Construction of Recombinant Human GM-CSF and GM-CSF-ApoA-I Fusion Protein and Evaluation of Their Biological Activity
Abstract
1. Introduction
2. Results
2.1. Creation of the Recombinant P. pastoris Strain Capable of Producing Authentic and Chimeric Forms of Human GM-CSF
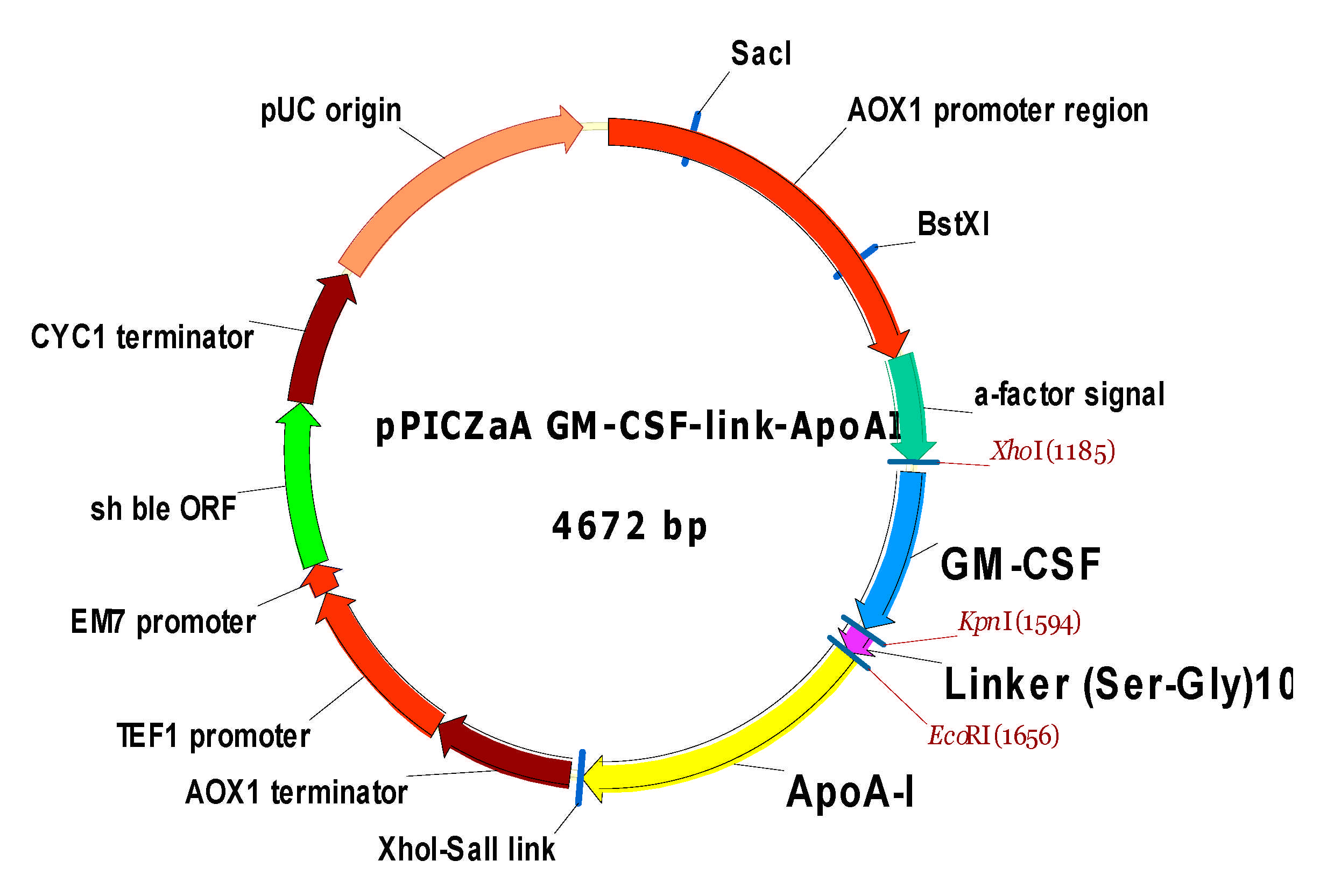
2.1.1. Creation of Recombinant Plasmids Encoding an Authentic and Chimeric Form of Mature Human GM-CSF
2.1.2. Transformation of P. pastoris Cells with Recombinant Plasmids and Screening of Transformants
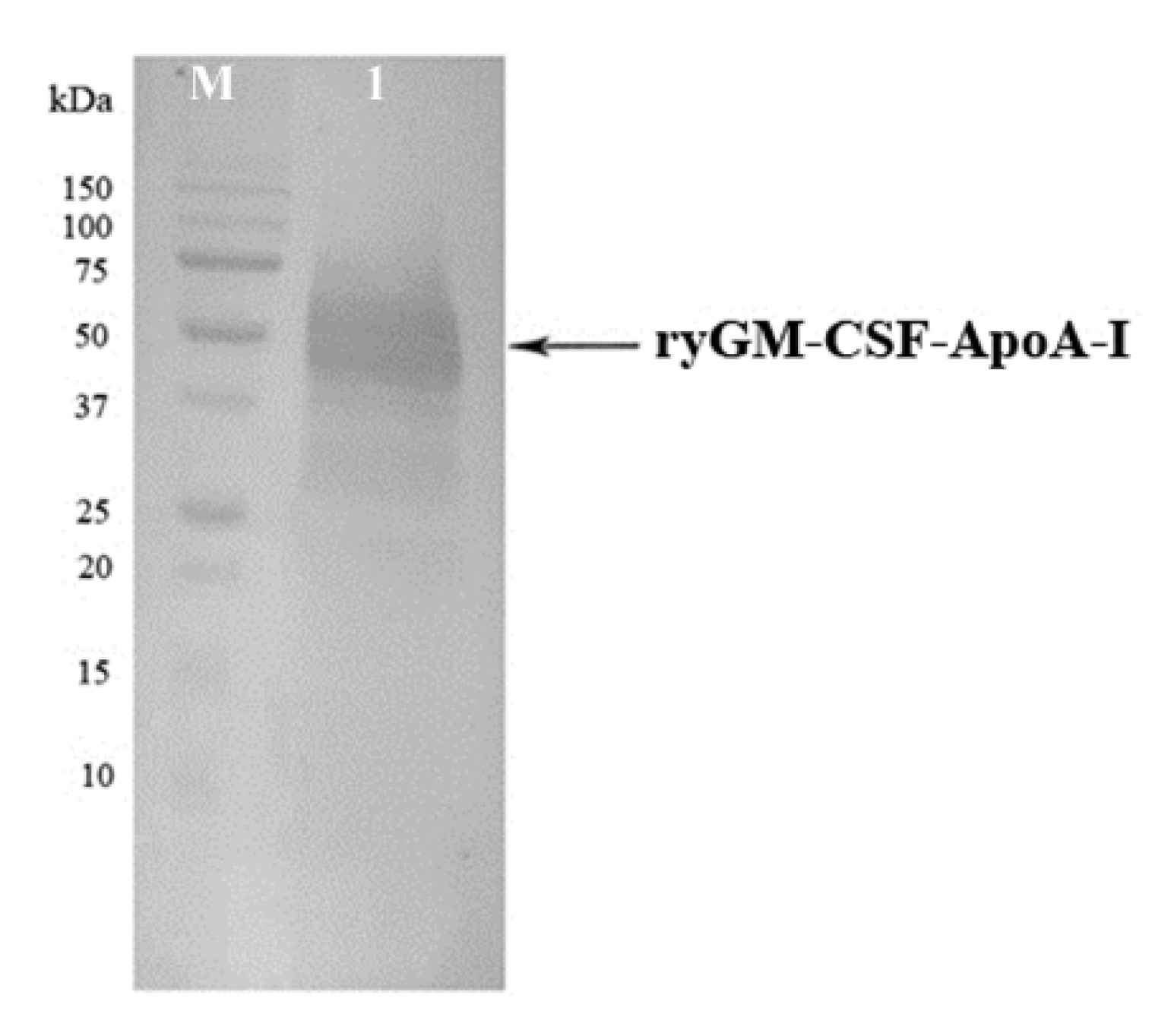
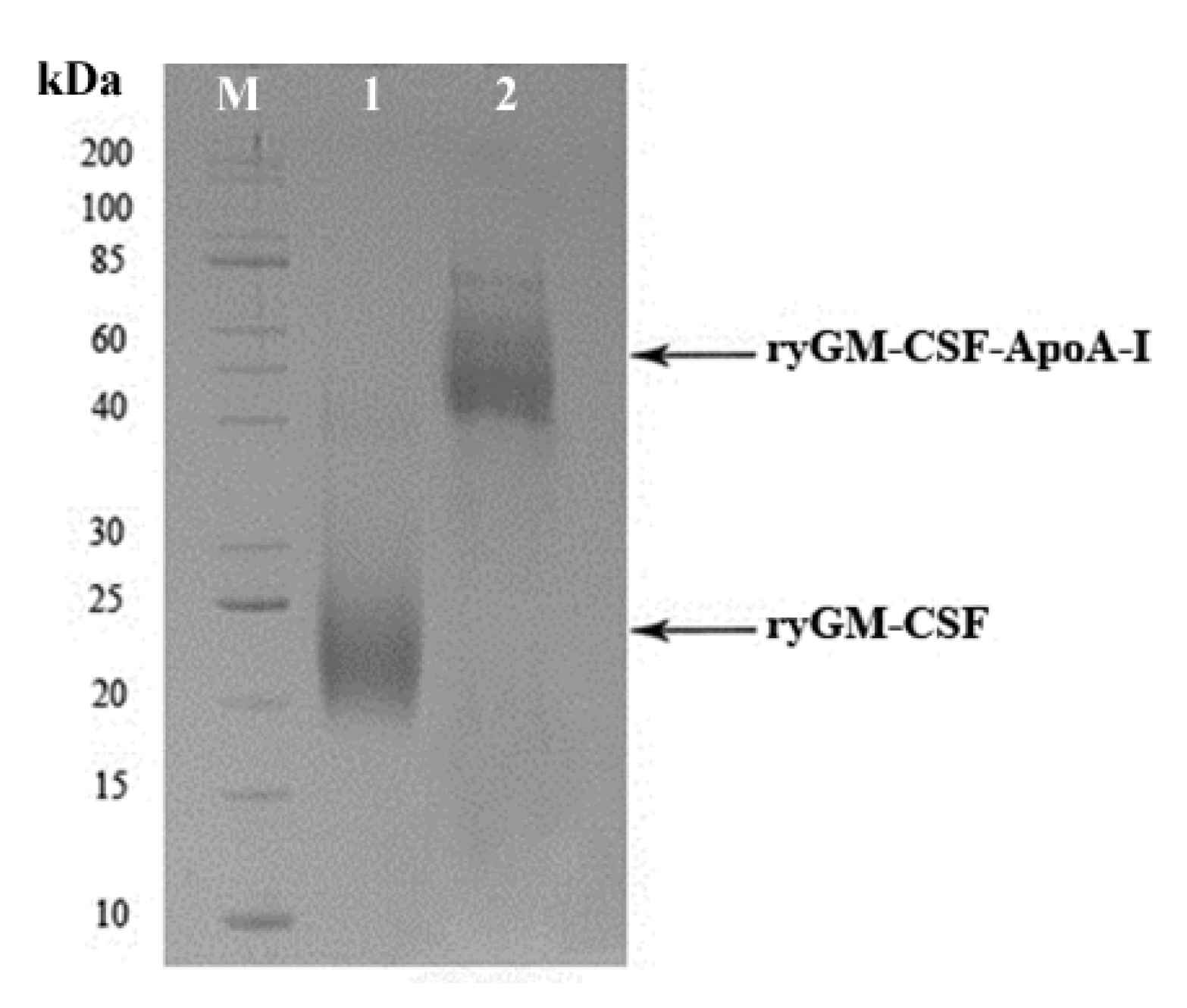
2.1.3. Cultivation of P. pastoris Strains Producing ryGM-CSF and ryGM-CSF-ApoA-I, and Purification of Recombinant Cytokines
2.2. Biological Activity of Recombinant Yeast GM-CSF and GM-CSF-ApoA-I
2.2.1. Erythroid Stimulating Activity of ryGM-CSF and ryGM-CSF-ApoA-I on Erythroleukemia Cells TF-1
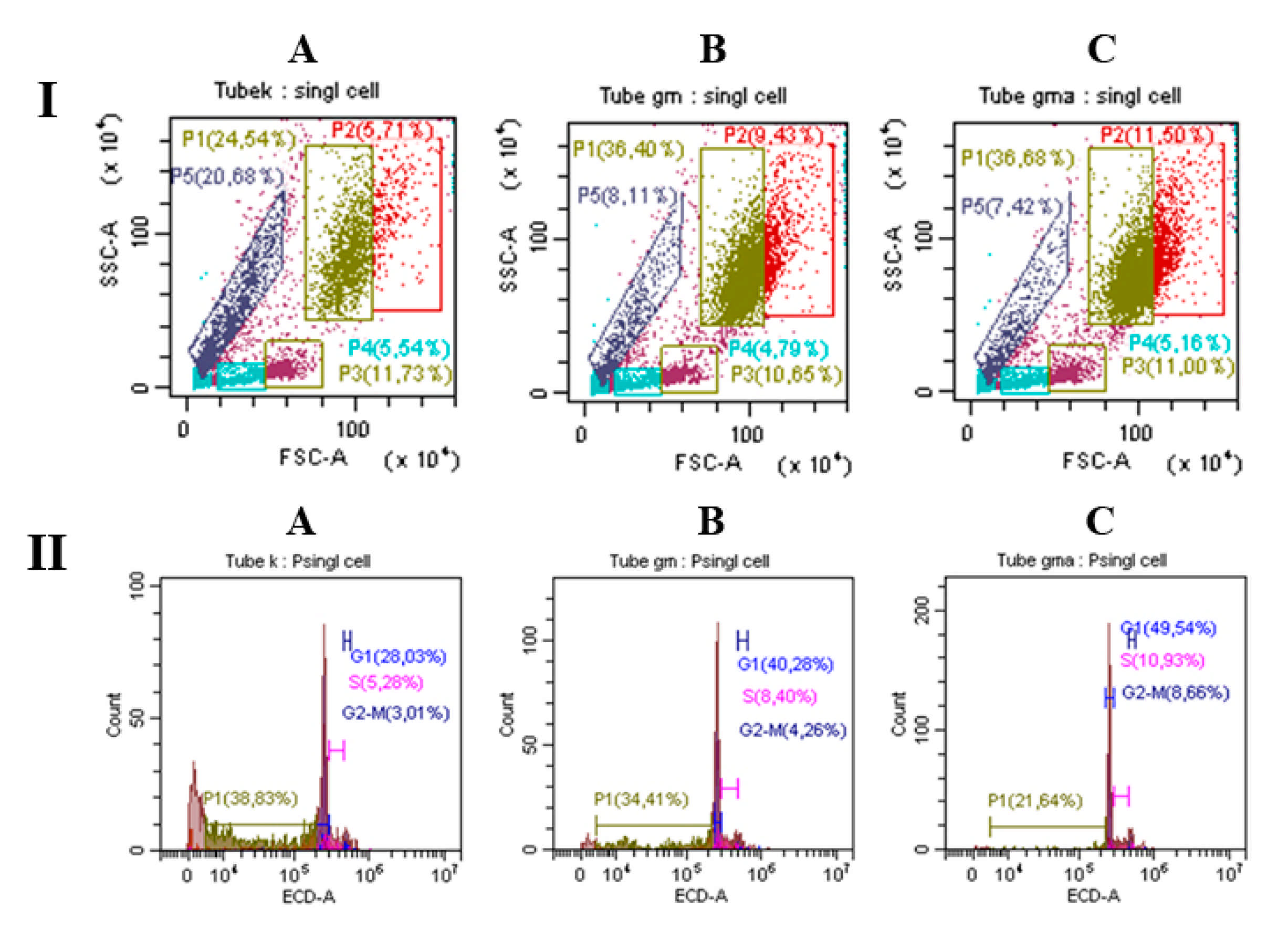
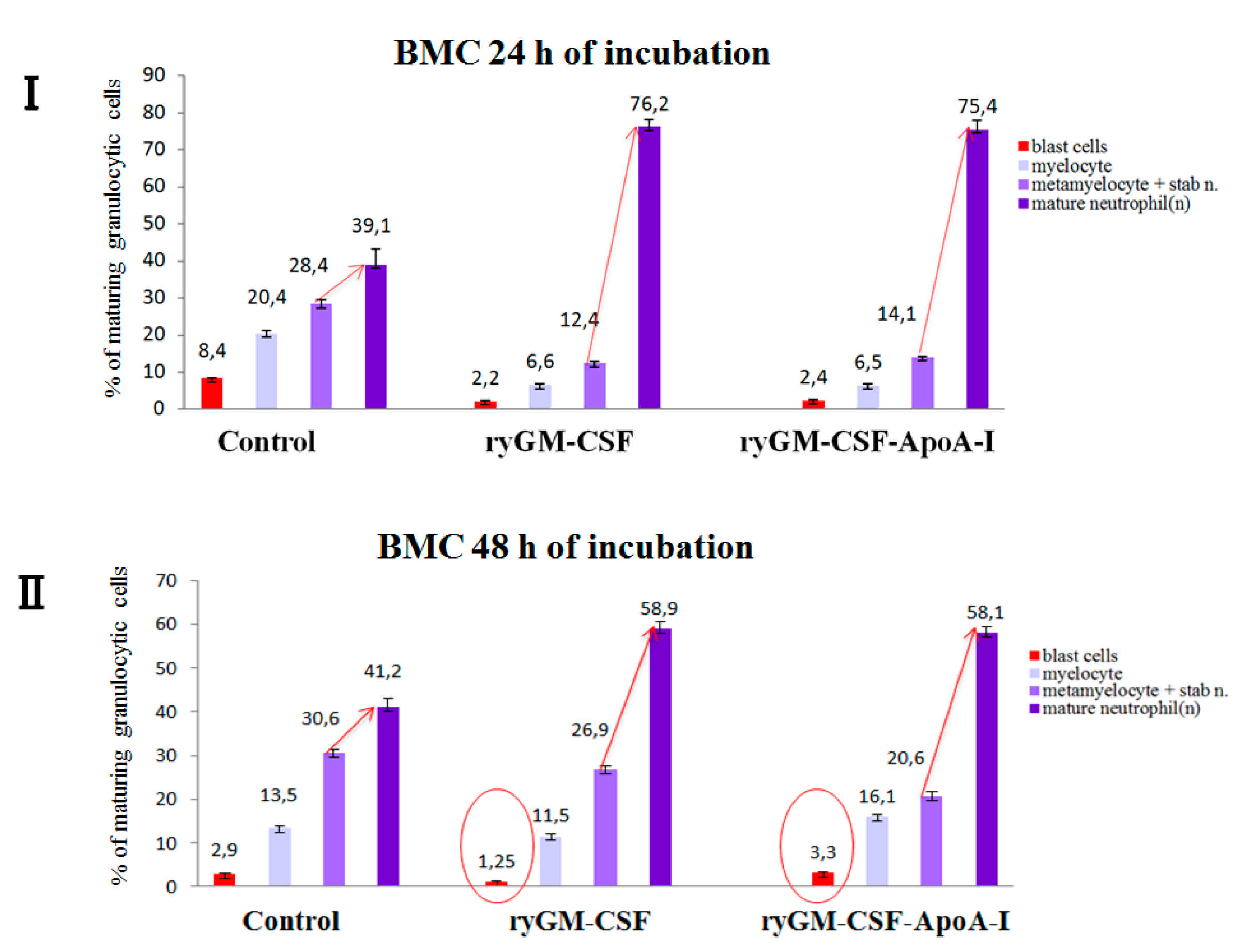
2.2.2. Myeloid-Stimulating Activity of ryGM-CSF and ryGM-CSF-ApoA-I on Human Bone Marrow Cell
Analysis of BMC Using Flow Cytometry
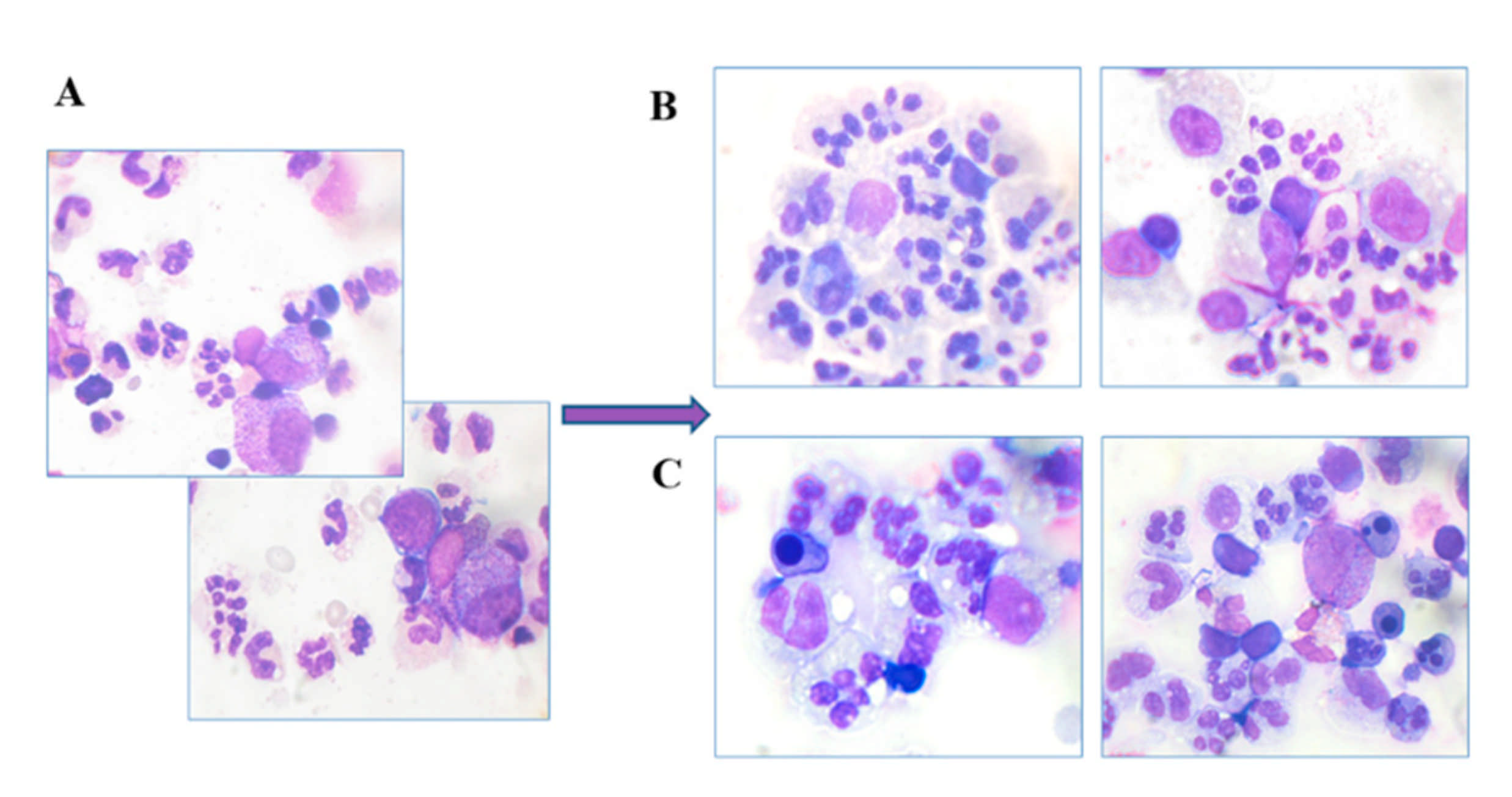
Myelography
3. Discussion
4. Materials and Methods
4.1. Bacterial and Yeast Strains, and the Plasmid Vector
4.2. Creation of the Recombinant P. pastoris Strain Capable of Producing Authentic and Chimeric Forms of Human GM-CSF
4.2.1. Design of a Synthetic Gene Encoding Mature Human GM-CSF
4.2.2. Construction of the pPICZαA/ryGM-CSF Plasmid
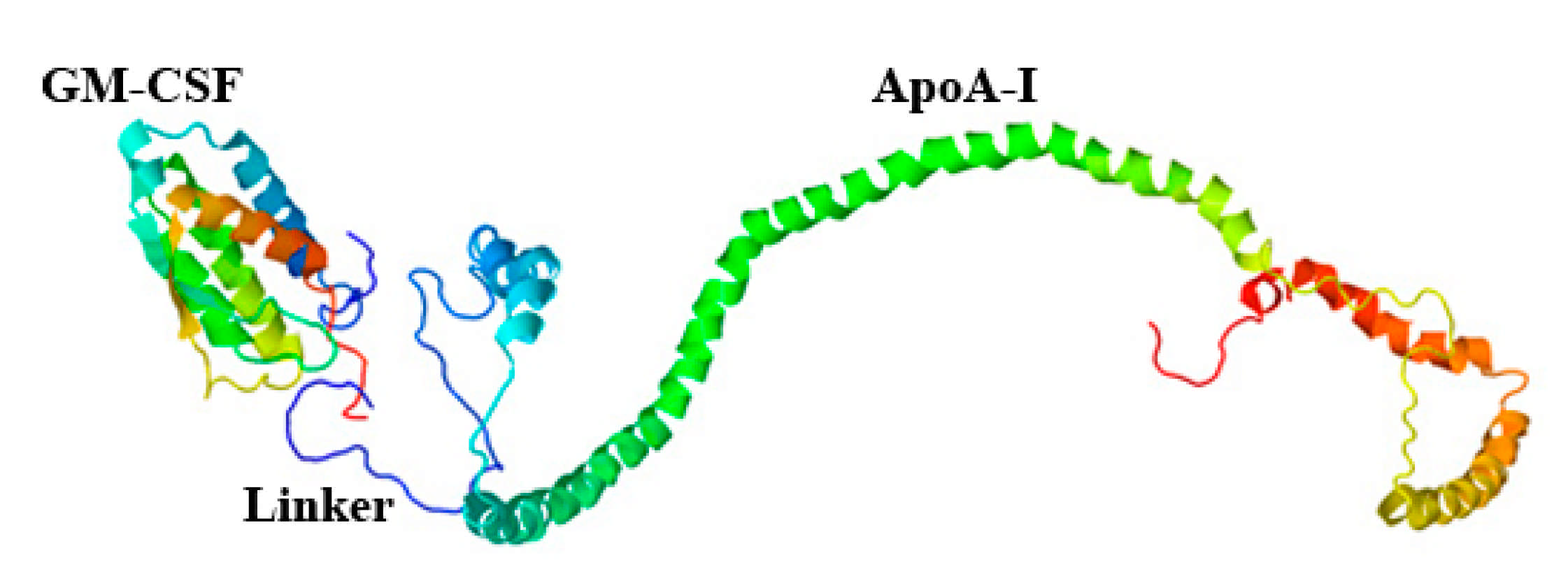
4.2.3. Construction of pPICZαA/ryGM-CSF- link-ApoA-I Plasmid
4.2.4. Transformation of E. coli Cells and Screening of Transformants
4.2.5. Transformation of P. pastoris Cells and the Selection of Recombinant Clones
4.2.6. Growing Yeast Cells Producing Target Proteins
4.3. Chromatographic Purification of Recombinant Cytokines
4.3.1. Purification of ryGM-CSF
4.3.2. Purification of ryGM-CSF-ApoA-I
4.3.3. Determination the Concentration of Recombinant Cytokines
5. Analysis of the Biological Activity of ryGM-CSF and ryGM-CSF-ApoA-I
5.1. XTT Assay
5.2. Myeloid Stimulating Activity of ryGM-CSF and ryGM-CSF-ApoA-I on Human Bone Marrow Cell
5.2.1. Cell Culture
5.2.2. Flow Cytometry
5.2.3. Myelography
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| ApoA-I | Apolipoprotein A-I |
| BMC | Bone Marrow Cells |
| BFU-E | Burst Forming Unit-Erythroid |
| CFU-MK | Colony-Forming Unit-Megakaryocyte |
| SDS-PAGE | Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis |
| LPS | Lipopolysaccharides |
