed]
- Guedes, I.A.; de Magalhaes, C.S.; Dardenne, L.E. Receptor-ligand molecular docking. Biophys. Rev. 2014, 6, 75–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammed, M.T.; Aki-Yalcin, E. Homology modeling in drug discovery: Overview, current applications, and future perspectives. Chem. Biol. Drug Des. 2019, 93, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poster, D.S.; Bruno, S.; Penta, J.; Neil, G.L.; McGovren, J.P. Acivicin. An antitumor antibiotic. Cancer Clin. Trials 1981, 4, 327–330. [Google Scholar] [PubMed]
- Dhillon, S. Zoledronic Acid (Reclast((R)), Aclasta((R))): A Review in Osteoporosis. Drugs 2016, 76, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Ameye, D.; Pringels, E.; Foreman, P.; Remon, J.P.; Adriaensens, P.; Storme, L.; Gelan, J. Correlation between the molecular morphology and the biocompatibility of bioadhesive carriers prepared from spray-dried starch/Carbopol® blends. Polymer 2005, 46, 2338–2345. [Google Scholar] [CrossRef]
- Kao, H.J.; Lin, H.R.; Lo, Y.L.; Yu, S.P. Characterization of pilocarpine-loaded chitosan/Carbopol nanoparticles. J. Pharm. Pharmacol. 2006, 58, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Lubrizol. Carbopol 981 NF Safety Data Sheet Pamphlet. version 3. 2018. Available online: https://www.lubrizol.com/Health/Pharmaceuticals/Excipients/Carbopol-Polymer-Products (accessed on 27 August 2021).
- Ibrahim, M.M.; Maria, D.N.; Wang, X.; Simpson, R.N.; Hollingsworth, T.J.; Jablonski, M.M. Enhanced corneal penetration of a poorly permeable drug using bioadhesive multiple microemulsion technology. Pharmaceutics 2020, 12, 704. [Google Scholar] [CrossRef] [PubMed]
- USP29-NF24. The United States Pharmacopeia, Pharmaceutical Dosage Forms Chapter; Online Version; United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2005; Available online: http://www.pharmacopeia.cn/ (accessed on 27 August 2021).
- Nourinia, R.; Ahmadieh, H.; Rezaei-Kanavi, M.; Shoeibi, N.; Kamrava, K.; Karimi, S. Safety of intravitreal zoledronic acid, an anti-angiogenic bisphosphonate, in a rat model. J. Ophthalmic Vis. Res. 2014, 9, 44–49. [Google Scholar] [PubMed]
- Gupta, S.; Onkar, A.; Vashisht, T. Zoledronic acid induced unilateral anterior uveitis. Indian J. Ophthalmol. 2020, 68, 2002–2003. [Google Scholar] [CrossRef] [PubMed]
- Han, L.S.; Weatherhead, R.G. Zoledronic acid associated orbital inflammation. Clin. Exp. Ophthalmol. 2020, 48, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, Z.; Li, Z.; Qian, X.; Lu, S.; Dong, M.; Zhou, Q.; Yan, N. Structure of the voltage-gated calcium channel Ca(v)1.1 at 3.6 A resolution. Nature 2016, 537, 191–196. [Google Scholar] [CrossRef] [PubMed]
| Parameters | PRG | Acivicin | Zoledronic Acid |
|---|---|---|---|
| pH | 5.3 ± 0.11 | 4.9 ± 0.15 | 5.5 ± 0.21 |
| Tmax (h) | 2.33 ± 0.67 | 2.67 ± 0.67 | 2.33 ± 0.88 |
| Tend (h) | 9.33 ± 0.67 | 7.33 ± 0.67 | 8.0 ± 0.00 |
| %IOP reduction at Tmax | 28.98 ± 1.8 | 13.46 ± 2.03 | 22.06 ± 2.5 |
| AUC %.h | 170 ± 16.4 | 52.16 ± 8.7 | 82.47 ± 10.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Ibrahim, M.M.; Chen, H.; Li, W.; Jablonski, M.M. In Silico Screening and In Vivo Evaluation of Potential CACNA2D1 Antagonists as Intraocular Pressure-Reducing Agents in Glaucoma Therapy. Pharmaceuticals 2021, 14, 887. https://doi.org/10.3390/ph14090887
Li H, Ibrahim MM, Chen H, Li W, Jablonski MM. In Silico Screening and In Vivo Evaluation of Potential CACNA2D1 Antagonists as Intraocular Pressure-Reducing Agents in Glaucoma Therapy. Pharmaceuticals. 2021; 14(9):887. https://doi.org/10.3390/ph14090887
Chicago/Turabian StyleLi, Hanxuan, Mohamed Moustafa Ibrahim, Hao Chen, Wei Li, and Monica M. Jablonski. 2021. "In Silico Screening and In Vivo Evaluation of Potential CACNA2D1 Antagonists as Intraocular Pressure-Reducing Agents in Glaucoma Therapy" Pharmaceuticals 14, no. 9: 887. https://doi.org/10.3390/ph14090887
APA StyleLi, H., Ibrahim, M. M., Chen, H., Li, W., & Jablonski, M. M. (2021). In Silico Screening and In Vivo Evaluation of Potential CACNA2D1 Antagonists as Intraocular Pressure-Reducing Agents in Glaucoma Therapy. Pharmaceuticals, 14(9), 887. https://doi.org/10.3390/ph14090887







