Quercetin and/or Ascorbic Acid Modulatory Effect on Phenobarbital-Induced Sleeping Mice Possibly through GABAA and GABAB Receptor Interaction Pathway
Abstract
:1. Introduction
2. Results
2.1. Animal Study
2.2. In Silico Study
2.2.1. GABA Homology Model
2.2.2. Interaction of Quercetin (QUR) with GABA Receptor
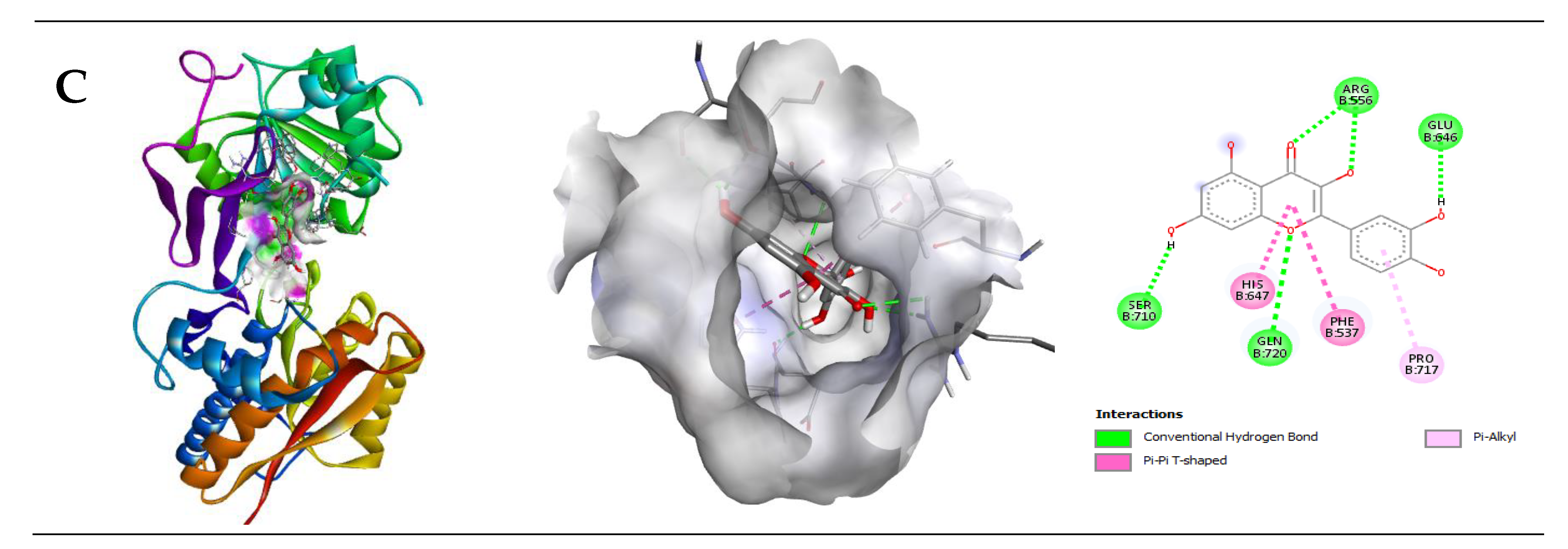
2.2.3. Interaction of Ascorbic Acid (AA) with GABA Receptor
2.2.4. MD Simulation Study
2.2.5. Binding Free Energy (MM-PBSA) Analysis
3. Discussion
4. Materials and Methods
4.1. Animal Model Study
4.1.1. Chemicals and Reagents
4.1.2. Experimental Animals
4.1.3. Phenobarbital-Induced Sleeping Test
4.1.4. Statistical Analysis
4.2. Molecular Docking (In Silico) Study
4.2.1. GABA Homology Model and Macromolecule
4.2.2. Ligand Preparation
4.2.3. Docking Protocol
4.2.4. Molecular Dynamic (MD) Simulation Study
4.2.5. Molecular Mechanics/Poisson-Boltzmann Surface Area (MM-PBSA) Calculations
5. Conclusions and Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.; Jang, E.Y.; Xiang, Y.; Kanba, S.; Kato, T.A.; Chong, M.; Lin, S.; Yang, S.; Avasthi, A.; Grover, S.; et al. Network analysis of the depressive symptom profiles in Asian patients with depressive disorders: Findings from the Research on Asian Psychotropic Prescription Patterns for Antidepressants (REAP-AD). Psychiatry Clin. Neurosci. 2020, 74, 344–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKenzie, C.S.; Reynolds, K.; Chou, K.L.; Pagura, J.; Sareen, J. Prevalence and correlates of Generalized Anxiety Disorder in a National Sample of Older Adults. Am. J. Geriatr. Psychiatry 2011, 19, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Patel, V.; Saxena, S.; Maj, M.; Maselko, J.; Phillips, M.R.; Rahman, A. No health without mental health. Lancet 2007, 370, 859–877. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Anderson, J.P. The General Health Policy Model: An Integrated Approach: Quality of Life Assessments in Clinical Trials; RavenPress: New York, NY, USA, 1990. [Google Scholar]
- Costa, J.P.; de Oliveira, G.A.L.; de Almeida, A.A.C.; Islam, M.T.; de Sousa, D.P.; de Freitas, R.M. Anxiolytic-like effects of phytol: Possible involvement of GABAergic transmission. Brain Res. 2014, 1547, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Markou, A.; Cryan, J.F. Stress, anxiety and depression: Toward new treatment strategies. Neuropharmacology 2012, 62, 1–2. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Nutt, D.J. Role of GABA in anxiety and depression. Depress. Anxiety 2007, 24, 495–517. [Google Scholar] [CrossRef] [PubMed]
- Enna, S.J.; Bowery, N.G. The GABA Receptors, 2nd ed.; Enna, S.J., Bowery, N., Eds.; Humana Press: New York, NY, USA, 1996; ISBN 9780896034587. [Google Scholar]
- Bowery, N.G.; Enna, S.J. γ-Aminobutyric acid receptors: First of the functional metabotropic heterodimers. J. Pharmacol. Exp. Ther. 2000, 292, 2–7. [Google Scholar]
- Atack, J.R. Anxio-selective Compounds Acting at the GABAA Receptor Benzodiazepine Binding Site. Curr. Drug Target CNS Neurol. Disord. 2003, 2, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Carbotte, R.M.; Denburg, S.D.; Denburg, J.A. Prevalence of Cognitive Impairment in Systemic Lupus Erythematosus. J. Nerv. Ment. Dis. 1986, 174, 357–364. [Google Scholar] [CrossRef]
- Hay, E.M.; Black, D.; Huddy, A.; Creed, F.; Tomenson, B.; Bernstein, R.M.; Holt, P.J. Psychiatric disorder and cognitive impairment in systemic lupus erythematosus. Arthritis Rheum. 1992, 35, 411–416. [Google Scholar] [CrossRef]
- Outset, T.; Golden, M.; Siberry, G.; Kiri, N.; Crum, R.M.; Petri, M. Depressive symptoms in patients with systemic lupus erythematosus: Association with central nervous system lupus and Sjögren’s syndrome. J. Rheumatol. 1994, 21, 2039–2045. [Google Scholar]
- Kozora, E.; Thompson, L.L.; West, S.G.; Kotzin, B.L. Analysis of cognitive and psychological deficits in systemic lupus erythematosus patients without overt central nervous system disease. Arthritis Rheum. 1996, 39, 2035–2045. [Google Scholar] [CrossRef] [PubMed]
