Flavonoids from the Genus Euphorbia: Isolation, Structure, Pharmacological Activities and Structure–Activity Relationships
Abstract
1. Introduction
2. Literature Sources and Search Strategy
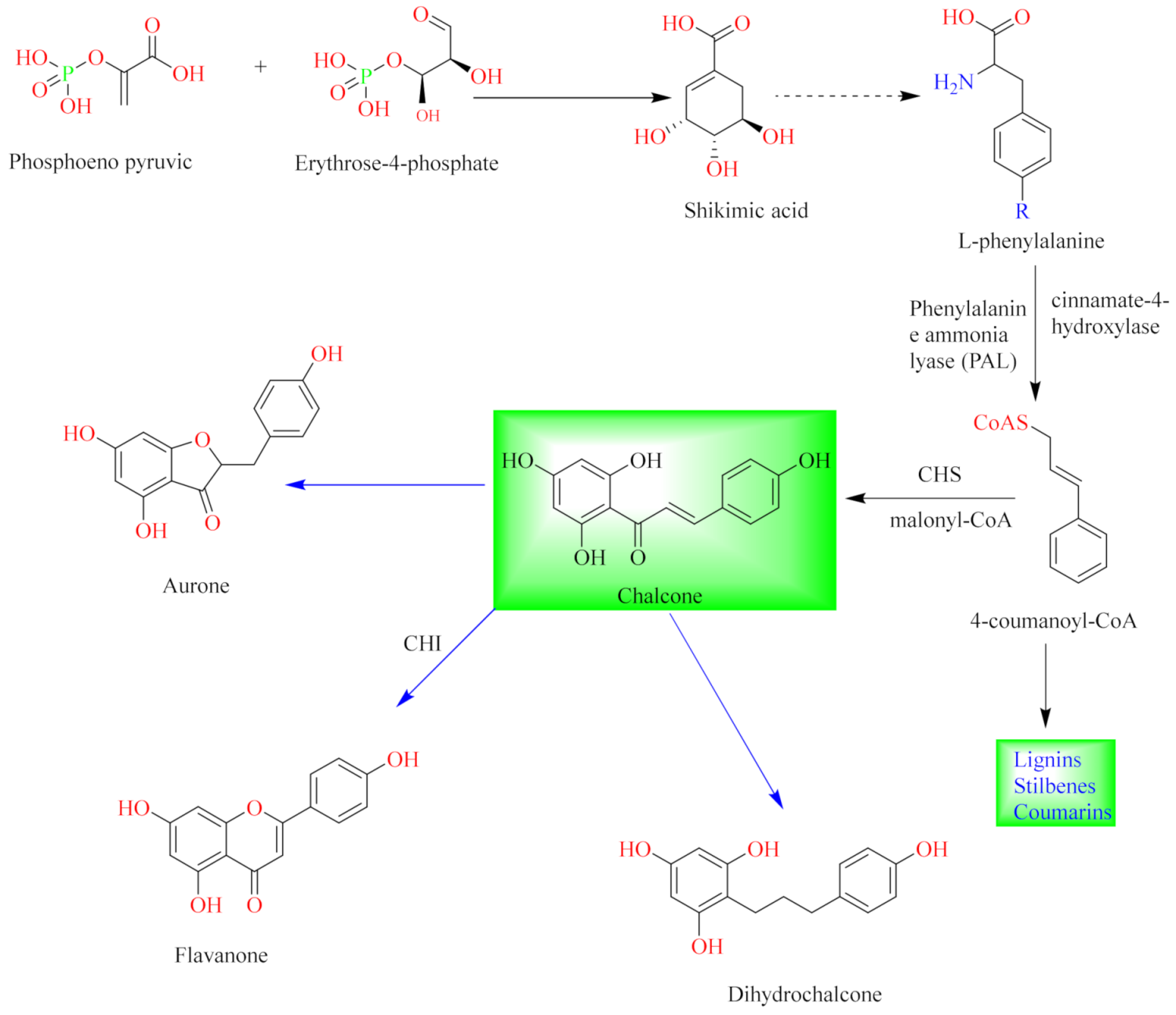
3. Flavonoids
4. Isolation of Euphorbia Flavonoids
5. Flavonoids Isolated from Euphorbia Species
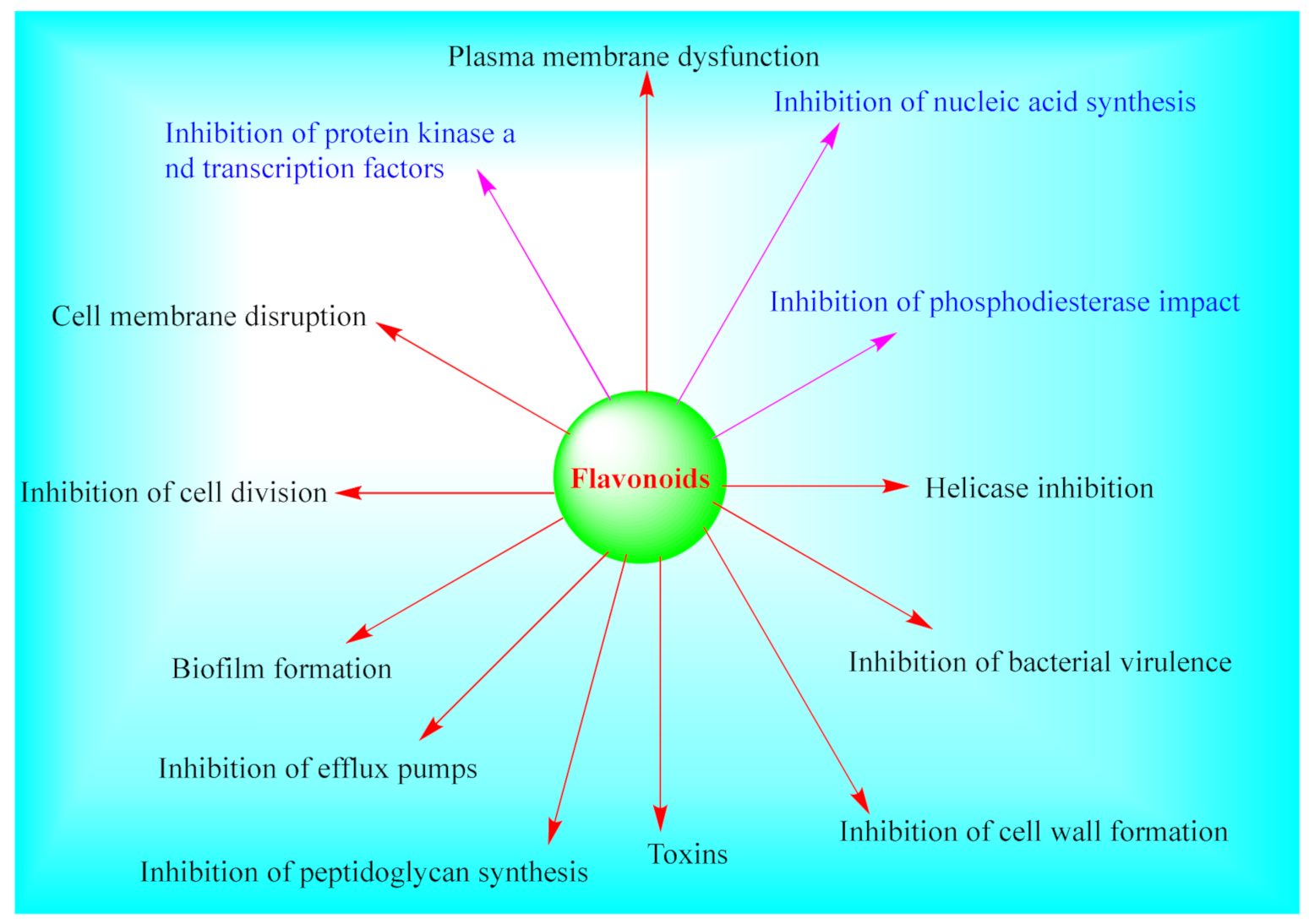
6. Biological Studies, Structure–Activity Relationship and Therapeutic Potential
6.1. Cytotoxic Studies
6.2. Antioxidant Activities
6.3. Anti-Hepatitis Activities
6.4. Anti-Venom Activities
6.5. Antimalarial Activities
6.6. Antibacterial and Antifungal Activities
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Montellano, B.O. Empirical Aztec medicine. Science 1975, 188, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Madeleine, E.; Olwen, M.; Grace, C.; Haris, S.L.; Niclas, N.; Henrik, T.; Nina, R. Global medicinal uses of Euphorbia L. (Euphorbiaceae). J. Ethnopharmacol. 2015, 76, 90–101. [Google Scholar] [CrossRef]
- Hooper, M. Major Herbs of Ayurveda; Elsevier Health Sciences: Amsterdam, The Netherlands, 2002; pp. 340–345. [Google Scholar]
- Kemboi, D.; Xolani, P.; Moses, L.; Jacqueline, T. A review of the ethnomedicinal uses, biological activities and triterpenoids of Euphorbia species. Molecules 2020, 25, 4019. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Mata, O.; Aguiar, R.S.; Paz, M.C.J.; Alencar, M.B.; Melo-Cavalcante, A.C. Therapeutic potential of essential oils focusing on diterpenes. Phytother. Res. 2016, 30, 1420–1444. [Google Scholar] [CrossRef]
- Mihail, A.; Viktorija, M.; Liljana, K.G.; Liljana, G.T. Review of the anticancer and cytotoxic activity of some species from the genus Euphorbia. Agric. Conspec. Sci. 2019, 84, 11–23. Available online: https://hrcak.srce.hr/file/318988 (accessed on 1 July 2020).
