Kynurenic Acid Accelerates Healing of Corneal Epithelium In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
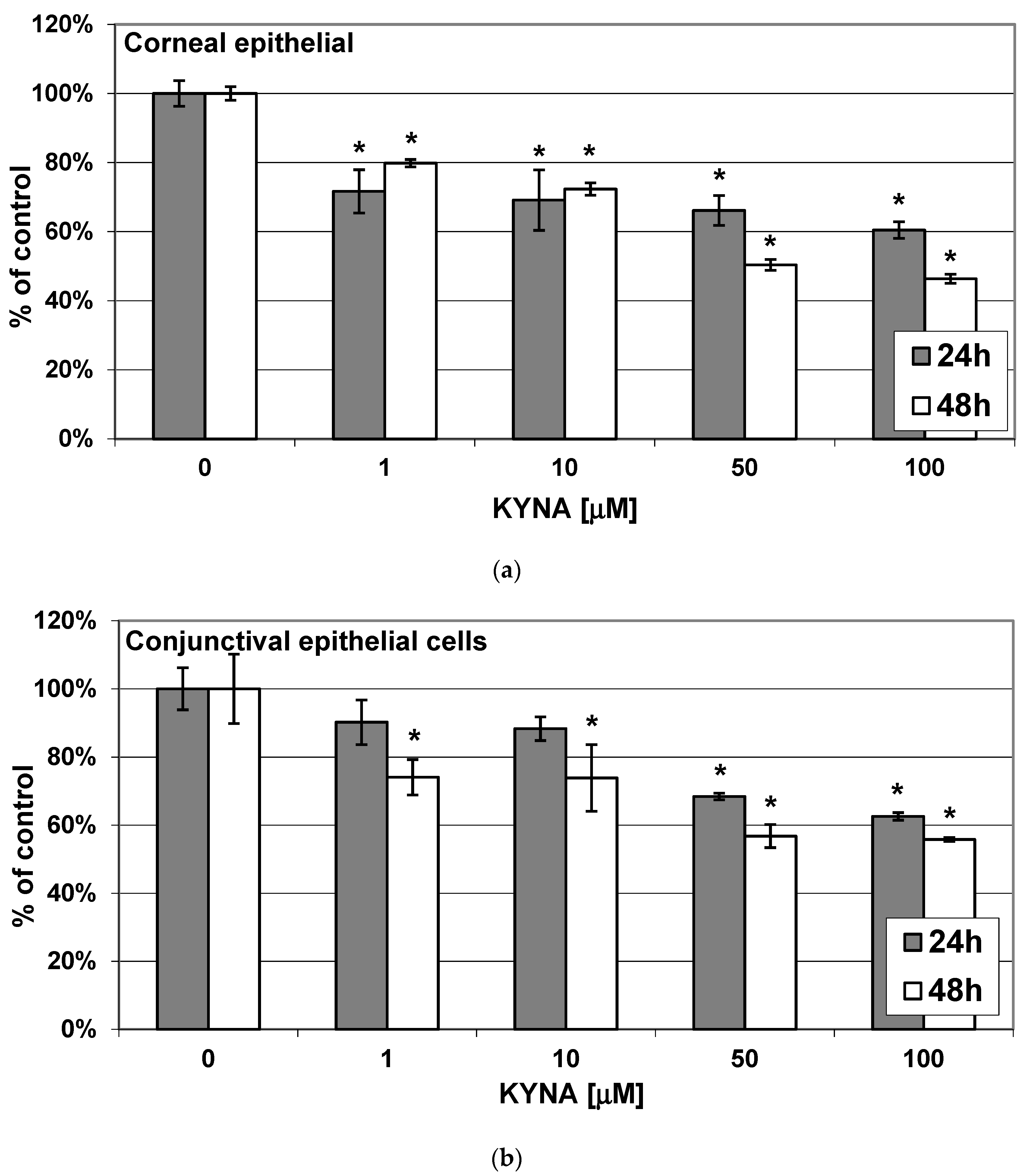
2.1. Effect of KYNA on Metabolic Activity of Corneal and Conjunctival Cells In Vitro
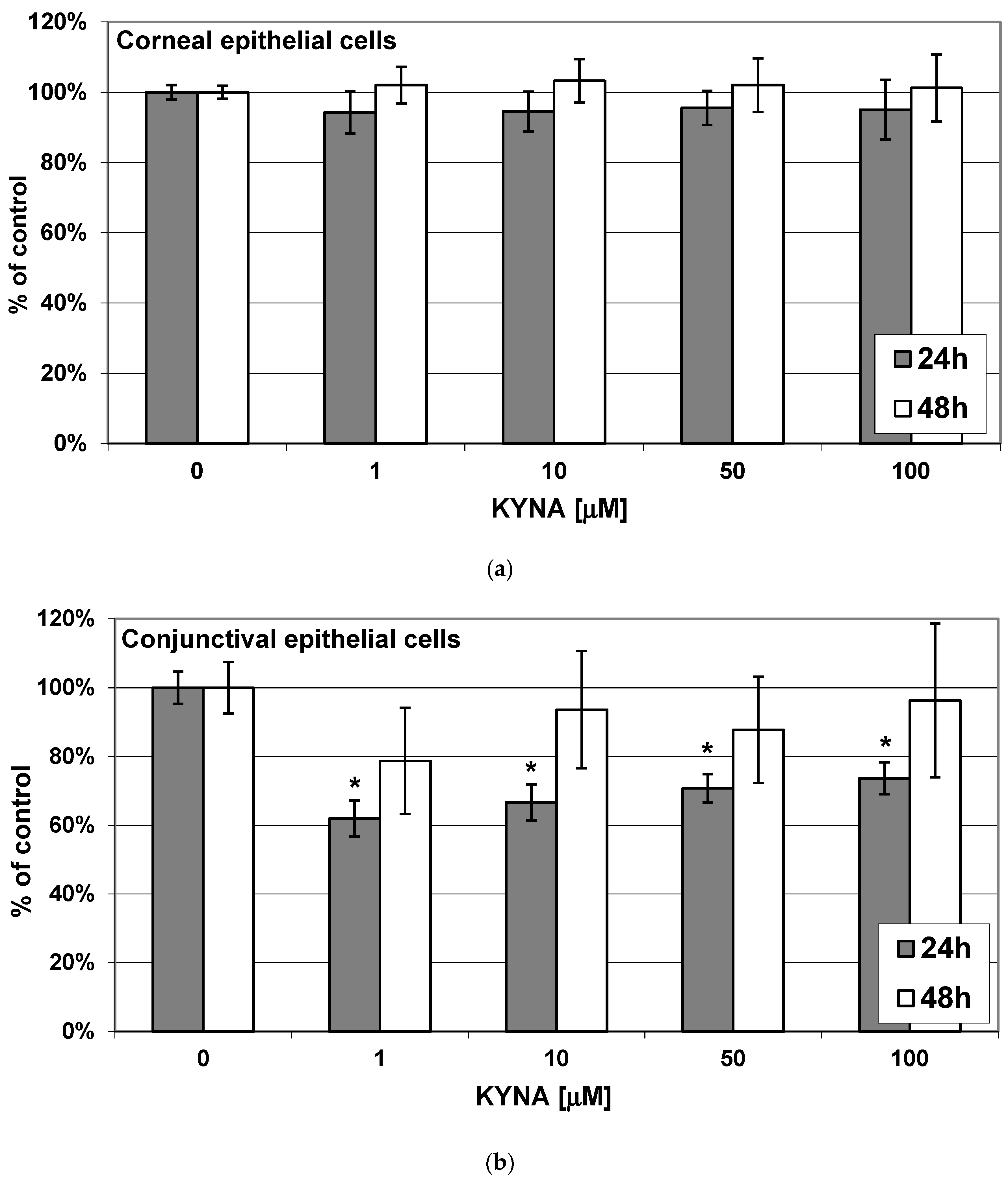
2.2. Effect of KYNA on Viability of Corneal and Conjunctival Cells In Vitro
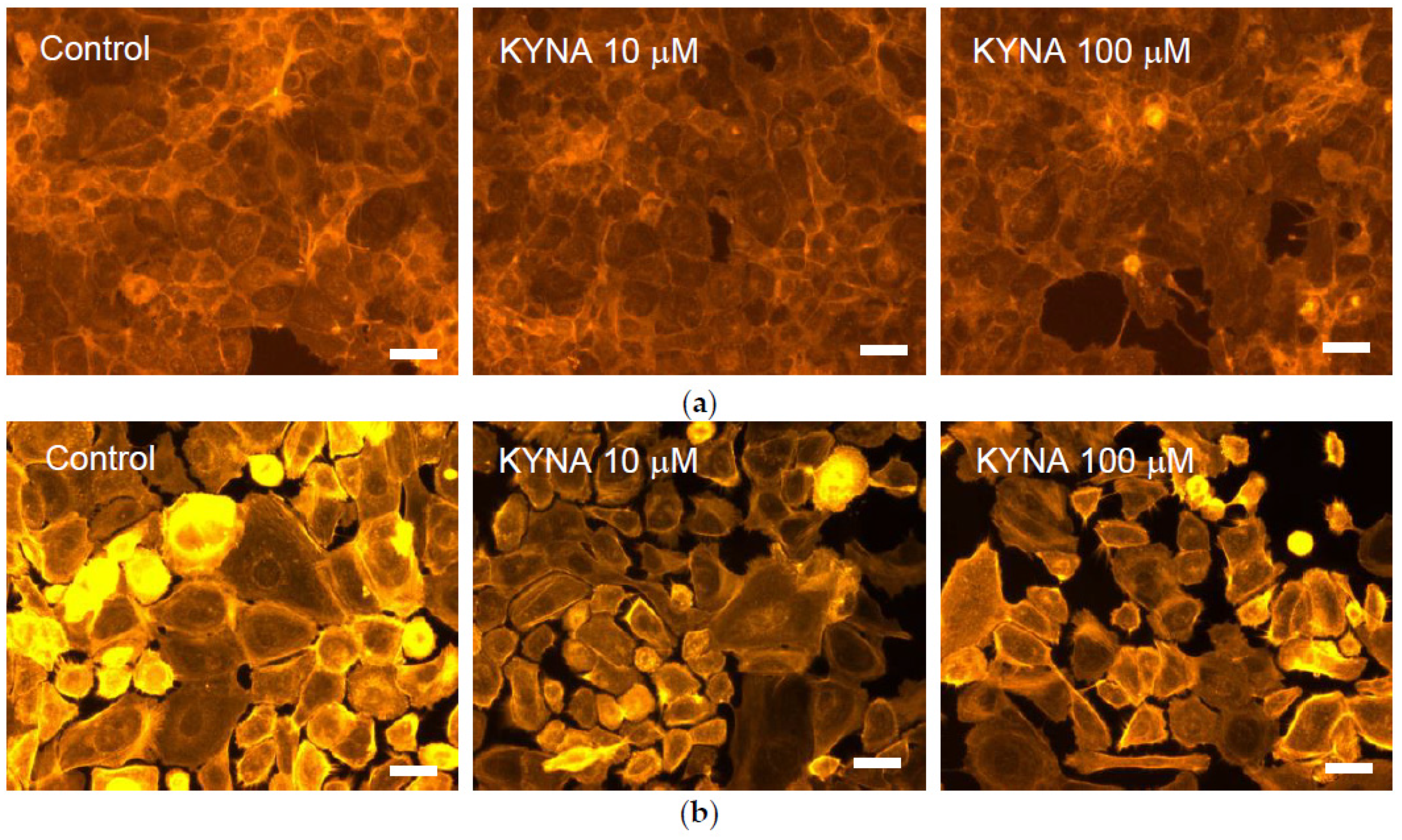
2.3. Effect of KYNA on Cellular Cytoskeleton F-Actin Organization of Corneal and Conjunctival Cells
2.4. Effect of KYNA on Transwell Migration Capacity of Corneal and Conjunctival Cells In Vitro
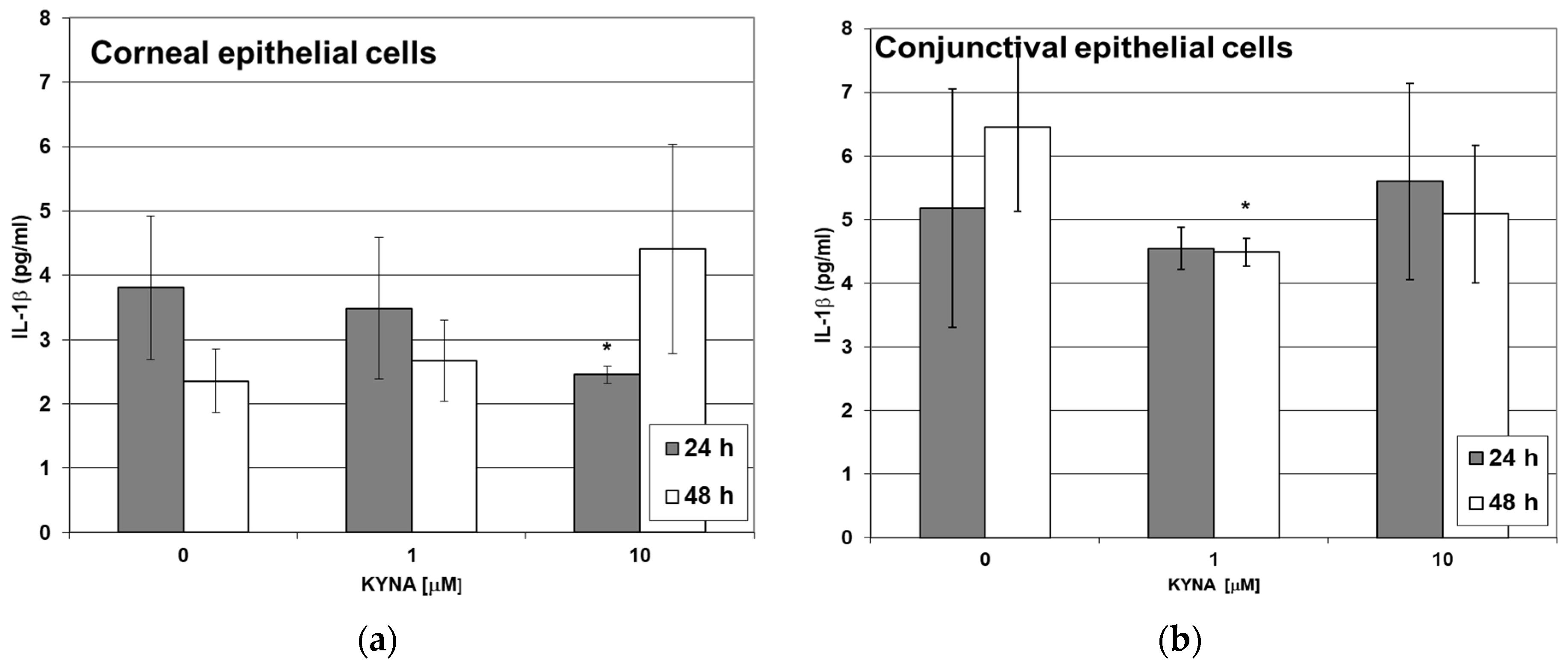
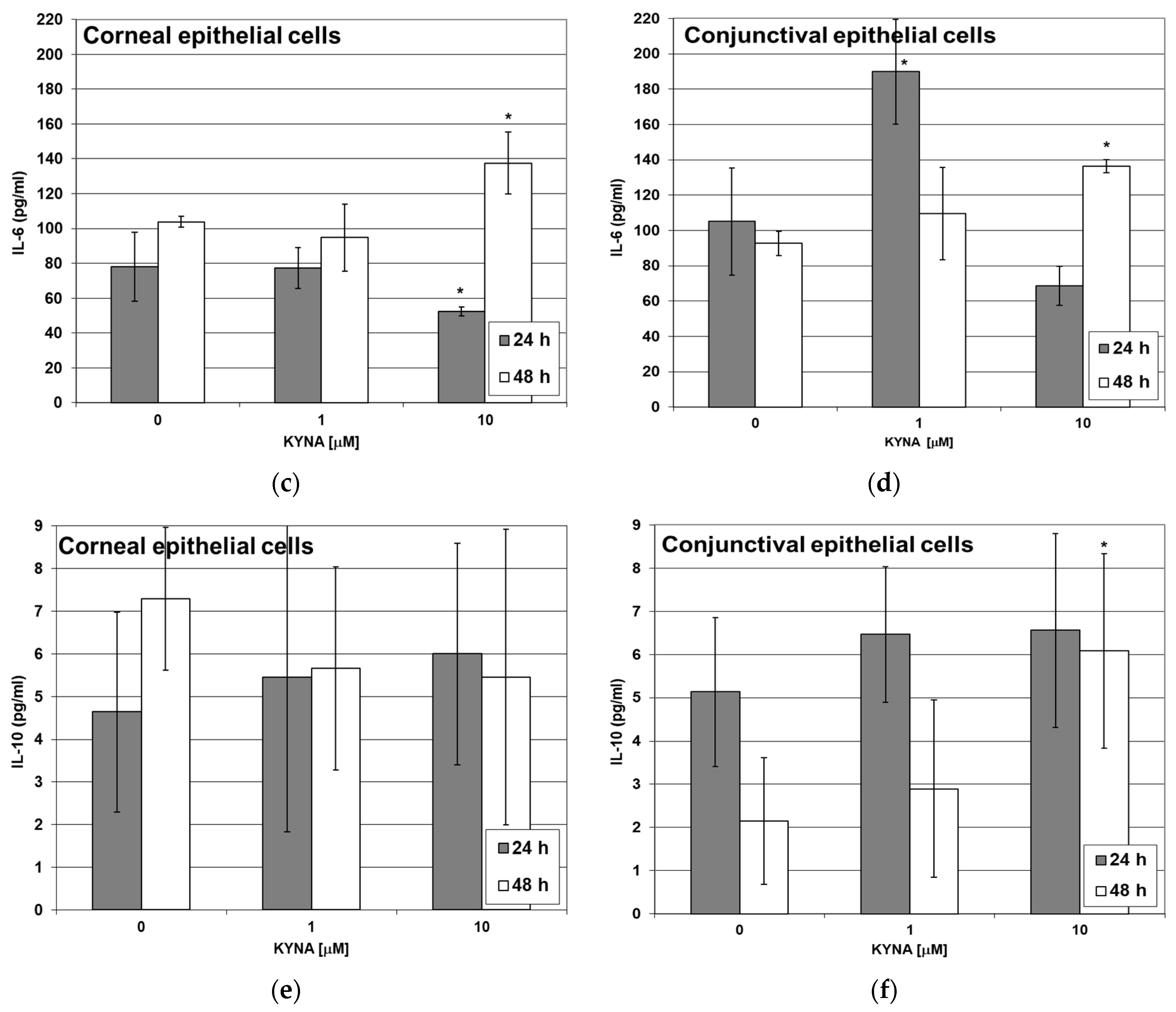
2.5. Effect of KYNA on Cytokine Release by Corneal and Conjunctival Cells In Vitro
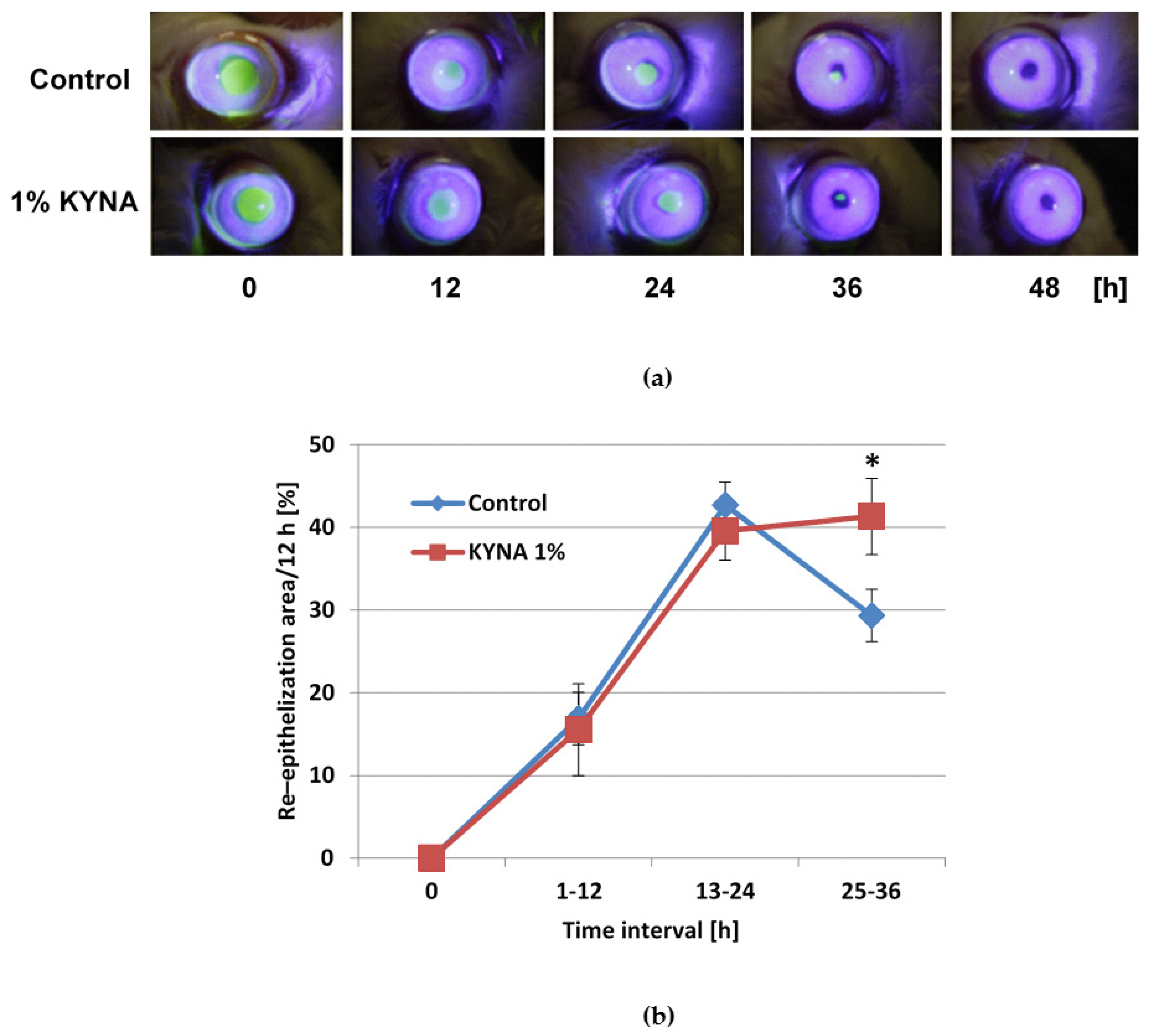
2.6. Effect of KYNA on Corneal Epithelialization In Vivo
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. In Vitro Studies
4.2.1. Cell Cultures
4.2.2. Experimental Schedule
4.2.3. Cell Metabolism and Viability—MTT Assay
4.2.4. Cell Viability-Neutral Red (NR) Uptake Assay
4.2.5. Cellular Cytoskeleton F-Actin Organization Analysis
4.2.6. Transwell Migration Test
4.2.7. ELISA Assay
4.3. In Vivo Studies
4.3.1. Animals
4.3.2. Anesthesia and Surgical Procedure
4.3.3. Treatment
4.3.4. Evaluation of Corneal Epithelialization
4.3.5. Image Analysis System
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freire, V.; Andollo, N.; Etxebarria, J.; Hernáez-Moya, R.; Durán, J.A.; Morales, M.C. Corneal Wound Healing Promoted by 3 Blood Derivatives: An in Vitro and in Vivo Comparative Study. Cornea 2014, 33, 614–620. [Google Scholar] [CrossRef]
- Geerling, G.; Daniels, J.T.; Dart, J.K.G.; Cree, I.A.; Khaw, P.T. Toxicity of Natural Tear Substitutes in a Fully Defined Culture Model of Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 948–956. