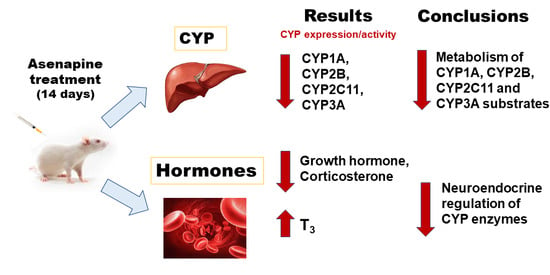
The Influence of Long-Term Treatment with Asenapine on Liver Cytochrome P450 Expression and Activity in the Rat. The Involvement of Different Mechanisms
Abstract
1. Introduction
2. Results
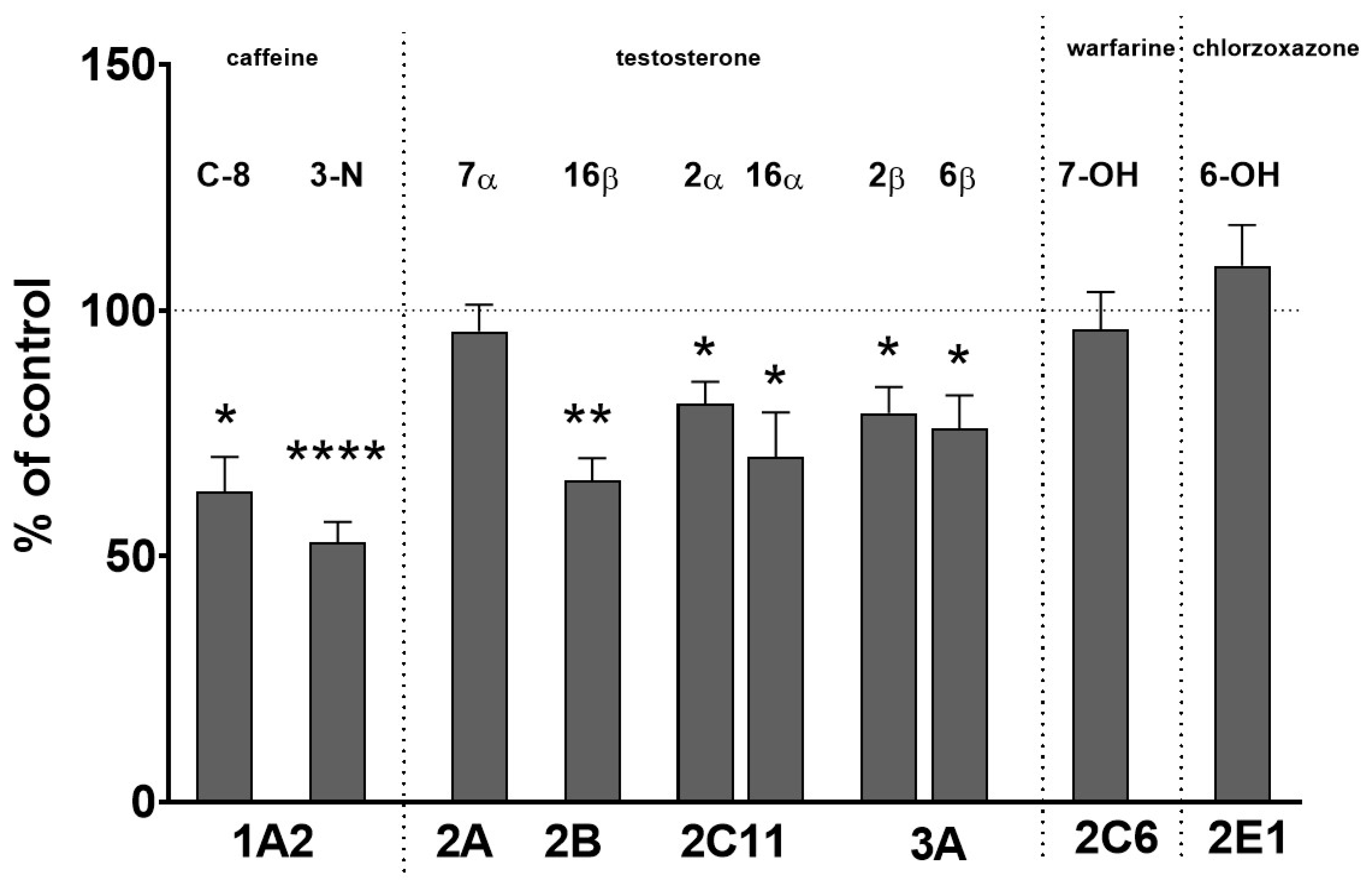
2.1. The Effect of Two-Week Treatment with Asenapine on the CYP Activity in Rat Liver Microsomes
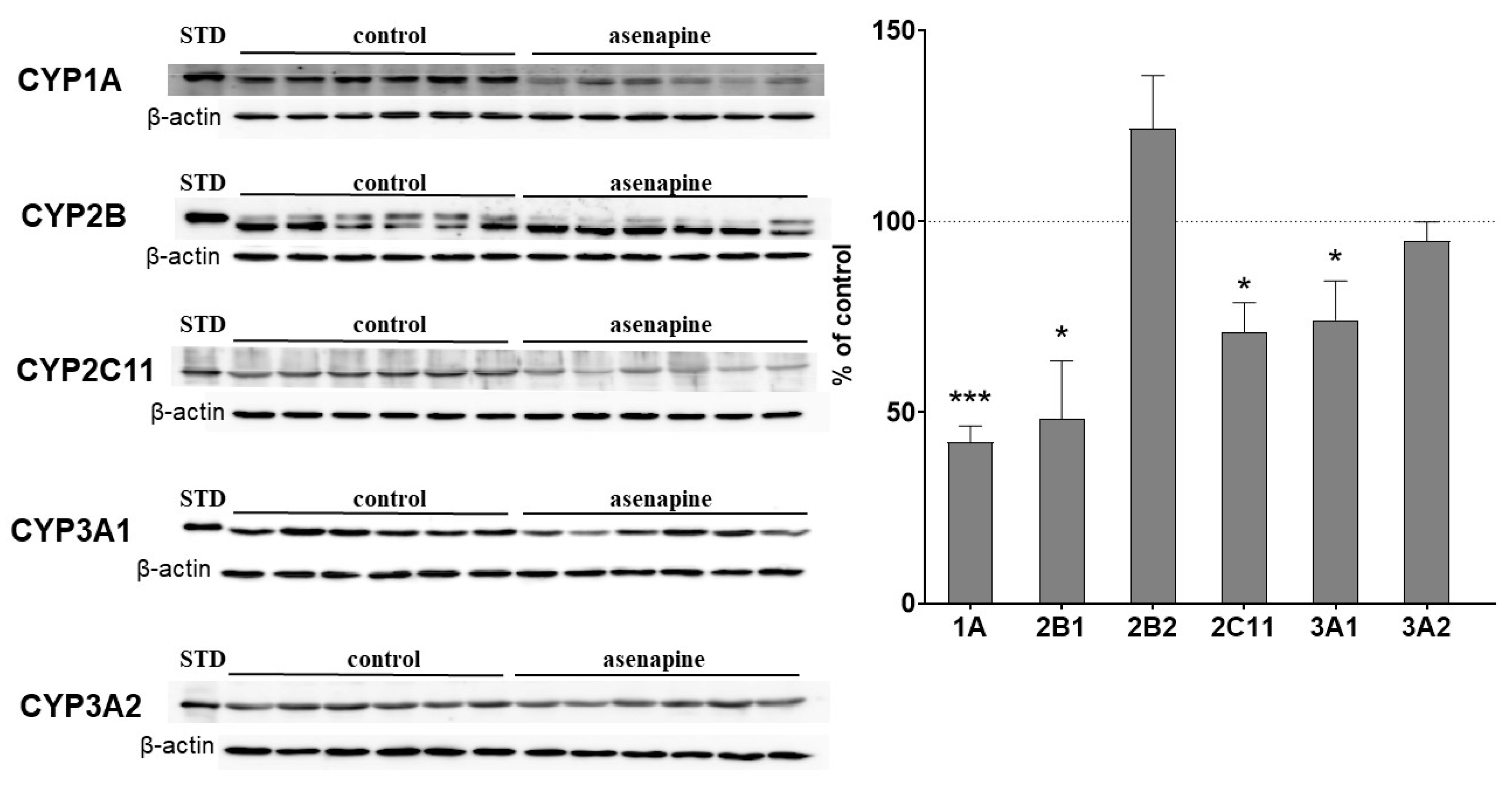
2.2. The Influence of Two-Week Treatment with Asenapine on the CYP Protein Level in Rat Liver Microsomes
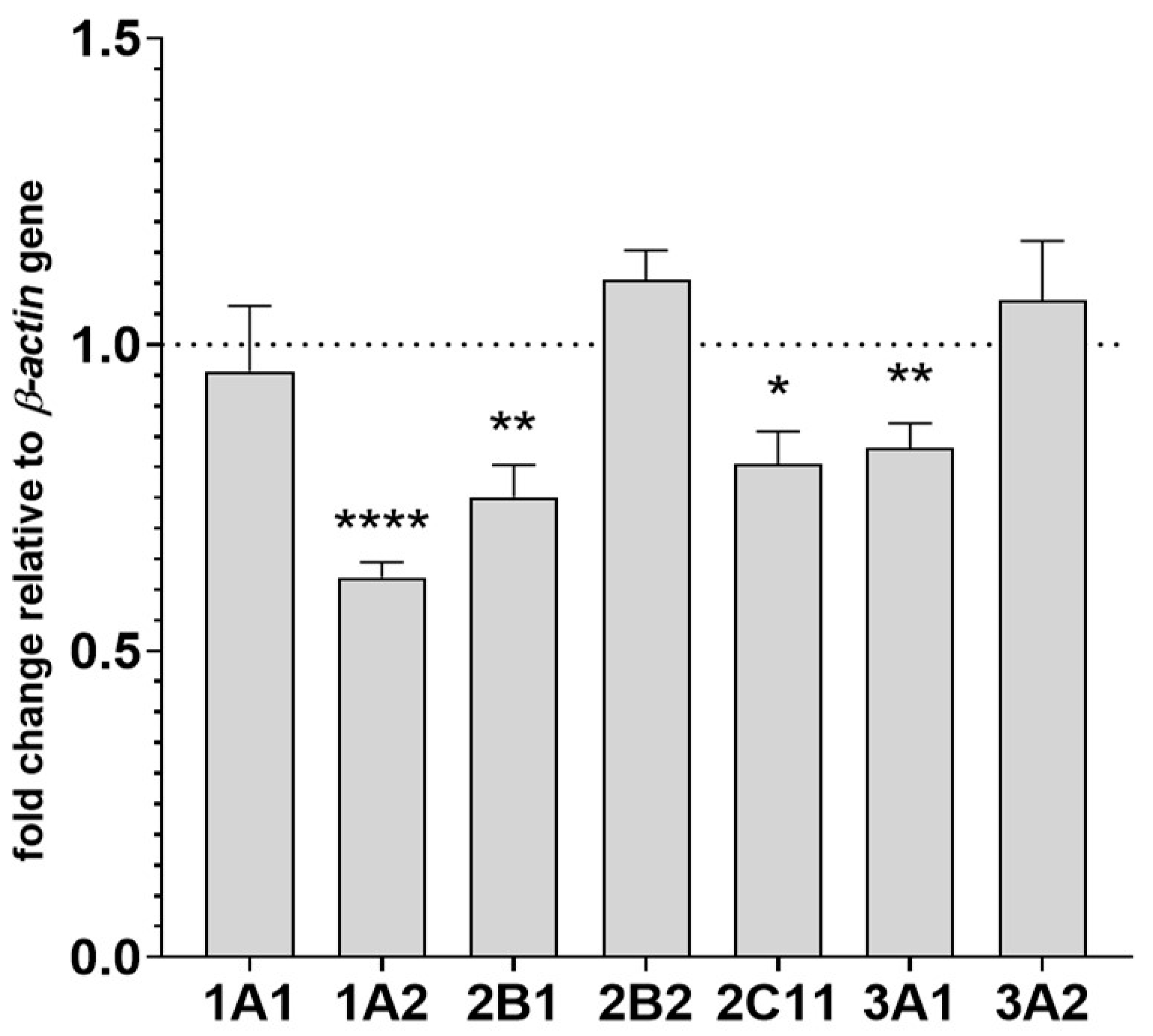
2.3. The Effect of Two-Week Treatment with Asenapine on CYP Gene Expression in the Liver
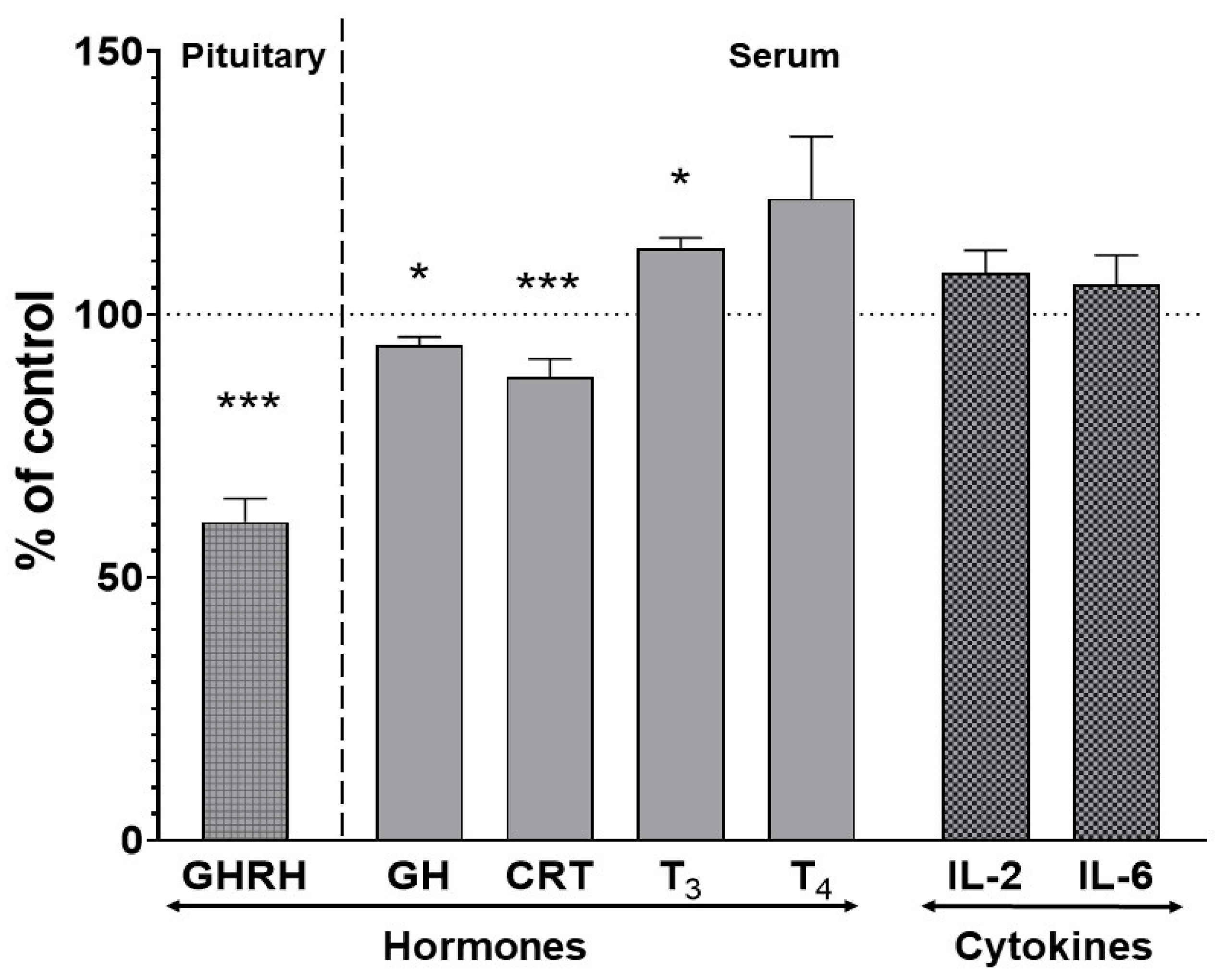
2.4. The Effect of Two-Week Treatment with Asenapine on the Pituitary GHRH and Serum Concentrations of Hormones and Cytokines
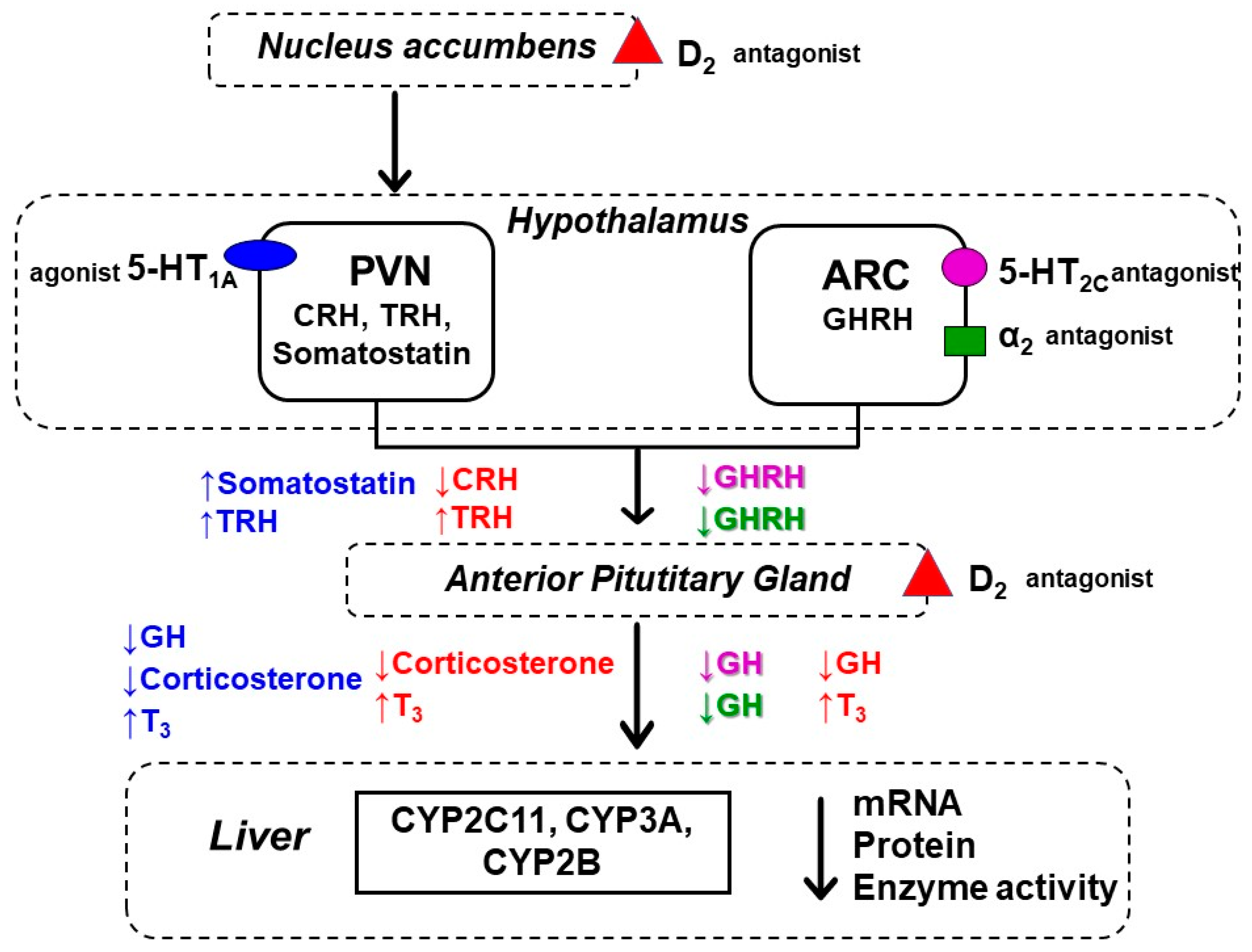
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animal Procedure and Preparation of Liver Microsomes
4.3. Determination of CYP Enzyme Activity in Liver Microsomes
4.4. An Analysis of CYP Proteins in Liver Microsomes
4.5. CYP mRNA Expression Assay in Liver Tissue
4.6. An Analysis of Hormones and Cytokines in the Pituitary and Blood Serum
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zanger, U.M.; Schwab, M. Cytochrome P450 Enzymes in Drug Metabolism: Regulation of Gene Expression, Enzyme Activities, and Impact of Genetic Variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Song, Y.; Li, C.; Liu, G.; Liu, R.; Chen, Y.; Li, W.; Cao, Z.; Zhao, B.; Lu, C.; Liu, Y. Drug-Metabolizing Cytochrome P450 Enzymes Have Multifarious Influences on Treatment Outcomes. Clin. Pharm. 2021, 60, 585–601. [Google Scholar] [CrossRef]
- Sikka, R.; Magauran, B.; Ulrich, A.; Shannon, M. Bench to Bedside: Pharmacogenomics, Adverse Drug Interactions, and the Cytochrome P450 System. Acad. Emerg. Med. 2005, 12, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Spina, E.; de Leon, J. Metabolic Drug Interactions with Newer Antipsychotics: A Comparative Review. Basic Clin. Pharmacol. Toxicol. 2007, 100, 4–22. [Google Scholar] [CrossRef]
