Abstract
Hexarelin, a synthetic hexapeptide, exerts cyto-protective effects at the mitochondrial level in cardiac and skeletal muscles, both in vitro and in vivo, may also have important neuroprotective bioactivities. This study examined the inhibitory effects of hexarelin on hydrogen peroxide (H2O2)-induced apoptosis in Neuro-2A cells. Neuro-2A cells were treated for 24 h with various concentrations of H2O2 or with the combination of H2O2 and hexarelin following which cell viability and nitrite (NO2−) release were measured. Cell morphology was also documented throughout and changes arising were quantified using Image J skeleton and fractal analysis procedures. Apoptotic responses were evaluated by Real-Time PCR (caspase-3, caspase-7, Bax, and Bcl-2 mRNA levels) and Western Blot (cleaved caspase-3, cleaved caspase-7, MAPK, and Akt). Our results indicate that hexarelin effectively antagonized H2O2-induced damage to Neuro-2A cells thereby (i) improving cell viability, (ii) reducing NO2− release and (iii) restoring normal morphologies. Hexarelin treatment also reduced mRNA levels of caspase-3 and its activation, and modulated mRNA levels of the BCL-2 family. Moreover, hexarelin inhibited MAPKs phosphorylation and increased p-Akt protein expression. In conclusion, our results demonstrate neuroprotective and anti-apoptotic effects of hexarelin, suggesting that new analogues could be developed for their neuroprotective effects.
1. Introduction
Growth hormone secretagogues (GHS) are a class of synthetic oligopeptides and non-peptidyl molecules endowed with endocrine and extra-endocrine properties. GHS preferentially recognize and bind the ghrelin receptor, known as growth hormone secretagogue receptor type-1a (GHS-R1a) [1]. The GHS-R1a is predominantly found in the hypothalamus and pituitary gland where it mediates the release of growth hormone (GH). The GHS-R1a is also implicated in regulation of gastrointestinal motility, as well as energy and glucose homeostasis [2].
In addition to their endocrine effects, GHS also target peripheral tissues; to illustrate, GHS improve muscle function in several pathological conditions by inhibiting the apoptosis pathway, reducing nitric oxide (NO) production, and counteracting inflammation [3,4].
Hexarelin (a synthetic hexapeptide) binds not only to the GHS-R1a but also the CD36 receptor and manifests varied beneficial effects in diseases associated with muscle wasting [5,6,7], chronic heart failure [8], excitotoxicity, neurological disorders, epilepsy and diabetes [9]. Despite the emerging biological importance of hexarelin, its signalling mechanisms have been only partially elucidated. Some studies have demonstrated that hexarelin modulates activation of different intracellular pathways, such as mitogen-activated protein kinases (MAPKs) and phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) [10,11], and, thereby, could indirectly influence intracellular calcium (Ca2+) concentrations [12]. Furthermore, hexarelin protects cells in vitro from apoptosis by inhibiting NO synthesis and reactive oxygen species (ROS) release, modulating caspases activity as well as the expression of proteins belonging to the BCL-2 family [6,12,13,14,15,16].
Oxidative stress is involved in the progression of many neuronal disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS), as well as in cancer, diabetes, and aging [17,18,19].
Hydrogen peroxide (H2O2) is the most abundant ROS generated through oxidative stress in mitochondria [20]. H2O2 is formed by dismutation of superoxide radical anions catalysed by superoxide dismutase (SOD); it is freely soluble in aqueous solutions and easily penetrates biological membranes [21]. The diffusibility of H2O2 in intercellular fluids and the intracellular space can enhance mitochondria damage and potentiate the intrinsic apoptotic pathway [21,22,23].
The neuroprotective and anti-apoptotic effects of hexarelin have not yet been assessed. Therefore, the primary aims of this study are to characterize the effects of hexarelin on H2O2-induced stress and to explore its neuroprotective mechanisms of action in a mouse neuroblastoma cell line (Neuro-2A cells), a well-established in vitro model, frequently used to study the neuroprotective activities of new drugs [24,25,26].
2. Results
2.1. Effects of Hexarelin on H2O2-Induced Toxicity in Neuro-2A Cells
Neuro-2A cells were treated with increasing concentrations of H2O2 (50–200 µM) for 24 h in order to assess its effects on cell replication and to identify the lower concentration that significantly and reproducibly inhibited cell growth. H2O2 reduced cell replication in a concentration-dependent manner (Figure 1A). As H2O2 at 100 µM significantly and reproducibly decreased cell replication (p < 0.001) compared with control, it was used in subsequent experiments. The effects of H2O2 were also confirmed by morphological observation of cells using Motic AE2000 Inverted Microscope. Neuro-2A cells exposed to H2O2 exhibited loss of confluence, disappearance of neurites, as well as grouping and shrinkage that were more marked with increasing H2O2 concentrations (Figure 1B).
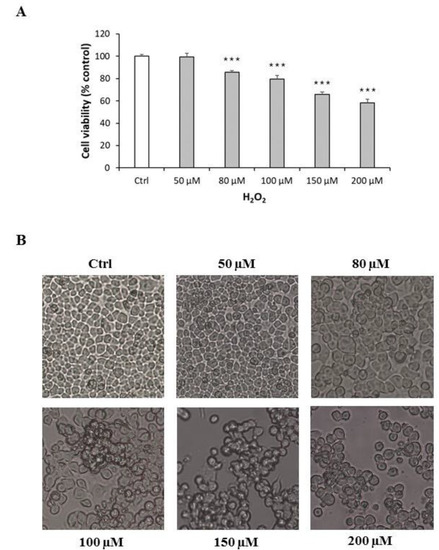
Figure 1.
Cytotoxic effect and morphological alterations of Neuro-2A cells in response to increasing concentration of H2O2. (A) Neuro-2A cells were treated for 24 h with different concentrations of H2O2 (0, 50, 80, 100, 150, 200 μM) and assessed for MTT assay. The assay was performed in at least three independent experiments (n = 21). Statistical significance: *** p < 0.001 vs. CTRL. (B) Morphological changes in Neuro-2A cells were observed by Motic AE2000 Inverted Microscope, at 10× magnification. Representative images of cells treated with increasing concentration of H2O2.
Exposure of Neuro-2A cells to various concentration of hexarelin (10 Nm–10 µM) alone for 24 h did not reduce cell viability; consequently, 1 µM hexarelin was used in subsequent experiments (Figure 2A). The viability of cells treated with the combination of H2O2 and hexarelin for 24 h was similar to that of control but significantly (p < 0.001) greater than that of cells treated with H2O2 alone (Figure 2B).
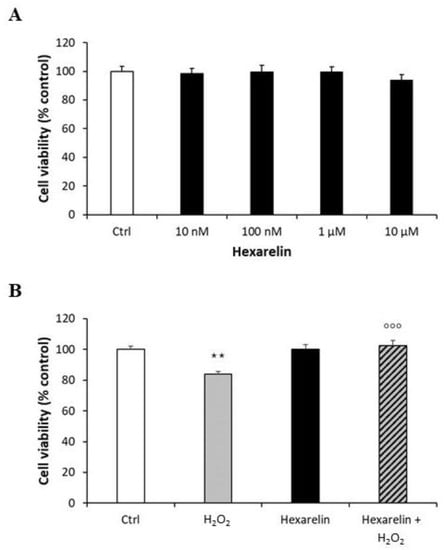
Figure 2.
Protective action of hexarelin in Neuro2A cells. (A) Neuro-2A cells were treated for 24 h with different concentrations of hexarelin (10 nM, 100 nM, 1 µM, and 10 µM), (B) with or without hexarelin (1 µM) and H2O2 (100 μM) and assessed for MTT assay. All assays were performed in at least three independent experiments (n = 21). Statistical significance: ** p < 0.01 vs. CTRL; °°° p < 0.001 vs. H2O2.
2.2. Effects of Hexarelin on NO2− Production on H2O2-Induced Neuro-2A Cells
Nitric oxide (NO) is a highly reactive cytotoxic free radical, which can be induced by oxidative stress. Extracellular nitrite (NO2−) concentrations, proportional to NO formation induced by H2O2, were measured by the Griess assay.
