Non-Pigmented Ciliary Epithelium-Derived Extracellular Vesicles Loaded with SMAD7 siRNA Attenuate Wnt Signaling in Trabecular Meshwork Cells In Vitro
Abstract
1. Introduction
2. Results
2.1. Establishing the Effect of the Electroporation Conditions on NPCE-Derived EVs Size, Concentration, and Membrane Integrity
2.1.1. TRPS Analysis of NPCE-Derived EVs Size and Concentration before and after Electroporation
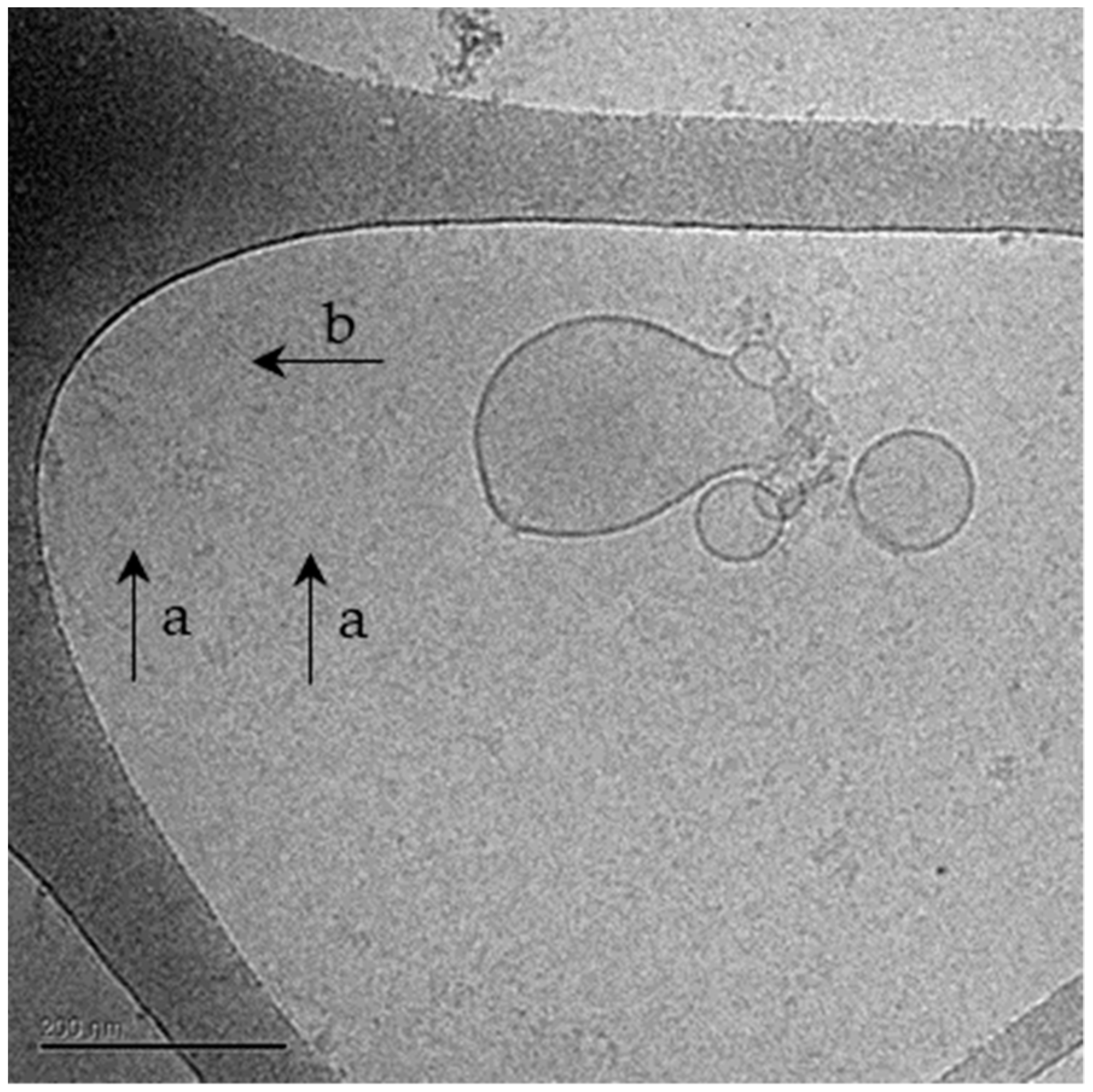
2.1.2. Cryo-TEM Analysis of NPCE-Derived EVs Membrane Integrity Following Electroporation
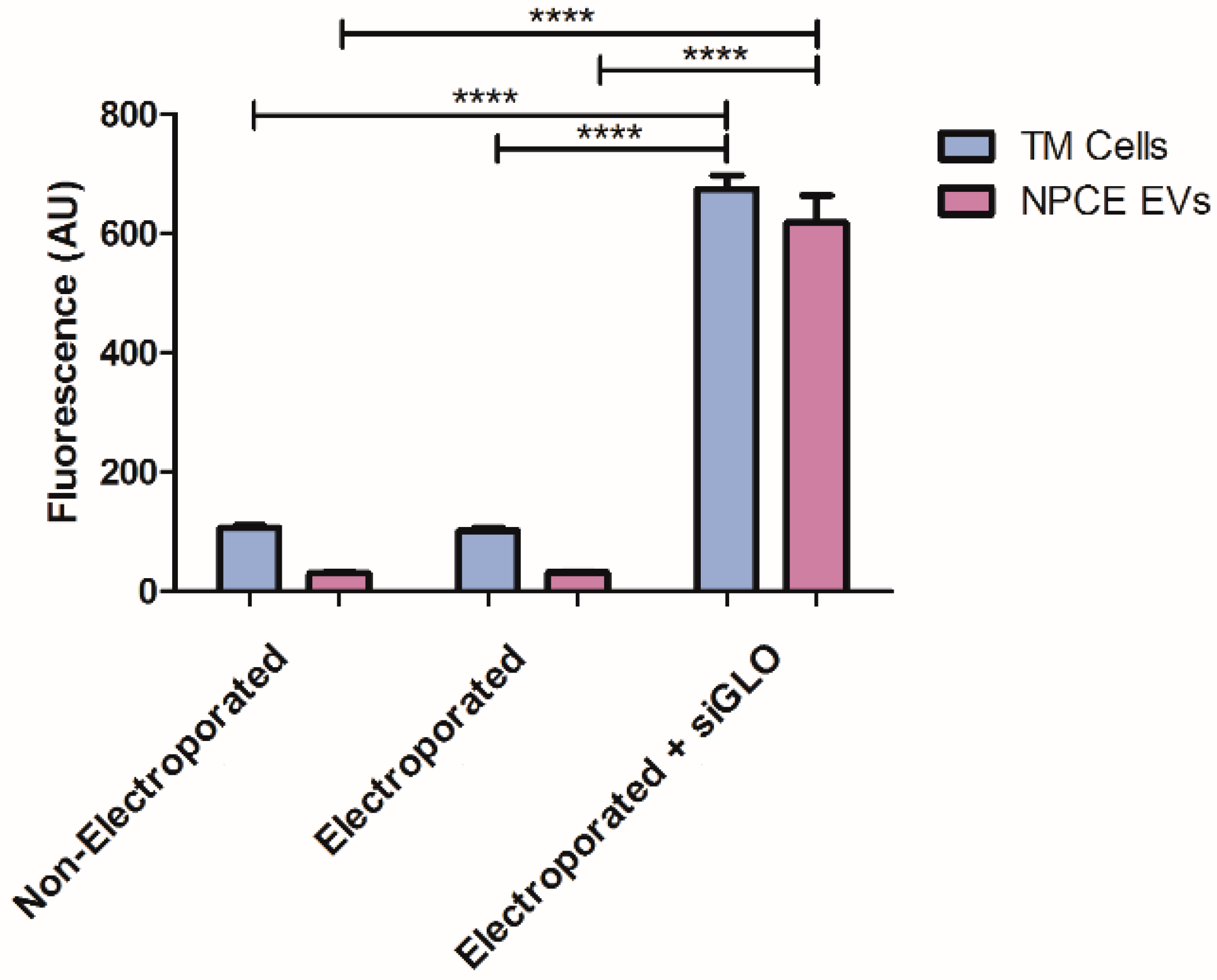
2.2. siRNA EVs Loading Assessment by Fluorescence Analysis
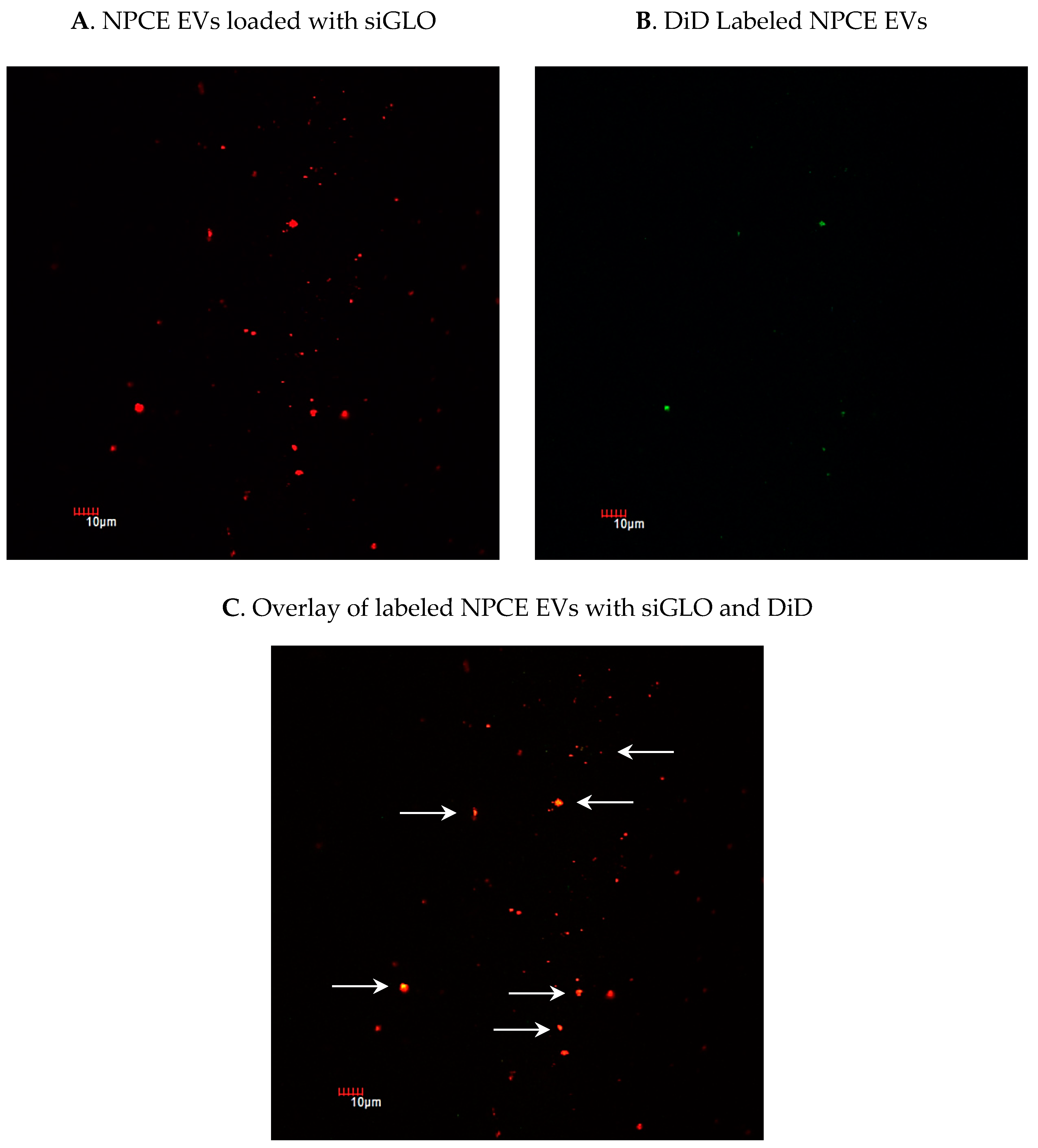
2.3. Detection of siRNA in NPCE-Derived EVs Using Confocal Microscopy
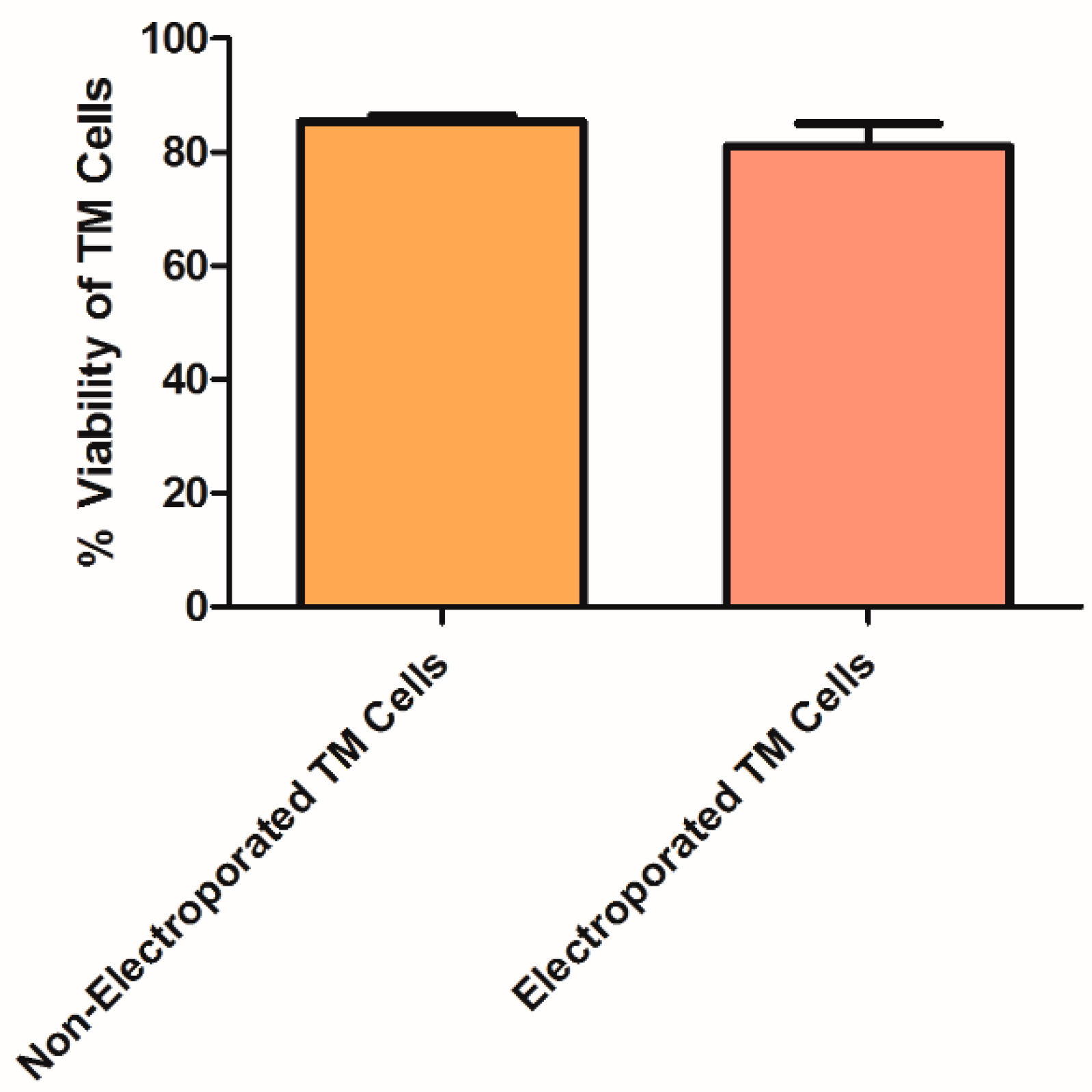
2.4. TM Cell Viability Following Electroporation
2.5. siRNA Loading Efficiency to TM Cells Analysis by qRT-PCR
2.6. Delivery of siRNA to TM Cells via NPCE-Derived EVs
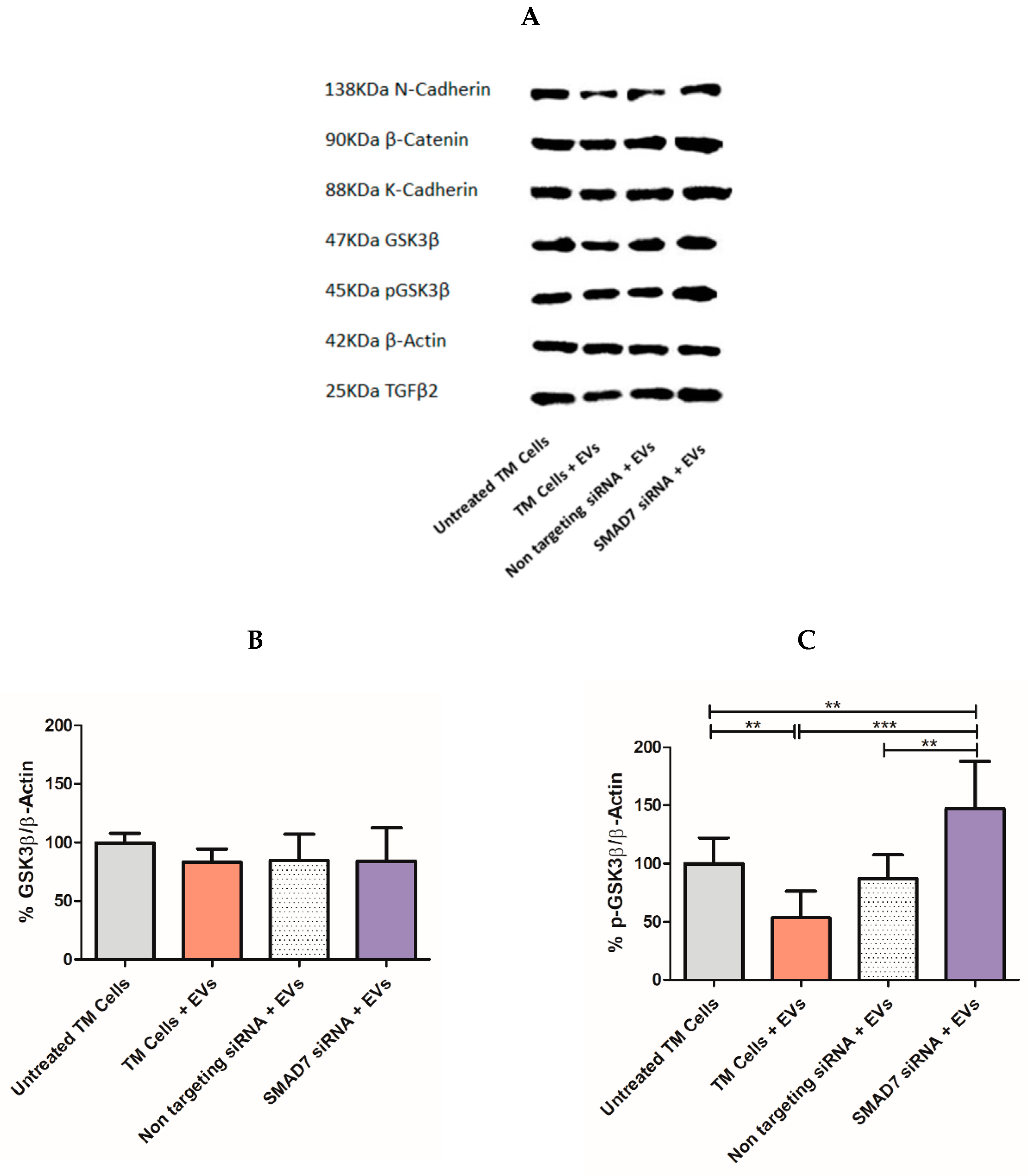
2.7. Tracking the Expression of Wnt-TGFβ2 Proteins in TM Cells Using SDS-PAGE Separation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Research Model
4.3. EVs Extraction
4.4. Tunable Resistive Pulse Sensing (TRPS)
4.5. Cryo-TEM
4.6. EVs Labeling with DiD
4.7. Tracking Electroporated EVs Labeled with siGLO
4.8. Confocal Microscopy
4.9. Electroporation of TM Cells with siRNAs
4.10. Electroporation of EVs with siRNA
4.11. TM Cell Viability
4.12. Incubation of TM Cells with EVs
4.13. Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
4.14. Protein Extraction from TM Cells
4.15. Western Blot Analysis
4.16. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwon, Y.H.; Fingert, J.H.; Kuehn, M.H.; Alward, W.L. Primary open-angle glaucoma. N. Engl. J. Med. 2009, 360, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Lim, S.H.; Gupta, P.; Aung, T.; Wong, T.Y.; Cheng, C.Y. Inter-relationship between ocular perfusion pressure, blood pressure, intraocular pressure profiles and primary open-angle glaucoma: The Singapore Epidemiology of Eye Diseases study. Br. J. Ophthalmol. 2018, 102, 1402–1406. [Google Scholar] [CrossRef]
- Schmidl, D.; Schmetterer, L.; Garhofer, G.; Popa-Cherecheanu, A. Pharmacotherapy of glaucoma. J. Ocul. Pharmacol. Ther. 2015, 31, 63–77. [Google Scholar] [CrossRef]
- Alward, W.L. Open angle glaucomas. In Glaucoma: The Requisites in Ophthalmology; Alward, W.L., Ed.; Mosby: St. Louis, MA, USA, 2000; pp. 128–140. [Google Scholar]
