Abstract
The present study aims at comparing the change in cytokine levels in schizophrenia patients treated with atypical antipsychotics, with or without metabolic syndrome (MetS). The study included 101 patients with schizophrenia, 38 with and 63 without MetS, who received risperidone, quetiapine, olanzapine or aripiprazole for six weeks. We analyzed the concentration of 21 cytokines in the serum patients. The treatment with atypical antipsychotics changed some proinflammatory cytokine levels. It led to increased IFN-α2 (p = 0.010), IL-1α (p = 0.024) and IL-7 (p = 0.017) levels in patients with MetS, whereas the same treatment led to decreased levels of IFN-γ (p = 0.011), IL-1β (p = 0.035), IL-12р40 (p = 0.011), IL-17A (p = 0.031), IL-6 (p = 0.043) and TNF-α (p = 0.012) in individuals without MetS. Our results demonstrated the effects of atypical antipsychotics on the immune–inflammatory parameters, depending on the metabolic disturbances in schizophrenia patients.
1. Introduction
Immunoinflammation plays an important role in the pathogenetic mechanisms of schizophrenia, as evidenced by the activation of microglia demonstrated in neuroimaging studies, which was followed by the formation of inflammatory mediators [1,2]. These modulators affect neighboring astrocytes and neurons, making a significant contribution to the homeostatic regulation of brain tissue [3,4]. In some patients, an acute psychotic episode is associated with mild systemic inflammation, which is reflected by increased concentrations of cytokines and other inflammatory markers in the peripheral blood. It is assumed that inflammation is not only a consequence of schizophrenia but may also be a risk factor for its development [5]. In addition, the immunomodulatory and anti-inflammatory effects of antipsychotics support the role of inflammation in schizophrenia [6,7,8]. However, the effects of individual psychotropic agents on the immune system and how this might contribute to their effectiveness largely remains unclear [9].
Various studies have tried to assess the effects of adding anti-inflammatory drugs (aspirin, celecoxib, minocycline, acetylcysteine, omega-3 fatty acids, cytokines and interferon (IFN)-γ) to antipsychotic therapy; however, most of these studies lack sufficient methodological rigidity [10,11]. Further developing this type of treatment of patients with schizophrenia requires not only research clarifying how the anti-inflammatory effects of these treatments affect the symptoms of schizophrenia themselves but, also, how they interact with the pharmacological effects of antipsychotics [12]. Besides, the unique mechanisms by which proinflammatory cytokines are involved in the etiopathology of schizophrenia should be investigated [13]. Exploring how systemic factors such as metabolic syndrome (MetS) are involved should also be taken into account.
MetS has a high prevalence in patients with schizophrenia due to predisposition, as well as due to the long-term use of antipsychotics [14]. The incidence of MetS in patients with schizophrenia taking antipsychotics varies from 28% [15] to 46% [16] and occurs already during the first psychotic episode, whereas its frequency subsequently depends on the duration of the disease [17].
There is evidence that patients with schizophrenia have a metabolic predisposition to diabetes that is exacerbated by obesity, leading to cardiovascular disease and other comorbidities. In turn, the influence of the inflammatory mediators on the brain not only contributes to the development of schizophrenia but, also, to the deterioration of health that accompanies this disease [18].
It was previously shown that the levels of inflammation markers are higher in patients with schizophrenia and MetS compared with the patients without it [19], and the levels of C-reactive protein, interleukin (IL)-6, leptin, IFN-γ and tumor necrosis factor (TNF)-α can serve as prognostic factors for the development of metabolic syndrome in patients with schizophrenia during treatment with antipsychotics [20,21].
Second-generation antipsychotics (SGA) are the most frequently used medications in the treatment of patients with schizophrenia in recent years. The spectrum of atypicality ranges from risperidone as the least atypical, followed by aripiprazole, amisulpride, lurasidone, ziprasidone, asenapine, quetiapine and olanzapine to clozapine, which is the most atypical of all antipsychotics [22]. They are more complex dopamine D2 receptor antagonists and involve other receptor targets that regulate dopamine and other neurotransmitters. Accordingly, atypical antipsychotics cause fewer movement side effects that are associated with a strong blockade of D2 receptors [22].
