Drug-Induced Photosensitivity—From Light and Chemistry to Biological Reactions and Clinical Symptoms
Abstract
1. Introduction: Photosensitivity as an Adverse Drug Reaction
2. Drug Photosafety Assessment
3. Chemical and Biological Basis of Photosensitivity
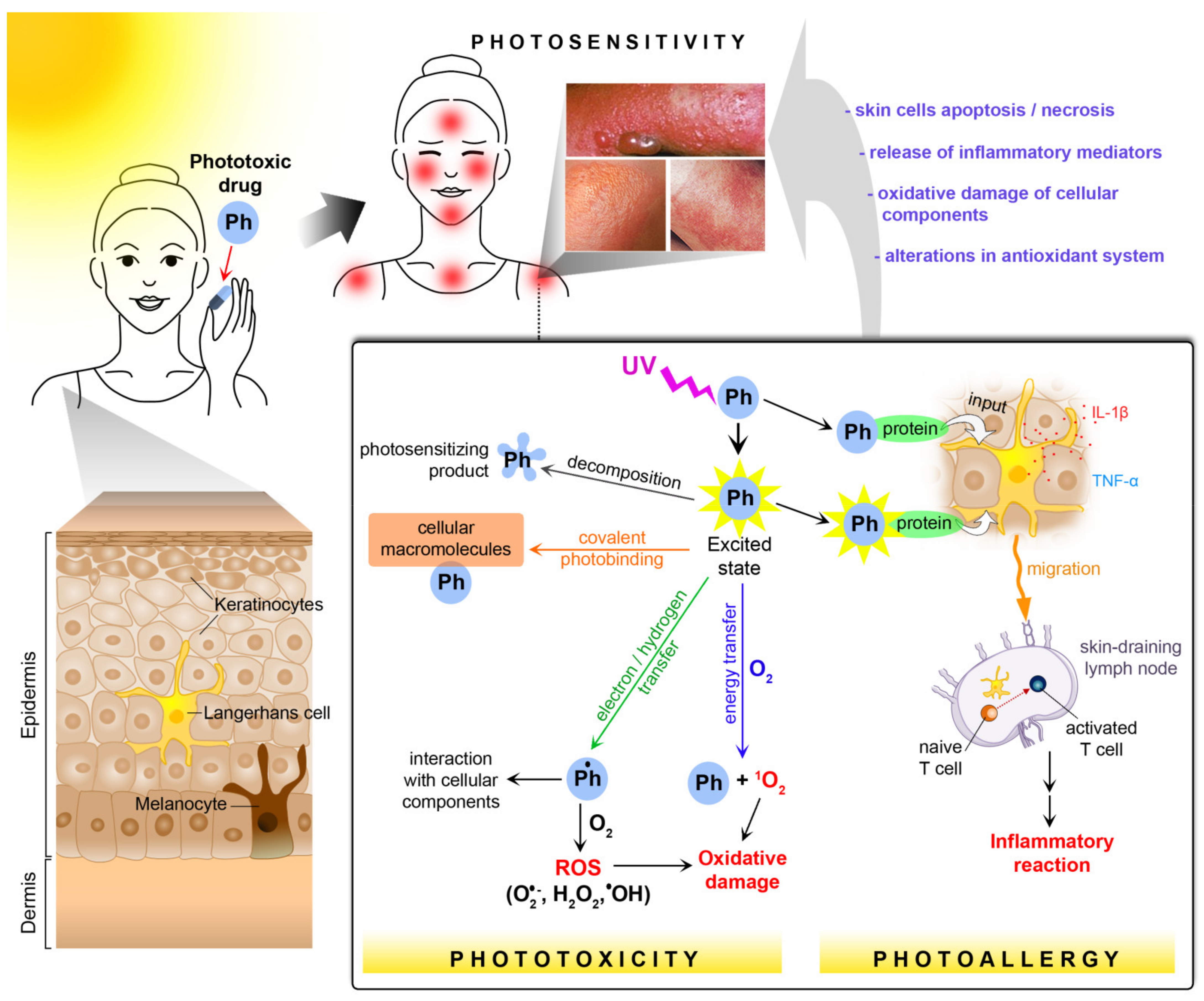
3.1. Photoallergy
3.2. Phototoxicity
3.3. Biochemical Effects of Oxidative Stress
3.4. Role of Melanin Biopolymers in Photosensitization
4. Characteristics of Drugs with Photosensitive Potential
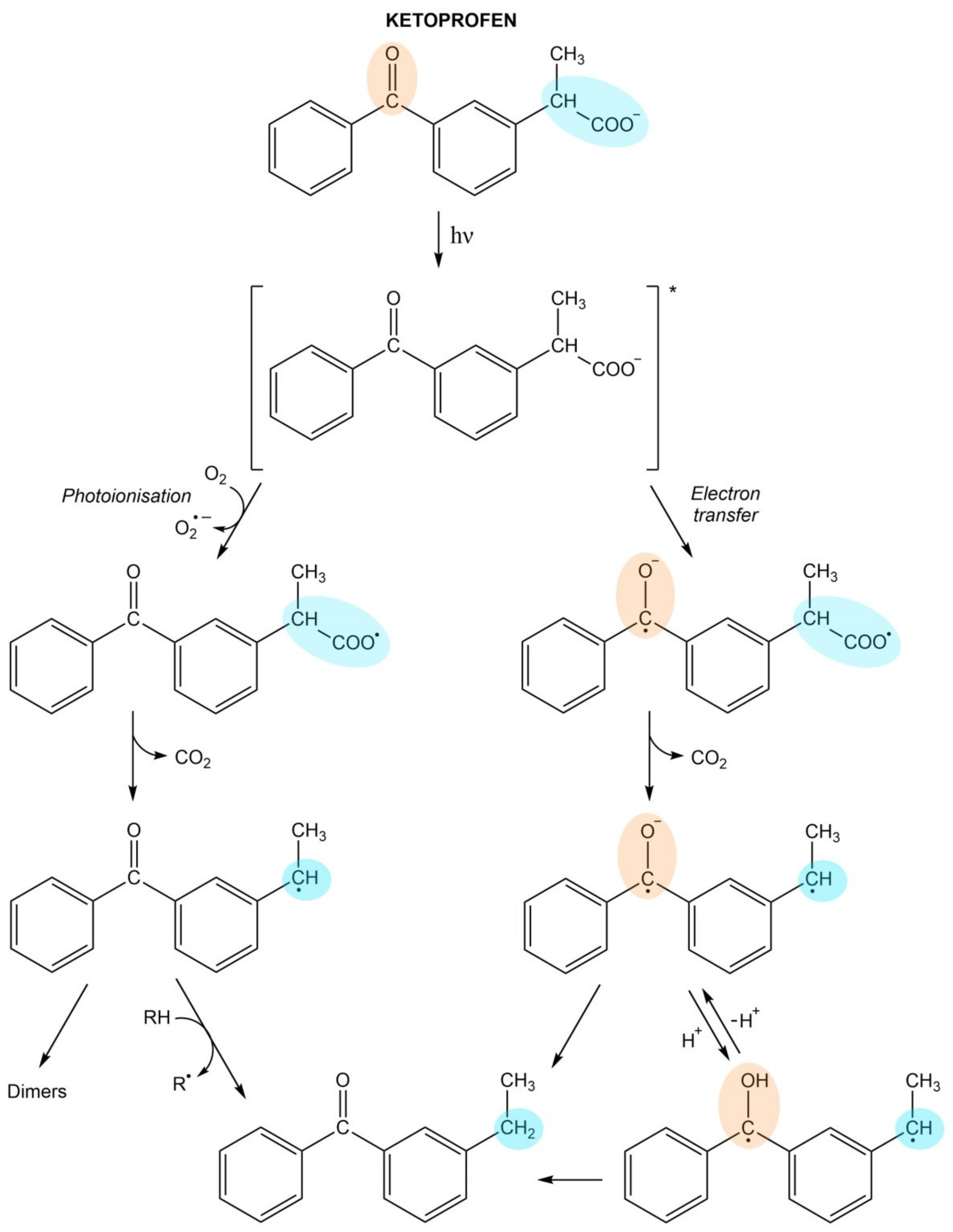
4.1. Nonsteroidal Anti-Inflammatory Drugs
- (a)
- arylpropionic acid analogs: benoxaprofen, ibuprofen, ketoprofen, carprofen, nabumetone, tiaprofenic acid, naproxen;
- (b)
- salicylic acid derivatives: aspirin;
- (c)
- anthranilic acid analogs: meclofenamic acid;
- (d)
- pyrazolidinedione derivatives: oxyphenbutazone, phenylbutazone.
Ketoprofen
4.2. Cardiovascular Drugs
- (a)
- diuretics: hydrochlorothiazide, chlorthalidone, indapamide, bumetanide, furosemide;
- (b)
- angiotensin-converting enzyme (ACE) inhibitors: ramipril, quinapril, enalapril;
- (c)
- angiotensin receptor blockers (ARBs): valsartan;
- (d)
- calcium channel blockers: amlodipine, nifedipine, diltiazem;
- (e)
- beta-blockers: tilisolol;
- (f)
- antiarrhythmic drugs: amiodarone;
- (g)
- antithrombotic agents: clopidogrel, triflusal;
- (h)
- others: rilmenidine, methyldopa.