- Muhammad, A.; Abdul, S.; Muhammad, J.; Farhat, U.; Muhammad, O.; Ikram, U.; Jawad, A.; Muhammad, S. Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Front. Aging Neurosci. 2019, 1–20. [Google Scholar] [CrossRef]
- Scholz, A. Euphorbiaceae. In Syllabus der Planzenfamilien; Engler, A., Ed.; Gebrüder Bornträger: Berlin, Germany, 1964; pp. 255–261. [Google Scholar]
- Webster, G.L. Classification of the Euphorbiaceae. Ann. Mol. Bot. Gard. 1994, 81, 3–32. [Google Scholar] [CrossRef]
- Andrea, V.; Judit, H. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Samec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2018. [Google Scholar] [CrossRef]
- Scarano, A.; Chieppa, M.; Santino, A. Looking at flavonoid biodiversity in horticultural crops: A colored mine with nutritional benefits. Plants 2018, 7, 98. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Sardesai, S.; Dose, A.I. Flavonoids: New frontier for immuno-regulation and breast cancer control. Antioxidants 2019, 8, 103. [Google Scholar] [CrossRef]
- Pretorius, J.C. Flavonoids: A review of its commercial application potential as anti-infective agents. Anti-Infect. Agents Curr. Med. Chem. 2003, 2, 335–353. [Google Scholar] [CrossRef]
- Zhao, K.; Yuan, Y.; Lin, B.; Miao, Z.; Li, Z.; Guo, Q.; Lu, N. LW-215, a newly synthesized flavonoid, exhibits potent anti-angiogenic activity in vitro and in vivo. J. Pharmacol. Sci. 2018, 642, 533–541. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Yong, H.; Kan, J.; Jin, C. Recent advances in flavonoid-grafted polysaccharides: Synthesis, structural characterization, bioactivities and potential applications. Int. J. Biol. Macromol. 2018, 116, 1011–1025. [Google Scholar] [CrossRef]
- Camero, C.M.; Germanò, M.P.; Rapisarda, A.; D’Angelo, V.; Amira, S.; Benchikh, F.; Braca, A.; De Leo, M. Anti-angiogenic activity of iridoids from Galium tunetanum. Rev. Bras. Farm. 2018, 28, 374–377. [Google Scholar] [CrossRef]
- Patel, K.; Kumar, V.; Rahman, M.; Verma, A.; Patel, D.K. New insights into the medicinal importance, physiological functions and bioanalytical aspects of an important bioactive compound of foods ‘Hyperin’: Health benefits of the past, the present, the future. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 31–42. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Guinó, E.; Alonso, M.H.; Vidal, C.; Barenys, M.; Soriano, A.; Moreno, V. Dietary flavonoids, lignans and colorectal cancer prognosis. Sci. Rep. 2015, 5, 14148. [Google Scholar] [CrossRef]
- Avtar, C.; Bhawna, G. Chemistry and Pharmacology of flavonoids—A review. Indian J. Pharm. Edu. Res. 2019, 53, 8–20. Available online: https://www.ijper.org/article/918 (accessed on 23 February 2021).
- Reale, G.; Russo, G.I.; di Mauro, M.; Regis, F.; Campisi, D.; Giudice, A.L.; Marranzano, M.; Ragusa, R.; Castelli, T.; Cimino, S. Association between dietary flavonoids intake and prostate cancer risk: A case-control study in Sicily. Complement. Ther. Med. 2018, 39, 14–18. [Google Scholar] [CrossRef]
- Rienks, J.; Barbaresko, J.; Nothlings, U. Association of isoflavone biomarkers with risk of chronic disease and mortality: A systematic review and meta-analysis of observational studies. Nutr. Rev. 2017, 75, 616–641. [Google Scholar] [CrossRef]
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J. Cell. Physiol. 2018, 233, 6544–6560. [Google Scholar] [CrossRef] [PubMed]
- Danihelová, M.; Viskupiˇcová, J.; Šturdík, E. Lipophilization of flavonoids for their food, therapeutic and cosmetic applications. Acta Chim. 2012, 5, 59–69. [Google Scholar] [CrossRef]
- Yang, Z.-G.; Jia, L.-N.; Shen, Y.; Ohmura, A.; Kitanaka, S. Inhibitory effects of constituents from Euphorbia lunulata on differentiation of 3T3-L1 cells and nitric oxide production in RAW264.7 cells. Molecules 2011, 16, 8305–8318. [Google Scholar] [CrossRef] [PubMed]
- Yuwei, W.; Xiao, Y.; Lingna, W.; Fang, Z.; Yongqing, Z. Research progress on chemical constituents and anticancer pharmacological activities of Euphorbia lunulata Bunge. Bio. Med. Res. Int. 2020, 1–11. [Google Scholar] [CrossRef]
- Caballero, B.; Trugo, L.; Finglas, P. Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: London, UK, 2003. [Google Scholar]
- Sotnikova, O.M.; Chagovets, R.K.; Litvinenko, V.I. New flavanone compounds from Euphorbia stepposa. Chem. Nat. Compd. 1968, 4, 71–74. [Google Scholar] [CrossRef]
- Ulubelen, A.; Öksüz, S.; Halfon, B.; Aynehchi, Y.; Mabry, T.J. Flavonoids from Euphorbia larica, E. virgata, E. chamaesyce and E. magalanta. J. Nat. Prod. 1983, 46, 598. [Google Scholar] [CrossRef]
- Dieter, T. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Ali, S.; Behrad, D.; Farzaneh, H.; Azadeh, M.; Antoni, S.; Seyed, F.; Leo, R.; Seyed, M.; Anupam, B. Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J. Gastroenterol. 2017, 23, 5097–5114. [Google Scholar] [CrossRef]
- Mariam, A.; Samson, M.; Elizabeth, V.; Sharon, V.; Peter, K.; Alena, L.; Dietrich, B. Flavonoids in cancer and apoptosis. Cancers 2019, 11, 28. [Google Scholar] [CrossRef]
- Bathelemy, N.; Ghislain, W.; Justin, K.; Pantaleon, A.; Tchoukoua, A.; Aimé, G.; Igor, K.; Bonaventure, T.; Berhanu, M.; Victor, K. Flavonoids and related compounds from the medicinal plants of Africa. J. Med. Plant. Res. 2019. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. Sci. World J. 2013, 16. [Google Scholar] [CrossRef]
- Shashank, K.; Abhay, K. Chemistry and biological activities of flavonoids: An Overview. Sci. World J. 2013, 1–16. [Google Scholar] [CrossRef]
- Nigel, C.; Renee, J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2011, 28, 1626. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, S.; Francis, G.; Becker, K. Phorbol esters: Structure, biological activity, and toxicity in animals. Int. J. Toxicol. 2007, 26, 279. [Google Scholar] [CrossRef]
- Shi, Q.W.; Su, X.H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295. [Google Scholar] [CrossRef]
- Rojas, R.; Tafolla-Arellano, J.C.; Martínez-Ávila, G.C.G. Euphorbia antisyphilitica Zucc: A Source of Phytochemicals with Potential Applications in Industry. Plants 2021, 10, 8. [Google Scholar] [CrossRef]
- Yang, T.; Jun, H.; Yu, Y.; Wen-Wen, L.; Cong-Yuan, X.; Jie-Kun, X.; Wei-Ku, Z. Euphorbia ebracteolata Hayata (Euphorbiaceae): A systematic review of its traditional uses, botany, phytochemistry, pharmacology, toxicology, and quality control. Phytochemistry 2021, 186, 112736. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Middleton, E. The flavonoids. Trends Pharmacol. Sci. 1984, 5, 335–338. [Google Scholar]
- Murphy, K.; Chronopoulos, A.; Singh, I. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am. J. Clin. Nutr. 2003, 77, 1466–1473. [Google Scholar] [CrossRef]
- Ke, L.; Hang, F.; Peipei, Y.; Lingguang, Y.; Qiang, X.; Xiang, L.; Liwei, S.; Yujun, L. Structure-activity relationship of eight high content flavonoids analyzed with a preliminary assign-score method and their contribution to antioxidant ability of flavonoids-rich extract from Scutellaria. Arab. J. Chem. 2018, 11, 159–170. [Google Scholar] [CrossRef]
- Syed, M.; Ghanadiana, A.; Suleiman, A.; Sumaira, H.; Omer, A.; Jean, J. Flavonol glycosides from Euphorbia microsciadia Bioss with their immunomodulatory activities. Iran. J. Pharm. Res. 2012, 11, 925–930. Available online: https://pubmed.ncbi.nlm.nih.gov/24250520/ (accessed on 14 January 2021).