It is assumed that antipsychotic treatment changes the patterns of gene expression in adipocytes and leads them to an inflammatory state, which is related to metabolic disorders in patients [23]. Thus, the ex vivo stimulation of primary mononuclear cells of peripheral blood from healthy donors with both olanzapine and aripiprazole reduced the mRNA levels of IL-1β, IL-6 and TNF-α and led to a decrease in the concentrations of the IL-6 and TNF-α proteins. The multiplex approach revealed the additional suppression of IL-2 and secretion of the macrophage inflammatory protein MIP-1β and interferon gamma-induced protein 10 (IP-10). Similarly, the stimulation of the transformed human monocytic leukemia cell line THP-1 with olanzapine and aripiprazole resulted in a significant decrease in the expression and secretion of IL-1β and TNF-α [24]. Schizophrenia patients with and without concomitant MetS treated with olanzapine or clozapine had significantly higher plasma IL-6, IL-10 and TNF-α levels compared to the normal controls, but markedly lower plasma silencing information regulator 2-related enzyme 1 (SIRT1) levels and higher plasma IL-6 levels were observed in patients with MetS compared to patients without MetS [25].
In another study, after 24 weeks of treatment with paliperidone, a significant increase in the waist circumference was found, which positively correlated with a change in the number of leukocytes [26]. Additionally, not only the long-term antipsychotic treatment of patients with schizophrenia leads to metabolic disorders; the use of atypical antipsychotics even for 6 weeks of therapy in patients without MetS contributes to a significant increase in visceral obesity, an increase in glucose levels and dyslipidemia [27], and after 6 months of treatment, the percentage of patients with schizophrenia and MetS increases from 5% to 27% [28].
The present study aims at comparing the change in the cytokine levels in schizophrenia patients treated with atypical antipsychotics, with or without MetS. We hypothesized that atypical antipsychotics have different effects on the serum cytokine levels in schizophrenic patients with and without MetS.
2. Results and Discussion
2.1. Baseline Characteristics of Study Participants
The demographics and disease characteristics, as well as the antipsychotic therapy of the study population, are summarized in Table 1. A total of 101 patients with schizophrenia who received SGA were enrolled in the study: 38 of them (37.6%) had MetS and 63 (62.4%) did not. Most patients were taking risperidone (43, 42.6%), quetiapine (22, 21.8%), olanzapine (14, 13.9%) or aripiprazole (11, 10.9%) as their baseline antipsychotic therapy. In the remaining cases, the patients received other SGA, but none received clozapine.

Table 1.
Sociodemographic, clinical and pharmacological characteristics of patients with schizophrenia, depending on the presence of MetS.
2.2. Cytokine Levels Depending on the Presence of MetS in Patients with Schizophrenia Receiving SGA
The treatment with atypical antipsychotics led to increased IFN-α2 (p = 0.010), IL-1α (p = 0.024) and IL-7 (p = 0.017) levels in patients with MetS, whereas the same treatment led to decreased levels of IFN-γ (p = 0.011), IL-1β (p = 0.035), IL-12р40 (p = 0.011), IL-17A (p = 0.031), IL-6 (p = 0.043) and TNF-α (p = 0.012) in individuals without MetS (Table 2).

Table 2.
Serum concentrations of the cytokines (pg/mL) in patients with and without MetS during 6 weeks of SGA therapy, Me (Q1; Q3).
When assessing the percentage of changes in the cytokines levels between patients with MetS and without it, statistically significant differences in the IFN-α2 (p = 0.028), IFN-γ (p = 0.007), IL-12p40 (p = 0.007), IL-17A (p = 0.009), IL-1α (p = 0.011), IL-1β (p = 0.008), IL-7 (p = 0.004) and TNF-α (p = 0.031) level were found (Figure 1).
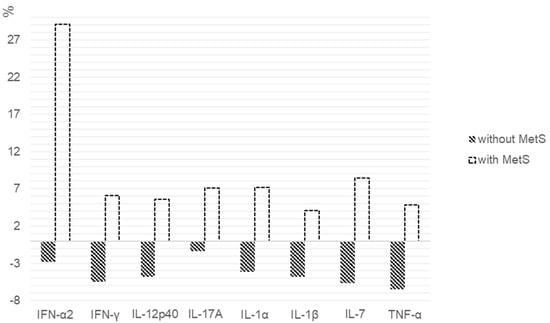
Figure 1.
Percentage change in the cytokine levels after 6 weeks of SGA therapy in patients with and without MetS. We calculated the individual percentage changes in the indicator for each patient separately using the formula: ((V2 − V1)/V1) × 100, where V1 is the initial value before therapy, and V2 is the final value after therapy. The data in the figure are presented as the median of the changes in the indicators. The figure shows only the statistically significant differences according to the Mann–Whitney U test.