4.2.1. Thiazides
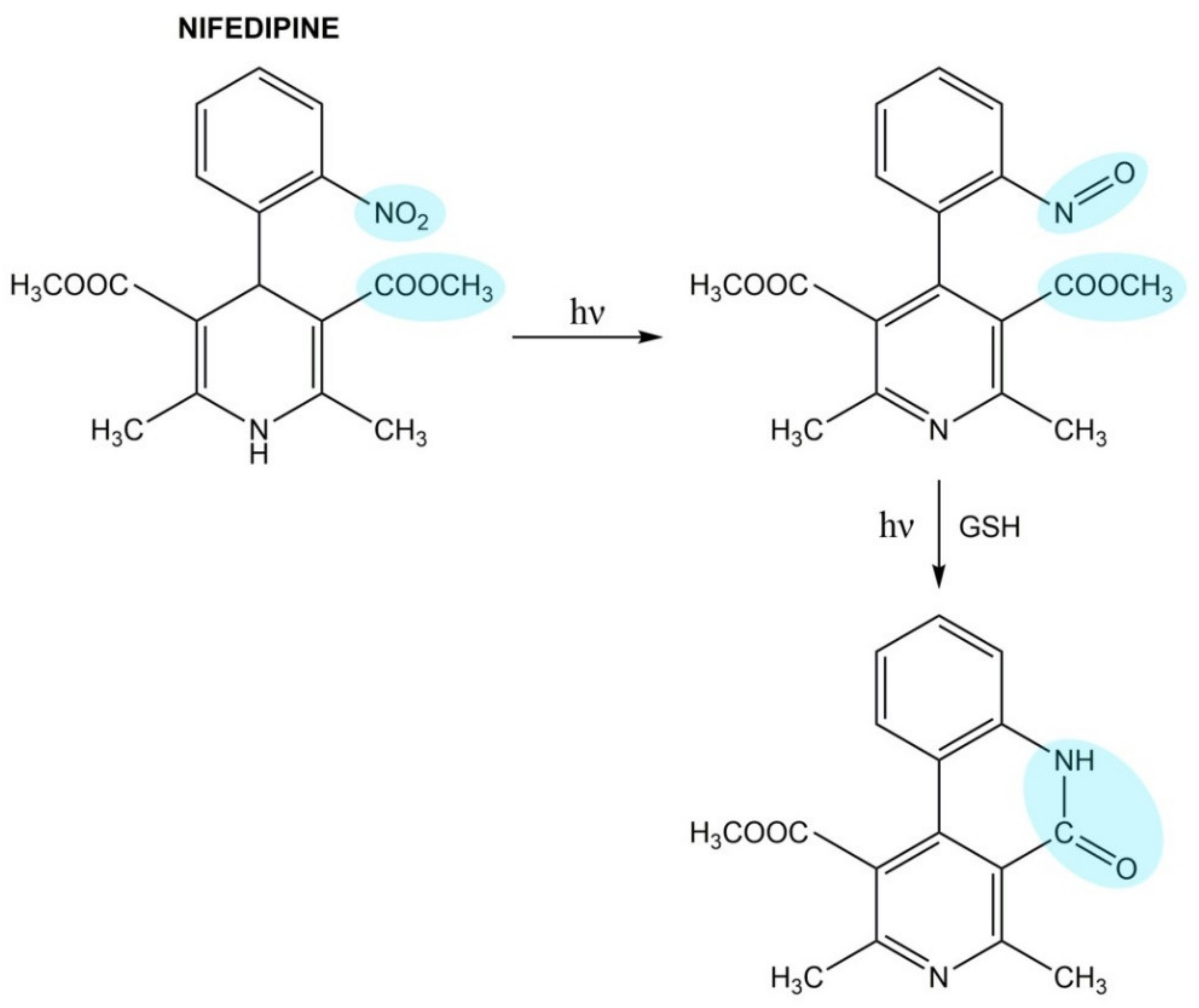
4.2.2. Calcium Channel Blockers
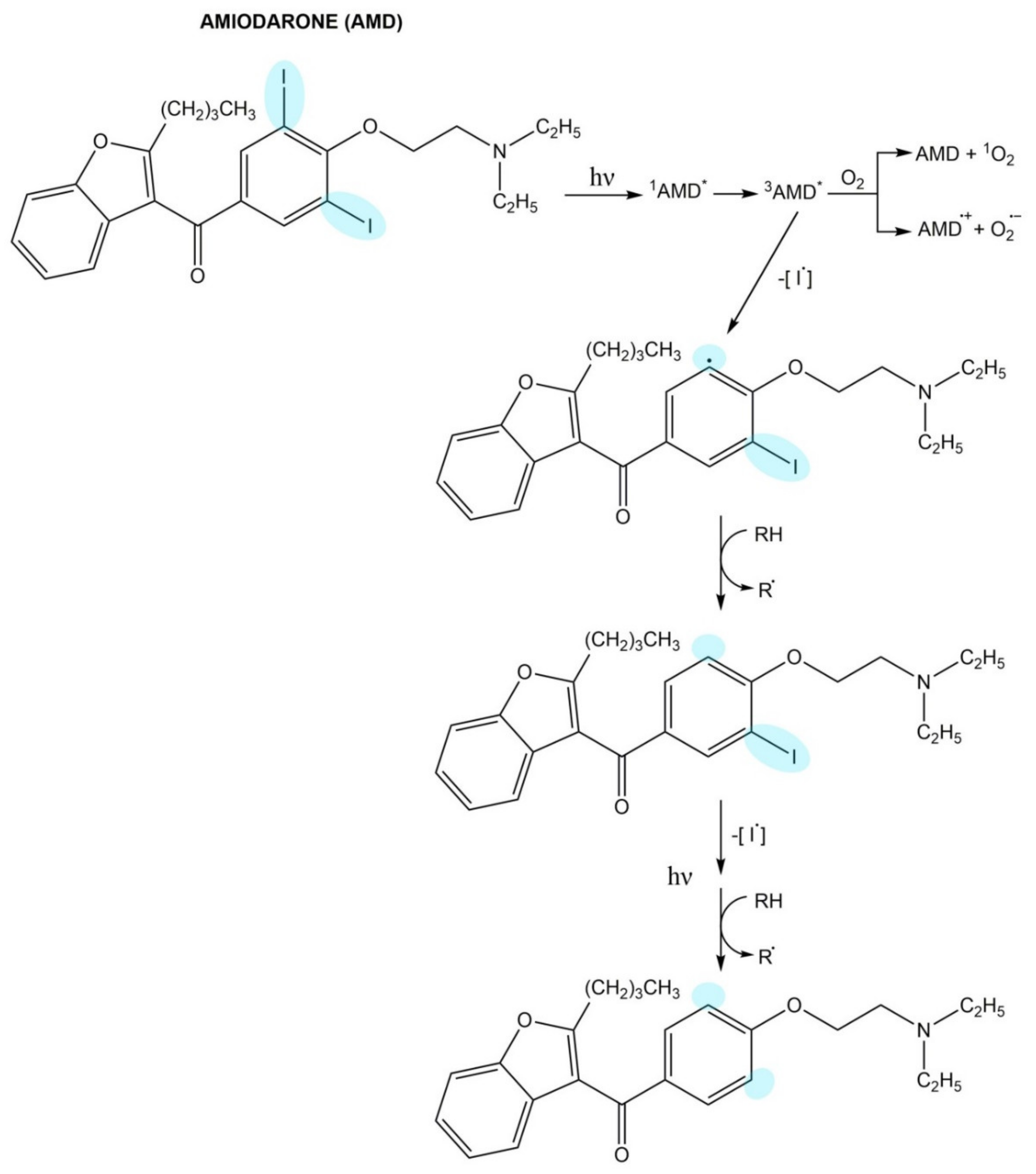
4.2.3. Amiodarone
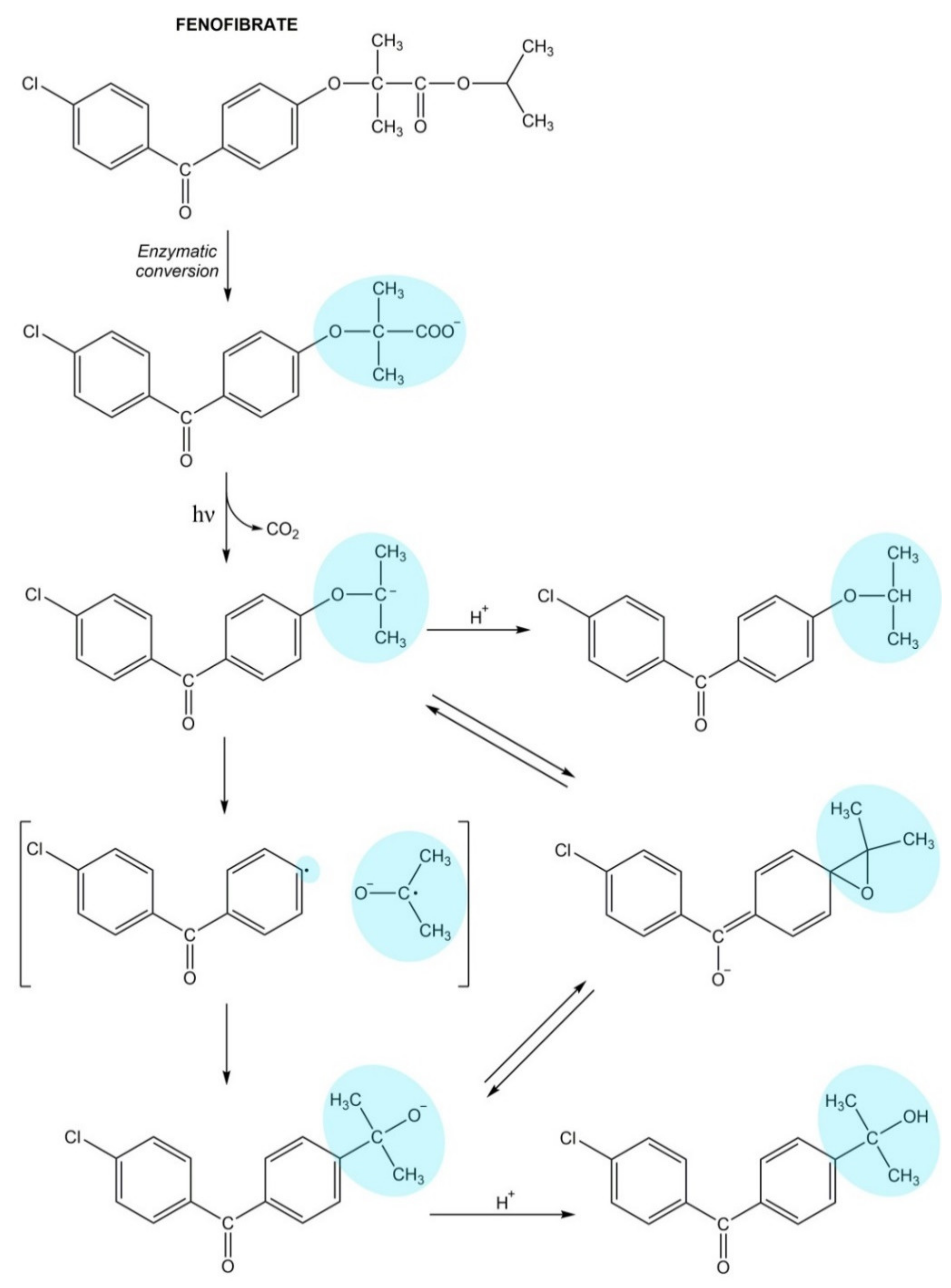
4.3. Antihyperlipidemic Drugs
Fenofibrate
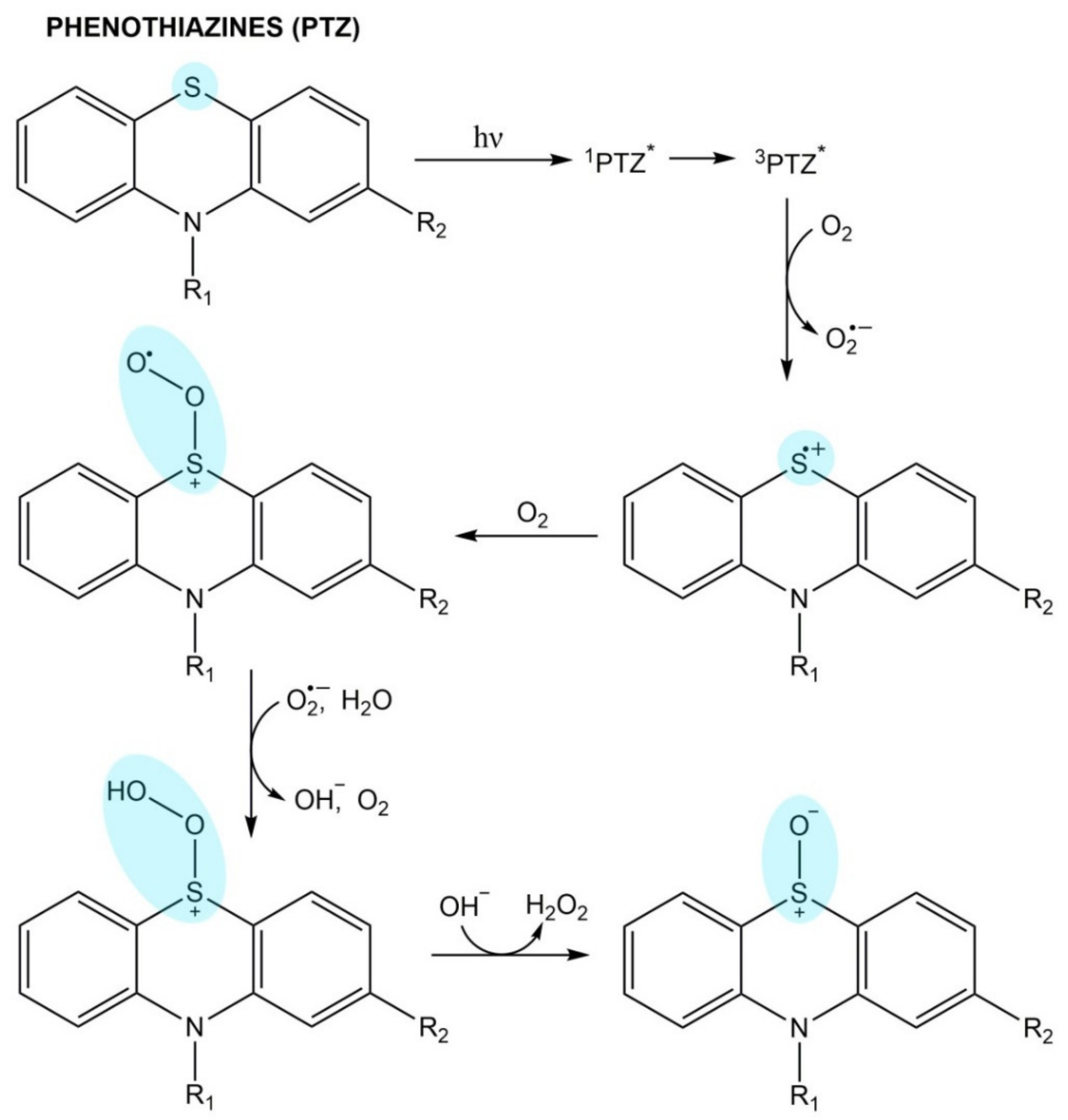
4.4. Psychotropic Drugs
- (a)
- antipsychotics: chlorpromazine, fluphenazine, perphenazine, thioridazine, chlorproethazine, flupenthixol, olanzapine, aripiprazole, clozapine;
- (b)
- antidepressants: imipramine, clomipramine, paroxetine, fluvoxamine, fluoxetine, citalopram, sertraline, escitalopram, phenelzine, venlafaxine;
- (c)
- anxiolytics: alprazolam, chlordiazepoxide.