- Caraci, F.; Crupi, R.; Drago, F.; Spina, E. Metabolic Drug Interactions between Antidepressants and Anticancer Drugs: Focus on Selective Serotonin Reuptake Inhibitors and Hypericum Extract. Curr. Drug Metab. 2011, 12, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Spina, E.; Barbieri, M.A.; Cicala, G.; Bruno, A.; de Leon, J. Clinically Relevant Drug Interactions between Newer Antidepressants and Oral Anticoagulants. Expert Opin. Drug Metab. Toxicol. 2020, 16, 31–44. [Google Scholar] [CrossRef]
- Linder, C.D.; Renaud, N.A.; Hutzler, J.M. Is 1-Aminobenzotriazole an Appropriate in Vitro Tool as a Nonspecific Cytochrome P450 Inactivator? Drug Metab. Dispos. 2009, 37, 10–13. [Google Scholar] [CrossRef]
- Stringer, R.A.; Weber, E.; Tigani, B.; Lavan, P.; Medhurst, S.; Sohal, B. 1-Aminobenzotriazole Modulates Oral Drug Pharmacokinetics through Cytochrome P450 Inhibition and Delay of Gastric Emptying in Rats. Drug Metab. Dispos. 2014, 42, 1117–1124. [Google Scholar] [CrossRef]
- De Montellano, P.R.O. 1-Aminobenzotriazole: A Mechanism-Based Cytochrome P450 Inhibitor and Probe of Cytochrome P450 Biology. Med. Chem. 2018, 8, 038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, J.; Chen, Z.; Dou, W. In Vitro Inhibitory Effects of Kaempferitrin on Human Liver Cytochrome P450 Enzymes. Pharm. Biol. 2019, 57, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Asquith, C.R.M.; Bennett, J.M.; Su, L.; Laitinen, T.; Elkins, J.M.; Pickett, J.E.; Wells, C.I.; Li, Z.; Willson, T.M.; Zuercher, W.J. Towards the Development of an In Vivo Chemical Probe for Cyclin G Associated Kinase (GAK). Molecules 2019, 24, 4016. [Google Scholar] [CrossRef]
- Zaretzki, J.; Matlock, M.; Swamidass, S.J. XenoSite: Accurately Predicting CYP-Mediated Sites of Metabolism with Neural Networks. J. Chem. Inf. Model. 2013, 53, 3373–3383. [Google Scholar] [CrossRef]
- Wójcikowski, J.; Daniel, W.A. The Brain Dopaminergic System as an Important Center Regulating Liver Cytochrome P450 in the Rat. Expert Opin. Drug Metab. Toxicol. 2009, 5, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Sadakierska-Chudy, A.; Haduch, A.; Rysz, M.; Gołembiowska, K.; Daniel, W.A. The Role of Brain Noradrenergic System in the Regulation of Liver Cytochrome P450 Expression. Biochem. Pharmacol. 2013, 86, 800–807. [Google Scholar] [CrossRef]
