Nanoparticles in Dentistry: A Comprehensive Review
Abstract
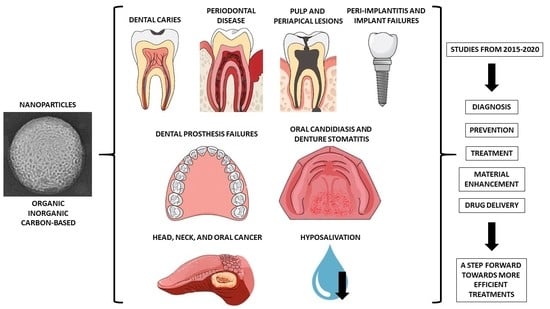
1. Introduction
2. Dental Caries
3. Periodontal Diseases
4. Pulp and Periapical Lesions
5. Peri-Implantitis and Implant Failures
6. Dental Prosthesis Failures
7. Oral Candidiasis and Denture Stomatitis
8. Head, Neck, and Oral Cancer
9. Hyposalivation
10. Oral Mucosa Drug Delivery
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yakop, F.; Abd Ghafar, S.A.; Yong, Y.K.; Saiful Yazan, L.; Mohamad Hanafiah, R.; Lim, V.; Eshak, Z. Silver nanoparticles Clinacanthus Nutans leaves extract induced apoptosis towards oral squamous cell carcinoma cell lines. Artif. Cells Nanomed. Biotechnol. 2018, 46, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, G.R.; Swamy, M.K. Potential applications of engineered nanoparticles in medicine and biology: An update. J. Biol. Inorg. Chem. 2018, 23, 1185–1204. [Google Scholar] [CrossRef]
- Anu Mary Ealia, S.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Meena, A.; Mali, H.S.; Patnaik, A.; Kumar, S.R. Comparative investigation of physical, mechanical and thermomechanical characterization of dental composite filled with nanohydroxyapatite and mineral trioxide aggregate. e-Polymers 2017, 17, 311–319. [Google Scholar] [CrossRef]
- Omidi, M.; Fatehinya, A.; Farahani, M.; Akbari, Z.; Shahmoradi, S.; Yazdian, F.; Tahriri, M.; Moharamzadeh, K.; Tayebi, L.; Vashaee, D. Characterization of Biomaterials; Elsevier: Singapore, 2017; pp. 97–115. [Google Scholar]
- Makkar, H.; Patri, G. Fabrication and appraisal of poly (Lactic-Co-Glycolic Acid)-moxifloxacin nanoparticles using vitamin E-tpgs: A potential intracanal drug delivery agent. J. Clin. Diagn. Res. 2017, 11, ZC05–ZC08. [Google Scholar] [CrossRef]
- Tanaka, M.; Okinaga, T.; Iwanaga, K.; Matsuo, K.; Toyono, T.; Sasaguri, M.; Ariyoshi, W.; Tominaga, K.; Enomoto, Y.; Matsumura, Y.; et al. Anticancer effect of novel platinum nanocomposite beads on oral squamous cell carcinoma cells. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Ketabat, F.; Pundir, M.; Mohabatpour, F.; Lobanova, L.; Koutsopoulos, S.; Hadjiiski, L.; Chen, X.; Papagerakis, P.; Papagerakis, S. Controlled drug delivery systems for oral cancer treatment-current status and future perspectives. Pharmaceutics 2019, 11, 302. [Google Scholar] [CrossRef]
- Cierech, M.; Kolenda, A.; Grudniak, A.M.; Wojnarowicz, J.; Wozniak, B.; Golas, M.; Swoboda-Kopec, E.; Lojkowski, W.; Mierzwinska-Nastalska, E. Significance of polymethylmethacrylate (PMMA) modification by zinc oxide nanoparticles for fungal biofilm formation. Int. J. Pharm. 2016, 510, 323–335. [Google Scholar] [CrossRef]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Aida, K.L.; Kreling, P.F.; Caiaffa, K.S.; Calixto, G.M.F.; Chorilli, M.; Spolidorio, D.M.; Santos-Filho, N.A.; Cilli, E.M.; Duque, C. Antimicrobial peptide-loaded liquid crystalline precursor bioadhesive system for the prevention of dental caries. Int. J. Nanomed. 2018, 13, 3081–3091. [Google Scholar] [CrossRef]
- Ghafar, H.; Khan, M.I.; Sarwar, H.S.; Yaqoob, S.; Hussain, S.Z.; Tariq, I.; Madni, A.U.; Shahnaz, G.; Sohail, M.F. Development and characterization of bioadhesive film embedded with lignocaine and calcium fluoride nanoparticles. AAPS PharmSciTech 2020, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Escudero, C.; Sediqi, N.; Smistad, G.; Hiorth, M. Fluoride loaded polymeric nanoparticles for dental delivery. Eur. J. Pharm. Sci. 2017, 104, 326–334. [Google Scholar] [CrossRef]
- Voltan, A.R.; Quindós, G.; Alarcón, K.P.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.; Chorilli, M. Fungal diseases: Could nanostructured drug delivery systems be a novel paradigm for therapy? Int. J. Nanomed. 2016, 11, 3715–3730. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, S.; Khan, S.; Hasan, S.; Khan, M.E.; Misba, L.; Khan, A.U. Calcium fluoride nanoparticles induced suppression of Streptococcus mutans biofilm: An in vitro and in vivo approach. Appl. Microbiol. Biotechnol. 2016, 100, 1901–1914. [Google Scholar] [CrossRef]
- Covarrubias, C.; Trepiana, D.; Corral, C. Synthesis of hybrid copper-chitosan nanoparticles with antibacterial activity against cariogenic Streptococcus mutans. Dent. Mater. J. 2018, 37, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Samprasit, W.; Kaomongkolgit, R.; Sukma, M.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Mucoadhesive electrospun chitosan-based nanofibre mats for dental caries prevention. Carbohydr. Polym. 2015, 117, 933–940. [Google Scholar] [CrossRef]
- Senthil Kumar, R.; Ravikumar, N.; Kavitha, S.; Mahalaxmi, S.; Jayasree, R.; Sampath Kumar, T.S.; Haneesh, M. Nanochitosan modified glass ionomer cement with enhanced mechanical properties and fluoride release. Int. J. Biol. Macromol. 2017, 104, 1860–1865. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Neo, J.; Esguerra, R.J.; Fawzy, A.S. Characterization of antibacterial and adhesion properties of chitosan-modified glass ionomer cement. J. Biomater. Appl. 2015, 30, 409–419. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Meera Priyadarshini, B.; Neo, J.; Fawzy, A.S. Characterization of chitosan/TiO(2) nano-powder modified glass-ionomer cement for restorative dental applications. J. Esthet. Restor. Dent. 2017, 29, 146–156. [Google Scholar] [CrossRef]
- Aliasghari, A.; Rabbani Khorasgani, M.; Vaezifar, S.; Rahimi, F.; Younesi, H.; Khoroushi, M. Evaluation of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: An in vitro study. Iran. J. Microbiol. 2016, 8, 93–100. [Google Scholar]
- Ashrafi, B.; Rashidipour, M.; Marzban, A.; Soroush, S.; Azadpour, M.; Delfani, S.; Ramak, P. Mentha piperita essential oils loaded in a chitosan nanogel with inhibitory effect on biofilm formation against S. mutans on the dental surface. Carbohydr. Polym. 2019, 212, 142–149. [Google Scholar] [CrossRef]
- Ikono, R.; Vibriani, A.; Wibowo, I.; Saputro, K.E.; Muliawan, W.; Bachtiar, B.M.; Mardliyati, E.; Bachtiar, E.W.; Rochman, N.T.; Kagami, H.; et al. Nanochitosan antimicrobial activity against Streptococcus mutans and Candida albicans dual-species biofilms. BMC Res. Notes 2019, 12, 383. [Google Scholar] [CrossRef]
- Ren, Q.; Ding, L.; Li, Z.; Wang, X.; Wang, K.; Han, S.; Li, W.; Zhou, X.; Zhang, L. Chitosan hydrogel containing amelogenin-derived peptide: Inhibition of cariogenic bacteria and promotion of remineralization of initial caries lesions. Arch. Oral Biol. 2019, 100, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Busscher, H.J.; Zhao, B.; Li, Y.; Zhang, Z.; van der Mei, H.C.; Ren, Y.; Shi, L. Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano 2016, 10, 4779–4789. [Google Scholar] [CrossRef] [PubMed]
- Sebelemetja, M.; Moeno, S.; Patel, M. Anti-acidogenic, anti-biofilm and slow release properties of Dodonaea viscosa var. angustifolia flavone stabilized polymeric nanoparticles. Arch. Oral Biol. 2020, 109, 104586. [Google Scholar] [CrossRef] [PubMed]
- Trigo Gutierrez, J.K.; Zanatta, G.C.; Ortega, A.L.M.; Balastegui, M.I.C.; Sanitá, P.V.; Pavarina, A.C.; Barbugli, P.A.; Mima, E.G.O. Encapsulation of curcumin in polymeric nanoparticles for antimicrobial photodynamic therapy. PLoS ONE 2017, 12, e0187418. [Google Scholar] [CrossRef]
- Zhao, Z.; Ding, C.; Wang, Y.; Tan, H.; Li, J. pH-Responsive polymeric nanocarriers for efficient killing of cariogenic bacteria in biofilms. Biomater. Sci. 2019, 7, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zhao, J.; Sun, J.; Hu, M.; Yang, X. Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano 2018, 12, 8882–8892. [Google Scholar] [CrossRef]
- Alvarez Echazú, M.I.; Olivetti, C.E.; Peralta, I.; Alonso, M.R.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development of pH-responsive biopolymer-silica composites loaded with Larrea divaricata Cav. extract with antioxidant activity. Colloids Surf. B Biointerfaces 2018, 169, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, R.; Palanisamy, S.; Chen, S.M.; Chelladurai, K.; Padmavathy, S.; Saravanan, M.; Prakash, P.; Ajmal Ali, M.; Al-Hemaid, F.M. Antimicrobial efficacy of green synthesized drug blended silver nanoparticles against dental caries and periodontal disease causing microorganisms. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 374–379. [Google Scholar] [CrossRef]
- Itohiya, H.; Matsushima, Y.; Shirakawa, S.; Kajiyama, S.; Yashima, A.; Nagano, T.; Gomi, K. Organic resolution function and effects of platinum nanoparticles on bacteria and organic matter. PLoS ONE 2019, 14, e0222634. [Google Scholar] [CrossRef]
- Vega-Jiménez, A.L.; Almaguer-Flores, A.; Flores-Castañeda, M.; Camps, E.; Uribe-Ramírez, M.; Aztatzi-Aguilar, O.G.; De Vizcaya-Ruiz, A. Bismuth subsalicylate nanoparticles with anaerobic antibacterial activity for dental applications. Nanotechnology 2017, 28, 435101. [Google Scholar] [CrossRef]
- Holden, M.S.; Black, J.; Lewis, A.; Boutrin, M.C.; Walemba, E.; Sabir, T.S.; Boskovic, D.S.; Wilson, A.; Fletcher, H.M.; Perry, C.C. Antibacterial activity of partially oxidized Ag/Au nanoparticles against the oral pathogen porphyromonas gingivalis W83. J. Nanomater. 2016, 2016, 9605906. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, D.N.; Lee, D.; Heo, M.; Rim, H.; Zhang, L.G.; Park, S.A.; Do, S.H.; Moon, J.H.; Kwon, I.K. One-Step fabrication of AgNPs embedded hybrid dual nanofibrous oral wound dressings. J. Biomed. Nanotechnol. 2016, 12, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jin, Y.; Wang, X.; Yao, S.; Li, Y.; Wu, Q.; Ma, G.; Cui, F.; Liu, H. An antimicrobial peptide-loaded gelatin/chitosan nanofibrous membrane fabricated by sequential layer-by-layer electrospinning and electrospraying techniques. Nanomaterials 2018, 8, 327. [Google Scholar] [CrossRef]
- Mahmoud, M.Y.; Sapare, S.; Curry, K.C.; Demuth, D.R.; Steinbach-Rankins, J.M. Rapid release polymeric fibers for inhibition of porphyromonas gingivalis adherence to streptococcus gordonii. Front. Chem. 2019, 7, 926. [Google Scholar] [CrossRef] [PubMed]
- Backlund, C.J.; Worley, B.V.; Sergesketter, A.R.; Schoenfisch, M.H. Kinetic-dependent Killing of Oral Pathogens with Nitric Oxide. J. Dent. Res. 2015, 94, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Brito, G.A.C.; Lima, M.L.S.; Silva Júnior, A.A.D.; Silva, E.D.S.; de Rezende, A.A.; Bortolin, R.H.; Galvan, M.; Pirih, F.Q.; Araújo Júnior, R.F.; et al. Metformin hydrochloride-loaded PLGA nanoparticle in periodontal disease experimental model using diabetic rats. Int. J. Mol. Sci. 2018, 19, 3488. [Google Scholar] [CrossRef]
- Mou, J.; Liu, Z.; Liu, J.; Lu, J.; Zhu, W.; Pei, D. Hydrogel containing minocycline and zinc oxide-loaded serum albumin nanopartical for periodontitis application: Preparation, characterization and evaluation. Drug Deliv. 2019, 26, 179–187. [Google Scholar] [CrossRef]
- Osorio, R.; Alfonso-Rodríguez, C.A.; Medina-Castillo, A.L.; Alaminos, M.; Toledano, M. Bioactive polymeric nanoparticles for periodontal therapy. PLoS ONE 2016, 11, e0166217. [Google Scholar] [CrossRef]
- Kalia, P.; Jain, A.; Radha Krishnan, R.; Demuth, D.R.; Steinbach-Rankins, J.M. Peptide-modified nanoparticles inhibit formation of Porphyromonas gingivalis biofilms with Streptococcus gordonii. Int. J. Nanomed. 2017, 12, 4553–4562. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, L.M.G.; Brandao, D.A.; Rocha, F.R.G.; Marsiglio, R.P.; Longo, I.B.; Primo, F.L.; Tedesco, A.C.; Guimaraes-Stabili, M.R.; Rossa Junior, C. Local administration of curcumin-loaded nanoparticles effectively inhibits inflammation and bone resorption associated with experimental periodontal disease. Sci. Rep. 2018, 8, 6652. [Google Scholar] [CrossRef] [PubMed]