| HAS | Human Serum Albumin |
| PI | Propidium Iodide |
| FBS | Fetal Bovine Serum |
References
- Metcalf, D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature 1989, 339, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Lifton, R.; Bennet, J.M. Clinical use of granulocyte_macrophage colony_stimulating factor and granulocyte colony_stimulating factor in neutropenia associated with malignancy. Hematol. Oncol. Clin. N. Am. 1996, 10, 825–839. [Google Scholar] [CrossRef]
- Gadish, M.; Kletter, Y.; Flidel, O.; Nagler, A.; Slavin, S.; Fabian, I. Effects of recombinant human granulocyte and granulocyte-macrophage colony-stimulating factors on neutrophil function following autologous bone marrow transplantation. Leuk. Res. 1991, 15, 1175–1182. [Google Scholar] [CrossRef]
- Schäbitz, W.R.; Krüger, C.; Pitzer, C.; Weber, D.; Laage, R.; Gassler, N.; Aronowski, J.; Mier, W.; Kirsch, F.; Dittgen, T.; et al. A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF). J. Cereb. Blood Flow Metab. 2008, 28, 29–43. [Google Scholar] [CrossRef]
- Sandy, S.R.; Tan, X.L.; Wright, D.K.; Liu, S.J.; Semple, B.D.; Johnston, L.; Jones, N.C.; Cook, A.D.; Hamilton, J.A.; O’Brien, T.J. Granulocyte-Macrophage Colony-Stimulating Factor Is Neuroprotective in Experimental Traumatic Brain Injury. J. Neurotrauma. 2014, 31, 976–983. [Google Scholar]
- Kiyota, T.; Machhi, J.; Lu, Y.; Dyavarshetty, B.; Nemati, M.; Yokoyama, I.; Mosley, R.L.; Gendelman, H.E. Granulocyte-macrophage colony-stimulating factor neuroprotective activities in Alzheimer’s disease mice. J. Neuroimmunol. 2018, 319, 80–92. [Google Scholar] [CrossRef]
- Yan, W.L.; Shen, K.Y.; Tien, C.Y.; Chen, Y.A.; Liu, S.J. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy 2017, 9, 347–360. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Ruby, C.E.; Hughes, T.; Slingluff, C.L., Jr. Current status of granulocyte–macrophage colony-stimulating factor in the immunotherapy of melanoma. J. Immunother. Cancer 2014, 2, 11. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Thiruppathi, M.; Elshabrawy, H.A.; Alharshawi, K.; Kumar, P.; Prabhakar, B.S. GM-CSF: An Immune Modulatory Cytokine that can Suppress Autoimmunity. Cytokine 2015, 75, 261–271. [Google Scholar] [CrossRef]
- Ganesh, B.B.; Cheatem, D.M.; Sheng, J.R.; Vasu, C.; Prabhakar, B.S. GM-CSF-induced CD11c1CD8a—dendritic cells facilitate Foxp31 and IL-101 regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int. Immunol. 2009, 21, 269–282. [Google Scholar] [CrossRef]