2.3. Cytokine Levels in Patients with Schizophrenia Depending on the Presence of MetS When Receiving Risperidone
The next stage of our study was to evaluate the effect of 6 weeks of risperidone treatment on the serum cytokine levels in patients with schizophrenia. A total 14 participants who take risperidone had a MetS, and 29 did not. In patients with MetS, 6 weeks of risperidone therapy led to increased IFN-α2 (p = 0.008), IL-1β (p = 0.041) and IL-7 (p = 0.046) levels. We did not find any changes in the cytokine levels in the individuals without MetS who received risperidone for 6 weeks (Table 3).

Table 3.
Serum concentrations of the cytokines (pg/mL) in patients with and without MetS during 6 weeks of risperidone therapy, Me (Q1; Q3).
The percentage changes in the cytokines levels, as shown in Figure 2. We found statistically significant differences in the IFN-α2 (p = 0.047), IL-1α (p = 0.017), IL-1β (p = 0.017) and IL-7 (p = 0.047) levels between the patients with and without MetS.
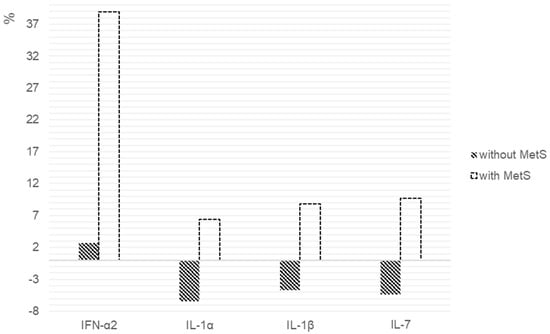
Figure 2.
Percentage changes in cytokine levels after 6 weeks of risperidone therapy in patients with and without MetS. We calculated the individual percentage changes in the indicators for each patient separately using the formula: ((V2 − V1)/V1) × 100, where V1 is the initial value before therapy, and V2 is the final value after therapy. The data in the figure are presented as the median of the changes in the indicators. The figure shows only the statistically significant differences according to the Mann–Whitney U test.
2.4. Cytokine Levels in Patients with Schizophrenia Depending on the Presence of MetS When Receiving Quetiapine or Olanzapine
Since quetiapine and olanzapine have approximately the same effect on the metabolic parameters [29], we pooled patients who received these drugs into one group to increase the sample size. In patients with MetS, 6 weeks of therapy led to an increased IFN-α2 (p = 0.050) level and, in patients without MetS, to decreased IL-6 (p = 0.041) and IL-12p40 (p = 0.050) (Table 4).

Table 4.
Serum concentrations of the cytokines (pg/mL) in patients with and without MetS during 6 weeks of quetiapine or olanzapine therapy, Me (Q1; Q3).
When assessing the percentages of the changes in cytokine levels between patients with MetS and without it, statistically significant differences in the IFN-α2 (p = 0.034), IFN-γ (p = 0.037), IL-12p40 (p = 0.011), IL-12p70 (p = 0.003), IL-17A (p = 0.002), IL-1β (p = 0.034) and IL-7 (p = 0.001) levels were found (Figure 3).
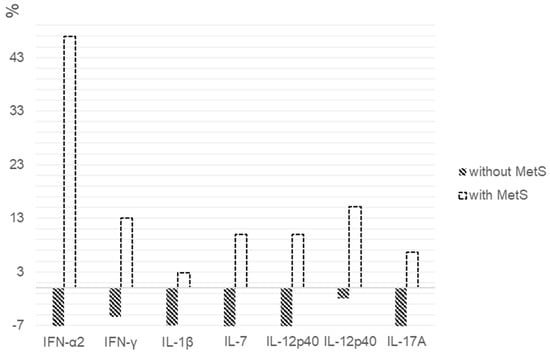
Figure 3.
Percentage changes in cytokine levels after 6 weeks of quetiapine or olanzapine therapy in patients with and without MetS. We calculated the individual percentage changes in the indicators for each patient separately using the formula: ((V2 − V1)/V1) × 100, where V1 is the initial value before therapy, and V2 is the final value after therapy. The data in the figure are presented as the median of the changes in the indicators. The figure shows only the statistically significant differences according to the Mann–Whitney U test.