Treatment of cells with 1 µM hexarelin alone had no significant effect on NO2− release compared with control; by comparison, H2O2-treatment (100 µM) alone significantly increased levels of NO2− (p < 0.05) compared with control (Figure 3). In sharp contrast, extracellular NO2− levels in cells co-incubated with 1 µM hexarelin and 100 µM H2O2 were significantly (p < 0.05) lower than in cells treated with H2O2 alone.
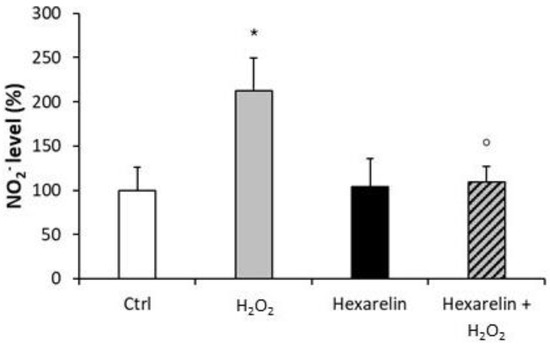
Figure 3.
Hexarelin reduced the extracellular NO2− release induced by H2O2. Neuro-2A cells were treated for 24 h with or without hexarelin and 100 µM H2O2. The culture media were used for Griess reaction to measure NO2− extracellular release. Data are expressed as mean ± SEM of 4 replicates (n = 24). Statistical significance: * p < 0.05 vs. CTRL; ° p < 0.05 vs. H2O2.
These results suggest that the anti-apoptotic properties of hexarelin may be mediated by a presumptive antioxidant mechanism.
2.3. Effects of Hexarelin on Morphological Changes Induced by H2O2 Treatment
Neuro-2A cells were stained as described in Materials and Methods and observed with a confocal laser-scanning microscope (LSM 710, ZEISS, Jena, Germany) in order to characterize morphological changes induced by treatments. A representative photomicrograph for each treatment is presented in Figure 4A. First, we quantified the number of Neuro-2A cells in a fixed area (Figure 4B) by the use of a specific macro for ImageJ software [27]. As shown in Figure 4B, H2O2 induced a significant reduction in the number of cells per field (p < 0.001) compared with control; by comparison, cell numbers were significantly greater when treated with the combination of H2O2 and hexarelin 1 µM (p < 0.05).
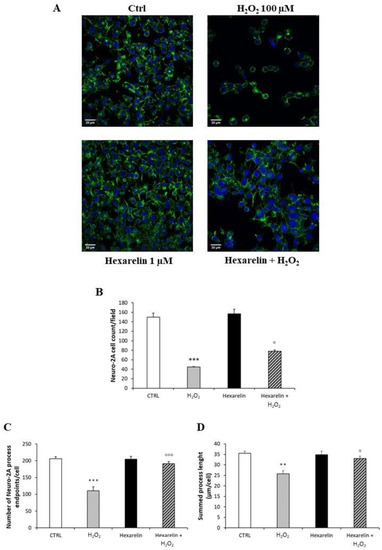
Figure 4.
Hexarelin reduces Neuro-2A cells de-ramification induced by H2O2. (A) Neuro-2A cells were seeded on poly-D-lysine pre-treated coverslips and incubated for 24 h with or without hexarelin and 100 µM H2O2. At the end of the treatment, cells were fixed and stained for phalloidin and DAPI. Images were captured with confocal laser scan microscope. Scale bar: 20 µm. (B) Graphical representation of the number of cells in the same areas per each treatment, (C) of the process endpoints/cells and (D) process length/cells. Data are expressed as mean ± SEM of 3 replicates (total number of cells analyzed = 160). Statistical significance: ** p < 0.01, *** p < 0.001 vs. CTRL; ° p < 0.05, °°° p < 0.001 vs. H2O2.
The number of cells in each field was used for normalizing data of skeleton analysis, which in turn was used to quantify endpoints and process length [28,29], since the loss of ramifications is a typical characteristic of morphological cytoskeletal changes in apoptosis. Briefly, Analyze Skeleton Plugin was applied to skeleton images obtained after a series of ImageJ plugin protocols of original photomicrographs, as described in Material and Methods, and shown in Figure 12. Process length (Figure 12B, orange) indicate the measure of processes elongation, while endpoints (Figure 12B, blue) are the termination of cellular ramifications.
Figure 4C,D reveal that H2O2 treatment alone caused both a reduction of cellular process endpoints and a reduction in summed process length per cell compared with control (p < 0.001 and p < 0.01, respectively); by comparison, these effects of H2O2 alone were significantly antagonized by hexarelin co-incubation (p < 0.001 and p < 0.05, respectively).
We used FracLac for ImageJ to investigate and quantify morphological changes of Neuro-2A cells as a consequence of treatments. Examples of cropped photomicrographs, binary and outline transformations are shown in Figure 5.
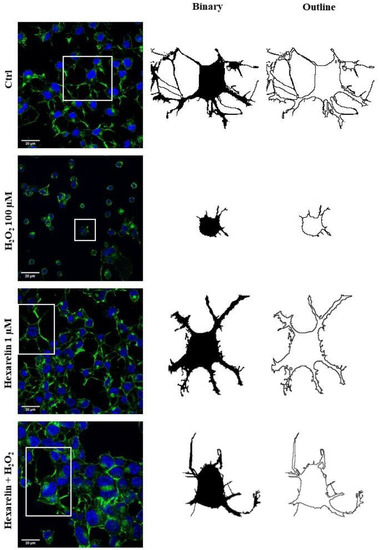
Figure 5.
Effects of hexarelin on morphology of Neuro-2A cells stimulated with H2O2. Representative photomicrographs of Neuro-2A cells incubated for 24 h with or without hexarelin and 100 µM H2O2, and examples of cell binarized and outlined. Cells were seeded on poly-D-lysine pre-treated coverslips and, at the end of the treatments, were fixed and stained with phalloidin and DAPI. Images were captured with a confocal laser scan microscope. Scale bar: 20 µm.
Fractal dimension (D) is an index of cell complexity pattern which is used to identify cellular forms ranging from simple rounded to complex branched (Figure 13B) [29,30]. As shown in Figure 6A, H2O2 significantly decreased D (p < 0.001) compared with the control, indicating a reduced branch complexity, according to skeleton analysis. Conversely, Neuro-2A cells treated with the combination of H2O2 with 1 µM hexarelin exhibited a significantly greater D value (p < 0.001) compared with cells treated with H2O2 alone and which was similar to the D value of controls.
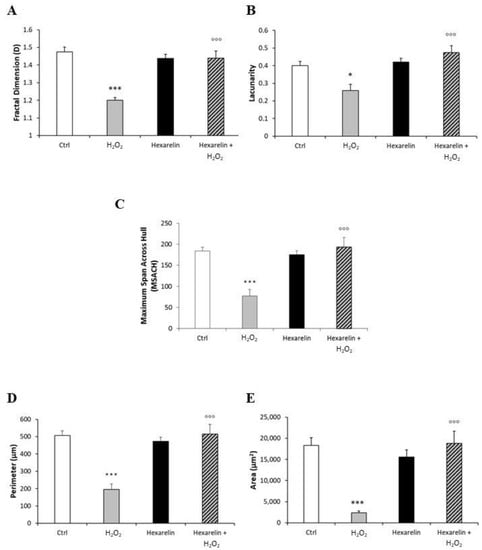
Figure 6.
Hexarelin modulates morphological changes of Neuro-2A cells induced by H2O2 treatment. (A) Fractal dimension, (B) lacunarity, (C) maximum span across hull, (D) perimeter, and (E) area graphical representation of Neuro-2A cells treated for 24 h with or without hexarelin and 100 µM H2O2. Total number of cells analyzed for each condition = 10. Data are expressed as mean ± SEM. Statistical significance: * p < 0.05, *** p < 0.001 vs. CTRL; °°° p < 0.001 vs. H2O2.
The lacunarity values of cells treated with 100 µM H2O2 alone, which ranged from 0.12 to 0.38, were significantly smaller than those of the control group. Lacunarity is a property of the soma based on the heterogeneity or translational and rotational invariance in a shape (Figure 13C); lower lacunarity values indicate a loss of shape heterogeneity [29,30]. Hexarelin antagonized the effects of H2O2, since cells treated with their combination showed values ranging 0.33 to 0.67, significantly greater (p < 0.001) compared to those treated with H2O2 alone (Figure 6B).