- Wijetunge, S.S.; Wen, J.; Yeh, C.K.; Sun, Y. Wheat germ agglutinin liposomes with surface grafted cyclodextrins as bioadhesive dual-drug delivery nanocarriers to treat oral cells. Colloids Surf. B Biointerfaces 2020, 185, 110572. [Google Scholar] [CrossRef] [PubMed]
- Moraes, G.S.; Santos, I.B.D.; Pinto, S.C.S.; Pochapski, M.T.; Farago, P.V.; Pilatti, G.L.; Santos, F.A. Liposomal anesthetic gel for pain control during periodontal therapy in adults: A placebo-controlled RCT. J. Appl. Oral Sci. 2020, 28, e20190025. [Google Scholar] [CrossRef]
- Klein-Júnior, C.A.; Reston, E.; Plepis, A.M.; Martins, V.C.; Pötter, I.C.; Lundy, F.; Hentschke, G.S.; Hentschke, V.S.; Karim, I.E. Development and evaluation of calcium hydroxide-coated, pericardium-based biomembranes for direct pulp capping. J. Investig. Clin. Dent. 2019, 10, e12380. [Google Scholar] [CrossRef]
- Elshinawy, M.I.; Al-Madboly, L.A.; Ghoneim, W.M.; El-Deeb, N.M. Synergistic effect of newly introduced root canal medicaments; ozonated olive oil and chitosan nanoparticles, against persistent endodontic pathogens. Front. Microbiol. 2018, 9, 1371. [Google Scholar] [CrossRef]
- Nair, N.; James, B.; Devadathan, A.; Johny, M.K.; Mathew, J.; Jacob, J. Comparative evaluation of antibiofilm efficacy of chitosan nanoparticle- and zinc oxide nanoparticle-incorporated calcium hydroxide-based sealer: An in vitro study. Contemp. Clin. Dent. 2018, 9, 434–439. [Google Scholar] [CrossRef]
- Teixeira, A.B.V.; de Castro, D.T.; Schiavon, M.A.; Dos Reis, A.C. Cytotoxicity and release ions of endodontic sealers incorporated with a silver and vanadium base nanomaterial. Odontology 2020, 108, 661–668. [Google Scholar] [CrossRef]
- Afkhami, F.; Elahy, S.; Mahmoudi-Nahavandi, A. Spectrophotometric analysis of crown discoloration following the use of silver nanoparticles combined with calcium hydroxide as intracanal medicament. J. Clin. Exp. Dent. 2017, 9, e842–e847. [Google Scholar] [CrossRef]
- Antunes, P.V.S.; Flamini, L.E.S.; Chaves, J.F.M.; Silva, R.G.; Cruz Filho, A.M.D. Comparative effects of final canal irrigation with chitosan and EDTA. J. Appl. Oral Sci. 2020, 28, e20190005. [Google Scholar] [CrossRef]
- Mathew, S.P.; Pai, V.S.; Usha, G.; Nadig, R.R. Comparative evaluation of smear layer removal by chitosan and ethylenediaminetetraacetic acid when used as irrigant and its effect on root dentine: An in vitro atomic force microscopic and energy-dispersive X-ray analysis. J. Conserv. Dent. 2017, 20, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Kesim, B.; Burak, A.K.; Ustun, Y.; Delikan, E.; Gungor, A. Effect of chitosan on sealer penetration into the dentinal tubules. Niger. J. Clin. Pract. 2018, 21, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Pivatto, K.; Pedro, F.L.M.; Guedes, O.A.; Silva, A.F.D.; Piva, E.; Pereira, T.M.; Rosa, W.; Borges, A.H. Cytotoxicity of chelating agents used in endodontics and their influence on MMPs of cell membranes. Braz. Dent. J. 2020, 31, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Raghu, R.; Pradeep, G.; Shetty, A.; Gautham, P.M.; Puneetha, P.G.; Reddy, T.V.S. Retrievability of calcium hydroxide intracanal medicament with three calcium chelators, ethylenediaminetetraacetic acid, citric acid, and chitosan from root canals: An in vitro cone beam computed tomography volumetric analysis. J. Conserv. Dent. 2017, 20, 25–29. [Google Scholar] [CrossRef]
- El Ashiry, E.A.; Alamoudi, N.M.; El Ashiry, M.K.; Bastawy, H.A.; El Derwi, D.A.; Atta, H.M. Tissue engineering of necrotic dental pulp of immature teeth with apical periodontitis in dogs: Radiographic and histological evaluation. J. Clin. Pediatr. Dent. 2018, 42, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Choi, S.K.; Sultan, A.S.; Chuncai, K.; Lin, X.; Dashtimoghadam, E.; Melo, M.A.; Weir, M.; Xu, H.; Tayebi, L.; et al. Nanomagnetic-mediated drug delivery for the treatment of dental disease. Nanomedicine 2018, 14, 919–927. [Google Scholar] [CrossRef]
- Uğur Aydin, Z.; Akpinar, K.E.; Hepokur, C.; Erdönmez, D. Assessment of toxicity and oxidative DNA damage of sodium hypochlorite, chitosan and propolis on fibroblast cells. Braz. Oral Res. 2018, 32, e119. [Google Scholar] [CrossRef]
- Yadav, P.; Chaudhary, S.; Saxena, R.K.; Talwar, S.; Yadav, S. Evaluation of antimicrobial and antifungal efficacy of chitosan as endodontic irrigant against enterococcus faecalis and candida albicans biofilm formed on tooth substrate. J. Clin. Exp. Dent. 2017, 9, e361–e367. [Google Scholar] [CrossRef]
- Kamble, A.B.; Abraham, S.; Kakde, D.D.; Shashidhar, C.; Mehta, D.L. Scanning electron microscopic evaluation of efficacy of 17% ethylenediaminetetraacetic acid and chitosan for smear layer removal with ultrasonics: An in vitro study. Contemp. Clin. Dent. 2017, 8, 621–626. [Google Scholar] [CrossRef]
- Sireesha, A.; Jayasree, R.; Vidhya, S.; Mahalaxmi, S.; Sujatha, V.; Kumar, T.S.S. Comparative evaluation of micron- and nano-sized intracanal medicaments on penetration and fracture resistance of root dentin-An in vitro study. Int. J. Biol. Macromol. 2017, 104, 1866–1873. [Google Scholar] [CrossRef]
- Del Carpio-Perochena, A.; Kishen, A.; Felitti, R.; Bhagirath, A.Y.; Medapati, M.R.; Lai, C.; Cunha, R.S. Antibacterial roperties of chitosan nanoparticles and propolis associated with calcium hydroxide against single- and multispecies biofilms: An in vitro and in situ study. J. Endod. 2017, 43, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, N.; Godiny, M.; Moradi, S.; Hemati Azandaryani, A.; Shahlaei, M. Chitosan/gelatin as a new nano-carrier system for calcium hydroxide delivery in endodontic applications: Development, characterization and process optimization. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 540–546. [Google Scholar] [CrossRef]
- Thota, M.M.; Sudha, K.; Malini, D.L.; Madhavi, S.B. Effect of different irrigating solutions on depth of penetration of sealer into dentinal tubules: A confocal microscopic study. Contemp. Clin. Dent. 2017, 8, 391–394. [Google Scholar] [CrossRef]
- Ozlek, E.; Rath, P.P.; Kishen, A.; Neelakantan, P. A chitosan-based irrigant improves the dislocation resistance of a mineral trioxide aggregate-resin hybrid root canal sealer. Clin. Oral Investig. 2020, 24, 151–156. [Google Scholar] [CrossRef]
- Paiola, F.G.; Lopes, F.C.; Mazzi-Chaves, J.F.; Pereira, R.D.; Oliveira, H.F.; Queiroz, A.M.; Sousa-Neto, M.D. How to improve root canal filling in teeth subjected to radiation therapy for cancer. Braz. Oral Res. 2018, 32, e121. [Google Scholar] [CrossRef]
- Flores-Arriaga, J.C.; Pozos-Guillén, A.J.; González-Ortega, O.; Escobar-García, D.M.; Masuoka-Ito, D.; Del Campo-Téllez, B.I.M.; Cerda-Cristerna, B.I. Calcium sustained release, pH changes and cell viability induced by chitosan-based pastes for apexification. Odontology 2019, 107, 223–230. [Google Scholar] [CrossRef]
- Savitha, A.; SriRekha, A.; Vijay, R.; Ashwija; Champa, C.; Jaykumar, T. An in vivo comparative evaluation of antimicrobial efficacy of chitosan, chlorhexidine gluconate gel and their combination as an intracanal medicament against Enterococcus faecalis in failed endodontic cases using real time polymerase chain reaction (qPCR). Saudi Dent. J. 2019, 31, 360–366. [Google Scholar] [CrossRef]
- Zhu, N.; Chatzistavrou, X.; Ge, L.; Qin, M.; Papagerakis, P.; Wang, Y. Biological properties of modified bioactive glass on dental pulp cells. J. Dent. 2019, 83, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhou, Y.; Yu, Y.; Zhou, X.; Du, W.; Wan, M.; Fan, Y.; Zhou, X.; Xu, X.; Zheng, L. Evaluation of chitosan hydrogel for sustained delivery of VEGF for odontogenic differentiation of dental pulp stem cells. Stem Cells Int. 2019, 2019, 1515040. [Google Scholar] [CrossRef]
- Bordini, E.A.F.; Cassiano, F.B.; Silva, I.S.P.; Usberti, F.R.; Anovazzi, G.; Pacheco, L.E.; Pansani, T.N.; Leite, M.L.; Hebling, J.; de Souza Costa, C.A.; et al. Synergistic potential of 1α,25-dihydroxyvitamin D3 and calcium-aluminate-chitosan scaffolds with dental pulp cells. Clin. Oral Investig. 2020, 24, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Balata, G.F.; Abdelhady, M.I.S.; Mahmoud, G.M.; Matar, M.A.; Abd El-Latif, A.N. Formulation of saudi propolis into biodegradable chitosan chips for vital pulpotomy. Curr. Drug Deliv. 2018, 15, 97–109. [Google Scholar] [CrossRef]
- Mittal, N.; Parashar, V. Regenerative evaluation of immature roots using PRF and artificial scaffolds in necrotic permanent teeth: A clinical study. J. Contemp. Dent. Pract. 2019, 20, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic evaluation of regenerative endodontic procedures with the Use of chitosan scaffolds in immature dog teeth with apical periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef]
- de Almeida, J.; Cechella, B.C.; Bernardi, A.V.; de Lima Pimenta, A.; Felippe, W.T. Effectiveness of nanoparticles solutions and conventional endodontic irrigants against enterococcus faecalis biofilm. Indian J. Dent. Res. 2018, 29, 347–351. [Google Scholar] [CrossRef]
- Halkai, K.R.; Halkai, R.; Mudda, J.A.; Shivanna, V.; Rathod, V. Antibiofilm efficacy of biosynthesized silver nanoparticles against endodontic-periodontal pathogens: An in vitro study. J. Conserv. Dent. 2018, 21, 662–666. [Google Scholar] [CrossRef]
- Zheng, T.; Huang, X.; Chen, J.; Feng, D.; Mei, L.; Huang, Y.; Quan, G.; Zhu, C.; Singh, V.; Ran, H.; et al. A liquid crystalline precursor incorporating chlorhexidine acetate and silver nanoparticles for root canal disinfection. Biomater. Sci. 2018, 6, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Sadek, R.W.; Moussa, S.M.; El Backly, R.M.; Hammouda, A.F. Evaluation of the efficacy of three antimicrobial agents used for regenerative endodontics: An in vitro study. Microb. Drug Resist. 2019, 25, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Halkai, K.R.; Mudda, J.A.; Shivanna, V.; Rathod, V.; Halkai, R. Antibacterial efficacy of biosynthesized silver nanoparticles against enterococcus faecalis biofilm: An in vitro study. Contemp. Clin. Dent. 2018, 9, 237–241. [Google Scholar] [CrossRef]
- Takamiya, A.S.; Monteiro, D.R.; Bernabé, D.G.; Gorup, L.F.; Camargo, E.R.; Gomes-Filho, J.E.; Oliveira, S.H.; Barbosa, D.B. In vitro and in vivo toxicity evaluation of colloidal silver nanoparticles used in endodontic treatments. J. Endod. 2016, 42, 953–960. [Google Scholar] [CrossRef]
- Chávez-Andrade, G.M.; Tanomaru-Filho, M.; Rodrigues, E.M.; Gomes-Cornélio, A.L.; Faria, G.; Bernardi, M.I.B.; Guerreiro-Tanomaru, J.M. Cytotoxicity, genotoxicity and antibacterial activity of poly(vinyl alcohol)-coated silver nanoparticles and farnesol as irrigating solutions. Arch. Oral Biol. 2017, 84, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, K.; Niazi, S.; Mylonas, P.; Mannocci, F.; Deb, S. The synthesis of nano silver-graphene oxide system and its efficacy against endodontic biofilms using a novel tooth model. Dent. Mater. 2019, 35, 1614–1629. [Google Scholar] [CrossRef]
