Broad-Spectrum Antibiotic Regimen Affects Survival in Patients Receiving Nivolumab for Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics and the Antibiotics Used
2.2. OS and PFS
2.3. Associated Factors with OS and PFS
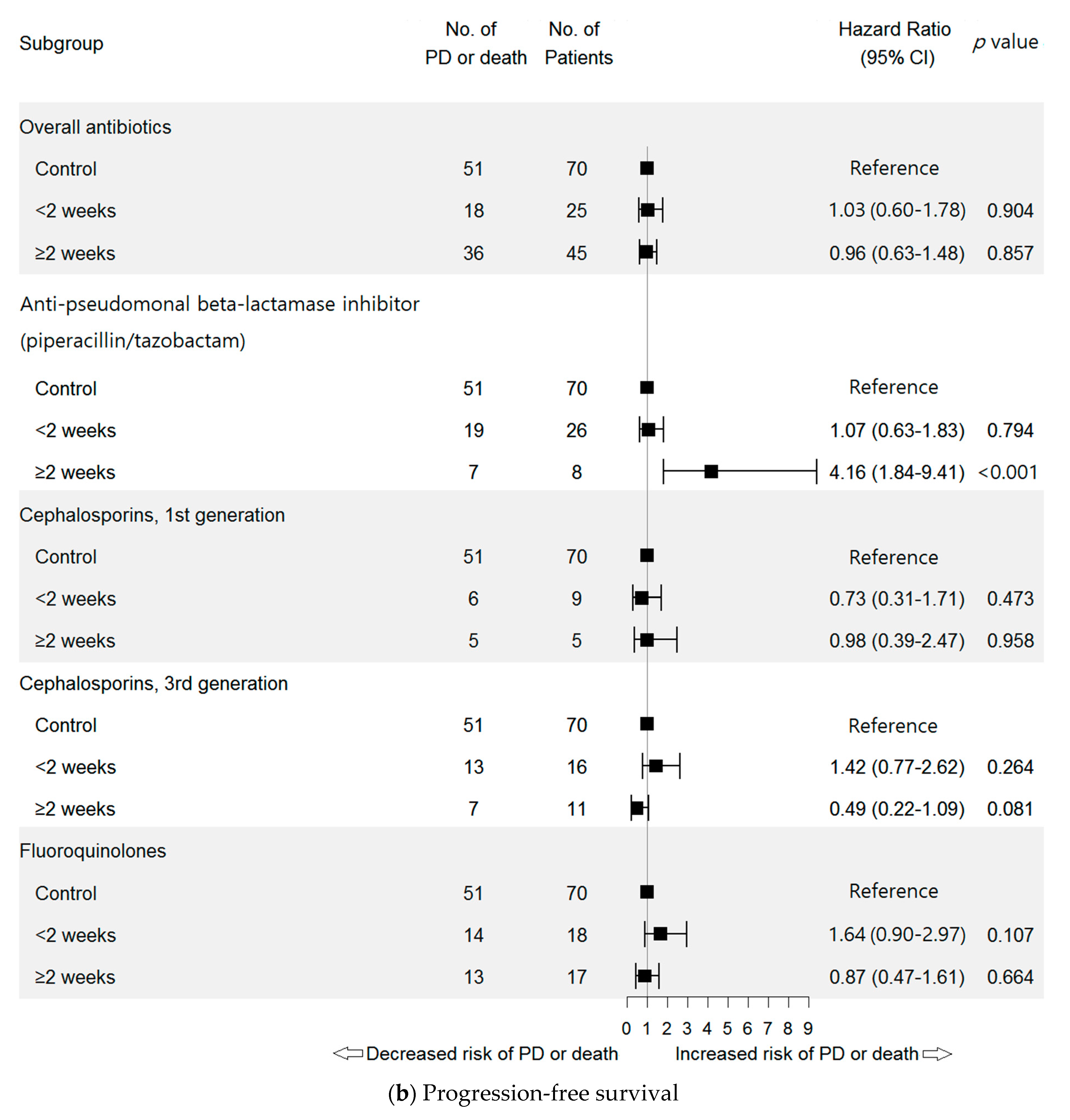
2.3.1. Antibiotic Class
2.3.2. Antibiotic Days of Therapy (DOT)
2.3.3. Defined Daily Dose (DDD) of Antibiotics
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.S.; Lee, K.H.; Cho, E.K.; Kim, D.W.; Kim, S.W.; Kim, J.H.; Cho, B.C.; Kang, J.H.; Han, J.Y.; Min, Y.J.; et al. Nivolumab in advanced non-small-cell lung cancer patients who failed prior platinum-based chemotherapy. Lung Cancer 2018, 122, 234–242. [Google Scholar] [CrossRef]
- Leal, T.A.; Ramalingam, S.S. Immunotherapy in previously treated non-small cell lung cancer (NSCLC). J. Thorac. Dis. 2018, 10, S422–S432. [Google Scholar] [CrossRef]
- Lim, S.M.; Kim, S.W.; Cho, B.C.; Kang, J.H.; Ahn, M.J.; Kim, D.W.; Kim, Y.C.; Lee, J.S.; Lee, J.S.; Lee, S.Y.; et al. Real World Experience of Nivolumab in Non-Small Cell Lung Cancer in Korea. Cancer Res. Treat. 2020, 52, 1112–1119. [Google Scholar] [CrossRef]
- Eoum, G.; Cho, Y.; Rhie, S.J. Evaluation of Nivolumab Use and Factors related to Treatment Outcomes in a Cancer Center of a Top Tier General Hospital. Korean J. Clin. Pharm. 2018, 28, 88–94. [Google Scholar] [CrossRef]
- Botticelli, A.; Cerbelli, B.; Lionetto, L.; Zizzari, I.; Salati, M.; Pisano, A.; Federica, M.; Simmaco, M.; Nuti, M.; Marchetti, P. Can IDO activity predict primary resistance to anti-PD-1 treatment in NSCLC? J. Transl. Med. 2018, 16, 219. [Google Scholar] [CrossRef]
- Derosa, L.; Hellmann, M.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.; Arbour, K.; Chaft, J.; et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Inamura, K. Roles of microbiota in response to cancer immunotherapy. Semin. Cancer Biol. 2020, 65, 164–175. [Google Scholar] [CrossRef]