- Bromek, E.; Wójcikowski, J.; Daniel, W.A. Involvement of the Paraventricular (PVN) and Arcuate (ARC) Nuclei of the Hypothalamus in the Central Noradrenergic Regulation of Liver Cytochrome P450. Biochem. Pharmacol. 2013, 86, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Bromek, E.; Daniel, W.A. The Regulation of Liver Cytochrome P450 Expression and Activity by the Brain Serotonergic System in Different Experimental Models. Expert Opin. Drug Metab. Toxicol. 2021, 17, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Wójcikowski, J.; Daniel, W.A. The Role of the Nervous System in the Regulation of Liver Cytochrome P450. Curr. Drug Metab. 2011, 12, 124–138. [Google Scholar] [CrossRef]
- Kot, M.; Haduch, A.; Papp, M.; Daniel, W.A. The Effect of Chronic Treatment with Lurasidone on Rat Liver Cytochrome P450 Expression and Activity in the Chronic Mild Stress Model of Depression. Drug Metab. Dispos. 2017, 45, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Stahl, S.M. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications; Cambridge University Press: Cambridge, UK, 2013; ISBN 978-1-139-83259-5. [Google Scholar]
- Patel, K.R.; Cherian, J.; Gohil, K.; Atkinson, D. Schizophrenia: Overview and Treatment Options. Pharm. Ther. 2014, 39, 638–645. [Google Scholar]
- Ferri, F.; Salone, A.; Ebisch, S.J.; De Berardis, D.; Romani, G.L.; Ferro, F.M.; Gallese, V. Action Verb Understanding in First-Episode Schizophrenia: Is There Evidence for a Simulation Deficit? Neuropsychologia 2012, 50, 988–996. [Google Scholar] [CrossRef]
- Ebisch, S.J.H.; Mantini, D.; Northoff, G.; Salone, A.; De Berardis, D.; Ferri, F.; Ferro, F.M.; Di Giannantonio, M.; Romani, G.L.; Gallese, V. Altered Brain Long-Range Functional Interactions Underlying the Link between Aberrant Self-Experience and Self-Other Relationship in First-Episode Schizophrenia. Schizophr. Bull. 2014, 40, 1072–1082. [Google Scholar] [CrossRef][Green Version]
- Citrome, L. Asenapine Review, Part II: Clinical Efficacy, Safety and Tolerability. Expert Opin. Drug Saf. 2014, 13, 803–830. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.; Byers, A.; Sereno, M.; Basra, M.K.; Dutta, S. Asenapine versus Placebo for Schizophrenia. Cochrane Database Syst. Rev. 2015, 2015, CD011458. [Google Scholar] [CrossRef]
