Deregulation of the Interleukin-7 Signaling Pathway in Lymphoid Malignancies
Abstract
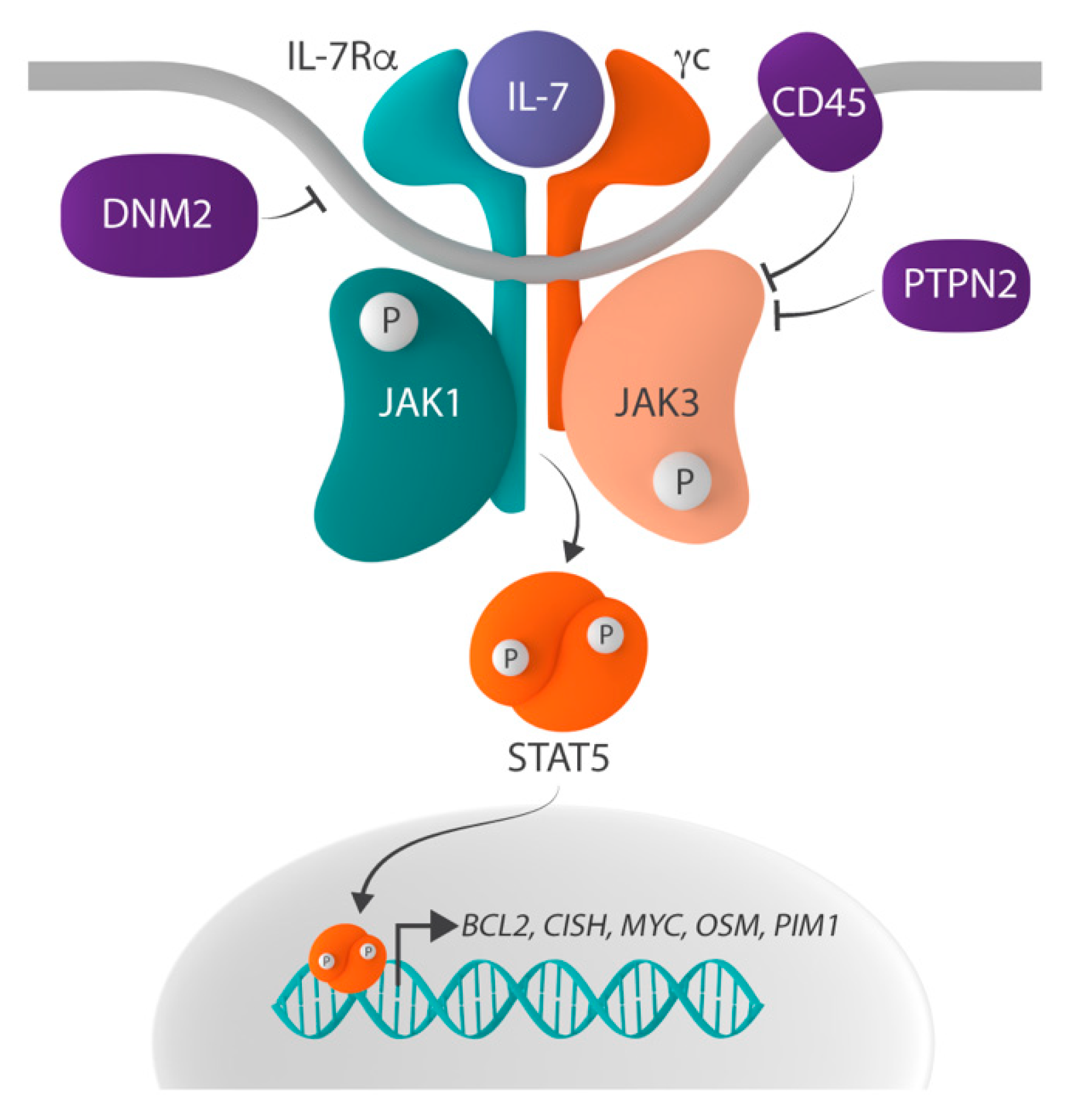
1. The Role of IL-7 Signaling in Lymphocyte Development
2. The Role of IL-7 Signaling in Acute Lymphoblastic Leukemia
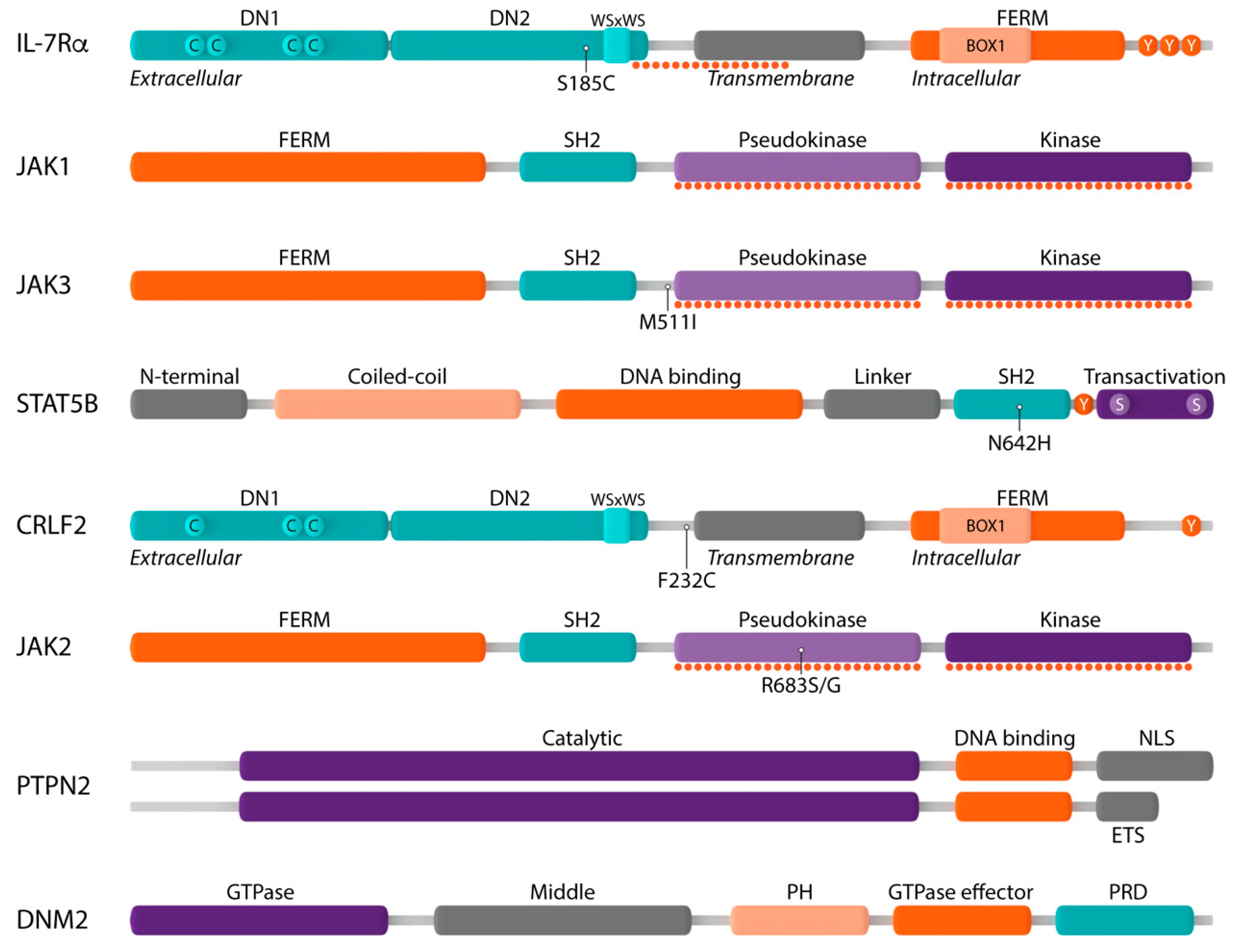
2.1. Mutations in the IL-7 Receptor
2.2. Mutations in the JAK1 and JAK3 Tyrosine Kinases
2.3. Mutation in the STAT Family Member STAT5B
2.4. Inactivation of the Protein Tyrosine Phosphatases PTPN2 and CD45
2.5. Alterations in DNM2
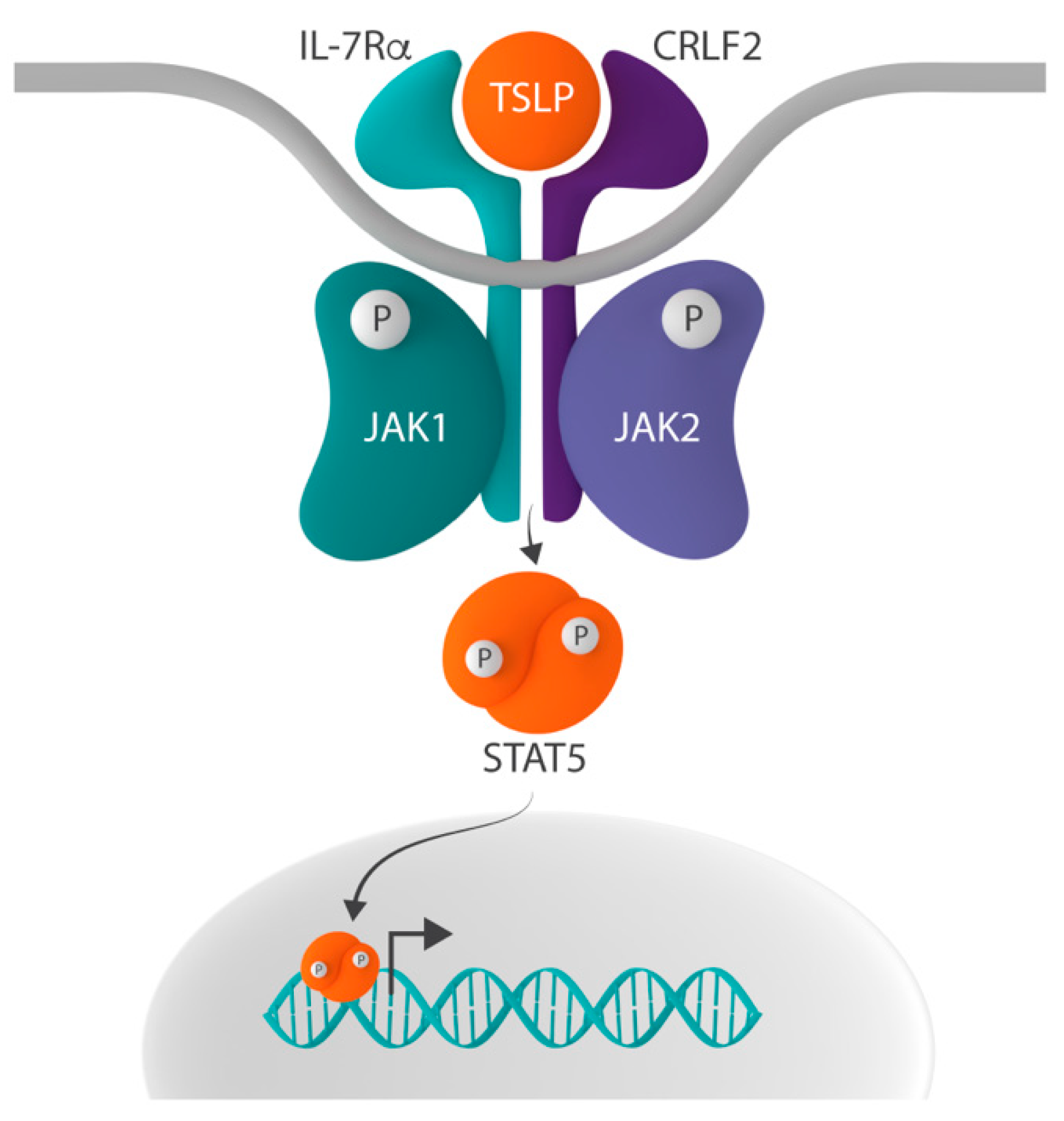
2.6. Alterations in the CRLF2 Receptor Chain
3. The Role of IL-7 Signaling in Other Lymphoid Malignancies
4. Therapeutic Targeting of the IL-7 Signaling Pathway in Lymphoid Malignancies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazzucchelli, R.; Durum, S.K. Interleukin-7 Receptor Expression: Intelligent Design. Nat. Rev. Immunol. 2007, 7, 144–154. [Google Scholar] [CrossRef]
- Oliveira, M.L.; Akkapeddi, P.; Ribeiro, D.; Melão, A.; Barata, J.T. IL-7R-Mediated Signaling in T-Cell Acute Lymphoblastic Leukemia: An Update. Adv. Biol. Regul. 2019, 71, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Von Freeden-Jeffry, U.; Vieira, P.; Lucian, L.A.; McNeil, T.; Burdach, S.E.G.; Murray, R. Lymphopenia in Interleukin (IL)-7 Gene-Deleted Mice Identifies IL-7 as a Nonredundant Cytokine. J. Exp. Med. 1995, 181, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Peschon, J.J.; Morrissey, P.J.; Grabstein, K.H.; Ramsdell, F.J.; Maraskovsky, E.; Gliniak, B.C.; Park, L.S.; Ziegler, S.F.; Williams, D.E.; Ware, C.B.; et al. Early Lymphocyte Expansion Is Severely Impaired in Interleukin 7 Receptor-Deficient Mice. J. Exp. Med. 1994, 180, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Cunningham-Rundles, C.; Ponda, P.P. Molecular Defects in T- and B-Cell Primary Immunodeficiency Diseases. Nat. Rev. Immunol. 2005, 5, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Puel, A.; Ziegler, S.F.; Buckley, R.H.; Leonard, W.J. Defective IL7R Expression in T-B+NK+ Severe Combined Immunodeficiency. Nat. Genet. 1998, 20, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Yi, H.; Rosenblatt, H.M.; Filipovich, A.H.; Adelstein, S.; Modi, W.S.; McBride, O.W.; Leonard, W.J. Interleukin-2 Receptor γ Chain Mutation Results in X-Linked Severe Combined Immunodeficiency in Humans. Cell 1993, 73, 147–157. [Google Scholar] [CrossRef]
- Buckley, R.H. Molecular Defects in Human Severe Combined Immunodeficiency and Approaches to Immune Reconstitution. Annu. Rev. Immunol. 2004, 22, 625–655. [Google Scholar] [CrossRef]
- Fry, T.J.; Mackall, C.L. Interleukin-7: From Bench to Clinic. Blood 2002, 99, 3892–3904. [Google Scholar] [CrossRef]
- Barata, J.T.; Cardoso, A.A.; Boussiotis, V.A. Interleukin-7 in T-Cell Acute Lymphoblastic Leukemia: An Extrinsic Factor Supporting Leukemogenesis? Leuk. Lymphoma 2005, 46, 483–495. [Google Scholar] [CrossRef]
- Fry, T.J.; Mackall, C.L. The Many Faces of IL-7: From Lymphopoiesis to Peripheral T Cell Maintenance. J. Immunol. 2005, 174, 6571–6576. [Google Scholar] [CrossRef] [PubMed]
- Munitic, I.; Williams, J.A.; Yang, Y.; Dong, B.; Lucas, P.J.; El Kassar, N.; Gress, R.E.; Ashwell, J.D. Dynamic Regulation of IL-7 Receptor Expression Is Required for Normal Thymopoiesis. Blood 2004, 104, 4165–4172. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Yu, Q.; Erman, B.; Appelbaum, J.S.; Montoya-Durango, D.; Grimes, H.L.; Singer, A. Suppression of IL7Ralpha Transcription by IL-7 and Other Prosurvival Cytokines: A Novel Mechanism for Maximizing IL-7-Dependent T Cell Survival. Immunity 2004, 21, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Kittipatarin, C.; Khaled, A.R. Interlinking Interleukin-7. Cytokine 2007, 39, 75–83. [Google Scholar] [CrossRef]
- Palmer, M.J.; Mahajan, V.S.; Trajman, L.C.; Irvine, D.J.; Lauffenburger, D.A.; Chen, J. Interleukin-7 Receptor Signaling Network: An Integrated Systems Perspective. Cell. Mol. Immunol. 2008, 5, 79–89. [Google Scholar] [CrossRef]
- Ribeiro, D.; Melão, A.; Barata, J.T. IL-7R-Mediated Signaling in T-Cell Acute Lymphoblastic Leukemia. Adv. Biol. Regul. 2013, 53, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Melão, A.; van Boxtel, R.; Santos, C.I.; Silva, A.; Silva, M.C.; Cardoso, B.A.; Coffer, P.J.; Barata, J.T. STAT5 Is Essential for IL-7–Mediated Viability, Growth, and Proliferation of T-Cell Acute Lymphoblastic Leukemia Cells. Blood Adv. 2018, 2, 2199–2213. [Google Scholar] [CrossRef]
- Levy, D.E.; Darnell, J.E. STATs: Transcriptional Control and Biological Impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, W.Q.; Hofmeister, R.R.; Young, H.A.; Hodge, D.R.; Keller, J.R.; Khaled, A.R.; Durum, S.K. Distinct Regions of the Interleukin-7 Receptor Regulate Different Bcl2 Family Members. Mol. Cell. Biol. 2004, 24, 6501–6513. [Google Scholar] [CrossRef] [PubMed]
- Le Saout, C.; Luckey, M.A.; Villarino, A.V.; Smith, M.; Hasley, R.B.; Myers, T.G.; Imamichi, H.; Park, J.H.; O’Shea, J.J.; Lane, H.C.; et al. IL-7-Dependent STAT1 Activation Limits Homeostatic CD4+ T Cell Expansion. JCI Insight 2017, 2, 1–18. [Google Scholar] [CrossRef]