- Brey, R.L.; Holliday, S.L.; Saklad, A.R.; Navarrete, M.G.; Hermosillo-Romo, D.; Stallworth, C.L.; Valdez, C.R.; Escalante, A.; DelRincón, I.; Gronseth, G.; et al. Neuropsychiatric syndromes in lupus: Prevalence using standardized definitions. Neurology 2002, 58, 1214–1220. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Cha, D.S.; Soczynska, J.K.; Woldeyohannes, H.O.; Gallaugher, L.A.; Kudlow, P.; Alsuwaidan, M.; Baskaran, A. Cognitive deficits and functional outcomes in major depressive disorder: Determinants, substrates, and treatment interventions: Review: Cognitive deficits and functional outcomes in MDD. Depress. Anxiety 2013, 30, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Vortioxetine: A Review in Cognitive Dysfunction in Depression. Drugs 2016, 76, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Korczyn, A.D.; Halperin, I. Depression and dementia. J. Neurol. Sci. 2009, 283, 139–142. [Google Scholar] [CrossRef]
- Rosenberg, P.B.; Mielke, M.M.; Appleby, B.S.; Oh, E.S.; Geda, Y.E.; Lyketsos, C.G. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am. J. Geriatr. Psychiatry 2013, 21, 685–695. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Sun, L.; Wang, J.; Sun, F.; Wang, W.; Wang, D.; Fan, X.; Liu, D.; Xu, Z.; Qiu, C.; et al. Role of Adiponectin-Notch pathway in cognitive dysfunction associated with depression and in the therapeutic effect of physical exercise. Aging Cell 2021, 20, e13387. [Google Scholar] [CrossRef]
- Murrough, J.W.; Iacoviello, B.; Neumeister, A.; Charney, D.S.; Iosifescu, D.V. Cognitive dysfunction in depression: Neurocircuitry and new therapeutic strategies. Neurobiol. Learn Mem. 2011, 96, 553–563. [Google Scholar] [CrossRef]
- Richelson, E. Multi-modality: A new approach for the treatment of major depressive disorder. Int. J. Neuropsychopharmacol. 2013, 16, 1433–1442. [Google Scholar] [CrossRef] [Green Version]
- Keefe, R.S.E.; McClintock, S.M.; Roth, R.M.; Doraiswamy, P.M.; Tiger, S.; Madhoo, M. Cognitive effects of pharmacotherapy for major depressive disorder: A systematic review. J. Clin. Psychiatry 2014, 75, 864–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortolato, B.; Miskowiak, K.W.; Köhler, C.A.; Maes, M.; Fernandes, B.S.; Berk, M.; Carvalho, A.F. Cognitive remission: A novel objective for the treatment of major depression? BMC Med. 2016, 14, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, R.W.; Kennedy, S.H.; Mclntyre, R.S.; Khullar, A. Cognitive dysfunction in major depressive disorder: Effects on psychosocial functioning and implications for treatment. Can. J. Psychiatry 2014, 59, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Pehrson, A.L.; Sanchez, C. Serotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunction. CNS Spectr. 2014, 19, 121–133. [Google Scholar] [CrossRef]
- Arnett, P.A.; Higginson, C.I.; Voss, W.D.; Randolph, J.J.; Grandey, A.A. Relationship between coping, cognitive dysfunction and depression in multiple sclerosis. Clin. Neuropsychol. 2002, 16, 341–355. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Xiao, H.X.; Syeda, K.; Vinberg, M.; Carvalho, A.F.; Mansur, R.B.; Maruschak, N.; Cha, D.S. The prevalence, measurement, and treatment of the cognitive dimension/domain in major depressive disorder. CNS Drugs 2015, 29, 577–589. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Lee, Y.; Mansur, R.B. Treating to target in major depressive disorder: Response to remission to functional recovery. CNS Spectr. 2015, 20, 17–31. [Google Scholar] [CrossRef]
- Mitte, K.; Noack, P.; Steil, R.; Hautzinger, M. Ameta-analytic review of the efficacy of drug treatment in generalized anxiety disorder. J. Clin. Psychopharmacol. 2005, 25, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, C.M.; Hanley, G.; Morgan, S. Patterns in the use of benzodiazepines in British Columbia: Examining the impact of increasing research and guideline cautions against long term use. Health Policy 2010, 97, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, B.; Lader, M. Do Benzodiazepines Still Deserve a Major Role in The Treatment of Psychiatric Disorders? A Critical Reappraisal. Eur. Psychiatry 2013, 28, 7–20. [Google Scholar] [CrossRef]