2.5. Cytokine Levels in Patients with Schizophrenia Depending on the Presence of MetS When Receiving Aripiprazole
All patients treated with aripiprazole had no MetS. We did not find any changes in the serum cytokines levels in the patients who received aripiprazole (Table 5).

Table 5.
Serum concentrations of the cytokines (pg/mL) in patients without MetS during 6 weeks of aripiprazole therapy, Me (Q1; Q3).
3. Materials and Methods
The study protocol was approved by the Local Bioethics Committee of the Mental Health Research Institute, Tomsk, Russian Federation (#187, from 24 April 2018). One hundred and one patients with schizophrenia, according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10: F20), 18–55 years of age, were included in the study during 2018–2020, after having signed informed consent forms. We did not include schizophrenia patients with acute and chronic infectious, inflammatory and autoimmune diseases or patients who used psychoactive substances or took medications that could affect the metabolic or immunological parameters. Most patients received SGAs in anti-relapse and maintenance dosages before admission to the hospital. They were often nonadherent, and were hospitalized due to schizophrenia exacerbation. We divided the schizophrenia patients into two groups, depending on the presence of MetS: 63 of the patients met the criteria for MetS, according to the International Diabetes Federation (IDF, 2005). MetS is diagnosed when a patient has a central obesity (waist circumference more than 94 cm in men and more than 80 cm in women) and any two of the following four signs: (1) the concentration of triglycerides in the serum is higher than 1.7 mmol/L (150 mg/dL), or lipid-lowering therapy is carried out; (2) the concentration of high-density lipoproteins in the serum is below 1.03 mmol/L (40 mg/dL) in men and 1.29 mmol/L (50 mg/dL) in women; (3) the arterial blood pressure level is systolic above 130 mmHg or diastolic above 85 mmHg (or when using treatment for previously diagnosed hypertension); (4) the serum glucose concentration is greater than 5.6 mmol/L (100 mg/dL) (or previously diagnosed type 2 diabetes). Anthropometric and biochemical studies were carried out as previously described [30]. The observation period was 6 weeks, during which the patients were in the hospital. The severity of the schizophrenia symptoms was assessed with the Positive and Negative Syndrome Scale [31] at the beginning of the observation.
Blood for the biochemical analysis was taken from all subjects in the first days of hospitalization and, after a 6-week treatment with SGAs, after 12-h overnight fasting. Blood was centrifuged for 30 min at 2000× g at 4 °C to isolate the serum. Quantitative analyses of the cytokine concentrations in the serum were executed using the multi-analyte panel HCYTMAG-60K-PX41 by MILLIPLEX® MAP (Merck, Darmstadt, Germany) on the multiplex analyzer MAGPIX (Luminex, Austin, TX, USA).
Statistical analysis of the data was performed using SPSS software (version 20) for Windows. We used several methods of group comparisons, such as the Shapiro–Wilk test, the Mann–Whitney U test, the Wilcoxon’s test and the chi-square test. Statistically significant differences were considered p-values less than 0.05.
4. Discussion
Metabolic disorders are frequently observed in patients with schizophrenia; they debuted from its early onset and showed an increase relative to its duration [17]. The differences in the schizophrenia duration between the groups obtained in this study agree with that observation. Additionally, patients with MetS had a longer duration of antipsychotic therapy. It is known that a long duration of illness and longer exposure to antipsychotics can be one of the risk factors for the development of MetS in patients with schizophrenia [32]. We found that patients with MetS had a significantly higher age and duration of schizophrenia in comparison with subjects without MetS. The results may be explained by the fact that the older the age of patients with schizophrenia, the more vulnerable they are to MetS development.
To the best of our knowledge, this is the first study that investigated the changes of the cytokine profile during SGA treatments in schizophrenia patients with the absence or presence of MetS. We studied the changes in the levels of 21 proinflammatory or anti-inflammatory cytokines.
We found that 6 weeks of therapy with SGAs did not affect the anti-inflammatory cytokines such as the IL-1 receptor antagonist (IL-1RA), IL-4 and IL-10, regardless of the presence or absence of MetS. The absence of SGA effects on the anti-inflammatory cytokines was demonstrated in a meta-analysis by Tourjman et al. (2013) [33]. In a 6-week study, taking SGA (risperidone or olanzapine) during the first episode of schizophrenia (FEP), patients with a normal BMI resulted in a decrease in IL-1RA and IL-10 [34]. Noto et al. [35] demonstrated a decrease of IL-4 and IL-10 in FEP patients who received risperidone over 10 weeks. In some in vitro and in vivo studies, a change in anti-inflammatory cytokines under the influence of antipsychotics was demonstrated [36,37]. It is tempting to speculate that the absence of the effect of antipsychotics on anti-inflammatory cytokines in our study had something to do with the adipose tissue protein–adiponectin. Adiponectin can enhance the production of anti-inflammatory mediators [38]. However, a long-term treatment with SGAs led to decreased adiponectin level [39], which, accordingly, canceled its ability to affect anti-inflammatory cytokines. In our study, patients with schizophrenia received antipsychotics for a long time and, as shown in previous research, had low adiponectin levels [30].