- Landbloom, R.; Mackle, M.; Wu, X.; Kelly, L.; Snow-Adami, L.; McIntyre, R.S.; Mathews, M.; Hundt, C. Asenapine for the Treatment of Adults with an Acute Exacerbation of Schizophrenia: Results from a Randomized, Double-Blind, Fixed-Dose, Placebo-Controlled Trial with Olanzapine as an Active Control. CNS Spectr. 2017, 22, 333–341. [Google Scholar] [CrossRef]
- Takao, N.; Murai, T.; Fujiwara, H. Treatment-Resistant Schizophrenia Characterised by Dopamine Supersensitivity Psychosis and Efficacy of Asenapine. BMJ Case Rep. 2021, 14, e242495. [Google Scholar] [CrossRef] [PubMed]
- Scheidemantel, T.; Korobkova, I.; Rej, S.; Sajatovic, M. Asenapine for Bipolar Disorder. Neuropsychiatr. Dis. Treat. 2015, 11, 3007–3017. [Google Scholar] [CrossRef]
- Vieta, E.; Montes, J.M. A Review of Asenapine in the Treatment of Bipolar Disorder. Clin. Drug Investig. 2018, 38, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Marazziti, D.; Mucci, F.; Falaschi, V.; Dell’Osso, L. Asenapine for the Treatment of Bipolar Disorder. Expert Opin. Pharm. 2019, 20, 1321–1330. [Google Scholar] [CrossRef]
- Stoner, S.C.; Pace, H.A. Asenapine: A Clinical Review of a Second-Generation Antipsychotic. Clin. Ther. 2012, 34, 1023–1040. [Google Scholar] [CrossRef]
- Findling, R.L.; Landbloom, R.P.; Mackle, M.; Pallozzi, W.; Braat, S.; Hundt, C.; Wamboldt, M.Z.; Mathews, M. Safety and Efficacy from an 8 Week Double-Blind Trial and a 26 Week Open-Label Extension of Asenapine in Adolescents with Schizophrenia. J. Child. Adolesc. Psychopharmacol. 2015, 25, 384–396. [Google Scholar] [CrossRef]
- Tarazi, F.I.; Stahl, S.M. Iloperidone, Asenapine and Lurasidone: A Primer on Their Current Status. Expert Opin. Pharm. 2012, 13, 1911–1922. [Google Scholar] [CrossRef]
- Citrome, L. Asenapine Review, Part I: Chemistry, Receptor Affinity Profile, Pharmacokinetics and Metabolism. Expert Opin. Drug Metab. Toxicol. 2014, 10, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Delcourte, S.; Abrial, E.; Etiévant, A.; Rovera, R.; Arnt, J.; Didriksen, M.; Haddjeri, N. Asenapine Modulates Mood-Related Behaviors and 5-HT1A/7 Receptors-Mediated Neurotransmission. CNS Neurosci. Ther. 2017, 23, 518–525. [Google Scholar] [CrossRef]
