Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes
Abstract
1. Introduction
Search Strategy
2. Anti-Inflammatory Action of Ginger Extracts
3. Ginger Constituents
3.1. Chemical Composition of Z. officinale Roscoe rhizome
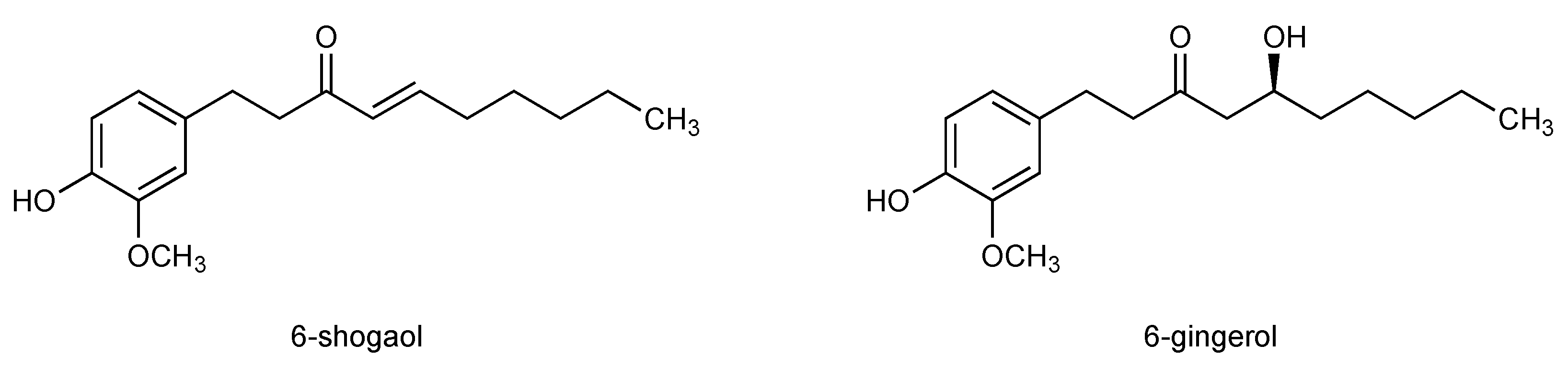
3.2. 6-Shogaol, The Pungent Principle in Dried Ginger Rhizome
4. Identification of Ginger Constituents Associated with Anti-Inflammatory Actions
5. Effects of 6-Shogaol on Inflammation-Related Processes
5.1. 6-Shogaol as Anti-Inflammatory Agent in Relevant In Vivo Models
5.2. 6-Shogaol Inhibits Inflammatory Processes In Vitro
5.3. Cell Protective Effects of 6-Shogaol against Inflammation-Related Oxidative Stress
5.4. The Impact of 6-Shogaol on the Inflammasome
5.5. Inhibition of Neuroinflammatory Actions by 6-Shogaol
5.6. The Anti-Inflammatory Potential of 6-Shogaol Derivatives
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bode, A.M.; Dong, Z. The amazing and mighty ginger. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Ernst, E.; Pittler, M.H. Efficacy of ginger for nausea and vomiting: A systematic review of randomized clinical trials. Br. J. Anaesth. 2000, 84, 367–371. [Google Scholar] [CrossRef]
- Hasani, H.; Arab, A.; Hadi, A.; Pourmasoumi, M.; Ghavami, A.; Miraghajani, M. Does ginger supplementation lower blood pressure? A systematic review and meta-analysis of clinical trials. Phytother. Res. PTR 2019, 33, 1639–1647. [Google Scholar] [CrossRef]
- Al-Nahain, A.; Jahan, R.; Rahmatullah, M. Zingiber officinale: A potential plant against rheumatoid arthritis. Arthritis 2014, 2014, 159089. [Google Scholar] [CrossRef]
- Assessment Report on Zingiber Officinale Roscoe. Available online: https://www.ema.europa.eu/en/documents/herbal-report/draft-assessment-report-zingiberis-rhizoma_en.pdf (accessed on 26 May 2021).