Phenothiazines
4.5. Antiretrovirals
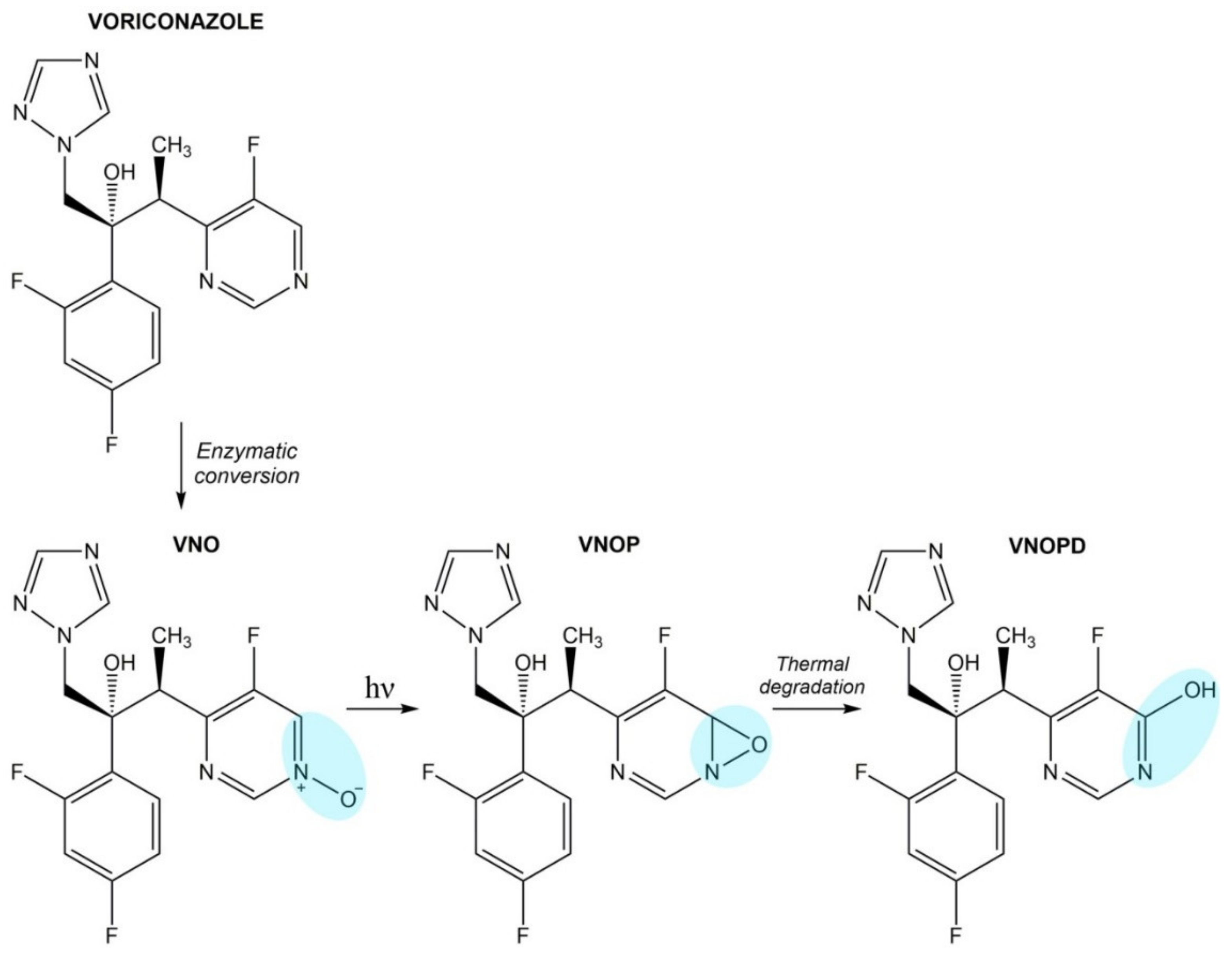
4.6. Antifungals
Voriconazole
4.7. Antibacterial Drugs
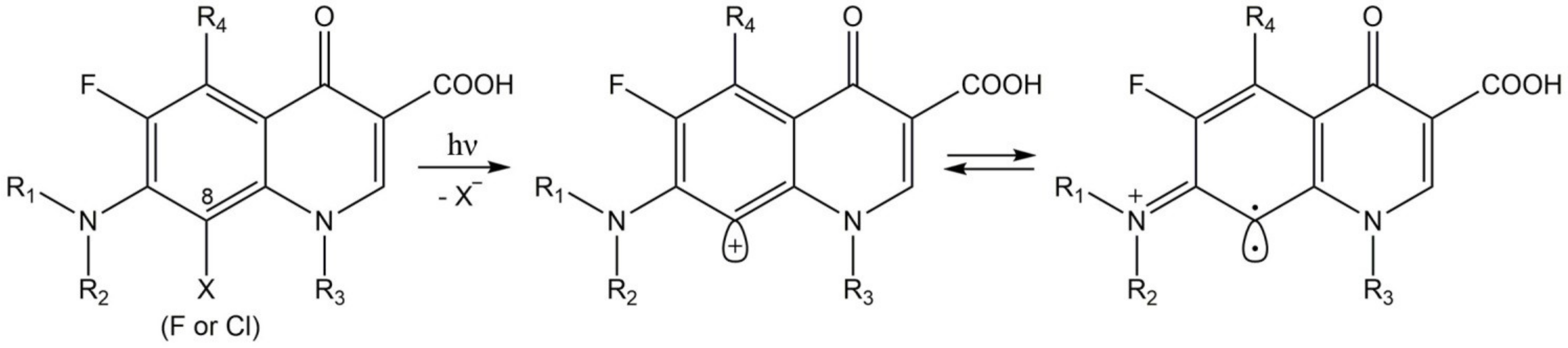
4.7.1. Fluroquinolones
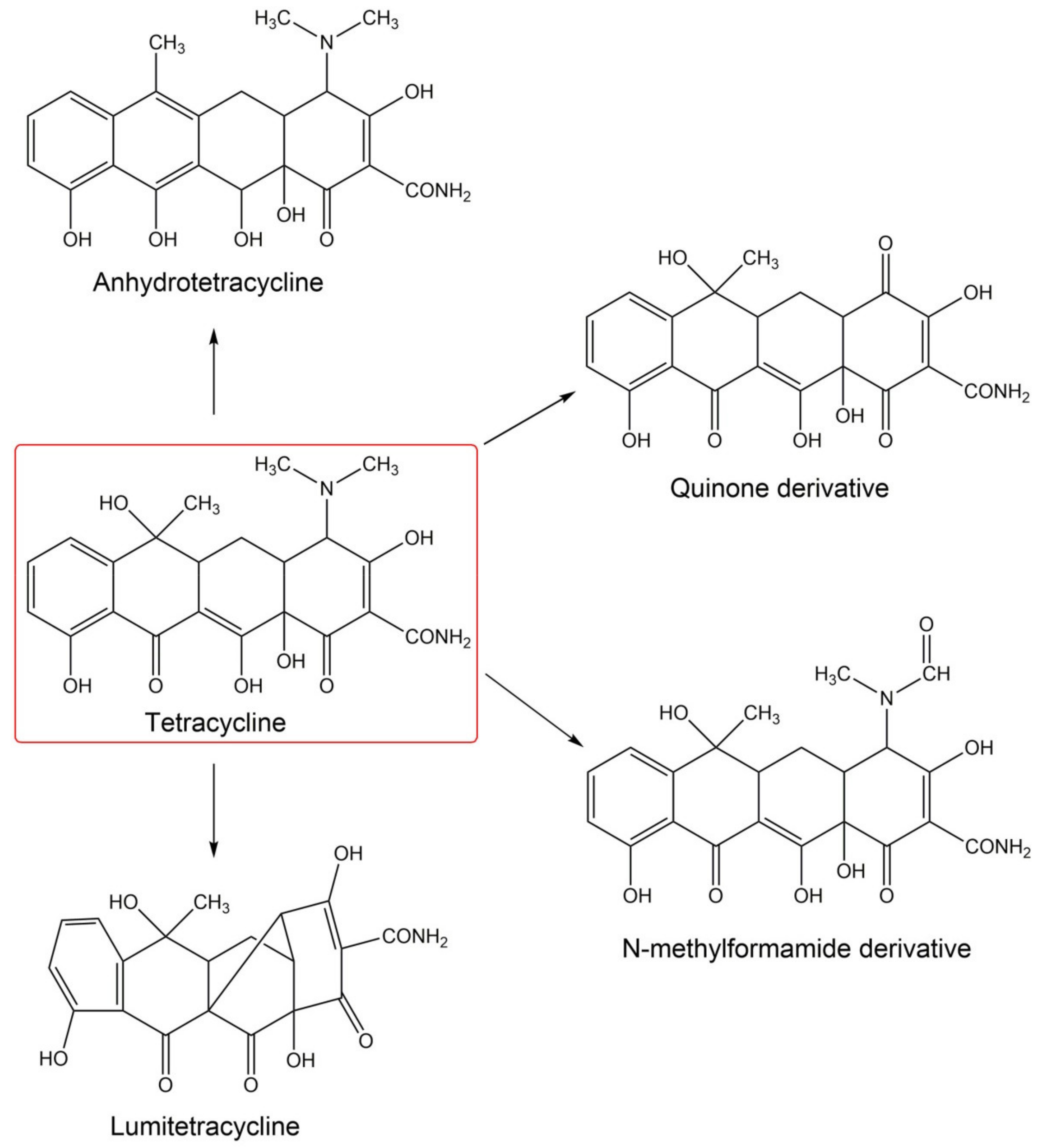
4.7.2. Tetracyclines
4.8. Antimalarials
4.9. Antineoplastic Agents
- (a)
- cytostatics: paclitaxel, fluorouracil, capecitabine, hydroxyurea, dacarbazine, vinblastine, 5-azacitidine;
- (b)
- tyrosine kinase inhibitors: imatinib, vandetanib, vemurafenib, pazopanib;
- (c)
- hormonal agents: flutamid, bicalutamide;
- (d)
- monoclonal antibodies: mogamulizumab, rovalpituzumab.