- Yan, D.; Liu, S.; Zhao, X.; Bian, H.; Yao, X.; Xing, J.; Sun, W.; Chen, X. Recombinant human granulocyte macrophage colony stimulating factor in deep second-degree burn wound healing. Medicine 2017, 96, e6881. [Google Scholar] [CrossRef]
- Mann, A.; Niekisch, K.; Schirmacher, P.; Blessing, M. Granulocyte-macrophage colony-stimulating factor is essential for normal wound healing. J. Investig. Dermatol. Symp. Proc. 2006, 11, 87–92. [Google Scholar] [CrossRef]
- Bianchi, L.; Ginebri, A.; Hagman, J.H.; Francesconi, F.; Carboni, I.; Chimenti, S. Local treatment of chronic cutaneous leg ulcers with recombinant human granulocyte-macrophage colony-stimulating factor. J. Eur. Acad. Dermatol. Venereol. 2002, 16, 595–598. [Google Scholar] [CrossRef]
- Fleetwood, A.J.; Cook, A.D.; Hamilton, J.A. Functions of granulocyte-macrophage colony-stimulating factor. Crit. Rev. Immunol. 2005, 25, 405–428. [Google Scholar] [CrossRef]
- Okamoto, M.; Nakai, M.; Nakayama, C.; Yanagi, H.; Matsui, H.; Noguchi, H.; Namiki, M.; Sakai, J.; Kadota, K.; Fukui, M.; et al. Purification and characterization of three forms of differently glycosylated recombinant human granulocyte–macrophage colony-stimulating factor. Arch. Biochem. Biophys. 1991, 286, 562–568. [Google Scholar] [CrossRef]
- Kaushansky, K.; Lopez, J.A.; Brown, C.B. Role of carbohydrate modification in the production and secretion of human granulocyte macrophage colony-stimulating factor in genetically engineered and normal mesenchymal cells. Biochemistry 1992, 31, 1881–1886. [Google Scholar] [CrossRef]
- Cebon, J.; Nicola, N.; Ward, M.; Gardner, I.; Dempsey, P.; Layton, J.; Duhrsen, U.; Burgess, A.W.; Nice, E.; Morstyn, G. Granulocyte–macrophage colony stimulating factor from human lymphocytes. The effect of glycosylation on receptor binding and biological activity. J. Biol. Chem. 1990, 265, 4483–4491. [Google Scholar] [CrossRef]
- Marini, G.; Forno, G.; Kratje, R.; Etcheverrigaray, M. Recombinant human granulocyte-macrophage colony-stimulating factor: Effect of glycosylation on pharmacokinetic parameters. Electron. J. Biotechnol. 2007, 10, 271–278. [Google Scholar] [CrossRef]
- Wadhwa, M.; Bird, C.; Fagerberg, J.; Gaines-Das, R.; Ragnhammar, P.; Mellstedt, H.; Thorpe, R. Production of neutralizing granulocyte-macrophage colony-stimulating factor (GM-CSF) antibodies in carcinoma patients following GM-CSF combination therapy. Clin. Exp. Immunol. 1996, 104, 351–358. [Google Scholar] [CrossRef]