Finally, we analyzed Neuro-2A cell size by: (i) the maximum span across the convex hull (MSACH), which is the maximum distance between two points across the convex hull (Figure 13E); (ii) perimeter, calculated as the number of pixels on the outline cell shape (Figure 13D); and (iii) area, quantified as the total number of pixels present in the filled shape of cell image (Figure 13A “Binary”).
H2O2 treatment alone significantly reduced MSACH, perimeter and area (Figure 6C–E; p < 0.001 for all); this reduction was attenuated by co-incubation with hexarelin.
The morphological results obtained with skeleton and fractal analysis in a 3D scatter plot are summarized in Figure 7. We have chosen to represent endpoints/cell, fractal dimension and lacunarity because they are independent variables and allow better appreciation of apoptosis-induced cytoskeletal changes.
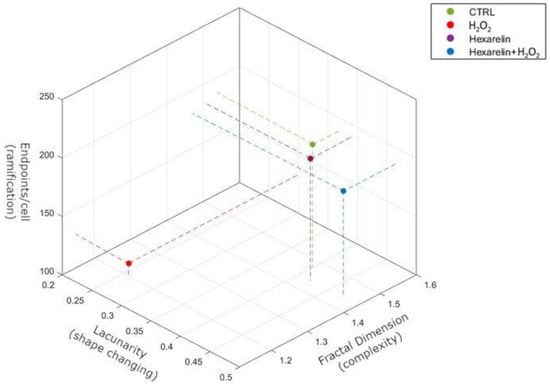
Figure 7.
Summary representation of Skeleton and FracLac analysis by 3D scatter plot. The figure summarizes the relationship between ramifications (endpoints/cell), shape changing (lacunarity) and complexity (fractal dimension). Pearson’s correlation demonstrates that fractal dimension significantly correlates with endpoints/cell (r = 0.9806, p = 0.0098) but not with lacunarity (r = 0.8134, p = 0.0981). Endpoints/cell directly correlates with both fractal dimension (r = 0.9806, p = 0.0098) and lacunarity (r = 0.7765, p = 0.0038).
2.4. Effects of Hexarelin on Caspases-3 and -7 and on BCL-2 Family mRNA Levels
Caspase-3 and caspase-7 activation after damage induced by H2O2 occur at an early stage of apoptotic cell death; therefore, we hypothesized that hexarelin could inhibit caspase-3 and caspase-7 mRNA levels.
First, we demonstrated that mRNA levels of both caspases were increased by H2O2 in a dose-dependent manner and that 100 µM H2O2 induced a significant increase in caspase-3 (p < 0.01) and caspase-7 (p < 0.001) mRNA levels (Figure 8A,B). Hexarelin alone did not affect mRNA levels of both caspases (Figure 8C,D). Notably, 1 µM hexarelin antagonized the increase in caspase-3 mRNA levels induced by 100 µM H2O2 (Figure 8D), whereas it did not oppose the effects of 100 µM H2O2 on caspase-7 mRNA levels (p < 0.001, Figure 8C).
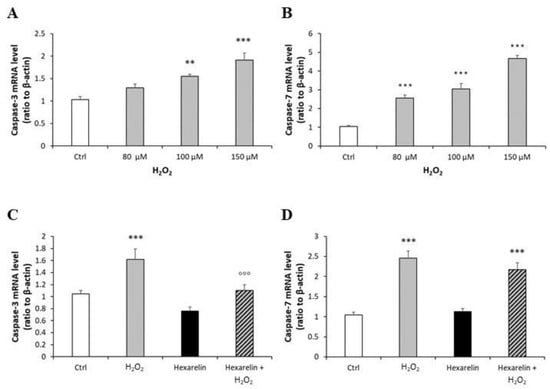
Figure 8.
Analysis of caspase-3 and caspase-7 mRNA levels following H2O2 exposure and their modulation induced by hexarelin. Neuro-2A cells were treated with different concentrations of H2O2 (80, 100, 150 μM) or co-incubated with 1 µM hexarelin and 100 µM H2O2 for 24 h. Caspase-3 (A,C) and caspase-7 (B,D) mRNA levels were measured by RT-PCR and normalized for the respective β-actin mRNA levels. Data are expressed as mean ± SEM of 3 replicates (n = 18). ** p < 0.01, *** p < 0.001 vs. CTRL; °°° p < 0.001 vs. H2O2.
Moreover, we also quantified the cellular content of activated caspase-3 and -7 proteins; both species are effector caspases, which are activated through proteolytic processing by upstream caspases to produce the mature subunit. Western blot analysis showed that H2O2 treatment significantly increased cleaved caspase-3 and cleaved caspase-7 (p < 0.001) (Figure 9A,B), whereas co-incubation with hexarelin blunted these effects only for caspase-3 activation (p < 0.05) (Figure 9A).
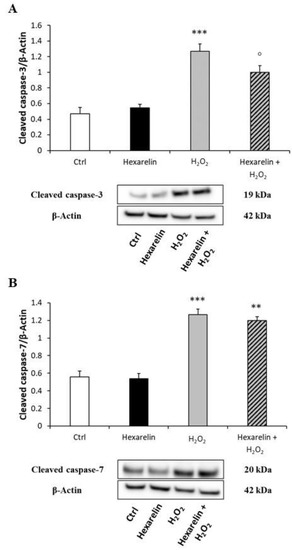
Figure 9.
Hexarelin inhibits apoptotic pathway through caspase-3 inactivation. Neuro-2A cells were treated with or without hexarelin and H2O2 for 24 h and assessed in Western blot for cleaved caspase-3/β-actin (A), and cleaved caspase-7/β-actin (B). All assays were performed in at least 3 independent experiments (n = 3). Statistical significance: ** p < 0.01, *** p < 0.001 vs. CTRL; ° p < 0.05 vs. H2O2.
Mitochondria also play a crucial role in the process of cell apoptosis with the activation of the BCL-2 protein family. H2O2, in a concentration-dependent manner, caused a significant increase in the pro-apoptotic Bax mRNA levels (Figure 10A) and tended to increase levels of the anti-apoptotic Bcl-2 mRNA (Figure 10B). Co-incubation for 24 h with hexarelin and H2O2 reveal the anti-apoptotic effects of hexarelin (Figure 10C,D). The mRNA levels of Bax (stimulates by H2O2) were significantly (p < 0.05) reduced by hexarelin co-incubation; by contrast, hexarelin co-incubation significantly (p < 0.01) increased Bcl-2 mRNA levels.
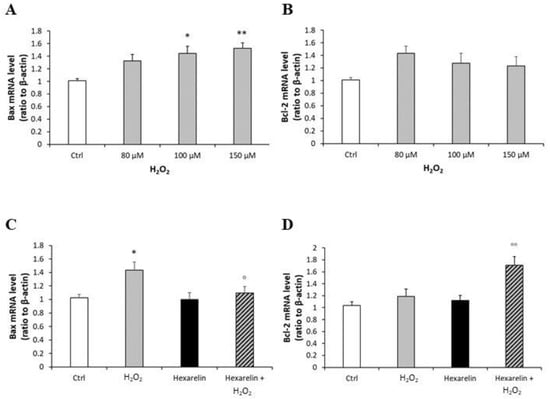
Figure 10.
Analysis of mRNA levels of apoptosis markers following H2O2 exposure and their modulation induced by hexarelin. Neuro-2A cells were treated with different concentrations of H2O2 (80, 100, 150 μM) or co-incubated with hexarelin 1 µM and H2O2 100 µM for 24 h. Bax (A,C) and Bcl-2 (B,D) mRNA levels were normalized for the respective β-actin mRNA levels. Data are expressed as mean ± SEM of 3 replicates (n = 18). * p < 0.05, ** p < 0.01 vs. CTRL; ° p < 0.05, °° p < 0.01 vs. H2O2.
2.5. Effects of Hexarelin on ERK 1/2, p38 and Akt Protein Levels in H2O2-Treated Neuro-2A Cells
We hypothesized that hexarelin could modify MAPK signalling as well. Among members of the MAPK family, ERK and p38 are known to be associated with cell death or survival, respectively [10].