- Martinez-Andrade, J.M.; Avalos-Borja, M.; Vilchis-Nestor, A.R.; Sanchez-Vargas, L.O.; Castro-Longoria, E. Dual function of EDTA with silver nanoparticles for root canal treatment-A novel modification. PLoS ONE 2018, 13, e0190866. [Google Scholar] [CrossRef] [PubMed]
- Baras, B.H.; Sun, J.; Melo, M.A.S.; Tay, F.R.; Oates, T.W.; Zhang, K.; Weir, M.D.; Xu, H.H.K. Novel root canal sealer with dimethylaminohexadecyl methacrylate, nano-silver and nano-calcium phosphate to kill bacteria inside root dentin and increase dentin hardness. Dent. Mater. 2019, 35, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Andrade, G.M.; Tanomaru-Filho, M.; Basso Bernardi, M.I.; de Toledo Leonardo, R.; Faria, G.; Guerreiro-Tanomaru, J.M. Antimicrobial and biofilm anti-adhesion activities of silver nanoparticles and farnesol against endodontic microorganisms for possible application in root canal treatment. Arch. Oral Biol. 2019, 107, 104481. [Google Scholar] [CrossRef] [PubMed]
- Moazami, F.; Sahebi, S.; Ahzan, S. Tooth discoloration induced by imidazolium based silver nanoparticles as an intracanal irrigant. J. Dent. 2018, 19, 280–286. [Google Scholar]
- Lee, D.K.; Kee, T.; Liang, Z.; Hsiou, D.; Miya, D.; Wu, B.; Osawa, E.; Chow, E.K.; Sung, E.C.; Kang, M.K.; et al. Clinical validation of a nanodiamond-embedded thermoplastic biomaterial. Proc. Natl. Acad. Sci. USA 2017, 114, E9445–E9454. [Google Scholar] [CrossRef]
- Yu, Q.; Li, J.; Zhang, Y.; Wang, Y.; Liu, L.; Li, M. Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci. Rep. 2016, 6, 26667. [Google Scholar] [CrossRef]
- Bukhari, S.; Kim, D.; Liu, Y.; Karabucak, B.; Koo, H. Novel endodontic disinfection approach using catalytic nanoparticles. J. Endod. 2018, 44, 806–812. [Google Scholar] [CrossRef]
- Wang, L.; Xie, X.; Li, C.; Liu, H.; Zhang, K.; Zhou, Y.; Chang, X.; Xu, H.H.K. Novel bioactive root canal sealer to inhibit endodontic multispecies biofilms with remineralizing calcium phosphate ions. J. Dent. 2017, 60, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Abdel Raheem, I.A.; Abdul Razek, A.; Elgendy, A.A.; Saleh, N.M.; Shaaban, M.I.; Abd El-Hady, F.K. Design, evaluation and antimicrobial activity of egyptian propolis-loaded nanoparticles: Intrinsic role as a novel and naturally based root canal nanosealer. Int. J. Nanomed. 2019, 14, 8379–8398. [Google Scholar] [CrossRef] [PubMed]
- Abdelmonem, R.; Younis, M.K.; Hassan, D.H.; El-Sayed Ahmed, M.A.E.; Hassanein, E.; El-Batouty, K.; Elfaham, A. Formulation and characterization of chlorhexidine HCl nanoemulsion as a promising antibacterial root canal irrigant: In-vitro and ex-vivo studies. Int. J. Nanomed. 2019, 14, 4697–4708. [Google Scholar] [CrossRef] [PubMed]
- Imura, K.; Hashimoto, Y.; Okada, M.; Yoshikawa, K.; Yamamoto, K. Application of hydroxyapatite nanoparticle-assembled powder using basic fibroblast growth factor as a pulp-capping agent. Dent. Mater. J. 2019, 38, 713–720. [Google Scholar] [CrossRef]
- Afkhami, F.; Akbari, S.; Chiniforush, N. Entrococcus faecalis elimination in root canals using silver nanoparticles, Photodynamic therapy, diode laser, or laser-activated nanoparticles: An in vitro study. J. Endod. 2017, 43, 279–282. [Google Scholar] [CrossRef]
- Camacho-Alonso, F.; Julián-Belmonte, E.; Chiva-García, F.; Martínez-Beneyto, Y. Bactericidal efficacy of photodynamic therapy and chitosan in root canals experimentally infected with enterococcus faecalis: An in vitro study. Photomed. Laser Surg. 2017, 35, 184–189. [Google Scholar] [CrossRef]
- Kushwaha, V.; Yadav, R.K.; Tikku, A.P.; Chandra, A.; Verma, P.; Gupta, P.; Shakya, V.K. Comparative evaluation of antibacterial effect of nanoparticles and lasers against endodontic microbiota: An in vitro study. J. Clin. Exp. Dent. 2018, 10, e1155–e1160. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Vaswani, S.D.; Najan, H.B.; Mehta, D.L.; Kamble, A.B.; Chaudhari, S.D. Scanning electron microscopic evaluation of smear layer removal at the apical third of root canals using diode laser, endoActivator, and ultrasonics with chitosan: An in vitro study. J. Conserv. Dent. 2019, 22, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Geethapriya, N.; Subbiya, A.; Padmavathy, K.; Mahalakshmi, K.; Vivekanandan, P.; Sukumaran, V.G. Effect of chitosan-ethylenediamine tetraacetic acid on Enterococcus faecalis dentinal biofilm and smear layer removal. J. Conserv. Dent. 2016, 19, 472–477. [Google Scholar] [CrossRef]
- Glenn, B.; Drum, M.; Reader, A.; Fowler, S.; Nusstein, J.; Beck, M. Does liposomal bupivacaine (exparel) significantly reduce postoperative pain/numbness in symptomatic teeth with a diagnosis of necrosis? A prospective, randomized, double-blind trial. J. Endod. 2016, 42, 1301–1306. [Google Scholar] [CrossRef]
- Bultema, K.; Fowler, S.; Drum, M.; Reader, A.; Nusstein, J.; Beck, M. Pain reduction in untreated symptomatic irreversible pulpitis using liposomal bupivacaine (exparel): A prospective, randomized, double-blind trial. J. Endod. 2016, 42, 1707–1712. [Google Scholar] [CrossRef]
- Al-Hashedi, A.A.; Laurenti, M.; Amine Mezour, M.; Basiri, T.; Touazine, H.; Jahazi, M.; Tamimi, F. Advanced inorganic nanocomposite for decontaminating titanium dental implants. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 761–772. [Google Scholar] [CrossRef]
- Fröber, K.; Bergs, C.; Pich, A.; Conrads, G. Biofunctionalized zinc peroxide nanoparticles inhibit peri-implantitis associated anaerobes and Aggregatibacter actinomycetemcomitans pH-dependent. Anaerobe 2020, 62, 102153. [Google Scholar] [CrossRef]
- Jadhav, K.; Hr, R.; Deshpande, S.; Jagwani, S.; Dhamecha, D.; Jalalpure, S.; Subburayan, K.; Baheti, D. Phytosynthesis of gold nanoparticles: Characterization, biocompatibility, and evaluation of its osteoinductive potential for application in implant dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 664–670. [Google Scholar] [CrossRef]
- Govindharajulu, J.P.; Chen, X.; Li, Y.; Rodriguez-Cabello, J.C.; Battacharya, M.; Aparicio, C. Chitosan-recombinamer layer-by-layer coatings for multifunctional implants. Int. J. Mol. Sci. 2017, 18, 369. [Google Scholar] [CrossRef]
- Divakar, D.D.; Jastaniyah, N.T.; Altamimi, H.G.; Alnakhli, Y.O.; Muzaheed; Alkheraif, A.A.; Haleem, S. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. Int. J. Biol. Macromol. 2018, 108, 790–797. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, L.; Shi, M.; Zhang, Y.; Wang, Q. Ti-GO-Ag nanocomposite: The effect of content level on the antimicrobial activity and cytotoxicity. Int. J. Nanomed. 2017, 12, 4209–4224. [Google Scholar] [CrossRef] [PubMed]
- Larsen, O.I.; Enersen, M.; Kristoffersen, A.K.; Wennerberg, A.; Bunaes, D.F.; Lie, S.A.; Leknes, K.N. Antimicrobial effects of three different treatment modalities on dental implant surfaces. J. Oral Implantol. 2017, 43, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, Y.; Zhang, H.; Xu, Z.; Zhao, L.; Wang, J.; Qiu, Y.; Liu, B. Improvements on biological and antimicrobial properties of titanium modified by AgNPs-loaded chitosan-heparin polyelectrolyte multilayers. J. Mater. Sci. Mater. Med. 2019, 30, 52. [Google Scholar] [CrossRef]
- Valverde, A.; Perez-Alvarez, L.; Ruiz-Rubio, L.; Pacha Olivenza, M.A.; Garcia Blanco, M.B.; Diaz-Fuentes, M.; Vilas-Vilela, J.L. Antibacterial hyaluronic acid/chitosan multilayers onto smooth and micropatterned titanium surfaces. Carbohydr. Polym. 2019, 207, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Roguska, A.; Belcarz, A.; Zalewska, J.; Holdynski, M.; Andrzejczuk, M.; Pisarek, M.; Ginalska, G. Metal TiO2 nanotube layers for the treatment of dental implant infections. ACS Appl. Mater. Interfaces 2018, 10, 17089–17099. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.; Versace, D.L.; Abbad-Andallousi, S.; Pires, R.; Azevedo, C.; Cenedese, P.; Dubot, P. Antibacterial properties of nanostructured Cu-TiO2 surfaces for dental implants. Biomater. Sci. 2017, 5, 455–462. [Google Scholar] [CrossRef]
- Sobolev, A.; Valkov, A.; Kossenko, A.; Wolicki, I.; Zinigrad, M.; Borodianskiy, K. Bioactive coating on Ti alloy with high osseointegration and antibacterial Ag nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 39534–39544. [Google Scholar] [CrossRef]
- Venugopal, A.; Muthuchamy, N.; Tejani, H.; Gopalan, A.I.; Lee, K.P.; Lee, H.J.; Kyung, H.M. Incorporation of silver nanoparticles on the surface of orthodontic microimplants to achieve antimicrobial properties. Korean J. Orthod. 2017, 47, 3–10. [Google Scholar] [CrossRef]
- Wohlfahrt, J.C.; Aass, A.M.; Koldsland, O.C. Treatment of peri-implant mucositis with a chitosan brush-A pilot randomized clinical trial. Int. J. Dent. Hyg. 2019, 17, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt, J.C.; Evensen, B.J.; Zeza, B.; Jansson, H.; Pilloni, A.; Roos-Jansaker, A.M.; Di Tanna, G.L.; Aass, A.M.; Klepp, M.; Koldsland, O.C. A novel non-surgical method for mild peri-implantitis- a multicenter consecutive case series. Int. J. Implant. Dent. 2017, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Cai, X.; Chen, R.; Zhang, H.; Jiang, T.; Wang, Y. A thermosensitive chitosan-based hydrogel for sealing and lubricating purposes in dental implant system. Clin. Implant. Dent. Relat. Res. 2019, 21, 324–335. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Mattioli-Belmonte, M.; Cimmarusti, M.T.; Panniello, A.; Dicarlo, M.; Milano, F.; Agostiano, A.; De Giglio, E.; Catucci, L. Liposome-modified titanium surface: A strategy to locally deliver bioactive molecules. Colloids Surf. B Biointerfaces 2017, 158, 387–396. [Google Scholar] [CrossRef]
- Song, J.; Chen, Q.; Zhang, Y.; Diba, M.; Kolwijck, E.; Shao, J.; Jansen, J.A.; Yang, F.; Boccaccini, A.R.; Leeuwenburgh, S.C. Electrophoretic deposition of chitosan coatings modified with gelatin nanospheres to tune the release of antibiotics. ACS Appl. Mater. Interfaces 2016, 8, 13785–13792. [Google Scholar] [CrossRef]
- Pokrowiecki, R.; Zaręba, T.; Szaraniec, B.; Pałka, K.; Mielczarek, A.; Menaszek, E.; Tyski, S. In vitro studies of nanosilver-doped titanium implants for oral and maxillofacial surgery. Int. J. Nanomed. 2017, 12, 4285–4297. [Google Scholar] [CrossRef] [PubMed]
- Lampé, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csik, A.; Dombrádi, Z.; Hajdu, P.; Hegedűs, V.; Rácz, R.; Varga, I.; et al. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int. J. Nanomed. 2019, 14, 4709–4721. [Google Scholar] [CrossRef]
- Choi, S.H.; Jang, Y.S.; Jang, J.H.; Bae, T.S.; Lee, S.J.; Lee, M.H. Enhanced antibacterial activity of titanium by surface modification with polydopamine and silver for dental implant application. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019847067. [Google Scholar] [CrossRef]
- Gosau, M.; Haupt, M.; Thude, S.; Strowitzki, M.; Schminke, B.; Buergers, R. Antimicrobial effect and biocompatibility of novel metallic nanocrystalline implant coatings. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Chidambaranathan, A.S.; Mohandoss, K.; Balasubramaniam, M.K. Comparative evaluation of antifungal effect of titanium, Zirconium and Aluminium nanoparticles coated Titanium plates against C. albicans. J. Clin. Diagn. Res. 2016, 10, Zc56–Zc59. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, M.; Ahila, S.C.; Muthu Kumar, B. The antibacterial influence of nanotopographic titanium, zirconium, and aluminum nanoparticles against Staphylococcus aureus and porphyromonas gingivalis: An in vitro study. Indian J. Dent. Res. 2019, 30, 37–42. [Google Scholar] [CrossRef]