- Dorr, R.T. Clinical properties of yeast-derived versus Escherichia coli-derived granulocyte-macrophage colony-stimulating factor. Clin. Ther. 1993, 15, 19–29. [Google Scholar]
- Ragnhammar, P.; Friesen, H.J.; Frödin, J.E.; Lefvert, A.K.; Hassan, M.; Osterborg, A.; Mellstedt, H. Induction of anti-recombinant human granulocyte-macrophage colony-stimulating factor (Escherichia coli-derived) antibodies and clinical effects in nonimmunocompromised patients. Blood 1994, 84, 4078–4087. [Google Scholar] [CrossRef]
- Ballou, C.E. Isolation, characterization, and properties of Saccharomyces cerevisiae mnn mutants with nonconditional protein glycosylation defects. Methods Enzymol. 1990, 185, 440–470. [Google Scholar]
- Cereghino, J.L.; Cregg, J.M. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 2000, 24, 45–66. [Google Scholar] [CrossRef]
- Becher, B.; Tugues, S.; Greter, M. GM-CSF: From Growth Factor to Central Mediator of Tissue Inflammation. Immunity 2016, 45, 963–973. [Google Scholar] [CrossRef]
- Hovgaard, D.; Mortensen, B.T.; Schifter, S.; Nissen, N.I. Clinical pharmacokinetic studies of a human haemopoietic growth factor, GM-CSF. Eur. J. Clin. Investig. 1992, 22, 45–49. [Google Scholar] [CrossRef]
- Lee, D.L.; Sharif, I.; Kodihalli, S.; Stewart, D.I.; Tsvetnitsky, V. Preparation and characterization of monopegylated human granulocyte-macrophage colony-stimulating factor. J. Interferon Cytokine Res. 2008, 28, 101–112. [Google Scholar] [CrossRef]
- Cox, G.N.; Lee, J.I.; Rosendahl, M.S.; Chlipala, E.; Doherty, D.H. Characterization of a Long-Acting Site-Specific PEGylated Murine GM-CSF Analog and Analysis of Its Hematopoietic Properties in Normal and Cyclophosphamide-Treated Neutropenic Rats. Protein J. 2020, 39, 160–173. [Google Scholar] [CrossRef]
- Veronese, F.M. Peptide and protein PEGylation: A review of problems and solutions. Biomaterials 2001, 22, 405–417. [Google Scholar] [CrossRef]
- Armstrong, J.K. The occurrence, induction, specificity and potential effect of antibodies against poly(ethylene glycol). PEGylated Protein Drugs Basic Sci. Clin. Appl. 2009, 147–168. [Google Scholar] [CrossRef]
- Judge, A.; McClintock, K.; Phelps, J.; MacLachlan, I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol. Ther. 2006, 13, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Baranyi, L.; Savay, S.; Milosevits, J.; Bunger, R.; Laverman, P.; Metselaar, J.M.; Storm, G.; Chanan-Khan, A.; Liebes, L.; et al. Role of complement activation in hypersensitivity reactions to doxil and hynic PEG liposomes: Experimental and clinical studies. J. Liposome Res. 2002, 12, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Curtis, B.M.; Williams, D.E.; Broxmeyer, H.E.; Dunn, J.; Farrah, T.; Jeffery, E.; Clevenger, W.; de Roos, P.; Martin, U.; Friend, D.; et al. Enhanced hematopoietic activity of a human granulocyte/macrophage colony-stimulating factor-interleukin 3 fusion protein. Proc. Natl. Acad. Sci. USA 1991, 88, 5809–5813. [Google Scholar] [CrossRef]
- Antignani, A.; Youle, R.J. The cytokine, granulocyte-macrophage colony-stimulating factor (GM-CSF), can deliver Bcl-XL as an extracellular fusion protein to protect cells from apoptosis and retain differentiation induction. J. Biol. Chem. 2007, 282, 11246–11254. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Galipeau, J. GM-CSF-based fusion cytokines as ligands for immune modulation. J. Immunol. 2011, 186, 5527–5532. [Google Scholar] [CrossRef]
- Heinzelman, P.; Priebe, M.C. Engineering superactive granulocyte macrophage colony-stimulating factor transferrin fusion proteins as orally-delivered candidate agents for treating neurodegenerative disease. Biotechnol. Prog. 2015, 31, 668–677. [Google Scholar] [CrossRef]
- Chuang, Y.M.; He, L.; Pinn, M.; Tsai, Y.C.; Cheng, M.A.; Farmer, E.; Karakousis, P.C.; Hung, C.F. Albumin fusion with granulocyte-macrophage colony-stimulating factor acts as an immunotherapy against chronic tuberculosis. Cell. Mol. Immunol. 2020. [Google Scholar] [CrossRef]
- Assmann, G.; Nofer, J.R. Atheroprotective effects of high-density lipoproteins. Annu. Rev. Med. 2003, 54, 321–341. [Google Scholar] [CrossRef]
- Suc, I.; Escargueil-Blanc, I.; Troly, M.; Salvayre, R.; Negre-Salvayre, A. HDL and apoA prevent cell death of endothelial cells induced by oxidized LDL. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2158–2166. [Google Scholar] [CrossRef]
- Mineo, C.; Deguchi, H.; Griffin, J.H.; Shaul, P.W. Endothelial and antithrombotic actions of HDL. Circ. Res. 2006, 98, 1352–1364. [Google Scholar] [CrossRef]
- Garner, B.; Waldeck, A.R.; Witting, P.K.; Rye, K.A.; Stocker, R. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J. Biol. Chem. 1998, 273, 6088–6095. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X. Prognostic Significance of Pretreatment Apolipoprotein A-I as a Noninvasive Biomarker in Cancer Survivors: A Meta-Analysis. Dis Markers 2018, 2018, 1034037. [Google Scholar] [CrossRef] [PubMed]
- Morin, E.E.; Guo, L.; Schwendeman, A.; Li, X.A. HDL in sepsis—Risk factor and therapeutic approach. Front. Pharmacol. 2015, 6, 244. [Google Scholar] [CrossRef]
