Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review
Abstract
1. Introduction
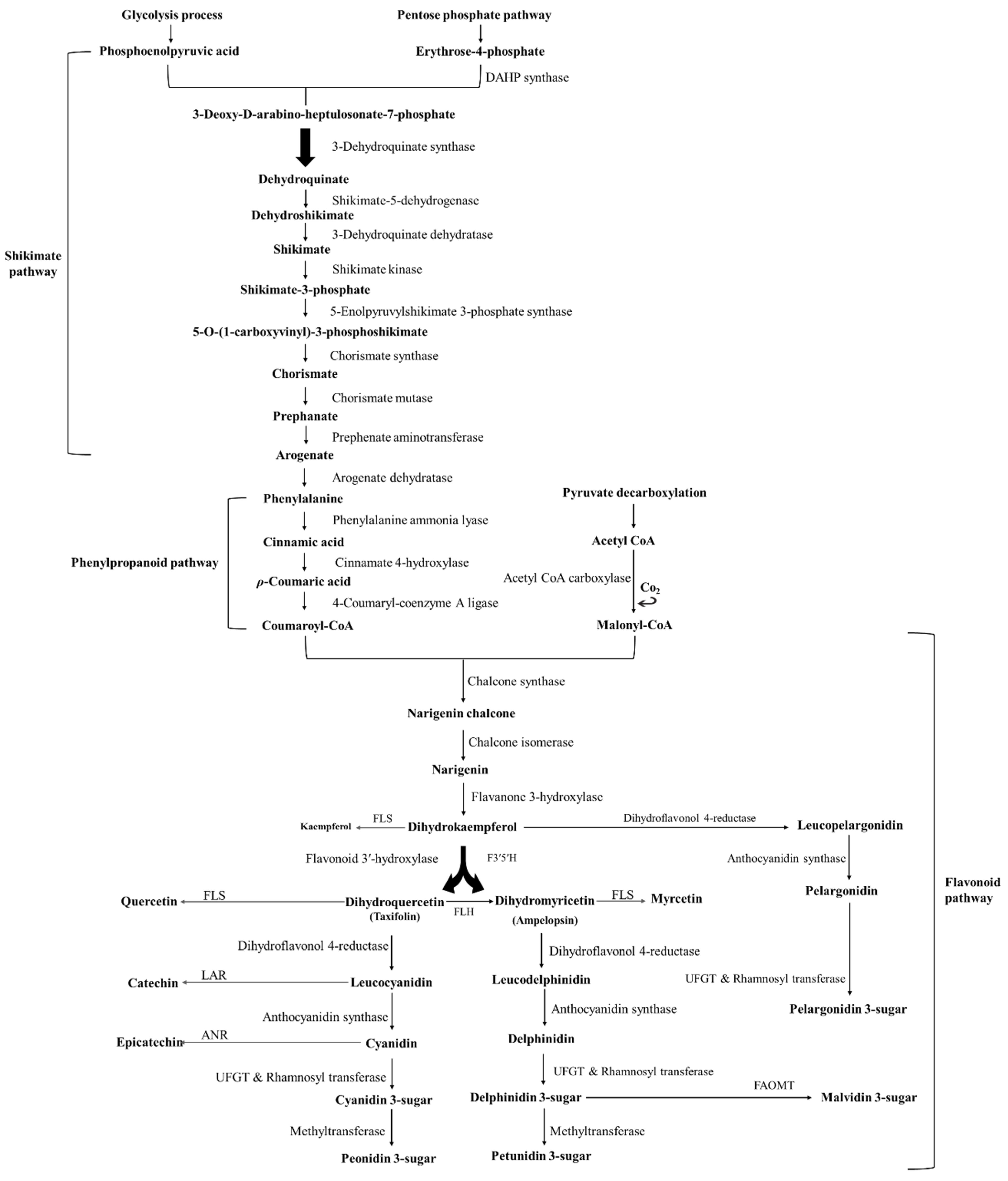
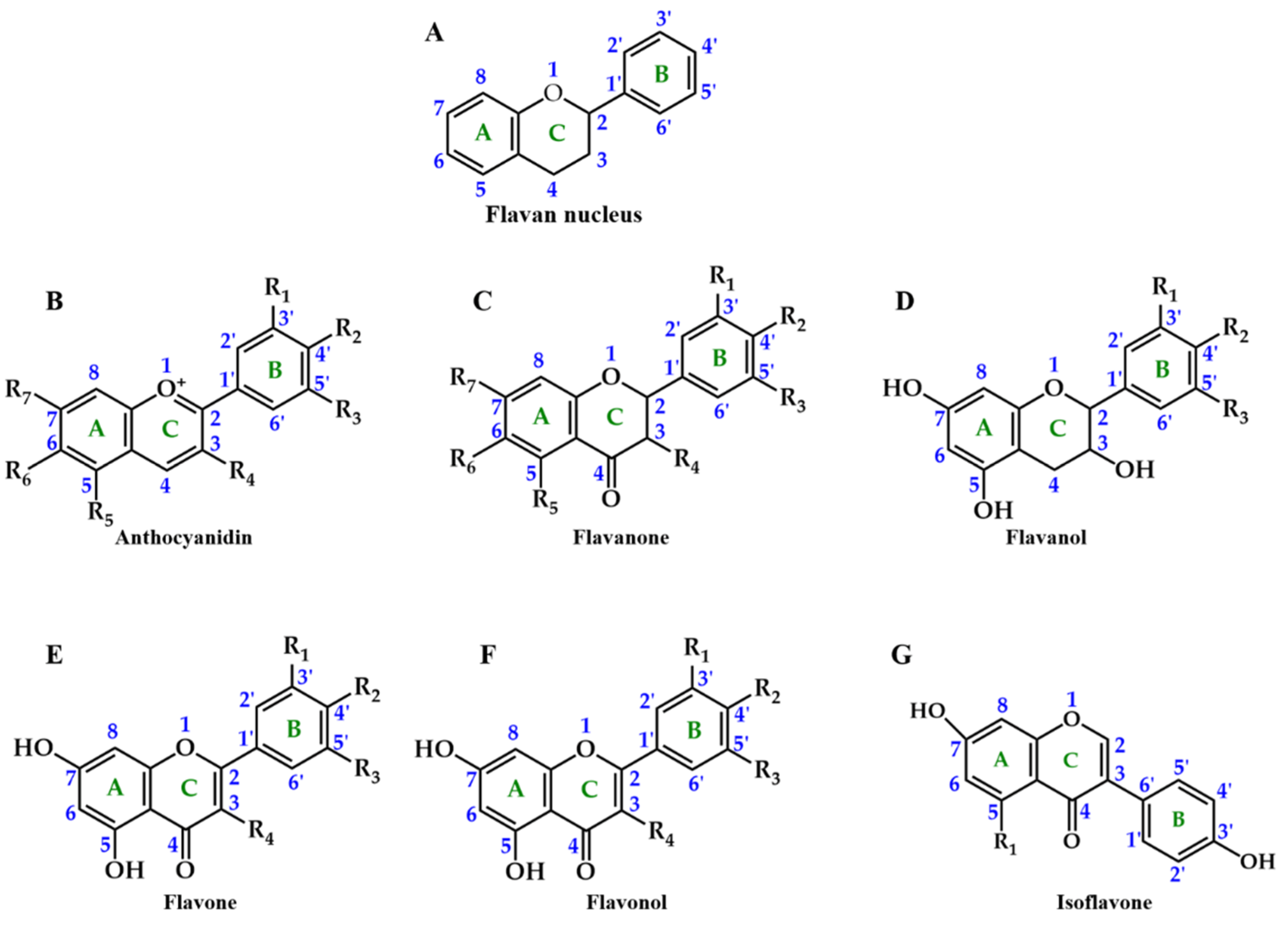
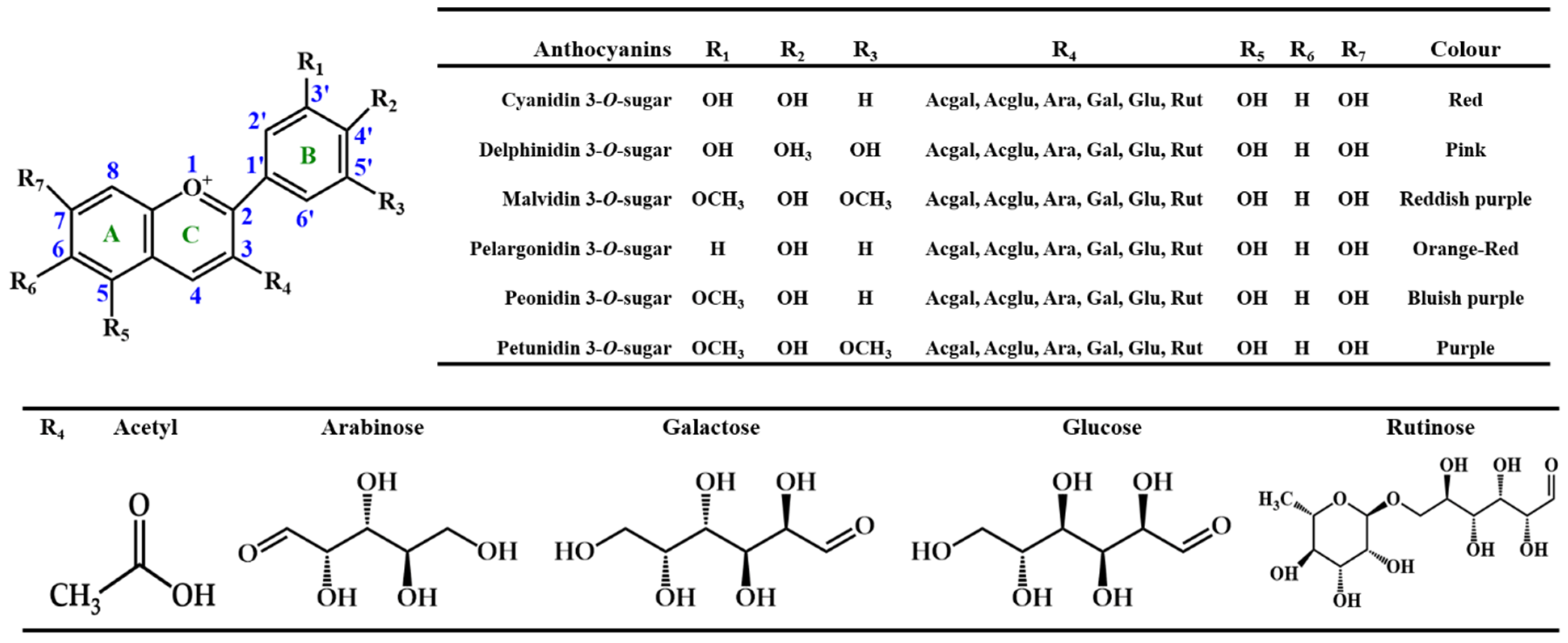
2. Chemical Structure and Function of Anthocyanins
3. Major Sources of Anthocyanins
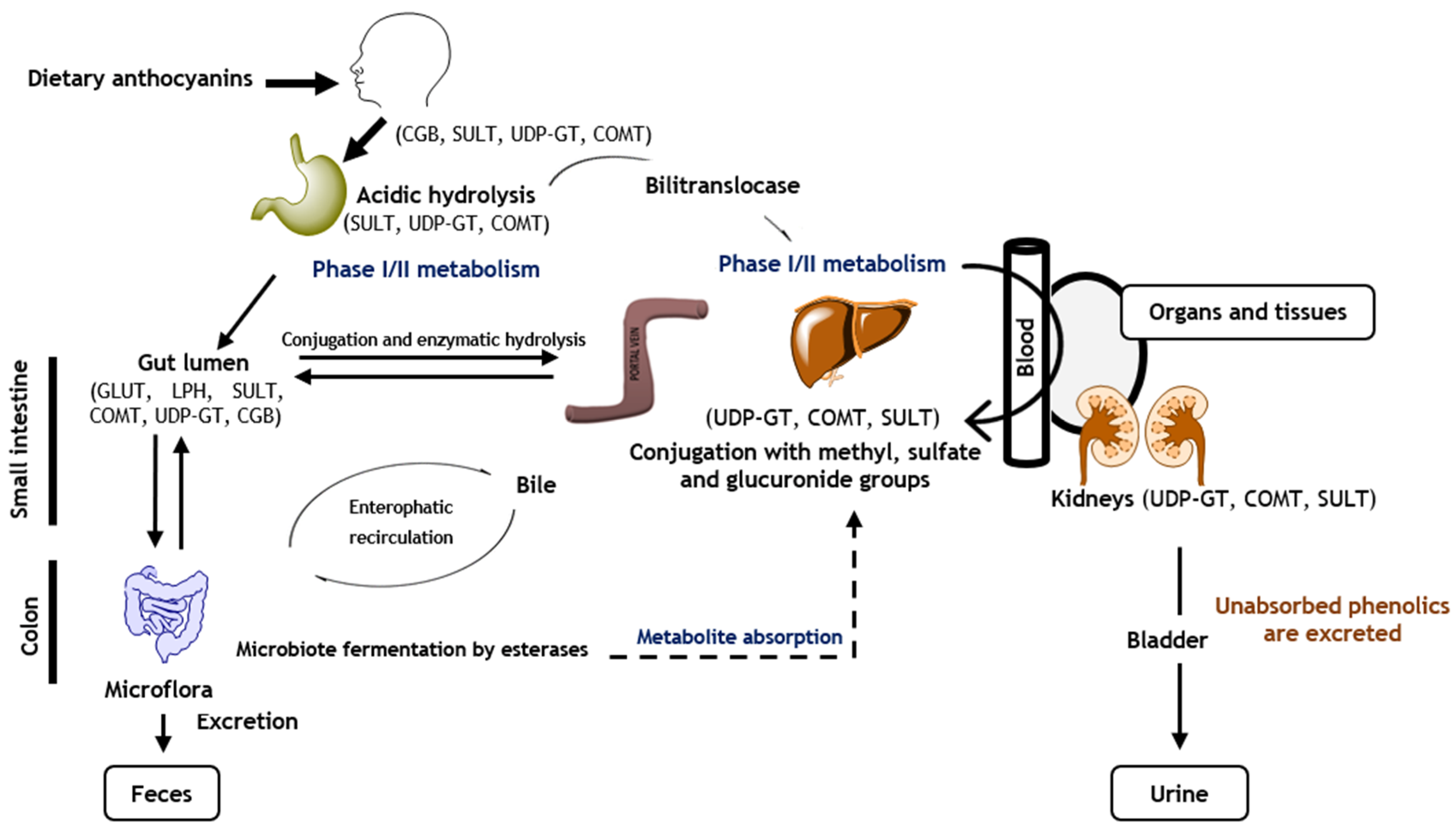
4. Anthocyanins’ Bioavailability and Metabolism
5. Anthocyanin Encapsulation
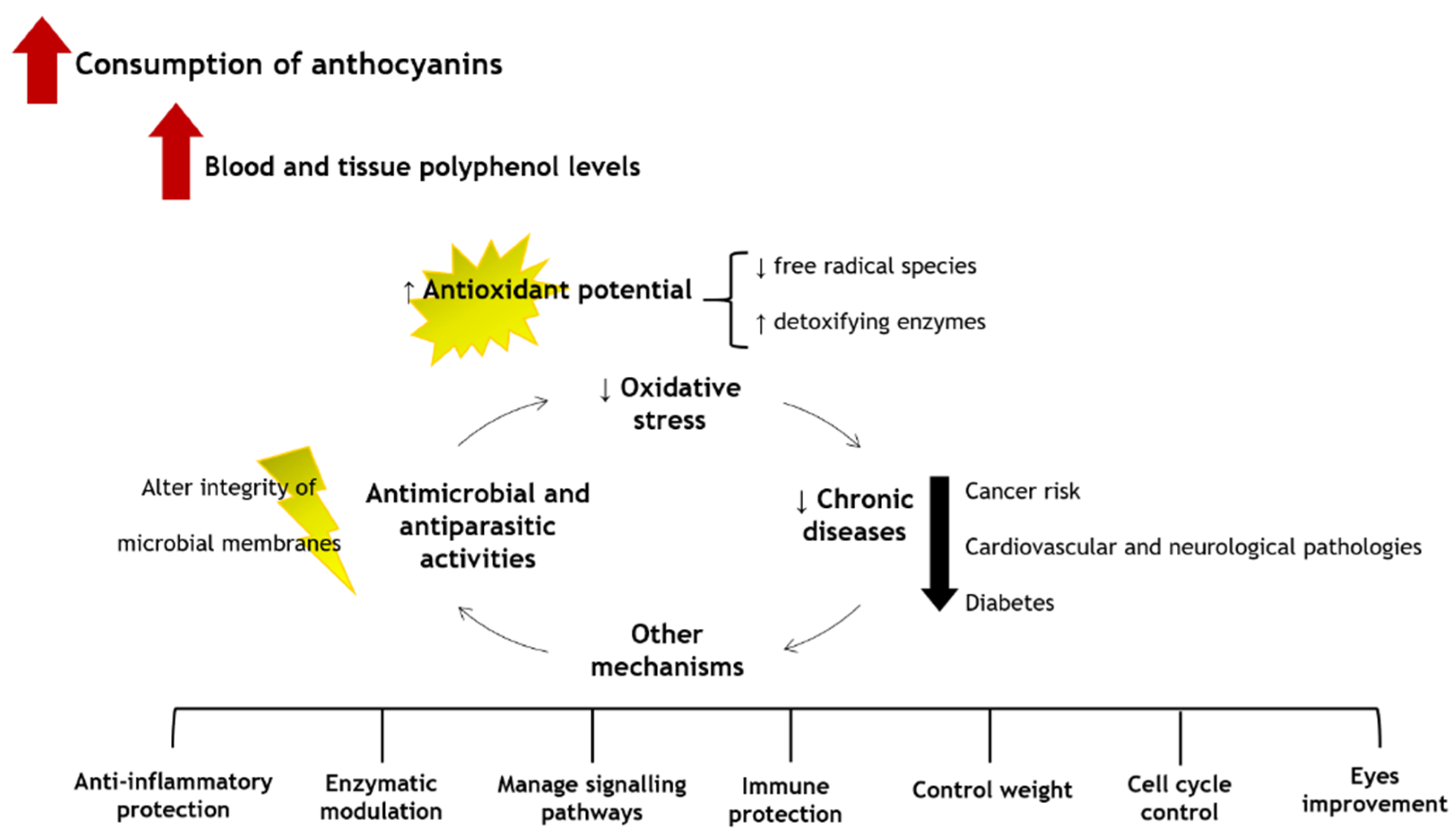
6. Putative Health Benefits
6.1. Antimicrobial Effects and Anti-Parasitic Activity
6.2. Antioxidant Properties
6.3. Anti-Inflammatory Properties
6.4. Anticancer Properties
6.5. Neurological Properties
6.6. Anti-Diabetic and Anti-Obesity Effects
6.7. Cardiovascular Properties
6.8. Eye Improvement
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mannino, G.; Perrone, A.; Campobenedetto, C.; Schittone, A. Phytochemical profile and antioxidative properties of Plinia trunciflora fruits: A new source of nutraceuticals. Food Chem. 2020, 307, 125515. [Google Scholar] [CrossRef] [PubMed]
- Ożarowski, M.; Karpiński, T.M.; Szulc, M.; Wielgus, K.; Kujawski, R.; Wolski, H.; Seremak-Mrozikiewicz, A. Plant phenolics and extracts in animal models of preeclampsia and clinical trials—Review of perspectives for novel therapies. Pharmaceuticals 2021, 14, 269. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Kuskoski, E.M.; Navas, M.J.; Asuero, A.G. Antioxidant capacity of anthocyanin pigments. In Flavonoids-From Biosynthesis to Human Health; Justino, G.C., Ed.; IntechOpen: London, UK, 2017; pp. 205–255. [Google Scholar]
- Aziz, M.A.; Sarwar, M.S.; Akter, T.; Uddin, M.S.; Xun, S.; Zhu, Y.; Islam, M.S.; Hongjie, Z. Polyphenolic molecules targeting STAT3 pathway for the treatment of cancer. Life Sci. 2021, 268, 118999. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Topor-Madry, R.; Szafraniec, K.; Pajak, A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition 2014, 30, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Bendokas, V.; Skemiene, K.; Trumbeckaite, S.; Passamonti, S.; Borutaite, V.; Liobikas, J. Anthocyanins: From plant pigments to health benefits at mitochondrial level. Crit. Rev. Food Sci. Nutr. 2020, 60, 3352–3365. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The influence of supplementation of anthocyanins on obesity-associated comorbidities: A concise review. Foods 2020, 9, 687. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; Rios, J.; Jilma-Stohlawetz, P.; Pacheco-Palencia, L.A.; Meibohm, B.; Talcott, S.T.; Derendorf, H. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich açai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J. Agric. Food Chem. 2008, 56, 7796–7802. [Google Scholar] [CrossRef]
- Lynn, A.; Mathew, S.; Moore, C.T.; Russell, J.; Robinson, E.; Soumpasi, V.; Barker, M.E. Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: A randomised controlled trial. Plant Foods Hum. Nutr. 2014, 69, 122–127. [Google Scholar] [CrossRef]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Aboo-Bakkar, Z.; Conway, M.; Adlam, A.-L.R.; Fulford, J. Enhanced task related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl. Physiol. Nutr. Metab. 2017, 42, 773–779. [Google Scholar] [CrossRef]
- Kwon, S.H.; Ahn, I.S.; Kim, S.-O.; Kong, C.S.; Chung, H.Y.; Do, M.S.; Park, K.Y. Anti-obesity and hypolipidemic effects of black soybean anthocyanins. J. Med. Food 2007, 10, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Hosking, H.; Pederick, W.; Singh, I.; Santhakumar, A.B. The effect of anthocyanin supplementation in modulating platelet function in sedentary population: A randomised, double-blind, placebo-controlled, cross-over trial. Br. J. Nutr. 2017, 118, 368–374. [Google Scholar] [CrossRef]
- Shim, S.H.; Kim, J.M.; Choi, C.Y.; Kim, C.Y.; Park, K.H. Ginkgo biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. J. Med. Food 2012, 15, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lyall, G.K.; Martinez-blazquez, J.A.; Vallejo, F.; Tomas-barberan, F.A.; Birch, K.M.; Boesch, C. Blood orange juice consumption increases flow-mediated dilation in adults with overweight and obesity: A randomized controlled trial. J. Nutr. 2020, 150, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Monica Giusti, M. Metal chelates of petunidin derivatives exhibit enhanced color and stability. Foods 2020, 9, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Mladěnka, P.; Říha, M.; Martin, J.; Gorová, B.; Matějíček, A.; Spilková, J. Fruit extracts of 10 varieties of elderberry (Sambucus nigra L.) interact differently with iron and copper. Phytochem. Lett. 2016, 18, 232–238. [Google Scholar] [CrossRef]
- Sinopoli, A.; Calogero, G.; Bartolotta, A. Computational aspects of anthocyanidins and anthocyanins: A review. Food Chem. 2019, 297, 124898. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Slimani, N.; Romieu, I.; Fedirko, V.; Magistris, M.S.; Ericson, U.; Amiano, P.; Trichopoulou, A.; et al. Estimated dietary intakes of flavonols, flavanones and flavones in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24 hour dietary recall cohort. Br. J. Nutr. 2011, 106, 1915–1925. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape berry secondary metabolites and their modulation by abiotic factors in a climate change scenario—A review. Front. Plant Sci. 2021, 12, 1–26. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Legua, P.; Domenech, A.; Martinez, J.J.; Sánchez-Rodríguez, L.; Hernández, F.; Carbonell-Barrachina, A.A.; Melgarejo, P. Bioactive and volatile compounds in sweet cherry cultivars. J. Food Nutr. Res. 2017, 5, 844–851. [Google Scholar] [CrossRef]
- Bresciani, L.; Martini, D.; Mena, P.; Tassotti, M.; Calani, L.; Brigati, G.; Brighenti, F.; Holasek, S.; Malliga, D.E.; Lamprecht, M.; et al. Absorption profile of (poly)phenolic compounds after consumption of three food supplements containing 36 different fruits, vegetables, and berries. Nutrients 2017, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Landete, J.M. Dietary intake of natural antioxidants: Vitamins and polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Ioana, S.; Xiao, J.; Leopold, N.; Ayvaz, Z.; Danciu, C.; Ayvaz, H.; Sttǎnilǎ, A.; Nistor, M.; Socaciu, C. Anthocyanins, vibrant color pigments, and their role in skin cancer prevention. Biomedicines 2020, 8, 336. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural antioxidant anthocyanins—A hidden therapeutic candidate in metabolic disorders with major focus in neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef]
- Ribnickya, D.M.; Roopchand, D.E.; Oren, A.; Grace, M.; Poulev, A.; Lila, M.A.; Havenaar, R.; Raskin, I. Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1). Food Chem. 2014, 142, 349–357. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- White, B.L.; Howard, L.R.; Prior, R.L. Proximate and polyphenolic characterization of cranberry pomace. J. Agric. Food Chem. 2010, 58, 4030–4036. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Campos, G.; Alves, G.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Physical and phytochemical composition of 23 Portuguese sweet cherries as conditioned by variety (or genotype). Food Chem. 2021, 335, 127637. [Google Scholar] [CrossRef] [PubMed]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Cévoli, R.; Castro-Espín, C.; Béraud, V.; Buckland, G.; Zamora-Ros, R. An overview of global flavonoid intake and its food sources. In Flavonoids—From Biosynthesis to Human Health; Justino, G.C., Ed.; InTech: London, UK, 2017; pp. 371–391. [Google Scholar]