- Abu-Hassan, D.W.; Acott, T.S.; Kelley, M.J. The trabecular meshwork: A basic review of form and function. J. Ocul. Biol. 2014, 2. [Google Scholar] [CrossRef]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52. [Google Scholar] [CrossRef]
- Mao, W.; Millar, J.C.; Wang, W.-H.; Silverman, S.M.; Liu, Y.; Wordinger, R.J.; Rubin, J.S.; Pang, I.-H.; Clark, A.F. Existence of the canonical Wnt signaling pathway in the human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7043–7051. [Google Scholar] [CrossRef]
- Lerner, N.; Beit-Yannai, E. Cross-talk between ciliary epithelium and trabecular meshwork cells in-vitro: A new insight into glaucoma. PLoS ONE 2014, 9, e112259. [Google Scholar] [CrossRef]
- Tabak, S.; Schreiber-Avissar, S.; Beit-Yannai, E. Extracellular vesicles have variable dose-dependent effects on cultured draining cells in the eye. J. Cell Mol. Med. 2018, 22, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, G.; Chatterjee, A.; Oh, S.S.; Oh, D.-J.; Kang, M.H.; Rhee, D.J. Canonical wnt signaling regulates extracellular matrix expression in the trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7433–7440. [Google Scholar] [CrossRef] [PubMed]
- Klingeborn, M.; Dismuke, W.M.; Rickman, C.B.; Stamer, W.D. Roles of exosomes in the normal and diseased eye. Prog. Retin. Eye Res. 2017, 59, 158–177. [Google Scholar] [CrossRef]
- Brembeck, F.H.; Rosário, M.; Birchmeier, W. Balancing cell adhesion and Wnt signaling, the key role of β-catenin. Curr. Opin. Genet. Dev. 2006, 16, 51–59. [Google Scholar] [CrossRef]
- McCrea, P.D.; Gu, D. The catenin family at a glance. J. Cell Sci. 2010, 123, 637–642. [Google Scholar] [CrossRef]
- Wecker, T.; Han, H.; Börner, J.; Grehn, F.; Schlunck, G. Effects of TGF-β2 on cadherins and β-catenin in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6456–6462. [Google Scholar] [CrossRef] [PubMed]
- Webber, H.C.; Bermudez, J.Y.; Millar, J.C.; Mao, W.; Clark, A.F. The Role of Wnt/beta-Catenin Signaling and K-Cadherin in the Regulation of Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.M.; Faralli, J.A.; Peters, J.M.; Newman, J.R.; Peters, D.M. Effect of heparin II domain of fibronectin on actin cytoskeleton and adherens junctions in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2924–2931. [Google Scholar] [CrossRef] [PubMed]