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, Y.W.; Ahn, J.S.; Ahn, J.T.; Kim, S.E.; Jeong, M.B.; Seo, M.S.; Kang, K.S.; Seo, K.M. Effects of Conditioned Media from Human Amniotic Epithelial Cells on Corneal Alkali Injuries in Rabbits. J. Vet. Sci. 2013, 14, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Wirostko, B.; Rafii, M.; Sullivan, D.A.; Morelli, J.; Ding, J. Novel Therapy to Treat Corneal Epithelial Defects: A Hypothesis with Growth Hormone. Ocul. Surf. 2015, 13, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Livny, E.; Livnat, T.; Yakimov, M.; Masoud, M.; Weinberger, D.; Bahar, I. Effect of Erythropoietin on Healing of Corneal Epithelial Defects in Rabbits. Ophthalmic. Res. 2013, 50, 129–133. [Google Scholar] [CrossRef]
- Matysik-Woźniak, A.; Paduch, R.; Turski, W.A.; Maciejewski, R.; Jünemann, A.G.; Rejdak, R. Effects of Tryptophan, Kynurenine and Kynurenic Acid Exerted on Human Reconstructed Corneal Epithelium in Vitro. Pharmacol. Rep. 2017, 69, 722–729. [Google Scholar] [CrossRef]
- Matysik-Woźniak, A.; Turski, W.A.; Turska, M.; Paduch, R.; Łańcut, M.; Piwowarczyk, P.; Czuczwar, M.; Rejdak, R. Tryptophan as a Safe Compound in Topical Ophthalmic Medications: In Vitro and In Vivo Studies. Ocul. Immunol. Inflamm. 2021, 1–11. [Google Scholar] [CrossRef]
- Liebig, J. Ueber Kynurensäure. Ann. Chem. Pharm. 1853, 86, 125–126. [Google Scholar] [CrossRef]
- Matysik-Woźniak, A.; Wnorowski, A.; Drączkowski, P.; Jóźwiak, K.; Turski, W.; Augustyn, N.; Chorągiewicz, T.; Rejdak, R. Tryptophan, Kynurenine and Kynurenic Acid in Human Tear Fluid. In Proceedings of the 8th International Symposium, CORNEA 2016, Wisla Poland, 3–5 March 2016; pp. 206–207. [Google Scholar]
- Turski, M.P.; Turska, M.; Paluszkiewicz, P.; Parada-Turska, J.; Oxenkrug, G.F. Kynurenic Acid in the Digestive System—New Facts, New Challenges. Int. J. Tryptophan Res. 2013, 6, 13–15. [Google Scholar] [CrossRef] [Green Version]
- Davis, I.; Liu, A. What Is the Tryptophan Kynurenine Pathway and Why Is It Important to Neurotherapeutics? Expert Rev. Neurother. 2015, 15, 719–721. [Google Scholar] [CrossRef] [Green Version]
