A Newfangled Collagenase Inhibitor Topical Formulation Based on Ethosomes with Sambucus nigra L. Extract
Abstract
1. Introduction
2. Results and Discussion
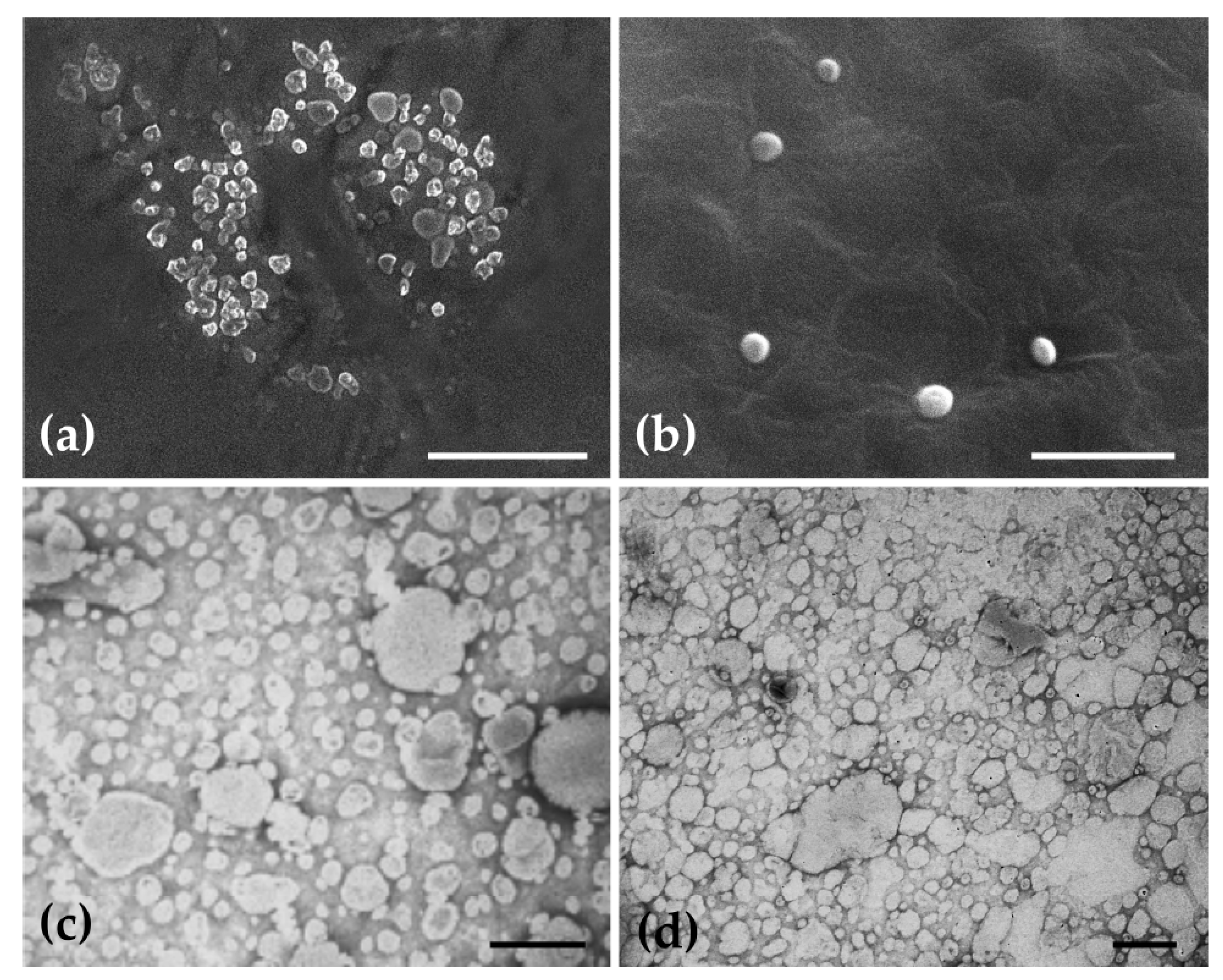
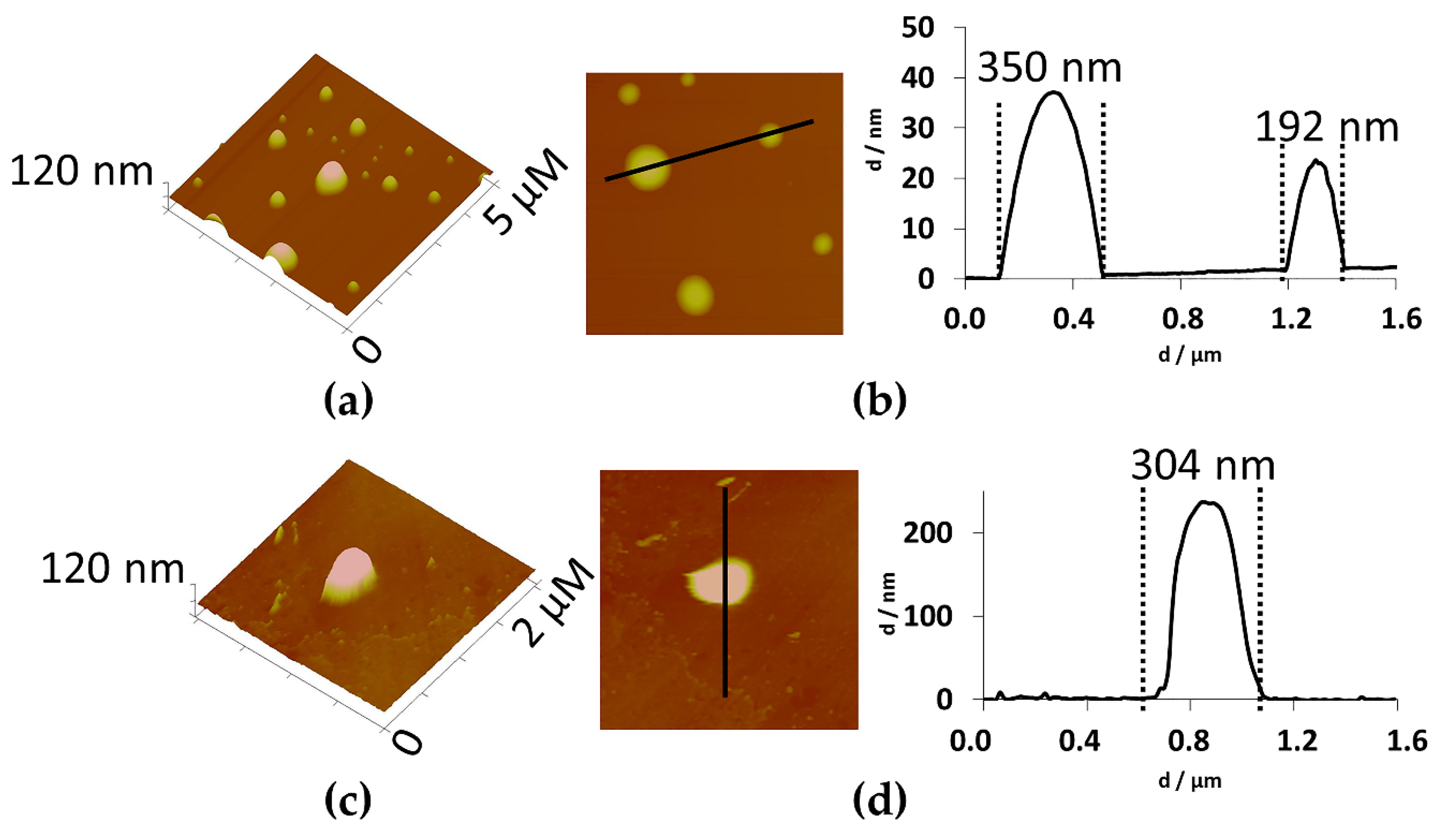
2.1. Physical Characterization of Ethosomes
2.2. Lipid Quantification of Ethosomes
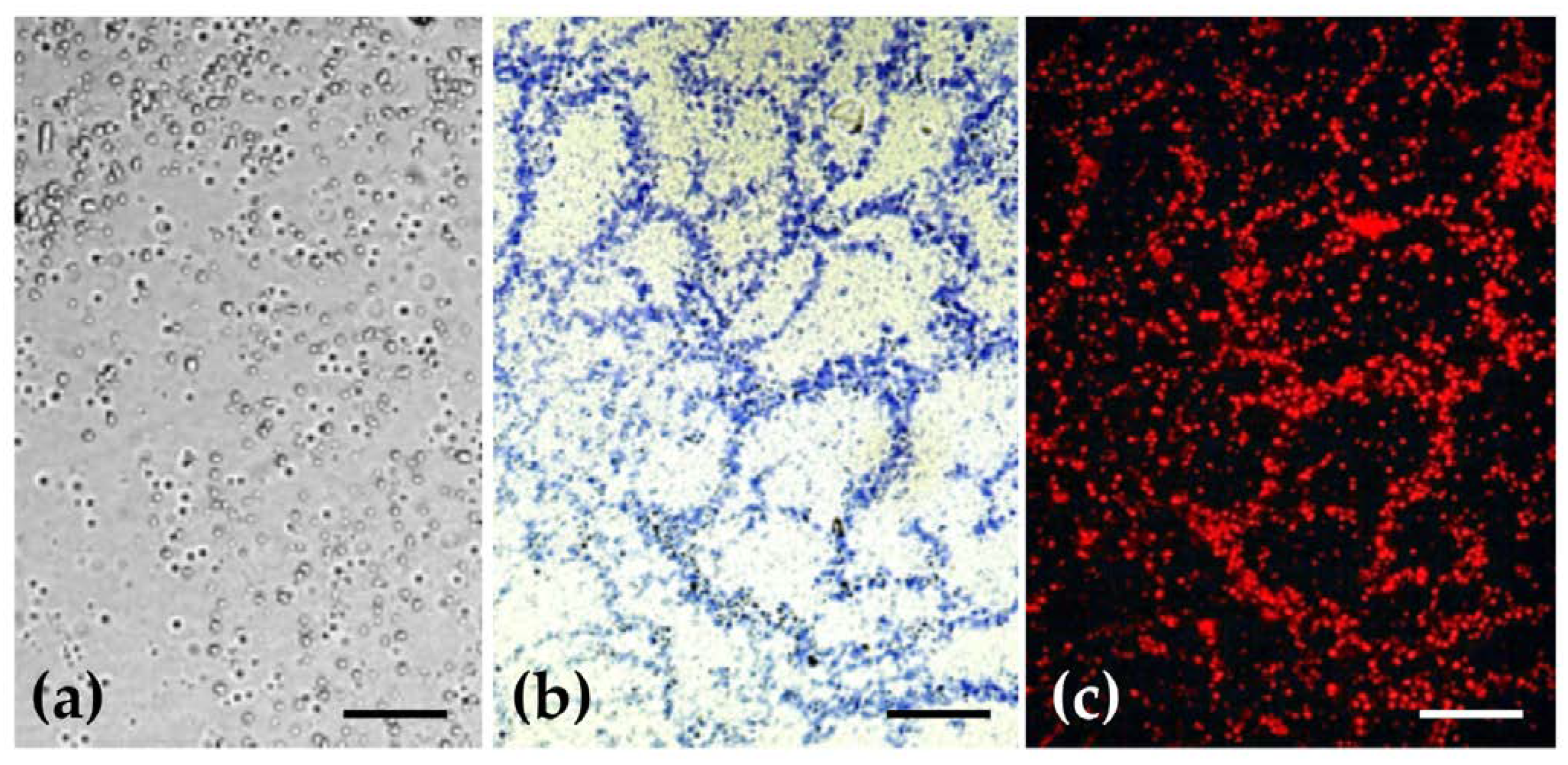
2.3. Histochemical Characterization of Ethosomes Lipidic Constitution and of Their Morphology
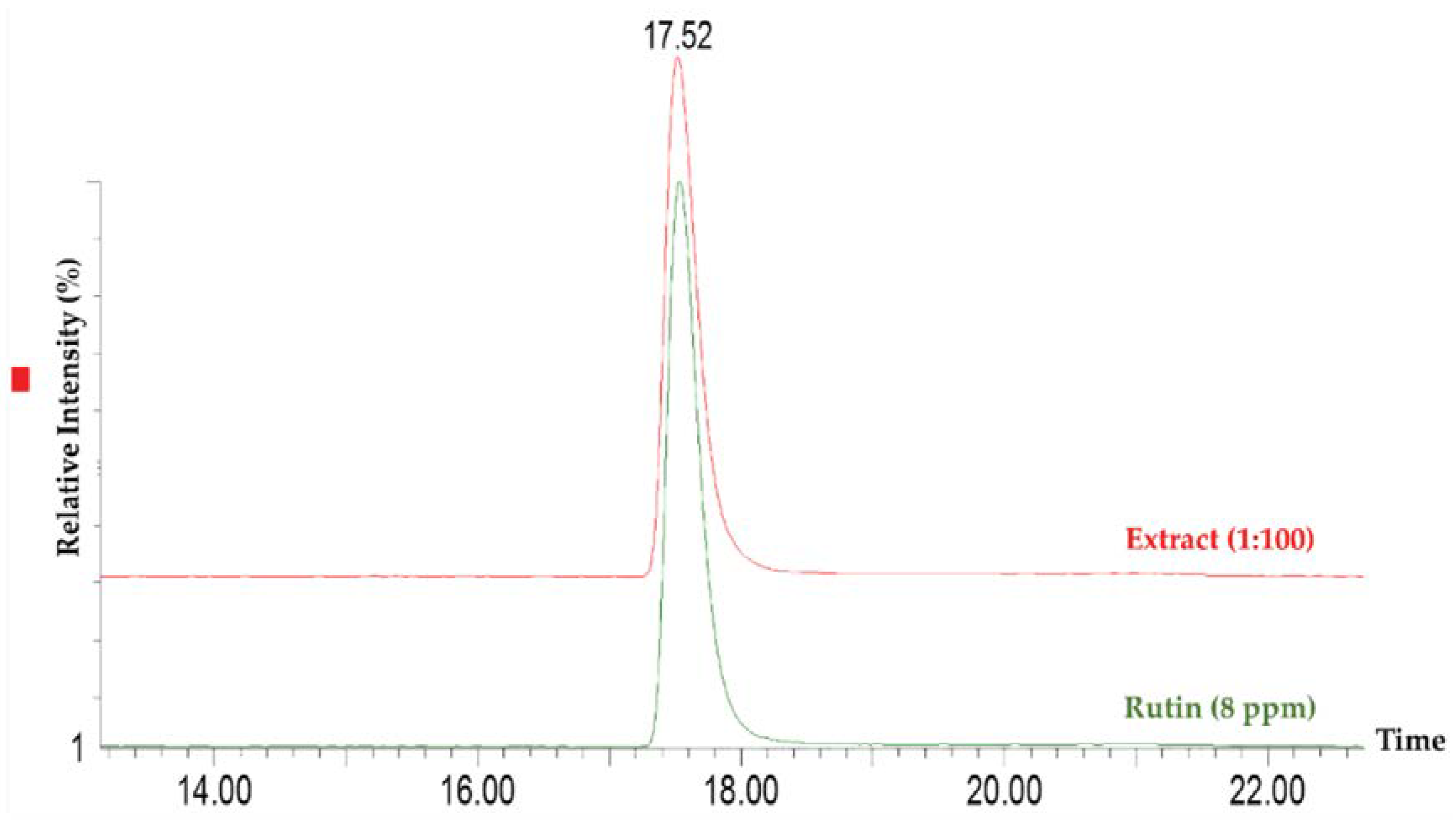
2.4. Entrapment Capacity of Extract in Ethosomes
2.5. Characterization of Rutin and Ethosomes Complexes
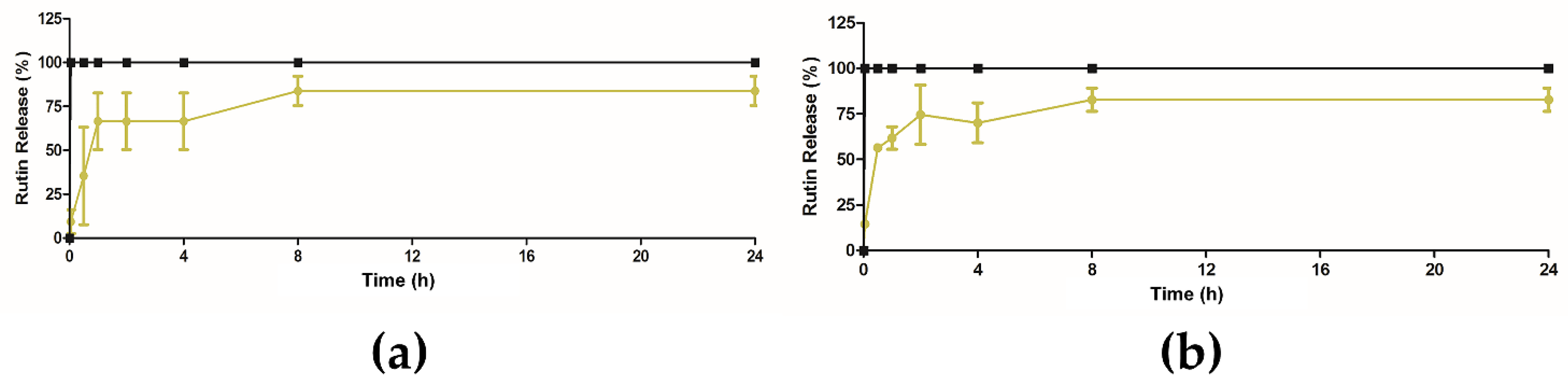
2.6. In Vitro Release Studies of Extract from Ethosomes
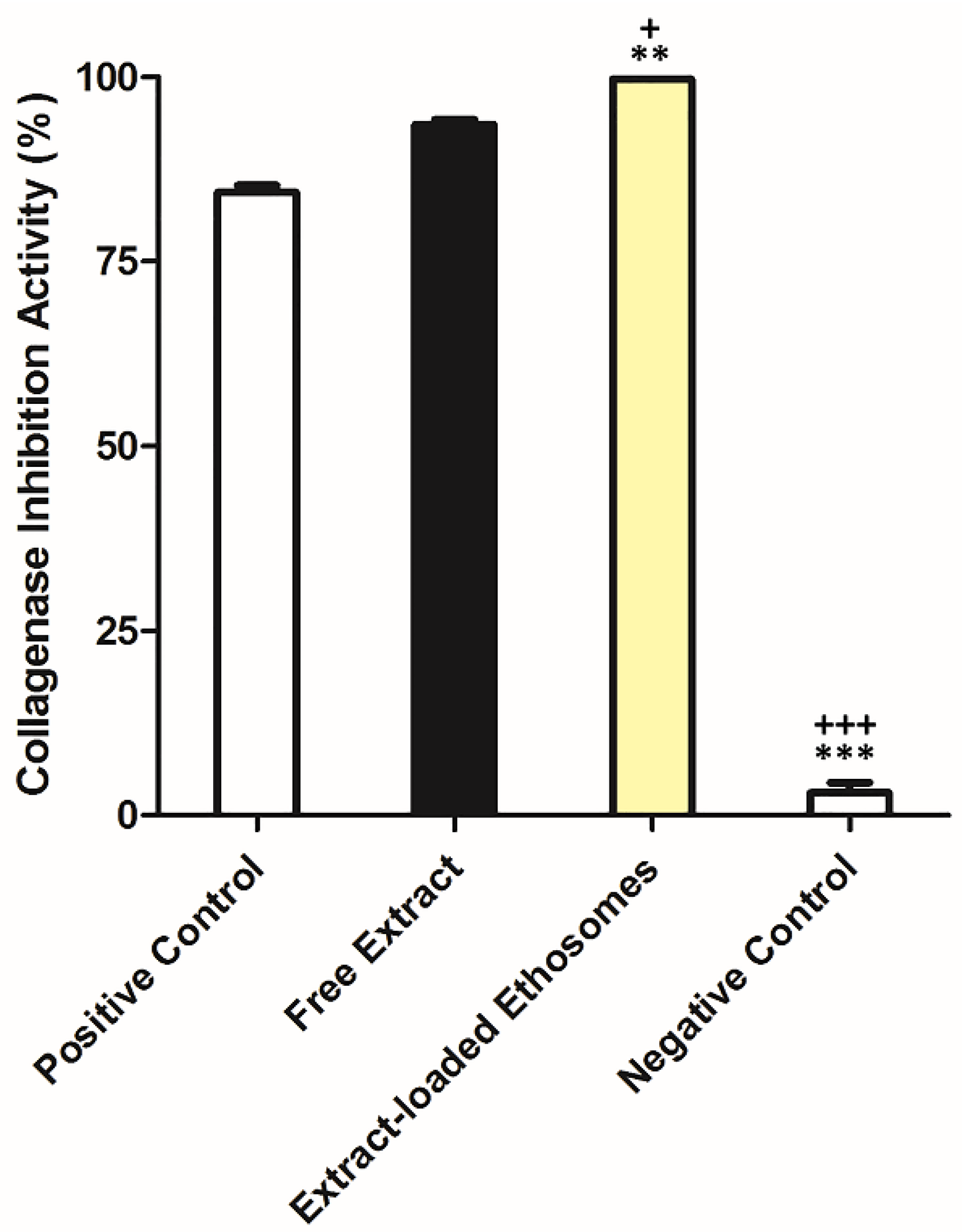
2.7. In Vitro Collagenase Inhibition Activity of Extract after Encapsulation
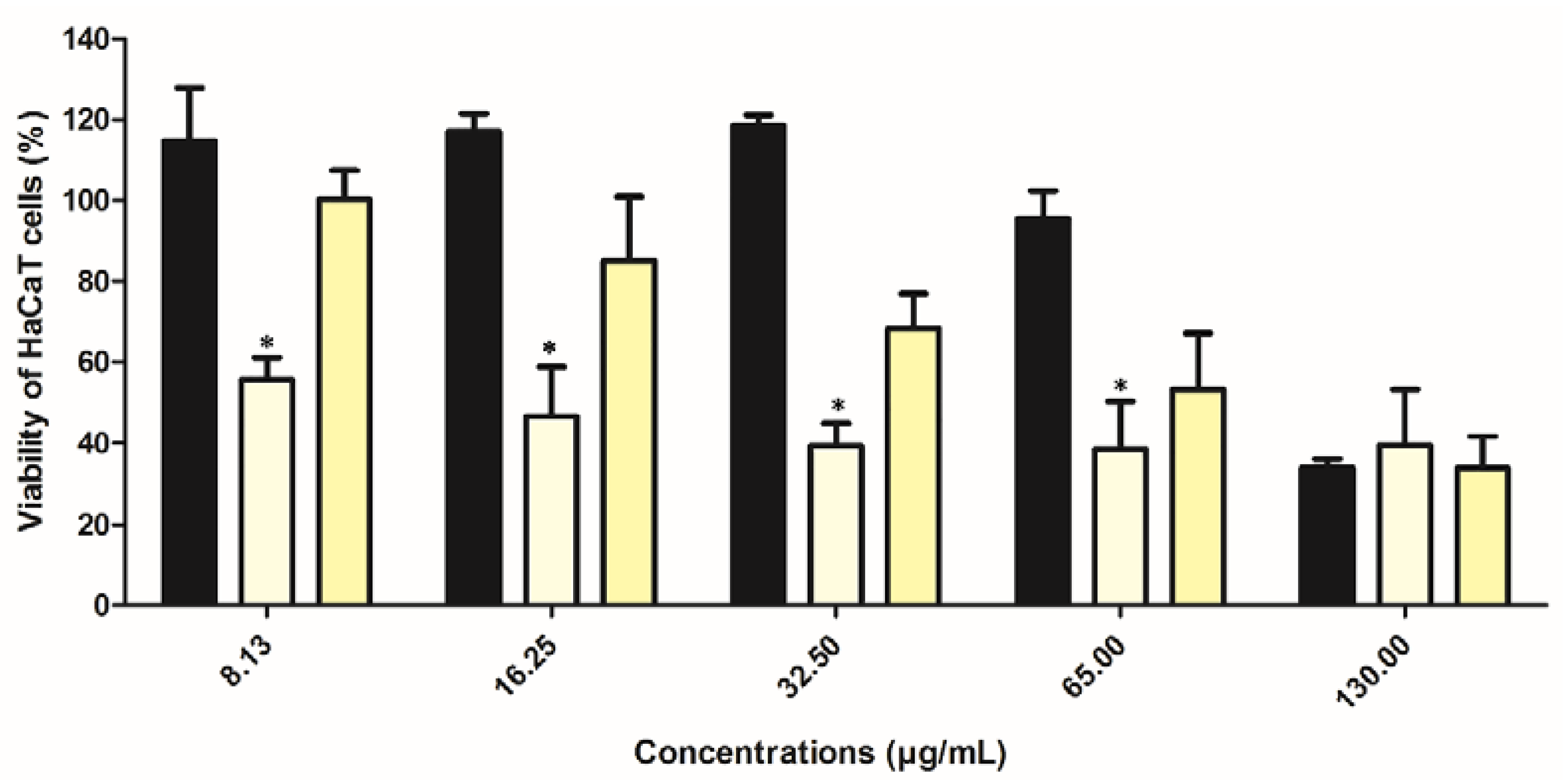
2.8. Preliminary In Vitro Safety Assessment of Extract in Ethosomes
2.9. Preliminary Stability Test of Semi-Solid Formulation with Free Extract and Ethosomes
2.9.1. Heating and Cooling Testing
2.9.2. Centrifugation Stress of Semi-Solid Formulation with Free Extract and Ethosomes
2.10. Accelerated Stability of Semi-Solid Formulation with Free Extract and Ethosomes
2.10.1. Tests Cycles of Heating and Cooling
2.10.2. Stability Test over 14 Days
2.10.3. Temperature Cycles
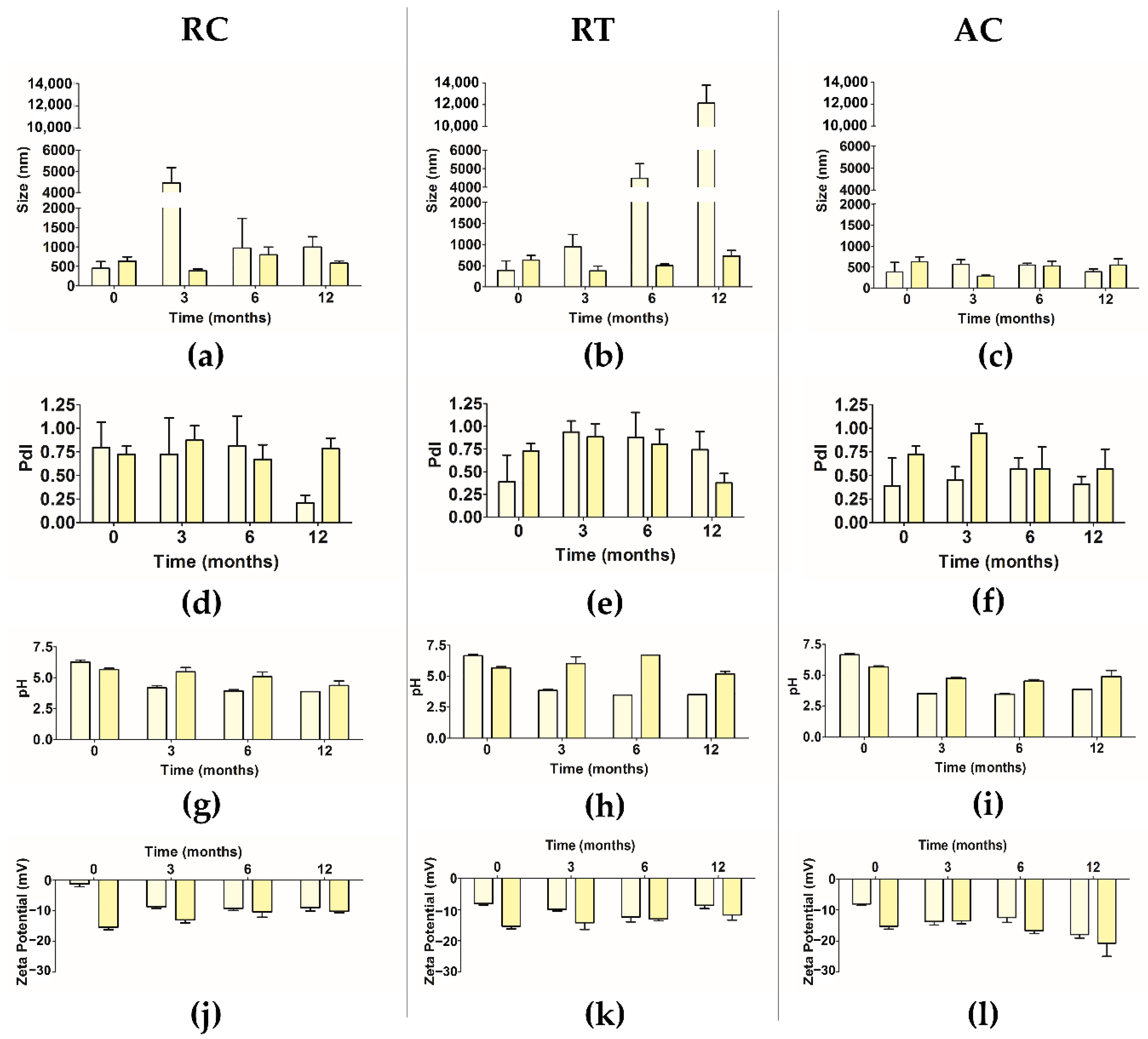
2.11. Stability over Three Months