- Picht, G.; Welge-Luessen, U.; Grehn, F.; Lütjen-Drecoll, E. Transforming growth factor β2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefe’s Arch. Clin. Exp. Ophthalmol. 2001, 239, 199–207. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Tamm, E.R. The role of TGF-β in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012, 347, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, P.L. Changes in aqueous humor dynamics with age and glaucoma. Prog. Retin. Eye Res. 2005, 24, 612–637. [Google Scholar]
- Platania, C.; Di Paola, L.; Leggio, G.M.; Romano, G.L.; Drago, F.; Salomone, S.; Bucolo, C. Molecular features of interaction between VEGFA and anti-angiogenic drugs used in retinal diseases: A computational approach. Front. Pharmacol. 2015, 6, 248. [Google Scholar] [CrossRef]
- Fleenor, D.L.; Shepard, A.R.; Hellberg, P.E.; Jacobson, N.; Pang, I.-H.; Clark, A.F. TGFβ2-induced changes in human trabecular meshwork: Implications for intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2006, 47, 226–234. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Stephan, D.A.; Russell, P.; Tamm, E.R. Gene expression profiling of TGFβ2-and/or BMP7-treated trabecular meshwork cells: Identification of Smad7 as a critical inhibitor of TGF-β2 signaling. Exp. Eye Res. 2009, 88, 1020–1032. [Google Scholar] [CrossRef]
- Bollinger, K.E.; Crabb, J.S.; Yuan, X.; Putliwala, T.; Clark, A.F.; Crabb, J.W. Quantitative proteomics: TGFβ2 signaling in trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8287–8294. [Google Scholar] [CrossRef]
- Alice, L.Y.; Birke, K.; Moriniere, J.; Welge-Lüssen, U. TGF-β2 induces senescence-associated changes in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5718–5723. [Google Scholar]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Su, Y.; Yang, C.-Y.C.; Li, Z.; Xu, F.; Zhang, L.; Wang, F.; Zhao, S. Smad7 siRNA inhibit expression of extracellular matrix in trabecular meshwork cells treated with TGF-β2. Mol. Vis. 2012, 18, 1881. [Google Scholar] [PubMed]
- Takai, Y.; Tanito, M.; Ohira, A. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Investig. Ophthalmol. Vis. Sci. 2012, 53, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H. Fine tuning and cross-talking of TGF-beta signal by inhibitory Smads. J. Biochem. Mol. Biol. 2005, 38, 9–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fuchshofer, R.; Tamm, E.R. Modulation of extracellular matrix turnover in the trabecular meshwork. Exp. Eye Res. 2009, 88, 683–688. [Google Scholar] [CrossRef]