Compared to the control group, exposure to 100 µM H2O2 alone significantly increased the p-ERK/t-ERK ratio (p < 0.05), whereas 1 µM hexarelin alone did not affect ERK protein levels. Notably, co-incubation with hexarelin and H2O2 induced a trend toward a reduction of p-ERK protein levels compared to the H2O2 alone group (Figure 11A).
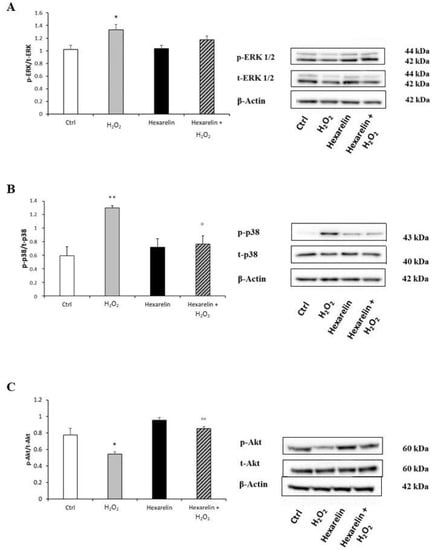
Figure 11.
Hexarelin modulates ERK, p38 and Akt activation. Neuro-2A cells were treated with or without hexarelin and H2O2 for 24 h and Western blot was used to measure p-ERK/t-ERK (A), p-p38/t-p38 (B), and p-Akt/t-Akt (C) levels. β-actin was used to control even protein loading in all lines. All assays were performed at least 3 independent experiments. Statistical significance: * p < 0.05, ** p < 0.01 vs. CTRL; ° p > 0.05, °° p < 0.01 vs. H2O2.
Incubation with 100 µM H2O2 alone significantly increased the p-p38/t-p38 ratio compared to controls, and this effect was significantly antagonized by co-incubation with hexarelin (p < 0.05) (Figure 11B).
Furthermore, levels of p-Akt, associated with cell survival after oxidative stress [31], were significantly reduced by H2O2 treatment (p < 0.05, Figure 11C). Notably, p-Akt levels in cells coincubated with hexarelin and H2O2 were significantly (p < 0.01) greater than those in cells treated with H2O2 alone.
3. Discussion
Our in vitro findings clearly demonstrate that hexarelin protects Neuro-2A cells from H2O2-induced damage.
Oxidative stress arises when the balance between oxidants and antioxidants is disrupted in favour of the former, resulting in potential damage to the organism [21,32]. Although each neurodegenerative disease (NDD) has its own distinct aetiology and differentially affects brain regions, NDDs share elements of oxidative stress, free radical generation and mitochondrial changes, leading to apoptosis [33].
Apoptosis is known to be one of the most sensitive biological markers for evaluating oxidative stress caused by imbalance between ROS generation and efficient activity of antioxidant systems [34,35]. Apoptotic cell death is an active process initiated by genetic programs and culminating in DNA fragmentation, characterized by morphological changes, including cell shrinkage, formation of membrane-packaged inclusions, called apoptotic bodies [23], activation of caspases, nucleases, inactivation of nuclear repair polymerases [36], and finally condensation of nuclei [37].
There are numerous inducers of oxidative stress that are capable of causing cytotoxicity and apoptosis in in vitro models. In our study, we used H2O2 because it is an established method for potency measurement of neuroprotective agents candidates [38,39].
In this study, H2O2 induced neuronal cytotoxicity in Neuro-2A cells in a dose-dependent manner, as demonstrated by both MTT assay and morphological observations, while increasing concentrations of hexarelin, which by itself did not affect cell viability. In order to determine the protective effects of GHS against H2O2-induced cytotoxicity, Neuro-2A cells were treated with 1 µM hexarelin. As expected, cell viability of the hexarelin treated group was similar to control cells; by contrast, H2O2-induced cell death was significantly attenuated by hexarelin.
The protective effects of hexarelin were further confirmed by Griess assay. Excessive levels of NO, an important mediator of cellular communication, implicated in the pathogenesis of NDDs [40] and in caspase-dependent cellular death [41], could be quantified by the measurement of extracellular NO2−, a primary stable product of NO breakdown. Previous studies reported that H2O2-induced insult resulted in increased production of NO in neuronal and glial cells [42,43] through the induction of inducible nitric oxide synthase. In this study, we demonstrated that in Neuro-2A cells H2O2 increased extracellular NO2− release, an effect that was blunted by the coincubation with hexarelin. The ability of hexarelin to reduce NO2− release suggests that its protective effects against H2O2 oxidative stress could be mediated through the modulation of apoptosis and downstream pathways.
Moreover, H2O2-stimulation of Neuro-2A cells induced morphological changes characteristic of an apoptotic phenotype, including de-ramification and reduction of process length, loss of cellular complexity and shape, as well as reduction of cell size. Both skeleton and fractal analysis suggested that hexarelin preserve the cellular complexity, ramification, dimension, heterogeneity and shape comparable to values observed in the control group.
Hexarelin is a synthetic hexapeptide ligand of the GHS-R1a, which is chemically more stable and functionally more potent when compared with ghrelin [44]; consequently, these characteristics make hexarelin a promising alternative to ghrelin [45]. Ghrelin has been demonstrated to protect several cell types such as adipocytes [46], osteoblasts [47], cardiomyocytes and endothelial cells [48] by inhibiting apoptotic stimuli. As well, ghrelin has been shown to have protective effects in vivo, in rats exposed to status epilepticus [49] or in a rat model of cisplatin-induced cachexia [4], and to promote neurogenesis [50].
Similarly to ghrelin, hexarelin has been shown to stimulate cell proliferation of adult hippocampal progenitor (AHP) cells and to protect against growth factor deprivation-induced apoptosis and necrosis [51], principally through the activation of PI3K/Akt pathway [50,52]. Interestingly, hexarelin also blunts the inflammatory process activated by neurodegenerative diseases, stroke, and tumor invasion [53] largely by modulating the release of pro-inflammatory mediators such as cytokines, reactive oxygen species, free radical species and nitric oxide, which could contribute to both neuronal dysfunction and cell death [54,55].
Hexarelin also exert cardioprotective effects, attenuating cardiomyocyte hypertrophy and apoptosis [56], and attenuates mitochondrial abnormalities reported in cancer cachexia [5,57], inducing biogenesis, mitochondrial mass and dynamics restoration, reducing expression of autophagy-related genes and ROS production. This study demonstrates anti-apoptotic effects of hexarelin via the inhibition of caspases activation. Neuro-2A cells treated for 24 h with increasing concentrations of H2O2 showed a significant activation of caspase-3 and -7. Treatment of cells with 1 µM hexarelin attenuated primarily the activation of caspase-3, both in terms of mRNA levels and protein activation. Our hypothesis was that the modulation of caspase mRNA levels induced by hexarelin was dependent on the intracellular pro-apoptotic signalling molecules belonging to the BCL-2 family.
The BCL-2 family consist of two groups of mediators including (i) anti-apoptotic group mainly represented by Bcl-2, and (ii) a pro-apoptotic group, represented principally by Bax. Both groups play important roles in mitochondrial related apoptosis pathways [58].
Therefore, we quantified, by RT-PCR, the effects of hexarelin on modulation of the expression in Bcl-2 and Bax mRNA levels. As expected, H2O2 treatment of Neuro-2A cells induced a concentration-dependent activation of pro-apoptotic Bax, and the inhibition of anti-apoptotic Bcl-2. Hexarelin treatment did not affect mRNA levels of apoptotic signalling molecules compared to the control group, demonstrating that hexarelin does not stimulate the apoptosis pathway. At the same time, the decrease in Bax mRNA levels and the increase in Bcl-2 mRNA levels, in the group incubated with the combination of hexarelin and H2O2 confirmed the anti-apoptotic effect of hexarelin.
In order to investigate the molecular pathways involved in hexarelin neuroprotection, we quantified the expression of MAPKs (ERK and p38) and PI3K/Akt. MAPKs activation contribute to neuronal dysfunction and are involved in NDDs [59,60]. Furthermore, ERK has been shown to participate in the regulation of cell growth and differentiation, and responses to cellular stress [61].