- Afzal, M.; Al-Hadidi, D.; Menon, M.; Pesek, J.; Dhami, M.S. Ginger: An ethnomedical, chemical and pharmacological review. Drug Metab. Drug Interact. 2001, 18, 159–190. [Google Scholar] [CrossRef]
- Khodaie, L.; Sadeghpoor, O. Ginger from ancient times to the new outlook. Jundishapur J. Nat. Pharm. Prod. 2015, 10, e18402. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Talaei, B.; Jalali, B.A.; Najarzadeh, A.; Mozayan, M.R. The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Med. 2014, 22, 9–16. [Google Scholar] [CrossRef]
- Shukla, Y.; Singh, M. Cancer preventive properties of ginger: A brief review. Food Chem. Toxicol. 2007, 45, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Yocum, G.T.; Hwang, J.J.; Mikami, M.; Danielsson, J.; Kuforiji, A.S.; Emala, C.W. Ginger and its bioactive component 6-shogaol mitigate lung inflammation in a murine asthma model. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L296–L303. [Google Scholar] [CrossRef]
- Aggarwal, B.; Prasad, S.; Sung, B.; Krishnan, S.; Guha, S. Prevention and treatment of colorectal cancer by natural agents from mother nature. Curr. Colorectal Cancer Rep. 2013, 9, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Ahui, M.L.; Champy, P.; Ramadan, A.; Pham Van, L.; Araujo, L.; Brou Andre, K.; Diem, S.; Damotte, D.; Kati-Coulibaly, S.; Offoumou, M.A.; et al. Ginger prevents th2-mediated immune responses in a mouse model of airway inflammation. Int. Immunopharmacol. 2008, 8, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, N.; Jain, R.; Jain, S.C.; Capasso, F. Ethnopharmacologic investigation of ginger (zingiber officinale). J. Ethnopharmacol. 1989, 27, 129–140. [Google Scholar] [CrossRef]
- Habib, S.H.; Makpol, S.; Abdul Hamid, N.A.; Das, S.; Ngah, W.Z.; Yusof, Y.A. Ginger extract (zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics 2008, 63, 807–813. [Google Scholar] [CrossRef]
- Grzanna, R.; Lindmark, L.; Frondoza, C.G. Ginger--an herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 2005, 8, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.C. Isolation and effects of some ginger components of platelet aggregation and eicosanoid biosynthesis. Prostaglandins Leukot. Med. 1986, 25, 187–198. [Google Scholar] [CrossRef]
- Srivastava, K.C. Effects of aqueous extracts of onion, garlic and ginger on platelet aggregation and metabolism of arachidonic acid in the blood vascular system: In vitro study. Prostaglandins Leukot. Med. 1984, 13, 227–235. [Google Scholar] [CrossRef]
- Srivastava, K.C.; Mustafa, T. Ginger (zingiber officinale) and rheumatic disorders. Med. Hypotheses 1989, 29, 25–28. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Thomson, M.; Al-Qattan, K.K.; Al-Sawan, S.M.; Alnaqeeb, M.A.; Khan, I.; Ali, M. The use of ginger (zingiber officinale rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 475–478. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Ezzat, M.I.; Okba, M.M.; Menze, E.T.; Abdel-Naim, A.B. The hidden mechanism beyond ginger (zingiber officinale rosc.) potent in vivo and in vitro anti-inflammatory activity. J. Ethnopharmacol. 2018, 214, 113–123. [Google Scholar] [CrossRef]
- Hwang, J.H.; Jung, H.W.; Oh, S.Y.; Kang, J.S.; Kim, J.P.; Park, J.K. Effects of zingiber officinale extract.on collagen-induced arthritis in mice and il-1β-induced inflammation in human synovial fibroblasts. Eur. J. Inflamm. 2017, 15, 168–178. [Google Scholar] [CrossRef]