- Huang, M.; Li, Z.; Dai, J.; Shahid, M.; Wong, E.H.F.; Meltzer, H.Y. Asenapine Increases Dopamine, Norepinephrine, and Acetylcholine Efflux in the Rat Medial Prefrontal Cortex and Hippocampus. Neuropsychopharmacology 2008, 33, 2934–2945. [Google Scholar] [CrossRef]
- Tarazi, F.I.; Neill, J.C. The Preclinical Profile of Asenapine: Clinical Relevance for the Treatment of Schizophrenia and Bipolar Mania. Expert Opin. Drug Discov. 2013, 8, 93–103. [Google Scholar] [CrossRef]
- Murray, M. Role of CYP Pharmacogenetics and Drug-Drug Interactions in the Efficacy and Safety of Atypical and Other Antipsychotic Agents. J. Pharm. Pharmacol. 2006, 58, 871–885. [Google Scholar] [CrossRef]
- English, B.A.; Dortch, M.; Ereshefsky, L.; Jhee, S. Clinically significant psychotropic drug-drug interactions in the primary care setting. Curr. Psychiatry Rep. 2012, 14, 376–390. [Google Scholar] [CrossRef]
- Kennedy, W.K.; Jann, M.W.; Kutscher, E.C. Clinically Significant Drug Interactions with Atypical Antipsychotics. CNS Drugs 2013, 27, 1021–1048. [Google Scholar] [CrossRef]
- Urichuk, L.; Prior, T.I.; Dursun, S.; Baker, G. Metabolism of Atypical Antipsychotics: Involvement of Cytochrome P450 Enzymes and Relevance for Drug-Drug Interactions. Curr. Drug Metab. 2008, 9, 410–418. [Google Scholar] [CrossRef]
- Conley, R.R.; Kelly, D.L. Drug-Drug Interactions Associated with Second-Generation Antipsychotics: Considerations for Clinicians and Patients. Psychopharmacol. Bull. 2007, 40, 77–97. [Google Scholar]
- Prior, T.I.; Baker, G.B. Interactions between the Cytochrome P450 System and the Second-Generation Antipsychotics. J. Psychiatry Neurosci. 2003, 28, 99–112. [Google Scholar] [PubMed]
- Spina, E.; Hiemke, C.; de Leon, J. Assessing Drug-Drug Interactions through Therapeutic Drug Monitoring When Administering Oral Second-Generation Antipsychotics. Expert Opin. Drug Metab. Toxicol. 2016, 12, 407–422. [Google Scholar] [CrossRef]