In this study, treatment of Neuro-2A cells with H2O2 led to cell death by up-regulating p-ERK and p-p38 protein expression. The up-regulation of MAPKs induced by H2O2 stimulation were blunted by hexarelin treatment. In addition, hexarelin alone did not affect the MAPKs proteins compared to the control.
PI3K/Akt is a key anti-apoptotic effector in the growth factor signalling pathway [62]. In particular, the phosphorylation of Thr-308 and Ser-473 of Akt serves a key role in mediating the anti-apoptotic actions of growth factors on cells and plays an important role in neuronal protection [63,64]. In this study, H2O2-induced oxidative stress significantly increased the de-phosphorylation of Akt, which stimulated the activation of the apoptotic pathway. Hexarelin treatment did not alter the p-Akt/t-Akt ratio compared to controls, but in cells treated for 24 h with both hexarelin and H2O2, Akt protein levels were significantly increased compared with cells treated with H2O2 alone.
In conclusion, our findings demonstrate that H2O2 caused early and late apoptotic pathways in Neuro-2A cells. Treatment of cells with hexarelin antagonized H2O2 cellular cytotoxicity, inhibited apoptosis and potentiated MAPKs and PI3K/Akt survival pathways.
This study demonstrates that hexarelin is capable of protecting Neuro-2A cells from H2O2-caused cytotoxicity effects; however, further investigations are required to clarify hexarelin molecular mechanisms of action, and whether its effects are mediated by the ghrelin receptor (GHS-R1a).
4. Materials and Methods
4.1. Chemicals
Hexarelin, Dulbecco’s Modified Eagle’s Medium (DMEM)-high glucose, hydrogen peroxide (H2O2), 3 (4,5 dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT), Griess reagent, poly-D-lysin hydrobromide, 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI), fluoromount aqueous mounting medium and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Penicillin, streptomycin, L-glutamine, trypsin-EDTA, phosphate-buffer saline (PBS) and fetal bovine serum (FBS) were obtained from Euroclone (Pero, Milan, Italy). Alexa Fluor 488 Phalloidin was purchased by ThermoFisher Scientific (Waltham, MA, USA). Prior to assay, hexarelin was dissolved in ultrapure water; both hexarelin and H2O2 were diluted in culture medium to final working concentrations.
4.2. Cell Culture
Immortalized Neuro-2A murine neuroblastoma cells were obtained from Interlab Cell Line Collection (ICLC, Genoa, Italy) and cultured in DMEM-high glucose (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% heat-inactivated FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin and 2 mM L-glutamine (all Euroclone, Pero, Italy) and Mycozap Prophylactic (Lonza, Walkersville, MD, USA) under standard cell culture conditions (37 °C, 5% CO2) [24,25]. Confluent cultures were washed with PBS, detached with trypsin-EDTA solution (all Euroclone, Pero, Italy), and used for experiments.
In each experiment, Neuro-2A cells were incubated with H2O2 alone or the combination of 100 µM H2O2 and 1 μM hexarelin for 24 h.
4.3. Cell Viability
Neuro-2A cells were seeded in 96-well culture plates at the density of 4 × 104 cells/well and cultured for 24 h at 37 °C. The day after seeding, the cells were treated with increasing concentrations (50–200 µM) of H2O2 or hexarelin (10 nM–10 µM) or with 100 µM H2O2 and hexarelin (1 µM). After 24 h of treatment, a 10 µL aliquot of 5 mg/mL MTT (M5655, Sigma-Aldrich, St. Louis, MO, USA) was added to each well and incubated at 37 °C for 3 h. Then, the culture medium was removed and a 200 µL aliquot of acidified isopropanol was added in order to dissolve the formazan crystals. Absorbance was read at 570 nm using a multilabel spectrophotometer VICTOR3 (Perkin Elmer, Waltham, MA, USA). Cell viability of control cells was set to 100% and the relative absorbances of experimental groups were converted to relative percentages (relative absorbance of experimental group/relative absorbance of control) × 100 = % of viable cells.
4.4. Griess Assay
NO production was evaluated measuring the nitrite (NO2−) content of culture media with the Griess reaction. Briefly, Neuro-2A cells were plated in 96-well culture plates and treated with 100 µM H2O2 and 1 µM hexarelin for 24 h. At the end of the treatment, 100 µL aliquots of medium were transferred to a new 96-well plate and were mixed with 100 µL of Griess reagent 1 × (G4410, Sigma-Aldrich, St. Louis, MO, USA). Absorbance was measured at 540 nm with the VICTOR3 spectrophotometer (Perkin Elmer, Waltham, MA, USA), after 15 minutes in the dark at room temperature. A standard curve with varied concentrations of sodium nitrite (S2252, Sigma-Aldrich, St. Louis, MO, USA) was conducted in parallel and used for quantification. NO2− of control cells was set to 100% and the relative absorbance of experimental groups were converted to relative percentages (relative absorbance of experimental group/relative absorbance of control) × 100 = % of NO2−. The final nitrite concentration is proportional to the NO metabolite present in the sample.
4.5. Observation of Morphological Changes
Neuro-2A cells were seeded in 6-well culture plates at a density of 80 × 104 cells/well and incubated at 37 °C for 24 h. Cells were incubated with various concentrations (50 to 200 µM) of H2O2 and photographed 24 h later using a Motic AE2000 Inverted Microscope (Motic, Hong Kong, China).
4.6. Actin Staining Assay
Neuro-2A cells (2 × 105 cells/well) were seeded on coverslips coated with poly-D-lysine (P0899, Sigma-Aldrich, St. Louis, MO, USA) for 24 h. Cells were incubated at 37 °C with 100 µM H2O2 for 24 h with or without hexarelin 1 µM, then washed with PBS and fixed with 4% paraformaldehyde (Titolchimica, Rovigo, Italy) for 10 min at room temperature. Cells were subsequently washed with PBS, incubated with cold acetone for 5 min at −20 °C and blocked in PBS with 1% BSA for 30 min at room temperature. In order to stain actin, Neuro-2A cells were incubated with 2 U/mL of Alexa Fluor 488 Phalloidin diluted in PBS with 1% BSA at room temperature for 20 min and then washed with PBS. Counterstaining of nuclei was made with 1 µg/mL of DAPI for 10 min at room temperature. After washing cells with PBS, fluoromount aqueous mounting medium was added, and the cells were observed under a confocal laser scanning microscope (LSM 710, ZEISS, Jena, Germany); images were captured at 40× and 63× magnification by ZEN software (ZEISS, Jena, Germany).
4.7. Morphological Analysis
Photomicrographs obtained at 40x magnification were used to evaluate the number of cells in the same area using a specifically designed macro with ImageJ software (National Institutes of Health, Bethesda, MD, USA) [27]. The same photomicrographs were used for skeleton analysis [28]. Skeleton analysis was applied to quantify the number of process endpoints and length normalized by the number of cells in the same area. Briefly, the photomicrographs were filtered to soften the background, enhance the contrast and remove noise, by using the application Fiji free software (https://imagej.net/fiji, accessed on 8 March 2021). All the images were then binarized, subsequently skeletonized and analysed with Analyze Skeleton (2D/3D) plugin (http://imagej.net/AnalyzeSkeleton, accessed on 8 March 2021). The overlay of skeleton to original image is reported to demonstrate that skeletons are representative of the original image; all the modification steps are illustrated in Figure 12.
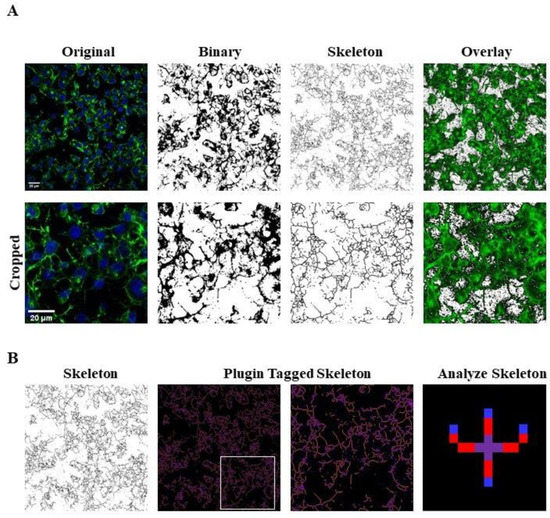
Figure 12.