- Lu, N.; Wang, Y.H.; Wang, Y.H.; Arima, K.; Hanabuchi, S.; Liu, Y.J. TSLP and IL-7 Use Two Different Mechanisms to Regulate Human CD4 + T Cell Homeostasis. J. Exp. Med. 2009, 206, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Rochman, Y.; Kashyap, M.; Robinson, G.W.; Sakamoto, K.; Gomez-Rodriguez, J.; Wagner, K.U.; Leonard, W.J. Thymic Stromal Lymphopoietin-Mediated STAT5 Phosphorylation via Kinases JAK1 and JAK2 Reveals a Key Difference from IL-7-Induced Signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 19455–19460. [Google Scholar] [CrossRef]
- Barata, J.T.; Silva, A.; Brandao, J.G.; Nadler, L.M.; Cardoso, A.A.; Boussiotis, V.A. Activation of PI3K Is Indispensable for Interleukin 7-Mediated Viability, Proliferation, Glucose Use, and Growth of T Cell Acute Lymphoblastic Leukemia Cells. J. Exp. Med. 2004, 200, 659–669. [Google Scholar] [CrossRef]
- Canté-Barrett, K.; Spijkers-Hagelstein, J.A.P.; Buijs-Gladdines, J.G.C.A.M.; Uitdehaag, J.C.M.; Smits, W.K.; Van Der Zwet, J.; Buijsman, R.C.; Zaman, G.J.R.; Pieters, R.; Meijerink, J.P.P. MEK and PI3K-AKT Inhibitors Synergistically Block Activated IL7 Receptor Signaling in T-Cell Acute Lymphoblastic Leukemia. Leukemia 2016, 30, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Winston, L.A.; Hunter, T. Intracellular Signalling: Putting JAKs on the Kinase MAP. Curr. Biol. 1996, 6, 668–671. [Google Scholar] [CrossRef]
- Benbernou, N.; Muegge, K.; Durum, S.K. Interleukin (IL)-7 Induces Rapid Activation of Pyk2, Which Is Bound to Janus Kinase 1 and IL-7Rα. J. Biol. Chem. 2000, 275, 7060–7065. [Google Scholar] [CrossRef]
- Kang, J.; Der, S.D. Cytokine Functions in the Formative Stages of a Lymphocyte’s Life. Curr. Opin. Immunol. 2004, 16, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wen, Q.L.; Aiello, F.B.; Mazzucchelli, R.; Asefa, B.; Khaled, A.R.; Durum, S.K. Cell Biology of IL-7, a Key Lymphotrophin. Cytokine Growth Factor Rev. 2005, 16, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Bousoik, E.; Montazeri Aliabadi, H. “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front. Oncol. 2018, 8, 1–20. [Google Scholar] [CrossRef]
- Vainchenker, W.; Constantinescu, S.N. JAK/STAT Signaling in Hematological Malignancies. Oncogene 2013, 32, 2601–2613. [Google Scholar] [CrossRef]
- Shuai, K.; Liu, B. Regulation of JAK-STAT Signalling in the Immune System. Nat. Rev. Immunol. 2003, 3, 900–911. [Google Scholar] [CrossRef]
- Pandey, A.; Ozaki, K.; Baumann, H.; Levin, S.D.; Puel, A.; Farr, A.G.; Ziegler, S.F.; Leonard, W.J.; Lodish, H.F. Cloning of a Receptor Subunit Required for Signaling by Thymic Stromal Lymphopoietin. Nat. Immunol. 2000, 1, 59–64. [Google Scholar] [CrossRef]
- Park, L.S.; Martin, U.; Garka, K.; Gliniak, B.; Di Santo, J.P.; Muller, W.; Largaespada, D.A.; Copeland, N.G.; Jenkins, N.A.; Farr, A.G.; et al. Cloning of the Murine Thymic Stromal Lymphopoietin (TSLP) Receptor: Formation of a Functional Heteromeric Complex Requires Interleukin 7 Receptor. J. Exp. Med. 2000, 192, 659–670. [Google Scholar] [CrossRef]
- Isaksen, D.E.; Baumann, H.; Trobridge, P.A.; Farr, A.G.; Levin, S.D.; Ziegler, S.F. Requirement for Stat5 in Thymic Stromal Lymphopoietin-Mediated Signal Transduction. J. Immunol. 1999, 163, 5971–5977. [Google Scholar]
- Ziegler, S.F.; Liu, Y.J. Thymic Stromal Lymphopoietin in Normal and Pathogenic T Cell Development and Function. Nat. Immunol. 2006, 7, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, R.; Khaled, A.R.; Benbernou, N.; Rajnavolgyi, E.; Muegge, K.; Durum, S.K. Interleukin-7: Physiological Roles and Mechanisms of Action. Cytokine Growth Factor Rev. 1999, 10, 41–60. [Google Scholar] [CrossRef]
- Sims, J.E.; Williams, D.E.; Morrissey, P.J.; Garka, K.; Foxworthe, D.; Price, V.; Friend, S.L.; Farr, A.; Bedell, M.A.; Jenkins, N.A.; et al. Molecular Cloning and Biological Characterization of a Novel Murine Lymphoid Growth Factor. J. Exp. Med. 2000, 192, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Levin, S.D.; Koelling, R.M.; Friend, S.L.; Isaksen, D.E.; Ziegler, S.F.; Perlmutter, R.M.; Farr, A.G. Thymic Stromal Lymphopoietin: A Cytokine That Promotes the Development of IgM+ B Cells in Vitro and Signals via a Novel Mechanism. J. Immunol. 1999, 162, 677–683. [Google Scholar]
- Vosshenrich, C.A.J.; Cumano, A.; Müller, W.; Di Santo, J.P.; Vieira, P. Thymic Stromal-Derived Lymphopoietin Distinguishes Fetal from Adult B Cell Development. Nat. Immunol. 2003, 4, 773–779. [Google Scholar] [CrossRef]
- Vosshenrich, C.A.J.; Cumano, A.; Müller, W.; Di Santo, J.P.; Vieira, P. Pre-B Cell Receptor Expression Is Necessary for Thymic Stromal Lymphopoietin Responsiveness in the Bone Marrow but Not in the Liver Environment. Proc. Natl. Acad. Sci. USA 2004, 101, 11070–11075. [Google Scholar] [CrossRef]