4.9.1. Fluorouracil
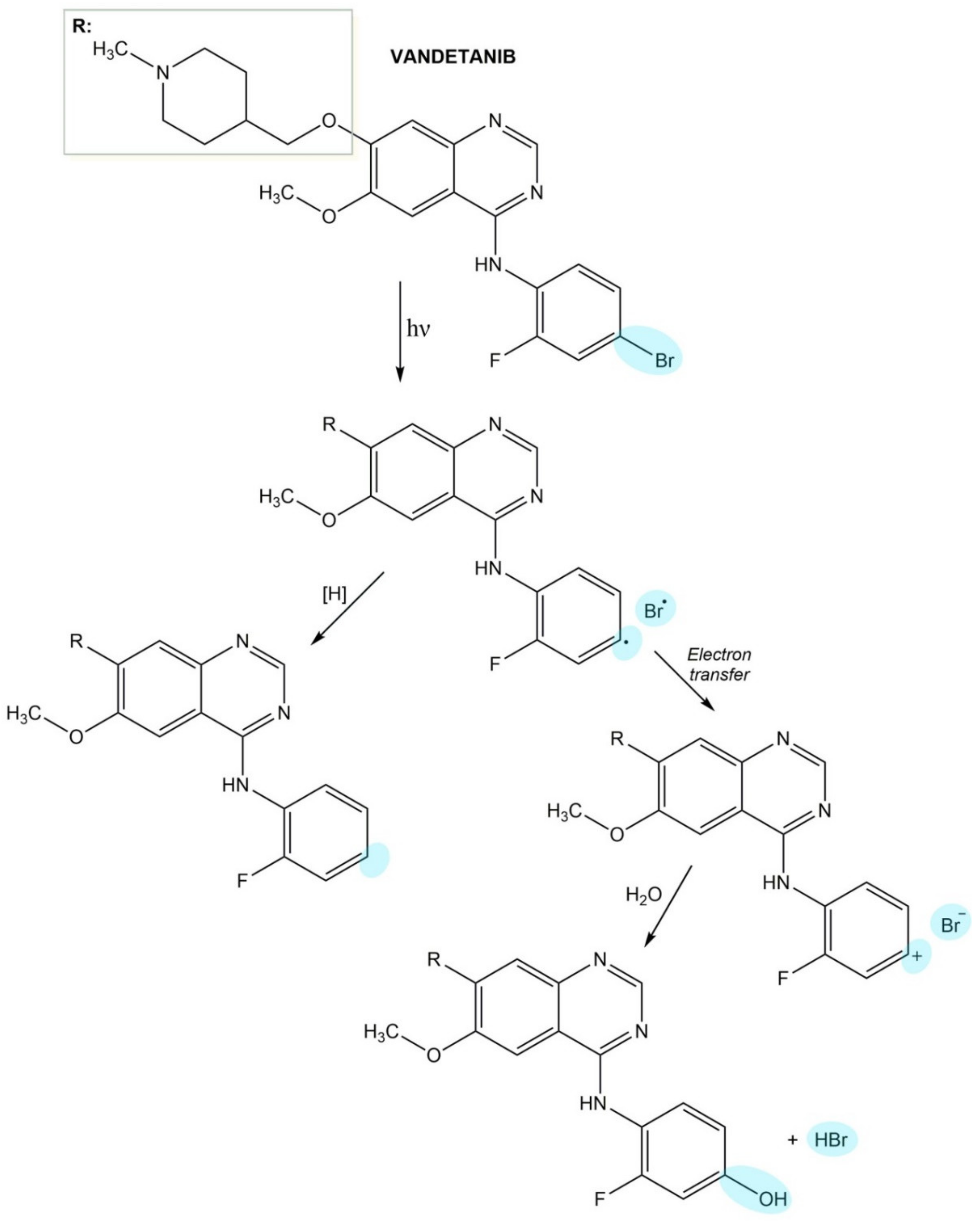
4.9.2. Vandetanib
4.10. Miscellaneous Drugs
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nayak, S.; Acharjya, B. Adverse cutaneous drug reaction. Ind. J. Dermatol. 2008, 53, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.F.; Pirmohamed, M. Biomarkers of adverse drug reactions. Exp. Biol. Med. 2018, 243, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Fiscus, V.; Hankinson, A.; Alweis, R. Minocycline-induced hyperpigmentation. J. Community Hosp. Intern. Med. Perspect. 2014, 4, 24063. [Google Scholar] [CrossRef] [PubMed]
- Hoetzenecker, W.; Nägeli, M.; Mehra, E.T.; Jensen, A.N.; Saulite, I.; Schmid-Grendelmeier, P.; Guenova, E.; Cozzio, A.; French, L.E. Adverse cutaneous drug eruptions: Current understanding. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 75–86. [Google Scholar] [CrossRef]
- Sharma, A.M.; Uetrecht, J. Bioactivation of drugs in the skin: Relationship to cutaneous adverse drug reactions. Drug Metab. Rev. 2014, 46, 1–18. [Google Scholar] [CrossRef]
- Marzano, A.V.; Borghi, A.; Cugno, M. Adverse drug reactions and organ damage: The skin. Eur. J. Intern. Med. 2016, 28, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kiepurska, N.; Paluchowska, E.; Owczarek, W.; Szkultecka-Dębek, M.; Jahnz-Różyk, K. The direct costs of drug-induced skin reactions. Ann. Agric. Environ. Med. 2017, 24, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.M.; Rosen, C.F. Drug-induced photosensitivity: Culprit drugs, management and prevention. Drug Saf. 2011, 34, 821–837. [Google Scholar] [CrossRef]
- Kutlubay, Z.; Sevim, A.; Engin, B.; Tüzün, Y. Photodermatoses, including phototoxic and photoallergic reactions (internal and external). Clin. Dermatol. 2014, 32, 73–79. [Google Scholar] [CrossRef]
- Hölzle, E.; Lehmann, P.; Neumann, N. Phototoxic and photoallergic reactions. J. Dtsch. Dermatol. Ges. 2009, 7, 643–649. [Google Scholar] [CrossRef]
- FDA. S10 Photosafety Evaluation of Pharmaceuticals Guidance for Industry. 2015. Available online: https://www.fda.gov/media/85076/download (accessed on 12 April 2021).
- EMA. S10 Guidance on Photosafety Evaluation of Pharmaceuticals. 2012. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/ich-guideline-s10-guidance-photosafety-evaluation-pharmaceuticals-step-3_en.pdf (accessed on 12 April 2021).
- ICH. Safety Guidelines S10 Photosafety Evaluation of Pharmaceuticals. 2013. Available online: https://database.ich.org/sites/default/files/S10_Guideline.pdf (accessed on 12 April 2021).
- Solano, F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- Shin, D.W. Various biological effects of solar radiation on skin and their mechanisms: Implications for phototherapy. Anim. Cells Syst. 2020, 24, 181–188. [Google Scholar] [CrossRef]
- Gould, J.W.; Mercurio, M.G.; Elmets, C.A. Cutaneous photosensitivity diseases induced by exogenous agents. J. Am. Acad. Dermatol. 1995, 33, 551–573. [Google Scholar] [CrossRef]
- Diffey, B.L. Human exposure to solar ultraviolet radiation. J. Cosmet. Dermatol. 2002, 1, 124–130. [Google Scholar] [CrossRef]
- Blumthaler, M.; Ambach, W.; Ellinger, R. Increase in solar UV radiation with altitude. J. Photochem. Photobiol. B 1997, 39, 130–134. [Google Scholar] [CrossRef]
- Fioletov, V.; Kerr, J.B.; Fergusson, A. The UV index: Definition, distribution and factors affecting it. Can. J. Public Health 2010, 101, 15–19. [Google Scholar] [CrossRef]
- IARC. Exposure to Artificial UV Radiation and Skin Cancer. 2006. Available online: https://www.iarc.who.int/wp-content/uploads/2018/07/ArtificialUVRadSkinCancer.pdf (accessed on 18 July 2021).
- ICNIRP. Protecting Workers from Ultraviolet Radiation. 2007. Available online: https://www.who.int/uv/publications/Protecting_Workers_UV_pub.pdf (accessed on 18 July 2021).
- Hietanen, M. Occupational Exposure to Artificial Sources of UVR and Prevention. Available online: https://oshwiki.eu/wiki/Occupational_exposure_to_artificial_sources_of_UVR_and_prevention (accessed on 18 July 2021).
- Dubakiene, R.; Kupriene, M. Scientific problems of photosensitivity. Medicina 2006, 42, 619–624. [Google Scholar] [PubMed]
- González, E.; González, S. Drug photosensitivity, idiopathic photodermatoses, and sunscreens. J. Am. Acad. Dermatol. 1997, 35, 871–885. [Google Scholar] [CrossRef]
- Harber, L.C.; Baer, R.L. Pathogenic mechanisms of drug-induced photosensitivity. J. Invest. Dermatol. 1972, 58, 327–342. [Google Scholar] [CrossRef]
- Vassileva, S.G.; Mateev, G.; Parish, L.C. Antimicrobial photosensitive reactions. Arch. Intern. Med. 1998, 158, 1993–2000. [Google Scholar] [CrossRef]
- Quintero, B.; Miranda, M.A. Mechanisms of photosensitization induced by drugs: A general survey. Ars Pharm. 2000, 41, 27–46. [Google Scholar]
- Ahmad, I.; Ahmed, S.; Anwar, Z.; Sheraz, M.A.; Sikorski, M. Photostability and photostabilization of drugs and drug products. Int. J. Photoenergy 2016, 1–19. [Google Scholar] [CrossRef]
- Monteiro, A.F.; Rato, M.; Martins, C. Drug-induced photosensitivity: Photoallergic and phototoxic reactions. Clin. Dermatol. 2016, 34, 571–581. [Google Scholar] [CrossRef]
- Stein, K.R.; Scheinfeld, N.S. Drug-induced photoallergic and phototoxic reactions. Expert Opin. Drug Saf. 2007, 6, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.A.; Castell, J.V.; Gómez-Lechón, M.J.; Hernández, D.; Martínez, L.A. Photobinding of drugs to cells as an indicator of potential photoallergy. Toxicol. In Vitro 1995, 9, 499–503. [Google Scholar] [CrossRef]
- Divkovic, M.; Pease, C.K.; Gerberick, G.F.; Basketter, D.A. Hapten-protein binding: From theory to practical application in the in vitro prediction of skin sensitization. Contact Dermat. 2005, 53, 189–200. [Google Scholar] [CrossRef]
- Boscá, F.; Cuquerella, M.C.; Marín, M.L.; Miranda, M.A. Photochemistry of 2-hydroxy-4-trifluoromethylbenzoic acid, major metabolite of the photosensitizing platelet antiaggregant drug triflusal. Photochem. Photobiol. 2001, 73, 463–468. [Google Scholar] [CrossRef]
- Caffieri, S.; Miolo, G.; Seraglia, R.; Dalzoppo, D.; Toma, F.M.; van Henegouwen, G.M.B. Photoaddition of fluphenazine to nucleophiles in peptides and proteins. Possible cause of immune side effects. Chem. Res. Toxicol. 2007, 20, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Nuin, E.; Pérez-Sala, D.; Lhiaubet-Vallet, V.; Andreu, I.; Miranda, M.A. Photosensitivity to triflusal: Formation of a photoadduct with ubiquitin demonstrated by photophysical and proteomic techniques. Front. Pharmacol. 2016, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.; Dearman, R.J.; Kimber, I. Langerhans cell migration in mice requires intact type I interleukin 1 receptor (IL-1RI) function. Arch. Dermatol. Res. 1999, 291, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Enk, A.H.; Katz, S.I. Early molecular events in the induction phase of contact sensitivity. Proc. Natl. Acad. Sci. USA 1992, 89, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Palermo, B.; Lenig, D.; Miettinen, M.; Matikainen, S.; Julkunen, I.; Forster, R.; Burgstahler, R.; Lipp, M.; Lanzavecchia, A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 1999, 29, 1617–1625. [Google Scholar] [CrossRef]
- Teunissen, M.B.M. Dynamic nature and function of epidermal Langerhans cells in vivo and in vitro: A review, with emphasis on human Langerhans cells. Histochem. J. 1992, 24, 697–716. [Google Scholar] [CrossRef]
- Toebak, M.J.; Gibbs, S.; Bruynzeel, D.P.; Scheper, R.J.; Rustemeyer, T. Dendritic cells: Biology of the skin. Contact Dermatitis 2009, 60, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.E.A.M.; Reis, V.M.S.D. Immunopathology of allergic contact dermatitis. An. Bras. Dermatol. 2011, 86, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Novak-Bilić, G.; Vučić, M.; Japundžić, I.; Meštrović-Štefekov, J.; Stanić-Duktaj, S.; Lugović-Mihić, L. Irritant and allergic contact dermatitis—Skin lesion characteristics. Acta Clin. Croat. 2018, 57, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, H.; Lim, K.M. Phototoxicity: Its mechanism and animal alternative test methods. Toxicol. Res. 2015, 31, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Svensson, C.K.; Cowen, E.W.; Gaspari, A.A. Cutaneous drug reactions. Pharmacol. Rev. 2001, 53, 357–379. [Google Scholar] [PubMed]
- Ibuki, Y.; Toyooka, T. Evaluation of chemical phototoxicity, focusing on phosphorylated histone H2AX. J. Radiat. Res. 2015, 56, 220–228. [Google Scholar] [CrossRef]
- Mang, R.; Stege, H.; Krutmann, J. Mechanisms of phototoxic and photoallergic reactions. In Contact Dermatitis; Johansen, J., Frosch, P., Lepoittevin, J.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Kulesza, M.; Dansonka-Mieszkowska, A.; Pieńkowska-Grela, B. Napraw albo zgiń—Rola białka p53 w życiu komórki. Biul. Pol. Tow. Onkol. Nowotw. 2019, 4, 220–231. [Google Scholar]
- Trouba, K.J.; Hamadeh, H.K.; Amin, R.P.; Germolec, D.R. Oxidative stress and its role in skin disease. Antioxid. Redox Signal. 2002, 4, 665–673. [Google Scholar] [CrossRef]
- Marrot, L.; Meunier, J.R. Skin DNA photodamage and its biological consequences. J. Am. Acad. Dermatol. 2008, 58, S139–S148. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, R.; Pancewicz, S.A.; Skrzydlewska, E.; Hermanowska-Szpakowicz, T. Właściwości biologiczne czynnika transkrypcji jądrowej NF-κB. Alerg. Astma Immunol. 2005, 10, 125–131. [Google Scholar]
- Nicolaou, A.; Pilkington, S.M.; Rhodes, L.E. Ultraviolet-radiation induced skin inflammation: Dissecting the role of bioactive lipids. Chem. Phys. Lipids 2011, 164, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrześniok, D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Postepy Hig. Med. Dosw. 2016, 70, 695–708. [Google Scholar] [CrossRef]
- D’Ischia, M.; Wakamatsu, K.; Cicoira, F.; di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef]
- Park, H.Y.; Kosmadaki, M.; Yaar, M.; Gilchrest, B.A. Cellular mechanisms regulating human melanogenesis. Cell. Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K.; Ozeki, H. Chemical analysis of melanins and its application to the study of the regulation of melanogenesis. Pigment Cell Res. 2000, 13, 103–109. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- ElObeid, A.S.; Kamal-Eldin, A.; Abdelhalim, M.A.K.; Haseeb, A.M. Pharmacological properties of melanin and its function in health. Basic Clin. Pharmacol. Toxicol. 2017, 120, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.; Bredeston, L.; Malanga, G.; Mordoh, J. Role of melanin as a scavenger of active oxygen species. Pigment Cell Res. 1993, 6, 348–353. [Google Scholar] [CrossRef]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef]