Skeleton Analysis application to quantify Neuro-2A cells morphology. (A) Representative photomicrograph (40× magnification) and the series of ImageJ plugin protocols which were applied to each photomicrograph for skeleton analysis. Original photomicrograph was modified enhancing the background, removing noise and using with FFT filter prior to be converted to binary images. Binary image was skeletonized. The overlay of skeletonized and original images is reported; cropped photomicrographs show the image details. Scale bar: 20 µm. (B) The workflow used to Analyze Skeleton plugin: skeletonized process in orange, endpoint in blue, and junction in purple.
Moreover, we applied fractal analysis using FracLac plugin for ImageJ (https://imagej.nih.gov/ij/plugins/fraclac/fraclac.html, (accessed on 8 March 2021) in order to evaluate the Neuro-2A shape and morphology by different parameters (fractal dimension, lacunarity, maximum span across the hull, perimeter and area) [30]. Photomicrographs obtained with 63 × oil immersion objective were modified similarly to skeleton analysis. Photomicrographs of cells were cropped and transformed to 8-bit grayscale images. Then, cell images were binarized and manually edited to obtain a single cell made of continuous set of pixels. To avoid bias, this modification was done taking into account the original image. Binary images were outlined and analyzed with Fractal Analysis plugin. All the modification steps and representative images of FracLac box counting analysis are shown in Figure 13.
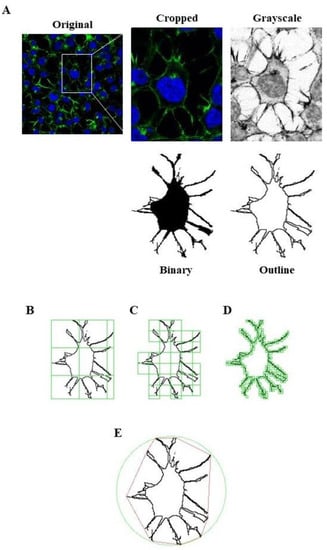
Figure 13.
FracLac Analysis application to quantify Neuro-2A cells morphology. (A) Representative process applied to obtain an outlined single cell for FracLac plugin. After selecting a cell in the photomicrograph (63× magnification), the image was cropped and modified to remove noise and enhance the background. The image was then processed to obtain an 8-bit grayscale microphotograph, and binarized. Binary image was manually edited to clear the background and to join all branches, and finally outlined. FracLac quantifies cell complexity and shape with a box counting method which permits to quantify fractal dimension (B), lacunarity (C), perimeter (D), and the maximum span across the convex hull (E) by drawing a convex hull (pink) and a bounding circle (green).
4.8. Real-Time PCR Analysis
In order to monitor the apoptosis pathway, Neuro-2A cells were plated in 24-well culture plates at a density of 2 × 105 cells/well, and treated for 24 h according to specific protocols. Following treatment, Neuro-2A cells were washed with PBS and total RNA was extracted using EuroGOLD Trifast reagent (Euroclone, Pero, MI, Italy), according to the manufacturer’s instructions and quantified using a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Reverse transcription was performed using iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) to equal amounts (140 ng) of RNA. Amplification of cDNA (21 ng) was performed in a total volume of 20 µL of iTaq Universal Probes Supermix (Bio-Rad), using Real-Time QuantStudio7 Flex (Thermo Fisher Scientific, Waltham, MA, USA). After 2 min at 50 °C and 10 min at 94.5 °C, 40 PCR cycles were performed using the following conditions: 15 s at 95 °C and 1 min at 60 °C. Relative mRNA concentrations of the target genes were normalized to the corresponding β-actin internal control and calculated using the 2−ΔΔCt method.
4.9. Western Blot Analysis
Neuro-2A cells were plated in 6-well culture plates at a density of 8 × 105 cells/well, incubated at 37 °C for 24 h and then treated as previously described. Following treatment, cells were rinsed with ice-cold PBS and lysed in RIPA buffer (Cell Signaling Technology, Danvers, MA, USA), supplemented with a protease-inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA), according to the manufacture’s protocol.
Total protein concentrations were determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts of protein (20 µg) were heated at 95 °C for 10 min, loaded on precast 4–12% gradient gels (Invitrogen, Carlsbad, CA, USA), separated by electrophoresis, and transferred to a polyvinylidene difluoride (PVDF) membrane (Thermo Fisher Scientific, Waltham, MA, USA). Non-specific binding was blocked with 5% dried fat-free milk dissolved in phosphate-buffered saline (PBS) supplemented with 0.1% Tween-20 (PBS-T) for 1 h at room temperature. After 3 washes in PBS-T, membranes were incubated with the primary antibody overnight at 4°C (Anti-cleaved caspase-3 (Asp175) (5A1E) rabbit antibody, #9664, Cell Signaling Technology, 1:1000; anti-cleaved caspase-7 (Asp198) rabbit antibody, #9491, Cell Signaling Technology, 1:1000; anti-Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) rabbit antibody, #9101, Cell Signaling Technology, 1:1000; anti-p44/42 MAPK (Erk1/2) rabbit antibody, #4695, Cell Signaling Technology, 1:1000; anti-Phospho-p38 MAPK (Thr180/Tyr182) rabbit antibody, #4511, Cell Signaling Technology, 1:1000; anti-p38 MAPK rabbit antibody, #9212, Cell Signaling Technology, 1:1000; anti-Phospho-Akt rabbit antibody, #4060, Cell Signaling Technology, 1:2000; anti-Akt rabbit antibody, #4685, Cell Signaling Technology, 1:1000; anti-actin rabbit antibody, #A2066, Sigma Aldrich, 1:2500), and then with a peroxidase-coupled goat anti-rabbit IgG (#31460, Thermo Scientific, 1:5000) for 1 h at room temperature.
Signals were developed with the extra sensitive chemiluminescent substrate LiteAblot TURBO (Euroclone, Pero, Milan, Italy) and detected with Amersham ImageQuant 800 (GE Healthcare, Chicago, IL, USA). Image J software was used to quantify protein bands.
4.10. Statistical Analysis
Statistical analysis was performed using the program GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Values are expressed as mean ± standard error of the mean (SEM). Experiments were independently replicated at least three times. One-way ANOVA followed by Tukey’s t-test was used for multiple comparisons. A p-value of less than 0.05 was considered significant.
Author Contributions
Conceptualization, R.M., A.T. and E.B.; cell biology investigation, R.M., L.R., E.B., L.M. and S.C.; formal analysis, R.M.; validation, R.M., L.R., E.B., L.M. and S.C.; imaging visualization, S.C. and R.M.; writing—original draft preparation, R.M., L.R. and L.M.; writing—review and editing, A.T., R.M., R.J.O. and V.L.; supervision, A.T., E.B., R.J.O. and V.L.; funding acquisition, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Fondo di Ateneo per la Ricerca of the University of Milano-Bicocca [FAR to AT].