- Al-Shami, A.; Spolski, R.; Kelly, J.; Fry, T.; Schwartzberg, P.L.; Pandey, A.; Mackall, C.L.; Leonard, W.J. A Role for Thymic Stromal Lymphopoietin in CD4(+) T Cell Development. J. Exp. Med. 2004, 200, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Reche, P.A.; Soumelis, V.; Gorman, D.M.; Clifford, T.; Liu, M.; Travis, M.; Zurawski, S.M.; Johnston, J.; Liu, Y.-J.; Spits, H.; et al. Human Thymic Stromal Lymphopoietin Preferentially Stimulates Myeloid Cells. J. Immunol. 2001, 167, 336–343. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human Epithelial Cells Trigger Dendritic Cell–Mediated Allergic Inflammation by Producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef]
- Brocker, T. Survival of Mature CD4 T Lymphocytes Is Dependent on Major Histocompatibility Complex Class II-Expressing Dendritic Cells. J. Exp. Med. 1997, 186, 1223–1232. [Google Scholar] [CrossRef]
- Ge, Q.; Palliser, D.; Eisen, H.N.; Chen, J. Homeostatic T Cell Proliferation in a T Cell-Dendritic Cell Coculture System. Proc. Natl. Acad. Sci. USA 2002, 99, 2983–2988. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Hanabuchi, S.; Soumelis, V.; Yuan, W.; Ho, S.; de Waal Malefyt, R.; Liu, Y.-J. Human Thymic Stromal Lymphopoietin Promotes Dendritic Cell-Mediated CD4+ T Cell Homeostatic Expansion. Nat. Immunol. 2004, 5, 426–434. [Google Scholar] [CrossRef]
- Watanabe, N.; Wang, Y.-H.; Lee, H.K.; Ito, T.; Wang, Y.-H.; Cao, W.; Liu, Y.-J. Hassall’s Corpuscles Instruct Dendritic Cells to Induce CD4+CD25+ Regulatory T Cells in Human Thymus. Nature 2005, 436, 1181–1185. [Google Scholar] [CrossRef]
- Carpino, N.; Thierfelder, W.E.; Chang, M.; Saris, C.; Turner, S.J.; Ziegler, S.F.; Ihle, J.N. Absence of an Essential Role for Thymic Stromal Lymphopoietin Receptor in Murine B-Cell Development. Mol. Cell. Biol. 2004, 24, 2584–2592. [Google Scholar] [CrossRef]
- Giliani, S.; Mori, L.; de Saint Basile, G.; Le Deist, F.; Rodriguez-Perez, C.; Forino, C.; Mazzolari, E.; Dupuis, S.; Elhasid, R.; Kessel, A.; et al. Interleukin-7 Receptor Alpha (IL-7Ralpha) Deficiency: Cellular and Molecular Bases. Analysis of Clinical, Immunological, and Molecular Features in 16 Novel Patients. Immunol. Rev. 2005, 203, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.; Ma, M.C.; Snow, J.W.; Miners, M.J.; Herndier, B.G.; Goldsmith, M.A. Haploinsufficiency Identifies STATS as a Modifier of IL-7-Induced Lymphomas. Oncogene 2005, 24, 5252–5257. [Google Scholar] [CrossRef] [PubMed]
- Osborne, L.C.; Duthie, K.A.; Seo, J.H.; Gascoyne, R.D.; Abraham, N. Selective Ablation of the YxxM Motif of IL-7Rα Suppresses Lymphomagenesis but Maintains Lymphocyte Development. Oncogene 2010, 29, 3854–3864. [Google Scholar] [CrossRef]
- Laouar, Y.; Crispe, I.N.; Flavell, R.A. Overexpression of IL-7Rα Provides a Competitive Advantage during Early T-Cell Development. Blood 2004, 103, 1985–1994. [Google Scholar] [CrossRef]
- Rich, B.E.; Campos-Torres, J.; Tepper, R.I.; Moreadith, R.W.; Leder, P. Cutaneous Lymphoproliferation and Lymphomas in Interleukin 7 Transgenic Mice. J. Exp. Med. 1993, 177, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Laranjeira, A.B.A.; Martins, L.R.; Cardoso, B.A.; Demengeot, J.; Andrés Yunes, J.; Seddon, B.; Barata, J.T. IL-7 Contributes to the Progression of Human T-Cell Acute Lymphoblastic Leukemias. Cancer Res. 2011, 71, 4780–4789. [Google Scholar] [CrossRef]
- Karawajew, L.; Ruppert, V.; Wuchter, C.; Kösser, A.; Schrappe, M.; Dörken, B.; Ludwig, W.D. Inhibition of in Vitro Spontaneous Apoptosis by IL-7 Correlates, with Bcl-2 up-Regulation, Cortical/Mature Immunophenotype, and Better Early Cytoreduction of Childhood T-Cell Acute Lymphoblastic Leukemia. Blood 2000, 96, 297–306. [Google Scholar] [CrossRef]
- Maude, S.L.; Tasian, S.K.; Vincent, T.; Hall, J.W.; Sheen, C.; Roberts, K.G.; Seif, A.E.; Barrett, D.M.; Chen, I.M.; Collins, J.R.; et al. Targeting JAK1/2 and MTOR in Murine Xenograft Models of Ph-like Acute Lymphoblastic Leukemia. Blood 2012, 120, 3510–3518. [Google Scholar] [CrossRef]
- Barata, J.T.; Durum, S.K.; Seddon, B. Flip the Coin: IL-7 and IL-7R in Health and Disease. Nat. Immunol. 2019, 20, 1584–1593. [Google Scholar] [CrossRef]
- González-García, S.; Mosquera, M.; Fuentes, P.; Palumbo, T.; Escudero, A.; Pérez-Martínez, A.; Ramírez, M.; Corcoran, A.E.; Toribio, M.L. IL-7R Is Essential for Leukemia-Initiating Cell Activity of T-Cell Acute Lymphoblastic Leukemia. Blood 2019, 134, 2171–2182. [Google Scholar] [CrossRef] [PubMed]
- Goossens, S.; Radaelli, E.; Blanchet, O.; Durinck, K.; Van Der Meulen, J.; Peirs, S.; Taghon, T.; Tremblay, C.S.; Costa, M.; Ghahremani, M.F.; et al. ZEB2 Drives Immature T-Cell Lymphoblastic Leukaemia Development via Enhanced Tumour-Initiating Potential and IL-7 Receptor Signalling. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Girardi, T.; Vereecke, S.; Sulima, S.O.; Khan, Y.; Fancello, L.; Briggs, J.W.; Schwab, C.; De Beeck, J.O.; Verbeeck, J.; Royaert, J.; et al. The T-Cell Leukemia-Associated Ribosomal RPL10 R98S Mutation Enhances JAK-STAT Signaling. Leukemia 2018, 32, 809–819. [Google Scholar] [CrossRef]
- Sharma, N.D.; Nickl, C.K.; Kang, H.; Ornatowski, W.; Brown, R.; Ness, S.A.; Loh, M.L.; Mullighan, C.G.; Winter, S.S.; Hunger, S.P.; et al. Epigenetic Silencing of SOCS5 Potentiates JAK-STAT Signaling and Progression of T-Cell Acute Lymphoblastic Leukemia. Cancer Sci. 2019, 110, 1931–1946. [Google Scholar] [CrossRef]