- Napolitano, A.; Panzella, L.; Monfrecola, G.; d’Ischia, M. Pheomelanin-induced oxidative stress: Bright and dark chemistry bridging red hair phenotype and melanoma. Pigment Cell Melanoma Res. 2014, 27, 721–733. [Google Scholar] [CrossRef]
- Ortonne, J.P. Photoprotective properties of skin melanin. Br. J. Dermatol. 2002, 146, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef]
- Simon, J.D.; Hong, L.; Peles, D.N. Insights into melanosomes and melanin from some interesting spatial and temporal properties. J. Phys. Chem. B 2008, 112, 13201–13217. [Google Scholar] [CrossRef] [PubMed]
- Maddodi, N.; Jayanthy, A.; Setaluri, V. Shining light on skin pigmentation: The darker and the brighter side of effects of UV radiation. Photochem. Photobiol. 2012, 88, 1075–1082. [Google Scholar] [CrossRef]
- Gupta, A.K.; Bharadwaj, M.; Mehrotra, R. Skin cancer concerns in people of color: Risk factors and prevention. Asian Pac. J. Cancer Prev. 2016, 17, 5257–5264. [Google Scholar] [CrossRef]
- Reinen, J.; van Sas, P.; van Huygevoort, T.; Rubio, L.; Scase, K.; Wenker, M. Development of a phototoxicity testing strategy for accurate photosafety evaluation of pharmaceuticals based on the assessment of possible melanin-binding effects. Int. J. Toxicol. 2018, 37, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Beberok, A.; Buszman, E.; Wrześniok, D.; Otręba, M.; Trzcionka, J. Interaction between ciprofloxacin and melanin: The effect on proliferation and melanization in melanocytes. Eur. J. Pharmacol. 2011, 669, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Buszman, E.; Wrześniok, D.; Trzcionka, J.; Miernik-Biela, E.; Stróż, M. Interaction of ketoprofen and paracetamol with melanin in vitro. Ann. Univ. Mariae Curie Sklodowska Med. 2009, 22, 81–86. [Google Scholar]
- Rok, J.; Rzepka, Z.; Respondek, M.; Beberok, A.; Wrześniok, D. Chlortetracycline and melanin biopolymer—The risk of accumulation and implications for phototoxicity: An in vitro study on normal human melanocytes. Chem. Biol. Interact. 2019, 303, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Buszman, E.; Beberok, A.; Delijewski, M.; Otręba, M.; Wrześniok, D. Modulation of melanogenesis and antioxidant status of melanocytes in response to phototoxic action of doxycycline. Photochem. Photobiol. 2015, 91, 1429–1434. [Google Scholar] [CrossRef]
- Buszman, E.; Wrześniok, D.; Otręba, M.; Beberok, A. The impact of ketoprofen on viability and melanization process in normal melanocytes HEMn-DP. Curr. Issues Pharm. Med. Sci. 2012, 25, 376–380. [Google Scholar] [CrossRef]
- Rok, J.; Rzepka, Z.; Kowalska, J.; Banach, K.; Beberok, A.; Wrześniok, D. Molecular and biochemical basis of minocycline-induced hyperpigmentation-the study on normal human melanocytes exposed to UVA and UVB radiation. Int. J. Mol. Sci. 2021, 22, 3755. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Rzepka, Z.; Maszczyk, M.; Beberok, A.; Wrześniok, D. Minocycline impact on redox homeostasis of normal human melanocytes HEMn-LP exposed to UVA radiation and hydrogen peroxide. Int. J. Mol. Sci. 2021, 22, 1642. [Google Scholar] [CrossRef]
- Onder, G.; Pellicciotti, F.; Gambassi, G.; Bernabei, R. NSAID-related psychiatric adverse events: Who is at risk? Drugs 2004, 64, 2619–2627. [Google Scholar] [CrossRef]
- Sánchez-Borges, M.; Capriles-Hulett, A.; Caballero-Fonseca, F. Risk of skin reactions when using ibuprofen-based medicines. Expert Opin. Drug Saf. 2005, 4, 837–848. [Google Scholar] [CrossRef]
- Bagheri, H.; Lhiaubet, V.; Montastruc, J.L.; Chouini-Lalanne, N. Photosensitivity to ketoprofen: Mechanisms and pharmacoepidemiological data. Drug Saf. 2000, 22, 339–349. [Google Scholar] [CrossRef]
- Canelas, M.M.; Cardoso, J.C.; Gonçalo, M.; Figueiredo, A. Photoallergic contact dermatitis from benzydamine presenting mainly as lip dermatitis. Contact Derm. 2010, 63, 85–88. [Google Scholar] [CrossRef]
- Cardoso, J.; Canelas, M.M.; Gonçalo, M.; Figueiredo, A. Photopatch testing with an extended series of photoallergens: A 5-year study. Contact Derm. 2009, 60, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Varela, P.; Amorim, I.; Massa, A.; Sanches, M.; Silva, E. Piroxicam-beta-cyclodextrin and photosensitivity reactions. Contact Derm. 1998, 38, 229. [Google Scholar] [CrossRef] [PubMed]
- Serrano, G.; Bonillo, J.; Aliaga, A.; Cuadra, J.; Pujol, C.; Pelufo, C.; Cervera, P.; Miranda, M.A. Piroxicam-induced photosensitivity and contact sensitivity to thiosalicylic acid. J. Am. Acad. Dermatol. 1990, 23, 479–483. [Google Scholar] [CrossRef]
- Gonçalo, M. Phototoxic and photoallergic reactions. In Contact Dermatitis; Johansen, J., Frosch, P., Lepoittevin, J.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Akat, P.B. Severe photosensitivity reaction induced by topical diclofenac. Indian J. Pharmacol. 2013, 45, 408–409. [Google Scholar] [CrossRef]
- Karlsson, I.; Persson, E.; Ekebergh, A.; Mårtensson, J.; Börje, A. Ketoprofen-induced formation of amino acid photoadducts: Possible explanation for photocontact allergy to ketoprofen. Chem. Res. Toxicol. 2014, 27, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.L.; Gardeazabal, J.; Manrique, P.; Ratón, J.A.; Urrutia, I.; Rodríguez-Sasiain, J.M.; Aguirre, C. Greater allergenicity of topical ketoprofen in contact dermatitis confirmed by use. Contact Derm. 2006, 54, 239–243. [Google Scholar] [CrossRef]
- Loh, T.Y.; Cohen, P.R. Ketoprofen-induced photoallergic dermatitis. Ind. J. Med. Res. 2016, 144, 803–806. [Google Scholar] [CrossRef]
- Monti, S.; Sortino, S.; de Guidib, G.; Marconi, G. Photochemistry of 2-(3-benzoylphenyl)propionic acid (ketoprofen) Part 1 A picosecond and nanosecond time resolved study in aqueous solution. J. Chem. Soc. Faraday Trans. 1997, 93, 2269–2275. [Google Scholar] [CrossRef]
- Martinez, L.J.; Scaiano, J.C. Transient intermediates in the laser flash photolysis of ketoprofen in aqueous solutions: Unusual photochemistry for the benzophenone chromophore. J. Am. Chem. Soc. 1997, 119, 11066–11070. [Google Scholar] [CrossRef]
- Nakazawa, T.; Shimo, T.; Chikamatsu, N.; Igarashi, T.; Nagata, O.; Yamamoto, M. Study on the mechanism of photosensitive dermatitis caused by ketoprofen in the guinea pig. Arch. Toxikol. 2006, 80, 442–448. [Google Scholar] [CrossRef]
- Shinoda, M.; Isozaki, T.; Suzuki, T. Photoreaction of ketoprofen with tryptophan and tyrosine in phosphate buffer solution. Photochem. Photobiol. 2014, 90, 92–98. [Google Scholar] [CrossRef]
- Atarashi, K.; Kabashima, K.; Akiyama, K.; Tokura, Y. Stimulation of Langerhans cells with ketoprofen plus UVA in murine photocontact dermatitis to ketoprofen. J. Dermatol. Sci. 2007, 47, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.S.; Mujtaba, S.F.; Dwivedi, A.; Yadav, N.; Verma, A.; Kushwaha, H.N.; Amar, S.K.; Goel, S.; Chopra, D. Singlet oxygen mediated DNA damage induced phototoxicity by ketoprofen resulting in mitochondrial depolarization and lysosomal destabilization. Toxicology 2013, 314, 229–237. [Google Scholar] [CrossRef]
- Selvaag, E.; Thune, P. Phototoxicity to sulphonamide-derived oral antidiabetics and diuretics: Investigations in hairless mice. Photodermatol. Photoimmunol. Photomed. 1997, 13, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bernal, S.; Alvarez-Pérez, A.; Rodríguez-Pazos, L.; Gutiérrez-González, E.; Rodríguez-Granados, M.; Toribio, J. Photosensitivity due to thiazides. Actas Dermosifiliogr. 2014, 105, 359–366. [Google Scholar] [CrossRef]
- Green, J.J.; Manders, S.M. Pseudoporphyria. J. Am. Acad. Dermatol. 2001, 44, 100–108. [Google Scholar] [CrossRef]
- Lowe, G.; Henderson, C.L.; Grau, R.H.; Hansen, C.B.; Sontheimer, R.D. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br. J. Dermatol. 2011, 164, 465–472. [Google Scholar] [CrossRef]
- Srivastava, M.; Rencic, A.; Diglio, G.; Santana, H.; Bonitz, P.; Watson, R.; Ha, E.; Anhalt, G.J.; Provost, T.T.; Nousari, C.H.; et al. Drug-induced, Ro/SSA-positive cutaneous lupus erythematosus. Arch. Dermatol. 2003, 139, 45–49. [Google Scholar] [CrossRef]
- Masuoka, E.; Bito, T.; Shimizu, H.; Nishigori, C. Dysfunction of melanocytes in photoleukomelanoderma following photosensitivity caused by hydrochlorothiazide. Photodermatol. Photoimmunol. Photomed. 2011, 27, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Baran, R.; Juhlin, L. Photoonycholysis. Photodermatol. Photoimmunol. Photomed. 2002, 18, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.A.; Coulson, I.H. Thiazide-induced lichenoid photosensitivity. Clin. Exp. Dermatol. 2002, 27, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Breier, F.; Feldmann, R.; Pelzl, M.; Gschnait, F. Pseudoporphyria cutanea tarda induced by furosemide in a patient undergoing peritoneal dialysis. Dermatology 1998, 197, 271–273. [Google Scholar] [CrossRef]
- Rutherford, T.; Sinclair, R. Photo-onycholysis due to indapamide. Australas. J. Dermatol. 2007, 48, 35–36. [Google Scholar] [CrossRef]
- Rodríguez Granados, M.T.; Abalde, T.; García Doval, I.; de la Torre, C. Systemic photosensitivity to quinapril. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 389–390. [Google Scholar] [CrossRef]
- Kanwar, A.J.; Dhar, S.; Ghosh, S. Photosensitive lichenoid eruption due to enalapril. Dermatology 1993, 187, 80. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.B.; Pettigrew, T.J. Angioedema and photosensitive rash induced by valsartan. Pharmacotherapy 1998, 18, 866–868. [Google Scholar]
- Bakkour, W.; Haylett, A.K.; Gibbs, N.K.; Chalmers, R.J.; Rhodes, L.E. Photodistributed telangiectasia induced by calcium channel blockers: Case report and review of the literature. Photodermatol. Photoimmunol. Photomed. 2013, 29, 272–275. [Google Scholar] [CrossRef]
- Basarab, T.; Yu, R.; Russell Jones, R. Calcium antagonist-induced photo-exposed telangiectasia. Br. J. Dermatol. 1997, 136, 974–975. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.; Pérez-Pérez, L.; Fernández-Redondo, V.; Toribio, J. Photoallergic dermatitis induced by diltiazem. Contact Derm. 2007, 56, 118–119. [Google Scholar] [CrossRef]
- Scherschun, L.; Lee, M.W.; Lim, H.W. Diltiazem-associated photodistributed hyperpigmentation: A review of 4 cases. Arch. Dermatol. 2001, 137, 179–182. [Google Scholar] [PubMed]