- Poynard, T.; Deckmyn, O.; Rudler, M.; Peta, V.; Ngo, Y.; Vautier, M.; Akhavan, S.; Calvez, V.; Franc, C.; Castille, J.M.; et al. Performance of serum apolipoprotein-A1 as a sentinel of Covid-19. PLoS ONE 2020, 15, e0242306. [Google Scholar] [CrossRef]
- Hilser, J.R.; Han, Y.; Biswas, S.; Gukasyan, J.; Cai, Z.; Zhu, R.; Tang, W.H.W.; Deb, A.; Lusis, A.J.; Hartiala, J.A.; et al. Association of serum HDL-cholesterol and apolipoprotein A1 levels with risk of severe SARS-CoV-2 infection. J. Lipid Res. 2021, 62, 100061. [Google Scholar] [CrossRef]
- De Nardo, D.; Labzin, L.I.; Kono, H.; Seki, R.; Schmidt, S.V.; Beyer, M.; Xu, D.; Zimmer, S.; Lahrmann, C.; Schildberg, F.A.; et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 2014, 15, 152–160. [Google Scholar] [CrossRef]
- Hyka, N.; Dayer, J.M.; Modoux, C.; Kohno, T.; Edwards, C.K.; Roux-Lombard, P.; Burger, D. Apolipoprotein A-I inhibits the production of interleukin-1 and tumor necrosis factor- by blocking contact-mediated activation of monocytes by T lymphocytes. Blood 2001, 97, 2381–2389. [Google Scholar] [CrossRef]
- Copaescu, A.; Smibert, O.; Gibson, A.; Phillips, E.J.; Trubiano, J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J. Allergy Clin. Immunol. 2020, 146, 518–534.e1. [Google Scholar] [CrossRef]
- Acton, S.; Rigotti, A.; Landschulz, K.T.; Xu, S.; Hobbs, H.H.; Krieger, M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996, 271, 518–520. [Google Scholar] [CrossRef]
- Zhu, R.Y.; Xin, X.; Dai, H.Y.; Li, Q.; Lei, J.Y.; Chen, Y.; Jin, J. Expression and purification of recombinant human serum albumin fusion protein with VEGF165b in Pichia pastoris. Protein Expr. Purif. 2012, 85, 32–37. [Google Scholar] [CrossRef]
- Zhao, H.L.; Xue, C.; Wang, Y.; Li, X.Y.; Xiong, X.H.; Yao, X.Q.; Liu, Z.M. Circumventing the heterogeneity and instability of human serum albumin- interferonalpha2b fusion protein by altering its orientation. J. Biotechnol. 2007, 131, 245–252. [Google Scholar] [CrossRef]
- Naseem, M.U.; Ahmed, N.; Khan, M.A.; Tahir, S.; Zafar, A.U. Production of potent long-lasting consensus interferon using albumin fusion technology in Pichia pastoris expression system. Protein Expr. Purif. 2020, 166, 105509. [Google Scholar] [CrossRef] [PubMed]
- Pykhtina, M.B.; Romanov, V.P.; Miroshnichenko, S.M.; Beklemishev, A.B. Construction of a Pichia pastoris strain efficiently producing recombinant human granulocyte-colony stimulating factor (rhG-CSF) and study of its biological activity on bone marrow cells. Mol. Biol. Rep. 2020, 47, 607–620. [Google Scholar] [CrossRef]