- Goodwin, R.G.; Friend, D.; Ziegler, S.F.; Jerzy, R.; Falk, B.A.; Gimpel, S.; Cosman, D.; Dower, S.K.; March, C.J.; Namen, A.E.; et al. Cloning of the Human and Murine Interleukin-7 Receptors: Demonstration of a Soluble Form and Homology to a New Receptor Superfamily. Cell 1990, 60, 941–951. [Google Scholar] [CrossRef]
- McElroy, C.A.; Dohm, J.A.; Walsh, S.T.R. Structural and Biophysical Studies of the Human IL-7/IL-7Rα Complex. Structure 2009, 17, 54–65. [Google Scholar] [CrossRef]
- Campos, L.W.; Pissinato, L.G.; Yunes, J.A. Deleterious and Oncogenic Mutations in the Il7ra. Cancers 2019, 11, 1952. [Google Scholar] [CrossRef]
- Zenatti, P.P.; Ribeiro, D.; Li, W.; Zuurbier, L.; Silva, M.C.; Paganin, M.; Tritapoe, J.; Hixon, J.A.; Silveira, A.B.; Cardoso, B.A.; et al. Oncogenic IL7R Gain-of-Function Mutations in Childhood T-Cell Acute Lymphoblastic Leukemia. Nat. Genet. 2011, 43, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Shochat, C.; Tal, N.; Bandapalli, O.R.; Palmi, C.; Ganmore, I.; te Kronnie, G.; Cario, G.; Cazzaniga, G.; Kulozik, A.E.; Stanulla, M.; et al. Gain-of-Function Mutations in Interleukin-7 Receptor-α (IL7R) in Childhood Acute Lymphoblastic Leukemias. J. Exp. Med. 2011, 208, 901–908. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, L.; Holmfeldt, L.; Wu, G.; Heatley, S.L.; Payne-Turner, D.; Easton, J.; Chen, X.; Wang, J.; Rusch, M.; et al. The Genetic Basis of Early T-Cell Precursor Acute Lymphoblastic Leukaemia. Nature 2012, 481, 157–163. [Google Scholar] [CrossRef]
- Roberts, K.G.; Li, Y.; Payne-Turner, D.; Harvey, R.C.; Yang, Y.L.; Pei, D.; McCastlain, K.; Ding, L.; Lu, C.; Song, G.; et al. Targetable Kinase-Activating Lesions in Ph-like Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2014, 371, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.G.; Morin, R.D.; Zhang, J.; Hirst, M.; Zhao, Y.; Su, X.; Chen, S.C.; Payne-Turner, D.; Churchman, M.L.; Harvey, R.C.; et al. Genetic Alterations Activating Kinase and Cytokine Receptor Signaling in High-Risk Acute Lymphoblastic Leukemia. Cancer Cell 2012, 22, 153–166. [Google Scholar] [CrossRef]
- Roberts, K.G.; Yang, Y.L.; Payne-Turner, D.; Lin, W.; Files, J.K.; Dickerson, K.; Gu, Z.; Taunton, J.; Janke, L.J.; Chen, T.; et al. Oncogenic Role and Therapeutic Targeting of ABL-Class and JAK-STAT Activating Kinase Alterations in Ph-like ALL. Blood Adv. 2017, 1, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Senkevitch, E.; Li, W.; Hixon, J.A.; Andrews, C.; Cramer, S.D.; Pauly, G.T.; Back, T.; Czarra, K.; Durum, S.K. Inhibiting Janus Kinase 1 and BCL-2 to Treat T Cell Acute Lymphoblastic Leukemia with IL7-Rα Mutations. Oncotarget 2018, 9, 22605–22617. [Google Scholar] [CrossRef]
- Treanor, L.M.; Zhou, S.; Janke, L.; Churchman, M.L.; Ma, Z.; Lu, T.; Chen, S.C.; Mullighan, C.G.; Sorrentino, B.P. Interleukin-7 Receptor Mutants Initiate Early t Cell Precursor Leukemia in Murine Thymocyte Progenitors with Multipotent Potential. J. Exp. Med. 2014, 211, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.W.; Zenatti, P.P.; Pissinato, L.G.; Rodrigues, G.O.L.; Artico, L.L.; Guimarães, T.R.; Archangelo, L.F.; Martínez, L.; Brooks, A.J.; Yunes, J.A. Oncogenic Basic Amino Acid Insertions at the Extracellular Juxtamembrane Region of IL7RA Cause Receptor Hypersensitivity. Blood 2019, 133, 1259–1263. [Google Scholar] [CrossRef]
- Russ, W.P.; Engelman, D.M. The GxxxG Motif: A Framework for Transmembrane Helix-Helix Association. J. Mol. Biol. 2000, 296, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Ridder, A.; Skupjen, P.; Unterreitmeier, S.; Langosch, D. Tryptophan Supports Interaction of Transmembrane Helices. J. Mol. Biol. 2005, 354, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Shochat, C.; Tal, N.; Gryshkova, V.; Birger, Y.; Bandapalli, O.R.; Cazzaniga, G.; Gershman, N.; Kulozik, A.E.; Biondi, A.; Mansour, M.R.; et al. Novel Activating Mutations Lacking Cysteine in Type i Cytokine Receptors in Acute Lymphoblastic Leukemia. Blood 2014, 124, 106–110. [Google Scholar] [CrossRef]
- Kiel, M.J.; Velusamy, T.; Rolland, D.; Sahasrabuddhe, A.A.; Chung, F.; Bailey, N.G.; Schrader, A.; Li, B.; Li, J.Z.; Ozel, A.B.; et al. Integrated Genomic Sequencing Reveals Mutational Landscape of T-Cell Prolymphocytic Leukemia. Blood 2014, 124, 1460–1472. [Google Scholar] [CrossRef]
- Chen, M.; Cheng, A.; Chen, Y.-Q.; Hymel, A.; Hanson, E.P.; Kimmel, L.; Minami, Y.; Taniguchi, T.; Changelian, P.S.; O’Shea, J.J. The Amino Terminus of JAK3 Is Necessary and Sufficient for Binding to the Common γ Chain and Confers the Ability to Transmit Interleukin 2-Mediated Signals. Proc. Natl. Acad. Sci. USA 1997, 94, 6910–6915. [Google Scholar] [CrossRef]
- Zhao, Y.; Wagner, F.; Frank, S.J.; Kraft, A.S. The Amino-Terminal Portion of the JAK2 Protein Kinase Is Necessary For Binding and Phosphorylation of the Granulocyte-Macrophage Colony-Stimulating Factor Receptor β Chain*. J. Biol. Chem. 1995, 270, 13814–13818. [Google Scholar] [CrossRef]