- Matysik-Woźniak, A.; Jünemann, A.; Turski, W.A.; Wnorowski, A.; Jóźwiak, K.; Paduch, R.; Okuno, E.; Moneta-wielgoś, J.; Chorągiewicz, T.; Maciejewski, R.; et al. The Presence of Kynurenine Aminotransferases in the Human Cornea: Evidence from Bioinformatics Analysis of Gene Expression and Immunohistochemical Staining. Mol. Vis. 2017, 23, 364–371. [Google Scholar] [PubMed]
- Andine, P.; Lehmann, A.; Ellren, K. The Excitatory Amino Acid Antagonist Kynurenic Acid Administered after Hypoxic-Ischemia in Neonatal Rats Offers Neuroprotection. Neurosci. Lett. 1988, 90, 208–212. [Google Scholar] [CrossRef]
- Stone, T.W.; Stoy, N.; Darlington, L.G. An Expanding Range of Targets for Kynurenine Metabolites of Tryptophan. Trends Pharmacol. Sci. 2013, 34, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Turski, M.P.; Kamiński, P.; Zgrajka, W.; Turska, M.; Turski, W.A. Potato—An Important Source of Nutritional Kynurenic Acid. Plant Foods Hum. Nutr. 2012, 67, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Resta, F.; Masi, A.; Sili, M.; Laurino, A.; Moroni, F.; Mannaioni, G. Kynurenic Acid and Zaprinast Induce Analgesia by Modulating HCN Channels through GPR35 Activation. Neuropharmacology 2016, 108, 136–143. [Google Scholar] [CrossRef]
- Lucas, D.R.; Newhouse, J.P. The Toxic Effect of Sodium L-Glutamate on the Inner Layers of the Retina. Arch. Ophthalmol. 1957, 58, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Turski, G.N.; Ikonomidou, C. Glutamate as a Neurotoxin. In Handbook of Neurotoxicity; Springer: New York, NY, USA, 2014. [Google Scholar]
- Ibitokun, B.O. Role of Glutamate in Corneal Nociception. Ph.D. Thesis, Oklahoma State University, Stillwater, OK, USA, 2012. [Google Scholar]
- Zhao, P.; Lane, T.R.; Gao, H.G.L.; Hurst, D.P.; Kotsikorou, E.; Le, L.; Brailoiu, E.; Reggio, P.H.; Abood, M.E. Crucial Positively Charged Residues for Ligand Activation of the GPR35 Receptor. J. Biol. Chem. 2014, 289, 3625–3638. [Google Scholar] [CrossRef] [Green Version]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic Acid Is a Potent Endogenous Aryl Hydrocarbon Receptor Ligand