One meta-analysis demonstrated the anti-inflammatory effects of antipsychotics in patients with schizophrenia, but the authors noted that most studies did not control for potential confounding factors, such as BMI and smoking [6]. We found, in our study, that 6 weeks of antipsychotic treatment led to an increase in some proinflammatory cytokines such as the IFN-α2, IL-1α and IL-7 levels in patients with MetS and to decreased IFN-γ, IL-1β, IL-12р40, IL-6 and TNF-α in patients without MetS. We assume that our findings are associated with a deterioration in the metabolic status, which, in turn, is closely related to the chronic disease [20,21,40]. Changes in the serum levels of proinflammatory cytokines during SGA treatment may reflect a direct anti-inflammatory effect of the antipsychotics and an increased production of proinflammatory cytokines, depending on the metabolic parameters of the patients.
We found a decrease in the IL-17A level during 6 weeks of SGA therapy in schizophrenia patients without MetS. We want to emphasize that IL-17A is produced by T-helper 17 lymphocytes (Th-17 cells) and is mainly associated with autoimmune diseases [41]. No difference in the IL-17A levels between healthy individuals and patients with FEP was demonstrated in a recent meta-analysis [42]. There is a limited number of studies on the effect of antipsychotics on Th-17 cells. In particular, Dimitrov et al. 2013 [43] showed a decrease of the IL-17A levels in a patient with a long-standing diagnosis of chronic schizophrenia taking antipsychotics. The addition of antipsychotics to the whole blood of healthy women led to an increase in the IL-17 levels [44]. Thus, antipsychotics might have a direct effect on the level of IL-17; however, the mechanisms of the revealed changes require further study.
Risperidone therapy led to an increase in IFN-α2, IL-1β and IL-7 in schizophrenia patients with MetS. In turn, no changes in the cytokine levels in patients without MetS were found. We obtained similar results for the changes in the cytokine levels in patients treated with quetiapine and olanzapine. These findings are generally consistent with the study of Song et al. [40]. They showed that the short-term risperidone treatment of drug-naïve schizophrenia patients was associated with an initial anti-inflammatory effect that was reduced after 6 months of treatment, potentially due to its weight gain side effect. A decrease of the proinflammatory cytokines in drug-naïve FEP patients who received risperidone therapy for 10 weeks was also found in another study [35]. An increase in the white blood cell count associated with a raise in the waist volume was found after 24 weeks of treatment paliperidone—a major active metabolite of risperidone [26].
We were unable to confirm the data received by Sobiś et al. [45] about the anti-inflammatory effects of aripiprazole in patients with chronic schizophrenia. Aripiprazole is associated with a minimal effect on the weight gain, glucose level and lipid metabolism [46]. We did not find any effect of aripiprazole on the serum cytokine levels, but we only studied 11 patients.
In conclusion, we suggest that the anti-inflammatory effect of antipsychotics may worsen in the presence of chronic persistent inflammation caused by metabolic syndrome; however, further in-depth studies are needed to elucidate the mechanisms of these phenomena. In general, the results of our study once again emphasized the need for the secure treatment of patients concerning the possible adverse effects of antipsychotic therapy. Ultimately, we are not treating only schizophrenia but that the treatment affects the patient as a whole, and by preventing MetS, we can avoid the development of other serious disorders, including diabetes and cardiovascular diseases.
Our study is limited by the inability to fully evaluate the long-term therapy that patients received before their current hospitalization due to a lack of relevant information. On the other hand, the study represents a typical clinical situation where patients are hospitalized who already received long-term antipsychotic therapy. We want to emphasize that their metabolic status requires special attention, taking into account the pharmacological profiles of the antipsychotics used.