- Salaie, R.N.; Besinis, A.; Le, H.; Tredwin, C.; Handy, R.D. The biocompatibility of silver and nanohydroxyapatite coatings on titanium dental implants with human primary osteoblast cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110210. [Google Scholar] [CrossRef]
- Martinez, E.F.; Ishikawa, G.J.; de Lemos, A.B.; Barbosa Bezerra, F.J.; Sperandio, M.; Napimoga, M.H. Evaluation of a Titanium surface treated with hydroxyapatite nanocrystals on osteoblastic cell behavior: An in vitro study. Int. J. Oral Maxillofac. Implants 2018, 33, 597–602. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cavalcanti, J.H.; Matos, P.C.; Depes de Gouvea, C.V.; Carvalho, W.; Calvo-Guirado, J.L.; Aragoneses, J.M.; Perez-Diaz, L.; Gehrke, S.A. In vitro assessment of the functional dynamics of titanium with surface coating of hydroxyapatite Nanoparticles. Materials 2019, 12, 840. [Google Scholar] [CrossRef]
- Suo, L.; Jiang, N.; Wang, Y.; Wang, P.; Chen, J.; Pei, X.; Wang, J.; Wan, Q. The enhancement of osseointegration using a graphene oxide/chitosan/hydroxyapatite composite coating on titanium fabricated by electrophoretic deposition. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 635–645. [Google Scholar] [CrossRef]
- Heo, D.N.; Ko, W.K.; Lee, H.R.; Lee, S.J.; Lee, D.; Um, S.H.; Lee, J.H.; Woo, Y.H.; Zhang, L.G.; Lee, D.W.; et al. Titanium dental implants surface-immobilized with gold nanoparticles as osteoinductive agents for rapid osseointegration. J. Colloid Interface Sci. 2016, 469, 129–137. [Google Scholar] [CrossRef]
- Kalyoncuoglu, U.T.; Yilmaz, B.; Koc, S.G.; Evis, Z.; Arpaci, P.U.; Kansu, G. Investigation of surface structure and biocompatibility of chitosan-coated zirconia and alumina dental abutments. Clin. Implant Dent. Relat. Res. 2018, 20, 1022–1029. [Google Scholar] [CrossRef]
- Zhong, X.; Song, Y.; Yang, P.; Wang, Y.; Jiang, S.; Zhang, X.; Li, C. Titanium surface priming with phase-transited lysozyme to establish a silver nanoparticle-loaded chitosan/hyaluronic acid antibacterial multilayer via layer-by-layer self-assembly. PLoS ONE 2016, 11, e0146957. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Zhang, J.Y.; Wang, Y.B.; Li, C.M.; Lu, Z.S.; Hu, X.F.; Xu, L.Q. Deposition of catechol-functionalized chitosan and silver nanoparticles on biomedical titanium surfaces for antibacterial application. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 649–656. [Google Scholar] [CrossRef]
- Guan, B.; Wang, H.; Xu, R.; Zheng, G.; Yang, J.; Liu, Z.; Cao, M.; Wu, M.; Song, J.; Li, N.; et al. Establishing antibacterial multilayer films on the surface of direct metal laser sintered Titanium primed with phase-transited lysozyme. Sci. Rep. 2016, 6, 36408. [Google Scholar] [CrossRef]
- Yuan, Z.; Huang, S.; Lan, S.; Xiong, H.; Tao, B.; Ding, Y.; Liu, Y.; Liu, P.; Cai, K. Surface engineering of titanium implants with enzyme-triggered antibacterial properties and enhanced osseointegration in vivo. J. Mater. Chem. B 2018, 6, 8090–8104. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, P.; Ge, Y.; Lan, F.; Zhou, X.; He, J.; Wu, Y. Dopamine self-polymerized along with hydroxyapatite onto the preactivated titanium percutaneous implants surface to promote human gingival fibroblast behavior and antimicrobial activity for biological sealing. J. Biomater. Appl. 2018, 32, 1071–1082. [Google Scholar] [CrossRef]
- Wang, J.; He, X.T.; Xu, X.Y.; Yin, Y.; Li, X.; Bi, C.S.; Hong, Y.L.; Chen, F.M. Surface modification via plasmid-mediated pLAMA3-CM gene transfection promotes the attachment of gingival epithelial cells to titanium sheets in vitro and improves biological sealing at the transmucosal sites of titanium implants in vivo. J. Mater. Chem. B 2019, 7, 7415–7427. [Google Scholar] [CrossRef]
- Palla-Rubio, B.; Araújo-Gomes, N.; Fernández-Gutiérrez, M.; Rojo, L.; Suay, J.; Gurruchaga, M.; Goñi, I. Synthesis and characterization of silica-chitosan hybrid materials as antibacterial coatings for titanium implants. Carbohydr. Polym. 2019, 203, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, M.A.; Cometa, S.; Dicarlo, M.; Baruzzi, F.; de Candia, S.; Gloria, A.; Giangregorio, M.M.; Mattioli-Belmonte, M.; De Giglio, E. Gallium-modified chitosan/poly(acrylic acid) bilayer coatings for improved titanium implant performances. Carbohydr. Polym. 2017, 166, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Belouka, S.M.; Strietzel, F.P. Sinus floor elevation and augmentation using synthetic nanocrystalline and nanoporous hydroxyapatite bone substitute materials: Preliminary histologic results. Int. J. Oral Maxillofac. Implants 2016, 31, 1281–1291. [Google Scholar] [CrossRef]
- Khaled, H.; Atef, M.; Hakam, M. Maxillary sinus floor elevation using hydroxyapatite nano particles vs tenting technique with simultaneous implant placement: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2019, 21, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, J.S.; Kim, J.E.; Lee, M.H.; Jeon, J.G.; Park, I.S.; Yi, H.K. Nanoparticle mediated PPARgamma gene delivery on dental implants improves osseointegration via mitochondrial biogenesis in diabetes mellitus rat model. Nanomedicine 2017, 13, 1821–1832. [Google Scholar] [CrossRef] [PubMed]
- Takanche, J.S.; Kim, J.E.; Kim, J.S.; Lee, M.H.; Jeon, J.G.; Park, I.S.; Yi, H.K. Chitosan-gold nanoparticles mediated gene delivery of c-myb facilitates osseointegration of dental implants in ovariectomized rat. Artif. Cells Nanomed. Biotechnol. 2018, 46, S807–S817. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Wang, X.; Xiao, S.; Zhang, G.; Li, M.; Wang, P.; Shi, Q.; Qiao, P.; E, L.; Liu, H. Osseointegration of layer-by-layer polyelectrolyte multilayers loaded with IGF1 and coated on titanium implant under osteoporotic condition. Int. J. Nanomed. 2017, 12, 7709–7720. [Google Scholar] [CrossRef]
- Iero, P.T.; Mulherin, D.R.; Jensen, O.; Berry, T.; Danesi, H.; Razook, S.J. A prospective, randomized, open-label study comparing an opioid-sparing postsurgical pain management protocol with and without liposomal bupivacaine for full-arch implant surgery. Int. J. Oral Maxillofac. Implant. 2018, 33, 1155–1164. [Google Scholar] [CrossRef]
- Totu, E.E.; Nechifor, A.C.; Nechifor, G.; Aboul-Enein, H.Y.; Cristache, C.M. Poly(methyl methacrylate) with TiO2 nanoparticles inclusion for stereolitographic complete denture manufacturing-the fututre in dental care for elderly edentulous patients? J. Dent. 2017, 59, 68–77. [Google Scholar] [CrossRef]
- Carlsson, G.E.; Omar, R. The future of complete dentures in oral rehabilitation. A critical review. J. Oral Rehabil. 2010, 37, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, F.A.; Abdel-Halim, M.S.; Gad, M.M.; Fouda, S.M.; Baba, N.Z.; AlRumaih, H.S.; Akhtar, S. Effect of nanodiamond addition on flexural strength, impact strength, and surface roughness of PMMA denture base. J. Prosthodont. 2019, 28, e417–e425. [Google Scholar] [CrossRef] [PubMed]
- Cevik, P.; Yildirim-Bicer, A.Z. The effect of silica and prepolymer nanoparticles on the mechanical properties of denture base acrylic resin. J. Prosthodont. 2018, 27, 763–770. [Google Scholar] [CrossRef]
- Gad, M.M.; Al-Thobity, A.M.; Rahoma, A.; Abualsaud, R.; Al-Harbi, F.A.; Akhtar, S. Reinforcement of PMMA denture base material with a mixture of ZrO2 nanoparticles and glass fibers. Int. J. Dent. 2019, 2019, 2489393. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R.; Rahoma, A.; Al-Thobity, A.M.; Al-Abidi, K.S.; Akhtar, S. Effect of zirconium oxide nanoparticles addition on the optical and tensile properties of polymethyl methacrylate denture base material. Int. J. Nanomed. 2018, 13, 283–292. [Google Scholar] [CrossRef]
- Oyar, P.; Sana, F.A.; Nasseri, B.; Durkan, R. Effect of green gold nanoparticles synthesized with plant on the flexural strength of heat-polymerized acrylic resin. Niger. J. Clin. Pract. 2018, 21, 1291–1295. [Google Scholar] [CrossRef]
- Karci, M.; Demir, N.; Yazman, S. Evaluation of flexural strength of different denture base materials reinforced with different nanoparticles. J. Prosthodont. 2019, 28, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Han, Z.; Huang, Z.; Karki, J.; Wang, C.; Zhu, B.; Zhang, X. Antibacterial activity, cytotoxicity and mechanical behavior of nano-enhanced denture base resin with different kinds of inorganic antibacterial agents. Dent. Mater. J. 2017, 36, 693–699. [Google Scholar] [CrossRef]
- Koroglu, A.; Sahin, O.; Kurkcuoglu, I.; Dede, D.O.; Ozdemir, T.; Hazer, B. Silver nanoparticle incorporation effect on mechanical and thermal properties of denture base acrylic resins. J. Appl. Oral Sci. 2016, 24, 590–596. [Google Scholar] [CrossRef]
- Begum, S.S.; Ajay, R.; Devaki, V.; Divya, K.; Balu, K.; Kumar, P.A. Impact strength and dimensional accuracy of heat-cure denture base resin reinforced with ZrO2 nanoparticles: An in vitro study. J. Pharm. Bioallied Sci. 2019, 11, S365–s370. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, T.; Akiba, N.; Inokoshi, M.; Shimada, M.; Minakuchi, S. Hydrophilic nano-silica coating agents with platinum and diamond nanoparticles for denture base materials. Dent. Mater. J 2017, 36, 333–339. [Google Scholar] [CrossRef]
- Gad, M.M.; Rahoma, A.; Al-Thobity, A.M.; ArRejaie, A.S. Influence of incorporation of ZrO2 nanoparticles on the repair strength of polymethyl methacrylate denture bases. Int. J. Nanomed. 2016, 11, 5633–5643. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.; ArRejaie, A.S.; Abdel-Halim, M.S.; Rahoma, A. The reinforcement effect of nano-zirconia on the transverse strength of repaired acrylic denture base. Int. J. Dent. 2016, 2016, 7094056. [Google Scholar] [CrossRef]
- Gad, M.M.; Al-Thobity, A.M.; Shahin, S.Y.; Alsaqer, B.T.; Ali, A.A. Inhibitory effect of zirconium oxide nanoparticles on Candida albicans adhesion to repaired polymethyl methacrylate denture bases and interim removable prostheses: A new approach for denture stomatitis prevention. Int. J. Nanomed. 2017, 12, 5409–5419. [Google Scholar] [CrossRef]
- Jiangkongkho, P.; Arksornnukit, M.; Takahashi, H. The synthesis, modification, and application of nanosilica in polymethyl methacrylate denture base. Dent. Mater. J 2018, 37, 582–591. [Google Scholar] [CrossRef]
- Anaraki, M.R.; Jangjoo, A.; Alimoradi, F.; Maleki Dizaj, S.; Lotfipour, F. Comparison of antifungal properties of acrylic resin reinforced with ZnO and Ag nanoparticles. Pharm. Sci. 2017, 23, 207–214. [Google Scholar] [CrossRef]
- Cierech, M.; Osica, I.; Kolenda, A.; Wojnarowicz, J.; Szmigiel, D.; Lojkowski, W.; Kurzydlowski, K.; Ariga, K.; Mierzwinska-Nastalska, E. Mechanical and physicochemical properties of newly formed ZnO-PMMA nanocomposites for denture bases. Nanomaterials 2018, 8, 305. [Google Scholar] [CrossRef]
- Salahuddin, N.; El-Kemary, M.; Ibrahim, E. Reinforcement of polymethyl methacrylate denture base resin with ZnO nanostructures. Int. J. Appl. Ceram. Technol. 2017, 15. [Google Scholar] [CrossRef]
- Anwander, M.; Rosentritt, M.; Schneider-Feyrer, S.; Hahnel, S. Biofilm formation on denture base resin including ZnO, CaO, and TiO2 nanoparticles. J. Adv. Prosthodont. 2017, 9, 482–485. [Google Scholar] [CrossRef]