That Synergistically Induces Interleukin-6 in the Presence of Inflammatory Signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Matysik-Woźniak, A.; Wnorowski, A.; Turski, W.A.; Jóźwiak, K.; Jünemann, A.; Rejdak, R. The Presence and Distribution of G Protein-Coupled Receptor 35 (GPR35) in the Human Cornea—Evidences from in Silico Gene Expression Analysis and Immunodetection. Exp. Eye Res. 2019, 179, 188–192. [Google Scholar] [CrossRef]
- Hu, H.H.; Deng, H.; Ling, S.; Sun, H.; Kenakin, T.; Liang, X.; Fang, Y. Integrative Biology. Integr. Biol. 2017, 9, 451–463. [Google Scholar] [CrossRef]
- Choudhary, M.; Malek, G. The Aryl Hydrocarbon Receptor: A Mediator and Potential Therapeutic Target for Ocular and Non-Ocular Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 6777. [Google Scholar] [CrossRef]
- Poormasjedi-Meibod, M.S.; Hartwell, R.; Kilani, R.T.; Ghahary, A. Anti-Scarring Properties of Different Tryptophan Derivatives. PLoS ONE 2014, 9, 3. [Google Scholar] [CrossRef]
- Oswald, D.J.; Lee, A.; Trinidad, M.; Chi, C.; Ren, R.; Rich, C.B.; Trinkaus-Randall, V. Communication between Corneal Epithelial Cells and Trigeminal Neurons Is Facilitated by Purinergic (P2) and Glutamatergic Receptors. PLoS ONE 2012, 7, e44574. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F.; Cozzi, A.; Mannaioni, G. Kynurenic Acid: A Metabolite with Multiple Actions and Multiple Targets in Brain and Periphery. J. Neural Transm. 2015, 119, 133–139. [Google Scholar] [CrossRef]
- Zhang, L. Expression and Role of Aryl Hydrocarbon Receptor in Aspergillus Fumigatus Keratitis. Int. J. Ophthalmol. 2020, 13, 199. [Google Scholar] [CrossRef]
- Ikuta, T.; Namiki, T.; Fujii-Kuriyama, Y.; Kawajiri, K. AhR Protein Trafficking and Function in the Skin. Biochem. Pharmacol. 2009, 77, 588–596. [Google Scholar] [CrossRef]