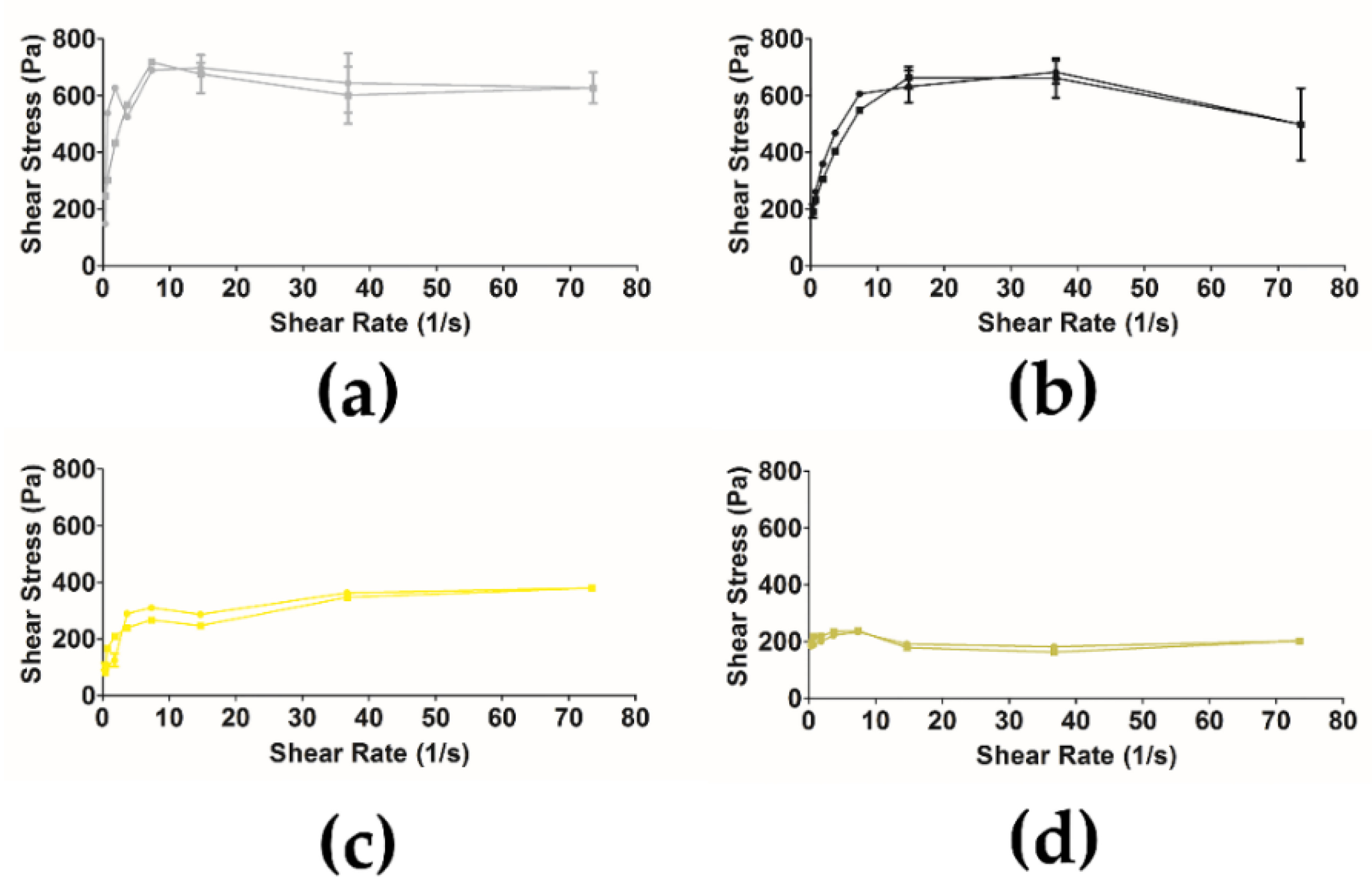
2.12. Rheology of Semi-Solid Formulation with Free Extract and Ethosomes
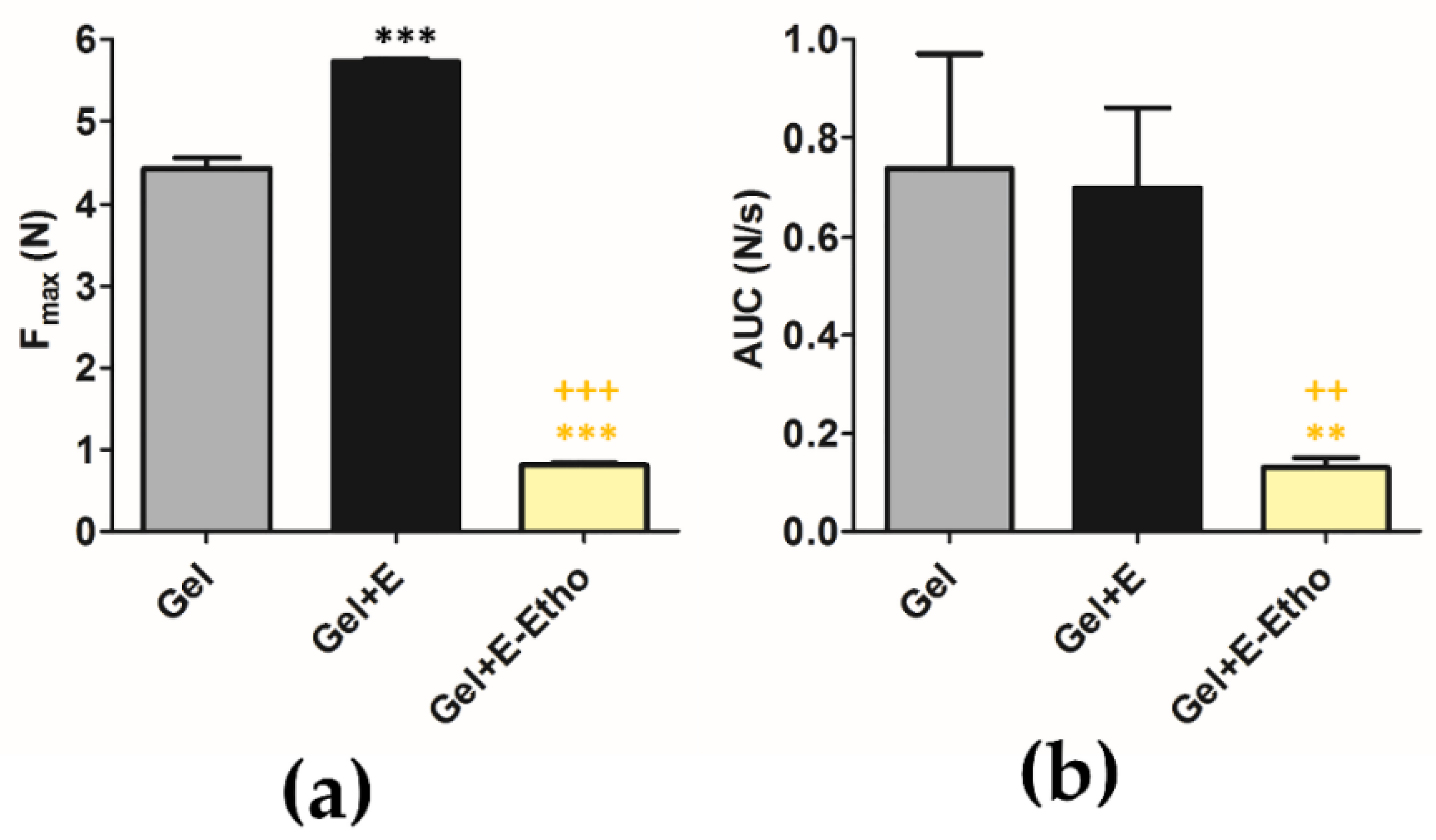
2.13. Texture of Semi-Solid Formulation with Free Extract and Ethosomes
2.14. Skin Compatibility Test of Semi-Solid Formulation with Free Extract and Ethosomes
3. Materials and Methods
3.1. Materials
3.1.1. Plant Material
3.1.2. Chemicals
3.1.3. Cell Lines
3.2. Methods
3.2.1. Extraction
3.2.2. Ethosomes Preparation Method
3.2.3. Lipid Quantification
3.2.4. Physical Characterization of Ethosomes: Size, Surface Charge and pH
3.2.5. Histochemical Characterization of the Lipidic Constitution of Ethosomes and Study of their Morphology
3.2.6. Entrapment Capacity of Extract in Ethosomes
3.2.7. Rutin and Ethosomes Complex Simulation
3.2.8. In Vitro Release Studies
3.2.9. In Vitro Collagenase Inhibition Activity
3.2.10. In Vitro Safety Assessment
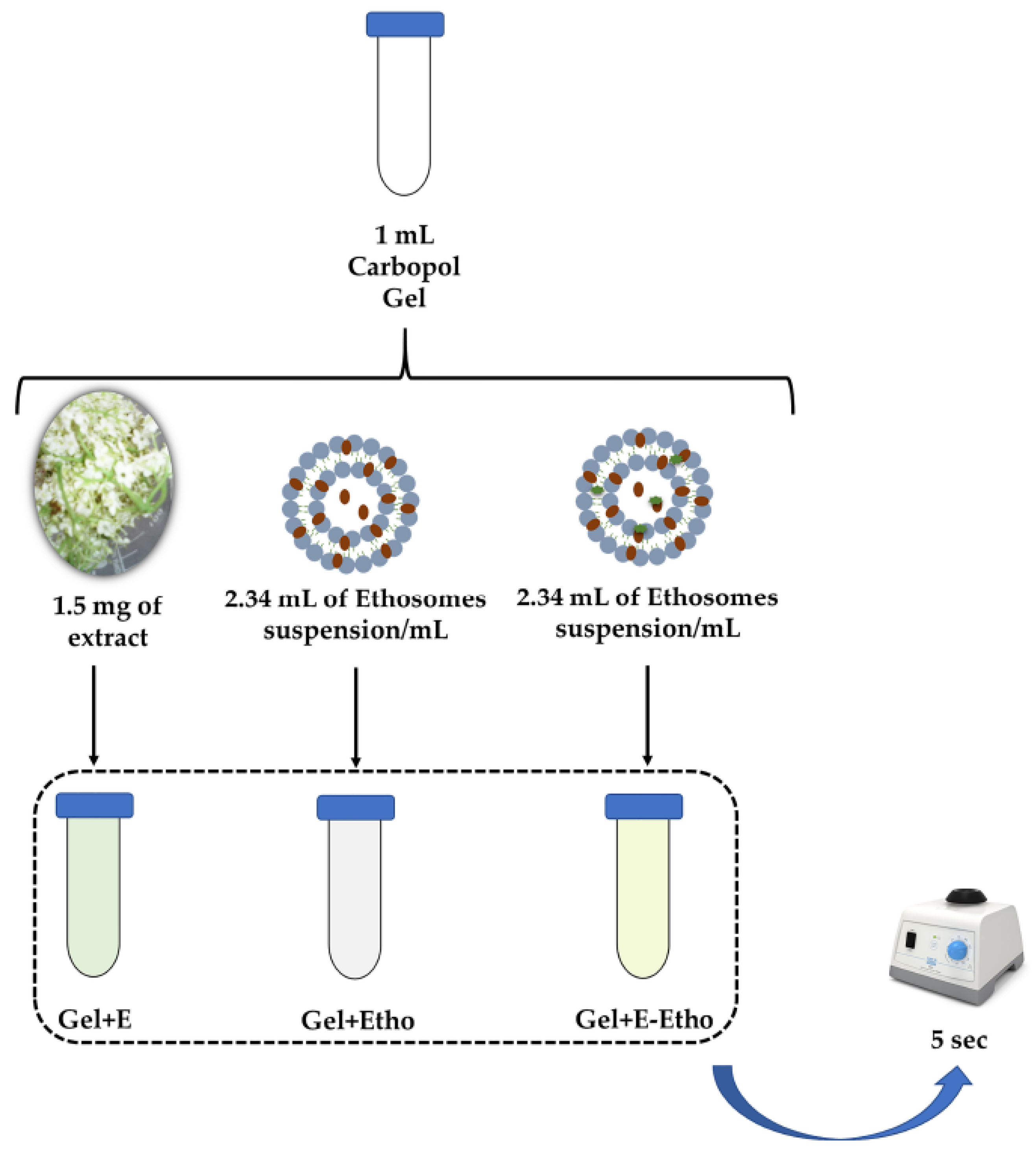
3.2.11. Inclusion of Extract-Loaded Ethosomes in a Semi-Solid Formulation
3.2.12. Characterization of the Semi-Solid Formulation for Skin Delivery of S. nigra Extract
- Organoleptic Characteristics
- pH Measurement
- Viscosity Measurement
- Preliminary Stability Assays of Semi-Solid Formulation with Free Extract and Ethosomes
- (1)
- Heating and Cooling
- (2)
- Centrifugation Stress