- Shui, L.; Yang, X.; Li, J.; Yi, C.; Sun, Q.; Zhu, H. Gut Microbiome as a Potential Factor for Modulating Resistance to Cancer Immunotherapy. Front. Immunol. 2020, 10, 2989. [Google Scholar] [CrossRef]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.V.; et al. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 3043–3054. [Google Scholar] [CrossRef]
- Palmore, T.N.; Parta, M.; Cuellar-Rodriguez, J.; Gea-Banacloche, J.C. Infections in the Cancer Patient. In DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology, 11th ed.; DeVita, V.T., Lawrence, T.S., Rosenberg, S.A., Eds.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2019; pp. 2036–2068. [Google Scholar]
- Pérez-Cobas, A.E.; Artacho, A.; Knecht, H.; Ferrús, M.L.; Friedrichs, A.; Ott, S.J.; Moya, A.; Latorre, A.; Gosalbes, M.J. Differential Effects of Antibiotic Therapy on the Structure and Function of Human Gut Microbiota. PLoS ONE 2013, 8, e80201. [Google Scholar] [CrossRef]
- Elkrief, A.; DeRosa, L.; Kroemer, G.; Zitvogel, L.; Routy, B. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: A new independent prognostic factor? Ann. Oncol. 2019, 30, 1572–1579. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The effect of antibiotics on the composition of the intestinal microbiota—A systematic review. J. Infect. 2019, 79, 471–489. [Google Scholar] [CrossRef]
- Vangay, P.; Ward, T.; Gerber, J.S.; Knights, D. Antibiotics, Pediatric Dysbiosis, and Disease. Cell Host Microbe 2015, 17, 553–564. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, G.; Li, W.; Li, X.; Zhao, C.; Jiang, T.; Jia, Y.; He, Y.; Li, A.; Su, C.; et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer 2019, 130, 10–17. [Google Scholar] [CrossRef]
- Routy, B.; Gopalakrishnan, V.; Daillère, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396. [Google Scholar] [CrossRef]
- Mielgo-Rubio, X.; Chara, L.; Sotelo-Lezama, M.; Castro, R.L.; Rubio-Martínez, J.; Velastegui, A.; Olier-Garate, C.; Falagan, S.; Gómez-Barreda, I.; Bautista-Sanz, P.; et al. MA10.01 Antibiotic Use and PD-1 Inhibitors: Shorter Survival in Lung Cancer, Especially When Given Intravenously. Type of Infection Also Matters. J. Thorac. Oncol. 2018, 13, S389. [Google Scholar] [CrossRef]