- Tektas, O.Y.; Lutjen-Drecoll, E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp. Eye Res. 2009, 88, 769–775. [Google Scholar] [CrossRef]
- Zenkel, M.; Krysta, A.; Pasutto, F.; Juenemann, A.; Kruse, F.E.; Schlotzer-Schrehardt, U. Regulation of lysyl oxidase-like 1 (LOXL1) and elastin-related genes by pathogenic factors associated with pseudoexfoliation syndrome. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8488–8495. [Google Scholar] [CrossRef]
- Zhang, S.; Fei, T.; Zhang, L.; Zhang, R.; Chen, F.; Ning, Y.; Han, Y.; Feng, X.H.; Meng, A.; Chen, Y.G. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol. Cell Biol. 2007, 27, 4488–4499. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Coca-Prados, M.; Escribano, J. New perspectives in aqueous humor secretion and in glaucoma: The ciliary body as a multifunctional neuroendocrine gland. Prog. Retin. Eye Res. 2007, 26, 239–262. [Google Scholar] [CrossRef] [PubMed]
- Stamer, W.D.; Hoffman, E.A.; Luther, J.M.; Hachey, D.L.; Schey, K.L. Protein profile of exosomes from trabecular meshwork cells. J. Proteomics 2011, 74, 796–804. [Google Scholar] [CrossRef]
- Dismuke, W.M.; Challa, P.; Navarro, I.; Stamer, W.D.; Liu, Y. Human aqueous humor exosomes. Exp. Eye Res. 2015, 132, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Lerner, N.; Avissar, S.; Beit-Yannai, E. Extracellular vesicles mediate signaling between the aqueous humor producing and draining cells in the ocular system. PLoS ONE 2017, 12, e0171153. [Google Scholar] [CrossRef] [PubMed]
- Lerner, N.; Schreiber-Avissar, S.; Beit-Yannai, E. Extracellular vesicle-mediated crosstalk between NPCE cells and TM cells result in modulation of Wnt signalling pathway and ECM remodelling. J. Cell. Mol. Med. 2020, 24, 4646–4658. [Google Scholar] [CrossRef]
- Tabak, S.; Hadad, U.; Schreiber-Avissar, S.; Beit-Yannai, E. Non-pigmented ciliary epithelium derived extracellular vesicles uptake mechanism by the trabecular meshwork. FASEB J. 2021, 35, e21188. [Google Scholar] [CrossRef] [PubMed]
- Beit-Yannai, E.; Tabak, S.; Stamer, W.D. Physical exosome: Exosome interactions. J. Cell. Mol. Med. 2018, 22, 2001–2006. [Google Scholar] [CrossRef]