- Danek, P.J.; Wójcikowski, J.; Daniel, W.A. Asenapine and Iloperidone Decrease the Expression of Major Cytochrome P450 Enzymes CYP1A2 and CYP3A4 in Human Hepatocytes. A Significance for Drug-Drug Interactions during Combined Therapy. Toxicol. Appl. Pharmacol. 2020, 406, 115239. [Google Scholar] [CrossRef]
- Wójcikowski, J.; Danek, P.J.; Basińska-Ziobroń, A.; Pukło, R.; Daniel, W.A. In Vitro Inhibition of Human Cytochrome P450 Enzymes by the Novel Atypical Antipsychotic Drug Asenapine: A Prediction of Possible Drug-Drug Interactions. Pharmacol. Rep. 2020, 72, 612–621. [Google Scholar] [CrossRef]
- Rane, A.; Liu, Z.; Levol, R.; Bjelfman, C.; Thyr, C.; Ericson, H.; Hansson, T.; Henderson, C.; Wolf, C.R. Differential Effects of Neuroleptic Agents on Hepatic Cytochrome P-450 Isozymes in the Male Rat. Biochim. Biophys. Acta 1996, 1291, 60–66. [Google Scholar] [CrossRef]
- Wójcikowski, J.; Haduch, A.; Daniel, W.A. Effect of Classic and Atypical Neuroleptics on Cytochrome P450 3A (CYP3A) in Rat Liver. Pharmacol. Rep. 2012, 64, 1411–1418. [Google Scholar] [CrossRef]
- Haduch, A.; Wójcikowski, J.; Daniel, W.A. Effect of Neuroleptics on Cytochrome P450 2C11 (CYP2C11) in Rat Liver. Pharmacol. Rep. 2011, 63, 1491–1499. [Google Scholar] [CrossRef]
- Dvorak, Z.; Pavek, P. Regulation of Drug-Metabolizing Cytochrome P450 Enzymes by Glucocorticoids. Drug Metab. Rev. 2010, 42, 621–635. [Google Scholar] [CrossRef]
- Konstandi, M. Psychophysiological Stress: A Significant Parameter in Drug Pharmacokinetics. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1317–1334. [Google Scholar] [CrossRef] [PubMed]
- Waxman, D.J.; Ram, P.A.; Pampori, N.A.; Shapiro, B.H. Growth Hormone Regulation of Male-Specific Rat Liver P450s 2A2 and 3A2: Induction by Intermittent Growth Hormone Pulses in Male but Not Female Rats Rendered Growth Hormone Deficient by Neonatal Monosodium Glutamate. Mol. Pharmacol. 1995, 48, 790–797. [Google Scholar]
- Waxman, D.J.; O’Connor, C. Growth Hormone Regulation of Sex-Dependent Liver Gene Expression. Mol. Endocrinol. 2006, 20, 2613–2629. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.G.; Plant, N.J.; Swales, K.E.; Ayrton, A.; El-Sankary, W. Receptor-Dependent Transcriptional Activation of Cytochrome P4503A Genes: Induction Mechanisms, Species Differences and Interindividual Variation in Man. Xenobiotica 2002, 32, 165–206. [Google Scholar] [CrossRef]