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fowkes, M.M.; LaLonde, T.; Yu, L.; Dhanvantari, S.; Kovacs, M.S.; Luyt, L.G. Peptidomimetic growth hormone secretagogue derivatives for positron emission tomography imaging of the ghrelin receptor. Eur. J. Med. Chem. 2018, 157, 1500–1511. [Google Scholar] [CrossRef]
- Arvat, E.; Broglio, F.; Aimaretti, G.; Benso, A.; Giordano, R.; Deghenghi, R.; Ghigo, E. Ghrelin and synthetic GH secretagogues. Best Pr. Res. Clin. Endocrinol. Metab. 2002, 16, 505–517. [Google Scholar] [CrossRef]
- Bresciani, E.; Rizzi, L.; Coco, S.; Molteni, L.; Meanti, R.; Locatelli, V.; Torsello, A. Growth Hormone Secretagogues and the Regulation of Calcium Signaling in Muscle. Int. J. Mol. Sci. 2019, 20, 4361. [Google Scholar] [CrossRef]
- Conte, E.; Bresciani, E.; Rizzi, L.; Cappellari, O.; De Luca, A.; Torsello, A.; Liantonio, A. Cisplatin-Induced Skeletal Muscle Dysfunction: Mechanisms and Counteracting Therapeutic Strategies. Int. J. Mol. Sci. 2020, 21, 1242. [Google Scholar] [CrossRef]
- Sirago, G.; Conte, E.; Fracasso, F.; Cormio, A.; Fehrentz, J.-A.; Martinez, J.; Musicco, C.; Camerino, G.M.; Fonzino, A.; Rizzi, L.; et al. Growth hormone secretagogues hexarelin and JMV2894 protect skeletal muscle from mitochondrial damages in a rat model of cisplatin-induced cachexia. Sci. Rep. 2017, 7, 13017. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Camerino, G.M.; Mele, A.; De Bellis, M.; Pierno, S.; Rana, F.; Fonzino, A.; Caloiero, R.; Rizzi, L.; Bresciani, E.; et al. Growth hormone secretagogues prevent dysregulation of skeletal muscle calcium homeostasis in a rat model of cisplatin-induced cachexia. J. Cachex Sarcopenia Muscle 2017, 8, 386–404. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.; Sun, Y.; Geng, Y.-J.; Yu, X.-Y.; Li, Y. The Molecular Mechanisms and Prevention Principles of Muscle Atrophy in Aging. Tissue Eng. 2018, 1088, 347–368. [Google Scholar] [CrossRef]
- Kamiji, M.M.; Inui, A. The role of ghrelin and ghrelin analogues in wasting disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Frago, L.M.; Baquedano, E.; Argente, J.; Chowen, J.A. Neuroprotective actions of ghrelin and growth hormone secretagogues. Front. Mol. Neurosci. 2011, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lim, E.; Kim, Y.; Li, E.; Park, S. Ghrelin attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. J. Endocrinol. 2010, 205, 263–270. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; Li, E.; Park, S. Ghrelin Protects Spinal Cord Motoneurons Against Chronic Glutamate Excitotoxicity by Inhibiting Microglial Activation. Korean J. Physiol. Pharmacol. 2012, 16, 43–48. [Google Scholar] [CrossRef]
- Liantonio, A.; Gramegna, G.; Carbonara, G.; Sblendorio, V.T.; Pierno, S.; Fraysse, B.; Giannuzzi, V.; Rizzi, L.; Torsello, A.; Camerino, D.C. Growth Hormone Secretagogues Exert Differential Effects on Skeletal Muscle Calcium Homeostasis in Male Rats Depending on the Peptidyl/Nonpeptidyl Structure. Endocrinology 2013, 154, 3764–3775. [Google Scholar] [CrossRef][Green Version]
- Seminara, R.S.; Jeet, C.; Biswas, S.; Kanwal, B.; Iftikhar, W.; Sakibuzzaman, M.; Rutkofsky, I.H. The Neurocognitive Effects of Ghrelin-induced Signaling on the Hippocampus: A Promising Approach to Alzheimer’s Disease. Cureus 2018, 10, e3285. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, E.; Rizzi, L.; Molteni, L.; Ravelli, M.; Liantonio, A.; Salah, K.B.H.; Fehrentz, J.-A.; Martinez, J.; Omeljaniuk, R.J.; Biagini, G.; et al. JMV2894, a novel growth hormone secretagogue, accelerates body mass recovery in an experimental model of cachexia. Endocrine 2017, 58, 106–114. [Google Scholar] [CrossRef]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.-J.; Xu, R.-K.; Xu, X.-B.; Cao, J.-M.; Ni, C.; Zhu, W.-L.; Asotra, K.; Chen, M.-C.; Chen, C. Hexarelin protects rat cardiomyocytes from angiotensin II-induced apoptosis in vitro. Am. J. Physiol. Circ. Physiol. 2004, 286, H1063–H1069. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, S.; Tripathi, V.K.; Bissoyi, A.; Garg, G.; Rizvi, S.I. Rapamycin Confers Neuroprotection Against Aging-Induced Oxidative Stress, Mitochondrial Dysfunction, and Neurodegeneration in Old Rats Through Activation of Autophagy. Rejuvenation Res. 2019, 22, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef]
- Nagase, M.; Yamamoto, Y.; Miyazaki, Y.; Yoshino, H. Increased oxidative stress in patients with amyotrophic lateral sclerosis and the effect of edaravone administration. Redox Rep. 2016, 21, 104–112. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, S.-J. Reactive oxygen/nitrogen species and their functional correlations in neurodegenerative diseases. J. Neural Transm. 2012, 119, 891–910. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.; Chen, Y.; Chen, B.; Huang, H.; Lv, J.; Hu, S.; Shen, J. Vaspin protects mouse mesenchymal stem cells from oxidative stress-induced apoptosis through the MAPK/p38 pathway. Mol. Cell. Biochem. 2019, 462, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Sathya, S.; Shanmuganathan, B.; Devi, K.P. Deciphering the anti-apoptotic potential of α-bisabolol loaded solid lipid nanoparticles against Aβ induced neurotoxicity in Neuro-2a cells. Colloids Surf. B Biointerfaces 2020, 190, 110948. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; Choya-Foces, C.; Hugo, M.; López, V. The Metabolite Urolithin-A Ameliorates Oxidative Stress in Neuro-2a Cells, Becoming a Potential Neuroprotective Agent. Antioxidants 2020, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, V.P.; Yogendra Prasad, K.; Ganesan, P.; Kumar, R. Mechanism of rutin mediated inhibition of insulin amyloid formation and protection of Neuro-2a cells from fibril-induced apoptosis. Mol. Biol. Rep. 2020, 47, 2811–2820. [Google Scholar] [CrossRef]
- Losurdo, M.; Pedrazzoli, M.; D’Agostino, C.; Elia, C.A.; Massenzio, F.; Lonati, E.; Mauri, M.; Rizzi, L.; Molteni, L.; Bresciani, E.; et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Transl. Med. 2020, 9, 1068–1084. [Google Scholar] [CrossRef]
- Morrison, H.; Young, K.; Qureshi, M.; Rowe, R.K.; Lifshitz, J. Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury. Sci. Rep. 2017, 7, 13211. [Google Scholar] [CrossRef] [PubMed]
- D’Aloia, A.; Molteni, L.; Gullo, F.; Bresciani, E.; Artusa, V.; Rizzi, L.; Ceriani, M.; Meanti, R.; Lecchi, M.; Coco, S.; et al. Palmitoylethanolamide Modulation of Microglia Activation: Characterization of Mechanisms of Action and Implication for Its Neuroprotective Effects. Int. J. Mol. Sci. 2021, 22, 3054. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arjona, M.D.M.; Grondona, J.M.; Granados-Durán, P.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis. Front. Cell. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Yune, T.Y.; Park, H.G.; Lee, J.Y.; Oh, T.H. Estrogen-Induced Bcl-2 Expression after Spinal Cord Injury Is Mediated through Phosphoinositide-3-Kinase/Akt-Dependent CREB Activation. J. Neurotrauma 2008, 25, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Nanetti, L.; Raffaelli, F.; Vignini, A.; Perozzi, C.; Silvestrini, M.; Bartolini, M.; Provinciali, L.; Mazzanti, L. Oxidative Stress in Ischaemic Stroke. Eur. J. Clin. Invest. 2011, 41, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995, 41, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Kassie, F.; Parzefall, W.; Knasmüller, S. Single cell gel electrophoresis assay: A new technique for human biomonitoring studies. Mutat. Res. Mutat. Res. 2000, 463, 13–31. [Google Scholar] [CrossRef]