- Tabak, S.; Schreiber-Avissar, S.; Beit-Yannai, E. Influence of Anti-Glaucoma Drugs on Uptake of Extracellular Vesicles by Trabecular Meshwork Cells. Int. J. Nanomed. 2021, 16, 1067–1081. [Google Scholar] [CrossRef]
- Tabak, S.; Schreiber-Avissar, S.; Beit-Yannai, E. Trabecular meshwork’s collagen network formation is inhibited by non-pigmented ciliary epithelium-derived extracellular vesicles. J. Cell. Mol. Med. 2021, 25, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Qu, J.L.; Qu, X.J.; Zhao, M.F.; Teng, Y.E.; Zhang, Y.; Hou, K.Z.; Jiang, Y.H.; Yang, X.H.; Liu, Y.P. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Dig. Liver Dis. 2009, 41, 875–880. [Google Scholar] [CrossRef]
- Skog, J.; Wurdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Kumar, M.; DeVaux, R.S.; Herschkowitz, J.I. Molecular and Cellular Changes in Breast Cancer and New Roles of lncRNAs in Breast Cancer Initiation and Progression. Prog. Mol. Biol. Transl. Sci. 2016, 144, 563–586. [Google Scholar] [CrossRef]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of siRNA. Int. J. Biomed. Sci. 2017, 13, 48–57. [Google Scholar]
- Guo, P.; Coban, O.; Snead, N.M.; Trebley, J.; Hoeprich, S.; Guo, S.; Shu, Y. Engineering RNA for targeted siRNA delivery and medical application. Adv. Drug Deliv. Rev. 2010, 62, 650–666. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, S.W.; Park, T.G. Molecular design of functional polymers for gene therapy. Prog. Polym. Sci. 2007, 32, 1239. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Han, Y.; Liang, L.H.; Ji, A. Nanoparticle-based delivery system for application of siRNA in vivo. Curr. Drug Metab. 2010, 11, 182–196. [Google Scholar] [CrossRef]
- Iorns, E.; Lord, C.J.; Turner, N.; Ashworth, A. Utilizing RNA interference to enhance cancer drug discovery. Nat. Rev. Drug Discov. 2007, 6, 556–568. [Google Scholar] [CrossRef]
- Bumcrot, D.; Manoharan, M.; Koteliansky, V.; Sah, D.W. RNAi therapeutics: A potential new class of pharmaceutical drugs. Nat. Chem. Biol. 2006, 2, 711–719. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Christiansen, G.; Gurevich, L.; Moos, T.; Duroux, M. Evaluation of electroporation-induced adverse effects on adipose-derived stem cell exosomes. Cytotechnology 2016, 68, 2125–2138. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, J.; Karlson, T.D.L.; Brisslert, M.; Vaziri Sani, F.; Telemo, E.; Sunnerhagen, P.; Valadi, H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012, 40, e130. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.T.; Borgheti-Cardoso, L.N.; Depieri, L.V.; de Macedo Mano, D.; Abelha, T.F.; Petrilli, R.; Bentley, M.V. Delivery systems and local administration routes for therapeutic siRNA. Pharm. Res. 2013, 30, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Stremersch, S.; Braeckmans, K.; de Smedt, S.C.; Hendrix, A.; Wood, M.J.A.; Schiffelers, R.M.; Raemdonck, K.; Vader, P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release 2013, 172, 229–238. [Google Scholar] [CrossRef]
- Ohno, S.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef]
- Hood, J.L.; Scott, M.J.; Wickline, S.A. Maximizing exosome colloidal stability following electroporation. Anal. Biochem. 2014, 448, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Lendeckel, W.; Tuschl, T. RNA interference is mediated by 21-and 22-nucleotide RNAs. Genes Dev. 2001, 15, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Bala, S.; Bukong, T.; Szabo, G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine 2014, 10, 1517–1527. [Google Scholar] [CrossRef]
- Limoni, S.K.; Moghadam, M.F.; Moazzeni, S.M.; Gomari, H.; Salimi, F. Engineered Exosomes for Targeted Transfer of siRNA to HER2 Positive Breast Cancer Cells. Appl. Biochem. Biotechnol. 2019, 187, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Soo, C.Y.; Song, Y.; Zheng, Y.; Campbell, E.C.; Riches, A.C.; Gunn-Moore, F.; Powis, S.J. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology 2012, 136, 192–197. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: Cambridge, MA, USA, 2013; Volume 2. [Google Scholar]
- Vader, P.; Kooijmans, S.A.; Stremersch, S.; Raemdonck, K. New considerations in the preparation of nucleic acid-loaded extracellular vesicles. Ther. Deliv. 2014, 5, 105–107. [Google Scholar] [CrossRef]
- Jhan, Y.-Y.; Prasca-Chamorro, D.; Zuniga, G.P.; Moore, D.M.; Kumar, S.A.; Gaharwar, A.K.; Bishop, C.J. Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery. Int. J. Pharm. 2020, 573, 118802. [Google Scholar] [CrossRef]
- Lamberton, J.S.; Christian, A.T. Varying the nucleic acid composition of siRNA molecules dramatically varies the duration and degree of gene silencing. Mol. Biotechnol. 2003, 24, 111–119. [Google Scholar] [CrossRef]
- Ramji, K.; Kulesza, D.W.; Chouaib, S.; Kaminska, B. Off-target effects of plasmid-transcribed shRNAs on NFκB signaling pathway and cell survival of human melanoma cells. Mol. Biol. Rep. 2013, 40, 6977–6986. [Google Scholar] [CrossRef]