- Kamonkhantikul, K.; Arksornnukit, M.; Takahashi, H. Antifungal, optical, and mechanical properties of polymethylmethacrylate material incorporated with silanized zinc oxide nanoparticles. Int. J. Nanomed. 2017, 12, 2353–2360. [Google Scholar] [CrossRef]
- Ashour, M.; El-Shennawy, M.; Althomali, Y.; Omar, A. Effect of Titanium dioxide nano particles incorporation on mechanical and physical properties on two different types of acrylic resin denture base. World J. Nano Sci. Eng. 2016, 06, 111–119. [Google Scholar] [CrossRef]
- Ghahremani, L.; Shirkavand, S.; Akbari, F.; Sabzikari, N. Tensile strength and impact strength of color modified acrylic resin reinforced with titanium dioxide nanoparticles. J. Clin. Exp. Dent. 2017, 9, e661–e665. [Google Scholar] [CrossRef] [PubMed]
- Ghahremanloo, A.; Movahedzadeh, M. The effect of silver nano particles on Candida albicans and streptococcus mutans in denture acrylic resins. J. Dent. Mater. Tech. 2016, 5, 23–30. [Google Scholar] [CrossRef]
- Li, Z.; Sun, J.; Lan, J.; Qi, Q. Effect of a denture base acrylic resin containing silver nanoparticles on Candida albicans adhesion and biofilm formation. Gerodontology 2016, 33, 209–216. [Google Scholar] [CrossRef]
- de Castro, D.T.; Valente, M.L.; Agnelli, J.A.; Lovato da Silva, C.H.; Watanabe, E.; Siqueira, R.L.; Alves, O.L.; Holtz, R.D.; dos Reis, A.C. In vitro study of the antibacterial properties and impact strength of dental acrylic resins modified with a nanomaterial. J. Prosthet. Dent. 2016, 115, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Ardestani, Z.; Falahati, M.; Sayah Alborzi, S.; Ashrafi Khozani, M.; Rostam Khani, F.; Bahador, A. The effect of nanochitosans particles on Candida biofilm formation. Curr. Med. Mycol. 2016, 2, 28–33. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Pinto Reis, C.; Vasques Roque, L.; Baptista, M.; Rijo, P. Innovative formulation of nystatin particulate systems in toothpaste for candidiasis treatment. Pharm. Dev. Technol. 2016, 21, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Atai, Z.; Atai, M.; Amini, J.; Salehi, N. In vivo study of antifungal effects of low-molecular-weight chitosan against Candida albicans. J. Oral Sci. 2017, 59, 425–430. [Google Scholar] [CrossRef]
- Mima, E.G.; Vergani, C.E.; Machado, A.L.; Massucato, E.M.; Colombo, A.L.; Bagnato, V.S.; Pavarina, A.C. Comparison of Photodynamic Therapy versus conventional antifungal therapy for the treatment of denture stomatitis: A randomized clinical trial. Clin. Microbiol. Infect. 2012, 18, E380–E388. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Aldawsari, H.M.; Bahmdan, R.H.; Sindi, A.M.; Kurakula, M.; Alrobaian, M.M.; Aldryhim, A.Y.; Alkhalidi, H.M.; Bahmdan, H.H.; Khallaf, R.A.; et al. Preparation, optimization, and evaluation of hyaluronic acid-based hydrogel loaded with miconazole self-nanoemulsion for the treatment of oral thrush. AAPS PharmSciTech 2019, 20, 297. [Google Scholar] [CrossRef]
- Roque, L.; Alopaeus, J.; Reis, C.; Rijo, P.; Molpeceres, J.; Hagesaether, E.; Tho, I.; Reis, C. Mucoadhesive assessment of different antifungal nanoformulations. Bioinspir. Biomim. 2018, 13, 055001. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Durnas, B.; Tokajuk, G.; Piktel, E.; Michalak, G.; Gu, X.; Kulakowska, A.; Savage, P.B.; Bucki, R. Formulation and candidacidal activity of magnetic nanoparticles coated with cathelicidin LL-37 and ceragenin CSA-13. Sci. Rep. 2017, 7, 4610. [Google Scholar] [CrossRef]
- de Carvalho, F.G.; Magalhaes, T.C.; Teixeira, N.M.; Gondim, B.L.C.; Carlo, H.L.; Dos Santos, R.L.; de Oliveira, A.R.; Denadai, A.M.L. Synthesis and characterization of TPP/chitosan nanoparticles: Colloidal mechanism of reaction and antifungal effect on C. albicans biofilm formation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109885. [Google Scholar] [CrossRef] [PubMed]
- Gondim, B.L.C.; Castellano, L.R.C.; de Castro, R.D.; Machado, G.; Carlo, H.L.; Valenca, A.M.G.; de Carvalho, F.G. Effect of chitosan nanoparticles on the inhibition of Candida spp. biofilm on denture base surface. Arch. Oral Biol. 2018, 94, 99–107. [Google Scholar] [CrossRef]
- Tan, Y.; Leonhard, M.; Moser, D.; Schneider-Stickler, B. Antibiofilm activity of carboxymethyl chitosan on the biofilms of non-Candida albicans Candida species. Carbohydr. Polym. 2016, 149, 77–82. [Google Scholar] [CrossRef]
- Tonglairoum, P.; Woraphatphadung, T.; Ngawhirunpat, T.; Rojanarata, T.; Akkaramongkolporn, P.; Sajomsang, W.; Opanasopit, P. Development and evaluation of N-naphthyl-N,O-succinyl chitosan micelles containing clotrimazole for oral candidiasis treatment. Pharm. Dev. Technol. 2017, 22, 184–190. [Google Scholar] [CrossRef]
- Fabio, C.A.; Yolanda, M.B.; Carmen, G.M.; Francisco, C.; Antonio Julian, B.; Leonor, P.L.; Jesus, S. Use of photodynamic therapy and chitosan for inactivacion of Candida albicans in a murine model. J. Oral Pathol. Med. 2016, 45, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Sakima, V.T.; Barbugli, P.A.; Cerri, P.S.; Chorilli, M.; Carmello, J.C.; Pavarina, A.C.; Mima, E.G.O. Antimicrobial photodynamic therapy mediated by curcumin-loaded polymeric nanoparticles in a murine model of oral Candidiasis. Molecules 2018, 23, 2075. [Google Scholar] [CrossRef] [PubMed]
- Cabrini Carmello, J.; Alves, F.; Basso, F.G.; de Souza Costa, C.A.; Tedesco, A.C.; Lucas Primo, F.; Mima, E.G.O.; Pavarina, A.C. Antimicrobial photodynamic therapy reduces adhesion capacity and biofilm formation of Candida albicans from induced oral candidiasis in mice. Photodiagn. Photodyn. Ther. 2019, 27, 402–407. [Google Scholar] [CrossRef]
- Mahattanadul, S.; Mustafa, M.W.; Kuadkaew, S.; Pattharachayakul, S.; Ungphaiboon, S.; Sawanyawisuth, K. Oral ulcer healing and anti-Candida efficacy of an alcohol-free chitosan-curcumin mouthwash. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7020–7023. [Google Scholar] [CrossRef]
- Mustafa, M.W.; Ungphaiboon, S.; Phadoongsombut, N.; Pangsomboon, K.; Chelae, S.; Mahattanadul, S. Effectiveness of an alcohol-free chitosan-curcuminoid mouthwash compared with chlorhexidine mouthwash in denture stomatitis treatment: A randomized trial. J. Altern. Complement. Med. 2019, 25, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Tejada, G.; Piccirilli, G.N.; Sortino, M.; Salomon, C.J.; Lamas, M.C.; Leonardi, D. Formulation and in-vitro efficacy of antifungal mucoadhesive polymeric matrices for the delivery of miconazole nitrate. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Tejada, G.; Barrera, M.G.; Piccirilli, G.N.; Sortino, M.; Frattini, A.; Salomon, C.J.; Lamas, M.C.; Leonardi, D. Development and evaluation of buccal films based on chitosan for the potential treatment of oral candidiasis. AAPS PharmSciTech 2017, 18, 936–946. [Google Scholar] [CrossRef]
- Kenechukwu, F.C.; Attama, A.A.; Ibezim, E.C. Novel solidified reverse micellar solution-based mucoadhesive nano lipid gels encapsulating miconazole nitrate-loaded nanoparticles for improved treatment of oropharyngeal candidiasis. J. Microencapsul. 2017, 34, 592–609. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.; He, Y.; Chen, Z.; Liu, B.; Emesto, C.S.; Yang, G.; Wang, W.; Zhang, J.; Lin, R. Preparation, characterization and toxicity evaluation of amphotericin B loaded MPEG-PCL micelles and its application for buccal tablets. Appl. Microbiol. Biotechnol. 2017, 101, 7357–7370. [Google Scholar] [CrossRef]
- Rencber, S.; Karavana, S.Y.; Yilmaz, F.F.; Erac, B.; Nenni, M.; Ozbal, S.; Pekcetin, C.; Gurer-Orhan, H.; Hosgor-Limoncu, M.; Guneri, P.; et al. Development, characterization, and in vivo assessment of mucoadhesive nanoparticles containing fluconazole for the local treatment of oral candidiasis. Int. J. Nanomed. 2016, 11, 2641–2653. [Google Scholar] [CrossRef]
- Niyogi, P.; Pattnaik, S.; Maharana, L.; Mohapatra, R.; Haldar, S. Temperature-dependent mucosal permeation kinetics of stigmasterol microspheres: In vivo mice model antioral candidiasis study. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1636–1654. [Google Scholar] [CrossRef]
- Roque, L.; Duarte, N.; Bronze, M.R.; Garcia, C.; Alopaeus, J.; Molpeceres, J.; Hagesaether, E.; Tho, I.; Rijo, P.; Reis, C. Development of a bioadhesive nanoformulation with Glycyrrhiza glabra L. extract against Candida albicans. Biofouling 2018, 34, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Wang, R.S.; Hsu, Y.C.; Chuang, C.C.; Chan, H.R.; Chiu, H.C.; Wang, Y.B.; Chen, K.Y.; Fu, E. Antifungal effect of tissue conditioners containing poly(acryloyloxyethyltrimethyl ammonium chloride)-grafted chitosan on Candida albicans growth in vitro. J. Dent. Sci. 2018, 13, 160–166. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Ghotaslou, R.; Kordi, S.; Khoramdel, A.; Aeenfar, A.; Kahjough, S.T.; Akbarzadeh, A. Antibacterial and antifungal effects of chitosan nanoparticles on tissue conditioners of complete dentures. Int. J. Biol. Macromol. 2018, 118, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Haider, A.; Zahid, S.; Khan, S.A.; Faryal, R.; Kaleem, M. In-vitro antifungal efficacy of tissue conditioner-chitosan composites as potential treatment therapy for denture stomatitis. Int. J. Biol. Macromol. 2019, 125, 761–766. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Ghotaslou, R.; Akbarzadeh, A.; Azima, N.; Aeinfar, A.; Khorramdel, A. Evaluation of antibacterial and antifungal properties of a tissue conditioner used in complete dentures after incorporation of ZnOAg nanoparticles. J. Dent. Res. Dent. Clin. Dent. Prospects 2019, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Herla, M.; Boening, K.; Meissner, H.; Walczak, K. Mechanical and surface properties of resilient denture liners modified with chitosan salts. Materials 2019, 12, 3518. [Google Scholar] [CrossRef]
- Namangkalakul, W.; Benjavongkulchai, S.; Pochana, T.; Promchai, A.; Satitviboon, W.; Howattanapanich, S.; Phuprasong, R.; Ungvijanpunya, N.; Supakanjanakanti, D.; Chaitrakoonthong, T.; et al. Activity of chitosan antifungal denture adhesive against common Candida species and Candida albicans adherence on denture base acrylic resin. J. Prosthet. Dent. 2020, 123, 181.e1–181.e7. [Google Scholar] [CrossRef] [PubMed]
- Zambom, C.R.; da Fonseca, F.H.; Crusca, E., Jr.; da Silva, P.B.; Pavan, F.R.; Chorilli, M.; Garrido, S.S. A novel antifungal system with potential for prolonged delivery of histatin 5 to limit growth of Candida albicans. Front. Microbiol. 2019, 10, 1667. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, Y.M.; Lee, J.K.; Kim, N.H.; Kim, E.J.; Heo, H.; Lee, M.Y.; Lee, J.R.; Jang, M.K. Targeting and synergistic action of an antifungal peptide in an antibiotic drug-delivery system. J. Control. Release 2017, 256, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.A.; Vinayagam, R.; Senthilkumar, V.; Paulpandi, M.; Murugan, K.; Xu, B.; Gothandam, K.M.; Kotakadi, V.S.; David, E. Phloretin loaded chitosan nanoparticles augments the pH-dependent mitochondrial-mediated intrinsic apoptosis in human oral cancer cells. Int. J. Biol. Macromol. 2019, 130, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Teng, Y.; Seyiti, P.; Yen, Y.; Qian, H.; Xie, C.; Li, R.; Lin, Z. Using omniscan-loaded nanoparticles as a tumor-targeted MRI contrast agent in oral squamous cell carcinoma by gelatinase-stimuli strategy. Nanoscale Res. Lett. 2019, 14, 395. [Google Scholar] [CrossRef]