- Flaveny, C.A.; Murray, I.A.; Perdew, G.H. Differential Gene Regulation by the Human and Mouse Aryl Hydrocarbon Receptor. Toxicol. Sci. 2010, 114, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Flaveny, C.; Reen, R.K.; Kusnadi, A.; Perdew, G.H. The Mouse and Human Ah Receptor Differ in Recognition of LXXLL Motifs. Arch. Biochem. Biophys. 2008, 471, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Tsukahara, T.; Hamouda, N.; Utsumi, D.; Matsumoto, K.; Amagase, K.; Kato, S. G Protein-Coupled Receptor 35 Contributes to Mucosal Repair in Mice via Migration of Colonic Epithelial Cells. Pharmacol. Res. 2017, 123, 27–39. [Google Scholar] [CrossRef]
- Słoniecka, M.; le Roux, S.; Boman, P.; Byström, B.; Zhou, Q.; Danielson, P. Expression Profiles of Neuropeptides, Neurotransmitters, and Their Receptors in Human Keratocytes In Vitro and In Situ. PLoS ONE 2015, 10, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2018, 8, 1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dua, H.S. The Conjunctiva in Corneal Epithelial Wound Healing. Br. J. Ophthalmol. 1998, 82, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Esposito, A. Focus on Molecules: Interleukin-1: A Master Regulator of the Corneal Response to Injury. Exp. Eye Res. 2009, 89, 124–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, T.; Nakamura, M.; Mishima, H.; Otori, T.; Hikida, M. Interleukin 6 Facilitates Corneal Epithelial Wound Closure in Vivo. Arch. Ophthalmol. 1992, 110, 1292–1294. [Google Scholar] [CrossRef]
- Arranz-Valsero, I.; Soriano-Romaní, L.; García-Posadas, L.; López-García, A.; Diebold, Y. IL-6 as a Corneal Wound Healing Mediator in an in Vitro Scratch Assay. Exp. Eye Res. 2014, 125, 183–192. [Google Scholar] [CrossRef]
- Notara, M.; Shortt, A.; Galatowicz, G.; Calder, V.; Daniels, J. IL6 and the Human Limbal Stem Cell Niche: A Mediator of Epithelial-Stromal Interaction. Stem Cell Res. 2010, 5, 188–200. [Google Scholar] [CrossRef]
- Ghasemi, H.; Ghazanfari, T.; Yaraee, R.; Owlia, P.; Hassan, Z.M.; Faghihzadeh, S. Roles of IL-10 in Ocular Inflammations: A Review. Ocul. Immunol. Inflamm. 2012, 20, 406–418. [Google Scholar] [CrossRef]