- Tanaka, M.; Yanagawa, Y.; Hirashima, N. Transfer of small interfering RNA by single-cell electroporation in cerebellar cell cultures. J. Neurosci. Methods 2009, 178, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, V.; Platania, C.B.M.; Lazzara, F.; Conti, F.; Pizzo, C.; Reibaldi, M.; Russo, A.; Fallico, M.; Ortisi, E.; Pignatelli, F. TGF-β Serum Levels in Diabetic Retinopathy Patients and the Role of Anti-VEGF Therapy. Int. J. Mol. Sci. 2020, 21, 9558. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.B.M.; Fisichella, V.; Fidilio, A.; Geraci, F.; Lazzara, F.; Leggio, G.M.; Salomone, S.; Drago, F.; Pignatello, R.; Caraci, F. Topical ocular delivery of TGF-β1 to the back of the eye: Implications in age-related neurodegenerative diseases. Int. J. Mol. Sci. 2017, 18, 2076. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- van der Meel, R.; Fens, M.H.; Vader, P.; Van Solinge, W.W.; Eniola-Adefeso, O.; Schiffelers, R.M. Extracellular vesicles as drug delivery systems: Lessons from the liposome field. J. Control. Release 2014, 195, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Ofir-Birin, Y.; Abou Karam, P.; Rudik, A.; Giladi, T.; Porat, Z.; Regev-Rudzki, N. Monitoring Extracellular Vesicle Cargo Active Uptake by Imaging Flow Cytometry. Front Immunol. 2018, 9, 1011. [Google Scholar] [CrossRef]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Moller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Xue, W.; Comes, N.; Borras, T. Presence of an established calcification marker in trabecular meshwork tissue of glaucoma donors. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3184–3194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3B1–A3B3. [Google Scholar] [CrossRef]


| Parameters | Untreated NPCE EVs | Condition 1 | Condition 2 | Condition 3 | Condition 4 |
|---|---|---|---|---|---|
| Size ± s.d. (Mean, nm) | 76.67 ± 11.06 | 77.33 ± 26.51 | 67.67 ± 14.15 | 66 ± 7.81 | 74 ± 1.41 |
| Concentration ± s.d. (Particles/)mL | 1.38 × 1012 ± 1.38 × 1012 | 2.25 × 1012 ± 2.3 × 1012 | 7.94 × 1011 ± 2.31 × 1011 | 1.63 × 1011 ± 4.67 × 1010 | 1.49 × 1011 ± 8.45 × 1010 |
| Electroporation Conditions | |||||
| Voltage (v) | Non-Electroporated | 400 | 400 | 400 | 400 |
| Capacitance (µF) | 125 | 125 | 125 | 125 | |
| EVs loading (µg/)µL | 0.5 | 0.5 | 0.25 | 0.25 | |