- Baldea, I.; Florea, A.; Olteanu, D.; Clichici, S.; David, L.; Moldovan, B.; Cenariu, M.; Achim, M.; Suharoschi, R.; Danescu, S.; et al. Effects of silver and gold nanoparticles phytosynthesized with Cornus mas extract on oral dysplastic human cells. Nanomedicine 2020, 15, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Narayanan, S.; Balasubramanian, G.; Sethuraman, S.; Krishnan, U.M. Dual drug loaded nanoliposomal chemotherapy: A promising strategy for treatment of head and neck squamous cell carcinoma. Eur. J. Pharm. Biopharm. 2016, 99, 73–83. [Google Scholar] [CrossRef]
- Suliman, S.; Mustafa, K.; Krueger, A.; Steinmüller-Nethl, D.; Finne-Wistrand, A.; Osdal, T.; Hamza, A.O.; Sun, Y.; Parajuli, H.; Waag, T.; et al. Nanodiamond modified copolymer scaffolds affects tumour progression of early neoplastic oral keratinocytes. Biomaterials 2016, 95, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Banerjee, P.P.; Sadhu, A.; Sengupta, A.; Chatterjee, S.; Sarkar, S.; Barman, S.; Chattopadhyay, A.; Battacharya, S.; Mondal, N.C.; et al. Silver nanoparticles as antibacterial and anticancer materials against human breast, cervical and oral cancer cells. J. Nanosci. Nanotechnol. 2017, 17, 968–976. [Google Scholar] [CrossRef]
- Maheswari, P.; Harish, S.; Navaneethan, M.; Muthamizhchelvan, C.; Ponnusamy, S.; Hayakawa, Y. Bio-modified TiO(2) nanoparticles with Withania somnifera, Eclipta prostrata and Glycyrrhiza glabra for anticancer and antibacterial applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110457. [Google Scholar] [CrossRef]
- Mehnath, S.; Arjama, M.; Rajan, M.; Annamalai, G.; Jeyaraj, M. Co-encapsulation of dual drug loaded in MLNPs: Implication on sustained drug release and effectively inducing apoptosis in oral carcinoma cells. Biomed. Pharmacother. 2018, 104, 661–671. [Google Scholar] [CrossRef]
- Neshastehriz, A.; Tabei, M.; Maleki, S.; Eynali, S.; Shakeri-Zadeh, A. Photothermal therapy using folate conjugated gold nanoparticles enhances the effects of 6MV X-ray on mouth epidermal carcinoma cells. J. Photochem. Photobiol. B 2017, 172, 52–60. [Google Scholar] [CrossRef]
- Poojari, R.; Kini, S.; Srivastava, R.; Panda, D. Intracellular interactions of electrostatically mediated layer-by-layer assembled polyelectrolytes based sorafenib nanoparticles in oral cancer cells. Colloids Surf. B Biointerfaces 2016, 143, 131–138. [Google Scholar] [CrossRef]
- Bonan, R.F.; Mota, M.F.; da Costa Farias, R.M.; da Silva, S.D.; Bonan, P.R.F.; Diesel, L.; Menezes, R.R.; da Cruz Perez, D.E. In vitro antimicrobial and anticancer properties of TiO(2) blow-spun nanofibers containing silver nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109876. [Google Scholar] [CrossRef] [PubMed]
- Furqan, M.; Huma, Z.; Ashfaq, Z.; Nasir, A.; Ullah, R.; Bilal, A.; Iqbal, M.; Khalid, M.H.; Hussain, I.; Faisal, A. Identification and evaluation of novel drug combinations of Aurora kinase inhibitor CCT137690 for enhanced efficacy in oral cancer cells. Cell Cycle 2019, 18, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I.; Chronopoulou, L.; Palocci, C.; Amalfitano, A.; Cantiani, M.; Cordaro, M.; Lajolo, C.; Callà, C.; Boninsegna, A.; Lucchetti, D.; et al. Controlled release of 18-β-glycyrrhetic acid by nanodelivery systems increases cytotoxicity on oral carcinoma cell line. Nanotechnology 2018, 29, 285101. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.X.; Wu, Y.N.; Wang, P.W.; Su, W.C.; Shieh, D.B. Iron release profile of silica-modified zero-valent iron NPs and their implication in cancer therapy. Int. J. Mol. Sci. 2019, 20, 4336. [Google Scholar] [CrossRef]
- Moosavi Nejad, S.; Takahashi, H.; Hosseini, H.; Watanabe, A.; Endo, H.; Narihira, K.; Kikuta, T.; Tachibana, K. Acute effects of sono-activated photocatalytic titanium dioxide nanoparticles on oral squamous cell carcinoma. Ultrason. Sonochem. 2016, 32, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Lee, S.Y.; Cho, H.J. Phloretin-loaded fast dissolving nanofibers for the locoregional therapy of oral squamous cell carcinoma. J. Colloid. Interface Sci. 2017, 508, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.C.; Chueh, F.S.; Hsiao, Y.T.; Cheng, Z.Y.; Lien, J.C.; Liu, K.C.; Peng, S.F.; Chung, J.G. Gefitinib and curcumin-loaded nanoparticles enhance cell apoptosis in human oral cancer SAS cells in vitro and inhibit SAS cell xenografted tumor in vivo. Toxicol. Appl. Pharmacol. 2019, 382, 114734. [Google Scholar] [CrossRef]
- Murata, T.; Kutsuna, T.; Kurohara, K.; Shimizu, K.; Tomeoku, A.; Arai, N. Evaluation of a new hydroxyapatite nanoparticle as a drug delivery system to oral squamous cell carcinoma cells. Anticancer Res. 2018, 38, 6715–6720. [Google Scholar] [CrossRef]
- Jin, B.Z.; Dong, X.Q.; Xu, X.; Zhang, F.H. Development and in vitro evaluation of mucoadhesive patches of methotrexate for targeted delivery in oral cancer. Oncol. Lett. 2018, 15, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yin, X.; Cai, Y.; Xu, W.; Song, C.; Wang, Y.; Zhang, J.; Kang, A.; Wang, Z.; Han, W. Antitumor effect of a Pt-loaded nanocomposite based on graphene quantum dots combats hypoxia-induced chemoresistance of oral squamous cell carcinoma. Int. J. Nanomed. 2018, 13, 1505–1524. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Q.; Chen, J.; Wang, Z.; Xin, H.; Zhang, D. Efficient delivery of therapeutic siRNA by Fe(3)O(4) magnetic nanoparticles into oral cancer cells. Pharmaceutics 2019, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Lam, T.; Chen, A.; Jensen, C.; Duncan, L.; Kong, F.C.; Kurago, Z.B.; Shay, C.; Teng, Y. Circumventing AKT-associated radioresistance in oral cancer by novel nanoparticle-encapsulated capivasertib. Cells 2020, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, L.; Huang, Y.; Liu, B.; Chi, H.; Shi, L.; Zhang, W.; Li, G.; Niu, Y.; Zhu, X. Synergistic therapy of chemotherapeutic drugs and MTH1 inhibitors using a pH-sensitive polymeric delivery system for oral squamous cell carcinoma. Biomater. Sci. 2017, 5, 2068–2078. [Google Scholar] [CrossRef]
- Shi, X.L.; Li, Y.; Zhao, L.M.; Su, L.W.; Ding, G. Delivery of MTH1 inhibitor (TH287) and MDR1 siRNA via hyaluronic acid-based mesoporous silica nanoparticles for oral cancers treatment. Colloids Surf. B Biointerfaces 2019, 173, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, S.; Wang, S.; Xu, Z.; Wei, L. Zinc oxide nanoparticles induce toxicity in CAL 27 oral cancer cell lines by activating PINK1/Parkin-mediated mitophagy. Int. J. Nanomed. 2018, 13, 3441–3450. [Google Scholar] [CrossRef]
- Kowshik, J.; Nivetha, R.; Ranjani, S.; Venkatesan, P.; Selvamuthukumar, S.; Veeravarmal, V.; Nagini, S. Astaxanthin inhibits hallmarks of cancer by targeting the PI3K/NF-κΒ/STAT3 signalling axis in oral squamous cell carcinoma models. IUBMB Life 2019, 71, 1595–1610. [Google Scholar] [CrossRef]
- Legge, C.J.; Colley, H.E.; Lawson, M.A.; Rawlings, A.E. Targeted magnetic nanoparticle hyperthermia for the treatment of oral cancer. J. Oral Pathol. Med. 2019, 48, 803–809. [Google Scholar] [CrossRef]
- Nayak, A.; Siddharth, S.; Das, S.; Nayak, D.; Sethy, C.; Kundu, C.N. Nanoquinacrine caused apoptosis in oral cancer stem cells by disrupting the interaction between GLI1 and β catenin through activation of GSK3β. Toxicol. Appl. Pharmacol. 2017, 330, 53–64. [Google Scholar] [CrossRef]
- Satapathy, S.R.; Nayak, A.; Siddharth, S.; Das, S.; Nayak, D.; Kundu, C.N. Metallic gold and bioactive quinacrine hybrid nanoparticles inhibit oral cancer stem cell and angiogenesis by deregulating inflammatory cytokines in p53 dependent manner. Nanomedicine 2018, 14, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Singh, M.; Kumar, R.; Belz, J.; Shanker, R.; Dwivedi, P.D.; Sridhar, S.; Singh, S.P. Synthesis and in vitro studies of PLGA-DTX nanoconjugate as potential drug delivery vehicle for oral cancer. Int. J. Nanomed. 2018, 13, 67–69. [Google Scholar] [CrossRef]
- Hembram, K.C.; Chatterjee, S.; Sethy, C.; Nayak, D.; Pradhan, R.; Molla, S.; Bindhani, B.K.; Kundu, C.N. Comparative and mechanistic study on the anticancer activity of quinacrine-based silver and gold hybrid nanoparticles in head and neck cancer. Mol. Pharm. 2019, 16, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Matos, B.N.; Pereira, M.N.; Bravo, M.O.; Cunha-Filho, M.; Saldanha-Araújo, F.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles loading oxaliplatin as a mucoadhesive topical treatment of oral tumors: Iontophoresis further enhances drug delivery ex vivo. Int. J. Biol. Macromol. 2020, 154, 1265–1275. [Google Scholar] [CrossRef]
- Dziedzic, A.; Kubina, R.; Bułdak, R.J.; Skonieczna, M.; Cholewa, K. Silver nanoparticles exhibit the dose-dependent anti-proliferative effect against human squamous carcinoma cells attenuated in the presence of berberine. Molecules 2016, 21, 365. [Google Scholar] [CrossRef]
- Srivastava, S.; Gupta, S.; Mohammad, S.; Ahmad, I. Development of α-tocopherol surface-modified targeted delivery of 5-fluorouracil-loaded poly-D, L-lactic-co-glycolic acid nanoparticles against oral squamous cell carcinoma. J. Cancer Res. Ther. 2019, 15, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Mohammad, S.; Pant, A.B.; Mishra, P.R.; Pandey, G.; Gupta, S.; Farooqui, S. Co-delivery of 5-fluorouracil and curcumin nanohybrid formulations for improved chemotherapy against oral squamous cell carcinoma. J. Maxillofac. Oral Surg. 2018, 17, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Rathinaraj, P.; Muthusamy, G.; Prasad, N.R.; Gunaseelan, S.; Kim, B.; Zhu, S. Folate-gold-bilirubin nanoconjugate induces apoptotic death in multidrug-resistant oral carcinoma cells. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 285–296. [Google Scholar] [CrossRef] [PubMed]
- El-Hamid, E.S.A.; Gamal-Eldeen, A.M.; Sharaf Eldeen, A.M. Liposome-coated nano doxorubicin induces apoptosis on oral squamous cell carcinoma CAL-27 cells. Arch. Oral Biol. 2019, 103, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, R.; Sahu, B.P.; Haloi, J.; Laloo, D.; Barooah, P.; Keppen, C.; Deka, M.; Medhi, S. Combinatorial therapeutic approach for treatment of oral squamous cell carcinoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 572–585. [Google Scholar] [CrossRef]
- Shtenberg, Y.; Goldfeder, M.; Prinz, H.; Shainsky, J.; Ghantous, Y.; Abu El-Naaj, I.; Schroeder, A.; Bianco-Peled, H. Mucoadhesive alginate pastes with embedded liposomes for local oral drug delivery. Int. J. Biol. Macromol. 2018, 111, 62–69. [Google Scholar] [CrossRef]