- Do, T.P.; Hegde, A.M.; Cherry, C.R.; Stroud, C.R.G.; Sharma, N.; Cherukuri, S.D.; Bowling, M.; Walker, P.R. Antibiotic use and overall survival in lung cancer patients receiving nivolumab. J. Clin. Oncol. 2018, 36, e15109. [Google Scholar] [CrossRef]
- Spakowicz, D.; Hoyd, R.; Muniak, M.; Husain, M.; Bassett, J.S.; Wang, L.; Tinoco, G.; Patel, S.H.; Burkart, J.; Miah, A.; et al. Inferring the role of the microbiome on survival in patients treated with immune checkpoint inhibitors: Causal modeling, timing, and classes of concomitant medications. BMC Cancer 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef]
- Pinato, D.J.; Howlett, S.; Ottaviani, D.; Urus, H.; Patel, A.; Mineo, T.; Brock, C.; Power, D.; Hatcher, O.; Falconer, A.; et al. Association of Prior Antibiotic Treatment With Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients With Cancer. JAMA Oncol. 2019, 5, 1774–1778. [Google Scholar] [CrossRef]
- Nord, C.; Brismar, B.; Kasholm-Tengve, B.; Tunevall, G. Effect of piperacillin/tazobactam treatment on human bowel microflora. J. Antimicrob. Chemother. 1993, 31 (Suppl. A), 61–65. [Google Scholar] [CrossRef]
- Tan, G.S.E.; Tay, H.L.; Tan, S.H.; Lee, T.H.; Ng, T.M.; Lye, D.C. Gut Microbiota Modulation: Implications for Infection Control and Antimicrobial Stewardship. Adv. Ther. 2020, 37, 4054–4067. [Google Scholar] [CrossRef]
- Kukla, M.; Adrych, K.; Dobrowolska, A.; Mach, T.; Reguła, J.; Rydzewska, G. Guidelines for Clostridium difficile infection in adults. Gastroenterol. Rev. 2020, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bhalodi, A.A.; Van Engelen, T.S.R.; Virk, H.S.; Wiersinga, W.J. Impact of antimicrobial therapy on the gut microbiome. J. Antimicrob. Chemother. 2019, 74 (Suppl. 1), i6–i15. [Google Scholar] [CrossRef]
- Pierrard, J.; Seront, E. Impact of the Gut Microbiome on Immune Checkpoint Inhibitor Efficacy—A Systematic Review. Curr. Oncol. 2019, 26, 395–403. [Google Scholar] [CrossRef]