- Fouda, A.M.; Berika, M.Y. Evaluation of the effect of hydroalcoholic extract of zingiber officinale rhizomes in rat collagen-induced arthritis. Basic Clin. Pharmacol. Toxicol. 2009, 104, 262–271. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Grabnar, I.; Verardo, R.; Klaric, E.; Marchionni, L.; Luidy-Imada, E.; Sut, S.; Agostinis, C.; Bulla, R.; Perissutti, B.; et al. Combined extracts of echinacea angustifolia dc. And zingiber officinale roscoe in softgel capsules: Pharmacokinetics and immunomodulatory effects assessed by gene expression profiling. Phytomedicine 2019, 65, 153090. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Naderi, Z.; Dehghan, A.; Nadjarzadeh, A.; Fallah Huseini, H. Effect of ginger supplementation on proinflammatory cytokines in older patients with osteoarthritis: Outcomes of a randomized controlled clinical trial. J. Nutr. Gerontol. Geriatr. 2016, 35, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Nigam, I.C.; Levi, L.; Handa, K. Essential oils and their constituents: XXII. Detection of new trace components in oil of ginger. Can. J. Chem. 1964, 42, 2610–2615. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol. Res. Pract. 2015, 2015, 142979. [Google Scholar] [CrossRef] [PubMed]
- Connell, D.; Sutherland, M. A re-examination of gingerol, shogaol, and zingerone, the pungent principles of ginger (zingiber officinale roscoe). Aust. J. Chem. 1969, 22, 1033–1043. [Google Scholar] [CrossRef]
- Connell, D. Natural pungent compounds. III. The paradols and associated compounds. Aust. J. Chem. 1970, 23, 369–376. [Google Scholar] [CrossRef]
- Nomura, H. The pungent principles of ginger. II. A new pungent principle, shogaol, occurring in ginger. Sci. Rep. Tohoku Imp. Univ. 1918, 7, 67–77. [Google Scholar]
- Nomura, H.; Tsurumi, S. The pungent principles of ginger. IV. Synthesis of shogaol. Sci. Rep. Tohoku Imp. Univ. 1927, 16, 565. [Google Scholar] [CrossRef]
- Shih, H.C.; Chern, C.Y.; Kuo, P.C.; Wu, Y.C.; Chan, Y.Y.; Liao, Y.R.; Teng, C.M.; Wu, T.S. Synthesis of analogues of gingerol and shogaol, the active pungent principles from the rhizomes of zingiber officinale and evaluation of their anti-platelet aggregation effects. Int. J. Mol. Sci. 2014, 15, 3926–3951. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, P.; Zhao, Y.; Yang, C.; Clark, A.; Leung, T.; Chen, X.; Sang, S. Synthesis, evaluation, and metabolism of novel [6]-shogaol derivatives as potent nrf2 activators. Free Radic. Biol. Med. 2016, 95, 243–254. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Banno, K. A new synthesis of the pungent principles of ginger-zingerone, gingerol and shogaol. Bull. Chem. Soc. Jpn. 1976, 49, 1453–1454. [Google Scholar]
- Rebellato, P.; Islam, M.S. [6]-shogaol induces ca(2)(+) signals by activating the trpv1 channels in the rat insulinoma ins-1e cells. JOP J. Pancreas 2014, 15, 33–37. [Google Scholar]
- Bhattarai, S.; Tran, V.H.; Duke, C.C. Stability of [6]-gingerol and [6]-shogaol in simulated gastric and intestinal fluids. J. Pharm. Biomed. Anal. 2007, 45, 648–653. [Google Scholar] [CrossRef]
- Bhattarai, S.; Tran, V.H.; Duke, C.C. The stability of gingerol and shogaol in aqueous solutions. J. Pharm. Sci. 2001, 90, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Wang, X.; Ji, R.; Liu, L.; Qiao, Y.; Lou, Z.; Ma, C.; Li, S.; Wang, H.; Ho, C.T. Occurrence, biological activity and metabolism of 6-shogaol. Food Funct. 2018, 9, 1310–1327. [Google Scholar] [CrossRef]
- Kiuchi, F.; Shibuya, M.; Sankawa, U. Inhibitors of prostaglandin biosynthesis from ginger. Chem. Pharm. Bull. 1982, 30, 754–757. [Google Scholar] [CrossRef]