- Li, B.; Tian, L.; Diao, Y.; Li, X.; Zhao, L.; Wang, X. Exogenous IL-10 Induces Corneal Transplantation Immune Tolerance by a Mechanism Associated with the Altered Th1/Th2 Cytokine Ratio and the Increased Expression of TGF-β. Mol. Med. Rep. 2014, 9, 2245–2250. [Google Scholar] [CrossRef] [Green Version]
- Csáti, A.; Edvinsson, L.; Vécsei, L.; Toldi, J.; Fülöp, F.; Tajti, J.; Warfvinge, K. Kynurenic Acid Modulates Experimentally Induced Inflammation in the Trigeminal Ganglion. J. Headache Pain 2015, 16, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Körtési, T.; Tuka, B.; Tajti, J.; Bagoly, T.; Fülöp, F.; Helyes, Z.; Vécsei, L. Kynurenic Acid Inhibits the Electrical Stimulation Induced Elevated Pituitary Adenylate Cyclase-Activating Polypeptide Expression in the TNC. Front. Neurol. 2018, 8, 745. [Google Scholar] [CrossRef] [Green Version]
- Lukacs, M.; Tajti, J.; Fulop, F.; Toldi, J.; Edvinsson, L.; Vecsei, L. Migraine, Neurogenic Inflammation, Drug Development—Pharmacochemical Aspects. Curr. Med. Chem. 2017, 24, 3649–3665. [Google Scholar] [CrossRef]
- Poormasjedi-Meibod, M.-S.; Pakyari, M.; Jackson, J.K.; Salimi Elizei, S.; Ghahary, A. Development of a Nanofibrous Wound Dressing with an Antifibrogenic Properties in Vitro and in Vivo Model. J. Biomed. Mater. Res. Part A 2016, 104, 487–497. [Google Scholar] [CrossRef]
- Toh, Z.H.; Lee, C.S.Y.; Chew, A.C.Y.; Perera, S. Time Heals All Wounds: Obstacles in Glaucoma Surgery from an Asian Perspective. Proc. Singap. Healthc. 2015, 24, 103–112. [Google Scholar] [CrossRef]
- Wejksza, K.; Rzeski, W.; Turski, W.A. Kynurenic Acid Protects against the Homo-Cysteine-Induced Impairment of Endothelial Cells. Pharmacol. Rep. 2009, 61, 751–756. [Google Scholar] [CrossRef]
- Rödl, J.B.; Bleich, S.; Schlötzer-Schrehardt, U.; Von Ahsen, N.; Kornhuber, J.; Naumann, G.O.; Kruse, F.E.; Jünemann, A.G. Increased Homocysteine Levels in Tear Fluid of Patients with Primary Open-Angle Glaucoma. Ophthalmic Res. 2008, 40, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ajith, T.A. Ranimenon Homocysteine in Ocular Diseases. Clin. Chim. Acta 2015, 450, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Papp, A.; Hartwell, R.; Evans, M.; Ghahary, A. The Safety and Tolerability of Topically Delivered Kynurenic Acid in Humans. A Phase 1 Randomized Double-Blind Clinical Trial. J. Pharm. Sci. 2018, 107, 1572–1576. [Google Scholar] [CrossRef]