- Toporkova, L.B.; Orlovskaya, I.A.; Sennikov, S.V.; Sakhno, L.V.; Kozlova, Y.N.; Lebedev, L.R.; Gileva, I.P. In vitro study of the biological properties of the recombinant granulocyte-macrophage colony-stimulating factor. Immunology 2009, 4, 203–205. [Google Scholar]
- Bae, C.S.; Yang, D.S.; Lee, J.; Park, Y.-H. Improved process for production of recombinant yeast-derived monomeric humanG-CSF. Appl. Microbiol. Biotechnol. 1999, 52, 338–344. [Google Scholar] [CrossRef]
- Bahrami, A.; Shojaosadati, S.A.; Khalilzadeh, R.; Mohammadian, J.; Farahani, E.V.; Masoumian, M.R. Prevention of human granulocyte colony-stimulating factor protein aggregation in recombinant Pichia pastoris fed-batch fermentation using additives. Biotechnol. Appl. Biochem. 2009, 52, 141–148. [Google Scholar] [CrossRef]
- Bretthauer, R.K.; Castellino, F.J. Glycosylation of Pichia pastoris-derived proteins. Biotechnol. Appl. Biochem. 1999, 30, 193–200. [Google Scholar]
- Donahue, R.E.; Emerson, S.G.; Wang, E.A.; Wong, G.G.; Clark, S.C.; Nathan, D.G. Demonstration of burst-promoting activity of recombinant human GM-CSF on circulating erythroid progenitors using an assay involving the delayed addition of erythropoietin. Blood 1985, 66, 1479–1481. [Google Scholar] [CrossRef]
- Usynin, I.F.; Dudarev, A.N.; Gorodetskaya, A.Y.; Miroshnichenko, S.M.; Tkachenko, T.A.; Tkachenko, V.I. Apolipoprotein A-I Stimulates Cell Proliferation in Bone Marrow Cell Culture. Bull. Exp. Biol. Med. 2018, 164, 308–311. [Google Scholar] [CrossRef]
- Miroshnichenko, S.; Usynin, I.; Dudarev, A.; Nimaev, V.; Solovieva, A. Apolipoprotein A-I Supports MSCs Survival under Stress Conditions. Int. J. Mol. Sci. 2020, 21, 4062. [Google Scholar] [CrossRef]
- Fioravanti, J.; González, I.; Medina-Echeverz, J.; Larrea, E.; Ardaiz, N.; González- Aseguinolaza, G.; Prieto, J.; Berraondo, P. Anchoring interferon alpha to apolipoprotein A-I reduces hematological toxicity while enhancing immunostimulatory properties. Hepatology 2011, 53, 1864–1873. [Google Scholar] [CrossRef]
- Trapnell, B.C.; Nakata, K.; Bonella, F.; Campo, I.; Griese, M.; Hamilton, J.; Wang, T.; Morgan, C.; Cottin, V.; McCarthy, C. Pulmonary alveolar proteinosis. Nat. Rev. Dis. Primers. 2019, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.M.; Figueroa, D.M.; Barochia, A.V.; Yao, X.; Levine, S.J. High-density Lipoproteins and Apolipoprotein A-I: Potential New Players in the Prevention and Treatment of Lung Disease. Front. Pharmacol. 2016, 7, 323. [Google Scholar] [CrossRef] [PubMed]