- Igwe, E.O.; Charlton, K.E.; Probst, Y.C. Usual dietary anthocyanin intake, sources and their association with blood pressure in a representative sample of Australian adults. J. Hum. Nutr. Diet. 2019, 32, 578–590. [Google Scholar] [CrossRef]
- Chinese Nutrition Society. Chinese DRIs Handbook; Standards Press of China: Beijing, China, 2013. [Google Scholar]
- Kim, K.; Vance, T.M.; Chun, O.K. Estimated intake and major food sources of flavonoids among US adults: Changes between 1999–2002 and 2007–2010 in NHANES. Eur. J. Nutr. 2016, 55, 833–843. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef]
- Frond, A.D.; Iuhas, C.I.; Stirbu, I.; Leopold, L.; Socaci, S.; Andreea, S.; Ayvaz, H.; Andreea, S.; Mihai, S.; Zorita, D.; et al. Phytochemical characterization of five edible purple-reddish vegetables: Anthocyanins, flavonoids, and phenolic acid derivatives. Molecules 2019, 24, 1536. [Google Scholar] [CrossRef] [PubMed]
- Kharadze, M.; Japaridze, I.; Kalandia, A.; Vanidze, M. Anthocyanins and antioxidant activity of red wines made from endemic grape varieties. Ann. Agrar. Sci. 2018, 16, 181–184. [Google Scholar] [CrossRef]
- Horincar, G.; Enachi, E.; Bolea, C.; Râpeanu, G.; Aprodu, I. Value-added lager beer enriched with eggplant (Solanum melongena L.) peel extract. Molecules 2020, 25, 731. [Google Scholar] [CrossRef]
- Zambrano-Moreno, E.L.; Chávez-Jáuregui, R.N.; Plaza, M.d.L.; Wessel-Beaver, L. Phenolic content and antioxidant capacity in organically and conventionally grown eggplant (Solanum melongena) fruits following thermal processing. Food Sci. Technol. 2015, 35, 414–420. [Google Scholar] [CrossRef]
- de Rosso, V.V.; Hillebrand, S.; Montilla, E.C.; Bobbio, F.O.; Winterhalter, P.; Mercadante, A.Z. Determination of anthocyanins from acerola (Malpighia emarginata DC.) and açai (Euterpe oleracea Mart.) by HPLC–PDA–MS/MS. J. Food Compos. Anal. 2008, 21, 291–299. [Google Scholar] [CrossRef]
- Polat, M.; Okatan, V.; Güclü, S.F.; Çolak, A.M. Determination of some chemical characteristics and total antioxidant capacity in apple varieties grown in Posof/Ardahan region. Int. J. Agric. Environ. Food Sci. 2018, 2, 131–134. [Google Scholar] [CrossRef]
- Wang, S.Y.; HsinShan, L. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Fan-Chiang, H.-J.; Wrolstad, R.E. Anthocyanin pigment composition of blackberries. JFS C Food Chem. Toxicol. 2005, 70, 198–202. [Google Scholar] [CrossRef]
- Jakobek, L.; Seruga, M.; Novak, I.; Medvidovic-Kosanovic, M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Leb. 2007, 103, 369–378. [Google Scholar]
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black currant anthocyanins attenuate weight gain and improve glucose metabolism in diet-induced obese mice with intact, but not disrupted, gut microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Kay, C.D.; Cottrell, T.; Holub, B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002, 50, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Siudem, P.; Paradowska, K.; Gralec, M.; Kaźmierski, S.; Wawer, I. Aronia melanocarpa fruits as a rich dietary source of chlorogenic acids and anthocyanins: 1H-NMR, HPLC-DAD, and chemometric studies. Molecules 2020, 25, 3234. [Google Scholar] [CrossRef] [PubMed]
- Jasutiene, L.; Cesonienė, I.; Sarkinas, A. Phenolics and anthocyanins in berries of European cranberry and their antimicrobial activity. Medicina 2009, 45, 992–999. [Google Scholar]
- Duymuş, H.G.; Göger, F.; Başer, K.H.C. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.E.; Altman, A.; Kerem, Z.; Flaishman, M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E. Polyphenol content in figs (Ficus carica L.): Effect of sun-drying. Int. J. Food Prop. 2015, 18, 521–535. [Google Scholar] [CrossRef]
- Silva, L.R.; Queiroz, M. Bioactive compounds of red grapes from Dão region (Portugal): Evaluation of phenolic and organic profile. Asian Pac. J. Trop. Biomed. 2016, 6, 315–321. [Google Scholar] [CrossRef]
- Kallithraka, S.; Aliaj, L.; Makris, D.P.; Kefalas, P. Anthocyanin profiles of major red grape (Vitis vinifera L.) varieties cultivated in Greece and their relationship with in vitro antioxidant characteristics. Int. J. Food Sci. Technol. 2009, 44, 2385–2393. [Google Scholar] [CrossRef]
- Khattab, R.; Brooks, M.S.-L.; Ghanem, A. Phenolic analyses of haskap berries (Lonicera caerulea L.): Spectrophotometry versus high performance liquid chromatography. Int. J. Food Prop. 2016, 19, 1708–1725. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Optimization of ultrasound-assisted extraction of anthocyanins from haskap berries (Lonicera caerulea L.) using Response Surface Methodology. Ultrason. Sonochem. 2015, 27, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Cantín, C.M.; Moreno, M.A.; Gogorcena, Y. Evaluation of the antioxidant capacity, phenolic compounds, and vitamin C content of different peach and nectarine [Prunus persica (L.) batsch] breeding progenies. J. Agric. Food Chem. 2009, 57, 4586–4592. [Google Scholar] [CrossRef]
- Usenik, V.; Štampar, F.; Veberič, R. Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chem. 2009, 114, 529–534. [Google Scholar] [CrossRef]
- Passafiume, R.; Perrone, A.; Sortino, G.; Gianguzzi, G.; Saletta, F.; Gentile, C.; Farina, V. Chemical-physical characteristics, polyphenolic content and total antioxidant activity of three Italian-grown pomegranate cultivars. NFS J. 2019, 16, 9–14. [Google Scholar] [CrossRef]
- Zhu, F.; Yuan, Z.; Zhao, X.; Yin, Y.; Feng, L. Composition and contents of anthocyanins in different pomegranate cultivars. Acta Hortic. 2015, 1089, 35–41. [Google Scholar]
- Mihaylova, D.; Popova, A.; Desseva, I.; Petkova, N.; Stoyanova, M.; Vrancheva, R.; Slavov, A.; Slavchev, A.; Lante, A. Comparative study of early- and mid-ripening peach (Prunus persica L.) varieties: Biological activity, macro-, and micro- nutrient profile. Foods 2021, 10, 164. [Google Scholar] [CrossRef]
- Bento, C.; Gonçalves, A.C.; Silva, B.; Silva, L.R. Assessing the phenolic profile, antioxidant, antidiabetic and protective effects against oxidative damage in human erythrocytes of peaches from Fundão. J. Funct. Foods 2018, 43, 224–233. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Anthocyanins contents, profiles, and color characteristics of red cabbage extracts from different cultivars and maturity stages. J. Agric. Food Chem. 2014, 62, 7524–7531. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Santisteban, A.; Gordillo, B.; Hernanz, D.; Heredia, F.J.; Escudero-Gilete, M.L. Comparative study of red berry pomaces (blueberry, red raspberry, red currant and blackberry) as source of antioxidants and pigments. Eur. Food Res. Technol. 2019, 245, 1–9. [Google Scholar] [CrossRef]
- Galvis Sánchez, A.C.; Gil-Izquierdo, A.; Gil, M.I. Comparative study of six pear cultivars in terms of their phenolic and vitamin C contents and antioxidant capacity. J. Sci. Food Agric. 2003, 83, 995–1003. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.J.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015, 89, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Booren, M.; Gray, I.; Dewitt, D.L. Cyclooxygenase active bioflavonoids from Balaton tart cherry and their structure activity relationships. Phytomedicine 2000, 7, 15–19. [Google Scholar] [CrossRef]
- Silva, F.L.; Escribano-Bailón, M.T.; Pérez Alonso, J.J.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT-Food Sci. Technol. 2007, 40, 374–382. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Bento, C.; Silva, B.M.; Silva, L.R. Sweet cherries from Fundão possess antidiabetic potential and protect human erythrocytes against oxidative damage. Food Res. Int. 2017, 95, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef]