| Pulse | one pulse | two pulses | one pulse | two pulses | |
| Untreated NPCE EVs | Condition 1 | Condition 2 | Condition 3 | Condition 4 | |
|---|---|---|---|---|---|
| Mean of Normal Shaped EVs (%) | 91.31 | 76.02 | 64.16 | 65.86 | 72.86 |
| Mean of Damaged EVs (%) | 8.69 | 23.98 | 35.84 | 34.14 | 27.14 |
| Treatments | Mean Ct Value (SMAD7) | Relative mRNA Levels (GAPDH) | Relative mRNA Levels |
|---|---|---|---|
| Untreated TM Cells | 24.041 | 23.223 | 1 ± 0.454 |
| Electroporated TM Cells | 25.128 | 25.976 | 3.146 ± 1.315 |
| Non-targeting siRNA + TM Cells | 24.038 | 23.941 | 1.562 ± 0.102 |
| SMAD7 siRNA + TM Cells | 26.470 | 24.575 | 0.47 ± 0.015 |
| Human Target Gene (25 nmol) | Primers | |
|---|---|---|
| Forward | Reverse | |
| SMAD7 | 5′-CCA ACT GCA GAC TGT CCA GA-3′ | 5′-TTC TCC TCC CAG TAT GCC AC-3′ |
| TGFβ2 | 5′-AAG AAG CGT GCT TTG GAT GCG G-3′ | 5′-ATG CTC CAG CAC AGA AGT TGG C-3′ |
| GSK-3β | 5′-CCG ACT AAC ACC ACT GGA AGC T -3′ | 5′-AGG ATG GTA GCC AGA GGT GGA T-3′ |
| β-Catenin | 5′-CAC AAG CAG AGT GCT GAA GGT G-3′ | 5′-GAT TCC TGA GAG TCC AAA GAC AG-3′ |
| GAPDH | 5′-GCA CCG TCA AGG CTG AGA AC-3′ | 5′-GGA TCT CGC TCC TGG AAG ATG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabak, S.; Feinshtein, V.; Schreiber-Avissar, S.; Beit-Yannai, E. Non-Pigmented Ciliary Epithelium-Derived Extracellular Vesicles Loaded with SMAD7 siRNA Attenuate Wnt Signaling in Trabecular Meshwork Cells In Vitro. Pharmaceuticals 2021, 14, 858. https://doi.org/10.3390/ph14090858
Tabak S, Feinshtein V, Schreiber-Avissar S, Beit-Yannai E. Non-Pigmented Ciliary Epithelium-Derived Extracellular Vesicles Loaded with SMAD7 siRNA Attenuate Wnt Signaling in Trabecular Meshwork Cells In Vitro. Pharmaceuticals. 2021; 14(9):858. https://doi.org/10.3390/ph14090858
Chicago/Turabian StyleTabak, Saray, Valeria Feinshtein, Sofia Schreiber-Avissar, and Elie Beit-Yannai. 2021. "Non-Pigmented Ciliary Epithelium-Derived Extracellular Vesicles Loaded with SMAD7 siRNA Attenuate Wnt Signaling in Trabecular Meshwork Cells In Vitro" Pharmaceuticals 14, no. 9: 858. https://doi.org/10.3390/ph14090858
APA StyleTabak, S., Feinshtein, V., Schreiber-Avissar, S., & Beit-Yannai, E. (2021). Non-Pigmented Ciliary Epithelium-Derived Extracellular Vesicles Loaded with SMAD7 siRNA Attenuate Wnt Signaling in Trabecular Meshwork Cells In Vitro. Pharmaceuticals, 14(9), 858. https://doi.org/10.3390/ph14090858