- Enari, M.; Sakahira, H.; Yokoyama, H.; Okawa, K.; Iwamatsu, A.; Nagata, S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nat. Cell Biol. 1998, 391, 43–50. [Google Scholar] [CrossRef]
- Fernandez, P.C.; Machado, J.; Heussler, V.T.; Botteron, C.; Palmer, G.H.; Dobbelaere, D.A. The Inhibition of NF-B Activation Pathways and the Induction of Apoptosis by Dithiocarbamates in T Cells Are Blocked by the Glutathione Precursor N-Acetyl-L-Cysteine. Biol. Chem. 1999, 380, 1383–1394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghaffari, H.; Ghassam, B.J.; Chandra Nayaka, S.; Ramachandra Kini, K.; Prakash, H.S. Antioxidant and Neuroprotective Activities of Hyptis suaveolens (L.) Poit. Against Oxidative Stress-Induced Neurotoxicity. Cell. Mol. Neurobiol. 2014, 34, 323–331. [Google Scholar] [CrossRef]
- Kumar, K.H.; Khanum, F. Hydroalcoholic Extract of Cyperus rotundus Ameliorates H2O2-Induced Human Neuronal Cell Damage via Its Anti-oxidative and Anti-apoptotic Machinery. Cell. Mol. Neurobiol. 2013, 33, 5–17. [Google Scholar] [CrossRef]
- Liu, B.; Gao, H.-M.; Wang, J.-Y.; Jeohn, G.-H.; Cooper, C.L.; Hong, J.-S. Role of Nitric Oxide in Inflammation-Mediated Neurodegeneration. Ann. N. Y. Acad. Sci. 2002, 962, 318–331. [Google Scholar] [CrossRef]
- Kim, P.K.; Zamora, R.; Petrosko, P.; Billiar, T.R. The regulatory role of nitric oxide in apoptosis. Int. Immunopharmacol. 2001, 1, 1421–1441. [Google Scholar] [CrossRef]
- Mahesh, R.; Bhuvana, S.; Begum, V.M.H. Effect ofTerminalia chebulaaqueous extract on oxidative stress and antioxidant status in the liver and kidney of young and aged rats. Cell Biochem. Funct. 2009, 27, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Han, Y. Insulin inhibits AMPA-induced neuronal damage via stimulation of protein kinase B (Akt). J. Neural Transm. 2005, 112, 179–191. [Google Scholar] [CrossRef]
- Mao, Y.; Tokudome, T.; Kishimoto, I. The cardiovascular action of hexarelin. J. Geriatr. Cardiol. 2014, 11, 253–258. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Chen, J.; Lin, C.; Shao, R.; Yan, C.; Chen, C. Hexarelin Protects Rodent Pancreatic Β-Cells Function from Cytotoxic Effects of Streptozotocin Involving Mitochondrial Signalling Pathways In Vivo and In Vitro. PLoS ONE 2016, 11, e0149730. [Google Scholar] [CrossRef]
- Kim, M.S.; Yoon, C.Y.; Jang, P.G.; Park, Y.J.; Shin, C.S.; Park, H.S.; Ryu, J.W.; Pak, Y.K.; Park, J.Y.; Lee, K.U.; et al. The Mitogenic and Antiapoptotic Actions of Ghrelin in 3T3-L1 Adipocytes. Mol. Endocrinol. 2004, 18, 2291–2301. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Her, S.J.; Park, S.J.; Kim, D.; Park, K.S.; Lee, H.K.; Han, B.H.; Kim, M.S.; Shin, C.S.; Kim, S.Y. Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3-E1 cells. Bone 2005, 37, 359–369. [Google Scholar] [CrossRef]
- Baldanzi, G.; Filigheddu, N.; Cutrupi, S.; Catapano, F.; Bonissoni, S.; Fubini, A.; Malan, D.; Baj, G.; Granata, R.; Broglio, F.; et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J. Cell Biol. 2002, 159, 1029–1037. [Google Scholar] [CrossRef]
- Lucchi, C.; Curia, G.; Vinet, J.; Gualtieri, F.; Bresciani, E.; Locatelli, V.; Torsello, A.; Biagini, G. Protective but Not Anticonvulsant Effects of Ghrelin and JMV-1843 in the Pilocarpine Model of Status epilepticus. PLoS ONE 2013, 8, e72716. [Google Scholar] [CrossRef] [PubMed]
- Barlind, A.; Karlsson, N.; Åberg, N.D.; Björk-Eriksson, T.; Blomgren, K.; Isgaard, J. The growth hormone secretagogue hexarelin increases cell proliferation in neurogenic regions of the mouse hippocampus. Growth Horm. IGF Res. 2010, 20, 49–54. [Google Scholar] [CrossRef]
- Johansson, I.; Destefanis, S.; Aberg, N.D.; Aberg, M.A.I.; Blomgren, K.; Zhu, C.; Ghé, C.; Granata, R.; Ghigo, E.; Muccioli, G.; et al. Proliferative and Protective Effects of Growth Hormone Secretagogues on Adult Rat Hippocampal Progenitor Cells. Endocrinology 2008, 149, 2191–2199. [Google Scholar] [CrossRef]
- Brywe, K.G.; Leverin, A.-L.; Gustavsson, M.; Mallard, C.; Granata, R.; Destefanis, S.; Volante, M.; Hagberg, H.; Ghigo, E.; Isgaard, J. Growth Hormone-Releasing Peptide Hexarelin Reduces Neonatal Brain Injury and Alters Akt/Glycogen Synthase Kinase-3β Phosphorylation. Endocrinology 2005, 146, 4665–4672. [Google Scholar] [CrossRef] [PubMed]
- Fetler, L. NEUROSCIENCE: Brain under Surveillance: The Microglia Patrol. Science 2005, 309, 392–393. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, I.; Tamiazzo, L.; Bresciani, E.; Rapetti, D.; Caporali, S.; Lattuada, D.; Locatelli, V.; Torsello, A. Desacyl-ghrelin and synthetic GH-secretagogues modulate the production of inflammatory cytokines in mouse microglia cells stimulated by β-amyloid fibrils. J. Neurosci. Res. 2009, 87, 2718–2727. [Google Scholar] [CrossRef]
- Griffin, W.S.; Sheng, J.G.; Royston, M.C.; Gentleman, S.M.; McKenzie, J.E.; Graham, D.I.; Roberts, G.W.; Mrak, R.E. Glial-Neuronal Interactions in Alzheimer’s Disease: The Potential Role of a ‘Cytokine Cycle’ in Disease Progression. Brain Pathol. 1998, 8, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Agbo, E.; Li, M.-X.; Wang, Y.-Q.; Saahene, R.-O.; Massaro, J.; Tian, G.-Z. Hexarelin Protects Cardiac H9C2 Cells from Angiotensin II-Induced Hypertrophy via the Regulation of Autophagy. Pharmazie 2019, 74, 485–491. [Google Scholar] [CrossRef]
- Carson, J.A.; Hardee, J.P.; Vanderveen, B.N. The emerging role of skeletal muscle oxidative metabolism as a biological target and cellular regulator of cancer-induced muscle wasting. Semin. Cell Dev. Biol. 2016, 54, 53–67. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Salvesen, G. Regulated Cell Death: Signaling and Mechanisms. Annu. Rev. Cell Dev. Biol. 2014, 30, 337–356. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Kim, J.-A.; Hong, S.-I.; Jung, Y.-H.; Kim, H.-C.; Lee, S.-Y.; Jang, C.-G. Loganin protects against hydrogen peroxide-induced apoptosis by inhibiting phosphorylation of JNK, p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem. Int. 2011, 58, 533–541. [Google Scholar] [CrossRef]
- He, M.T.; Lee, A.Y.; Park, C.H.; Cho, E.J. Protective effect of Cordyceps militaris against hydrogen peroxide-induced oxidative stress in vitro. Nutr. Res. Pract. 2019, 13, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, M.; Kim, S.-J. Insulin involved Akt/ERK and Bcl-2/Bax pathways against oxidative damages in C6 glial cells. J. Recept. Signal Transduct. 2016, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chae, S.; Lee, J.H.; Hyun, J.W. The cytoprotective effect of butin against oxidative stress is mediated by the up-regulation of manganese superoxide dismutase expression through a PI3K/Akt/Nrf2-dependent pathway. J. Cell. Biochem. 2012, 113, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Pei, D.-S.; Zhang, Q.-G.; Guan, Q.-H.; Zhang, G.-Y. The neuroprotection of insulin on ischemic brain injury in rat hippocampus through negative regulation of JNK signaling pathway by PI3K/Akt activation. Brain Res. 2005, 1052, 1–9. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Li, F.; Maiese, K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog. Neurobiol. 2005, 75, 207–246. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).