- Tjendraputra, E.; Tran, V.H.; Liu-Brennan, D.; Roufogalis, B.D.; Duke, C.C. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg. Chem. 2001, 29, 156–163. [Google Scholar] [CrossRef]
- Flynn, D.L.; Rafferty, M.F.; Boctor, A.M. Inhibition of human neutrophil 5-lipoxygenase activity by gingerdione, shogaol, capsaicin and related pungent compounds. Prostaglandins Leukot. Med. 1986, 24, 195–198. [Google Scholar] [CrossRef]
- Wang, Z.; Hasegawa, J.; Wang, X.; Matsuda, A.; Tokuda, T.; Miura, N.; Watanabe, T. Protective effects of ginger against aspirin-induced gastric ulcers in rats. Yonago Acta Med. 2011, 54, 11–19. [Google Scholar] [PubMed]
- Guahk, G.H.; Ha, S.K.; Jung, H.S.; Kang, C.; Kim, C.H.; Kim, Y.B.; Kim, S.Y. Zingiber officinale protects hacat cells and c57bl/6 mice from ultraviolet b-induced inflammation. J. Med. Food 2010, 13, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.S.; Simon, O.R. Six-shogaol inhibits production of tumour necrosis factor alpha, interleukin-1 beta and nitric oxide from lipopolysaccharide-stimulated raw 264.7 macrophages. West Indian Med. J. 2009, 58, 295–300. [Google Scholar] [PubMed]
- Levy, A.S.; Simon, O.; Shelly, J.; Gardener, M. 6-shogaol reduced chronic inflammatory response in the knees of rats treated with complete freund’s adjuvant. BMC Pharm. 2006, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.; Han, N.Y.; Lee, M.J.; Cho, H.J.; Jung, H.S. [6]-shogaol inhibits the production of proinflammatory cytokines via regulation of nf-kappab and phosphorylation of jnk in hmc-1 cells. Immunopharmacol. Immunotoxicol. 2013, 35, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Hsieh, M.C.; Hsu, P.C.; Ho, S.Y.; Lai, C.S.; Wu, H.; Sang, S.; Ho, C.T. 6-shogaol suppressed lipopolysaccharide-induced up-expression of inos and cox-2 in murine macrophages. Mol. Nutr. Food Res. 2008, 52, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hsieh, M.C.; Lo, C.Y.; Liu, C.B.; Sang, S.; Ho, C.T.; Pan, M.H. 6-shogaol is more effective than 6-gingerol and curcumin in inhibiting 12-o-tetradecanoylphorbol 13-acetate-induced tumor promotion in mice. Mol. Nutr. Food Res. 2010, 54, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, G.; Suresh, K. [6]-shogaol attenuates inflammation, cell proliferation via modulate nf-kappab and ap-1 oncogenic signaling in 7,12-dimethylbenz[a]anthracene induced oral carcinogenesis. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 98, 484–490. [Google Scholar] [CrossRef]
- Han, S.J.; Kim, M.; D’Agati, V.D.; Lee, H.T. 6-shogaol protects against ischemic acute kidney injury by modulating nf-kappab and heme oxygenase-1 pathways. Am. J. Physiol. Ren. Physiol. 2019, 317, F743–F756. [Google Scholar] [CrossRef]
- Na, J.Y.; Song, K.; Lee, J.W.; Kim, S.; Kwon, J. Pretreatment of 6-shogaol attenuates oxidative stress and inflammation in middle cerebral artery occlusion-induced mice. Eur. J. Pharm. 2016, 788, 241–247. [Google Scholar] [CrossRef]
- Ahn, S.I.; Lee, J.K.; Youn, H.S. Inhibition of homodimerization of toll-like receptor 4 by 6-shogaol. Mol. Cells 2009, 27, 211–215. [Google Scholar] [CrossRef]
- Luettig, J.; Rosenthal, R.; Lee, I.M.; Krug, S.M.; Schulzke, J.D. The ginger component 6-shogaol prevents tnf-alpha-induced barrier loss via inhibition of pi3k/akt and nf-kappab signaling. Mol. Nutr. Food Res. 2016, 60, 2576–2586. [Google Scholar] [CrossRef]
- Villalvilla, A.; da Silva, J.A.; Largo, R.; Gualillo, O.; Vieira, P.C.; Herrero-Beaumont, G.; Gomez, R. 6-shogaol inhibits chondrocytes’ innate immune responses and cathepsin-k activity. Mol. Nutr. Food Res. 2014, 58, 256–266. [Google Scholar] [CrossRef]