- Hornakova, T.; Springuel, L.; Devreux, J.; Dusa, A.; Constantinescu, S.N.; Knoops, L.; Renauld, J.C. Oncogenic JAK1 and JAK2-Activating Mutations Resistant to ATP-Competitive Inhibitors. Haematologica 2011, 96, 845–853. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Staerk, J.; Kallin, A.; Demoulin, J.B.; Vainchenker, W.; Constantinescu, S.N. JAK1 and Tyk2 Activation by the Homologous Polycythemia Vera JAK2 V617F Mutation: Cross-Talk with IGF1 Receptor. J. Biol. Chem. 2005, 280, 41893–41899. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.M.; Lambert, Q.T.; Daniel, K.G.; Reuther, G.W. Transforming JAK1 Mutations Exhibit Differential Signalling, FERM Domain Requirements and Growth Responses to Interferon-γ. Biochem. J. 2010, 432, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Flex, E.; Petrangeli, V.; Stella, L.; Chiaretti, S.; Hornakova, T.; Knoops, L.; Ariola, C.; Fodale, V.; Clappier, E.; Paoloni, F.; et al. Somatically Acquired JAK1 Mutations in Adult Acute Lymphoblastic Leukemia. J. Exp. Med. 2008, 205, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Sandoval, C.; Baeg, G.H. Identification of Mutant Alleles of JAK3 in Pediatric Patients with Acute Lymphoblastic Leukemia. Leuk. Lymphoma 2015, 56, 1502–1506. [Google Scholar] [CrossRef]
- Mullighan, C.G.; Zhang, J.; Harvey, R.C.; Collins-Underwood, J.R.; Schulman, B.A.; Phillips, L.A.; Tasian, S.K.; Loh, M.L.; Su, X.; Liu, W.; et al. JAK Mutations in High-Risk Childhood Acute Lymphoblastic Leukemia. Proc. Natl. Acad. Sci. USA 2009, 106, 9414–9418. [Google Scholar] [CrossRef]
- Asnafi, V.; Le Noir, S.; Lhermitte, L.; Gardin, C.; Legrand, F.; Vallantin, X.; Malfuson, J.V.; Ifrah, N.; Dombret, H.; MacIntyre, E. JAK1 Mutations Are Not Frequent Events in Adult T-ALL: A GRAALL Study. Br. J. Haematol. 2010, 148, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, B.; Hu, L.; Ning, H.; Jiang, M.; Wang, D.; Liu, T.; Zhang, B.; Chen, H. Identification of a Novel Functional JAK1 S646P Mutation in Acute Lymphoblastic Leukemia. Oncotarget 2017, 8, 34687–34697. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeong, E.G.; Kim, M.S.; Nam, H.K.; Min, C.K.; Lee, S.; Chung, Y.J.; Yoo, N.J.; Lee, S.H. Somatic Mutations of JAK1 and JAK3 in Acute Leukemias and Solid Cancers. Clin. Cancer Res. 2008, 14, 3716–3721. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.; Schwab, C.; Broux, M.; Geerdens, E.; Degryse, S.; Demeyer, S.; Lahortiga, I.; Elliott, A.; Chilton, L.; La Starza, R.; et al. Targeted Sequencing Identifies Associations between IL7R-JAK Mutations and Epigenetic Modulators in T-Cell Acute Lymphoblastic Leukemia. Haematologica 2015, 100, 1301–1310. [Google Scholar] [CrossRef]
- Liu, Y.; Easton, J.; Shao, Y.; Maciaszek, J.; Wang, Z.; Wilkinson, M.R.; McCastlain, K.; Edmonson, M.; Pounds, S.B.; Shi, L.; et al. The Genomic Landscape of Pediatric and Young Adult T-Lineage Acute Lymphoblastic Leukemia. Nat. Genet. 2017, 49, 1211–1218. [Google Scholar] [CrossRef]
- Elliott, N.E.; Cleveland, S.M.; Grann, V.; Janik, J.; Waldmann, T.A.; Davé, U.P. FERM Domain Mutations Induce Gain of Function in JAK3 in Adult T-Cell Leukemia/Lymphoma. Blood 2011, 118, 3911–3921. [Google Scholar] [CrossRef]
- Bains, T.; Heinrich, M.C.; Loriaux, M.M.; Beadling, C.; Nelson, D.; Warrick, A.; Neff, T.L.; Tyner, J.W.; Dunlap, J.; Corless, C.L.; et al. Newly Described Activating JAK3 Mutations in T-Cell Acute Lymphoblastic Leukemia. Leukemia 2012, 26, 2144–2146. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.; Cools, J. The Origin of Relapse in Pediatric T-Cell Acute Lymphoblastic Leukemia. Haematologica 2015, 100, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
- Degryse, S.; De Bock, C.E.; Cox, L.; Demeyer, S.; Gielen, O.; Mentens, N.; Jacobs, K.; Geerdens, E.; Gianfelici, V.; Hulselmans, G.; et al. JAK3 Mutants Transform Hematopoietic Cells through JAK1 Activation, Causing T-Cell Acute Lymphoblastic Leukemia in a Mouse Model. Blood 2014, 124, 3092–3100. [Google Scholar] [CrossRef]
- Losdyck, E.; Hornakova, T.; Springuel, L.; Degryse, S.; Gielen, O.; Cools, J.; Constantinescu, S.N.; Flex, E.; Tartaglia, M.; Renauld, J.C.; et al. Distinct Acute Lymphoblastic Leukemia (ALL)-Associated Janus Kinase 3 (JAK3) Mutants Exhibit Different Cytokine-Receptor Requirements and JAK Inhibitor Specificities. J. Biol. Chem. 2015, 290, 29022–29034. [Google Scholar] [CrossRef]
- Steven Martinez, G.; Ross, J.A.; Kirken, R.A. Transforming Mutations of Jak3 (A573V and M511I) Show Differential Sensitivity to Selective Jak3 Inhibitors. Clin. Cancer Drugs 2016, 3, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; McVicar, D.W.; Johnston, J.A.; Blake, T.B.; Chen, Y.Q.; Lal, B.K.; Lloyd, A.R.; Kelvin, D.J.; Staples, J.E.; Ortaldo, J.R. Molecular Cloning of L-JAK, a Janus Family Protein-Tyrosine Kinase Expressed in Natural Killer Cells and Activated Leukocytes. Proc. Natl. Acad. Sci. USA 1994, 91, 6374–6378. [Google Scholar] [CrossRef]