- Diep, T.; Pook, C.; Yoo, M. Phenolic and anthocyanin compounds and antioxidant activity of Tamarillo (Solanum betaceum Cav.). Antioxidants 2020, 9, 169. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, Q.; Lin, J.; Li, X.; Sun, C.; Chen, K. Physicochemical characterisation of four cherry species (Prunus spp.) grown in China. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef]
- Borghesi, E.; González-Miret, M.L.; Escudero-Gilete, M.L.; Malorgio, F.; Heredia, F.J.; Meléndez-Martínez, A.J. Effects of salinity stress on carotenoids, anthocyanins, and color of diverse tomato genotypes. J. Agric. Food Chem. 2011, 59, 11676–11682. [Google Scholar] [CrossRef]
- Blando, F.; Berland, H.; Maiorano, G.; Durante, M.; Mazzucato, A.; Picarella, M.E.; Nicoletti, I.; Gerardi, C.; Mita, G.; Andersen, Ø.M. Nutraceutical characterization of anthocyanin-rich fruits produced by “Sun Black” tomato line. Front. Nutr. 2019, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.; Carle, R.; Schieber, A. Quantification of anthocyanins in black carrot extracts (Daucus carota ssp. sativus var. atrorubens Alef.) and evaluation of their color properties. Eur. Food Res. Technol. 2004, 219, 479–486. [Google Scholar] [CrossRef]
- Shi-Lin, Z.; Peng, D.; Yu-Chao, X.; Jian-Jun, W. Quantification and analysis of anthocyanin and flavonoids compositions, and antioxidant activities in onions with three different colors. J. Integr. Agric. 2016, 15, 2175–2181. [Google Scholar]
- Legua, P.; Melgarejo, P.; Martínez, J.J.; Martínez, R.; Hernández, F. Evaluation of Spanish pomegranate juices: Organic acids, sugars, and anthocyanins. Int. J. Food Prop. 2012, 15, 481–494. [Google Scholar] [CrossRef]
- Lapidot, T.; Harel, S.; Granit, R.; Kanner, J. Bioavailability of red wine anthocyanins as detected in human urine. J. Agric. Food Chem. 1998, 46, 4297–4302. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Seymour, E.M.; Llanes, D.E.U.; Kaufman, P.B.; Bolling, S.F. Chemical profile and antioxidant capacities of tart cherry products. Food Chem. 2009, 115, 20–25. [Google Scholar] [CrossRef]
- Oliveira, H.; Roma-Rodrigues, C.; Santos, A.; Veigas, B.; Brás, N.; Faria, A.; Calhau, C.; De Freitas, V.; Baptista, P.V.; Mateus, N.; et al. GLUT1 and GLUT3 involvement in anthocyanin gastric transport- Nanobased targeted approach. Sci. Rep. 2019, 789, 1–14. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion on the re-evaluation of anthocyanins (E 163) as a food additive. EFSA J. 2013, 11, 3145. [Google Scholar]
- Matsumoto, H.; Inaba, H.; Kishi, M.; Tominaga, S.; Hirayama, M.; Tsuda, T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J. Agric. Food Chem. 2001, 49, 1546–1551. [Google Scholar] [CrossRef]
- Cao, G.; Muccitelli, H.U.; Sánchez-Moreno, C.; Prior, R.L. Anthocyanins are absorbed in glycated forms in elderly women: A pharmacokinetic study. Am. J. Clin. Nutr. 2001, 73, 920–926. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Huang, Y.; Xiao, D.; Park, E.; Edirisinghe, I.; Burton-Freeman, B. Pharmacokinetic characterization and bioavailability of strawberry anthocyanins relative to meal intake. J. Agric. Food Chem. 2016, 64, 4891–4899. [Google Scholar] [CrossRef]
- Nielsen, I.L.F.; Dragsted, L.O.; Ravn-haren, G.; Freese, R.; Rasmussen, S.E. Absorption and excretion of black currant anthocyanins in humans and watanabe heritable hyperlipidemic rabbits. J. Agric. Food Chem. 2003, 51, 2813–2820. [Google Scholar] [CrossRef]
- Bitsch, R.; Netzel, M.; Frank, T.; Strass, G.; Bitsch, I. Bioavailability and biokinetics of anthocyanins from red grape juice and red wine. J. Biomed. Biotechnol. 2004, 2004, 293–298. [Google Scholar] [CrossRef]
- Milbury, P.E.; Vita, J.A.; Blumberg, J.B. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J. Nutr. 2010, 140, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; Bell, P.G.; Lodge, J.K.; Constantinou, C.L.; Jenkinson, S.E.; Bass, R.; Howatson, G. Phytochemical uptake following human consumption of Montmorency tart cherry (L. Prunus cerasus) and influence of phenolic acids on vascular smooth muscle cells in vitro. Eur. J. Nutr. 2016, 55, 1695–1705. [Google Scholar] [CrossRef]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Coletta, W.; Tamburrelli, C.; D’Imperio, M.; Crescente, M.; Silvestri, C.; Rapisarda, P.; Reforgiato Recupero, G.; De Curtis, A.; Iacoviello, L.; et al. Four-week ingestion of blood orange juice results in measurable anthocyanin urinary levels but does not affect cellular markers related to cardiovascular risk: A randomized cross-over study in healthy volunteers. Eur. J. Nutr. 2012, 51, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Romaszko, E.; Piskula, M.K. Bioavailability of cyanidin glycosides from natural chokeberry (Aronia melanocarpa) juice with dietary-relevant dose of anthocyanins in humans. J. Agric. Food Chem. 2010, 58, 12130–12136. [Google Scholar] [CrossRef] [PubMed]
- McGhie, T.K.; Ainge, G.D.; Barnett, L.E.; Cooney, J.M.; Jensen, D.J. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. J. Agric. Food Chem. 2003, 51, 4539–4548. [Google Scholar] [CrossRef]
- Morazzoni, P.; Bombardelli, E. Vaccinium myrtillus L. Fitoterapia 1996, 67, 3–29. [Google Scholar]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Oliveira, H.; Perez-Gregório, R.; Freitas, V.; Mateus, N.; Fernandes, I. Comparison of the in vitro gastrointestinal bioavailability of acylated and non-acylated anthocyanins: Purple-fleshed sweet potato vs red wine. Food Chem. 2018, 276, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The interaction of anthocyanins with bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296, 631–636. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Han, F.; Oliveira, H.; Brás, N.F.; Fernandes, I.; Cruz, L.; de Freitas, V.; Mateus, N. In vitro gastrointestinal absorption of red wine anthocyanins–Impact of structural complexity and phase II metabolization. Food Chem. 2020, 317, 126398. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Herrera, R.; Moya-león, M.A. Anthocyanins: Food sources and benefits to consumer’s health. In Handbook of Anthocyanins; Warner, L.M., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 363–384. [Google Scholar]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Brncic, S.R.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; De Pascual-Teresa, S. A review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Kulozik, U.; Schwarz, K.; Richling, E. Encapsulation of anthocyanins from bilberries–Effects on bioavailability and intestinal accessibility in humans. Food Chem. 2018, 248, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Norberto, S.; Silva, S.; Meireles, M.; Faria, A.; Pintado, M.; Calhau, C. Blueberry anthocyanins in health promotion: A metabolic overview. J. Funct. Foods 2013, 5, 1518–1528. [Google Scholar] [CrossRef]
- Azzini, E.; Bugianesi, R.; Romano, F.; Di Venere, D.; Miccadei, S.; Durazzo, A.; Foddai, M.S.; Catasta, G.; Linsalata, V.; Maiani, G. Absorption and metabolism of bioactive molecules after oral consumption of cooked edible heads of Cynara scolymus L. (cultivar Violetto di Provenza) in human subjects: A pilot study. Br. J. Nutr. 2007, 97, 963–969. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Bioactivity and cell metabolism of in vitro digested sweet cherry (Prunus avium) phenolic compounds. Int. J. Food Sci. Nutr. 2018, 70, 335–348. [Google Scholar] [CrossRef]