Author Contributions
Conceptualization, A.S.B., E.G.K. and S.A.I.; methodology, E.G.K., A.N.K. and S.A.I.; software, A.S.B.; validation, I.A.M.; formal analysis, A.S.B.; investigation, A.S.B., I.A.M. and V.I.G.; resources, A.S.B. and E.G.K.; data curation, S.A.I. and A.N.K.; writing—original draft preparation, A.S.B., I.A.M., V.I.G. and E.G.K.; writing—review and editing, A.J.M.L., E.G.K., A.N.K. and S.A.I.; visualization, I.A.M. and V.I.G.; supervision, S.A.I. and N.A.B.; project administration, N.A.B. and funding acquisition, S.A.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation, grant #18-15-00011.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Local Bioethics Committee of the Mental Health Research Institute, Tomsk, Russian Federation (#187, from 24 April 2018).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
Authors can confirm that all relevant data are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marques, T.; Ashok, A.; Pillinger, T.; Veronese, M.; Turkheimer, F.; Dazzan, P.; Sommer, I.; Howes, O. Neuroinflammation in schizophrenia: Meta-analysis of in vivo microglial imaging studies. Psychol. Med. 2019, 49, 2186–2196. [Google Scholar] [CrossRef]
- Van Kesteren, C.; Gremmels, H.; De Witte, L.; Hol, E.; Van Gool, A.; Falkai, P.; Kahn, R.; Sommer, I. Immune involvement in the pathogenesis of schizophrenia: A meta-analysis on postmortem brain studies. Transl. Psychiatry 2017, 7, 1075. [Google Scholar] [CrossRef]
- Müller, N. Inflammation in schizophrenia: Pathogenetic aspects and therapeutic considerations. Schizophr. Bull. 2018, 44, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Upthegrove, R.; Khandaker, G. Cytokines, oxidative stress and cellular markers of inflammation in schizophrenia. Curr. Top. Behav. Neurosci. 2019, 44, 49–66. [Google Scholar] [CrossRef]
- Miller, B.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol. Psychiatry 2011, 70, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Monji, A.; Mizoguchi, Y.; Hashioka, S.; Horikawa, H.; Seki, Y.; Kasai, M.; Utsumi, H.; Kanba, S. Anti-Inflammatory properties of antipsychotics via microglia modulations: Are antipsychotics a ’fire extinguisher’ in the brain of schizophrenia? Mini Rev. Med. Chem. 2011, 11, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, A.; Islam, J.; Khan, J.; Turkheimer, F.; Vernon, A. Effects of Antipsychotic Drugs: Cross Talk Between the Nervous and Innate Immune System. CNS Drugs 2020, 34, 1229–1251. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, D.; Ciufolini, S.; Mondelli, V. Effects of psychotropic drugs on inflammation: Consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacology 2016, 233, 1575–1589. [Google Scholar] [CrossRef]
- Debnath, M.; Venkatasubramanian, G. Recent advances in psychoneuroimmunology relevant to schizophrenia therapeutics. Curr. Opin. Psychiatry 2013, 26, 433–439. [Google Scholar] [CrossRef]
- Müller, N.; Riedel, M.; Schwarz, M. Psychotropic effects of COX-2 inhibitors--a possible new approach for the treatment of psychiatric disorders. Pharmacopsychiatry 2004, 37, 266–269. [Google Scholar] [CrossRef]
- Hong, J.; Bang, M. Anti-inflammatory Strategies for Schizophrenia: A Review of Evidence for Therapeutic Applications and Drug Repurposing. Clin. Psychopharmacol. Neurosci. 2020, 18, 10–24. [Google Scholar] [CrossRef]
- Na, K.S.; Jung, H.Y.; Kim, Y.K. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 277–286. [Google Scholar] [CrossRef]
- Mitchell, A.; Vancampfort, D.; Sweers, K.; van Winkel, R.; Yu, W.; De Hert, M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr. Bull. 2013, 39, 306–318. [Google Scholar] [CrossRef]
- Sugawara, N.; Yasui-Furukori, N.; Sato, Y.; Umeda, T.; Kishida, I.; Yamashita, H.; Saito, M.; Furukori, H.; Nakagami, T.; Hatakeyama, M.; et al. Prevalence of metabolic syndrome among patients with schizophrenia in Japan. Schizophr. Res. 2010, 123, 244–250. [Google Scholar] [CrossRef]