- Boyer, M.; Katta, R.; Markus, R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol. Online J. 2003, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, H.; Horiki, S.; Horio, T. Clinical and experimental photosensitivity reaction to tilisolol hydrochloride. Photodermatol. Photoimmunol. Photomed. 1994, 10, 255–258. [Google Scholar]
- Vassallo, P.; Trohman, R.G. Prescribing amiodarone: An evidence-based review of clinical indications. JAMA 2007, 298, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, K.; Walecka, I.; Rudnicka, L.; Gnatowski, M.; Kosior, D.A. Cutaneous adverse reactions of amiodarone. Med. Sci. Monit. 2014, 20, 2369–2372. [Google Scholar] [CrossRef]
- Yones, S.S.; O’Donoghue, N.B.; Palmer, R.A.; Menagé, H.D.P.; Hawk, J.L.M. Persistent severe amiodarone-induced photosensitivity. Clin. Exp. Dermatol. 2005, 30, 500–502. [Google Scholar] [CrossRef]
- Harris, L.; McKenna, W.J.; Rowland, E.; Holt, D.W.; Storey, G.C.A.; Krikler, D.M. Side effects of long-term amiodarone therapy. Circulation 1983, 67, 45–51. [Google Scholar] [CrossRef]
- Joshi, K.M.; Gill, M.K. Amiodarone: A potential risk factor for retinal phototoxicity. Am. J. Ophthalmol. Case Rep. 2016, 5, 119–123. [Google Scholar] [CrossRef]
- Martínez Leboráns, L.; Cubells Sánchez, L.; Zaragoza Ninet, V.; Pérez Ferriols, A. Atypical photosensitivity associated with triflusal. Contact Derm. 2016, 75, 245–247. [Google Scholar] [CrossRef]
- Dogra, S.; Kanwar, A.J. Clopidogrel bisulphate-induced photosensitive lichenoid eruption: First report. Br. J. Dermatol. 2003, 148, 609–610. [Google Scholar] [CrossRef]
- Mota, A.V.; Vasconcelos, C.; Correia, T.M.; Barros, M.A.; Mesquita-Guimarães, J. Rilmenidine-induced photosensitivity reaction. Photodermatol. Photoimmunol. Photomed. 1998, 14, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, L.; Le Marchand, D.; Grognard, C.; Hocine, R.; Lorette, G. Photosensitivity to methyldopa. Arch. Dermatol. 1988, 124, 326–327. [Google Scholar] [CrossRef]
- Matsuo, I.; Fujita, H.; Hayakawa, K.; Ohkido, M. Lipid peroxidative potency of photosensitized thiazide diuretics. J. Invest. Dermatol. 1986, 87, 637–641. [Google Scholar] [CrossRef]
- Kunisada, M.; Masaki, T.; Ono, R.; Morinaga, H.; Nakano, E.; Yogianti, F.; Okunishi, K.; Sugiyama, H.; Nishigori, C. Hydrochlorothiazide enhances UVA-induced DNA damage. Photochem. Photobiol. 2013, 89, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Han, K.D.; Bark, K.M.; Heo, E.P.; Lee, J.K.; Kang, J.S.; Kim, T.H. Increased phototoxicity of hydrochlorothiazide by photodegradation. Photodermatol. Photoimmunol. Photomed. 2000, 16, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Igarashi, N.; Yamada, S.; Tsuda, Y. High-throughput reactive oxygen species (ROS) assay: An enabling technology for screening the phototoxic potential of pharmaceutical substances. J. Pharm. Biomed. Anal. 2008, 46, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Al-Ajmi, H.S.; Dawe, R.S.; Renwick, A.G.; Macklin, B.S.; Ferguson, J.; Gibbs, N.K. The effect of whole-body sunbed ultraviolet A exposure on the pharmacokinetics of the photolabile drug nifedipine. Photodermatol. Photoimmunol. Photomed. 2000, 16, 111–115. [Google Scholar] [CrossRef]
- De Vries, H.; van Henegouwen, G.M.B. Photoreactivity of nifedipine in vitro and in vivo. J. Photochem. Photobiol. B 1998, 43, 217–221. [Google Scholar] [CrossRef]
- Boscá, F.; Miranda, M.A. Photosensitizing drugs containing the benzophenone chromophore. J. Photochem. Photobiol. B 1998, 43, 1–26. [Google Scholar] [CrossRef]
- Marguery, M.C.; Chouini-Lalanne, N.; Drugeon, C.; Gadroy, A.; Bayle, P.; Journe, F.; Bazex, J.; D’Incan, M. UV-B phototoxic effects induced by atorvastatin. Arch. Dermatol. 2006, 142, 1082–1084. [Google Scholar] [CrossRef]
- Granados, M.T.; de la Torre, C.; Cruces, M.J.; Piñeiro, G. Chronic actinic dermatitis due to simvastatin. Contact Derm. 1998, 38, 294–295. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pazos, L.; Sánchez-Aguilar, D.; Rodríguez-Granados, M.; Pereiro-Ferreirós, M.; Toribio, J. Erythema multiforme photoinduced by statins. Photodermatol. Photoimmunol. Photomed. 2010, 26, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Holme, S.A.; Pearse, A.D.; Anstey, A.V. Chronic actinic dermatitis secondary to simvastatin. Photodermatol. Photoimmunol. Photomed. 2002, 18, 313–314. [Google Scholar] [CrossRef]
- Mohammed, F.; Wally, L.L.; Karaban, J.E.; Reddy, V.B.; Lertratanakul, Y. Fenofibrate-induced lichenoid drug eruption: A rare culprit. Case Rep. Dermatol. 2017, 9, 236–242. [Google Scholar] [CrossRef]
- Tsai, K.C.; Yang, J.H.; Hung, S.J. Fenofibrate-induced photosensitivity—A case series and literature review. Photodermatol. Photoimmunol. Photomed. 2017, 33, 213–219. [Google Scholar] [CrossRef]
- Marguery, M.C.; Chouini-Lalanne, N.; Ader, J.C.; Paillous, N. Comparison of the DNA damage photoinduced by fenofibrate and ketoprofen, two phototoxic drugs of parent structure. Photochem. Photobiol. 1998, 68, 679–684. [Google Scholar] [CrossRef]
- Vayá, I.; Andreu, I.; Monje, V.T.; Jiménez, M.C.; Miranda, M.A. Mechanistic studies on the photoallergy mediated by fenofibric acid: Photoreactivity with serum albumins. Chem. Res. Toxicol. 2016, 29, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Terencio, M.C.; Guillén, I.; Gómez-Lechón, M.J.; Miranda, M.A.; Castell, J.V. Release of inflammatory mediators (PGE2, IL-6) by fenofibric acid-photosensitized human keratinocytes and fibroblasts. Photochem. Photobiol. 1998, 68, 331–336. [Google Scholar]
- Diemer, S.; Eberlein-König, B.; Przybilla, B. Evaluation of the phototoxic properties of some hypolipidemics in vitro: Fenofibrate exhibits a prominent phototoxic potential in the UVA and UVB region. J. Dermatol. Sci. 1996, 13, 172–177. [Google Scholar] [CrossRef]
- Lhiaubet, V.; Paillous, N.; Chouini-Lalanne, N. Comparison of DNA damage photoinduced by ketoprofen, fenofibric acid and benzophenone via electron and energy transfer. Photochem. Photobiol. 2001, 74, 670–678. [Google Scholar] [CrossRef]
- Miolo, G.; Levorato, L.; Gallocchio, F.; Caffieri, S.; Bastianon, C.; Zanoni, R.; Reddi, E. In vitro phototoxicity of phenothiazines: Involvement of stable UVA photolysis products formed in aqueous medium. Chem. Res. Toxicol. 2006, 19, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Llambrich, A.; Lecha, M. Photoinduced lichenoid reaction by thioridazine. Photodermatol. Photoimmunol. Photomed. 2004, 20, 108–109. [Google Scholar] [CrossRef]
- Barbaud, A.; Collet, E.; Martin, S.; Granel, F.; Trechot, P.; Lambert, D.; Schmutz, J.L. Contact sensitization to chlorproethazine can induce persistent light reaction and cross-photoreactions to other phenothiazines. Contact Derm. 2001, 44, 373. [Google Scholar] [CrossRef]
- Giomi, B.; Difonzo, E.M.; Lotti, L.; Massi, D.; Francalanci, S. Allergic and photoallergic conditions from unusual chlorpromazine exposure: Report of three cases. Int. J. Dermatol. 2011, 50, 1276–1278. [Google Scholar] [CrossRef]
- Gacías, L.; Linares, T.; Escudero, E.; Soto-Mera, M.T.; Abalde, M.T. Perphenazine as a cause of mother-to-daughter contact dermatitis and photocontact dermatitis. J. Investig. Allergol. Clin. Immunol. 2013, 23, 60–61. [Google Scholar]
- Romita, P.; Foti, C.; Stingeni, L. Photoallergy to promazine hydrochloride. Contact Derm. 2017, 77, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.C.; Vilaça, S.; Lobo, I.; Sanches, M.; Costa, V.; Selores, M. Photoallergic reaction to cyamemazine. Dermatol. Online J. 2013, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Bourrain, J.L.; Paillet, C.; Woodward, C.; Beani, J.C.; Amblard, P. Diagnosis of photosensitivity to flupenthixol by photoprick testing. Photodermatol. Photoimmunol. Photomed. 1997, 13, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, S.; Karagiorga, T.; Stratigos, A.; Volonakis, K.; Kontochristopoulos, G.; Rigopoulos, D. Photo-onycholysis caused by olanzapine and aripiprazole. J. Clin. Psychopharmacol. 2008, 28, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.E.; Bhawan, J.; Stefanato, C.M.; McCalmont, T.H.; Cohen, L.M. Imipramine-induced hyperpigmentation: Four cases and a review of the literature. J. Am. Acad. Dermatol. 1999, 40, 159–166. [Google Scholar] [CrossRef]
- Thédenat, B.; Loche, F.; Albes, B.; Marguery, M.C.; Bazex, J. Acute generalized exanthematous pustulosis with photodistribution pattern induced by sertraline. Dermatology 2001, 203, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Doffoel-Hantz, V.; Boulitrop-Morvan, C.; Sparsa, A.; Bonnetblanc, J.M.; Dalac, S.; Bédane, C. Photosensitivity associated with selective serotonin reuptake inhibitors. Clin. Exp. Dermatol. 2009, 34, e763–e765. [Google Scholar] [CrossRef] [PubMed]
- Ram-Wolf, C.; Mahé, E.; Saiag, P. Escitalopram photo-induced erythroderma. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1015–1017. [Google Scholar] [CrossRef]
- Inalöz, H.S.; Kirtak, N.; Herken, H.; Ozgöztaşi, O.; Aynacioğlu, A.S. Citalopram-induced photopigmentation. J. Dermatol. 2001, 28, 742–745. [Google Scholar]
- Pazzagli, L.; Banfi, R.; Borselli, G.; Semmola, M.V. Photosensitivity reaction to fluoxetine and alprazolam. Pharm. World Sci. 1998, 20, 136. [Google Scholar] [CrossRef] [PubMed]
- Röhrs, S.; Geiser, F.; Conrad, R. Citalopram-induced subacute cutaneous lupus erythematosus—First case and review concerning photosensitivity in selective serotonin reuptake inhibitors. Gen. Hosp. Psychiatry 2012, 34, 541–545. [Google Scholar] [CrossRef]
- Vaccaro, M.; Borgia, F.; Barbuzza, O.; Guarneri, B. Photodistributed eruptive telangiectasia: An uncommon adverse drug reaction to venlafaxine. Br. J. Dermatol. 2007, 157, 822–824. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kawada, A.; Ohnishi, Y.; Tajima, S.; Ishibashi, A. Photosensitivity due to alprazolam with positive oral photochallenge test after 17 days administration. J. Am. Acad. Dermatol. 1999, 40, 832–833. [Google Scholar] [CrossRef]
- Chignell, C.F.; Motten, A.G.; Buettner, G.R. Photoinduced free radicals from chlorpromazine and related phenothiazines: Relationship to phenothiazine-induced photosensitization. Environ. Health Perspect. 1985, 64, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Piñero-Santiago, L.E.; García, C.; Lhiaubet-Vallet, V.; Trzcionka, J.; Oyola, R.; Torres, K.; Leguillú, J.; Miranda, M.A. Photooxidation mechanism of levomepromazine in different solvents. Photochem. Photobiol. 2013, 89, 1479–1489. [Google Scholar] [CrossRef]