- de Bock, C.E.; Demeyer, S.; Degryse, S.; Verbeke, D.; Sweron, B.; Gielen, O.; Vandepoel, R.; Vicente, C.; Bempt, M.V.; Dagklis, A.; et al. HOXA9 Cooperates with Activated JAK/STAT Signaling to Drive Leukemia Development. Cancer Discov. 2018, 8, 616–631. [Google Scholar] [CrossRef]
- Degryse, S.; Bornschein, S.; de Bock, C.E.; Leroy, E.; Vanden Bempt, M.; Demeyer, S.; Jacobs, K.; Geerdens, E.; Gielen, O.; Soulier, J.; et al. Mutant JAK3 Signaling Is Increased by Loss of Wild-Type JAK3 or by Acquisition of Secondary JAK3 Mutations in T-ALL. Blood 2018, 131, 421–425. [Google Scholar] [CrossRef]
- Hornakova, T.; Staerk, J.; Royer, Y.; Flex, E.; Tartaglia, M.; Constantinescu, S.N.; Knoops, L.; Renauld, J.C. Acute Lymphoblastic Leukemia-Associated JAK1 Mutants Activate the Janus Kinase/STAT Pathway via Interleukin-9 Receptor α Homodimers. J. Biol. Chem. 2009, 284, 6773–6781. [Google Scholar] [CrossRef]
- Clevenger, C.V. Roles and Regulation of Stat Family Transcription Factors in Human Breast Cancer. Am. J. Pathol. 2004, 165, 1449–1460. [Google Scholar] [CrossRef]
- Kontro, M.; Kuusanmäki, H.; Eldfors, S.; Burmeister, T.; Andersson, E.I.; Bruserud, Ø.; Brümmendorf, T.H.; Edgren, H.; Gjertsen, B.T.; Itälä-Remes, M.; et al. Novel Activating STAT5B Mutations as Putative Drivers of T-Cell Acute Lymphoblastic Leukemia. Leukemia 2014, 28, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Bandapalli, O.R.; Schuessele, S.; Kunz, J.B.; Rausch, T.; Stütz, A.M.; Tal, N.; Geron, I.; Gershman, N.; Izraeli, S.; Eilers, J.; et al. The Activating STAT5B N642H Mutation Is a Common Abnormality in Pediatric T-Cell Acutelymphoblastic Leukemia and Confers a Higher Risk of Relapse. Haematologica 2014, 99, 188–192. [Google Scholar] [CrossRef]
- Govaerts, I.; Jacobs, K.; Vandepoel, R.; Cools, J. JAK/STAT Pathway Mutations in T-ALL, Including the STAT5B N642H Mutation, Are Sensitive to JAK1/JAK3 Inhibitors. HemaSphere 2019, 3, 5–8. [Google Scholar] [CrossRef]
- Pham, H.T.T.; Maurer, B.; Prchal-Murphy, M.; Grausenburger, R.; Grundschober, E.; Javaheri, T.; Nivarthi, H.; Boersma, A.; Kolbe, T.; Elabd, M.; et al. STAT5B N642H Is a Driver Mutation for T Cell Neoplasia. J. Clin. Investig. 2018, 128, 387–401. [Google Scholar] [CrossRef]
- Pike, K.A.; Tremblay, M.L. TC-PTP and PTP1B: Regulating JAK-STAT Signaling, Controlling Lymphoid Malignancies. Cytokine 2016, 82, 52–57. [Google Scholar] [CrossRef]
- Kleppe, M.; Lahortiga, I.; El Chaar, T.; De Keersmaecker, K.; Mentens, N.; Graux, C.; Van Roosbroeck, K.; Ferrando, A.A.; Langerak, A.W.; Meijerink, J.P.P.; et al. Deletion of the Protein Tyrosine Phosphatase Gene PTPN2 in T-Cell Acute Lymphoblastic Leukemia. Nat. Genet. 2010, 42, 530–535. [Google Scholar] [CrossRef]
- Alcantara, M.; Simonin, M.; Lhermitte, L.; Touzart, A.; Dourthe, M.E.; Latiri, M.; Grardel, N.; Cayuela, J.M.; Chalandon, Y.; Graux, C.; et al. Clinical and Biological Features of PTPN2-Deleted Adult and Pediatric T-Cell Acute Lymphoblastic Leukemia. Blood Adv. 2019, 3, 1981–1988. [Google Scholar] [CrossRef]
- Kleppe, M.; Soulier, J.; Asnafi, V.; Mentens, N.; Hornakova, T.; Knoops, L.; Constantinescu, S.; Sigaux, F.; Meijerink, J.P.; Vandenberghe, P.; et al. PTPN2 Negatively Regulates Oncogenic JAK1 in T-Cell Acute Lymphoblastic Leukemia. Blood 2011, 117, 7090–7098. [Google Scholar] [CrossRef] [PubMed]
- Ruela-de-Sousa, R.R.; Queiroz, K.C.S.; Peppelenbosch, M.P.; Fuhler, G.M. Reversible Phosphorylation in Haematological Malignancies: Potential Role for Protein Tyrosine Phosphatases in Treatment? Biochim. Biophys. Acta Rev. Cancer 2010, 1806, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Kishihara, K.; Penninger, J.; Wallace, V.A.; Kündig, T.M.; Kawal, K.; Wakeham, A.; Timms, E.; Pfeffer, K.; Ohashi, P.S.; Thomas, M.L.; et al. Normal B Lymphocyte Development but Impaired T Cell Maturation in CD45-Exon6 Protein Tyrosine Phosphatase-Deficient Mice. Cell 1993, 74, 143–156. [Google Scholar] [CrossRef]
- Byth, K.F.; Conroy, L.A.; Howlett, S.; Smith, A.J.; May, J.; Alexander, D.R.; Holmes, N. CD45-Null Transgenic Mice Reveal a Positive Regulatory Role for CD45 in Early Thymocyte Development, in the Selection of CD4+CD8+ Thymocytes, and B Cell Maturation. J. Exp. Med. 1996, 183, 1707–1718. [Google Scholar] [CrossRef]
- Kung, C.; Pingel, J.T.; Heikinheimo, M.; Klemola, T.; Varkila, K.; Yoo, L.I.; Vuopala, K.; Poyhonen, M.; Uhari, M.; Rogers, M.; et al. Mutations in the Tyrosine Phosphatase CD45 Gene in a Child with Severe Combined Immunodeficiency Disease. Nat. Med. 2000, 6, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Irie-Sasaki, J.; Sasaki, T.; Matsumoto, W.; Opavsky, A.; Cheng, M.; Welstead, G.; Griffiths, E.; Krawczyk, C.; Richardson, C.D.; Aitken, K.; et al. CD45 Is a JAK Phosphatase and Negatively Regulates Cytokine Receptor Signalling. Nature 2001, 409, 349–354. [Google Scholar] [CrossRef]
- Wu, L.; Bijian, K.; Shen, S.S. CD45 Recruits Adapter Protein DOK-1 and Negatively Regulates JAK-STAT Signaling in Hematopoietic Cells. Mol. Immunol. 2009, 46, 2167–2177. [Google Scholar] [CrossRef]