- Galdino, P.M.; Nascimento, M.V.M.; Florentino, I.F.; Lino, R.C.; Fajemiroye, J.O.; Chaibub, B.A.; Paula, J.R.; Lima, T.C.M.; Costa, E.A. The anxiolytic-like effect of an essential oil derived from Spiranthera odoratissima A. St. Hil. leaves and its major component, β-caryophyllene, in male mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fajemiroye, J.O.; Adam, K.; Jordan, K.Z.; Alves, C.E.; Aderoju, A.A. Evaluation of anxiolytic and antidepressant-like activity of aqueous leaf extract of Nymphaea lotus Linn. In mice. Iran. J. Pharm. Res. 2018, 17, 613–626. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Productsas Sources of New Drugsover the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Pallauf, K.; Duckstein, N.; Rimbach, G. A literature review of flavonoids and lifespan in model organisms. Proc. Nutr. Soc. 2017, 76, 145–162. [Google Scholar] [CrossRef] [Green Version]
- Erukainure, O.L.; Hafizur, R.M.; Kabir, N.; Choudhary, M.I.; Atolani, O.; Banerjee, P.; Chukwuma, C.I.; Muhammad, A.; Amonsou, E.; Islam, M. Suppressive Effects of Clerodendrumvolubile P Beauv. [Labiatae] Methanolic Extract and Its Fractions on Type 2 Diabetes and Its Complications. Front. Pharmacol. 2018, 9, 8. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Mesaik, M.A.; Atolani, O.; Muhammad, A.; Chukwuma, C.I.; Islam, M.S. Pectolinarigenin from the leaves of Clerodendrum volubile shows potent immunomodulatory activity by inhibiting T−cell proliferation and modulating respiratory oxidative burst in phagocytes. Biomed. Pharmacother. 2017, 93, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, K.; Jiang, K.; Tao, S.; Li, Y.; Chen, W.; Kou, S.; Gu, C.; Li, Z.; Guo, L.; et al. A Review of Flavonoids from Cassia Species and their Biological Activity. Curr. Pharm. Biotechnol. 2016, 17, 1134–1146. [Google Scholar] [CrossRef]
- Faggio, C.; Sureda, A.; Morabito, S.; Sanches-Silva, A.; Mocan, A.; Nabavi, S.F.; Nabavi, S.M. Flavonoids and platelet aggregation: A brief review. Eur. J. Pharmacol. 2017, 807, 91–101. [Google Scholar] [CrossRef]
- Lamson, D.W.; Brignall, M.S. Antioxidants and cancer, part 3: Quercetin. Altern. Med. Rev. A J. Clin. Ther. 2000, 5, 196–208. [Google Scholar]
- Perez-Vizcaino, F.; Duarte, J.; Jimenez, R.; Santos-Buelga, C.; Osuna, A. Antihypertensive effects of the flavonoid quercetin. Pharm. Rep. 2009, 61, 67–75. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Deng, G.; Liang, Q.; Chen, D.-F.; Guo, R.; Lai, R.-C. Antioxidant Activity of Quercetin and Its Glucosides from propolis: A Theoretical Study. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; McGeer, E.G.; McGeer, P.L. Quercetin, not caffeine, is a major neuroprotective component in coffee. Neurobiol. Aging 2016, 46, 113–123. [Google Scholar] [CrossRef]
- Islam, S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N.; et al. Neuropharmacological Effects of Quercetin: A Literature-Based Review. Front. Pharmacol. 2021, 12, 3389. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Selvakumar, K.; Bavithra, S.; Ganesh, L.; Krishnamoorthy, G.; Venkataraman, P.; Arunakaran, J. Polychlorinated biphenyls induced oxidative stress-mediated neurodegeneration in hippocampus and behavioral changes of adult rats: Anxiolytic-like effects of quercetin. Toxicol. Lett. 2013, 222, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Chauhan, G.; Shri, R. Anti-depressant like effects of quercetin 4’-O-glucoside from Allium cepa via regulation of brain oxidative stress and monoamine levels in mice subjected to unpredictable chronic mild stress. Nutr. Neurosci. 2021, 24, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Kaur, T.; Goel, R.K. Adjuvant quercetin therapy for combined treatment of epilepsy and co-morbid depression. Neurochem. Int. 2017, 104, 27–33. [Google Scholar] [CrossRef]