- Monostory, K.; Kohalmy, K.; Prough, R.A.; Kóbori, L.; Vereczkey, L. The Effect of Synthetic Glucocorticoid, Dexamethasone on CYP1A1 Inducibility in Adult Rat and Human Hepatocytes. FEBS Lett. 2005, 579, 229–235. [Google Scholar] [CrossRef]
- Audet-Walsh, E.; Auclair-Vincent, S.; Anderson, A. Glucocorticoids and Phenobarbital Induce Murine CYP2B Genes by Independent Mechanisms. Expert Opin. Drug Metab. Toxicol. 2009, 5, 1501–1511. [Google Scholar] [CrossRef]
- Audet-Walsh, E.; Anderson, A. Dexamethasone Induction of Murine CYP2B Genes Requires the Glucocorticoid Receptor. Drug Metab. Dispos. 2009, 37, 580–588. [Google Scholar] [CrossRef]
- Liddle, C.; Goodwin, B.J.; George, J.; Tapner, M.; Farrell, G.C. Separate and Interactive Regulation of Cytochrome P450 3A4 by Triiodothyronine, Dexamethasone, and Growth Hormone in Cultured Hepatocytes. J. Clin. Endocrinol. Metab. 1998, 83, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Murayama, N.; Shimada, M.; Yamazoe, Y.; Kato, R. Difference in the Susceptibility of Two Phenobarbital-Inducible Forms, P450IIB1 and P450IIB2, to Thyroid Hormone- and Growth Hormone-Induced Suppression in Rat Liver: Phenobarbital-Inducible P450IIB2 Suppression by Thyroid Hormone Acting Directly, but Not through the Pituitary System. Mol. Pharmacol. 1991, 39, 811–817. [Google Scholar] [PubMed]
- Yamazoe, Y.; Murayama, N.; Shimada, M.; Kato, R. Thyroid Hormone Suppression of Hepatic Levels of Phenobarbital-Inducible P-450b and P-450e and Other Neonatal P-450s in Hypophysectomized Rats. Biochem. Biophys. Res. Commun. 1989, 160, 609–614. [Google Scholar] [CrossRef]
- Brtko, J.; Dvorak, Z. Role of Retinoids, Rexinoids and Thyroid Hormone in the Expression of Cytochrome P450 Enzymes. Curr. Drug Metab. 2011, 12, 71–88. [Google Scholar] [CrossRef]
- Frånberg, O.; Wiker, C.; Marcus, M.M.; Konradsson, A.; Jardemark, K.; Schilström, B.; Shahid, M.; Wong, E.H.F.; Svensson, T.H. Asenapine, a Novel Psychopharmacologic Agent: Preclinical Evidence for Clinical Effects in Schizophrenia. Psychopharmacology 2008, 196, 417–429. [Google Scholar] [CrossRef]