- Jin, Y.; Dong, H.; Xia, L.; Yang, Y.; Zhu, Y.; Shen, Y.; Zheng, H.; Yao, C.; Wang, Y.; Lu, S. The Diversity of Gut Microbiome is Associated with Favorable Responses to Anti–Programmed Death 1 Immunotherapy in Chinese Patients with NSCLC. J. Thorac. Oncol. 2019, 14, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Iizumi, T.; Battaglia, T.; Ruiz, V.; Perez, G.I.P. Gut Microbiome and Antibiotics. Arch. Med. Res. 2017, 48, 727–734. [Google Scholar] [CrossRef]
- Galli, G.; Triulzi, T.; Proto, C.; Signorelli, D.; Imbimbo, M.; Poggi, M.; Fucà, G.; Ganzinelli, M.; Vitali, M.; Palmieri, D.; et al. Association between antibiotic-immunotherapy exposure ratio and outcome in metastatic non small cell lung cancer. Lung Cancer 2019, 132, 72–78. [Google Scholar] [CrossRef]
- Wilson, H.L.; Daveson, K.; Del Mar, C.B. Optimal antimicrobial duration for common bacterial infections. Aust. Prescr. 2019, 42, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Han, J.H.; Landsburg, D.; Pegues, D.; Reesey, E.; Gilmar, C.; Gorman, T.; Bink, K.; Moore, A.; Kelly, B.J.; et al. Impact of Levofloxacin for the Prophylaxis of Bloodstream Infection on the Gut Microbiome in Patients With Hematologic Malignancy. Open Forum Infect. Dis. 2019, 6, ofz252. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Yi, M.; Yu, S.; Qin, S.; Liu, Q.; Xu, H.; Zhao, W.; Chu, Q.; Wu, K. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 47. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [PubMed]
- The 2019 WHO AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use; World Health Organization: Geneva, Switzerland, 2019; Available online: www.who.int/medicines/news/2019/WHO_releases2019AWaRe_classification_antibiotics/en/ (accessed on 23 March 2021).
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Polk, R.E.; Fox, C.; Mahoney, A.; Letcavage, J.; MacDougall, C. Measurement of Adult Antibacterial Drug Use in 130 US Hospitals: Comparison of Defined Daily Dose and Days of Therapy. Clin. Infect. Dis. 2007, 44, 664–670. [Google Scholar] [CrossRef]