- De-freitsa, T.J.B.; Silva, A.J.; Kuster, R. Isolation and characterization of polyphenols from Euphorbia heterophylla L. (Euphorbiaceae) leaves. Rev. Fitos 2019, 13, 49–60. [Google Scholar] [CrossRef]
- Zhang, W.J. Study on Chemical Constituents and Antitumor Activity of Euphorbia Lunulata Bge; Huaqiao University: Quanzhou, China, 2016. [Google Scholar]
- Laila, A.-G. Study on Flavonoids and Triterpenoids content of some Euphorbiaceae plants. J. Life Sci. 2011, 5, 100–107. Available online: https://www.researchgate.net/publication/273351317 (accessed on 14 January 2021).
- Sheliya, M.A.; Rayhana, B.; Ali, A.; Pillai, K.K.; Aeri, V.; Sharmam, R. Inhibition of α-glucosidase by new prenylated flavonoids from Euphorbia hirta l. herbs. J. Ethnopharmacol. 2015, 176, 1–8. [Google Scholar] [CrossRef]
- Maria, D.; Luziene, A.C.; Emily, C.; Bruno, O.; M´arcia, V.; Lívia, N.C.; Renata, M. Bioactivity flavonoids from roots of Euphorbia tirucalli L. Phytochem. Lett. 2021, 41, 186–192. [Google Scholar] [CrossRef]
- Seham, S.; Raba, M.; Ahmed, F.; Nadia, M.; Sameh, F.; Mohamed, N.; Usama, R.A.; Elham, A. Cytotoxic activity and metabolic profiling of fifteen Euphorbia Species. Metabolites 2021, 11, 15. [Google Scholar] [CrossRef]
- Safwat, N.A.; Kashef, M.T.; Aziz, R.K.; Amer, K.F.; Ramadan, M.A. Quercetin 3-O-glucoside recovered from the wild Egyptian Sahara plant, Euphorbia paralias L., inhibits glutamine synthetase and has antimycobacterial activity. Tuberculosis 2017, 108, 106–113. [Google Scholar] [CrossRef]
- Chaabi, M.; Freund-Michel, V.; Frossard, N.; Randriantsoa, A.; Andriantsitohaina, R.; Lobstein, A. Anti-proliferative effect of Euphorbia stenoclada in human airway smooth muscle cells in culture. J. Ethnopharmacol. 2007, 109, 134–139. Available online: https://www.sciencedirect.com/science/article/pii/S0378874106003539 (accessed on 14 January 2021). [CrossRef]
- Li, R.; Wang, J.; Wu, H.X.; Li, L.; Wang, N.L. Isolation, identification and activity determination of antioxidant active components in Euphorbia lunulata Bunge. J. Shenyang Pharm. Univ. 2011, 28, 25–29. [Google Scholar]
- Sevil, O.; Faliha, G.; Long-Ze, L.; Roberto, R.; Gil, J.M.; Pezzuto, M.; Geoffrey, A.C. Aleppicatines A and B from Euphorbia allepica. Phytochemistry 1996, 42, 473–478. [Google Scholar]
- Wen, Z.; Yue-Wei, G. Chemical studies on the constituents of the chinese medicinal herb Euphorbia helioscopia L. Chem. Pharm. Bull. 2006, 54, 1037–1039. Available online: https://pubmed.ncbi.nlm.nih.gov/16819227/ (accessed on 14 January 2021).
- Dumkow, K. Kaempferol-3-glucuronide and quercetin-3-glucuronide, principal flavonoids of Euphorbia lathyris L. and their separation on acetylated polyamide. Z. Naturforsch 1969, 24, 358. [Google Scholar] [CrossRef]
- Ying, T.; Li-Min, S.; Xi-Qiao, L.; Bin Li, Q.; Jun-Xing, D. Anti-HBV active flavone glucosides from Euphorbia humifusa Willd. Fitoterapia 2010, 81, 799–802. [Google Scholar] [CrossRef]
- Liu, X.; Ye, W.; Yu, B.; Zhao, S.; Wu, H.; Che, C. Two new flavonol glycosides from Gymnema sylvestre and Euphorbia ebracteolata. Carbohydr. Res. 2004, 339, 891–895. [Google Scholar] [CrossRef]
- Tadahiro, N.; Li-Yan, W.; Kouji, K.; Susumu, K. Flavonoids that mimic human ligands from the whole plants of Euphorbia lunulata. Chem. Pharm. Bull. 2005, 53, 305–308. Available online: https://pubmed.ncbi.nlm.nih.gov/15744103/ (accessed on 24 January 2021).
- Veena, S.; Pracheta, J. Extraction, isolation and identification of flavonoid from Euphorbia neriifolia leaves. Arab. J. Chem. 2017, 10, 509–514. [Google Scholar] [CrossRef]
- Cheng, J.; Han, W.; Wang, Z.; Shao, Y.; Wang, Y.; Zhang, Y.; Li, Z.; Xu, X.; Zhang, Y. Hepatocellular carcinoma growth is inhibited by Euphorbia helioscopia L. extract in nude mice xenografts. Bio. Med. Res. Int. 2015, 1–9. [Google Scholar] [CrossRef]
- Abu-reidah, I.M.; Ali-shtayeh, M.S.; Jamous, R.M.; Arr´aez-rom´an, D.; Segura-carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- Markham, K.R.; Ternai, B. 13C NMR of flavonoids-ii flavonoids other than flavone and flavonol aglycones. Tetrahedron 1976, 32, 2607–2612. [Google Scholar] [CrossRef]
- Ahn, B.T.; Lee, K.S. Phenolic compounds from Euphorbia ebracteolata. Saengyak Hakhoechi 1996, 27, 136–141. [Google Scholar]
- Heba, I.; Abd, E.-M. Flavonoid and phenolic acid compounds in Euphorbia bivonae Steud roots. Curr. Sci. Int. 2015, 4, 668–676. Available online: http://www.curresweb.com/csi/csi/2015/668-676.pdf (accessed on 2 February 2021).
- Liu, C.; Sun, H.; Wang, W.T. Study on chemical constituents of Euphorbia lunulata bge. J. Chin. Med. Mater. 2015, 3, 514–517. [Google Scholar]
- Hassana, G.K.; Omera, M.A.; Babadoust, S.; Najata, D.D. Flavonoids from Euphorbia condylocarpa roots. Int. J. Chem. Bio. Sci. 2014, 6, 56–60. Available online: http://www.iscientific.org/wp-content/uploads/2018/02/8-IJCBS-14-06-05.pdf (accessed on 2 February 2021).