- Lee, J.; Nurjono, M.; Wong, A.; Salim, A. Prevalence of metabolic syndrome among patients with schizophrenia in Singapore. Ann. Acad. Med. Singap. 2012, 41, 457–462. [Google Scholar]
- De Hert, M.; van Winkel, R.; Van Eyck, D.; Hanssens, L.; Wampers, M.; Scheen, A.; Peuskens, J. Prevalence of diabetes, metabolic syndrome and metabolic abnormalities in schizophrenia over the course of the illness: A cross-sectional study. Clin. Pract. Epidemiol. Ment. Health 2006, 2, 14. [Google Scholar] [CrossRef]
- Leonard, B.; Schwarz, M.; Myint, A. The metabolic syndrome in schizophrenia: Is inflammation a contributing cause? J. Psychopharmacol. 2012, 26, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Lasić, D.; Bevanda, M.; Bošnjak, N.; Uglešić, B.; Glavina, T.; Franić, T. Metabolic syndrome and inflammation markers in patients with schizophrenia and recurrent depressive disorder. Psychiatr. Danub. 2014, 6, 214–219. [Google Scholar]
- Kelly, C.W.; McEvoy, J.P.; Miller, B.J. Total and differential white blood cell counts, inflammatory markers, adipokines, and incident metabolic syndrome in phase 1 of the clinical antipsychotic trials of intervention effectiveness study. Schizophr. Res. 2019, 209, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Pandurangi, A.K.; Buckley, P.F. Inflammation, Antipsychotic Drugs, and Evidence for Effectiveness of Anti-inflammatory Agents in Schizophrenia. Curr. Top. Behav. Neurosci. 2020, 44, 227–244. [Google Scholar] [CrossRef]
- Carli, M.; Kolachalam, S.; Longoni, B.; Pintaudi, A.; Baldini, M.; Aringhieri, S.; Fasciani, I.; Annibale, P.; Maggio, R.; Scarselli, M. Atypical Antipsychotics and Metabolic Syndrome: From Molecular Mechanisms to Clinical Differences. Pharmaceuticals 2021, 14, 238. [Google Scholar] [CrossRef]
- Sárvári, A.; Veréb, Z.; Uray, I.; Fésüs, L.; Balajthy, Z. Atypical antipsychotics induce both proinflammatory and adipogenic gene expression in human adipocytes in vitro. Biochem. Biophys. Res. Commun. 2014, 450, 1383–1389. [Google Scholar] [CrossRef]
- Stapel, B.; Sieve, I.; Falk, C.S.; Bleich, S.; Hilfiker-Kleiner, D.; Kahl, K.G. Second generation atypical antipsychotics olanzapine and aripiprazole reduce expression and secretion of inflammatory cytokines in human immune cells. J. Psychiatr. Res. 2018, 105, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Yu, L.; Wang, D.; Chen, Y.; Wang, Y.; Wu, Z.; Liu, R.; Ren, J.; Tang, W.; Zhang, C. Association Between SIRT1, Cytokines, and Metabolic Syndrome in Schizophrenia Patients with Olanzapine or Clozapine Monotherapy. Front. Psychiatry 2020, 11, 602121. [Google Scholar] [CrossRef] [PubMed]
- Na, K.S.; Kim, W.H.; Jung, H.Y.; Ryu, S.G.; Min, K.J.; Park, K.C.; Kim, Y.S.; Yoon, J.S.; Ahn, Y.M.; Kim, C.E. Relationship between inflammation and metabolic syndrome following treatment with paliperidone for schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 39, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Kornetova, E.G.; Kornetov, A.N.; Mednova, I.A.; Dubrovskaya, V.V.; Boiko, A.S.; Bokhan, N.A.; Loonen, A.J.M.; Ivanova, S.A. Changes in body fat and related biochemical parameters associated with atypical antipsychotic drug treatment in schizophrenia patients with or without metabolic syndrome. Front. Psychiatry 2019, 10, 803. [Google Scholar] [CrossRef]
- Jaberi, N.; Faramarzi, E.; Farahbakhsh, M.; Ostadarahimi, A.; Asghari Jafarabadi, M.; Fakhari, A. Prevalence of metabolic syndrome in schizophrenia patients treated with antipsychotic medications. Casp. J. Intern. Med. 2020, 11, 310–314. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Reynolds, G.P.; Yue, W.; Deng, W.; Yan, H.; Tan, L.; Wang, C.; Yang, G.; Lu, T.; et al. Chinese Antipsychotics Pharmacogenomics Consortium. Metabolic Effects of 7 Antipsychotics on Patients with Schizophrenia: A Short-Term, Randomized, Open-Label, Multicenter, Pharmacologic Trial. J. Clin. Psychiatry. 2020, 81, 19m12785. [Google Scholar] [CrossRef]