- Tarazi, F.I.; Shahid, M. Asenapine Maleate: A New Drug for the Treatment of Schizophrenia and Bipolar Mania. Drugs Today 2009, 45, 865–876. [Google Scholar] [CrossRef]
- Marston, H.M.; Young, J.W.; Martin, F.D.C.; Serpa, K.A.; Moore, C.L.; Wong, E.H.F.; Gold, L.; Meltzer, L.T.; Azar, M.R.; Geyer, M.A.; et al. Asenapine Effects in Animal Models of Psychosis and Cognitive Function. Psychopharmacology 2009, 206, 699. [Google Scholar] [CrossRef][Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Liu, L.; Miao, M.; Zhong, Z.; Xu, P.; Chen, Y.; Liu, X. Chronic Administration of Caderofloxacin, a New Fluoroquinolone, Increases Hepatic CYP2E1 Expression and Activity in Rats. Acta Pharmacol. Sin. 2016, 37, 561–570. [Google Scholar] [CrossRef]
- Pearce, R.E.; McIntyre, C.J.; Madan, A.; Sanzgiri, U.; Draper, A.J.; Bullock, P.L.; Cook, D.C.; Burton, L.A.; Latham, J.; Nevins, C.; et al. Effects of Freezing, Thawing, and Storing Human Liver Microsomes on Cytochrome P450 Activity. Arch. Biochem. Biophys. 1996, 331, 145–169. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danek, P.J.; Bromek, E.; Daniel, W.A. The Influence of Long-Term Treatment with Asenapine on Liver Cytochrome P450 Expression and Activity in the Rat. The Involvement of Different Mechanisms. Pharmaceuticals 2021, 14, 629. https://doi.org/10.3390/ph14070629
Danek PJ, Bromek E, Daniel WA. The Influence of Long-Term Treatment with Asenapine on Liver Cytochrome P450 Expression and Activity in the Rat. The Involvement of Different Mechanisms. Pharmaceuticals. 2021; 14(7):629. https://doi.org/10.3390/ph14070629
Chicago/Turabian StyleDanek, Przemysław J., Ewa Bromek, and Władysława A. Daniel. 2021. "The Influence of Long-Term Treatment with Asenapine on Liver Cytochrome P450 Expression and Activity in the Rat. The Involvement of Different Mechanisms" Pharmaceuticals 14, no. 7: 629. https://doi.org/10.3390/ph14070629
APA StyleDanek, P. J., Bromek, E., & Daniel, W. A. (2021). The Influence of Long-Term Treatment with Asenapine on Liver Cytochrome P450 Expression and Activity in the Rat. The Involvement of Different Mechanisms. Pharmaceuticals, 14(7), 629. https://doi.org/10.3390/ph14070629