- Accelerated Stability Assays of Semi-Solid Formulation with Free Extract and Ethosomes
- (1)
- Tests Cycles of Heating and Cooling
- (2)
- Stability Test over 14 Days
- (3)
- Temperature Cycles
- Stability over Three Months
- Rheological Properties
- Texture Analysis
3.2.13. Human Skin’s Compatibility Testing
3.2.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mota, A.H.; Santos-Rebelo, A.; Almeida, A.J.; Reis, C.P. Therapeutic Implications of Nanopharmaceuticals in Skin Delivery. In Nanopharmaceuticals: Principles and Applications; Yata, V.K., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2021; Volume 1, pp. 205–272. ISBN 9783030449247. [Google Scholar]
- Mota, A.H.; Rijo, P.; Molpeceres, J.; Reis, C.P. Broad overview of engineering of functional nanosystems for skin delivery. Int. J. Pharm. 2017, 532, 710–728. [Google Scholar] [CrossRef]
- Mota, A.H.; Sousa, A.; Figueira, M.; Amaral, M.; Sousa, B.; Rocha, J.; Fattal, E.; Almeida, A.J.; Reis, C.P. Natural-based consumer health nanoproducts: Medicines, cosmetics, and food supplements. In Handbook of Functionalized Nanomaterials for Industrial Applications; Hussain, C.M., Ed.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 527–578. ISBN 9780128167885. [Google Scholar]