- Hao, L.; Zhu, G.; Lu, Y.; Wang, M.; Jules, J.; Zhou, X.; Chen, W. Deficiency of cathepsin k prevents inflammation and bone erosion in rheumatoid arthritis and periodontitis and reveals its shared osteoimmune role. FEBS Lett. 2015, 589, 1331–1339. [Google Scholar] [CrossRef]
- Sang, S.; Hong, J.; Wu, H.; Liu, J.; Yang, C.S.; Pan, M.H.; Badmaev, V.; Ho, C.T. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from zingiber officinale relative to gingerols. J. Agric. Food Chem. 2009, 57, 10645–10650. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Jang, H.D. 6-shogaol attenuates h2o2-induced oxidative stress via upregulation of nrf2-mediated gamma-glutamylcysteine synthetase and heme oxygenase expression in hepg2 cells. Food Sci. Biotechnol. 2016, 25, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Chen, F.; Tang, Y.; Sun, Y.; Veeraraghavan, V.P.; Mohan, S.K.; Cui, C. 6-shogaol, a active constiuents of ginger prevents uvb radiation mediated inflammation and oxidative stress through modulating nrf2 signaling in human epidermal keratinocytes (hacat cells). J. Photochem. Photobiol. B 2019, 197, 111518. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Chang, Y.H. Comparison of inhibitory capacities of 6-, 8- and 10-gingerols/shogaols on the canonical nlrp3 inflammasome-mediated il-1beta secretion. Molecules 2018, 23, 466. [Google Scholar]
- Ha, S.K.; Moon, E.; Ju, M.S.; Kim, D.H.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. 6-shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology 2012, 63, 211–223. [Google Scholar] [CrossRef]
- Han, Q.; Yuan, Q.; Meng, X.; Huo, J.; Bao, Y.; Xie, G. 6-shogaol attenuates lps-induced inflammation in bv2 microglia cells by activating ppar-gamma. Oncotarget 2017, 8, 42001–42006. [Google Scholar] [CrossRef]
- Martin, H. Role of ppar-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutat. Res. 2010, 690, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.; Kim, S.; Choi, D.S.; Kwon, Y.B.; Kwon, J. Anti-inflammatory effects of [6]-shogaol: Potential roles of hdac inhibition and hsp70 induction. Food Chem. Toxicol. 2011, 49, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- Adcock, I.M. Hdac inhibitors as anti-inflammatory agents. Br. J. Pharmacol. 2007, 150, 829–831. [Google Scholar] [CrossRef]
- Gan, F.F.; Kaminska, K.K.; Yang, H.; Liew, C.Y.; Leow, P.C.; So, C.L.; Tu, L.N.; Roy, A.; Yap, C.W.; Kang, T.S.; et al. Identification of michael acceptor-centric pharmacophores with substituents that yield strong thioredoxin reductase inhibitory character correlated to antiproliferative activity. Antioxid. Redox Signal. 2013, 19, 1149–1165. [Google Scholar] [CrossRef] [PubMed]
- Gan, F.F.; Ling, H.; Ang, X.; Reddy, S.A.; Lee, S.S.; Yang, H.; Tan, S.H.; Hayes, J.D.; Chui, W.K.; Chew, E.H. A novel shogaol analog suppresses cancer cell invasion and inflammation, and displays cytoprotective effects through modulation of nf-kappab and nrf2-keap1 signaling pathways. Toxicol. Appl. Pharmacol. 2013, 272, 852–862. [Google Scholar] [CrossRef]
- Du, Y.T.; Zheng, Y.L.; Ji, Y.; Dai, F.; Hu, Y.J.; Zhou, B. Applying an electrophilicity-based strategy to develop a novel nrf2 activator inspired from dietary [6]-shogaol. J. Agric. Food Chem. 2018, 66, 7983–7994. [Google Scholar] [CrossRef] [PubMed]
| Organism/Model | Dose | Form | Administration Route | Effects |
|---|---|---|---|---|
| Rat/paw edema | 50 and 100 mg/kg | extract | oral | Reduction of carrageenan-induced paw edema formation by 22 or 38% |