- Amani, S.A.; Nabilah, A.; John, E.; Reham, M.; Mohamed, E. Antiulcerogenic activities of the extracts and isolated flavonoids of Euphorbia cuneata Vahl. Phytother. Res. 2013, 27, 126–130. [Google Scholar] [CrossRef]
- Shang, T.M.; Wang, L.; Liang, X.T. Study on the chemical constituents of Euphorbia Lunulata. J. Chem. 1979, 37, 119–128. [Google Scholar]
- Lee, S.C.; Ahn, B.T.; Park, W.Y.; Lee, S.H.; Ro, J.S.; Lee, K.S.; Ryu, E.K. Pharmacognostic study on Euphorbia ebracteolata. Flavonoid constituents. Korean J. Pharmacogn. 1992, 23, 126–131. [Google Scholar]
- Tarek, B.; Hichema, A.; Khalfallaha, A.; Kabouchea, Z.; Kabouchea, I.; Jaime, B.; Christian, B. A New alkaloid and flavonoids from the aerial parts of Euphorbia guyoniana. Nat. Prod. Commun. 2010, 5, 1–3. Available online: https://pubmed.ncbi.nlm.nih.gov/20184016/ (accessed on 2 February 2021).
- Wu, Y.; Qu, W.; Geng, D.; Liang, J.-Y.; Luo, Y.-L. Phenols and flavonoids from the aerial part of Euphorbia hirta. Chin. J. Nat. Med. 2012, 10, 40–42. [Google Scholar] [CrossRef]
- Abida, T.; Ismat, N.; Hifsa, M. Isolation of flavonols from Euphorbia wallichii by preparative High Performance Liquid Chromatography. Nat. Sci. 2009, 7, 1–3. Available online: http://www.sciencepub.net/nature (accessed on 2 February 2021).
- Mueller, R.; Pohl, R. Flavonol glycosides of Euphorbia amygdaloides and their quantitative determination at various stages of plant development. 5. Flavonoids of native Euphorbiaceae. Planta Med. 1970, 18, 114–129. [Google Scholar] [CrossRef]
- Chai, G.S. Study on the chemical constituents of the seeds of Euphorbia Fischeriana Steud and Euphorbia Esula Linn. Qiqihar University, Qiqihar, China. Chemo-prevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Salehi, B.; Iriti, M.; Vitalini, E.; Antolak, H.; Pawlikowska, E.; Kręgiel, D.; Sharifi-rad, J.; Oyeleye, S.I.; Ademiluyi, A.O.; Czopek, K.; et al. Euphorbia-derived natural products with potential for use in health maintenance. Biomolecules 2019, 9, 337. [Google Scholar] [CrossRef]
- Ozbílgín, S.; Saltan, G. Uses of some Euphorbia species in traditional medicine in turkey and their biological activities. Turk. J. Pharm. Sci. 2012, 9, 241–256. [Google Scholar]
- Liu, Z.G.; Li, Z.L.; Bai, J.; Meng, D.L.; Li, N.; Pei, Y.H.; Zhao, F.; Hua, H.M. Anti-inflammatory diterpenoids from the roots of Euphorbia ebracteolata. J. Nat. Prod. 2014, 77, 792–799. [Google Scholar] [CrossRef]
- Halaweish, F.; Kronberg, S.; Rice, J. Rodent and ruminant ingestive response to flavonoids in Euphorbia esula. J. Chem. Ecol. 2003, 5, 1073–1082. [Google Scholar] [CrossRef]
- Kadiyala, G.; Anbarasu, K.; Kadali, R.; Jayanthi, S.; Vishwanath, B.S.; Gurunathan, J. Quercetin-3-O-rhamnoside from Euphorbia hirta protects against snake venom induced toxicity. Biochim. Biophys. Acta 2016, 1860, 1528–1540. [Google Scholar] [CrossRef]
- Noori, M.; Chehreghani, A.; Kaveh, M. Flavonoids of 17 species of Euphorbia (Euphorbiaceae). Iran. Toxicol. Environ. Chem. 2009, 91, 631–641. [Google Scholar] [CrossRef]
- Byung, T.A.; Kapjin, O.; Jai, S.R.; Kyong, S.L. A New Flavonoid from Euphorbia ebracteolata. Planta Med. 1996, 62, 383–384. [Google Scholar]
- Reham, H.; Norbert, K.; Peter, W.; Ágnes, K.; István, Z.; Péter, O.; László, T.; Judit, H.; Andrea, V. Isolation and pharmacological investigation of compounds from Euphorbia matabelensis. Nat. Prod. Commun. 2019, 1–5. [Google Scholar] [CrossRef]
- Martinez, V.M.; Ramirez, A.T.; Lazcano, M.; Bye, R. Anti-inflammatory Active Compounds from then-hexane extract of Euphorbia hirta. J. Mex. Chem. Soc. 1999, 43, 103–105. [Google Scholar]
- Bahar, A.; Tawfeq, A.; Jaber, S.m.; Kehel, T. Isolation, antihypertensive activity and structure activity relationship of flavonoids from medicinal plants. Indian J. Chem. 2005, 44, 400–404. Available online: http://nopr.niscair.res.in/bitstream/123456789/8947/1/IJCB%2044B(2)%20400-404.pdf (accessed on 2 February 2021).
- Ko, W.C.; Wang, H.L.; Lei, C.B.; Shih, C.H.; Chung, M.I.; Lin, C.N. Mechanisms of relaxant action of 3-O-methylquercetin in isolated guinea pig trachea. Planta Medica 2002, 68, 30–35. [Google Scholar] [CrossRef]
- Haggag, E.G.; Abou-Moustafa, M.A.; Boucher, W.; Theoharides, T.C. The effect of herbal water-extract on histamine release from mast cells and on allergic asthma. J. Herb. Pharmacother. 2003, 3, 41–54. Available online: https://pubmed.ncbi.nlm.nih.gov/15277119/ (accessed on 2 February 2021). [CrossRef]
- Jiang, S. The Inhibition of Lung Cancer Active Ingredient Extracts of Euphorbia Lunulata and Its Mechanism; Ocean University of China: Qingdao, China, 2011. [Google Scholar]
- Gao, F.; Fu, Z.; Tian, H.; He, Z. The Euphorbia lunulata extract inhibits proliferation of human hepatoma Hep-G2 cells and induces apoptosis. J. BUON 2013, 18, 491–495. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23818367 (accessed on 2 February 2021).
- Hameed, A.; Hafizur, R.M.; Hussain, N. Eriodictyol stimulates insulin secretion through cAMP/PKA signaling pathway in mice islets. Eur. J. Pharmacol. 2018, 820, 245–255. [Google Scholar] [CrossRef]
- Campos, P.M.; Prudente, A.S.; Horinouchi, C.D. Inhibitory effect of GB-2a (I3-naringenin-II8-eriodictyol) on melanogenesis. J. Ethnopharmacol. 2015, 174, 224–229. [Google Scholar] [CrossRef]
- Lee, J.K. Anti-inflammatory effects of eriodictyol in lipopolysaccharide-stimulated raw 264.7 murine macrophages. Arch. Pharm. Res. 2011, 34, 671–679. [Google Scholar] [CrossRef]
- Whelan, L.C.; Ryan, M.F. Ethanolic extracts of Euphorbia and other ethnobotanical species as inhibitors of human tumour cellgrowth. Phytomedicine 2003, 10, 53–58. [Google Scholar] [CrossRef]
- Wongprayoon, P.; Charoensuksai, P. Cytotoxic and anti-migratory activities from hydroalcoholic extract of Euphorbia lactea Haw against HN22 cell line. Thai J. Pharm. Sci. 2018, 13, 69–77. [Google Scholar] [CrossRef]
- Villanueva, J.; Quirós, L.M.; Castañón, S. Purification and partial characterization of a ribosome-inactivating protein from the latex of Euphorbia trigona Miller with cytotoxic activity toward human cancer cell lines. Phytomedicine 2015, 22, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Waczuk, E.P.; Kamdem, J.P.; Abolaji, A.O.; Meinerz, D.F.; Bueno, D.C.; do Nascimento Gonzaga, T.K.; do Canto Dorow, T.S.; Boligon, A.A.; Athayde, M.L.; da Rocha, J.B.T. Euphorbia tirucalli aqueous extract induces cytotoxicity, genotoxicity and changes in antioxidant gene expression in human leukocytes. Toxicol. Res. 2015, 4, 739–748. [Google Scholar] [CrossRef]
- Hou, D.X.; Kumamoto, T. Flavonoids as protein kinase inhibitors for cancer chemoprevention: Direct binding and molecular modeling. Antioxid. Redox Signal. 2010, 13, 691–719. [Google Scholar] [CrossRef] [PubMed]
- Lolli, G.; Cozza, G.; Mazzorana, M.; Tibaldi, E.; Cesaro, L.; Donella-Deana, A.; Pinna, L.A. Inhibition of protein kinase CK2 by flavonoids and tyrphostins. A structural insight. Biochemistry 2012, 51, 6097–6107. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Kosaka, Y.; Mizuguchi, M. Structural insight into the interactions between death-associated protein kinase 1 and natural flavonoids. J. Med. Chem. 2015, 58, 7400–7408. [Google Scholar] [CrossRef]
- Peng, H.L.; Huang, W.C.; Cheng, S.C.; Liou, C.J. Fisetin inhibits the generation of inflammatory mediators in interleukin-1β-induced human lung epithelial cells by suppressing the Nf-κb and Erk1/2 pathways. Int. Immunopharmacol. 2018, 60, 202–210. [Google Scholar] [CrossRef]
- Atashpour, S.; Fouladdel, S.; Movahhed, T.K.; Barzegar, E.; Ghahremani, M.H.; Ostad, S.N.; Azizi, E. Quercetin induces cell cycle arrest and apoptosis in CD133+ cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran. J. Basic Med. Sci. 2015, 18, 635. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26351552 (accessed on 10 February 2021).