- Fessler, M.B. A New Frontier in Immunometabolism. Cholesterol in Lung Health and Disease. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. 5), S399–S405. [Google Scholar] [CrossRef] [PubMed]
- VanLenten, B.J.; Wagner, A.C.; Navab, M.; Anantharamaiah, G.M.; Hui, E.K.; Nayak, D.P.; Fogelman, A.M. D-4F, an apolipoprotein A-I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human type II pneumocytes. Circulation 2004, 110, 3252–3258. [Google Scholar] [CrossRef]
- Sharma, S.; Umar, S.; Potus, F.; Iorga, A.; Wong, G.; Meriwether, D.; Breuils-Bonnet, S.; Mai, D.; Navab, K.; Ross, D.; et al. Apolipoprotein A-I mimetic peptide 4F rescues pulmonary hypertension by inducing microRNA-193-3p. Circulation 2014, 130, 776–785. [Google Scholar] [CrossRef]
- Nandedkar, S.D.; Weihrauch, D.; Xu, H.; Shi, Y.; Feroah, T.; Hutchins, W.; Rickaby, D.A.; Duzgunes, N.; Hillery, C.A.; Konduri, K.S.; et al. D-4F, an apoA-1 mimetic, decreases airway hyperresponsiveness, inflammation, and oxidative stress in a murine model of asthma. J. Lipid. Res. 2011, 52, 499–508. [Google Scholar] [CrossRef]
- Jiao, Y.L.; Wu, M.P. Apolipoprotein A-I diminishes acute lung injury and sepsis in mice induced by lipoteichoic acid. Cytokine 2008, 43, 83–87. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pykhtina, M.; Miroshnichenko, S.; Romanov, V.; Grazhdantseva, A.; Kochneva, G.; Beklemishev, A. Construction of Recombinant Human GM-CSF and GM-CSF-ApoA-I Fusion Protein and Evaluation of Their Biological Activity. Pharmaceuticals 2021, 14, 459. https://doi.org/10.3390/ph14050459
Pykhtina M, Miroshnichenko S, Romanov V, Grazhdantseva A, Kochneva G, Beklemishev A. Construction of Recombinant Human GM-CSF and GM-CSF-ApoA-I Fusion Protein and Evaluation of Their Biological Activity. Pharmaceuticals. 2021; 14(5):459. https://doi.org/10.3390/ph14050459
Chicago/Turabian StylePykhtina, Mariya, Svetlana Miroshnichenko, Vladimir Romanov, Antonina Grazhdantseva, Galina Kochneva, and Anatoly Beklemishev. 2021. "Construction of Recombinant Human GM-CSF and GM-CSF-ApoA-I Fusion Protein and Evaluation of Their Biological Activity" Pharmaceuticals 14, no. 5: 459. https://doi.org/10.3390/ph14050459
APA StylePykhtina, M., Miroshnichenko, S., Romanov, V., Grazhdantseva, A., Kochneva, G., & Beklemishev, A. (2021). Construction of Recombinant Human GM-CSF and GM-CSF-ApoA-I Fusion Protein and Evaluation of Their Biological Activity. Pharmaceuticals, 14(5), 459. https://doi.org/10.3390/ph14050459