- Mota, A.H.; Duarte, N.; Serra, A.T.; Ferreira, A.; Bronze, M.R.; Custódio, L.; Gaspar, M.M.; Simões, S.; Rijo, P.; Ascensão, L.; et al. Further Evidence of Possible Therapeutic Uses of Sambucus nigra L. Extracts by the Assessment of the In Vitro and In Vivo Anti-Inflammatory Properties of Its PLGA and PCL-Based Nanoformulations. Pharmaceutics 2020, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.H.; Andrade, J.M.; Rodrigues, M.J.; Custódio, L.; Bronze, M.R.; Duarte, N.; Baby, A.; Rocha, J.; Gaspar, M.M.; Simões, S.; et al. Synchronous insight of in vitro and in vivo biological activities of Sambucus nigra L. extracts for industrial uses. Ind. Crops Prod. 2020, 154, 1–11. [Google Scholar] [CrossRef]
- Leverett, J.C.; Chandra, A.; Rana, J.; Fast, D.J.; Missler, S.R.; Flower, D.M. Extracts of durian fruit for use in skin care compositions. U.S. Patent Application No. 11/655,015, 24 May 2007. [Google Scholar]
- Liotta, A.; Unnur, P.; Universio, P. Role of collagenases in tumor cell invasion. Cancer Metastasis Rev. 1982, 288, 277–288. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Maroni, P.; Özer, N.; Zingg, J.M.; Azzi, A. Age-dependent increase of collagenase expression can be reduced by α-tocopherol via protein kinase C inhibition. Free Radic. Biol. Med. 1999, 27, 729–737. [Google Scholar] [CrossRef]
- Krutmann, J. Skin Aging. In Nutrition Health Skin; Springer: Berlin/Heidelberg, Germany, 2010; pp. 15–24. ISBN 9783642122637. [Google Scholar]