- Rodrigues, T.; dos Santos, C.G.; Riposati, A.; Barbosa, L.R.; di Mascio, P.; Itri, R.; Baptista, M.S.; Nascimento, O.R.; Nantes, I.L. Photochemically generated stable cation radical of phenothiazine aggregates in mildly acid buffered solutions. J. Phys. Chem. B 2006, 110, 12257–12265. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, I.O.; Pavani, C.; Sales, E.M.; Itri, R.; Wainwright, M.; Baptista, M.S. Membrane damage efficiency of phenothiazinium photosensitizers. Photochem. Photobiol. 2014, 90, 801–813. [Google Scholar] [CrossRef]
- Elisei, F.; Latterini, L.; Aloisi, G.G.; Mazzucato, U.; Viola, G.; Miolo, G.; Vedaldi, D.; Dall’Acqua, F. Excited-state properties and in vitro phototoxicity studies of three phenothiazine derivatives. Photochem. Photobiol. 2002, 75, 11–21. [Google Scholar] [CrossRef]
- Onoue, S.; Kato, M.; Inoue, R.; Seto, Y.; Yamada, S. Photosafety screening of phenothiazine derivatives with combined use of photochemical and cassette-dosing pharmacokinetic data. Toxicol. Sci. 2014, 137, 469–477. [Google Scholar] [CrossRef][Green Version]
- Viola, G.; Latterini, L.; Vedaldi, D.; Aloisi, G.G.; Dall’Acqua, F.; Gabellini, N.; Elisei, F.; Barbafina, A. Photosensitization of DNA strand breaks by three phenothiazine derivatives. Chem. Res. Toxicol. 2003, 16, 644–651. [Google Scholar] [CrossRef]
- Drago, F.; Gasparini, G.; Marenco, S.; Picciotto, A.; Parodi, A. Porphyrin elevation in a patient on treatment with simeprevir: Could it be a possible explanation for simeprevir-associated photosensitivity? Am. J. Gastroenterol. 2016, 111, 1368. [Google Scholar] [CrossRef]
- Verma, R.; Vasudevan, B.; Shankar, S.; Pragasam, V.; Suwal, B.; Venugopal, R. First reported case of tenofovir-induced photoallergic reaction. Ind. J. Pharmacol. 2012, 44, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Furue, M. Photosensitive drug eruption induced by efavirenz in a patient with HIV infection. Intern. Med. 2004, 43, 533. [Google Scholar] [CrossRef]
- Isaacs, T.; Ngwanya, M.R.; Dlamini, S.; Lehloenya, R.J. Annular erythema and photosensitivity as manifestations of efavirenz-induced cutaneous reactions: A review of five consecutive cases. J. Antimicrob. Chemother. 2013, 68, 2871–2874. [Google Scholar] [CrossRef] [PubMed]
- Treudler, R.; Husak, R.; Raisova, M.; Orfanos, C.E.; Tebbe, B. Efavirenz-induced photoallergic dermatitis in HIV. AIDS 2001, 15, 1085–1086. [Google Scholar] [CrossRef]
- Yoshimoto, E.; Konishi, M.; Takahashi, K.; Murakawa, K.; Maeda, K.; Mikasa, K.; Yamashina, Y. The first case of efavirenz-induced photosensitivity in a Japanese patient with HIV infection. Intern. Med. 2004, 43, 630–631. [Google Scholar] [CrossRef]
- Alvarez-Fernández, J.G.; Castaño-Suárez, E.; Cornejo-Navarro, P.; de la Fuente, E.G.; de Frutos, F.J.O.; Iglesias-Diez, L. Photosensitivity induced by oral itraconazole. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, R.; Hafner, M.F.S.; Miguel, B.A.F.; Kawakami, N.T.; Nakagome, B.H.Y. Allergic contact dermatitis caused by topical ketoconazole: A relevant issue? Review of ketoconazole-positive patch tests. Contact Derm. 2018, 78, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, N.S.; Wetter, D.A. Bullous phototoxicity from voriconazole. J. Emerg. Med. 2014, 46, e83–e84. [Google Scholar] [CrossRef] [PubMed]
- Tolland, J.P.; McKeown, P.P.; Corbett, J.R. Voriconazole-induced pseudoporphyria. Photodermatol. Photoimmunol. Photomed. 2007, 23, 29–31. [Google Scholar] [CrossRef]
- Rubenstein, M.; Levy, M.L.; Metry, D. Voriconazole-induced retinoid-like photosensitivity in children. Pediatr. Dermatol. 2004, 21, 675–678. [Google Scholar] [CrossRef]
- Racette, A.J.; Roenigk, H.H., Jr.; Hansen, R.; Mendelson, D.; Park, A. Photoaging and phototoxicity from long-term voriconazole treatment in a 15-year-old girl. J. Am. Acad. Dermatol. 2005, 52, S81–S85. [Google Scholar] [CrossRef]
- Miller, D.D.; Cowen, E.W.; Nguyen, J.C.; McCalmont, T.H.; Fox, L.P. Melanoma associated with long-term voriconazole therapy: A new manifestation of chronic photosensitivity. Arch. Dermatol. 2010, 146, 300–304. [Google Scholar] [CrossRef]
- Cowen, E.W.; Nguyen, J.C.; Miller, D.D.; McShane, D.; Arron, S.T.; Prose, N.S.; Turner, M.L.; Fox, L.P. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J. Am. Acad. Dermatol. 2010, 62, 31–37. [Google Scholar] [CrossRef]
- McCarthy, K.L.; Playford, E.G.; Looke, D.F.; Whitby, M. Severe photosensitivity causing multifocal squamous cell carcinomas secondary to prolonged voriconazole therapy. Clin. Infect. Dis. 2007, 44, e55–e56. [Google Scholar] [CrossRef]
- Williams, K.; Mansh, M.; Chin-Hong, P.; Singer, J.; Arron, S.T. Voriconazole-associated cutaneous malignancy: A literature review on photocarcinogenesis in organ transplant recipients. Clin. Infect. Dis. 2014, 58, 997–1002. [Google Scholar] [CrossRef]
- Ramachandran, S.M.; Leventhal, J.S.; Franco, L.G.; Mir, A.; Walters, R.F.; Franks, A.G., Jr. Topical drug-induced subacute cutaneous lupus erythematosus isolated to the hands. Lupus Sci. Med. 2017, 4, e000207. [Google Scholar] [CrossRef] [PubMed]
- Morlière, P.; Silva, A.; Seixas, R.; Boscá, F.; Mazière, J.C.; Ferreira, J.; Santus, R.; Filipe, P. Photosensitisation by voriconazole-N-oxide results from a sequence of solvent and pH-dependent photochemical and thermal reactions. J. Photochem. Photobiol. B 2018, 187, 1–9. [Google Scholar] [CrossRef]
- Ona, K.; Oh, D.H. Voriconazole N-oxide and its ultraviolet B photoproduct sensitize keratinocytes to ultraviolet A. Br. J. Dermatol. 2015, 173, 751–759. [Google Scholar] [CrossRef]
- Moore, D.E. Drug-induced cutaneous photosensitivity: Incidence, mechanism, prevention and management. Drug Saf. 2002, 25, 345–372. [Google Scholar] [CrossRef]
- Goetze, S.; Hiernickel, C.; Elsner, P. Phototoxicity of doxycycline: A systematic review on clinical manifestations, frequency, cofactors, and prevention. Skin Pharmacol. Physiol. 2017, 30, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.K.; Prendiville, J.; Peacock, D.L.; Wong, L.T.; Davidson, A.G. An unusual presentation of doxycycline-induced photosensitivity. Pediatrics 2000, 106, E13. [Google Scholar] [CrossRef]
- Yap, L.M.; Foley, P.A.; Crouch, R.B.; Baker, C.S. Drug-induced solar urticaria due to tetracycline. Australas. J. Dermatol. 2000, 41, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Owens, R.C., Jr.; Ambrose, P.G. Antimicrobial safety: Focus on fluoroquinolones. Clin. Infect. Dis. 2005, 41, S144–S157. [Google Scholar] [CrossRef]
- Borgia, F.; Vaccaro, M.; Guarneri, F.; Cannavò, S.P. Photodistributed telangiectasia following use of cefotaxime. Br. J. Dermatol. 2000, 143, 674–675. [Google Scholar] [CrossRef]
- Lee, A.Y.; Jung, S.Y. Two patients with isoniazid-induced photosensitive lichenoid eruptions confirmed by photopatch test. Photodermatol. Photoimmunol. Photomed. 1998, 14, 77–78. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Bihari, S.; Prakash, S. Pyrazinamide-induced phototoxicity: A case report and review of literature. Ind. J. Dermatol. 2010, 55, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Kar, B.R. Dapsone-induced photosensitivity: A rare clinical presentation. Photodermatol. Photoimmunol. Photomed. 2008, 24, 270–271. [Google Scholar] [CrossRef] [PubMed]
- De, D.; Dogra, S.; Kaur, I. Dapsone induced acute photosensitivity dermatitis; A case report and review of literature. Lepr. Rev. 2007, 78, 401–404. [Google Scholar] [CrossRef]
- Cuquerella, M.C.; Miranda, M.A.; Bosca, F. Generation of detectable singlet aryl cations by photodehalogenation of fluoroquinolones. J. Phys. Chem. B 2006, 110, 6441–6443. [Google Scholar] [CrossRef] [PubMed]
- Fasani, E.; Mella, M.; Caccia, D.; Tassi, S.; Fagnoni, M.; Albini, A. The photochemistry of lomefloxacin. An aromatic carbene as the key intermediate in photodecomposition. Chem. Commun. 1997, 14, 1329–1330. [Google Scholar] [CrossRef]
- Thompson, A.M. Ocular toxicity of fluoroquinolones. Clin. Exp. Ophthalmol. 2007, 35, 566–577. [Google Scholar] [CrossRef]
- Martínez, L.J.; Sik, R.H.; Chignell, C.F. Fluoroquinolone antimicrobials: Singlet oxygen, superoxide and phototoxicity. Photochem. Photobiol. 1998, 67, 399–403. [Google Scholar] [CrossRef]
- Shimoda, K. Mechanisms of quinolone phototoxicity. Toxicol. Lett. 1998, 102-103, 369–373. [Google Scholar] [CrossRef]
- De Guidi, G.; Bracchitta, G.; Catalfo, A. Photosensitization reactions of fluoroquinolones and their biological consequences. Photochem. Photobiol. 2011, 87, 1214–1229. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; Banach, K.; Rok, J.; Beberok, A.; Rzepka, Z.; Wrześniok, D. Molecular and biochemical basis of fluoroquinolones-induced phototoxicity-the study of antioxidant system in human melanocytes exposed to UV-A radiation. Int. J. Mol. Sci. 2020, 21, 9714. [Google Scholar] [CrossRef] [PubMed]
- Viola, G.; Facciolo, L.; Canton, M.; Vedaldi, D.; Dall’Acqua, F.; Aloisi, G.G.; Amelia, M.; Barbafina, A.; Elisei, F.; Latterini, L. Photophysical and phototoxic properties of the antibacterial fluoroquinolones levofloxacin and moxifloxacin. Chem. Biodivers 2004, 1, 782–801. [Google Scholar] [CrossRef]
- Soldevila, S.; Cuquerella, M.C.; Bosca, F. Understanding of the photoallergic properties of fluoroquinolones: Photoreactivity of lomefloxacin with amino acids and albumin. Chem. Res. Toxicol. 2014, 27, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.L. Chemical and biological dynamics of tetracyclines. Adv. Dent. Res. 1998, 12, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, C.; Qu, J.; Yang, M. Photodegradation of tetracycline and formation of reactive oxygen species in aqueous tetracycline solution under simulated sunlight irradiation. J. Photochem. Photobiol. A 2008, 197, 81–87. [Google Scholar] [CrossRef]
- Drexel, R.E.; Olack, G.; Jones, C.; Chmurny, G.N.; Santini, R.; Morrison, H. Lumitetracycline: A novel new tetracycline photoproduct. J. Org. Chem. 1990, 55, 2471–2478. [Google Scholar] [CrossRef]