- Yang, P.-W.; Lu, Z.-Y.; Pan, Q.; Chen, T.-T.; Feng, X.-J.; Wang, S.-M.; Pan, Y.-C.; Zhu, M.-H.; Zhang, S.-H. MicroRNA-6809–5p mediates luteolin-induced anticancer effects against hepatoma by targeting flotillin. Phytomedicine 2019, 57, 18–29. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Tang, G.H.; Lou, L.L.; Li, W.; Zhang, B.; Liu, B.; Yin, S. Prenylated flavonoids as potent phosphodiesterase-4 inhibitors from Morus alba: Isolation, modification, and structure-activity relationship study. Eur. J. Med. Chem. 2018, 144, 758–766. [Google Scholar] [CrossRef]
- Choene, M.; Motadi, L. Validation of the antiproliferative effects of Euphorbia tirucalli extracts in breast cancer cell lines. Mol. Biol. 2016, 50, 98–110. [Google Scholar] [CrossRef]
- Devi, K.P.; Rajavel, T.; Nabavi, S.F.; Setzer, W.N.; Ahmadi, A.; Mansouri, K.; Nabavi, S.M. Hesperidin: A promising anticancer agent from nature. Ind. Crops Prod. 2015, 76, 582–589. [Google Scholar] [CrossRef]
- Ersoz, M.; Erdemir, A.; Duranoglu, D.; Uzunoglu, D.; Arasoglu, T.; Derman, S.; Mansuroglu, B. Comparative evaluation of hesperetin loaded nanoparticles for anticancer activity against C6 glioma cancer cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 319–329. [Google Scholar] [CrossRef]
- Alsayari, A.; Muhsinah, A.B.; Hassan, M.Z.; Ahsan, M.J.; Alshehri, J.A.; Begum, N. Aurone: A biologically attractive scaffold as anticancer agent. Eur. J. Med. Chem. 2019, 166, 417–431. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Kumar, R.; Caruso, I.P.; Ullah, A.; Cornelio, M.L.; Fossey, M.A. Exploring the binding mechanism of flavonoid quercetin to phospholipase A2: Fluorescence spectroscopy and computational approach. Eur. J. Exp. Biol. 2017, 7, 33. [Google Scholar] [CrossRef]
- Novo, M.; Hessel, H.; Fabri, C.; Ramos da Cruz Costa, C.; de Oliveira Toyama, D.; Domingues Passero, L.F.; Dalastra Laurenti, M.; Hikari Toyama, M. Evaluation of rhamnetin as an inhibitor of the pharmacological effect of secretory phospholipase A2. Molecules 2017, 22, 1441. [Google Scholar] [CrossRef]
- González Mosquera, D.M.; Hernández Ortega, Y.; Fernández, P.L.; González, Y.; Doens, D.; Vander Heyden, Y.; Pieters, L. Flavonoids from Boldoa purpurascens inhibit proinflammatory cytokines (TNF-α and IL-6) and the expression of COX-2. Phytother. Res. 2018, 32, 1750–1754. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Q.; Zhao, W.; Zhang, J.; Liu, W.; Huang, M.; Zeng, X. Oligomeric proanthocyanidins attenuate airway inflammation in asthma by inhibiting dendritic cells maturation. Mol. Immunol. 2017, 91, 209–217. [Google Scholar] [CrossRef]
- Lin, X.; Lin, C.H.; Zhao, T.; Zuo, D.; Ye, Z.; Liu, L.; Lin, M.T. Quercetin protects against heat stroke-induced myocardial injury in male rats: Antioxidative and anti-inflammatory mechanisms. Chemico-Biol. Interact. 2017, 265, 47–54. [Google Scholar] [CrossRef]
- Galleggiante, V.; De Santis, S.; Cavalcanti, E.; Scarano, A.; De Benedictis, M.; Serino, G.; Chieppa, M. Dendritic cells modulate iron homeostasis and inflammatory abilities following quercetin exposure. Curr. Pharm. Des. 2017, 23, 2139–2146. [Google Scholar] [CrossRef]
- Gong, J.H.; Shin, D.; Han, S.Y.; Kim, J.L.; Kang, Y.H. Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma. J. Nutr. 2011, 142, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Patel, A.B.; Panagiotidou, S.; Theoharides, T.C. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J. Allergy Clin. Immunol. 2015, 135, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zhang, B.; Asadi, S.; Sismanopoulos, N.; Butcher, A.; Fu, X.; Theoharides, T.C. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jung, W.S.; Kim, M.E.; Lee, H.W.; Youn, H.Y.; Seon, J.K.; Lee, J.S. Genistein inhibits pro-inflammatory cytokines in human mast cell activation through the inhibition of the ERK pathway. Int. J. Mol. Med. 2014, 34, 1669–1674. [Google Scholar] [CrossRef]
- Veena, S.; Pracheta, J. Protective assessment of Euphorbia neriifolia and its isolated flavonoid against N nitrosodiethylamine-induced Hepatic carcinogenesis in male mice: A histopathological analysis. Toxicol. Int. 2015, 1–8. [Google Scholar] [CrossRef][Green Version]
- Lizhen, Z.; Chu, W.; Qiuxia, M.; Qin, T.; Yu, N.; Wei, N. Phytochemicals of Euphorbia lathyris L. and their antioxidant activities. Molecules 2017, 22, 1335. [Google Scholar] [CrossRef]
- Kerry, N.L.; Abbey, M. Red wine and fractionated phenolic compounds prepared from red wine inhibits low density lipoprotein oxidation in vitro. Atherosclerosis 1997, 135, 93–102. Available online: https://pubmed.ncbi.nlm.nih.gov/9395277/ (accessed on 1 March 2021). [CrossRef]
- Hanan, M.; Ali, S.; Afaf, E.A.; Sayed, A.E.; Wagdi, I.A.; Wafaa, H.B.; Hanaa, M. Nephroprotective and antioxidant activities of ethyl acetate fraction of Euphorbia geniculata Ortega family Euphorbiaceae. Arab. J. Chem. 2020, 13, 7843–7850. [Google Scholar] [CrossRef]
- Claudia, M.; Furlan, K.; Pereira, S.; Martha, D.; Sedano-Partida, L.; Barbosa, D.; Deborah, Y.A.C.; Maria, L.; Giuseppina, N.; Paul, E.; et al. Flavonoids and antioxidant potential of nine Argentinian species of Croton (Euphorbiaceae). Braz. J. Bot. 2015, 38, 693–702. [Google Scholar] [CrossRef]
- Mishra, B.; Priyadarsini, K.; Kumar, M.; Unnikrishnan, M.; Mohan, H. Effect of O-glycosilation on the antioxidant activity, free radical reactions of a plant flavonoid, chrysoeriol. Bioorg. Med. Chem. 2003, 11, 2677–2685. [Google Scholar] [CrossRef]
- You, H.J.; Ahn, H.J.; Ji, G.E. Transformation of rutin to antiproliferative quercetin-3-glucoside by Aspergillus niger. J. Agric. Food Chem. 2010, 58, 10886–10892. [Google Scholar] [CrossRef]