- Oancea, A.M.; Onofrei, C.; Turturică, M.; Bahrim, G.; Râpeanu, G.; Stănciuc, N. The kinetics of thermal degradation of polyphenolic compounds from elderberry (Sambucus nigra L.) extract. Food Sci. Technol. Int. 2018, 24, 361–369. [Google Scholar] [CrossRef]
- Carlsen, C.; Stapelfeldt, H. Light sensitivity of elderberry extract. Quantum yields for photodegradation in aqueous solution. Food Chem. 1997, 60, 383–387. [Google Scholar] [CrossRef]
- Rijo, P.; Matias, D.; Fernandes, A.; Simões, M.; Nicolai, M.; Reis, C. Antimicrobial plant extracts encapsulated into polymeric beads for potential application on the skin. Polymers 2014, 6, 479–490. [Google Scholar] [CrossRef]
- Sudhakar, C.K.; Upadhyay, N.; Jain, S.; Charyulu, R.N. Ethosomes as Non-invasive Loom for Transdermal Drug Delivery System. In Nanomedicine and Drug Delivery-Adavnces in Nanoscience and Nanotechnology; Sebastian, M., Ninan, N., Haghi, A.K., Eds.; CRC Press/Taylor & Francis: Oakville, ON, Canada, 2012; Volume 1, pp. 1–16. ISBN 9781466560079. [Google Scholar]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 1–19. [Google Scholar] [CrossRef]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-Based Cosmeceuticals. ISRN Dermatol. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- A Guidebook to Particle Size Analysis; Horiba Scientific: Piscataway, NJ, USA, 2016; Available online: https://docplayer.net/18477598-A-guidebook-to-particle-size-analysis.html (accessed on 13 January 2017).