- Samanta, A.; Guchhait, N.; Bhattacharya, S.C. Photophysical Aspects of Biological Photosensitizer Kynurenic Acid from the Perspective of Experimental and Quantum Chemical Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 713–715. [Google Scholar] [CrossRef]
- Zhuravleva, Y.S.; Sherin, P.S. Influence of PH on Radical Reactions between Kynurenic Acid and Amino Acids Tryptophan and Tyrosine. Part, I. Amino Acids in Free State. Free. Radic. Biol. Med. 2021, 172, 331–339. [Google Scholar] [CrossRef]
- Zelentsova, E.A.; Sherin, P.S.; Snytnikova, O.A.; Kaptein, R.; Vauthey, E.; Tsentalovich, Y.P. Photochemistry of Aqueous Solutions of Kynurenic Acid and Kynurenine Yellow. Photochem. Photobiol. Sci. 2013, 12, 546–558. [Google Scholar] [CrossRef] [Green Version]
- Zarnowski, T.; Rejdak, R.; Zagorski, Z.; Juenemann, A.G.M.; Zrenner, E.; Kocki, T.; Urbanska, E.M.; Turski, W.A. Content of Kynurenic Acid and Activity of Kynurenine Aminotransferases in Mammalian Eyes. Ophthalmic Res. 2004, 36, 124–128. [Google Scholar] [CrossRef]
- Flieger, J.; Święch-Zubilewicz, A.; Śniegocki, T.; Dolar-Szczasny, J.; Pizoń, M. Determination of Tryptophan and Its Major Metabolites in Fluid from the Anterior Chamber of the Eye in Diabetic Patients with Cataract by Liquid Chromotography Mass Spectrometry (LC-MS/MS). Molecules 2018, 23, 3012. [Google Scholar] [CrossRef] [Green Version]
- Moshirfar, M.; Chew, J.; Werner, L.; Meyer, J.J.; Hunter, B.; Stevens, S.; Jensen, M.; Kleinmann, G.; Mamalis, N. Comparison of the Effects of Fourth-Generation Fluoroquinolones on Corneal Re-Epithelialization in Rabbit Eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 1455–1461. [Google Scholar] [CrossRef]