- Moradzadeh Khiavi, M.; Anvari, E.; Hamishehkar, H.; Abdal, K. Assessment of the blood parameters, cardiac and liver Enzymes in oral squamous cell carcinoma following treated with injectable doxorubicin-loaded nano-particles. Asian Pac. J. Cancer Prev. 2019, 20, 1973–1977. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wen, Y.; You, X.; Zhang, F.; Shah, V.; Chen, X.; Tong, D.; Wei, X.; Yin, L.; Wu, J.; et al. Development of a reactive oxygen species (ROS)-responsive nanoplatform for targeted oral cancer therapy. J. Mater. Chem. B 2016, 4, 4675–4682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, G.; Li, Z.; Shi, S.; Chen, B.; Li, C.; Zhang, L.; Wang, Y. PEGylated doxorubicin nanoparticles mediated by HN-1 peptide for targeted treatment of oral squamous cell carcinoma. Int. J. Pharm. 2017, 525, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, L.; Zhu, M.; Wan, G.; Li, C.; Zhang, J.; Wang, Y.; Wang, Y. Reactive oxygen species-responsive nanoparticles based on peglated prodrug for targeted treatment of oral tongue squamous cell carcinoma by combining photodynamic therapy and chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 29260–29272. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, X.; Zhang, K.; Sun, B.; Wang, L.; Meng, L.; Liu, Q.; Zheng, C.; Yang, B.; Sun, H. Codelivery of doxorubicin and MDR1-siRNA by mesoporous silica nanoparticles-polymerpolyethylenimine to improve oral squamous carcinoma treatment. Int. J. Nanomed. 2018, 13, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Feng, J.; Qiu, L.; Gao, Z.; Li, P.; Pang, L.; Zhang, Z. SDF-1-loaded PLGA nanoparticles for the targeted photoacoustic imaging and photothermal therapy of metastatic lymph nodes in tongue squamous cell carcinoma. Int. J. Pharm. 2019, 554, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, J.; Zhu, B.; Xu, Q. Development of a multifunctional gold nanoplatform for combined chemo-photothermal therapy against oral cancer. Nanomedicine 2020, 15, 661–676. [Google Scholar] [CrossRef]
- Ankri, R.; Ashkenazy, A.; Milstein, Y.; Brami, Y.; Olshinka, A.; Goldenberg-Cohen, N.; Popovtzer, A.; Fixler, D.; Hirshberg, A. Gold nanorods based air scanning electron microscopy and diffusion reflection imaging for mapping tumor margins in squamous cell carcinoma. ACS Nano 2016, 10, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Viveka, T.S.; Arvind, K.; Shyamsundar, V.; Kanchan, M.; Alex, S.A.; Chandrasekaran, N.; Vijayalakshmi, R.; Mukherjee, A. A facile gold nanoparticle-based ELISA system for detection of osteopontin in saliva: Towards oral cancer diagnostics. Clin. Chim. Acta 2018, 477, 166–172. [Google Scholar] [CrossRef]
- Kim, C.S.; Ingato, D.; Wilder-Smith, P.; Chen, Z.; Kwon, Y.J. Stimuli-disassembling gold nanoclusters for diagnosis of early stage oral cancer by optical coherence tomography. Nano Converg. 2018, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yan, B.; Xue, L.; Li, Y.; Luo, X.; Ji, P. Surface-enhanced Raman spectroscopy of blood serum based on gold nanoparticles for the diagnosis of the oral squamous cell carcinoma. Lipids Health Dis. 2017, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Yan, B.; Li, Y.; Tan, Y.; Luo, X.; Wang, M. Surface-enhanced Raman spectroscopy of blood serum based on gold nanoparticles for tumor stages detection and histologic grades classification of oral squamous cell carcinoma. Int. J. Nanomed. 2018, 13, 4977–4986. [Google Scholar] [CrossRef] [PubMed]
- Fălămaș, A.; Rotaru, H.; Hedeșiu, M. Surface-enhanced Raman spectroscopy (SERS) investigations of saliva for oral cancer diagnosis. Lasers Med. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, A.; Shukla, A.; Kaswan, J.; Arora, K.; Ramirez-Vick, J.; Singh, P.; Singh, S.P. Anti-IL8/AuNPs-rGO/ITO as an Immunosensing Platform for Noninvasive Electrochemical Detection of Oral Cancer. ACS Appl. Mater. Interfaces 2017, 9, 27462–27474. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, B.; Zhou, J.; Shi, X.; Sha, Y.; Wu, H. Assessment of lingual sentinel lymph nodes metastases using dual-modal indirect CT/MR lymphography with gold-gadolinium-based nanoprobes in a tongue VX(2) carcinoma model. Acta Otolaryngol. 2018, 138, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Chen, C.W.; Chan, M.H.; Chang, Y.C.; Chang, W.M.; Chi, L.H.; Yu, H.M.; Lin, Y.F.; Tsai, D.P.; Liu, R.S.; et al. MMP2-sensing up-conversion nanoparticle for fluorescence biosensing in head and neck cancer cells. Biosens. Bioelectron. 2016, 80, 131–139. [Google Scholar] [CrossRef]
- Wu, W.J.; Zheng, L.; Zhang, J.G. The use of carbon nanoparticles to track occult lingual lymph nodes in early-stage tongue squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2019, 48, 1153–1155. [Google Scholar] [CrossRef] [PubMed]
- Moisoiu, V.; Stefancu, A.; Gulei, D.; Boitor, R.; Magdo, L.; Raduly, L.; Pasca, S.; Kubelac, P.; Mehterov, N.; Chiș, V.; et al. SERS-based differential diagnosis between multiple solid malignancies: Breast, colorectal, lung, ovarian and oral cancer. Int. J. Nanomed. 2019, 14, 6165–6178. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosens. Bioelectron. 2016, 78, 497–504. [Google Scholar] [CrossRef]
- Chundayil Madathil, G.; Iyer, S.; Thankappan, K.; Gowd, G.S.; Nair, S.; Koyakutty, M. A novel surface enhanced Raman catheter for rapid detection, classification, and grading of oral cancer. Adv. Healthc. Mater. 2019, 8, e1801557. [Google Scholar] [CrossRef]
- Kumar, S.; Panwar, S.; Kumar, S.; Augustine, S.; Malhotra, B.D. Biofunctionalized nanostructured yttria modified non-invasive impedometric biosensor for efficient detection of oral cancer. Nanomaterials 2019, 9, 1190. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Shay, C.; Zhao, X.; Xiong, Y.; Wang, X.; Teng, Y. Simultaneously inactivating Src and AKT by saracatinib/capivasertib co-delivery nanoparticles to improve the efficacy of anti-Src therapy in head and neck squamous cell carcinoma. J. Hematol. Oncol. 2019, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Liu, D.; Chen, L.; Zhang, J.; Ru, L.; Chen, Z.; Gao, Z.; Wang, X. CD44-targeted magnetic nanoparticles kill head and neck squamous cell carcinoma stem cells in an alternating magnetic field. Int. J. Nanomed. 2019, 14, 7549–7560. [Google Scholar] [CrossRef]
- Mizrachi, A.; Shamay, Y.; Shah, J.; Brook, S.; Soong, J.; Rajasekhar, V.K.; Humm, J.L.; Healey, J.H.; Powell, S.N.; Baselga, J.; et al. Tumour-specific PI3K inhibition via nanoparticle-targeted delivery in head and neck squamous cell carcinoma. Nat. Commun. 2017, 8, 14292. [Google Scholar] [CrossRef]
- Damiani, V.; Falvo, E.; Fracasso, G.; Federici, L.; Pitea, M.; De Laurenzi, V.; Sala, G.; Ceci, P. Therapeutic efficacy of the novel stimuli-sensitive nano-ferritins containing doxorubicin in a head and neck cancer model. Int. J. Mol. Sci. 2017, 18, 1555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhuang, L.; Lin, Y.; Yan, M.; Lv, J.; Li, X.; Lin, H.; Zhu, P.; Lin, Q.; Xu, Y. Novel drug delivery system based on hollow mesoporous magnetic nanoparticles for head and neck cancers--targeted therapy in vitro and in vivo. Am. J. Cancer Res. 2020, 10, 350–364. [Google Scholar] [PubMed]
- Lang, L.; Shay, C.; Xiong, Y.; Thakkar, P.; Chemmalakuzhy, R.; Wang, X.; Teng, Y. Combating head and neck cancer metastases by targeting Src using multifunctional nanoparticle-based saracatinib. J. Hematol. Oncol. 2018, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, L.; Wei, J.; Zhang, C.; Zhou, Z.; Wu, L.; Liu, W. Cellular uptake and anticancer activity of salvianolic acid B phospholipid complex loaded nanoparticles in head and neck cancer and precancer cells. Colloids Surf. B Biointerfaces 2016, 147, 65–72. [Google Scholar] [CrossRef]
- Teraoka, S.; Kakei, Y.; Akashi, M.; Iwata, E.; Hasegawa, T.; Miyawaki, D.; Sasaki, R.; Komori, T. Gold nanoparticles enhance X-ray irradiation-induced apoptosis in head and neck squamous cell carcinoma in vitro. Biomed. Rep. 2018, 9, 415–420. [Google Scholar] [CrossRef]
- Lecaros, R.L.; Huang, L.; Lee, T.C.; Hsu, Y.C. Nanoparticle delivered VEGF-A siRNA enhances photodynamic therapy for head and neck cancer treatment. Mol. Ther. 2016, 24, 106–116. [Google Scholar] [CrossRef]
- Takeuchi, I.; Kamiki, Y.; Makino, K. Therapeutic efficacy of rebamipide-loaded PLGA nanoparticles coated with chitosan in a mouse model for oral mucositis induced by cancer chemotherapy. Colloids Surf. B Biointerfaces 2018, 167, 468–473. [Google Scholar] [CrossRef]
- Choi, J.S.; Han, S.H.; Hyun, C.; Yoo, H.S. Buccal adhesive nanofibers containing human growth hormone for oral mucositis. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Togo, C.; Makino, K. Rebamipide-containing film using chitosan and HPMC for oral mucositis induced by cancer chemotherapy. Anticancer Res. 2019, 39, 6531–6536. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Dean, S.M.; Brown, C.T.; Smith, R.J.; Lei, P.; Andreadis, S.T.; Baker, O.J. Synergistic effects of laminin-1 peptides, VEGF and FGF9 on salivary gland regeneration. Acta Biomater. 2019, 91, 186–194. [Google Scholar] [CrossRef]
- Nam, K.; Maruyama, C.L.; Wang, C.S.; Trump, B.G.; Lei, P.; Andreadis, S.T.; Baker, O.J. Laminin-111-derived peptide conjugated fibrin hydrogel restores salivary gland function. PLoS ONE 2017, 12, e0187069. [Google Scholar] [CrossRef]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Ther. Clin. Risk Manag. 2015, 11, 45–51. [Google Scholar] [CrossRef]
- Escobar, A.; Aitken, J. Xerostomia: An update of causes and treatments. In Salivary Glands-New Approaches in Diagnostics and Treatment; IntechOpen: London, UK, 2019. [Google Scholar]
- Adamczak, M.I.; Martinsen, Ø.G.; Smistad, G.; Hiorth, M. Water sorption properties of HM-pectin and liposomes intended to alleviate dry mouth. Int. J. Pharm. 2016, 506, 201–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adamczak, M.I.; Hagesaether, E.; Smistad, G.; Hiorth, M. An in vitro study of mucoadhesion and biocompatibility of polymer coated liposomes on HT29-MTX mucus-producing cells. Int. J. Pharm. 2016, 498, 225–233. [Google Scholar] [CrossRef]
- Adamczak, M.I.; Martinsen, Ø.G.; Smistad, G.; Hiorth, M. Polymer coated mucoadhesive liposomes intended for the management of xerostomia. Int. J. Pharm. 2017, 527, 72–78. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Duan, W.; Tran, T.T.D. Recent developments of nanoparticle-delivered dosage forms for buccal delivery. Int. J. Pharm. 2019, 571, 118697. [Google Scholar] [CrossRef]
- Hua, S. Advances in nanoparticulate drug delivery approaches for sublingual and buccal administration. Front. Pharmacol. 2019, 10, 1328. [Google Scholar] [CrossRef]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Development and evaluation of buccal films impregnated with selegiline-loaded nanospheres. Drug Deliv. 2016, 23, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhubiab, B.E. In vitro and in vivo evaluation of nano-based films for buccal delivery of zolpidem. Braz. Oral Res. 2016, 30, e126. [Google Scholar] [CrossRef]
- Castro, P.M.; Baptista, P.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Combination of PLGA nanoparticles with mucoadhesive guar-gum films for buccal delivery of antihypertensive peptide. Int. J. Pharm. 2018, 547, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Abd El Azim, H.; Nafee, N.; Ramadan, A.; Khalafallah, N. Liposomal buccal mucoadhesive film for improved delivery and permeation of water-soluble vitamins. Int. J. Pharm. 2015, 488, 78–85. [Google Scholar] [CrossRef]
- Mašek, J.; Lubasová, D.; Lukáč, R.; Turánek-Knotigová, P.; Kulich, P.; Plocková, J.; Mašková, E.; Procházka, L.; Koudelka, Š.; Sasithorn, N.; et al. Multi-layered nanofibrous mucoadhesive films for buccal and sublingual administration of drug-delivery and vaccination nanoparticles-important step towards effective mucosal vaccines. J. Control. Release 2017, 249, 183–195. [Google Scholar] [CrossRef]
- Al-Nemrawi, N.K.; Alsharif, S.S.M.; Alzoubi, K.H.; Alkhatib, R.Q. Preparation and characterization of insulin chitosan-nanoparticles loaded in buccal films. Pharm. Dev. Technol. 2019, 24, 967–974. [Google Scholar] [CrossRef]