- Ratei, R.; Sperling, C.; Karawajew, L.; Schott, G.; Schrappe, M.; Harbott, J.; Riehm, H.; Ludwig, W.D. Immunophenotype and Clinical Characteristics of CD45-Negative and CD45-Positive Childhood Acute Lymphoblastic Leukemia. Ann. Hematol. 1998, 77, 107–114. [Google Scholar] [CrossRef]
- Porcu, M.; Kleppe, M.; Gianfelici, V.; Geerdens, E.; De Keersmaecker, K.; Tartaglia, M.; Foà, R.; Soulier, J.; Cauwelier, B.; Uyttebroeck, A.; et al. Mutation of the Receptor Tyrosine Phosphatase PTPRC (CD45) in T-Cell Acute Lymphoblastic Leukemia. Blood 2012, 119, 4476–4479. [Google Scholar] [CrossRef]
- Tremblay, C.S.; Brown, F.C.; Collett, M.; Saw, J.; Chiu, S.K.; Sonderegger, S.E.; Lucas, S.E.; Alserihi, R.; Chau, N.; Toribio, M.L.; et al. Loss-of-Function Mutations of Dynamin 2 Promote T-ALL by Enhancing IL-7 Signalling. Leukemia 2016, 30, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.C.; Collett, M.; Tremblay, C.S.; Rank, G.; De Camilli, P.; Booth, C.J.; Bitoun, M.; Robinson, P.J.; Kile, B.T.; Jane, S.M.; et al. Loss of Dynamin 2 GTPase Function Results in Microcytic Anaemia. Br. J. Haematol. 2017, 178, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Li, M.; Zhao, G.; Xiao, L.; Gu, Y.; Zhou, X.; Yu, M.D.; Li, J.; Dovat, S.; Song, C. Novel Dynamin 2 Mutations in Adult T-Cell Acute Ymphoblastic Leukemia. Oncol. Lett. 2016, 12, 2746–2751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Russell, L.J.; Capasso, M.; Vater, I.; Akasaka, T.; Bernard, O.A.; Calasanz, M.J.; Chandrasekaran, T.; Chapiro, E.; Gesk, S.; Griffiths, M.; et al. Deregulated Expression of Cytokine Receptor Gene, CRLF2, Is Involved in Lymphoid Transformation in B-Cell Precursor Acute Lymphoblastic Leukemia. Blood 2009, 114, 2688–2698. [Google Scholar] [CrossRef]
- Harvey, R.C.; Mullighan, C.G.; Chen, I.M.; Wharton, W.; Mikhail, F.M.; Carroll, A.J.; Kang, H.; Liu, W.; Dobbin, K.K.; Smith, M.A.; et al. Rearrangement of CRLF2 Is Associated with Mutation of JAK Kinases, Alteration of IKZF1, Hispanic/Latino Ethnicity, and a Poor Outcome in Pediatric B-Progenitor Acute Lymphoblastic Leukemia. Blood 2010, 115, 5312–5321. [Google Scholar] [CrossRef] [PubMed]
- Yoda, A.; Yoda, Y.; Chiaretti, S.; Bar-Natan, M.; Mani, K.; Rodig, S.J.; West, N.; Xiao, Y.; Brown, J.R.; Mitsiades, C.; et al. Functional Screening Identifies CRLF2 in Precursor B-Cell Acute Lymphoblastic Leukemia. Proc. Natl. Acad. Sci. USA 2010, 107, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, L.; Vendramini, E.; Ganmore, I.; Cazzaniga, G.; Schmitz, M.; Chalker, J.; Shiloh, R.; Iacobucci, I.; Shochat, C.; Zeligson, S.; et al. Down Syndrome Acute Lymphoblastic Leukemia, a Highly Heterogeneous Disease in Which Aberrant Expression of CRLF2 Is Associated with Mutated JAK2: A Report from the International BFM Study Group. Blood 2010, 115, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Collins-Underwood, J.R.; Phillips, L.A.A.; Loudin, M.G.; Liu, W.; Zhang, J.; Ma, J.; Coustan-Smith, E.; Harvey, R.C.; Willman, C.L.; et al. Rearrangement of CRLF2 in B-Progenitor-and Down Syndrome-Associated Acute Lymphoblastic Leukemia. Nat. Genet. 2009, 41, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Chapiro, E.; Russell, L.; Lainey, E.; Kaltenbach, S.; Ragu, C.; Della-Valle, V.; Hanssens, K.; MacIntyre, E.A.; Radford-Weiss, I.; Delabesse, E.; et al. Activating Mutation in the TSLPR Gene in B-Cell Precursor Lymphoblastic Leukemia. Leukemia 2010, 24, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Tal, N.; Shochat, C.; Geron, I.; Bercovich, D.; Izraeli, S. Interleukin 7 and Thymic Stromal Lymphopoietin: From Immunity to Leukemia. Cell. Mol. Life Sci. 2014, 71, 365–378. [Google Scholar] [CrossRef]
- Van Bodegom, D.; Zhong, J.; Kopp, N.; Dutta, C.; Kim, M.S.; Bird, L.; Weigert, O.; Tyner, J.; Pandey, A.; Yoda, A.; et al. Differences in Signaling through the B-Cell Leukemia Oncoprotein CRLF2 in Response to TSLP and through Mutant JAK2. Blood 2012, 120, 2853–2863. [Google Scholar] [CrossRef]
- Schluns, K.S.; Kieper, W.C.; Jameson, S.C.; Lefrançois, L. Interleukin-7 Mediates the Homeostasis of Naïve and Memory CD8 T Cells in Vivo. Nat. Immunol. 2000, 1, 426–432. [Google Scholar] [CrossRef]
- Dalloul, A.; Laroche, L.; Bagot, M.; Djavad Mossalayi, M.; Fourcade, C.; Thacker, D.J.; Hogge, D.E.; Merle-Béral, H.; Debré, P.; Schmitt, C. Interleukin-7 Is a Growth Factor for Sézary Lymphoma Cells. J. Clin. Investig. 1992, 90, 1054–1060. [Google Scholar] [CrossRef]
- Digel, W.; Schmid, M.; Heil, G.; Conrad, P.; Gillis, S.; Porzsolt, F. Human Interleukin-7 Induces Proliferation of Neoplastic Cells from Chronic Lymphocytic Leukemia and Acute Leukemias. Blood 1991, 78, 753–759. [Google Scholar] [CrossRef]
- Cattaruzza, L.; Gloghini, A.; Olivo, K.; Di Francia, R.; Lorenzon, D.; De Filippi, R.; Carbone, A.; Colombatti, A.; Pinto, A.; Aldinucci, D. Functional Coexpression of Interleukin (IL)-7 and Its Receptor (IL-7R) on Hodgkin and Reed-Sternberg Cells: Involvement of IL-7 in Tumor Cell Growth and Microenvironmental Interactions of Hodgkin’s Lymphoma. Int. J. Cancer 2009, 125, 1092–1101. [Google Scholar] [CrossRef]