- Juliana, F.; Jacyra, A.S.; Júlia, M.F.; Angela, K.C.; Yamara, A.S.M.; Elizabeth, C.G.; Denise, V.T.; Arnóbio, A.S.; Silvana, M.Z.; Matheus, F. Comparison of two Jatropha species (Euphorbiaceae) used popularly to treat snakebites in Northeastern Brazil: Chemical profile, inhibitory activity against Bothrops erythromelas venom and antibacterial activity. J. Ethnopharmacol. 2018, 213, 12–20. [Google Scholar] [CrossRef]
- Ajayi, E.I.O.; Adeleke, M.A.; Adewumi, T.Y.; Adeyemi, A.A. Antiplasmodial activities of ethanol extracts of Euphorbia hirta whole plant and Vernonia amygdalina leaves in Plasmodium berghei-infected mice. J. Taibah Univ. Sci. 2017, 11, 831–835. [Google Scholar] [CrossRef]
- Ashraf, A.; sarfraz, R.A.; Rashid, M.A.; Shahid, M. Antioxidant, antimicrobial, antitumor, and cytotoxic activities of an important medicinal plant (Euphorbia royleana) from Pakistan. J. Food Drug Anal. 2015, 23, 109–115. [Google Scholar] [CrossRef]
- Boumaza, S.; Bouchenak, O.; Yahiaoui, K.; Toubal, S.; El haddad, D.; Arab, K. Effect of the aqueous extract of Euphorbia guyoniana (euphorbiaceae) on pathogenic bacteria from land-based sources. Appl. Ecol. Environ. Res. 2018, 16, 3767–3781. [Google Scholar] [CrossRef]
- Beatriz, G.; Hélcio, S.; Paulo, N.; Bandeira, T.; Helena, S.; Rodrigues, C.; Matos, F.; Nascimento, G.C.; de Carvalho, R.; Braz-Filhoe, M.R.; et al. Coutinhoa Evaluation of antibacterial and enhancement of antibiotic action by the flavonoid kaempferol 7-O-β-D-(6″-O-cumaroyl)-glucopyranoside isolated from Croton piauhiensis müll. Microb. Pathog. 2020, 143, 104144. [Google Scholar] [CrossRef]
- Ali, R.; Mat Houghton, P.J.; Raman, A.; Hoult, J.R.S. Antimicrobial and anti-inflammatory activities of extracts and constituents of Oroxylum indicum (L.) Vent. Phytomedicine 1998, 5, 375–381. [Google Scholar] [CrossRef]
- Alcaráz, L.E.; Blanco, S.E.; Puig, O.N.; Tomás, F.; Ferretti, F.H. Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains. J. Theor. Biol. 2000, 205, 231–240. [Google Scholar] [CrossRef]
- Edziri, H.; Mastouri, M.; Mahjoub, M.A.; Mighri, Z.; Mahjoub, A.; Verschaeve, L. Antibacterial, antifungal and cytotoxic activities of two flavonoids from Retama raetam flowers. Molecules 2012, 17, 7284–7293. [Google Scholar] [CrossRef]
- Jamil, S. Antimicrobial Flavonoids from Artocarpus anisophyllus Miq and Artocarpus lowii King. J. Teknol. 2014, 71, 95–99. [Google Scholar] [CrossRef]
- Farooq, S.; Wahab, A.T.; Fozing, C.; Rahman, A.U.; Choudhary, M.I. Artonin I inhibits multidrug resistance in Staphylococcus aureus and potentiates the action of inactive antibiotics in vitro. J. Appl. Microbiol. 2014, 117, 996–1011. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss. J. Ethnopharmacol. 2005, 101, 243–248. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Ohemeng, K.A.; Schwender, C.F.; Fu, K.P.; Barrett, J.F. DNA gyrase inhibitory and antibacterial activity of some flavones. Bioorg. Med. Chem. Lett. 1993, 3, 225–230. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]


| No | Compound Structure and Name | Classification | Species Name | Plant Part, Solvent | Biological Effect | Reference |
|---|---|---|---|---|---|---|
| 1. |  quercetin 3-O-β-d-rutinoside | Glucosidic flavonol | E. microsciadia | Aerial, EtOH | Antiproliferative | [45] |
| 2. | 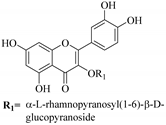 myricetin 3-O-β-d-galactopyranoside | Glucosidic flavonol | E. microsciadia | Aerial, EtOH | Antiproliferative | [45] |
| 3. |  quercetin 3-O-β-d-galactopyranoside | Glucosidic flavonol | E. microsciadia, E. heterophylla | Aerial, EtOH | Antiproliferative | [45,46] |
| 4. |  ampelopsin | Flavonol | E. tirucalli | Whole plant, EtOH | Antibacterial, antifungal | [50] |
| 5. | 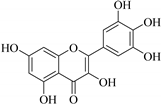 myricetin | Flavonol | E. tirucalli | Whole plant, EtOH | Antibacterial, antifungal | [49] |
| 6. |  hirtacoumaroflavonoside (7-O-(p-coumaroyl)-5,7,4-trihydroxy-6-(3,3-dimethylallyl)-flavonol-3-O-β-d-glucopyr-anosyl-(2″→1″′)-O-α-l-rhamnopyranoside) | Carbohydrate flavonol | E. hirta | Roots, MeOH | Inhibitory activity (α-glucosidase) | [49] |
| 7. |  dimethoxyquercitrin | Glucosidic flavonol | E. hirta | Roots, MeOH | Inhibitory activity (α-glucosidase) | [49] |
| 8. |  2-(3,4-dihydroxy-5-methoxy-phenyl)-3,5-dihydroxy-6,7-dimethoxychromen-4-one | Flavonol | E. neriifolia | EtOH, leaves | Ant-oxidant | [61] |
| 9. |  2′,4,4′-trihydroxychalcone | Chalcone | E. helioscopia | Whole plant, EtOH | Not evaluated | [56] |
| 10. |  3,5,3′-trihydroxy-6,7-dimethoxy-4′ (7”-hydroxygeranyl-1”-ether) flavone | Flavone | E. paralais | Whole plant, MeoH | Not evaluated | [48] |
| 11. |  3,5,7-trihydroxi-2-(3′,4′,5′ trihidroxefenil)-2,3 dihidrobenzopiran-4-ona | Flavonol | E. tirucalli, E. helioscopia | Roots, EtOH | Antimicrobial | [50,56,62] |
| 12. |  3,5,7-trihydroxy-8- methoxyflavone | Flavone | E. lunulata | Aerial, EtOH | [26,47] | |
| 13. |  3,5,7-trihydroxy-2-2(3′, 4′, 5′-trihydroxyphenyl) benzopyran-4-one | Flavonol | E. tirucalli | Roots, EtOH | Antimicrobial | [50,63] |
| 14. | 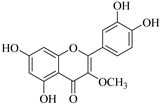 3-O-methylquercetin | Flavonol | E. lunulata | Aerial, EtOH | Antiproliferative | [25,26] |
| 15. |  kaempferol 3-O-rutinoside | Glucosidic flavonol | E. larica, E. virgata, E. mgalanta, E. helioscopia, E. bivonae, E. ebracteolata | Whole plant, EtOH | Not evaluated | [56,64,65,66] |
| 16. | 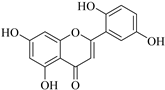 5,7,2′,5′-tetrahydroxyflavone | Flavone | E. lunulata | Aerial, EtOH | Antiproliferative | [25,26] |
| 17. |  hirtaflavonoside-B (5,7,3′,4′ –trihyroxy-6-(3,3 –dimethyl allyl)-8-9-iso-butenyl)-flavonol-3-O-β-d-glucosidase) | Glucosidic flavonol | E. hirta | Roots, MeOH | Inhibitory activity (α-glucosidase) | [49] |
| 18. |  6-methoxyapigenin | Flavone | E. larica, E. lunulata | Aerial, EtOH | Antiproliferative | [29,67] |
| 19. | 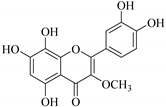 5,7,8,3′,4′-pentahydroxy-3-methoxyflavone | Flavone | E.retusa | Whole plant, MeoH | Not evaluated | [48] |