- Tetz, M.R.; Auffarth, G.U.; Sperker, M.; Blum, M.; Völcker, H. Photographic Image Analysis System of Posterior Capsule Opacification. J. Cataract Refract. Surg. 1997, 23, 1351–1355. [Google Scholar] [CrossRef]

| pRSV-T | HC0597 | |||
|---|---|---|---|---|
| Number of Cell Counts | % of Control | Number of Cell Counts | % of Control | |
| Control | 13 ± 2.6 | 100 | 10 ± 1.5 | 100 |
| KYNA | 27 ± 7.0 * | 208 * | 12 ± 4.0 | 120 |
| Time [h] | Erosion Area [%] | |
|---|---|---|
| Control | KYNA 1% | |
| 0 | 100 ± 3.6 | 100 ± 3.2 |
| 12 | 83 ± 3.4 | 84 ± 3.8 |
| 24 | 40 ± 2.5 | 45 ± 2.1 |
| 36 | 12 ± 4.5 | 4 ± 1.4 * |
| 48 | 3 ± 2.8 | 0 |
| Effect | Corneal Epithelial Cells | Conjunctival Epithelial Cells |
|---|---|---|
| Metabolism reduction | YES | YES |
| Viability reduction | NO | YES |
| Cytoskeleton impairment | NO | YES |
| Migratory activity enhancement | YES | NO |
| Based on own presented results. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matysik-Woźniak, A.; Turski, W.A.; Turska, M.; Paduch, R.; Łańcut, M.; Piwowarczyk, P.; Czuczwar, M.; Rejdak, R. Kynurenic Acid Accelerates Healing of Corneal Epithelium In Vitro and In Vivo. Pharmaceuticals 2021, 14, 753. https://doi.org/10.3390/ph14080753
Matysik-Woźniak A, Turski WA, Turska M, Paduch R, Łańcut M, Piwowarczyk P, Czuczwar M, Rejdak R. Kynurenic Acid Accelerates Healing of Corneal Epithelium In Vitro and In Vivo. Pharmaceuticals. 2021; 14(8):753. https://doi.org/10.3390/ph14080753
Chicago/Turabian StyleMatysik-Woźniak, Anna, Waldemar A. Turski, Monika Turska, Roman Paduch, Mirosław Łańcut, Paweł Piwowarczyk, Mirosław Czuczwar, and Robert Rejdak. 2021. "Kynurenic Acid Accelerates Healing of Corneal Epithelium In Vitro and In Vivo" Pharmaceuticals 14, no. 8: 753. https://doi.org/10.3390/ph14080753
APA StyleMatysik-Woźniak, A., Turski, W. A., Turska, M., Paduch, R., Łańcut, M., Piwowarczyk, P., Czuczwar, M., & Rejdak, R. (2021). Kynurenic Acid Accelerates Healing of Corneal Epithelium In Vitro and In Vivo. Pharmaceuticals, 14(8), 753. https://doi.org/10.3390/ph14080753