- Dharmawansa, K.V.S.; Hoskin, D.W.; Rupasinghe, H.P. Chemopreventive effect of dietary anthocyanins against gastrointestinal cancers: A review of recent advances and perspectives. Int. J. Mol. Sci. 2020, 21, 6555. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, Z.; Lam, K.L.; Zeng, S.; Tan, B.K.; Hu, J. Role of intestinal microecology in the regulation of energy metabolism by dietary polyphenols and their metabolites. Food Nutr. Res. 2019, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ling, W.; Yang, Y.; Chen, Y.; Tian, Z.; Du, Z.; Chen, J.; Xie, Y.; Liu, Z.; Yang, L. Role of purified anthocyanins in improving cardiometabolic risk factors in chinese men and women with prediabetes or early untreated diabetes—A randomized controlled trial. Nutrients 2017, 9, 1104. [Google Scholar] [CrossRef]
- Kim, M.J.; Rehman, S.U.; Amin, F.U.; Kim, M.O. Enhanced neuroprotection of anthocyanin-loaded PEG-gold nanoparticles against Aβ1-42-induced neuroinflammation and neurodegeneration via the NF-KB /JNK/GSK3β signaling pathway. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2533–2544. [Google Scholar] [CrossRef]
- Amin, F.U.; Shah, S.A.; Badshah, H.; Khan, M.; Kim, M.O. Anthocyanins encapsulated by PLGA@PEG nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Aβ1-42-induced oxidative stress. J. Nanobiotechnol. 2017, 15, 12. [Google Scholar] [CrossRef]
- Thibado, S.; Thornthwaite, J.; Ballard, T.; Goodman, B. Anticancer effects of bilberry anthocyanins compared with NutraNanoSphere encapsulated bilberry anthocyanins. Mol. Clin. Oncol. 2017, 8, 330–335. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. Antimicrobial activity of anthocyanins and catechins against foodborne pathogens Escherichia coli and Salmonella. Food Control 2019, 106, 106712. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, T.; Wei, C.; Lan, W.; Zhao, Y.; Pan, Y.; Wu, V.C.H. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 2018, 94, 155–161. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, M.; Woo, H.; Tharmalingam, N.; Lee, G.; Rhee, K.J.; Eom, Y. Bin; Han, S.I.; Seo, W.D.; Kim, J.B. Inhibitory effects of anthocyanins on secretion of Helicobacter pylori CagA and VacA toxins. Int. J. Med. Sci. 2012, 9, 838–842. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bendokas, V.; Šarkinas, A.; Jasinauskienë, D.; Anisimovienë, N.; Morkûnaitë-Haimi, Š.; Stanys, V.; Šikšnianas, T. Antimicrobial activity of berries extracts of four Ribes species, their phenolic content and anthocyanin composition. Folia Hortic. 2018, 30, 249–257. [Google Scholar] [CrossRef]
- Chen, H.; Yu, W.; Chen, G.; Meng, S.; Xiang, Z.; He, N. Antinociceptive and antibacterial properties of anthocyanins and flavonols from fruits of black and non-black mulberries. Molecules 2018, 23, 4. [Google Scholar] [CrossRef]
- Majiene, D.; Liobikas, J.; Trumbeckaite, S.; Kopustinskiene, D.M.; Bendokas, V.; Sasnauskas, A.; Šikšnianas, T.; Liegiute, S.; Anisimoviene, N. Antioxidative and antimicrobial activity of anthocyanin-rich extracts from fruits of blackcurrant and cherry. Acta Hortic. 2014, 1040, 173–178. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Mendes, M.; Morais, R.M.; Calhau, C.; Pintado, M.M. Antimicrobial, antiadhesive and antibiofilm activity of an ethanolic, anthocyanin-rich blueberry extract purified by solid phase extraction. J. Appl. Microbiol. 2016, 121, 693–703. [Google Scholar] [CrossRef]
- Carvalho, F.B.; Gutierres, J.M.; Bohnert, C.; Zago, A.M.; Abdalla, F.H.; Vieira, J.M.; Palma, H.E.; Oliveira, S.M.; Spanevello, R.M.; Duarte, M.M.; et al. Anthocyanins suppress the secretion of proinflammatory mediators and oxidative stress, and restore ion pump activities in demyelination. J. Nutr. Biochem. 2015, 26, 378–390. [Google Scholar] [CrossRef]
- Rashid, K.; Wachira, F.N.; Nyariki, J.N.; Isaac, A.O. Kenyan purple tea anthocyanins and coenzyme-Q10 ameliorate post treatment reactive encephalopathy associated with cerebral human African trypanosomiasis in murine model. Parasitol. Int. 2014, 63, 417–426. [Google Scholar] [CrossRef]
- Yoon, B.I.; Bae, W.J.; Choi, Y.S.; Kim, S.J.; Ha, U.S.; Hong, S.H.; Sohn, D.W.; Kim, S.W. Anti-inflammatory and antimicrobial effects of anthocyanin extracted from black soybean on chronic bacterial prostatitis rat model. Chin. J. Integr. Med. 2018, 24, 621–626. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Rodrigues, M.; Santos, A.O.; Alves, G.; Silva, L.R. Antioxidant status, antidiabetic properties and effects on Caco-2 cells of colored and non-colored enriched extracts of sweet cherry fruits. Nutrients 2018, 10, 1688. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety-Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ichiyanagi, T.; Komiyama, T.; Hatano, Y.; Konishi, T. Superoxide radical- and peroxynitrite-scavenging activity of anthocyanins; structure-activity relationship and their synergism. Free Radic. Res. 2006, 40, 993–1002. [Google Scholar] [CrossRef]
- Tarozzi, A.; Marchesi, A.; Hrelia, S.; Angeloni, C.; Andrisano, V.; Fiori, J.; Cantelli-Forti, G.; Hrelia, P. Protective effects of Cyanidin-3-O-β-glucopyranoside against UVA-Induced Oxidative Stress in Human Keratinocytes. Photochem. Photobiol. 2005, 81, 623–629. [Google Scholar] [CrossRef]
- Heinonen, I.M.; Meyer, A.S.; Frankel, E.N. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation. J. Agric. Food Chem. 1998, 46, 4107–4112. [Google Scholar] [CrossRef]
- Elisia, I.; Hu, C.; Popovich, D.G.; Kitts, D.D. Antioxidant assessment of an anthocyanin-enriched blackberry extract. Food Chem. 2007, 101, 1052–1058. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Afaq, F.; Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.H.; Khan, N.; Zaid, M.A.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J. Investig. Dermatol. 2007, 127, 222–232. [Google Scholar] [CrossRef]
- Hassimotto, N.M.A.; Lajolo, F.M. Antioxidant status in rats after long-term intake of anthocyanins and ellagitannins from blackberries. J. Sci. Food Agric. 2011, 91, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Nurulhuda, M.H.; Ismail, A.; Abas, F.; Hamid, M.; Roowi, S. Antioxidative and cardioprotective properties of anthocyanins from defatted Dabai extracts. Evid. Based Complement. Altern. Med. 2013, 2013, 434057. [Google Scholar] [CrossRef]
- Azzini, E.; Intorre, F.; Vitaglione, P.; Napolitano, A.; Foddai, M.S.; Durazzo, A.; Fumagalli, A.; Catasta, G.; Rossi, L.; Venneria, E.; et al. Absorption of strawberry phytochemicals and antioxidant status changes in humans. J. Berry Res. 2010, 1, 81–89. [Google Scholar] [CrossRef]
- Kuntz, S.; Kunz, C.; Herrmann, J.; Borsch, C.H.; Abel, G.; Fröhling, B.; Dietrich, H.; Rudloff, S. Anthocyanins from fruit juices improve the antioxidant status of healthy young female volunteers without affecting anti-inflammatory parameters: Results from the randomised, double-blind, placebo-controlled, cross-over ANTHONIA (ANTHOcyanins in Nutrition. Br. J. Nutr. 2014, 112, 925–936. [Google Scholar] [CrossRef]