| 20. |  acacetin | Flavone | E. bivonae | Roots, MeOH | Not evaluated | [66] |
| 21. | 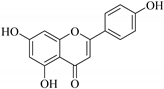 apigenin | Flavone | E. lunulata, E. condylocarpa | Aerial, EtOH | Antiproliferative, antioxidant, anti-tumour, anti-inflammatory, antibacterial, antiproliferative | [25,26,68] |
| 22. | 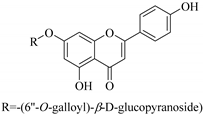 apigenin-7-O-(6′′- O-galloyl)-β-d-glucopyranoside | Glucosidic flavone | E. humifusa | Whole plant, EtOH | Anti-HBV | [58] |
| 23. |  apigenin-7-O-β-d-glucoside | Glucosidic flavone | E. lunulata, E. humifusa | Aerial, EtOH | Anti-HBV | [26,54,58] |
| 24. |  aromadendrin | Flavonol | E. cuneate | Aerial, EtOH | Antiulcerogenic | [69] |
| 25. | 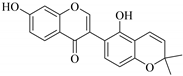 glabrone | Flavone | E. helioscopia | Whole plant, EtOH | Not evaluated | [56] |
| 26. |  hyperoside | Flavone | E. lunulata | Aerial, EtOH | Antiproliferative | [26,70] |
| 27. |  hyperin | Glucosidic flavonol | E. lunulata | Whole plant, C3H6O | Antiproliferative | [60] |
| 28. |  isoquercetin | Glucosidic flavonol | E. lunulata, E. tirucalli, E. ebracteolata | Aerial, EtOH | Antiproliferative | [25,26,50,71] |
| 29. |  jaceosidin | Flavone | E. lunulata | Aerial, EtOH | Antiproliferative | [26,67] |
| 30. |  kaempferol | Flavonol | E. guyoniana, E. allepica, E. charnaesyce, E. rnagalanta, E. virgate, E. lunulata, E. hirta, E. wallichii | Aerial; C3H6O:MeOH, whole plant; Me2CO2, Leaf; EtOH | Not evaluated | [26,29,55,72,73,74] |
| 31. |  kaempferol 3-O-glucoside | Glucosidic flavonol | E. guyoniana, E. rnagalanta, E. charnaesyce, E. virgata | Aerial, C3H6O:MeOH, Leaf; EtOH | Not evaluated | [29,72] |
| 32. |  kaempferol 3-O-β-d-glucopyranoside | Glucosidic flavonol | E. altotibetic, E. retusa | Whole plant | [54] | |
| 33. | 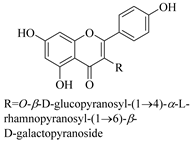 kaempferol 3-O-β-d-glucopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→6)-β-d-galactopyranoside | Carbohydrate flavonol | E. ebracteolata | Aerial, EtOH | Not evaluated | [59] |
| 34. |  isorhamnetin | Flavanone | E. hirta, E. guyoniana, E. charnaesyce, E. rnagalanta, E. amygdaloides | Aerial, C3H6O:MeOH, Leaf; EtOH | Not evaluated | [29,72,75] |
| 35. |  kaempferol-3-O-β-d-glucuronide | Glucosidic flavonol | E. lathyris | Aerial, EtOH | Cytotoxic | [57] |
| 36. |  kaempferol-3-glucuronide | Glucosidic flavonol | E. lathyris | Aerial, MeoH | Not evaluated | [51,57] |
| 37. | 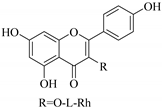 kaempferol-3-l-rhamnoside | Carbohydrate flavonol | E. lunulata | Aerial, EtOH | Antiproliferative | [26,70] |
| 38. |  kaempferol-3-O-(6′′-galloyl)-β-d-glucoside | Carbohydrate flavonol | E. lunulata, E. fischeriana, E. esula | Aerial, EtOH | Antiproliferative | [26,76] |
| 39. |  kaempferol-3-O-α-rhamnoside-O-β-d-glucopyranoside | Carbohydrate flavonol | E. bivonae | Roots, MeOH | Not evaluated | [66] |
| 40. | 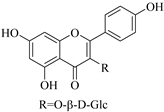 kaempferol-3-O-β-d-glucoside | Glucosidic flavonol | E. lunulata, E. fischeriana, E. esula | Aerial, EtOH | Antiproliferative | [26,76] |
| 41. | 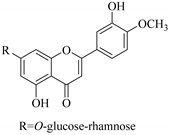 4′-O-methoxy-luteolin-7-O-rhamnoglucoside | Carbohydrate flavonol | E. cuneate | Aerial, EtOH | Antiulcerogenic | [69] |
| 42. | 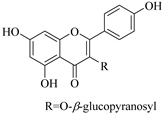 kaempferol-3-β-d glucopyranosyl | Glucosidic flavonol | E.retusa | Whole plant, MeoH | Not evaluated | [48] |
| 43. | 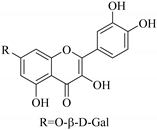 kaempferol-7-O-β-d-glucoside | Glucosidic flavonol | E. lunulata, E. fischeriana, E. esula | Aerial, EtOH | Antiproliferative | [26,76] |
| 44. |  licochalcone A | Chalcone | E. helioscopia | Whole plant, EtOH | Not evaluated | [56] |
| 45. |  licochalcone B | Chalcone | E. helioscopia | Whole plant, EtOH | Not evaluated | [56] |
| 46. |  luteolin | Flavone | E. lunulata, E. hirta, E. humifusa, E. bivonae | Aerial, whole plant, roots, EtOH, | Antiproliferative, Anti-HBV | [25,26,58,66,73] |
| 47. | 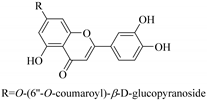 luteolin-7-O-(6′′-O-coumaroyl)-β-d-glucopyranoside | Glucosidic flavonol | E. humifusa | Whole plant, EtOH | Anti-HBV | [58] |
| 48. |  luteolin-7-O-(6′′-O-trans- feruloyl)-β-d-glucopyranoside | Glucosidic flavonol | E. humifusa | Whole plant, EtOH | Anti-HBV | [58] |
| 49. |  luteolin-7-O-β-d-glucopyranoside | Glucosidic flavonol | E. humifusa | Whole plant, EtOH | Anti-HBV | [58] |
| 50. | 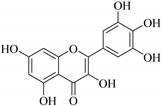 myricetin | Flavonol | E. lunulata, E. wallichii | Aerial, EtOH | Antiproliferative | [25,26,74] |
| 51. |  myricetin-3-O-(2′′,3′′-digalloyl)-β-d-galactopyranoside | Carbohydrate flavone | E. lunulata | Aerial, EtOH | Antiproliferative | [25,26] |
| 52. |  myricetin-3-O-(2′′-galloyl)-β-d-galactopyranoside | Carbohydrate flavone | E. lunulata | Aerial, EtOH | Antiproliferative | [25,26] |
| 53. | 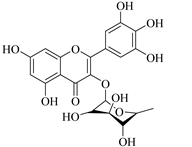 myricitrin | Glucosidic flavone | E. lunulata | Aerial, EtOH | Antiproliferative | [25,26] |
| 54. |  naringenin-7-O-β-d-glucoside | Glucosidic flavone | E. lunulata | Aerial, EtOH | [26,54] | |
| 55. |  quercetin | Flavonol | E. guyoniana, E. stenoclada, E. hirta, E. neriifolia, E. charnaesyce, E. rnagalanta, E. virgate, E. lunulata, E. humifusa, E. helioscopia, E. tirucalli | Aerial, leaves, C3H6O:MeOH, Leaf; EtOH | Antiproliferative, anti-HBV, antidiarrheal, anticancer, antimalarial, antibacterial, antifungal | [26,29,50,53,58,62,67,72,77] |
| 56. |  quercetin 3-O-(2′,3′-digalloyl)-β-d-galactopyranoside | Carbohydrate flavonol | E. lunulata | Whole plant, C3H6O | Antiproliferative | [60] |
| 57. | 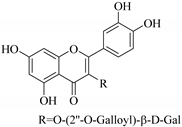 quercetin 3-O-(2′-galloyl)-β-d-galactopyranoside | Carbohydrate flavonol | E. lunulata | Whole plant, C3H6O | Antiproliferative | [60] |
| 58. |  quercetin 3-O-(2″,3″-digalloyl)-β-d-galactopyranoside | Carbohydrate flavonol | E. lunulata | Roots, MeOH | Antiproliferation activity | [78] |