- Waldmann, T.A.; Chen, J. Disorders of the JAK/STAT Pathway in T Cell Lymphoma Pathogenesis: Implications for Immunotherapy. Annu. Rev. Immunol. 2017, 35, 533–550. [Google Scholar] [CrossRef]
- Crescenzo, R.; Abate, F.; Lasorsa, E.; Tabbo’, F.; Gaudiano, M.; Chiesa, N.; Di Giacomo, F.; Spaccarotella, E.; Barbarossa, L.; Ercole, E.; et al. Convergent Mutations and Kinase Fusions Lead to Oncogenic STAT3 Activation in Anaplastic Large Cell Lymphoma. Cancer Cell 2015, 27, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Koo, G.C.; Tan, S.Y.; Tang, T.; Poon, S.L.; Allen, G.E.; Tan, L.; Chong, S.C.; Ong, W.S.; Tay, K.; Tao, M.; et al. Janus Kinase 3-Activating Mutations Identifi Ed in Natural Killer/T-Cell Lymphoma. Cancer Discov. 2012, 2, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Bouchekioua, A.; Scourzic, L.; De Wever, O.; Zhang, Y.; Cervera, P.; Aline-Fardin, A.; Mercher, T.; Gaulard, P.; Nyga, R.; Jeziorowska, D.; et al. JAK3 Deregulation by Activating Mutations Confers Invasive Growth Advantage in Extranodal Nasal-Type Natural Killer Cell Lymphoma. Leukemia 2014, 28, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Rajala, H.L.M.; Porkka, K.; MacIejewski, J.P.; Loughran, T.P.; Mustjoki, S. Uncovering the Pathogenesis of Large Granular Lymphocytic Leukemia-Novel STAT3 and STAT5b Mutations. Ann. Med. 2014, 46, 114–122. [Google Scholar] [CrossRef]
- Küçük, C.; Jiang, B.; Hu, X.; Zhang, W.; Chan, J.K.C.; Xiao, W.; Lack, N.; Alkan, C.; Williams, J.C.; Avery, K.N.; et al. Activating Mutations of STAT5B and STAT3 in Lymphomas Derived from Γδ-T or NK Cells. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Kleppe, M.; Tousseyn, T.; Geissinger, E.; Atak, Z.K.; Aerts, S.; Rosenwald, A.; Wlodarska, I.; Cools, J. Mutation Analysis of the Tyrosine Phosphatase PTPN2 in Hodgkin’s Lymphoma and T-Cell Non-Hodgkin’s Lymphoma. Haematologica 2011, 96, 1723–1727. [Google Scholar] [CrossRef]
- Cramer, S.D.; Aplan, P.D.; Durum, S.K. Therapeutic Targeting of IL-7Rα Signaling Pathways in ALL Treatment. Blood 2016, 128, 473–478. [Google Scholar] [CrossRef]
- Alsadeq, A.; Lenk, L.; Vadakumchery, A.; Cousins, A.; Vokuhl, C.; Khadour, A.; Vogiatzi, F.; Seyfried, F.; Meyer, L.H.; Cario, G.; et al. IL7R Is Associated with CNS Infiltration and Relapse in Pediatric B-Cell Precursor Acute Lymphoblastic Leukemia. Blood 2018, 132, 1614–1617. [Google Scholar] [CrossRef]
- Yasunaga, M.; Manabe, S.; Matsumura, Y. Immunoregulation by IL-7R-Targeting Antibody-Drug Conjugates: Overcoming Steroid-Resistance in Cancer and Autoimmune Disease. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hixon, J.A.; Andrews, C.; Kashi, L.; Kohnhorst, C.L.; Senkevitch, E.; Czarra, K.; Barata, J.T.; Li, W.; Schneider, J.P.; Walsh, S.T.R.; et al. New Anti-IL-7Rα Monoclonal Antibodies Show Efficacy against T Cell Acute Lymphoblastic Leukemia in Pre-Clinical Models. Leukemia 2020, 34, 35–49. [Google Scholar] [CrossRef]
- Akkapeddi, P.; Fragoso, R.; Hixon, J.A.; Ramalho, A.S.; Oliveira, M.L.; Carvalho, T.; Gloger, A.; Matasci, M.; Corzana, F.; Durum, S.K.; et al. A Fully Human Anti-IL-7Rα Antibody Promotes Antitumor Activity against T-Cell Acute Lymphoblastic Leukemia. Leukemia 2019, 33, 2155–2168. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.R.; Reed, C.; Eisenberg, A.R.; Tseng, J.C.; Twizere, J.C.; Daakour, S.; Yoda, A.; Rodig, S.J.; Tal, N.; Shochat, C.; et al. Targeting Oncogenic Interleukin-7 Receptor Signalling with N-Acetylcysteine in T Cell Acute Lymphoblastic Leukaemia. Br. J. Haematol. 2015, 168, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Martin, C.; Meyer, L.K.; Huang, B.J.; Shimano, K.A.; Zinter, M.S.; Nguyen, J.V.; Smith, G.A.; Taunton, J.; Winter, S.S.; Roderick, J.R.; et al. JAK/STAT Pathway Inhibition Overcomes IL7-Induced Glucocorticoid Resistance in a Subset of Human T-Cell Acute Lymphoblastic Leukemias. Leukemia 2017, 31, 2568–2576. [Google Scholar] [CrossRef]
- Degryse, S.; de Bock, C.E.; Demeyer, S.; Govaerts, I.; Bornschein, S.; Verbeke, D.; Jacobs, K.; Binos, S.; Skerrett-Byrne, D.A.; Murray, H.C.; et al. Mutant JAK3 Phosphoproteomic Profiling Predicts Synergism between JAK3 Inhibitors and MEK/BCL2 Inhibitors for the Treatment of T-Cell Acute Lymphoblastic Leukemia. Leukemia 2018, 32, 788–800. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lodewijckx, I.; Cools, J. Deregulation of the Interleukin-7 Signaling Pathway in Lymphoid Malignancies. Pharmaceuticals 2021, 14, 443. https://doi.org/10.3390/ph14050443
Lodewijckx I, Cools J. Deregulation of the Interleukin-7 Signaling Pathway in Lymphoid Malignancies. Pharmaceuticals. 2021; 14(5):443. https://doi.org/10.3390/ph14050443
Chicago/Turabian StyleLodewijckx, Inge, and Jan Cools. 2021. "Deregulation of the Interleukin-7 Signaling Pathway in Lymphoid Malignancies" Pharmaceuticals 14, no. 5: 443. https://doi.org/10.3390/ph14050443
APA StyleLodewijckx, I., & Cools, J. (2021). Deregulation of the Interleukin-7 Signaling Pathway in Lymphoid Malignancies. Pharmaceuticals, 14(5), 443. https://doi.org/10.3390/ph14050443