- Del Bo’, C.; Riso, P.; Campolo, J.; Møller, P.; Loft, S.; Klimis-Zacas, D.; Brambilla, A.; Rizzolo, A.; Porrini, M. A single portion of blueberry (Vaccinium corymbosum L) improves protection against DNA damage but not vascular function in healthy male volunteers. Nutr. Res. 2013, 33, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Bialasiewicz, P.; Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Krol, M.; Glusac, J.; Nowak, P.; Markowski, J.; Rutkowski, K.P.; et al. Addition of strawberries to the usual diet decreases resting chemiluminescence of fasting blood in healthy subjects—possible health-promoting effect of these fruits Consumption. J. Am. Coll. Nutr. 2013, 4, 274–287. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Flieller, E.B.; Dillon, K.J.; Leverett, B.D. Black currant nectar reduces muscle damage and inflammation following a bout of high-intensity eccentric contractions. J. Diet. Suppl. 2014, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; Van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sport. 2010, 20, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.D.; Lyall, K.A.; Wells, R.W.; Sawyer, G.M.; Lomiwes, D.; Ngametua, N.; Hurst, S.M. Daily consumption of an anthocyanin-rich extract made from New Zealand blackcurrants for 5 weeks supports exercise recovery through the management of oxidative stress and inflammation: A randomized placebo controlled pilot study. Front. Nutr. 2020, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhou, J.; Liu, W.; Tao, W.; He, J.; Jin, W.; Guo, H.; Yang, N.; Li, Y. The anti-inflammatory potential of protein-bound anthocyanin compounds from purple sweet potato in LPS-induced RAW264.7 macrophages. Food Res. Int. 2020, 137, 109647. [Google Scholar] [CrossRef]
- Fernandez-Panchon, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant activity of phenolic compounds: From in vitro results to in vivo evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, U.; Baraniak, B. Antioxidant and potentially anti-inflammatory activity of anthocyanin fractions from pomace obtained from enzymatically treated raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, 6234. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, F.; Esposito, D.; Grace, M.H.; Pirovani, M.E.; Lila, M.A. Anti-inflammatory and wound healing properties of polyphenolic extracts from strawberry and blackberry fruits. Food Res. Int. 2019, 121, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.K.; Lee, J.W.; Choi, H.; Yim, S.V. Aronia melanocarpa fruit bioactive fraction attenuates lps-induced inflammatory response in human bronchial epithelial cells. Antioxidants 2020, 9, 816. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.R.; Pereira, R.; Figueiredo, I.; Freitas, V.; Dinis, T.C.P.; Almeida, L.M. Comparison of anti-inflammatory activities of an anthocyanin-rich fraction from Portuguese blueberries (Vaccinium corymbosum L.) and 5-aminosalicylic acid in a TNBS-induced colitis rat model. PLoS ONE 2017, 12, e0174116. [Google Scholar] [CrossRef] [PubMed]
- Tall, J.M.; Seeram, N.P.; Zhao, C.; Nair, M.G.; Meyer, R.A.; Raja, S.N. Tart cherry anthocyanins suppress inflammation-induced pain behavior in rat. Behav. Brain Res. 2004, 153, 181–188. [Google Scholar] [CrossRef]
- Palungwachira, P.; Tancharoen, S.; Phruksaniyom, C.; Klungsaeng, S.; Srichan, R.; Kikuchi, K.; Nararatwanchai, T. Antioxidant and anti-inflammatory properties of anthocyanins extracted from Oryza sativa L. in primary dermal fibroblasts. Oxid. Med. Cell. Longev. 2019, 2019, 2089817. [Google Scholar] [CrossRef]
- Jacob, R.A.; Spinozzi, G.M.; Vicky, A.; Kelley, D.S.; Prior, R.L.; Hess-Pierce, B.; Kader, A.A. Consumption of cherries lowers plasma urate in healthy women. J. Nutr. 2003, 133, 1826–1829. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Rasooly, R.; Jacob, R.A.; Kader, A.A.; Mackey, B.E. Consumption of Bing sweet cherries lowers circulating concentrations of inflammation markers in healthy men and women. J. Nutr. 2006, 136, 981–986. [Google Scholar] [CrossRef]
- Beltrán-Debón, R.; Alonso-Villaverde, C.; Aragonès, G.; Rodríguez-Medina, I.; Rull, A.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Camps, J.; Joven, J. The aqueous extract of Hibiscus sabdariffa calices modulates the production of monocyte chemoattractant protein-1 in humans. Phytomedicine 2010, 17, 186–191. [Google Scholar] [CrossRef]
- Biedermann, L.; Mwinyi, J.; Scharl, M.; Frei, P.; Zeitz, J.; Kullak-Ublick, G.A.; Vavricka, S.R.; Fried, M.; Weber, A.; Humpf, H.U.; et al. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis-An open pilot study. J. Crohn’s Colitis 2013, 7, 271–279. [Google Scholar] [CrossRef]
- do Rosario, V.A.; Chang, C.; Spencer, J.; Alahakone, T.; Roodenrys, S.; Francois, M.; Weston-Green, K.; Hölzel, N.; Nichols, D.S.; Kent, K.; et al. Anthocyanins attenuate vascular and inflammatory responses to a high fat high energy meal challenge in overweight older adults: A cross-over, randomized, double-blind clinical trial. Clin. Nutr. 2020, 40, 879–889. [Google Scholar] [CrossRef]
- Xia, M.; Ling, W.; Zhu, H.; Wang, Q.; Ma, J.; Hou, M.; Tang, Z.; Li, L.; Ye, Q. Anthocyanin prevents CD40-activated proinflammatory signaling in endothelial cells by regulating cholesterol distribution. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Momin, R.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Vilkickyte, G.; Raudone, L.; Petrikaite, V. Phenolic fractions from Vaccinium vitis-idaea L. and their antioxidant and anticancer activities assessment. Antioxidants 2020, 9, 1261. [Google Scholar] [CrossRef]
- Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; González-Paramás, A.M.; Santos-Buelga, C.; Mezzetti, B.; Quiles, J.L.; Battino, M.; Giampieri, F.; et al. Strawberry (cv. Romina) methanolic extract and anthocyanin-enriched fraction improve lipid profile and antioxidant status in HepG2 cells. Int. J. Mol. Sci. 2017, 18, 1149. [Google Scholar] [CrossRef]
- Masci, A.; Coccia, A.; Lendaro, E.; Mosca, L.; Paolicelli, P.; Cesa, S. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 2016, 202, 59–69. [Google Scholar] [CrossRef]
- Lage, N.N.; Anne, M.; Layosa, A.; Arbizu, S.; Chew, B.P.; Pedrosa, M.L.; Mertens-Talcott, S.; Talcott, S.; Noratto, G.D. Dark sweet cherry (Prunus avium) phenolics enriched in anthocyanins exhibit enhanced activity against the most aggressive breast cancer subtypes without toxicity to normal breast cells. J. Funct. Foods 2020, 64, 103710. [Google Scholar] [CrossRef]
- Hogan, S.; Chung, H.; Zhang, L.; Li, J.; Lee, Y.; Dai, Y.; Zhou, K. Antiproliferative and antioxidant properties of anthocyanin-rich extract from açai. Food Chem. 2010, 118, 208–214. [Google Scholar] [CrossRef]
- Long, H.L.; Zhang, F.F.; Wang, H.L.; Yang, W.S.; Hou, H.T.; Yu, J.K.; Liu, B. Mulberry anthocyanins improves thyroid cancer progression mainly by inducing apoptosis and autophagy cell death. Kaohsiung J. Med. Sci. 2018, 34, 255–262. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jo, Y.; Shin, H.; Lee, J.; Chae, S.U.; Bae, S.K.; Na, K. Anthocyanin-fucoidan nanocomplex for preventing carcinogen induced cancer: Enhanced absorption and stability. Int. J. Pharm. 2020, 586, 119597. [Google Scholar] [CrossRef]
- Sousa, C.; Moita, E.; Valentão, P.; Fernandes, F.; Monteiro, P.; Andrade, P.B. Effects of colored and noncolored phenolics of Echium plantagineum L. bee pollen in Caco-2 cells under oxidative stress induced by tert -butyl hydroperoxide. J. Agric. Food Chem. 2015, 63, 2083–2091. [Google Scholar] [CrossRef]
- Simas Frauches, N.; Montenegro, J.; Amaral, T.; Abreu, J.P.; Laiber, G.; Junior, J.; Borguini, R.; Santiago, M.; Pacheco, S.; Nakajima, V.M.; et al. Antiproliferative activity on human colon adenocarcinoma cells and in vitro antioxidant effect of anthocyanin-rich extracts from peels of species of the Myrtaceae family. Molecules 2021, 26, 564. [Google Scholar] [CrossRef]
- Stoner, G.D.; Wang, L.-S. Chemoprevention of esophageal squamous cell carcinoma with berries. Top. Curr. Chem. 2013, 329, 1–20. [Google Scholar] [PubMed]