| Mouse/Th2-mediated airway inflammation in OVA-sensitized animals | 360 mg/kg | extract | i.p. | Reduced amount of eosinophils, neutrophils, and monocytes in BALF and lung tissue |
| Rat/cervix cancer | 100 mg/kg | extract | oral | Less NFκB distribution in tissue |
| Rat/liver cancer | 100 mg/kg | extract | oral | Decreased NFκB expression |
| Rat/ breast cancer/liver cancer cells | 100 mg/kg | extract | oral | Reduced TNF levels |
| Rat/saline administration | 50 and 500 mg/kg | extract | oral or i.p. | Reduced PGE2 serum levels |
| Rat/saline administration | 500 mg/kg | extract | oral | Reduced TXB2 serum levels |
| Rat/paw edema | 25, 50, 100 and 200 mg/kg | extract | oral | Reduced carrageenan-induced paw volume, levels of PGE2, TNF, IL-6, IL-1β, IFNγ, MCP-1, MIP-2, RANTES, and MPO activity and NO levels |
| Rat/gastric ulcer | 200 mg/kg | powder | oral | Block of aspirin-induced gastric mucosal lesion formation, abrogation of ulcer with distorted gastric glands, damaged mucosal epithelium, and the formation of cell debris Restoration of normal physiological volume of gastric juice and acidity; reduction of mucosal iNOS activity, TNF, and IL-1β plasma levels |
| Mouse/arthritis | 100 and 200 mg/kg | extract | oral | Reduction of collagen II-induced IL-4, IFN-γ, and IL-17 protein and MMP1, 3 and 13 mRNA |
| Rat/arthritis | 50, 100 and 200 mg/kg | extract | i.p. | Reduction of collagen II-induced joint temperature and paw thickness, serum levels of cytokines IL-1β, IL-2, IL-6, TNF, and anti-type II collagen antibodies, increased paw removal latency |
| Mouse/UVB-irradiated hyperplasia | 1 and 2.5% | extract | oral | UVB-induced reduction of leukocyte infiltration, levels of IL-1β and IL-6 |
| Organism/Model | Dose | Administration Route | Effects |
|---|---|---|---|
| Rat/mono-arthritis model | 6.2 mg/kg | oral | Reduction of edema swelling volume, lymphocyte and monocyte infiltration |
| Mouse/TPA-induced skin model | 1 and 2.5 µmol | topical application | Reduction of iNOS and COX-2 |
| Rat PCA model | 1 and 5 mg/kg | oral | Reduction of DNP-HAS-induced PCA by 72% and 45% |
| Mouse/ischemic acute kidney injury model | 20 mg/kg | i.p. | Involvement of NFκB and HO-1reduction of creatinine, blood urea nitrogen and mRNA of neutrophil gelatinase-associated lipocalin, neutrophil infiltration, andIL-6, MCP-1, MIP-2, and KC mRNA |
| Hamster, buccal pouch carcinogenesis model | 20 mg/kg | oral | Reduction of DMBA-induced IKK, p65, COX, and iNOS levelsBlock of IκBα degradation and IL-6,IL-1 and TNFReduction of NFκB and AP-1 mRNA expression and c-jun, c-fos protein levels |
| Mouse, middle cerebral artery occlusion-induced brain damage model | 5 and 20 mg/kg | oral | Reduction of brain infarct volume, MDA and ROS production, IL-1β, TNF, COX-2 and iNOS, ERK, JNK, and p38 activation |
| Cell Type | Concentration | Effects |
|---|---|---|
| Human HaCaT cells | 0.1, 1, and 10 µM | Reduced release of IL-1β, TNF, IL-6, IL-8 |
| Human polymorphonuclear neutrophils | Increasing concentrations | DPPH scavenging: IC50: 8 µM |
| 6 µM | Reduction of fMLP-induced oxidative burst | |
| Murine RAW 264.7 macrophages | 1, 3, 6 µM | Reduction of LPS-induced nitrite and PGE2 release |
| Murine RAW 264.7 macrophages | 5 µM | Reduction of LPS-triggered exposition of arachidonic acid and of LPS/IFN-γ-induced NO synthesis |
| Murine RAW 264.7 macrophages | 2, 10, and 20 µM | Reduction of LPS-induced TNF, IL-1β, and NO |