- Moghbelinejad, S.; Alizadeh, S.; Mohammadi, G.; Khodabandehloo, F.; Rashvand, Z.; Najafipour, R.; Nassiri-Asl, M. The effects of quercetin on the gene expression of the GABAA receptor α5 subunit gene in a mouse model of kainic acid-induced seizures. J. Physiol. Sci. 2017, 67, 339–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, X.; Zhang, Y.; Huang, B.; Wang, X.; Wang, G.; Zhu, Y.; Liu, Y.; Liang, J.; Jia, Y.; Wang, K. Natural product in carvillateine aggravates epileptic seizures by inhibiting GABAA currents. Eur. J. Pharmacol. 2019, 858, 172496. [Google Scholar] [CrossRef]
- Knight, J.; Madduma-Liyanage, K.; Mobley, J.A.; Assimos, D.G.; Holmes, R.P. Ascorbic acid intake and oxalate synthesis. Urolithiasis 2016, 44, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Yen, G.-C.; Duh, P.-D.; Tsai, H.-L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Cort, W.M. Antioxidant Properties of Ascorbic Acid in Foods; American Chemical Society: Washington, DC, USA, 1982; pp. 533–550. ISBN 9780841206328. [Google Scholar]
- Sorice, A.; Guerriero, E.; Capone, F.; Colonna, G.; Castello, G.; Costantini, S. Ascorbic Acid: Its Role in Immune System and Chronic Inflammation Diseases. Mini-Rev. Med. Chem. 2014, 14, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Fraga, D.B.; Rodrigues, A.L. Ascorbic Acid to Manage Psychiatric Disorders. CNS Drugs 2017, 31, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhong, S.; Liao, X.; Chen, J.; He, T.; Lai, S.; Jia, Y. A Meta-Analysis of Oxidative Stress Markers in Depression. PLoS ONE 2015, 10, e0138904. [Google Scholar] [CrossRef]
- Shivavedi, N.; Kumar, M.; Tej, G.N.V.C.; Nayak, P.K. Metformin and ascorbic acid combination therapy ameliorate type 2 diabetes mellitus and comorbid depression in rats. Brain Res. 2017, 1674, 1–9. [Google Scholar] [CrossRef]
- Kocot, J.; Luchowska-Kocot, D.; Kiełczykowska, M.; Musik, I.; Kurzepa, J. Does Vitamin C Influence Neurodegenerative Diseases and Psychiatric Disorders? Nutrients 2017, 9, 659. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.M.; Seo, J.S.; Go, T.-H.; Nahm, S.-S.; Chang, B.-J. Ascorbic Acid Supplementation Prevents the Detrimental Effects of Prenatal and Postnatal Lead Exposure on the Purkinje Cell and Related Proteins in the Cerebellum of Developing Rats. Biol. Trace Elem. Res. 2018, 190, 446–456. [Google Scholar] [CrossRef]
- Gromiha, M.M.; Nagarajan, R.; Selvaraj, S. Protein Structural Bioinformatics: An Overview. In Encyclopedia of Bioinformatics and Computational Biology; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 445–459. [Google Scholar]
- Muhammed, M.T.; Aki-Yalcin, E. Homology modeling in drug discovery: Overview, current applications, and future perspectives. Chem. Biol. Drug Des. 2019, 93, 12–20. [Google Scholar] [CrossRef] [Green Version]
- dos Santos Nascimento, I.J.; de Aquino, T.M.; da Silva Santos-Júnior, P.F.; de Araújo-Júnior, J.X.; da Silva-Júnior, E.F. Molecular Modeling Applied to Design of Cysteine Protease Inhibitors–A Powerful Tool for the Identification of Hit Compounds Against Neglected Tropical Diseases. Front. Comput. Chem. 2020, 5, 63–110. [Google Scholar]
- Laskowski, R.A.; MacArthur, M.W.; Thornton, J. PROCHECK: Validation of protein-structure coordinates. In International Tables for Crystallography; International Union of Crystallography (IUCr): Chester, UK, 2012; pp. 684–687. [Google Scholar]
- Tan, K.R.; Rudolph, U.; Lüscher, C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci. 2011, 34, 188–197. [Google Scholar] [CrossRef] [Green Version]
- Calero, C.I.; Vickers, E.; Cid, G.M.; Aguayo, L.G.; VonGersdorff, H.; Calvo, D.J. Allosteric Modulation of Retinal GABA Receptors by Ascorbic Acid. J. Neurosci. 2011, 31, 9672–9682. [Google Scholar] [CrossRef]