- ATC Classification Index with DDDs; WHO Collaborating Centre for Drug Statistics Methodology: Oslo, Norway, 2020; Available online: www.whocc.no/atc_ddd_index/ (accessed on 23 March 2021).
- Hutchinson, J.M.; Patrick, D.M.; Marra, F.; Ng, H.; Bowie, W.R.; Heule, L.; Muscat, M.; Monnet, D.L. Measurement of Antibiotic Consumption: A Practical Guide to the Use of the Anatomical Therapeutic Chemical Classification and Defined Daily Dose System Methodology in Canada. Can. J. Infect. Dis. 2004, 15, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Jainaf, N.R.A.M.; Parimalakrishnan, S.; Ramakrishna, R.M. Study on drug utilization pattern of antihypertensive medications on out-patients and inpatients in a tertiary care teaching hospital: A cross sectional Study. Afr. J. Pharm. Pharmacol. 2015, 9, 383–396. [Google Scholar] [CrossRef][Green Version]

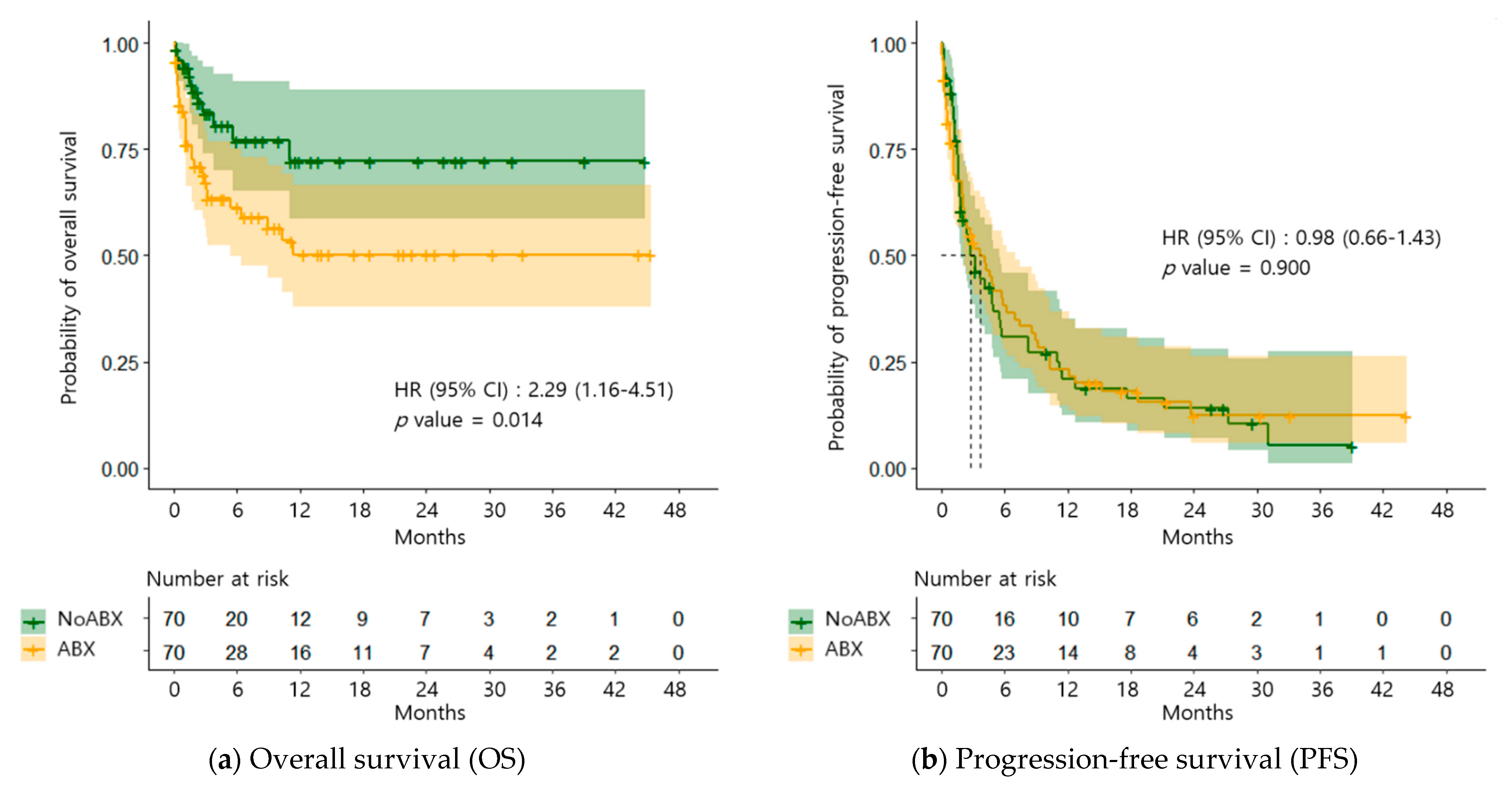

| Variables | Total n = 140 | NoABX n = 70 | ABX n = 70 | p-Value |
|---|---|---|---|---|
| Age | ||||
| Mean ± SD | 62.5 ± 11.1 | 63.2 ± 9.4 | 61.7 ± 12.6 | 0.693 |
| <65 years, n (%) | 78 (55.7) | 40 (57.1) | 38 (54.3) | 0.865 |
| ≥65 years, n (%) | 62 (44.3) | 30 (42.9) | 32 (45.7) | |
| Sex, n (%) | ||||
| Female | 40 (28.6) | 20 (28.6) | 20 (28.6) | 1.000 |
| Male | 100 (71.4) | 50 (71.4) | 50 (71.4) | |
| Stage, n (%) | ||||
| 2 | 3 (2.1) | 1 (1.4) | 2 (2.9) | 0.634 |
| 3 | 18 (12.9) | 11 (15.7) | 7 (10.0) | |
| 4 | 107 (76.4) | 51 (72.9) | 56 (80.0) | |
| Recurrence | 12 (8.6) | 7 (10.0) | 5 (7.1) | |
| ECOG performance status, n (%) | ||||
| 0 | 136 (97.1) | 69 (98.6) | 67 (95.7) | 0.620 |
| 1 | 4 (2.9) | 1 (1.4) | 3 (4.3) | |
| Tumor subtype, n (%) | ||||
| Adenocarcinoma | 98 (70.0) | 50 (71.4) | 48 (68.6) | 0.854 |
| Squamous cell carcinoma | 42 (30.0) | 20 (28.6) | 22 (31.4) | |
| Number of regimens before nivolumab | ||||
| <3, n (%) | 80 (57.1) | 41 (58.6) | 39 (55.7) | 0.864 |
| ≥3, n (%) | 60 (42.9) | 29 (41.4) | 31 (44.3) | |
| PD-L1 expression, n (%) | ||||
| High (≥50%) | 36 (25.7) | 19 (27.1) | 17 (24.3) | 0.918 |
| Moderate (≥10%, <50%) | 38 (27.1) | 19 (27.1) | 19 (27.1) | |
| Low (<10%) | 66 (47.1) | 32 (45.7) | 34 (48.6) | |