- Stonera, G.D.; Wanga, L.-S.; Zikrib, N.; Chenc, T.; Hechtd, S.S.; Huang, C.; Sardoc, C.; Lechner, J.F. Cancer prevention with freeze-dried berries and berry components. Semin. Cancer Biol. 2008, 17, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Lippert, E.; Ruemmele, P.; Obermeier, F.; Goelder, S.; Kunst, C.; Rogler, G.; Dunger, N.; Messmann, H.; Hartmann, A.; Endlicher, E. Anthocyanins prevent colorectal cancer development in a mouse model. Digestion 2017, 95, 275–280. [Google Scholar] [CrossRef]
- Kim, M.J.; Paramanantham, A.; Lee, W.S.; Yun, J.W.; Chang, S.H.; Kim, D.C.; Park, H.S.; Choi, Y.H.; Kim, G.S.; Ryu, C.H.; et al. Anthocyanins derived from Vitis coignetiae Pulliat contributes anti-cancer effects by suppressing NF-κB pathways in Hep3B human hepatocellular carcinoma cells and In Vivo. Molecules 2020, 25, 5445. [Google Scholar] [CrossRef]
- Aqil, F.; Jeyabalan, J.; Kausar, H.; Munagala, R.; Singh, I.P.; Gupta, R. Lung cancer inhibitory activity of dietary berries and berry polyphenolics. J. Berry Res. 2016, 6, 105–114. [Google Scholar] [CrossRef]
- Chen, P.-N.; Chu, S.-C.; Chiou, H.-L.; Chiang, C.-L.; Yang, S.-F.; Hsieh, Y.-S. Cyanidin 3-glucoside and peonidin 3-glucoside inhibit tumor cell growth and induce apoptosis in vitro and suppress tumor growth in vivo. Nutr. Cancer 2005, 53, 232–243. [Google Scholar] [CrossRef]
- Hafeez, B.; Siddiqui, I.A.; Asim, M.; Malik, A.; Afaq, F.; Adhami, V.M.; Saleem, M.; Din, M.; Mukhtar, H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: Involvement of nuclear factor-κB signaling. Cancer Res. 2008, 68, 8564–8572. [Google Scholar] [CrossRef]
- Ha, U.S.; Bae, W.J.; Kim, S.J.; Yoon, B.I.; Hong, S.H.; Lee, J.Y.; Hwang, T.K.; Hwang, S.Y.; Wang, Z.; Kim, S.W. Anthocyanin induces apoptosis of du-145 cells in vitro and inhibits xenograft growth of prostate cancer. Yonsei Med. J. 2015, 56, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.; Bin, Y.; Xiaoping, Y.; Long, Y.; Chunye, C.; Mantian, M.; Wenhua, L. Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr. Cancer 2010, 62, 1128–1136. [Google Scholar] [CrossRef]
- Liu, W.; Xu, J.; Liu, Y.; Yu, X.; Tang, X.I.; Wang, Z.H.I.; Li, X.I.N. Anthocyanins potentiate the activity of trastuzumab in human epidermal growth factor receptor 2-positive breast cancer cells in vitro and in vivo. Mol. Med. Rep. 2014, 10, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Condello, M.; Pellegrini, E.; Spugnini, E.P.; Baldi, A.; Amadio, B.; Vincenzi, B.; Occhionero, G.; Delfine, S.; Mastrodonato, F.; Meschini, S. Anticancer activity of “Trigno M”, extract of Prunus spinosa drupes, against in vitro 3D and in vivo colon cancer models. Biomed. Pharmacother. 2019, 118, 109281. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.J.; Yeh, P.H.; Lin, J.P.; Huang, A.C.; Lien, J.C.; Lin, H.Y.; Chung, J.G. Anthocyanins from black rice (Oryza sativa) promote immune responses in leukemia through enhancing phagocytosis of macrophages in vivo. Exp. Ther. Med. 2017, 14, 59–64. [Google Scholar] [CrossRef]
- Razina, T.G.; Zueva, E.P.; Ulrich, A.V.; Rybalkina, O.Y.; Chaikovskii, A.V.; Isaikina, N.V.; Kalinkina, G.I.; Zhdanov, V.V.; Zyuz’Kov, G.N. Antitumor effects of Sorbus aucuparia L. extract highly saturated with anthocyans and their mechanisms. Bull. Exp. Biol. Med. 2016, 162, 93–97. [Google Scholar] [CrossRef]
- Kresty, L.A.; Mallery, S.R.; Stoner, G.D. Black raspberries in cancer clinical trials: Past, present and future. J. Berry Res. 2016, 6, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Lazzè, M.C.; Savio, M.; Pizzala, R.; Cazzalini, O.; Perucca, P.; Scovassi, A.I.; Stivala, L.A.; Bianchi, L. Anthocyanins induce cell cycle perturbations and apoptosis in different human cell lines. Carcinogenesis 2004, 25, 1427–1433. [Google Scholar] [CrossRef]
- Ma, H.; Johnson, S.L.; Liu, W.; Dasilva, N.A.; Meschwitz, S.; Dain, J.A.; Seeram, N.P. Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-β-amyloid aggregation, and mic. Int. J. Mol. Sci. 2018, 19, 461. [Google Scholar] [CrossRef]
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.; Simon, J.E.; Ann, M.; Rochet, J.-C. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinson’s disease. Brain Res. 2014, 1555, 60–77. [Google Scholar] [CrossRef]
- Ullah, I.; Park, H.Y.; Kim, M.O. Anthocyanins protect against kainic acid-induced excitotoxicity and apoptosis via ROS-activated AMPK pathway in hippocampal neurons. CNS Neurosci. Ther. 2014, 20, 327–338. [Google Scholar] [CrossRef]
- Whyte, A.R.; Cheng, N.; Fromentin, E.; Williams, C.M. A randomized, double-blinded, placebo-controlled study to compare the safety and efficacy of low dose enhanced wild blueberry powder and wild blueberry extract (ThinkBlueTM) in maintenance of episodic and working memory in older adults. Nutrients 2018, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Bendokas, V.; Stanys, V.; Mažeikienė, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the field to the antioxidants in the body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Kelishadi, R.; Rafieian-Kopaei, M.; Najafi, S.; Najafi, M.; Sahebkar, A. Investigation of the lipid-modifying and antiinflammatory effects of Cornus mas L. supplementation on dyslipidemic children and adolescents. Pediatr. Cardiol. 2013, 34, 1729–1735. [Google Scholar] [CrossRef]
- Noordin, L.; Wan Mohamad Noor, W.N.I.; Safuan, S.; Wan Ahmad, W.A.N. Therapeutic effects of anthocyanin-rich Hibiscus sabdariffa L. extract on body mass index, lipid profile and fatty liver in obese-hypercholesterolaemic rat model. Int. J. Basic Clin. Pharmacol. 2019, 9, 1. [Google Scholar] [CrossRef]
- Güder, A.; Gür, M.; Engin, M.S. Antidiabetic and antioxidant properties of bilberry (Vaccinium myrtillus Linn.) fruit and their chemical composition. J. Agric. Sci. Technol. 2015, 17, 401–414. [Google Scholar]
- Akkarachiyasit, S.; Yibchok-Anun, S.; Wacharasindhu, S.; Adisakwattana, S. In vitro inhibitory effects of cyanidin-3-rutinoside on pancreatic α-amylase and its combined effect with acarbose. Molecules 2011, 16, 2075–2083. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Shin, T.S.; Kim, M.Y.; Cho, N.J.; Kim, J.D. Anthocyanins from Cornus kousa ethanolic extract attenuate obesity in association with anti-angiogenic activities in 3T3-L1 cells by down-regulating adipogeneses and lipogenesis. PLoS ONE 2018, 13, e0208556. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Yibchok-Anun, S.; Charoenlertkul, P.; Wongsasiripat, N. Cyanidin-3-rutinoside alleviates postprandia hyperglycemia and its synergism with acarbose by inhibition of intestinal α-glucosidase. J. Clin. Biochem. Nutr. 2011, 49, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, D.; Zhang, Y.; Sun, R.; Xia, M. Anthocyanin increases adiponectin secretion and protects against diabetes-related endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2014, 306, 975–988. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, Z.; Yin, J.; Long, H.; Zheng, X. Anti-obesity effects of artificial planting blueberry (Vaccinium ashei) anthocyanin in high-fat diet- treated mice. Int. J. Food Sci. Nutr. 2016, 67, 257–264. [Google Scholar] [CrossRef]
- Wu, T.; Liu, R. Anthocyanins in black rice, soybean and purple corn increase fecal butyric acid and prevent liver inflammation in high fat diet-induced obese mice. Food Funct. 2017, 8, 3178–3186. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, D.; Shi, L.; Liu, X.; Zhang, Y.; Tong, C.; Song, D.; Hou, M. Blueberry anthocyanins-enriched extracts attenuate cyclophosphamide-induced cardiac injury. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef]