- Sun, J.Y.; Yang, J.Y.; Wang, F.; Wang, J.Y.; Song, W.; Su, G.Y.; Dong, Y.X.; Wu, C.F. Lesions of nucleus accumbens affect the morphine-induced release of ascorbic acid and GABA but not of glutamate in rats: Release of AA, Glu and GABA induced by morphine. Addict. Biol. 2011, 16, 540–550. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Yang, J.-Y.; Wang, F.; Hou, Y.; Dong, Y.-X.; Wu, C.-F. GABAA receptors in VTA mediate the morphine-induced release of ascorbic acid in rat nucleus accumbens. Brain Res. 2011, 1368, 52–58. [Google Scholar] [CrossRef]
- Naseer, M.I.; Lee, H.Y.; Kim, M.O. Neuroprotective effect of vitamin C against the ethanol and nicotine modulation of GABAB receptor and PKA-α expression in prenatal rat brain. Synapse 2010, 64, 467–477. [Google Scholar] [CrossRef]
- Grigor’ev, I.P.; Neokesariĭskiĭ, A.A. Effect of ascorbic acid on the binding of 3H-GABA and 3H-glutamic acid to synaptosomes of the rat cerebral cortex. Biull. Eksp. Biol. Med. 1986, 102, 288–289. [Google Scholar] [PubMed]
- Rosa, P.B.; Neis, V.B.; Ribeiro, C.M.; Moretti, M.; Rodrigues, A.L.S. Antidepressant-like effects of ascorbic acid and ketamine involve modulation of GABAA and GABAB receptors. Pharmacol. Rep. 2016, 68, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Haroon, B.; Tahir, A.; Ashfaq, A.; Min, J.K.; Noman, B.A.; Shahid, A.S.; Gwang, Y.H.; Hae, Y.L.; Myeong, O.K. Co-treatment with anthocyanins and vitamin ameliorates ethanol-induced neurodegeneration via modulation of GABAB receptor signaling in the adult rat brain. CNS Neurol. Disord. Drug Targets 2015, 14, 791–803. [Google Scholar]
- Ullah, I.; Badshah, H.; Naseer, M.I.; Lee, H.Y.; Kim, M.O. Thymoquinone and vitamin C attenuates pentylenetetrazole-induced seizures via activation of the GABAB1 receptor in the adult rats cortex and hippocampus. Neuromolecular Med. 2015, 17, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Jeon, M.-T.; Jung, U.J.; Kim, D.W.; Moon, G.J.; Kim, S.R. Perspective: Therapeutic Potential of Flavonoids as Alternative Medicines in Epilepsy. Adv. Nutr. 2019, 10, 778–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Naidu, P.S.; Kulkarni, S.K. Reversal of Aging and Chronic Ethanol-induced Cognitive Dysfunction by Quercetin a Bioflavonoid. Free. Radic. Res. 2003, 37, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Naidu, P.S.; Kulkarni, S.K.; Singh, A. Reversal of Reserpine-Induced Orofacial Dyskinesia and Cognitive Dysfunction by Quercetin. Pharmacology 2004, 70, 59–67. [Google Scholar] [CrossRef]
- Rinwa, P.; Kumar, A. Quercetin along with piperine prevents cognitive dysfunction, oxidative stress and neuroinflammation associated with mouse model of chronic unpredictable stress. Arch. Pharm. Res. 2017, 40, 1166–1175. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Moghbelinejad, S.; Abbasi, E.; Yonsei, F.; Haghighi, M.-R.; Lotfizadeh, M.; Bazahang, P. Effects of quercetin on oxidative stress and memory retrieval link in dled rats. Epilepsy Behav. 2013, 28, 151–155. [Google Scholar] [CrossRef]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Bhutada, C.; Tawari, S.; Dixit, P.; Mundhada, D. Ameliorative effect of quercetin on memory dysfunction in streptozotocin-induced diabetic rats. Neurobiol. Learn Mem. 2010, 94, 293–302. [Google Scholar] [CrossRef]
- Santos, Í.M.D.S.; Freitas, R.L.M.D.; Saldanha, G.B.; Tomé, A.D.R.; Jordán, J.; Freitas, R.M.D. Alterations on monoamines concentration in rat hippocampus produced by lipoic acid. Arq. Neuropsiquiatr. 2010, 68, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Delrobaei, F.; Fatemi, I.; Shamsizadeh, A.; Allahtavakoli, M. Ascorbic acid attenuates cognitive impairment and brain oxidative stress in ovariectomized mice. Pharmacol. Rep. 2019, 71, 133–138. [Google Scholar] [CrossRef]