- Cândido, T.M.; De Oliveira, C.A.; Ariede, M.B.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Safety and Antioxidant Efficacy Profiles of Rutin-Loaded Ethosomes for Topical Application. AAPS PharmSciTech 2018, 19, 1773–1780. [Google Scholar] [CrossRef]
- Eaton, P.; Quaresma, P.; Soares, C.; Neves, C.; de Almeida, M.P.; Pereira, E.; West, P. A direct comparison of experimental methods to measure dimensions of synthetic nanoparticles. Ultramicroscopy 2017, 182, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, J.; Thomas, A.; Ou-Yang, D.; Muzykantov, V.R. The shape of things to come: Importance of design in nanotechnology for drug delivery. Ther. Deliv. 2012, 3, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Scopel, M.; Mentz, L.A.; Henriques, A.T. Comparative analysis of sambucus nigra and sambucus australis flowers: Development and validation of an HPLC method for raw material quantification and preliminary stability study. Planta Medica 2010, 76, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Testoni, L.D.; De Souza, A.B.; De Krueger, C.M.A.; Quintão, N.L.M.; Couto, A.G.; Bresolin, T.M.B. Quantification of sambucus nigra (Adoxaceae) markers related to tincture stability. Nat. Prod. Commun. 2019, 14, 59–62. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Olech, M.; Oniszczuk, T.; Wojtunik-Kulesza, K.; Wójtowicz, A. Extraction methods, LC-ESI-MS/MS analysis of phenolic compounds and antiradical properties of functional food enriched with elderberry flowers or fruits. Arab. J. Chem. 2019, 12, 4719–4730. [Google Scholar] [CrossRef]
- Barupal, A.K.; Gupta, V.; Ramteke, S. Preparation and Characterization of Ethosomes for Topical delivery of Aceclofenac. Indian J. Pharm. Sci. 2010, 72, 582–586. [Google Scholar]
- Iizhar, S.A.; Syed, I.A.; Satar, R.; Ansari, S.A. In vitro assessment of pharmaceutical potential of ethosomes entrapped with terbinafine hydrochloride. J. Adv. Res. 2016, 7, 453–461. [Google Scholar] [CrossRef]
- Dhiman, A.; Singh, D.; Fatima, K.; Zia, G. Development of Rutin Ethosomes for Enhanced Skin Permeation. Int. J. Tradit. Med. Appl. 2019, 1, 4–10. [Google Scholar] [CrossRef]
- Jacob, M.; Agrawal, N.; Paul, D. Development, characterization & in vitro skin permeation of rutin ethosomes as a novel vesicular carrier. Int. J. Biomed. Res. 2017, 8, 559–566. [Google Scholar]
- Park, S.N.; Lee, H.J.; Gu, H.A. Enhanced skin delivery and characterization of rutin-loaded ethosomes. Korean J. Chem. Eng. 2014, 31, 485–489. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Aggarwal, G.; Manchanda, S.; Narula, A. Development of Topical Gel of Methotrexate Incorporated Ethosomes and Salicylic Acid for the Treatment of Psoriasis. Pharm. Nanotechnol. 2019, 7, 362–374. [Google Scholar] [CrossRef]
- Marto, J.; Baltazar, D.; Duarte, A.; Fernandes, A.; Gouveia, L.; Militão, M.; Salgado, A.; Simões, S.; Oliveira, E.; Ribeiro, H.M. Topical gels of etofenamate: In vitro and in vivo evaluation. Pharm. Dev. Technol. 2015, 20, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Andleeb, M.; Shoaib Khan, H.M.; Daniyal, M. Development, Characterization and Stability Evaluation of Topical Gel Loaded with Ethosomes Containing Achillea millefolium L. Extract. Front. Pharmacol. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Pannu, A.; Goyal, R.K.; Ojha, S.; Nandave, M. Naringenin: A Promising Flavonoid for Herbal Treatment of Rheumatoid Arthritis and Associated Inflammatory Disorders. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 343–354. ISBN 9780128138205. [Google Scholar]
- Zofia, N.Ł.; Martyna, Z.D.; Aleksandra, Z.; Tomasz, B. Comparison of the Antiaging and Protective Properties of Plants from the Apiaceae Family. Oxid. Med. Cell. Longev. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar]
- Van Hoogevest, P.; Wendel, A. Review Article The use of natural and synthetic phospholipids as pharmaceutical excipients Ã. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT Cells as a Reliable In Vitro Differentiation Model to Dissect the Inflammatory/Repair Response of Human Keratinocytes. Mediat. Inflamm. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Marques, P.; Marto, J.; Gonçalves, L.M.; Pacheco, R.; Fitas, M.; Pinto, P.; Serralheiro, M.L.M.; Ribeiro, H. Cynara scolymus L.: A promising Mediterranean extract for topical anti-aging prevention. Ind. Crops Prod. 2017, 109, 699–706. [Google Scholar] [CrossRef]
- Islam, M.T.; Ciotti, S.; Ackermann, C. Rheological Characterization of Topical Carbomer Gels Neutralized to Different pH. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Mutual influence of pH and temperature on the swelling of ionizable and thermosensitive hydrogels. Macromolecules 1992, 25, 5528–5530. [Google Scholar] [CrossRef]
- Barry, B.W.; Meyer, M.C. The rheological properties of carbopol gels I. Continuous shear and creep properties of carbopol gels. Int. J. Pharm. 1979, 2, 1–25. [Google Scholar] [CrossRef]
- Kunitz, M. Syneresis and swelling of gelatin. J. Gen. Physiol. 1928, 12, 289–312. [Google Scholar] [CrossRef]
- Dave, V.; Bhardwaj, N.; Gupta, N.; Tak, K. Herbal ethosomal gel containing luliconazole for productive relevance in the field of biomedicine. 3 Biotech 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Madan, P.; Lin, S. Formulation and rheological evaluation of ethosome-loaded carbopol hydrogel for transdermal application. Drug Dev. Ind. Pharm. 2016, 42, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Walicka, A.; Falicki, J. Rheology of Drugs for Topical and Transdermal Delivery. Int. J. Appl. Mech. Eng. 2019, 24, 179–198. [Google Scholar] [CrossRef]
- Ortan, A.; Parvu, C.D.; Ghica, M.V.; Popescu, L.M.; Ionita, L. Rheological study of a liposomal hydrogel based on carbopol. Rom. Biotechnol. Lett. 2011, 16, 47–54. [Google Scholar]
- Pawar, J.; Narkhede, R.; Amin, P.; Tawde, V. Design and Evaluation of Topical Diclofenac Sodium Gel Using Hot Melt Extrusion Technology as a Continuous Manufacturing Process with Kolliphor® P407. AAPS PharmSciTech 2017, 18, 2303–2315. [Google Scholar] [CrossRef]
- Bonacucina, G.; Cespi, M.; Misici-Falzi, M.; Palmieri, G.F. Rheological, adhesive and release characterisation of semisolid Carbopol/tetraglycol systems. Int. J. Pharm. 2006, 307, 129–140. [Google Scholar] [CrossRef]
- Rijo, P.; Falé, P.L.; Serralheiro, M.L.; Simões, M.F.; Gomes, A.; Reis, C. Optimization of medicinal plant extraction methods and their encapsulation through extrusion technology. Meas. J. Int. Meas. Confed. 2014, 58, 249–255. [Google Scholar] [CrossRef]
- Rouser, G.; Fleischer, S.; Yamamoto, A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 1970, 5, 494–496. [Google Scholar] [CrossRef]
- Gaspar, M.M.; Cruz, A.; Penha, A.F.; Reymão, J.; Sousa, A.C.; Eleutério, C.V.; Domingues, S.A.; Fraga, A.G.; Filho, A.L.; Cruz, M.E.M.; et al. Rifabutin encapsulated in liposomes exhibits increased therapeutic activity in a model of disseminated tuberculosis. Int. J. Antimicrob. Agents 2008, 31, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.O.; Rijo, P.; Molpeceres, J.; Figueiredo, I.V.; Ascensão, L.; Fernandes, A.S.; Roberto, A.; Reis, C.P. Polymeric nanoparticles modified with fatty acids encapsulating betamethasone for anti-inflammatory treatment. Int. J. Pharm. 2015, 493, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, S.; Karthika, T.N.; Mishra, A.K.; Chandra, T.S. Spectrofluorimetric method for the estimation of total lipids in Eremothecium ashbyii fungal filaments using Nile blue and avoiding interference of autofluorescent riboflavin. J. Microbiol. Methods 2003, 55, 99–103. [Google Scholar] [CrossRef]
- Mota, A.H.; Direito, R.; Carrasco, M.P.; Rijo, P.; Ascensão, L.; Silveira Viana, A.; Rocha, J.; Eduardo-Figueira, M.; João Rodrigues, M.; Custódio, L.; et al. Combination of hyaluronic acid and PLGA particles as hybrid systems for viscosupplementation in osteoarthritis. Int. J. Pharm. 2019, 559, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Choonara, Y.E.; Pillay, V. In silico analytico-mathematical interpretation of biopolymeric assemblies: Quantification of energy surfaces and molecular attributes via atomistic simulations. Bioeng. Transl. Med. 2018, 3, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rebelo, A.; Kumar, P.; Pillay, V.; Choonara, Y.E.; Eleutério, C.; Figueira, M.; Viana, A.S.; Ascensão, L.; Molpeceres, J.; Rijo, P.; et al. Development and mechanistic insight into the enhanced cytotoxic potential of parvifloron D albumin nanoparticles in EGFR-overexpressing pancreatic cancer cells. Cancers 2019, 11, 1733. [Google Scholar] [CrossRef]
- Mota, A.H.; Andrade, J.M.; Ntungwe, E.N.; Pereira, P.; Cebola, M.J.; Bernardo-Gil, M.G.; Molpeceres, J.; Rijo, P.; Viana, A.S.; Ascensão, L.; et al. Green extraction of Sambucus nigra L. for potential application in skin nanocarriers. Green Mater. 2020, 8, 181–193. [Google Scholar] [CrossRef]
- Jackson, J.K.; Zhao, J.; Wong, W.; Burt, H.M. The inhibition of collagenase induced degradation of collagen by the galloyl-containing polyphenols tannic acid, epigallocatechin gallate and epicatechin gallate. J. Mater. Sci. Mater. Med. 2010, 21, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rebelo, A.; Garcia, C.; Eleutério, C.; Bastos, A.; Coelho, S.C.; Coelho, M.A.N.; Molpeceres, J.; Viana, A.S.; Ascensão, L.; Pinto, J.F.; et al. Development of parvifloron D-loaded smart nanoparticles to target pancreatic cancer. Pharmaceutics 2018, 10, 216. [Google Scholar] [CrossRef]
- Delfim Fernando Gonçalves dos Santos Absorção percutânea-geles de carbopol contendo fentiazac, Faculdade de Farmacia do Porto. 1995. Available online: http://hdl.handle.net/10216/10158 (accessed on 31 October 2019).