- Song, F.; Zhu, Y.; Shi, Z.; Tian, J.; Deng, X.; Ren, J.; Andrews, M.C.; Ni, H.; Ling, W.; Yang, Y. Plant food anthocyanins inhibit platelet granule secretion in hypercholesterolaemia: Involving the signalling pathway of PI3K–Akt. Thromb. Haemost. 2014, 112, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, N.; Tsuruma, K.; Shimazawa, M.; Yokota, S.; Hara, H. Inhibitory actions of bilberry anthocyanidins on angiogenesis. Phyther. Res. 2009, 24, S42–S47. [Google Scholar] [CrossRef] [PubMed]
- Gaiz, A.; Kundur, A.R.; Colson, N.; Shibeeb, S.; Singh, I. Assessment of in vitro effects of anthocyanins on platelet function. Altern. Ther. Health Med. 2020, 26, 12–17. [Google Scholar] [PubMed]
- Hassellund, S.S.; Flaa, A.; Kjeldsen, S.E.; Seljeflot, I.; Karlsen, A.; Erlund, I.; Rostrup, M. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: A double-blind randomized placebo-controlled crossover study. J. Hum. Hypertens. 2013, 27, 100–106. [Google Scholar] [CrossRef]
- McAnulty, L.S.; Collier, S.R.; Landram, M.J.; Whittaker, D.S.; Isaacs, S.E.; Klemka, J.M.; Cheek, S.L.; Arms, J.C.; McAnulty, S.R. Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr. Res. 2014, 34, 577–584. [Google Scholar] [CrossRef]
- Habanova, M.; Saraiva, J.A.; Haban, M.; Schwarzova, M.; Chlebo, P.; Predna, L.; Gažo, J.; Wyka, J. Intake of bilberries (Vaccinium myrtillus L.) reduced risk factors for cardiovascular disease by inducing favorable changes in lipoprotein profiles. Nutr. Res. 2016, 36, 1415–1422. [Google Scholar] [CrossRef]
- Arevström, L.; Bergh, C.; Landberg, R.; Wu, H.; Rodriguez-Mateos, A.; Waldenborg, M.; Magnuson, A.; Blanc, S.; Fröbert, O. Freeze-dried bilberry (Vaccinium myrtillus) dietary supplement improves walking distance and lipids after myocardial infarction: An open-label randomized clinical trial. Nutr. Res. 2019, 62, 13–22. [Google Scholar] [CrossRef]
- Draijer, R.; de Graaf, Y.; Slettenaar, M.; de Groot, E.; Wright, C.I. Consumption of a polyphenol-rich grape-wine extract lowers ambulatory blood pressure in mildly hypertensive subjects. Nutrients 2015, 7, 3138–3153. [Google Scholar] [CrossRef]
- Igwe, E.O.; Charlton, K.E.; Roodenrys, S.; Kent, K.; Fanning, K.; Netzel, M.E. Anthocyanin-rich plum juice reduces ambulatory blood pressure but not acute cognitive function in younger and older adults: A pilot crossover dose-timing study. Nutr. Res. 2017, 47, 28–43. [Google Scholar] [CrossRef]
- Xie, L.; Vance, T.; Kim, B.; Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Lee, J.Y.; Chun, O.K.; Bolling, B.W. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: A randomized controlled trial. Nutr. Res. 2017, 37, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Bakkar, Z.A.; Fulford, J.; Gates, P.E.; Jackman, S.R.; Jones, A.M.; Bond, B.; Bowtell, J.L. Montmorency cherry supplementation attenuates vascular dysfunction induced by prolonged forearm occlusion in overweight, middle-aged men. J. Appl. Physiol. 2019, 126, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Lamport, D.J.; Butler, L.T.; Williams, C.M. A study of glycaemic effects following acute anthocyanin-rich blueberry supplementation in healthy young adults. Food Funct. 2017, 8, 3104–3110. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xia, M.; Yang, Y.; Liu, F.; Li, Z.; Hao, Y.; Mi, M.; Jin, T.; Ling, W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin. Chem. 2011, 57, 1524–1533. [Google Scholar] [CrossRef]
- Kianbakht, S.; Abasi, B.; Hashem Dabaghian, F. Improved lipid profile in hyperlipidemic patients taking vaccinium arctostaphylos fruit hydroalcoholic extract: A randomized double-blind placebo-controlled clinical trial. Phyther. Res. 2014, 28, 432–436. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Willems, M.E.T. Blackcurrant alters physiological responses and femoral artery diameter during sustained isometric contraction. Nutrients 2017, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Milbury, P.E.; Graf, B.; Curran-Celentano, J.M.; Blumberg, J.B. Bilberry (Vaccinium myrtillus) anthocyanins modulate heme oxygenase-1 and glutathione S-transferase-pi expression in ARPE-19 cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Sundalius, N.M. Examination of Blueberry Anthocyanins in Prevention of Age-Related Macular Degeneration through Retinal Pigment Epithelial Cell Culture Study; Louisiana State University and Agricultural and Mechanical College: Baton Rouge, LA, USA, 2008. [Google Scholar]
- Matsumoto, H.; Kamm, K.E.; Stull, J.T.; Azuma, H. Delphinidin-3-rutinoside relaxes the bovine ciliary smooth muscle through activation of ETB receptor and NO/cGMP pathway. Exp. Eye Res. 2005, 80, 313–322. [Google Scholar] [CrossRef]
- Iida, H.; Nakamura, Y.; Matsumoto, H.; Takeuchi, Y.; Harano, S.; Ishihara, M.; Katsumi, O. Effect of black-currant extract on negative lens-induced ocular growth in chicks. Ophthalmic Res. 2010, 44, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Nakaishi, H.; Matsumoto, H.; Tominaga, S.; Hirayama, M. Effects of black currant anthocyanoside intake on dark adaptation and VDT work-induced transient refractive alteration in healthy humans. Altern. Med. Rev. 2000, 5, 553–562. [Google Scholar] [PubMed]
| Source | Maximum Anthocyanin Amount in FW (mg C3G/100 g) | Maximum Anthocyanin Amount in DW (mg C3G/100 g) | Dominant Anthocyanins | Reference |
|---|---|---|---|---|
| Fruits | ||||
| Açaí | 282.5–303.7 | Cy 3,5-hexose pentose, Cy 3-O-glucoside, Cy 3-O-rutinoside, Pg 3-O-glucoside, Pn 3-O-glucoside, Pn 3-O-rutinoside, Cy 3-(acetyl)hexose | [51] | |
| Acerola | 6.5–8.4 | Cy 3-rhamnoside, Pg 3-rhamnoside Cy, Pg | [51] | |
| Apple | 30.07–71.49 * | Cy 3-galactoside, Cy 3-O-glucoside, Cy 3-arabinoside, Pn 3-galactoside, Cy 7-arabinoside, Cy3-xyloside | [46,52] | |
| Blackberries | 70.3–201 | 909.3 | Cy 3-O-glucoside, Cy 3-O-rutinoside, Cy 3-xyloside, Cy 3-malonylglucoside, Cy 3-dioxalylglucoside | [53,54] |
| Black currants | 218.93 | 32,300 1 | Dp 3-O-glucoside, Dp 3-O- rutinoside, Cy 3-O-glucoside, Cy 3-O- rutinoside | [55,56] |
| Blueberries | 406.90 | 11601 | Dp 3-O-galactoside, Dp 3-O-glucoside, Cy-3-O-galactoside, Dp 3-O-arabinoside, Cy-3-glucoside, Pt-3-galactoside, Cy 3-O-arabinoside, Pt 3-O-glucoside, Pn 3-O-arabinoside, Mv 3-O-galactoside, Mv 3-O-glucoside | [55,57] |
| Chokeberries | 357.20 | Cy 3-O-galactoside, Cy 3-arabinoside, Cy 3-O-glucoside, Cy xyloside | [55,58] | |
| Cranberries | 40.7–207.3 1 | Cy 3-O-galactoside Cy 3-O-glucoside, Cy arabinoside, Pn galactoside, Pn 3-O-glucoside, Pn arabinoside | [59] | |
| Elderberries | 317.51 | 408.6–1066.6 | Cy 3-O-glucoside, Cy 3-O-sambubioside | [55,60] |
| Fig | 0.3–10.9 | 4.6–83 | Cy 3-O-glucoside, Cy 3-O-rutinoside, Cy 3-sambubioside-5-glucoside, Cy 3,5-diglucoside | [61,62] |
| Grapes | 38.70–186.02 1 | 135,960–533,630 1 | Cy, Dp, Mv, Pn, Pt 3-O-glycosides; Mv, Pn, Pt 3-O-coumarylglucosides | [63,64] |
| Haskaps | 449–697 | 2273 | Cy 3,5-di-glucoside; Cy 3-galactoside, Cy 3-O-glucoside, Cy 3-O-rutinoside, Pg 3-O-glucoside; Pn 3-O-glucoside | [65,66] |
| Nectarine | 0.22 | Cy 3-O-glucoside, Cy 3-O-rutinoside | [46,67] | |
| Plums | 7.4–36.6 1 | Cy 3-xyloside, Cy 3-O-glucoside, Cy 3-O-rutinoside, Pn 3-O-rutinoside, Pn 3-O-glucoside, Cy 3-galactoside, Cy 3-(6’’-acetoyl)glucoside | [46,68] | |
| Pomegranate | 1500–2000 | Dp 3,5-diglucoside, Cy 3-O-glucoside, Cy 3,5-diglucoside, Pg 3-O-glucoside, Pg 3,5-diglucoside | [69,70] | |
| Peaches | 0.27–2.50 | 0.28–15.34 1 | Cy 3-O-rutinoside and glucoside | [71,72] |