- Tomé, A.D.R.; Ferreira, P.M.P.; Freitas, R.M.D. Inhibitory action of antioxidants (ascorbic acid or α-tocopherol) on seizures and brain damage induced by pilocarpine in rats. Arq. Neuropsiquiatr. 2010, 68, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Binfaré, R.W.; Rosa, A.O.; Lobato, K.R.; Santos, A.R.S.; Rodrigues, A.L.S. Ascorbic acid administration produces an antidepressant-like effect: Evidence for the involvement of monoaminergic neurotransmission. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Budni, J.; Freitas, A.E.; Neis, V.B.; Ribeiro, C.M.; Oliveira Balen, G. TNF-alpha-induced depressive like phenotype and p38 (MAPK) activation are abolished by ascorbic acid treatment. Eur. Neuropsychopharmacol. 2015, 25, 902–912. [Google Scholar] [CrossRef]
- Fogaça, M.V.; Duman, R.S. Cortical GABAergic dysfunction in stress and depression: New insights for therapeutic interventions. Front. Cell Neurosci. 2019, 13, 87. [Google Scholar] [CrossRef] [Green Version]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, S.A.; Wamsteeker, J.I.; Kurz, E.U.; Bains, J.S. Altered chloride homeostasis removes the synaptic inhibitory constraint of the stress axis. Nat. Neurosci. 2009, 12, 438–443. [Google Scholar] [CrossRef]
- Crestani, F.; Lorez, M.; Baer, K.; Essrich, C.; Benke, D.; Laurent, J.P.; Belzung, C.; Fritschy, J.M.; Lüscher, B.; Mohler, H. Decreased GABAA- receptor clustering results in enhanced anxiety and a bias for threat cues. Nat. Neurosci. 1999, 2, 833–839. [Google Scholar] [CrossRef]
- Chandra, D.; Korpi, E.R.; Miralles, C.P.; DeBlas, A.L.; Homanics, G.E. GABAA receptor gamma 2 subunit knockdown mice have enhanced anxiety-like behavior butun altered hypnotic response to benzodiazepines. BMC Neurosci. 2005, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Shen, Q.; Lal, R.; Luellen, B.A.; Earnheart, J.C.; Andrews, A.M.; Luscher, B. gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic–pituitary–adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol. Psychiatry 2010, 68, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.S.; Rudolph, U. Anxiety and depression: Mouse genetics and pharmacological approaches to the role of GABA(A) receptor subtypes. Neuropharmacology 2012, 62, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilabert-Juan, J.; Castillo-Gómez, E.; Guirado, R.; Molto, M.D.; Nacher, J. Chronic stress alters inhibitory networks in the medial prefrontal cortex of adult mice. Brain Struct. Funct. 2013, 218, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Xu, A.; Cui, S.; Sun, M.-R.; Xue, Y.-C.; Wang, J.-H. Impaired GABA synthesis, uptake and release are associated with depression-like behaviors induced by chronic mild stress. Transl. Psychiatry 2016, 6, e910. [Google Scholar] [CrossRef] [PubMed]
- Northoff, G.; Sibille, E. Why are cortical GABA neurons relevant to internal focus in depression? Across-level model linking cellular, biochemical and neural network findings. Mol. Psychiatry 2014, 19, 966–977. [Google Scholar] [CrossRef] [Green Version]
- McKlveen, J.M.; Morano, R.L.; Fitzgerald, M.; Zoubovsky, S.; Cassella, S.; Scheimann, J.; Ghosal, S.; Mahbod, P.; Packard, B.A.; Myers, B.; et al. Chronic Stress Increases Prefrontal Inhibition: A Mechanism for Stress-Induced Prefrontal Dysfunction. Biol. Psychiatry 2016, 80, 754–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godfrey, K.E.; Gardner, A.C.; Kwon, S.; Chea, S.; Muthukumaraswamy, S.D. Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: A systematic review and meta-analysis. J. Psychiatr. Res. 2018, 105, 33–44. [Google Scholar] [CrossRef]
- Hasler, G.; Vanderveen, J.W.; Tumonis, T.; Meyers, N.; Shen, J.; Drevets, W.C. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 2007, 64, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Calcaterra, N.E.; Barrow, J.C. Classics in Chemical Neuroscience: Diazepam (Valium). ACS Chem. Neurosci. 2014, 5, 253–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitwam, J.G.; Amrein, R. Pharmacology of flumazenil. Acta Anaesthesiol. Scand. 1995, 39, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Goldfrank, L.R.; Hoffman, R.S.; Howland, M.A.; Lewin, N.A. Goldfrank’s Toxicologic Emergencies; McGraw Hill Professional: New York, NY, USA, 2006. [Google Scholar]