| Variables * | Overall Survival | Progression-Free Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | |||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Anti-pseudomonal beta-lactamase inhibitor (piperacillin/tazobactam) | 3.31 (1.77–6.18) | <0.001 | 3.31 (1.77–6.18) | <0.001 | 1.43 (0.91–2.23) | 0.119 | 3.40 (1.82–6.34) | <0.001 |
| Beta-lactamase inhibitors | 0.28 (0.04–2.11) | 0.220 | 0.47 (0.19–1.16) | 0.100 | - | - | ||
| Cephalosporins, 1st generation | 1.17 (0.46–2.98) | 0.749 | 0.83 (0.44–1.54) | 0.549 | ||||
| Cephalosporins, 2nd generation | 0.50 (0.12–2.09) | 0.345 | 0.78 (0.39–1.56) | 0.489 | ||||
| Cephalosporins, 3rd generation | 1.67 (0.85–3.28) | 0.140 | - | - | 0.84 (0.52–1.37) | 0.492 | ||
| Fluoroquinolones | 2.88 (1.54–5.37) | <0.001 | - | - | 1.24 (0.80–1.92) | 0.338 | ||
| Glycopeptides | 3.22 (1.26–8.25) | 0.015 | - | - | 1.40 (0.65–3.03) | 0.391 | ||
| Sulfamethoxazole/ trimethoprim | 2.24 (0.99–5.06) | 0.053 | - | - | 1.08 (0.58–2.03) | 0.802 | ||
| Variables | Overall Survival | Progression-Free Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| DDD, Mean ± SD | r | p-Value | DDD, Mean ± SD | r | p-Value | |||
| Survival n = 100 | Death n = 40 | Progression- Free Survival n = 35 | Progressed Disease or Death n = 105 | |||||
| Overall antibiotics | 13.1 ± 33.7 | 16.4 ± 33.3 | 0.15 | 0.068 | 15.7 ± 29.1 | 16.6 ± 34.7 | 0.01 | 0.896 |
| Anti-pseudomonal beta-lactamase inhibitor (piperacillin/tazobactam) | 1.7 ± 4.9 | 2.8 ± 6.5 | 0.27 | 0.001 | 2.2 ± 5.5 | 3.0 ± 6.8 | 0.05 | 0.576 |
| Anti-tuberculosis medications | - | 0.3 ± 3.9 | 0.13 | 0.114 | - | 0.3 ± 3.9 | 0.05 | 0.566 |
| Beta-lactamase inhibitors | 0.4 ± 2.7 | 0.3 ± 2.3 | −0.06 | 0.514 | 0.1 ± 0.5 | 0.4 ± 2.7 | 0.05 | 0.544 |
| Carbapenem (meropenem) | 0.5 ± 4.6 | 0.5 ± 4.2 | 0.02 | 0.810 | 1.3 ± 7.8 | 0.2 ± 1.9 | −0.11 | 0.196 |
| Cephalosporins, 1st generation | 0.7 ± 2.8 | 0.7 ± 2.8 | 0.01 | 0.891 | 0.2 ± 0.8 | 0.8 ± 3.1 | 0.09 | 0.280 |
| Cephalosporins, 2nd generation | 0.3 ± 1.3 | 0.3 ± 1.2 | −0.04 | 0.608 | 0.0 ± 0.2 | 0.4 ± 1.3 | 0.13 | 0.134 |