| Red cabbages | 109–185 | 1111–1780 | Cy 3-diglucoside-5-glucoside, Cy 3-(sinapoyl)(sinapoyl)-diglucoside-5-glucoside, Cy 3-(ρ-coumaroyl)-diglucoside-5-glucoside | [73,74] |
| Red currants | 19.78 | 149.91 1 | Cyanidin-3-O-sambusoside, Cy 3-O-glucoside, Cy 3-O-rutinoside | [55,75] |
| Red pears | 1.2–12.0 1 | Cy 3-O-galactoside, Cy 3-O-glucoside, Cy pentoside, Cy 3-O-arabinoside, Cy 3-O-rutinoside | [76] | |
| Red raspberries | 23.17–68.0 | 260.9–571.8 | Cy 3-O-sophoroside, Cy 3-O-(2’’-O-glucosyl)rutinoside, Cy 3-O-glucoside, Cy 3-O-rutinoside, Cy 3-O-(2’’-O-xylosyl)rutinoside, Pg 3-O-sophoroside, Pg 3-O-glucoside, Cy 3,5-O-diglucoside | [53,55,77] |
| Strawberries | 20–60 1 | 31.9–315.2 2 | Pg 3-O-glucoside, Pg 3-O-glucoside, Cy 3-O-glucoside, Cy 3-O-rutinoside, Pg 3-O-glucoside, Pg 3-O-rutinoside, Pg 3-(malonoyl)glucoside, Pg 3-(6’’-acetoyl)glucoside | [46,78,79] |
| Sweet cherries | 2.06–462.77 1 | 107.70–218.36 1 | Cy, Dp, Pg, Pn 3-O-rutinosides and glucosides, Cy 3-coumaroyl-diglucoside, Cy 3-O-sambubioside, Cy 3-5-diglucoside, Cy 3-sophoroside Cy 3-O-arabinoside Mv 3-O-glucoside-acetaldehyde | [40,80,81] |
| Tamarillo | 29.70–486.84 1 | Dp 3-O-rutinoside, Cy 3-O-rutinoside, Cy 3-O-glucoside, Pg 3-O-rutinoside | [82] | |
| Tart cherries | 65.06–82.40 | 114.59 | Cy, Cy 3-O-sophoroside, Cy 3-glucosylrutinoside, Cy 3-O-glucoside, Cy 3-O-rutinoside, Pn 3-O-rutinoside | [55,83] |
| Tomato | 7.1 1 | 5.48–29.86 3 | Dp glycoside, Dp rutinoside, Dp ρ-coumaroyl-rutinoside Mv glycoside, Mv rutinoside, Mv ρ-coumaroyl-rutinoside-glycoside, Pt rutinoside, Pt ρ-coumaroyl-rutinoside, Pt ρ-coumaroyl-rutinoside-glycoside | [84,85] |
| Vegetables | ||||
| Black carrot | 22.45 * | 1.74–4.54 1 | Cy 3-(ρ-coumaroyl)-diglucoside-5-glucoside | [47,86] |
| Eggplant | 6.31 | 138 4 | Dp 3-(ρ-coumaroylrutinoside)-5-glucoside, Dp 3-O-glucoside, Dp 3-glucosyl-rhamnoside, Pt -3-O-rutinoside, Cy -3-O-rutinoside | [49,50] |
| Purple sweet potato | 42.37 * | Pn 3-O-sophoroside-5-O-glucoside, Pn 3-O-glucoside, Cy 3-ρ-hydroxybenzoylsophoroside-5-glucoside, Pn 3-ρ-hydroxybenzoylsophoroside-5-glucoside, Cy 3-caffeoylsophoroside-5-glucoside, Pn 3-caffeoylsophoroside-5-glucoside, Cyanidin-3-caffeoyl- ρ-hydroxybenzoylsophoroside-5-glucoside, Pn 3-dicaffeoylsophoroside-5-glucoside, Pn 3-caffeoyl-ρ-hydroxybenzoylsophoroside-5-glucoside, Pn 3-caffeoy-feruloylsophoroside-5-glucosie | [47] | |
| Red Chicory | 39.20 * | Cy 3-O-glucoside, Cy 3-O-(6”-malonyl-glucoside) | [47] | |
| Red onion | 29.99 | Cy 3-O-glucoside, Cy 3-O-laminaribioside, Cy 3-(6’’-malonyl-glucoside), Cy 3-(6”-malonyl- laminaribioside), Cy 3-xylosylglucosylgalactoside, Dp 3,5-diglycosides | [47,87] | |
| Beverages | ||||
| Blackberry juice | 12.3–107 | Cy 3-O-glycoside, Cy 3-O-rutinoside, Cy 3-xyloside, Cy malonylglucoside, Cy dioxalylglucoside | [54] | |
| Pomegranate juice | 7.2–20 1 | Cy 3-O-glucoside, Cy 3,5-diglucoside, Dp 3,5-diglucoside, Cy 3,5-diglucoside, Pg 3,5-diglucoside, Dp 3-glucoside, Cy 3- O-glucoside; Pg 3-O-glucoside | [88] | |
| Red wine | 32.71–87.17 1 | Cy, Dp, Mv, Pn, Pt 3-O-glycosides, Pn 3-O-acetylglucoside, Mv 3-O-acetylglucoside, Mv 3-O-coumarylglucoside, Pn 3-O-ρ-coumarylglucoside; | [48,89] | |
| Tart cherry juice | 72.2 | Cy 3-sophoroside, Cy 3-glucosylrutinoside, Cy 3-O-glucoside, Cy 3-O-rutinoside, Cy, Pg, Pn 3-O-glucoside | [90] | |
| Intake | n (a) | Total Anthocyanins Intake | Cmax (b) | tmax (h) (c) | AUC (d) | Urinary Excretion (%) | Reference |
|---|---|---|---|---|---|---|---|
| Foods | |||||||
| Blueberries (100 g) | 5 | 1200 mg | 13.1 ng/mL | 4 | [57] | ||
| Elderberries (12 g) | 4 | 720 mg | 97.4 nmol/L | 1.2 h | [94] | ||
| Red raspberries (300 g) | 9 | 292 µmol | 0.1–180 nmol/L | 1–1.5 | 0.007% (1–1.5 h) | [77] | |
| Beverages | |||||||
| Red wine (300 mL) | 6 | 218 mg | 6 | 1.5–5.1% (12 h) | [89] | ||
| Red grape juice (400 mL) | 9 | Cy 3-O-glucoside | 0.42 ng/mL | 0.5 | 0.60 ng × h/mL (3 h) | 0.09% (7 h) | [97] |
| Dp 3-O-glucoside | 6.12 ng/mL | 0.5 | 11.9 ng × h/mL (3 h) | 0.20% (7 h) | |||
| Mv 3-O-glucoside | 48.8 ng/mL | 0.5 | 71.7 ng × h/mL (3 h) | 0.18% (7 h) | |||
| Pn 3-O-glucoside | 27.3 ng/mL | 0.5 | 49.7 ng × h/mL (3 h) | 0.29% (7 h) | |||
| Pt 3-O-glucoside | 16.1 ng/mL | 0.5 | 31.5 ng × h/mL (3 h) | 0.32% (7 h) | |||
| Σ = 283.5 mg | 100.1 ng/mL | 0.5 | 168.4 ng × h/mL | ||||
| Black currant juice (150 mL) | 8 | Cy 3-O-glucoside: 0.165 mg | 5.0 nmol/L | 1.34 | 11.0–19.6 ng × h/mL (4 h) | 0.060% (8 h) | [96] |
| Cy 3-O-rutinoside: 1.24 mg | 46.3 nmol/L | 3.45 | 19.6–24.9 ng × h/mL (4 h) | 0.098% (8 h) | |||
| Dp 3-O-glucoside: 0.488 mg | 22.7 nmol/L | 4.19 | 11.0–16.3 ng × h/mL (4 h) | 0.066 (8 h) | |||
| Dp 3-O-rutinoside: 1.68 mg | 73.4 nmol/L | 3.18 | 16.3–24.9 ng × h/mL (4 h) | 0.11% (8 h) | |||
| Açaí Juice (7 mL/kg of body weight) | 12 | 165.9 mg/L | 1138.51 ng/L | 2 | 3314.04 ng × h/L (12 h) | [11] | |
| Cranberry Juice (480 mL) | 15 | Cy 3-O-galactoside (18.7 mg) | 1.38 nmol/L | 1.27 | 3.91 nmol × h/L (4 h) | 0.007% (4 h) | [98] |
| Cy 3-O-glucoside (1.58 mg) | 0.93 nmol/L | 1.13 | 1.99 nmol × h/L (4 h) | 0.007% (4 h) | |||
| Cy 3-O-arabinoside (16.47 mg) | 3.61 nmol/L | 1.47 | 9.16 nmol × h/L (4 h) | 0.010% (4 h) | |||
| Mv 3-O-glucoside | 0.56 nmol/L | 0.93 | 1.25 nmol × h/L (4 h) | ||||
| Pn 3-O-galactoside (30.83 mg) | 4.64 nmol/L | 1.47 | 12.00 nmol × h/L (4 h) | 0.015% (4 h) | |||
| Pn 3-O-glucoside (5.85 mg) | 0.71 nmol/L | 1.40 | 1.85 nmol × h/L (4 h) | 0.029% (4 h) | |||
| Pn 3-O-arabinose (21.03 mg) | 1.78 nmol/L | 1.27 | 4.13 nmol × h/L (4 h) | 0.010% (4 h) | |||
| Σ = 94.47 mg | |||||||
| Tart cherry juice (60 mL) | 12 | 62.47 mg/L | 2.75 µg × h/mL | 1 | 106.4 µg × h/mL | [99] | |
| Grape/blueberry juice (330 mL) | 10 | 3,4-dihydroxybenzoic acid | 7.6 nmol/L | 1 | 568 nmol × min/L | [100] | |
| Cy 3-O-glucoside | 0.10 nmol/L | 1 | 6 nmol × min/L | ||||
| Dp 3-O-glucoside | 0.18 nmol/L | 1.1 | 10 nmol × min/L | ||||
| Mv 3-O-glucoside | 1.5 nmol/L | 1.1 | 103 nmol × min/L | ||||
| Mv 3-O-glucuronide | 1.1 nmol/L | 2 | 114 nmol × min/L | ||||
| Pn 3-O-glucuronide | 1.1 nmol/L | 1.8 | 114 nmol × min/L | ||||
| Pn 3-O-glucoside | 1.7 nmol/L | 1 | 52 nmol × min/L | ||||
| Pt 3-O-glucoside | 0.8 nmol/L | 1 | 12 nmol × min/L | ||||
| Σ = 841 mg/L | 1.21 nmol/L | ||||||
| Orange juice | 18 | 53.09 mg/L | 0.63 nmol/L | 0.96 | 8.99 nmol × h/L (8 h) | 43–53% (2 h) | [101] |
| Chokeberry juice (0.8 mg/kg of body weight) | 13 | 32.7 nmol/L | 1.3 | 109.4 nmol × h/L (1 h) | 0.25 (24 h) | [102] | |
| Strawberry juice (34.7 mg) | 14 | Cy 3-O-glucoside: 7.8 µmol | 0.6 nmol/L | 2.1 | 1.7 nmol × h/L (10 h) | [95] | |
| Pg glucuronide | 38.0 nmol/L | 1.7 | 123.8 nmol × h/L (10 h) | ||||
| Pg-3-O-glucoside: 58.8 µmol | 5.2 nmol/L | 1.3 | 15.0 nmol × h/L (10 h) | ||||
| Pg 3-O-rutinoside: 9.7 µmol | 0.4 nmol/L | 1.9 | 1.4 nmol × h/L (10 h) | ||||
| Σ = 76.6 µmol | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals 2021, 14, 690. https://doi.org/10.3390/ph14070690
Gonçalves AC, Nunes AR, Falcão A, Alves G, Silva LR. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals. 2021; 14(7):690. https://doi.org/10.3390/ph14070690
Chicago/Turabian StyleGonçalves, Ana C., Ana R. Nunes, Amílcar Falcão, Gilberto Alves, and Luís R. Silva. 2021. "Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review" Pharmaceuticals 14, no. 7: 690. https://doi.org/10.3390/ph14070690
APA StyleGonçalves, A. C., Nunes, A. R., Falcão, A., Alves, G., & Silva, L. R. (2021). Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals, 14(7), 690. https://doi.org/10.3390/ph14070690