- Kraisit, P.; Limmatvapirat, S.; Luangtana-Anan, M.; Sriamornsak, P. Buccal administration of mucoadhesive blend films saturated with propranolol loaded nanoparticles. Asian J. Pharm. Sci. 2018, 13, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Baptista, P.; Zuccheri, G.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Film-nanoparticle composite for enhanced oral delivery of alpha-casozepine. Colloids Surf. B Biointerfaces 2019, 181, 149–157. [Google Scholar] [CrossRef]
- Abozaid, D.; Ramadan, A.; Barakat, H.; Khalafallah, N. Acyclovir lipid nanocapsules gel for oromucosal delivery: A preclinical evidence of efficacy in the chicken pouch membrane model. Eur. J. Pharm. Sci. 2018, 121, 228–235. [Google Scholar] [CrossRef]
- Marques, A.C.; Rocha, A.I.; Leal, P.; Estanqueiro, M.; Lobo, J.M.S. Development and characterization of mucoadhesive buccal gels containing lipid nanoparticles of ibuprofen. Int. J. Pharm. 2017, 533, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Muniz, B.V.; Baratelli, D.; Di Carla, S.; Serpe, L.; da Silva, C.B.; Guilherme, V.A.; Ribeiro, L.N.M.; Cereda, C.M.S.; de Paula, E.; Volpato, M.C.; et al. Hybrid hydrogel composed of polymeric nanocapsules co-loading lidocaine and prilocaine for topical intraoral anesthesia. Sci. Rep. 2018, 8, 17972. [Google Scholar] [CrossRef] [PubMed]
- Mahdizadeh Barzoki, Z.; Emam-Djomeh, Z.; Mortazavian, E.; Akbar Moosavi-Movahedi, A.; Rafiee Tehrani, M. Formulation, in vitro evaluation and kinetic analysis of chitosan-gelatin bilayer muco-adhesive buccal patches of insulin nanoparticles. J. Microencapsul. 2016, 33, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Hazzah, H.A.; Farid, R.M.; Nasra, M.M.; El-Massik, M.A.; Abdallah, O.Y. Lyophilized sponges loaded with curcumin solid lipid nanoparticles for buccal delivery: Development and characterization. Int. J. Pharm. 2015, 492, 248–257. [Google Scholar] [CrossRef] [PubMed]
- El-Nahas, A.E.; Allam, A.N.; El-Kamel, A.H. Mucoadhesive buccal tablets containing silymarin Eudragit-loaded nanoparticles: Formulation, characterisation and ex vivo permeation. J. Microencapsul. 2017, 34, 463–474. [Google Scholar] [CrossRef]
- Mura, P.; Cirri, M.; Mennini, N.; Casella, G.; Maestrelli, F. Polymeric mucoadhesive tablets for topical or systemic buccal delivery of clonazepam: Effect of cyclodextrin complexation. Carbohydr. Polym. 2016, 152, 755–763. [Google Scholar] [CrossRef]
| Nanoparticle | Association with Other Substances/Materials | Oral Disease/Problem | Potential Applications | Main Findings |
|---|---|---|---|---|
| CaF2NPs | No | Dental caries | Prevention and treatment | Biofilm formation prevention, inhibition of exopolysaccharide production by Streptococcus mutans, remineralization of tooth enamel [15]. |
| Yes—chitosan bioadhesive films | Increase in the residence time of CaF2NPs in the oral environment [12]. | |||
| Chitosan | No | Dental caries | Prevention and treatment | Anti-growth and anti-adherence activity against cariogenic bacteria in vitro [21]. |
| Yes—sodium fluoride | Slow and continuous fluoride release, with increased release in acidic conditions [13]. | |||
| Yes—glass ionomer cement | Increase in the material’s resistance and fluoride release, bactericidal activity [18]. | |||
| Yes—glass ionomer cement and titanium oxide (TiO2) NPs | Antimicrobial activity with inhibition of biofilm growth, improvement of some physical characteristics [20]. | |||
| Yes—copper NPs | Bactericidal activity against S. mutans [16]. | |||
| Yes—amelogenin-derived peptide QP5 | Antibacterial activity and tooth remineralization [24]. | |||
| PEG-PLGA NPs | Yes—Dodonaea viscosa var. angustifolia derived flavone | Dental caries | Prevention and treatment | Bactericidal and anti-biofilm effect against S. mutans [26]. |
| AgNPs | Yes—azithromycin and clarithromycin | Periodontal disease | Treatment | Antimicrobial efficacy against periodontal disease causing microorganisms [31]. |
| Yes—electrospun nanofibers | Excellent antibacterial activity [35]. | |||
| PtNPs | No | Periodontal disease | Treatment | Antibacterial activity against S. mutans, Enterococcus faecalis, and Porphyromonas gingivalis [32]. |
| Bismuth subsalicylate NPs | No | Periodontal disease | Treatment | Antibacterial effect against Aggregatibacter actinomycetemcomitans, Capnocytophaga gingivalis, and P. gingivalis [33]. |
| PLGA NPs | Yes—BAR peptide | Periodontal disease | Treatment | Disruption of the preformed biofilms more effectively [42]. |
| Yes—metformin hydrochloride | These NPs decreased inflammation and bone loss [39]. | |||
| Calcium and zinc-loaded NPs | No | Periodontal disease | Treatment | Periodontal regeneration and precipitation of calcium phosphate deposits [41]. |
| PLA/PLGA NPs | Yes—curcumin | Periodontal disease | Treatment | Inhibition of inflammation and bone resorption [43]. |
| Liposomes | Yes—lidocaine/prilocaine | Periodontal disease | Treatment | This formulation could be a good option to increase patient compliance during treatment [45]. |
| Chitosan | No | Pulp and periapical lesions | Treatment | Chitosan can be used as an intracanal medication [61]; as an effective chelating agent with less alteration in radicular dentine than EDTA [52]; and as as a root canal irrigant with less undesirable effects than NaOCl and chlorhexidine [59]. |
| Yes—EDTA | This association can simultaneously disinfect root canals and remove the smear layer [98]. | |||
| AgNPs | No | Pulp and periapical lesions | Treatment | AgNPs are effective against some endodontic-periodontal pathogens [76], and can be used as endodontic irrigants [75]. |
| Yes—EDTA | EDTA-AgNPs have antimicrobial activity and could be used for effective smear layer removal [83]. | |||
| Diamond NPs | Yes—gutta percha | Pulp and periapical lesions | Treatment | Decrease in the size of periapical lesions, with no adverse events after 6 months [87]. |
| AuNP and iron oxide NPs | No | Pulp and periapical lesions | Treatment | Inhibition of pathogenic biofilm formation [88,89]. |
| PLGA NPs | Yes—propolis | Pulp and periapical lesions | Treatment | Prolonged release of propolis, enhanced cytocompatibility, and antimicrobial activity [91]. |
| Yes—moxifloxacin | Sustained antibacterial effect in low doses against E. faecalis [6]. | |||
| AgNPs | No | Peri-implantitis and implant failures | Prevention | AgNPs can be used for the coating of titanium surfaces given their antimicrobial activitiy [120]. |
| TiO2NPs | No | Peri-implantitis and implant failures | Prevention | Titanium discs coated with TiO2NPs showed significant antibacterial [124] and anticandidal [123] activities. |
| AuNPs | No | Peri-implantitis and implant failures | Prevention | These NPs showed significantly enhanced osteogenic differentiation and influence on the osseous interface formation [129], and can be used as a bone inductive adjuvant [103]. |
| Chitosan | Yes—AgNPs | Peri-implantitis and implant failures | Prevention | These associations can prevent the surface adhesion of bacteria [132,133]. |
| Yes—hyaluronic acid | ||||
| Yes—collagen | Improvement of peri-implant tissue attachment [136]. | |||
| Bismuth NPs | No | Peri-implantitis and implant failures | Treatment | Bismuth-coated titanium surfaces may improve the treatment of peri-implantitis and peri-implant mucositis [122]. |
| Zirconium oxide (ZrO2) NPs | Yes—PMMA | Dental prosthesis failures | Prevention | The incorporation of these NPs into PMMA can increase the dimensional accuracy [155] and tensile strength of the denture base acrylic [150], decrease the impact strength [155], and improve the flexural [157] and transverse strength [158]. |
| Silica NPs | Yes—PMMA | Dental prosthesis failures | Prevention | Decrease in the flexural strenght [148,152]. |
| Zinc oxide (ZnO) NPs | Yes—PMMA | Dental prosthesis failures | Prevention | Increase in the hardness, thermal stability, glass transition temperature, and the hydrophilicity of PMMA composites [162,163]. |
| AgNPs | Yes—PMMA | Dental prosthesis failures | Prevention | These NPs affected the transverse strength of the denture base acrylic resins in a concentration-dependent manner and decreased the glass transition temperature [154]. |
| TiO2NPs | Yes—PMMA | Dental prosthesis failures | Prevention | TiO2NPs can significantly increase the impact strength, tensile strength, and microhardness of acrylic resin [166,167]. |
| Chitosan | No | Oral candidiasis and denture stomatitis | Treatment | Chitosan mouthwash significantly decreased the erythematous area, burning sensation, time required for clinical improvement, and number of blastospores and mycelia [174]. |
| Yes—curcumin | Chitosan-curcumin mouthwash may serve as a safe and potential topical therapeutic alternative [187]. | |||
| Yes—miconazole | Chitosan-based films are a promising approach to deliver miconazole nitrate [189]. | |||
| Yes—tissue conditioners | These modified materials present a significant antifungal and antibacterial activity [197,198]. | |||
| Yes—denture adhesive | Chitosan has shown properties suitable for the development of an antifungal denture adhesive that could inhibit C. albicans adherence [200]. | |||
| Liposomes | Yes—0WHistatin 5 | Oral candidiasis and denture stomatitis | Treatment | This system was able to limit the growth of C. albicans [201]. |
| Yes—doxorubicin | Head, neck, and oral cancer | Treatment | This formulation resulted in a higher percentage of apoptotic cells than doxorubicin alone [239]. | |
| Solid lipid NPs | Yes—paclitaxel and ascorbic acid | Head, neck, and oral cancer | Treatment | This combination can be a novel approach for the treatment of oral squamous cell carcinoma [240]. |
| Carbon NPs | No | Head, neck, and oral cancer | Diagnosis | These NPs were able to track occult lingual lymph nodes in early-stage tongue squamous cell carcinoma [258]. |
| AuNPs | No | Head, neck, and oral cancer | Diagnosis | The incorporation of AuNPs have improved the sensitivity of different analyses, such as ELISA [250], optical coherence tomography [251], and surface enhanced Raman spectroscopy [252]. |
| Treatment | When combined with X-ray irradiation, AuNPs can induce apoptosis [270]. | |||
| Superparamagnetic iron oxide NPs | Yes—anti-CD44 antibody | Head, neck, and oral cancer | Treatment | Inhibition of tumor growth [264]. |
| Liposomes | No | Hyposalivation | Treatment | Effectiveness in water adsorption, desorption, and diffusion [279]. |
| Yes—pectin, alginate and chitosan | Increase in the stability [279] and water sorption [281] of liposomes. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes, G.; Zambom, C.; Siqueira, W.L. Nanoparticles in Dentistry: A Comprehensive Review. Pharmaceuticals 2021, 14, 752. https://doi.org/10.3390/ph14080752
Moraes G, Zambom C, Siqueira WL. Nanoparticles in Dentistry: A Comprehensive Review. Pharmaceuticals. 2021; 14(8):752. https://doi.org/10.3390/ph14080752
Chicago/Turabian StyleMoraes, Gustavo, Carolina Zambom, and Walter L. Siqueira. 2021. "Nanoparticles in Dentistry: A Comprehensive Review" Pharmaceuticals 14, no. 8: 752. https://doi.org/10.3390/ph14080752
APA StyleMoraes, G., Zambom, C., & Siqueira, W. L. (2021). Nanoparticles in Dentistry: A Comprehensive Review. Pharmaceuticals, 14(8), 752. https://doi.org/10.3390/ph14080752