- Reis, C.; Antunes, A.F.; Rijo, P.; Baptista, M.; Mota, J.P.; Monteiro Rodrigues, L. A novel topical association with zinc oxide, chamomile and aloe vera extracts-stability and safety studies. Biomed. Biopharm. Res. 2015, 12, 251–264. [Google Scholar] [CrossRef]
- EMA/CPMC/ICH/2736/99 ICH Topic Q1A (R2). 2003. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-r2-stability-testing-new-drug-substances-products-step-5_en.pdf (accessed on 6 August 2020).
- Braden, M. Tissue Conditioners: II. Rheologic Properties. J. Dent. Res. 1970, 49, 496–501. [Google Scholar] [CrossRef]
- Reis, C.P.; Gomes, A.; Rijo, P.; Candeias, S.; Pinto, P. Evaluation of a New Topical Treatment for Acne with Azelaic Acid-Loaded Nanoparticles. Microsc. Microanal. 2013, 19, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Marzulli, F.N.; Maibach, H.I. Contact allergy: Predictive testing in man. Contact Dermatitis 1976, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- PhD Trials Patch Test study protocol nº PT.02.01/final03, RNEC nº 127504.
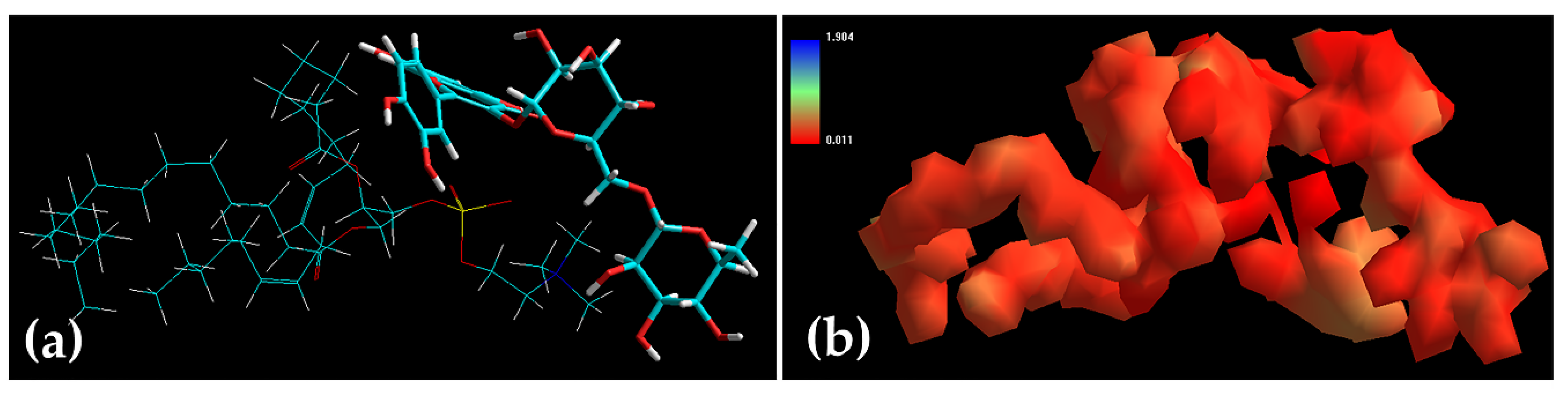
| Energy | Rutin | SPC | SPC-Rutin | ΔE 1 |
|---|---|---|---|---|
| Total 2 | 23.09 | 1.10 | 18.61 | −5.58 8 |
| Bond 3 | 1.16 | 1.39 | 2.49 | −0.05 8 |
| Angle 4 | 7.57 | 35.69 | 42.85 | −0.41 8 |
| Dihed 5 | 14.32 | 10.28 | 27.60 | 3.00 9 |
| vdW 6 | 0.04 | −4.25 | −14.35 | −10.15 8 |
| Elec 7 | 0.00 | −42.01 | −39.97 | 2.04 9 |
| Time (Days) | Gel | Gel + E | Gel + Etho | Gel + E-Etho | ||||||||
| OC | pH | Viscosity | OC | pH | Viscosity | OC | pH | Viscosity | OC | pH | Viscosity | |
| Preliminary Stability Testing (Heating and Cooling) | ||||||||||||
| 0 | N | 7.68 ± 0.01 | 134,050 ± 14,453 | N | 6.39 ± 0.03 | 159,800 ± 200 | N | 7.39 ± 0.02 | 183,933 ± 14,609 | N | 8.77 ± 0.01 | 155,533 ± 1922 |
| 2 | N | 7.65 ± 0.01 | 128,333 ± 1246 | N | 6.35 ± 0.21 | 123,933 ± 306 | N | 7.37 ± 0.02 | 129,667 ± 874 | N | 8.55 ± 0.04 | 28,200 ± 200 |
| 4 | N | 7.59 ± 0.02 | 124,867 ± 566 | N | 6.33 ± 0.12 | 107,333 ± 416 | N | 7.21 ± 0.03 | 100,067 ± 2055 | N | 8.50 ± 0.01 | 53,400 ± 872 |
| 7 | N | 7.45 ± 0.01 | 111,717 ± 58 | N | 6.13 ± 0.03 | 108,333 ± 643 | N | 7.27 ± 0.01 | 122,700 ± 200 | N | 8.45 ± 0.02 | 29,800 ± 200 |
| Preliminary Stability Testing (Centrifugation Stress) | ||||||||||||
| Before | N | 6.89 ± 0.01 | 192622 ± 518 | N | 6.60 ± 0.03 | 162,533 ± 306 | N | 5.38 ± 0.02 | 28,000 ± 200 | N | 7.11 ± 0.04 | 53,600 ± 529 |
| After | N | 6.96 ± 0.02 | 190933 ± 115 | N | 6.63 ± 0.01 | 150,733 ± 306 | N | 5.39 ± 0.03 | 27,867 ± 115 | N | 7.00 ± 0.01 | 49,000 ± 200 |
| Accelerated Stability Testing (Heating and Cooling) | ||||||||||||
| 0 | N | 7.58 ± 0.18 | 158,267 ± 2203 | N | 6.19 ± 0.02 | 156,933 ± 3252 | N | 7.50 ± 0.02 | 20,800 ± 529 | |||
| 2 | N | 6.72 ± 0.02 | 175,467 ± 25015 | N | 6.16 ± 0.08 | 133,800 ± 200 | N | 7.63 ± 0.04 | 38,733 ± 306 | |||
| 4 | N | 6.78 ± 0.02 | 193,800 ± 200 | N | 6.10 ± 0.02 | 98,600 ± 200 | N | 7.59 ± 0.02 | 31,200 ± 200 | |||
| 6 | N | 6.72 ± 0.02 | 133,400 ± 200 | N | 6.11 ± 0.01 | 128,933 ± 1890 | N | 7.61 ± 0.02 | 30,200 ± 200 | |||
| 8 | N | 6.73 ± 0.01 | 194,867 ± 5601 | N | 6.07 ± 0.04 | 62,600 ± 721 | N | 7.64 ± 0.01 | 40,867 ± 306 | |||
| 10 | N | 6.70 ± 0.01 | 114,600 ± 529 | N | 6.09 ± 0.17 | 57,800 ± 200 | N | 7.67 ± 0.04 | 25,000 ± 200 | |||
| 12 | N | 6.66 ± 0.02 | 96,467 ± 416 | N | 6.43 ± 0.16 | 73,467 ± 306 | N | 7.62 ± 0.02 | 28,533 ± 115 | |||
| Time (days) | Gel | Gel + E | Gel + Etho | Gel + E-Etho | ||||||||
| OC | pH | Viscosity | OC | pH | Viscosity | OC | pH | Viscosity | OC | pH | Viscosity | |
| Accelerated Stability Testing (14 Days)—RC | ||||||||||||
| 0 | N | 8.88 ± 0.14 | 168,333 ± 306 | N | 6.34 ± 0.01 | 104,000 ± 200 | N | 6.77 ± 0.03 | 33,467 ± 306 | |||
| 3 | N | 9.17 ± 0.08 | 66,533 ± 306 | N | 6.33 ± 0.02 | 151,200 ± 200 | N | 6.72 ± 0.01 | 42,200 ± 200 | |||
| 7 | N | 9.01 ± 0.06 | 68,000 ± 200 | N | 6.18 ± 0.01 | 118,333 ± 306 | N | 6.73 ± 0.03 | 41,333 ± 987 | |||
| 14 | N | 8.89 ± 0.02 | 50,200 ± 200 | N | 6.26 ± 0.17 | 99,933 ± 306 | N | 6.70 ± 0.02 | 64,733 ± 503 | |||
| Accelerated Stability Testing (14 Days)—RT | ||||||||||||
| 0 | N | 6.31 ± 0.06 | 194,533 ± 503 | N | 6.78 ± 0.02 | 167,200 ± 400 | N | 8.50 ± 0.01 | 21,800 ± 200 | |||
| 3 | N | 6.61 ± 0.02 | 185,467 ± 306 | N | 6.68 ± 0.03 | 138,200 ± 200 | N | 8.44 ± 0.02 | 29,400 ± 200 | |||
| 7 | N | 6.50 ± 0.03 | 121,200 ± 200 | N | 6.60 ± 0.04 | 109,600 ± 800 | N | 8.34 ± 0.01 | 129,400 ± 3470 | |||
| 14 | N | 6.54 ± 0.03 | 138,867 ± 416 | N | 6.55 ± 0.02 | 101,133 ± 306 | N | 8.03 ± 0.05 | 81,600 ± 4386 | |||
| Accelerated Stability Testing (14 Days)—AC | ||||||||||||
| 0 | N | 5.89 ± 0.04 | 159,267 ± 2444 | N | 7.42 ± 0.02 | 145,200 ± 200 | N | 8.02 ± 0.02 | 127,333 ± 503 | |||