- Mednova, I.A.; Boiko, A.S.; Kornetova, E.G.; Parshukova, D.A.; Semke, A.V.; Bokhan, N.A.; Loonen, A.J.M.; Ivanova, S.A. Adipocytokines and Metabolic Syndrome in Patients with Schizophrenia. Metabolites 2020, 10, 410. [Google Scholar] [CrossRef]
- Kay, S.R.; Opler, L.A.; Fiszbein, A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Papanastasiou, E. The prevalence and mechanisms of metabolic syndrome in schizophrenia: A review. Ther. Adv. Psychopharmacol. 2013, 3, 33–51. [Google Scholar] [CrossRef]
- Tourjman, V.; Kouassi, É.; Koué, M.È.; Rocchetti, M.; Fortin-Fournier, S.; Fusar-Poli, P.; Potvin, S. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: A meta-analysis. Schizophr. Res. 2013, 151, 43–47. [Google Scholar] [CrossRef]
- De Witte, L.; Tomasik, J.; Schwarz, E.; Guest, P.C.; Rahmoune, H.; Kahn, R.S.; Bahn, S. Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophr. Res. 2014, 154, 23–29. [Google Scholar] [CrossRef]
- Noto, C.; Ota, V.K.; Gouvea, E.S.; Rizzo, L.B.; Spindola, L.; Honda, P.H.; Cordeiro, Q.; Belangero, S.I.; Bressan, R.A.; Gadelha, A.; et al. Effects of risperidone on cytokine profile in drug-naive first-episode psychosis. Int. J. Neuropsychopharmacol. 2015, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Wu, S.; Tsai, T.C.; Wang, L.K.; Tsai, F.M. Regulation of macrophage immune responses by antipsychotic drugs. Immunopharmacol. Immunotoxicol. 2013, 35, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Jaehne, E.J.; Corrigan, F.; Toben, C.; Jawahar, M.; Baune, B. The effect of the antipsychotic drug quetiapine and its metabolite norquetiapine on acute inflammation, memory and anhedonia. Pharmacol. Biochem. Behav. 2015, 135, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Wolf, D.; Rumpold, H.; Enrich, B.; Tilg, H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem. Biophys. Res. Commun. 2004, 323, 630–635. [Google Scholar] [CrossRef]
- Chen, C.Y.; Goh, K.K.; Chen, C.H.; Lu, M.L. The Role of Adiponectin in the Pathogenesis of Metabolic Disturbances in Patients with Schizophrenia. Front. Psychiatry 2020, 11, 605124. [Google Scholar] [CrossRef]
- Song, X.; Fan, X.; Li, X.; Zhang, W.; Gao, J.; Zhao, J.; Harrington, A.; Ziedonis, D.; Lv, L. Changes in pro-inflammatory cytokines and body weight during 6-month risperidone treatment in drug naive, first-episode schizophrenia. Psychopharmacology 2014, 231, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhang, Y.; Fan, W.; Tang, W.; Zhang, C. Interleukin-17 alteration in first-episode psychosis: A meta-analysis. Mol. Neuropsychiatry 2017, 3, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.H.; Lee, S.; Yantis, J.; Valdez, C.; Paredes, R.M.; Braida, N.; Velligan, D.; Walss-Bass, C. Differential correlations between inflammatory cytokines and psychopathology in veterans with schizophrenia: Potential role for IL-17 pathway. Schizophr. Res. 2013, 151, 29–35. [Google Scholar] [CrossRef]
- Himmerich, H.; Schönherr, J.; Fulda, S.; Sheldrick, A.J.; Bauer, K.; Sack, U. Impact of antipsychotics on cytokine production in-vitro. J. Psychiatr. Res. 2011, 45, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Sobiś, J.; Rykaczewska-Czerwińska, M.; Świętochowska, E.; Gorczyca, P. Therapeutic effect of aripiprazole in chronic schizophrenia is accompanied by anti-inflammatory activity. Pharmacol. Rep. 2015, 67, 353–359. [Google Scholar] [CrossRef]
- Yogaratnam, J.; Biswas, N.; Vadivel, R.; Jacob, R. Metabolic complications of schizophrenia and antipsychotic medications-an updated review. East Asian Arch. Psychiatry 2013, 23, 21–28. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).