| 59. |  quercetin 3-O-(2″-galloyl)-β-d-galactopyranoside | Carbhydrate flavonol | E. lunulata | Roots, MeOH | Antiproliferation activity | [78] |
| 60. | 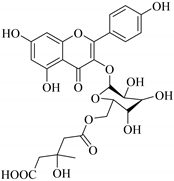 quercetin 3-O-6′′-(3-hydroxyl-3-methylglutaryl)-β-d-glucopyranoside | Glucosidic flavonol | E. ebracteolata | Aerial, EtOH | Not evaluated | [59] |
| 61. |  quercetin 3-O-6′-(3hydroxyl-3-methylglutaryl)-β-d-glucopyranoside | Glucosidic flavonol | E. ebracteolata | Aerial, MeOH | Not evaluated | [78,79] |
| 62. |  quercetin 3-O-glucoside | Glucosidic flavonol | E. guyoniana, E. charnaesyce, E. virgate, E. paralias, E. condylocarpa | Aerial, whole plant, C3H6O:MeOH, Leaf; EtOH | Anticancer against colon, breast, hepato cellular and lung cancer cell lines, inhibitory activities | [29,52,68,72] |
| 63. | 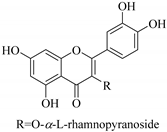 quercetin 3-O-α-l-rhamnopyranoside | Carbohydrate flavonol | E. heterophylla | Aerial, EtOH | Not evaluated | [46] |
| 64. | 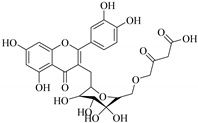 quercetin 3-O-β-d-6′′-malonate | Carbohydrate flavonol | E. heterophylla | Aerial, EtOH | Not evaluated | [46] |
| 65. |  quercetin 3-O-β-d-glucopyranoside | Glucosidic flavonol | E. paralias, E. humifusa, E. microsciadia, E. heterophylla, E. ebracteolata | Whole plant, Aerial, EtOH | Ant-HBV | [45,52,58,71] |
| 66. |  quercetin-3-O-β-glucuronic | Glucosidic flavonol | E. lunulata, E. esula | Aerial, EtOH | Antiproliferative | [26,80] |
| 67. |  quercetin-3-l-rhamnoside | Carbohydrate flavonol | E. lunulata | Aerial, EtOH | Antiproliferative | [26,70] |
| 68. |  quercetin-3-O- (2′′-galloyl)-β-d-galactopyranoside | Glucosidic flavonol | E. lunulata | Aerial, EtOH | Antiproliferative | [26,70] |
| 69. |  quercetin-3-O-(2′′,3′′- digalloyl)-β-d-galactopyranoside | Glucosidic flavonol | E. lunulata | Aerial, EtOH | Antiproliferative | [26,70] |
| 70. |  quercetin-3-O-(6′′-galloyl)-β-d-galactopyranoside | Carbohydrate flavonol | E. lunulata | Aerial, EtOH | [26,54] | |
| 71. |  quercetin-3-O-α-l-rhamnosyl (1→6)-β-d-galactoside | Carbohydrate flavonol | E. humifusa | Whole plant, EtOH | Anti-HBV | [58] |
| 72. |  quercetin-3-O-α-rhamnoside | Carbohydrate flavonol | E. hirta | Whole plant, MeOH | Anti-snake venom activity | [81] |
| 73. | 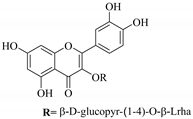 quercetin-3-O-β- quercetin-3-O-β- d-glucopyranosyl- (1-4)-O-α-l-rhamnopyranoside | Carboydrate flavonol | E.drancunculoides | Aerial, EtOH | Cytotoxic | [82] |
| 74. | 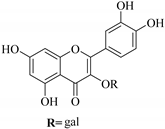 quercetin-3-O-β-d-galactoside | Glucosidic flavonol | E. humifusa, | Whole plant, EtOH, | Anti-HBV, cytotoxic | [58] |
| 75. | 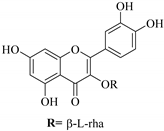 quercetin-3-O-β-l-rhamnoside | Carbohydrate flavonol | E. lunulata, E. fischeriana, E. esula | Aerial, EtOH | Antiproliferative | [26,76] |
| 76. |  quercetin-7-O-β-d-glucoside | Glucosidic flavonol | E. lunulata, E. fischeriana, E. esula | Aerial, EtOH | Antiproliferative | [26,76] |
| 77. |  quercetin 3-O-6′′-(3-hydroxy-3-methylglutaryl)-β-d-glucopyranoside | Glucosidic flavonol | E. ebracteolata | leaves | [83] | |
| 78. |  quercitrin | Glucosidic flavonol | E. stenoclada, E. tirucalli | Aerial, MeOH, EtOH | Antiproliferative, antibacterial, antifungal | [50,53] |
| 79. |  pinocembrin | Flavone | E. hirta | Aerial, EtOH | Not evaluated | [75] |
| 80. |  rhamnetin-3-α-arabinofuranoside | Carbohydrate flavonol | E. lathyris, E-amygdaloides | Aerial, EtOH | Not evaluated | [51,75] |
| 81. |  rutin | Glucosidic flavonol | E. guyoniana, E. charnaesyce, E. rnagalanta, E. virgate, E. tirucalli, E. ebracteolata | Aerial, C3H6O:MeOH, Leaf; EtOH | Antibacterial, antifungal | [29,50,71,72] |
| 82. |  eriodictyol | Flavone | E. matabelensis | Stems, MeOH | Antiproliferative | [84] |
| 83. | 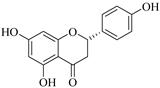 naringenin | Flavone | E. matabelensis | Stems, MeOH | Antiproliferative | [84] |
| 84. |  5,7,3′,4′ -trihyroxy-6-(3,3 –dimethyl allyl)-8-9-iso-butenyl)-flavonol-3-C-β-d-glucosidase | Glucosidic flavonol | E. hirta | Aerial, MeOH | Inhibitory activity (α-glucosidase) | [85] |
| 85. | 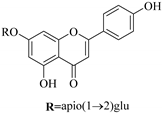 apigenin-7-O-β-d-apiofuranosyl(1→2)-β-d-glucopyranoside | Glucosidic flavonol | E. humifusa | Whole plant, EtOH | Anti-HBV | [58] |
| 86. | 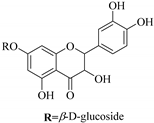 isoaromadendrin-7-O-β-d-glucopyranoside (isosinemsin) | Glucosidic flavonol | E. cuneata | Whole plant, EtOH | Hypertensive | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magozwi, D.K.; Dinala, M.; Mokwana, N.; Siwe-Noundou, X.; Krause, R.W.M.; Sonopo, M.; McGaw, L.J.; Augustyn, W.A.; Tembu, V.J. Flavonoids from the Genus Euphorbia: Isolation, Structure, Pharmacological Activities and Structure–Activity Relationships. Pharmaceuticals 2021, 14, 428. https://doi.org/10.3390/ph14050428
Magozwi DK, Dinala M, Mokwana N, Siwe-Noundou X, Krause RWM, Sonopo M, McGaw LJ, Augustyn WA, Tembu VJ. Flavonoids from the Genus Euphorbia: Isolation, Structure, Pharmacological Activities and Structure–Activity Relationships. Pharmaceuticals. 2021; 14(5):428. https://doi.org/10.3390/ph14050428
Chicago/Turabian StyleMagozwi, Douglas Kemboi, Mmabatho Dinala, Nthabiseng Mokwana, Xavier Siwe-Noundou, Rui W. M. Krause, Molahlehi Sonopo, Lyndy J. McGaw, Wilma A. Augustyn, and Vuyelwa Jacqueline Tembu. 2021. "Flavonoids from the Genus Euphorbia: Isolation, Structure, Pharmacological Activities and Structure–Activity Relationships" Pharmaceuticals 14, no. 5: 428. https://doi.org/10.3390/ph14050428
APA StyleMagozwi, D. K., Dinala, M., Mokwana, N., Siwe-Noundou, X., Krause, R. W. M., Sonopo, M., McGaw, L. J., Augustyn, W. A., & Tembu, V. J. (2021). Flavonoids from the Genus Euphorbia: Isolation, Structure, Pharmacological Activities and Structure–Activity Relationships. Pharmaceuticals, 14(5), 428. https://doi.org/10.3390/ph14050428