| Cephalosporins, 3rd generation | 1.6 ± 4.7 | 1.7 ± 4.5 | −0.05 | 0.595 | 2.3 ± 6.2 | 1.5 ± 3.8 | −0.07 | 0.392 |
| Cephalosporin, 4th generation (cefepime) | - | 0.2 ± 2.0 | 0.13 | 0.114 | - | 0.2 ± 2.3 | 0.05 | 0.566 |
| Fluoroquinolones | 4.1 ± 11.0 | 4.9 ± 11.0 | 0.12 | 0.156 | 7.1 ± 15.0 | 4.2 ± 9.3 | −0.11 | 0.181 |
| Glycopeptides | 0.1 ± 0.6 | 0.3 ± 1.6 | 0.21 | 0.012 | 0.1 ± 0.5 | 0.4 ± 1.8 | 0.08 | 0.335 |
| Imidazole (metronidazole) | - | 0.5 ± 5.9 | 0.13 | 0.114 | - | 0.7 ± 6.8 | 0.05 | 0.566 |
| Macrolides | 0.1 ± 1.0 | 0.3 ± 2.6 | 0.12 | 0.142 | 0.2 ± 1.4 | 0.4 ± 2.9 | 0.02 | 0.794 |
| Sulfamethoxazole/trimethoprim | 3.1 ± 23.0 | 3.0 ± 20.0 | −0.01 | 0.918 | 0.9 ± 4.4 | 3.7 ± 22.9 | 0.06 | 0.481 |
| Tetracycline (doxycycline) | 0.8 ± 7.7 | - | −0.05 | 0.529 | 2.2 ± 13.0 | - | −0.15 | 0.083 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geum, M.J.; Kim, C.; Kang, J.E.; Choi, J.H.; Kim, J.S.; Son, E.S.; Lim, S.M.; Rhie, S.J. Broad-Spectrum Antibiotic Regimen Affects Survival in Patients Receiving Nivolumab for Non-Small Cell Lung Cancer. Pharmaceuticals 2021, 14, 445. https://doi.org/10.3390/ph14050445
Geum MJ, Kim C, Kang JE, Choi JH, Kim JS, Son ES, Lim SM, Rhie SJ. Broad-Spectrum Antibiotic Regimen Affects Survival in Patients Receiving Nivolumab for Non-Small Cell Lung Cancer. Pharmaceuticals. 2021; 14(5):445. https://doi.org/10.3390/ph14050445
Chicago/Turabian StyleGeum, Min Jung, Chungsoo Kim, Ji Eun Kang, Jae Hee Choi, Jae Song Kim, Eun Sun Son, Sun Min Lim, and Sandy Jeong Rhie. 2021. "Broad-Spectrum Antibiotic Regimen Affects Survival in Patients Receiving Nivolumab for Non-Small Cell Lung Cancer" Pharmaceuticals 14, no. 5: 445. https://doi.org/10.3390/ph14050445
APA StyleGeum, M. J., Kim, C., Kang, J. E., Choi, J. H., Kim, J. S., Son, E. S., Lim, S. M., & Rhie, S. J. (2021). Broad-Spectrum Antibiotic Regimen Affects Survival in Patients Receiving Nivolumab for Non-Small Cell Lung Cancer. Pharmaceuticals, 14(5), 445. https://doi.org/10.3390/ph14050445