| 3 | N | 6.15 ± 0.03 | 181,200 ± 200 | N | 7.35 ± 0.02 | 118,200 ± 200 | N | 7.79 ± 0.01 | 27,600 ± 200 | |||
| 7 | N | 6.21 ± 0.01 | 136,933 ± 306 | N | 7.08 ± 0.01 | 103,533 ± 611 | N | 7.78 ± 0.01 | 33,200 ± 200 | |||
| 14 | N | 6.27 ± 0.02 | 121,800 ± 200 | N | 7.01 ± 0.02 | 105,200 ± 200 | N | 7.67 ± 0.01 | 35,933 ± 306 | |||
| Accelerated Stability Testing (14 Days)—AC * | ||||||||||||
| 0 | N | 6.57 ± 0.01 | 158,667 ± 1617 | N | 6.74 ± 0.01 | 163,000 ± 1929 | N | 7.40 ± 0.01 | 71,533 ± 2759 | |||
| 3 | N | 6.60 ± 0.02 | 190,800 ± 721 | N | 6.77 ± 0.01 | 109,600 ± 917 | N | 7.11 ± 0.01 | 92,867 ± 643 | |||
| 7 | N | 6.60 ± 0.03 | 119,867 ± 306 | N | 6.77 ± 0.03 | 87,800 ± 200 | N | 7.09 ± 0.02 | 32,667 ± 306 | |||
| 14 | N | 6.58 ± 0.02 | 113,867 ± 115 | N | 6.80 ± 0.01 | 102,800 ± 600 | N | 7.09 ± 0.01 | 33,400 ± 200 | |||
| Accelerated Stability Testing (14 Days)—Temperature Cycles | ||||||||||||
| Before | N | 6.60 ± 0.32 | 161,000 ± 1217 | N | 6.56 ± 0.01 | 180,733 ± 22,689 | N | 6.55 ± 0.09 | 108,333 ± 416 | |||
| After | N | 7.21 ± 0.04 | 171,200 ± 20101 | N | 6.61 ± 0.03 | 156,533 ± 306 | N | 6.48 ± 0.01 | 38,467 ± 306 | |||
| Time (Days) | Gel | Gel + E | Gel + Etho | Gel + E-Etho | ||||||||
| OC | pH | Viscosity | OC | pH | Viscosity | OC | pH | Viscosity | OC | pH | Viscosity | |
| Accelerated Stability Testing (3 Months)—RC | ||||||||||||
| 0 | N | 8.88 ± 0.14 | 168,333 ± 306 | N | 6.34 ± 0.01 | 104,000 ± 200 | N | 6.77 ± 0.03 | 33,467 ± 306 | |||
| 30 | N | 8.79 ± 0.01 | 44,733 ± 416 | N | 6.09 ± 0.01 | 70,067 ± 611 | N | 6.62 ± 0.04 | 40,600 ± 200 | |||
| 60 | N | 8.77 ± 0.03 | 45,667 ± 757 | N | 6.20 ± 0.01 | 53,000 ± 1058 | N | 6.73 ± 0.04 | 36,533 ± 1332 | |||
| 90 | N | 8.80 ± 0.01 | 186,467 ± 8612 | N | 6.30 ± 0.01 | 126,000 ± 9035 | N | 6.90 ± 0.01 | 113,133 ± 1553 | |||
| Accelerated Stability Testing (3 Months)—RT | ||||||||||||
| 0 | N | 6.31 ± 0.06 | 194,533 ± 503 | N | 6.78 ± 0.02 | 167,200 ± 400 | N | 8.50 ± 0.01 | 21,800 ± 200 | |||
| 30 | N | 6.56 ± 0.03 | 42,933 ± 416 | N | 6.51 ± 0.01 | 50,533 ± 115 | N | 7.94 ± 0.01 | 97,800 ± 529 | |||
| 60 | N | 6.50 ±0.02 | 105,000 ± 6630 | N | 6.58 ± 0.04 | 109,133 ± 3775 | N | 7.44 ± 0.02 | 95,267 ± 8425 | |||
| 90 | N | 6.51 ± 0.01 | 132,600 ± 1929 | N | 6.67 ± 0.01 | 186,933 ± 2023 | N | 7.17 ± 0.03 | 56,400 ± 200 | |||
| Accelerated Stability Testing (3 Months)—AC | ||||||||||||
| 0 | N | 5.89 ± 0.04 | 159,267 ± 2444 | N | 7.42 ± 0.02 | 145,200 ± 200 | N | 8.02 ± 0.02 | 127,333 ± 503 | |||
| 30 | N | 6.33 ± 0.03 | 60,867 ± 416 | N | 6.87 ± 0.06 | 54,400 ± 200 | N | 7.52 ± 0.01 | 30,467 ± 833 | |||
| 60 | N | 6.36 ± 0.03 | 55,800 ± 2163 | N | 6.80 ± 0.01 | 72,467 ± 5623 | N | 7.37 ± 0.01 | 27,867 ± 702 | |||
| 90 | N | 6.47 ± 0.02 | 145,733 ± 1026 | N | 6.96 ± 0.02 | 168,733 ± 16,931 | N | 6.95 ± 0.04 | 100,600 ± 1562 | |||
| Samples | Control Time after Patch Removal | Reactive Subjects (n) | Types of Reaction | Mean Daily Irritation Score (Mdis) |
|---|---|---|---|---|
| Gel + E | 15 min | 0 | None | 0 ± 0 |
| 24 h | 0 | None | 0 ± 0 | |
| 48 h | 0 | None | 0 ± 0 | |
| Gel + E-Etho | 15 min | 0 | None | 0 ± 0 |
| 24 h | 0 | None | 0 ± 0 | |
| 48 h | 0 | None | 0 ± 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, A.H.; Prazeres, I.; Mestre, H.; Bento-Silva, A.; Rodrigues, M.J.; Duarte, N.; Serra, A.T.; Bronze, M.R.; Rijo, P.; Gaspar, M.M.; et al. A Newfangled Collagenase Inhibitor Topical Formulation Based on Ethosomes with Sambucus nigra L. Extract. Pharmaceuticals 2021, 14, 467. https://doi.org/10.3390/ph14050467
Mota AH, Prazeres I, Mestre H, Bento-Silva A, Rodrigues MJ, Duarte N, Serra AT, Bronze MR, Rijo P, Gaspar MM, et al. A Newfangled Collagenase Inhibitor Topical Formulation Based on Ethosomes with Sambucus nigra L. Extract. Pharmaceuticals. 2021; 14(5):467. https://doi.org/10.3390/ph14050467
Chicago/Turabian StyleMota, Ana Henriques, Inês Prazeres, Henrique Mestre, Andreia Bento-Silva, Maria João Rodrigues, Noélia Duarte, Ana Teresa Serra, Maria Rosário Bronze, Patrícia Rijo, Maria Manuela Gaspar, and et al. 2021. "A Newfangled Collagenase Inhibitor Topical Formulation Based on Ethosomes with Sambucus nigra L. Extract" Pharmaceuticals 14, no. 5: 467. https://doi.org/10.3390/ph14050467
APA StyleMota, A. H., Prazeres, I., Mestre, H., Bento-Silva, A., Rodrigues, M. J., Duarte, N., Serra, A. T., Bronze, M. R., Rijo, P., Gaspar, M. M., Viana, A. S., Ascensão, L., Pinto, P., Kumar, P., Almeida, A. J., & Reis, C. P. (2021). A Newfangled Collagenase Inhibitor Topical Formulation Based on Ethosomes with Sambucus nigra L. Extract. Pharmaceuticals, 14(5), 467. https://doi.org/10.3390/ph14050467