- Ishola, I.O.; Chatterjee, M.; Tota, S.; Tadigopulla, N.; Adeyemi, O.O.; Palit, G.; Shukla, R. Antidepressant and anxiolytic effects of amentoflavone isolated from Cnestis ferruginea in mice. Pharmacol. Biochem. Behav. 2012, 103, 322–331. [Google Scholar] [CrossRef]
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; Klein, T.E. Pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef]
- Sanacora, G.; Saricicek, A. GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action. CNS Neurol. Disord. Drug Targets 2007, 6, 127–140. [Google Scholar] [CrossRef]
- Ponnulakshmi, R.; Shyamaladevi, B.; Vijayalakshmi, P.; Selvaraj, J. In Silico and in vivo analysis to identify the antidiabetic activity of beta-sitosterol in adipose tissue of high-fat diet and sucrose induced type-2 diabetic experimental rats. Toxicol. Mech. Methods 2019, 29, 276–290. [Google Scholar] [CrossRef]
- Carlini, E.A.; Burgos, V. Screening farmacologico de ansioliticos: Metadologia laboratorial e comparacao entre diazepam e clorobenzepam. Rev. Assoc. Bras. Psiquiatr. 1979, 1, 25–31. [Google Scholar]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Schwede, T. SWISS-MODEL: Modeling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, 252–258. [Google Scholar] [CrossRef]
- UniProt Consortium. UniProt: A hub for protein information. Nucleic Acids Res. 2015, 43, D204–D212. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Ali, M.T.; Shawan, M.M.A.K.; Sarwar, M.G.; Khan, M.A.K.; Halim, M.A. Halogen-directed drug design for A Alzheimer’s disease: A combined density functional and molecular docking study. Springer Plus 2016, 5, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekins, S.; Mestres, J.; Testa, B. In Silico pharmacology for drug discovery: Methods for virtuallig and screening and profiling. Br. J. Pharmacol. 2007, 152, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Fukunishi, Y.; Nakamura, H. Prediction of ligand binding sites of proteins by molecular docking calculation for a random ligand library: Prediction of Ligand-Binding Sites. Protein Sci. 2011, 20, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.; Roy, K. How far can virtual screening take us in drug discovery? Expert Opin. Drug Discov. 2013, 8, 245–261. [Google Scholar] [CrossRef]
- Al-Khafaji, K.; TaskinTok, T. Molecular dynamics simulation, free energy landscape and binding free energy computations in exploration the anti-invasive activity of amygdale in against metastasis. Comput. Methods Programs Biomed. 2020, 195, 105660. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High-performance molecular simulations through multi-level parallelism from laptops to supercomputers. Software X 2015, 1–2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM: A program for macromolecular energy, minimization, and dynamics calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Zoete, V.; Cuendet, M.A.; Grosdidier, A.; Michielin, O. Swiss Param: A fast force field generation tool for small organic molecules. J. Comput. Chem. 2011, 32, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, K.; Tok, T.T. Understanding the mechanism of amygdalin’s multifunctional anti-cancer action using computational approach. J. Biomol. Struct. Dyn. 2021, 39, 1600–1610. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R.; Open Source Drug Discovery Consortium; Lynn, A. g_mmpbsa—AGROMACS tool for high -throughput MM-PBSA calculations. J. Chem. Inf. Model 2014, 54, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Lynn, A.M.; Gupta, V. Standardization of virtual-screening and post-processing protocols relevant to in –silico drug discovery. 3 Biotech 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]







| Treatment Groups | Latency (min) | Duration of Sleep (min) |
|---|---|---|
| NC | 4.35 ± 0.32 | 47.13 ± 4.56 |
| DZP | 2.36 ± 0.21 acd | 68.03 ± 3.91 acd |
| QUR | 27.00 ± 7.91 | 52.20 ± 4.02 a |
| AA | 19.20 ± 1.52 c | 57.60 ± 3.40 ac |
| DZP + QUR | 16.80 ± 1.24 cd | 57.20 ± 4.26 ac |