| Murine RAW 264.7 macrophages | 10–20 µM | Reduction of LPS-triggered mRNA, protein and activation of iNOS and COX-2; reduction of nitrite and PGE2 |
| 6 and 10 µM | Reduction of NFκB nuclear translocation and IκBα degradation and phosphorylation; inhibition of ERK phosphorylation and PI3K/Akt activation | |
| Human 293T cells | 20 and 30 µM | Reduction of MyD88- and IKKβ-induced NFκB activity |
| Murine hematopoietic cell line Ba/F3 | 20 and 30 µM | Block of LPS-activated degradation of IRAK-1 |
| 30 µM | Block of LPS-induced TLR4 dimerization | |
| Primary rat cortical neuron-glia cells | 10 µM | Reduction of LPS-induced NO, iNOS, COX-2 protein, PGE2, IL-1β, and TNF; inhibition of LPS-triggered p38, JNK and ERK phosphorylation and NFκB activity, IκBα phosphorylation and degradation |
| Murine microglia cell line BV-2 | 10 µM | Reduction of LPS-induced iNOS, COX-2 |
| Human mast cells (HMC-1) | 0.1 and 1 µM | Reduction of TPA/A23187-induced IL-6, IL-8, and TNF release |
| 50 and 100 µM | Reduction of nuclear NFκB and cytosolic IκBα phosphorylation | |
| 10 µM | Inhibition of JNK activation | |
| Rat peritoneal mast cells | 0.1 µM | Reduction of compound 48/80-induced histamine release |
| Murine microglia cell line BV-2 | 5, 10, and 20 µM | Reduction of LPS-activated TNF, IL-1β, PGE2, and IL-6 release, NFκB phosphorylation and translocation into the nucleus, IκBα degradation and phosphorylation; Increase of PPARγ |
| Human proximal tubular cell line HK-2 | 50, 100, and 150 µM | Reduction of TNF-induced TNF, IL-6, IL-8, MCP-1, MIP-2, and ICAM-1 mRNA; reduction of H2O2-induced IL-8, MIP-2, TNF, ICAM-1 |
| 150 µM | Inhibition of TNF-activated nuclear NFκB, pIKK, pIκBα, and IκBα degradation | |
| Primary mouse proximal tubule | 50, 100, and 150 µM | Reduction of LPS/TNF-induced TNF, IL-6, IL-8, MCP-1, MIP-2, and ICAM-1 |
| Human HepG2 cells | 1, 5, and 10 µM | Reduction of H2O2-induced cellular oxidative stress; Increase of GSH, GCS, and ARE activity |
| 5 and 10 µM | Block of GST Upregulation of pJNK, Nrf2, and HO-1 | |
| Human THP-1 macrophages | 5 and 20 µM | Reduction of LPS/ATP-triggered IL-1β and secretion and mRNA; inhibition of NLRP3 and active caspase-1 |
| Human HT29/B6 | 100 µM | Reduction of TNF-induced Akt, IκBα and NFκB phosphorylation; induction of ERK and p38 |
| 75, 100, and 125 µM | Increase of TER and prevention of fluorescein permeability and claudin 1, down-regulation of claudin 2 | |
| Murine chondrogenic cell line ATDC5 | 5 µM | Reduction of LPS/IL-1β-induced NO, LPS-induced MCP-1, IL-6, MyD88, ERK phosphorylation and iNOS |
| Human primary chondrocytes | 5 µM | Reduction of cathepsin K activity |
| Primary rat astrocytes | 10 µM | Reduction LPS-triggered IL-1β and IL-6 release, iNOS and COX-2 protein, LPS-induced HDAC1 protein and up-regulation of HSP70; restoration of acetyl histone 3 protein after LPS degradation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bischoff-Kont, I.; Fürst, R. Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes. Pharmaceuticals 2021, 14, 571. https://doi.org/10.3390/ph14060571
Bischoff-Kont I, Fürst R. Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes. Pharmaceuticals. 2021; 14(6):571. https://doi.org/10.3390/ph14060571
Chicago/Turabian StyleBischoff-Kont, Iris, and Robert Fürst. 2021. "Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes" Pharmaceuticals 14, no. 6: 571. https://doi.org/10.3390/ph14060571
APA StyleBischoff-Kont, I., & Fürst, R. (2021). Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes. Pharmaceuticals, 14(6), 571. https://doi.org/10.3390/ph14060571