- Shea, C.R.; Olack, G.A.; Morrison, H.; Chen, N.; Hasan, T. Phototoxicity of lumidoxycycline. J. Invest. Dermatol. 1993, 101, 329–333. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Wang, Z.; Tao, T.; Hu, C. Photoproducts of tetracycline and oxytetracycline involving self-sensitized oxidation in aqueous solutions: Effects of Ca2+ and Mg2+. J. Environ. Sci. 2011, 23, 1634–1639. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Wang, Z.; Tao, T.; Wei, D.; Hu, C. Photolysis of chlortetracycline in aqueous solution: Kinetics, toxicity and products. J. Environ. Sci. 2012, 24, 254–260. [Google Scholar] [CrossRef]
- Wammer, K.H.; Slattery, M.T.; Stemig, A.M.; Ditty, J.L. Tetracycline photolysis in natural waters: Loss of antibacterial activity. Chemosphere 2011, 85, 1505–1510. [Google Scholar] [CrossRef]
- Jiao, S.; Zheng, S.; Yin, D.; Wang, L.; Chen, L. Aqueous photolysis of tetracycline and toxicity of photolytic products to luminescent bacteria. Chemosphere 2008, 73, 377–382. [Google Scholar] [CrossRef]
- Epstein, J.H. Phototoxicity and photoallergy. Semin. Cutan. Med. Surg. 1999, 18, 274–284. [Google Scholar] [CrossRef]
- Aronson, J.K. (Ed.) Meyler’s side effects of drugs. In The International Encyclopedia of Adverse Drug Reactions and Interactions, 15th ed.; Elsevier: New York, NY, USA, 2006; pp. 3330–3342. [Google Scholar]
- Nicolaidou, E.; Katsambas, A.D. Pigmentation disorders: Hyperpigmentation and hypopigmentation. Clin. Dermatol. 2014, 32, 66–72. [Google Scholar] [CrossRef]
- Trzcionka, J.; Buszman, E. Oddziaływanie antybiotyków tetracyklinowych z melaniną w aspekcie ich fototoksycznego działania na skórę. Ann. Acad. Med. Siles. 2003, 54–55, 55–63. [Google Scholar]
- Rok, J.; Buszman, E.; Delijewski, M.; Otręba, M.; Beberok, A.; Wrześniok, D. Effect of tetracycline and UV radiation on melanization and antioxidant status of melanocytes. J. Photochem. Photobiol. B 2015, 148, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Wrześniok, D.; Beberok, A.; Otręba, M.; Delijewski, M.; Buszman, E. Phototoxic effect of oxytetracycline on normal human melanocytes. Toxicol. In Vitro 2018, 48, 26–32. [Google Scholar] [CrossRef]
- Agudo-Mena, J.L.; Romero-Pérez, D.; Encabo-Durán, B.; Álvarez-Chinchilla, P.J.; Silvestre-Salvador, J.F. Photoallergic contact dermatitis caused by quinidine sulfate in a caregiver. Contact Derm. 2017, 77, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Nacher, M.; McGready, R.; Lermoo, C.; Wichiponpiboon, J.; Nosten, F. Photoallergy to quinine. Trop. Doct. 2005, 35, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Lisi, P.; Assalve, D.; Hansel, K. Phototoxic and photoallergic dermatitis caused by hydroxychloroquine. Contact Derm. 2004, 50, 255–256. [Google Scholar] [CrossRef]
- Walker, G.; Lane, N.; Parekh, P. Photosensitive lichenoid drug eruption to capecitabine. J. Am. Acad. Dermatol. 2014, 71, e52–e53. [Google Scholar] [CrossRef]
- Almagro, B.M.; Steyls, M.C.; Navarro, N.L.; Domínguez, E.G.; Acosta, E.H.; Pérez, M.A.G.; Ceballos, E.H. Occurrence of subacute cutaneous lupus erythematosus after treatment with systemic fluorouracil. J. Clin. Oncol. 2011, 29, e613–e615. [Google Scholar] [CrossRef] [PubMed]
- Weger, W.; Kränke, B.; Gerger, A.; Salmhofer, W.; Aberer, E. Occurrence of subacute cutaneous lupus erythematosus after treatment with fluorouracil and capecitabine. J. Am. Acad. Dermatol. 2008, 59, S4–S6. [Google Scholar] [CrossRef]
- Beutler, B.D.; Cohen, P.R. Nab-paclitaxel-associated photosensitivity: Report in a woman with non-small cell lung cancer and review of taxane-related photodermatoses. Dermatol. Pract. Concept. 2015, 5, 121–124. [Google Scholar] [CrossRef]
- Hussain, S.; Anderson, D.N.; Salvatti, M.E.; Adamson, B.; McManus, M.; Braverman, A.S. Onycholysis as a complication of systemic chemotherapy: Report of five cases associated with prolonged weekly paclitaxel therapy and review of the literature. Cancer 2000, 88, 2367–2371. [Google Scholar] [CrossRef]
- Yanamandra, U.; Sahu, K.K.; Malhotra, P.; Varma, S. Photodermatosis secondary to hydroxyurea. BMJ Case Rep. 2014, bcr2014205974. [Google Scholar] [CrossRef] [PubMed]
- Keating, M.; Dasanu, C.A. Severe phototoxic reaction secondary to subcutaneous 5-azacitidine. J. Oncol. Pharm. Pract. 2017, 23, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Gabeff, R.; Dutartre, H.; Khammari, A.; Boisrobert, A.; Nguyen, J.M.; Quereux, G.; Brocard, A.; Saint-Jean, M.; Peuvrel, L.; Dreno, B. Phototoxicity of B-RAF inhibitors: Exclusively due to UVA radiation and rapidly regressive. Eur. J. Dermatol. 2015, 25, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Jew, O.S.; Provini, L.E.; Treat, J.R. Severe vemurafenib-induced photosensitivity in a 6-year-old boy. Pediatr. Dermatol. 2019, 36, e62–e63. [Google Scholar] [CrossRef]
- Brazzelli, V.; Muzio, F.; Manna, G.; Moggio, E.; Vassallo, C.; Orlandi, E.; Fiandrino, G.; Lucioni, M.; Borroni, G. Photoinduced dermatitis and oral lichenoid reaction in a chronic myeloid leukemia patient treated with imatinib mesylate. Photodermatol. Photoimmunol. Photomed. 2012, 28, 2–5. [Google Scholar] [CrossRef]
- Rousselot, P.; Larghero, J.; Raffoux, E.; Calvo, F.; Tulliez, M.; Giraudier, S.; Rybojad, M. Photosensitization in chronic myelogenous leukaemia patients treated with imatinib mesylate. Br. J. Haematol. 2003, 120, 1091–1092. [Google Scholar] [CrossRef]
- Udompanich, S.; Chanprapaph, K.; Rajatanavin, N. Phototoxic reaction induced by pazopanib. Case Rep. Dermatol. 2018, 10, 251–256. [Google Scholar] [CrossRef]
- Giacchero, D.; Ramacciotti, C.; Arnault, J.P.; Brassard, M.; Baudin, E.; Maksimovic, L.; Mateus, C.; Tomasic, G.; Wechsler, J.; Schlumberger, M.; et al. A new spectrum of skin toxic effects associated with the multikinase inhibitor vandetanib. Arch. Derm. 2012, 148, 1418–1420. [Google Scholar] [CrossRef]
- Caro-Gutiérrez, D.; Floristán Muruzábal, M.U.; de la Fuente, E.G.; Franco, A.P.; López Estebaranz, J.L. Photo-induced erythema multiforme associated with vandetanib administration. J. Am. Acad. Dermatol. 2014, 71, e142–e144. [Google Scholar] [CrossRef]
- Kong, H.H.; Fine, H.A.; Stern, J.B.; Turner, M.L.C. Cutaneous pigmentation after photosensitivity induced by vandetanib therapy. Arch. Derm. 2009, 145, 923–925. [Google Scholar] [CrossRef]
- Fava, P.; Quaglino, P.; Fierro, M.T.; Novelli, M.; Bernengo, M.G. Therapeutic hotline. A rare vandetanib-induced photo-allergic drug eruption. Dermatol. Ther. 2010, 23, 553–555. [Google Scholar] [CrossRef]
- Lee, K.; Oda, Y.; Sakaguchi, M.; Yamamoto, A.; Nishigori, C. Drug-induced photosensitivity to bicalutamide—Case report and review of the literature. Photodermatol. Photoimmunol. Photomed. 2016, 32, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Rafael, J.P.; Manuel, G.G.; Antonio, V.; Carlos, M.J. Widespread vitiligo after erythroderma caused by photosensitivity to flutamide. Contact Derm. 2004, 50, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Tatsuno, K.; Kitano, S.; Miyazawa, H.; Ishibe, J.; Aoshima, M.; Shimauchi, T.; Fujiyama, T.; Ito, T.; Tokura, Y. Mogamulizumab-induced photosensitivity in patients with mycosis fungoides and other T-cell neoplasms. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.L.; Bridges, A.G. Phototoxic drug reaction with the novel agent rovalpituzumab tesirine. Int. J. Dermatol. 2018, 57, e17–e19. [Google Scholar] [CrossRef] [PubMed]
- Miolo, G.; Marzano, C.; Gandin, V.; Palozzo, A.C.; Dalzoppo, D.; Salvador, A.; Caffieri, S. Photoreactivity of 5-fluorouracil under UVB light: Photolysis and cytotoxicity studies. Chem Res Toxicol. 2011, 24, 1319–1326. [Google Scholar] [CrossRef]
- Dall’acqua, S.; Vedaldi, D.; Salvador, A. Isolation and structure elucidation of the main UV-A photoproducts of vandetanib. J. Pharm. Biomed. Anal. 2013, 84, 196–200. [Google Scholar] [CrossRef]
- Gómez-Bernal, S.; Loureiro, M.; Rodríguez-Granados, M.T.; Toribio, J. Systemic photosensitivity due to a contraceptive patch. Photodermatol. Photoimmunol. Photomed. 2010, 26, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Kagaya, M.; Yamada, Y.; Matsusaka, H.; Jimbow, K. UVB photosensitivity due to ranitidine. Dermatology 2000, 201, 71–73. [Google Scholar] [CrossRef]
- Shukla, A.; Mahapatra, A.; Gogtay, N.; Khopkar, U. Esomeprazole-induced photoallergic dermatitis. J. Postgrad. Med. 2010, 56, 229–231. [Google Scholar] [CrossRef]
- Droitcourt, C.; Adamski, H.; Polat, A.; Polard, E.; Kerjouan, M.; Arnouat, B.; Garrec, M.L.; Oger, E.; Dupuy, A.; Jouneau, S. Pirfenidone photosensitization in patients with idiopathic pulmonary fibrosis: A case series. Br. J. Dermatol. 2018, 178, e222–e223. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-Y.; Shim, W.-H.; Kim, J.-M.; Kim, G.-W.; Kim, H.-S.; Ko, H.-C.; Kim, M.-B.; Kim, B.-S. Pirfenidone-induced photo-allergic reaction in a patient with idiopathic pulmonary fibrosis. Photodermatol. Photoimmunol. Photomed. 2017, 33, 209–212. [Google Scholar] [CrossRef] [PubMed]
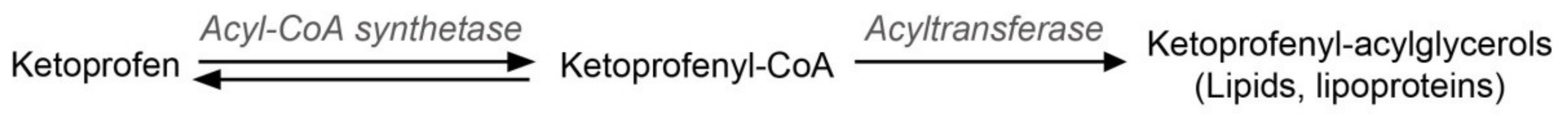
| Source | Emitted Spectrum of Wavelengths |
|---|---|
| Germicidal lamps for sterilization and disinfection | UVC |
| Welding arcs | Full spectrum of ultraviolet (UVA, UVB, UVC) |
| High power metal halide and tungsten halogen lamps | Full spectrum of ultraviolet (UVA, UVB, UVC) |
| UV lasers and light emitting diodes (LEDs) | UVA/UVB/UVC |
| Fluorescent lamps | UVA + UVB |
| Sunlamps, sunbeds, and tanning beds | UVA or UVA + UVB |
| Phototherapy lamps used for medical and dental conditions | UVA or UVB |
| UV photocuring | UVA |
| UVA “blacklight” lamps | UVA |
| UVA lamps used for material inspection in industry, checking banknotes, and as insect traps | UVA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, J.; Rok, J.; Rzepka, Z.; Wrześniok, D. Drug-Induced Photosensitivity—From Light and Chemistry to Biological Reactions and Clinical Symptoms. Pharmaceuticals 2021, 14, 723. https://doi.org/10.3390/ph14080723
Kowalska J, Rok J, Rzepka Z, Wrześniok D. Drug-Induced Photosensitivity—From Light and Chemistry to Biological Reactions and Clinical Symptoms. Pharmaceuticals. 2021; 14(8):723. https://doi.org/10.3390/ph14080723
Chicago/Turabian StyleKowalska, Justyna, Jakub Rok, Zuzanna Rzepka, and Dorota Wrześniok. 2021. "Drug-Induced Photosensitivity—From Light and Chemistry to Biological Reactions and Clinical Symptoms" Pharmaceuticals 14, no. 8: 723. https://doi.org/10.3390/ph14080723
APA StyleKowalska, J., Rok, J., Rzepka, Z., & Wrześniok, D. (2021). Drug-Induced Photosensitivity—From Light and Chemistry to Biological Reactions and Clinical Symptoms. Pharmaceuticals, 14(8), 723. https://doi.org/10.3390/ph14080723