| DZP + AA | 7.40 ± 2.22 cd | 58.00 ± 1.46 a |
| DZP + AA + QUR | 13.60 ± 1.44 cd | 69.00 ± 4.71 acd |
| FLU | 42.38 ± 0.23 | 06.99 ± 3.67 |
| QUR + FLU | 38.89 ± 4.45 cd | 04.58 ± 2.29 |
| AA + FLU | 32.61 ± 6.30 | 06.02 ± 2.31 |
| Protein (Receptor) | Binding Affinity (Kcal/mol) | No of H-Bond | H-Bond Residues | Bond Length (Å) | Other Bond Residues |
|---|---|---|---|---|---|
| GABA (A5) | −6.9 | 1 | Glu327 | 2.94 | Ile391, Trp320 |
| GABA (B1) | −8.4 | 3 | Arg571 Glu745 Ser813 | 2.88 2.39 1.98 | Ala819, Leu633 |
| GABA (B2) | −8.2 | 4 | Arg556 Gln720 Glu646 Ser710 | 2.14 2.90 2.19 2.12 | His647, Phe537, Pro717 |
| Protein (Receptor) | Binding Affinity (Kcal/mol) | No. of H-Bond | H-Bond Residues | Bond Length (Å) | Other Bond Residues |
|---|---|---|---|---|---|
| GABA (A2) | −5.0 | 4 | Ile37 Glu39 Ser36 Thr38 | 2.41 2.12 1.89 2.37 | |
| GABA (B1) | −5.5 | 3 | Arg652 Glu745 Leu573 | 2.70 2.43 2.49 | Leu635 |
| GABA (B2) | −5.2 | 4 | Arg556 Gln720 His647 Leu539 | 2.84 2.51 2.75 2.44 |
| Complex Name | ∆GvdW (kJ/mol) | ∆Gelec (kJ/mol) | ∆Gpol (kJ/mol) | ∆Gnonpol (kJ/mol) | ∆E (MM-PBSA) (kJ/mol) |
|---|---|---|---|---|---|
| GABA A2-AA | −65.844 | −48.201 | 97.412 | −9.442 | −26.074 |
| GABA A2-QUR | −68.791 | −7.920 | 53.211 | −10.038 | −33.538 |
| GABA A5-AA | −48.923 | −45.689 | 70.275 | −7.732 | −32.068 |
| GABA A5-QUR | −61.891 | −38.976 | 82.465 | −8.669 | −27.071 |
| GABA B1-AA | −61.664 | −31.617 | 78.994 | −7.740 | −22.027 |
| GABA B1-QUR | −76.362 | −78.516 | 142.703 | −12.908 | −25.083 |
| GABA B2-AA | −75.571 | −46.703 | 122.131 | −10.228 | −14.629 |
| GABA B2-QUR | −82.083 | −52.631 | 128.317 | −10.453 | −16.850 |
| Treatment Groups | Description | Dose |
|---|---|---|
| Gr.-I: NC | Distilled water | 10 mL/kg |
| Gr.-II: DZP | Diazepam (Standard 1: Benzodiazepine receptor agonist) | 2 mg/kg |
| Gr.-III: QUR | Quercetin (Test sample 1) | 50 mg/kg |
| Gr.-IV: AA | Ascorbic acid (Test sample 2) | 25 mg/kg |
| Gr.-V: DZP + QUR | Diazepam + Quercetin | 2 mg/kg + 50 mg/kg |
| Gr.-VI: DZP + AA | Diazepam + Ascorbic acid | 2 mg/kg + 25 mg/kg |
| Gr.-VII: DZP + AA + QUR | Diazepam + Ascorbic acid + Quercetin | 2 mg/kg + 25 mg/kg + 50 mg/kg |
| Gr.-VIII: FLU | Flumazenil (Standard 2: Benzodiazepine receptor antagonist) | 2 mg/kg |
| Gr.-X: QUR + FLU | Quercetin + Flumazenil | 50 mg/kg + 2 mg/kg |
| Gr.-IX: AA + FLU | Ascorbic acid + Flumazenil | 25 mg/kg + 2 mg/kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, R.; Al-Khafaji, K.; Khan, R.A.; Sarkar, C.; Islam, M.S.; Dey, D.; Jain, D.; Faria, F.; Akbor, R.; Atolani, O.; et al. Quercetin and/or Ascorbic Acid Modulatory Effect on Phenobarbital-Induced Sleeping Mice Possibly through GABAA and GABAB Receptor Interaction Pathway. Pharmaceuticals 2021, 14, 721. https://doi.org/10.3390/ph14080721
Hossain R, Al-Khafaji K, Khan RA, Sarkar C, Islam MS, Dey D, Jain D, Faria F, Akbor R, Atolani O, et al. Quercetin and/or Ascorbic Acid Modulatory Effect on Phenobarbital-Induced Sleeping Mice Possibly through GABAA and GABAB Receptor Interaction Pathway. Pharmaceuticals. 2021; 14(8):721. https://doi.org/10.3390/ph14080721
Chicago/Turabian StyleHossain, Rajib, Khattab Al-Khafaji, Rasel Ahmed Khan, Chandan Sarkar, Md. Shahazul Islam, Dipta Dey, Divya Jain, Farhana Faria, Rukaya Akbor, Olubunmi Atolani, and et al. 2021. "Quercetin and/or Ascorbic Acid Modulatory Effect on Phenobarbital-Induced Sleeping Mice Possibly through GABAA and GABAB Receptor Interaction Pathway" Pharmaceuticals 14, no. 8: 721. https://doi.org/10.3390/ph14080721
APA StyleHossain, R., Al-Khafaji, K., Khan, R. A., Sarkar, C., Islam, M. S., Dey, D., Jain, D., Faria, F., Akbor, R., Atolani, O., Oliveira, S. M. R., Siyadatpanah, A., Pereira, M. d. L., & Islam, M. T. (2021). Quercetin and/or Ascorbic Acid Modulatory Effect on Phenobarbital-Induced Sleeping Mice Possibly through GABAA and GABAB Receptor Interaction Pathway. Pharmaceuticals, 14(8), 721. https://doi.org/10.3390/ph14080721












