The Repurposing of Acetylsalicylic Acid as a Photosensitiser to Inactivate the Growth of Cryptococcal Cells
Abstract
1. Introduction
2. Results
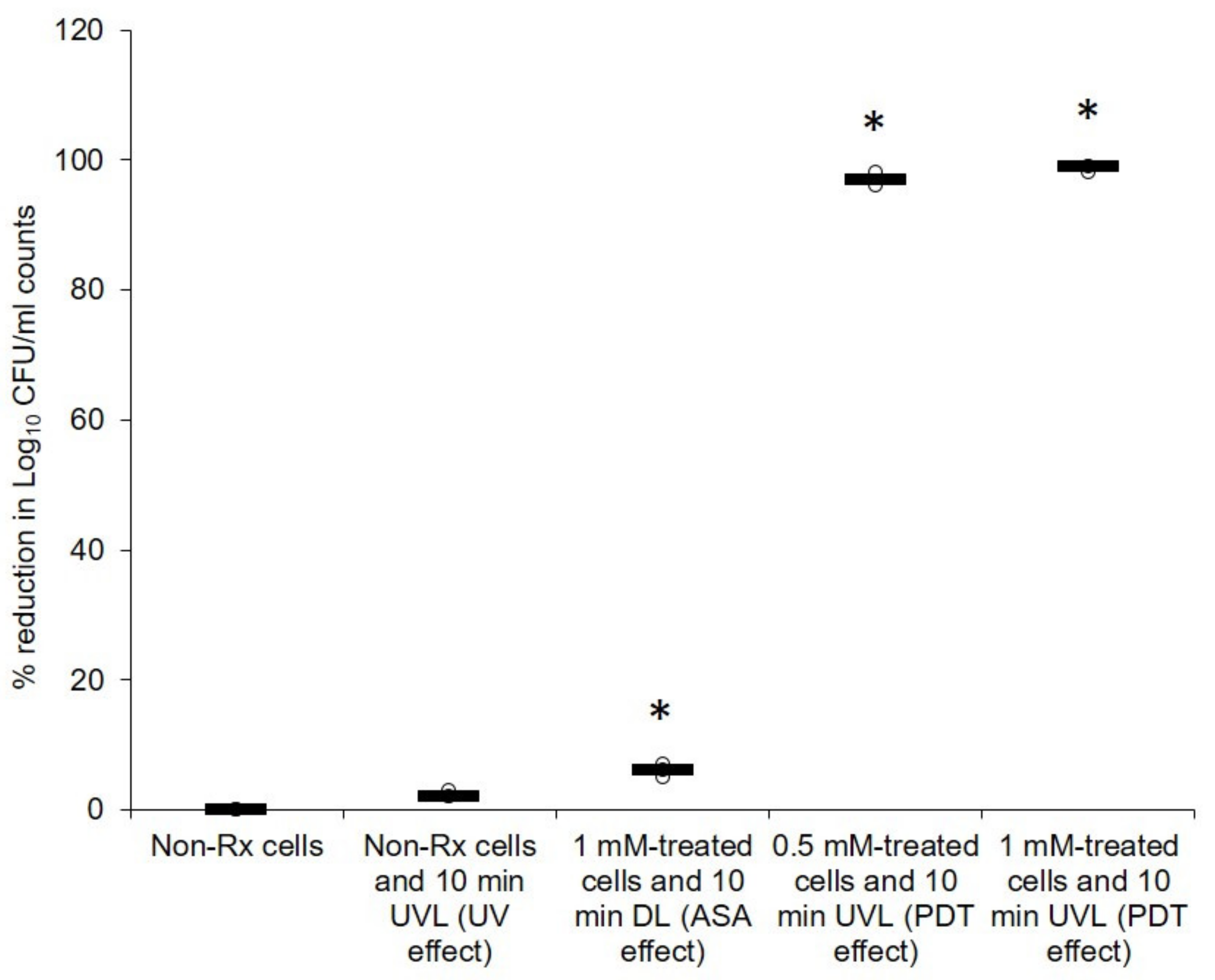
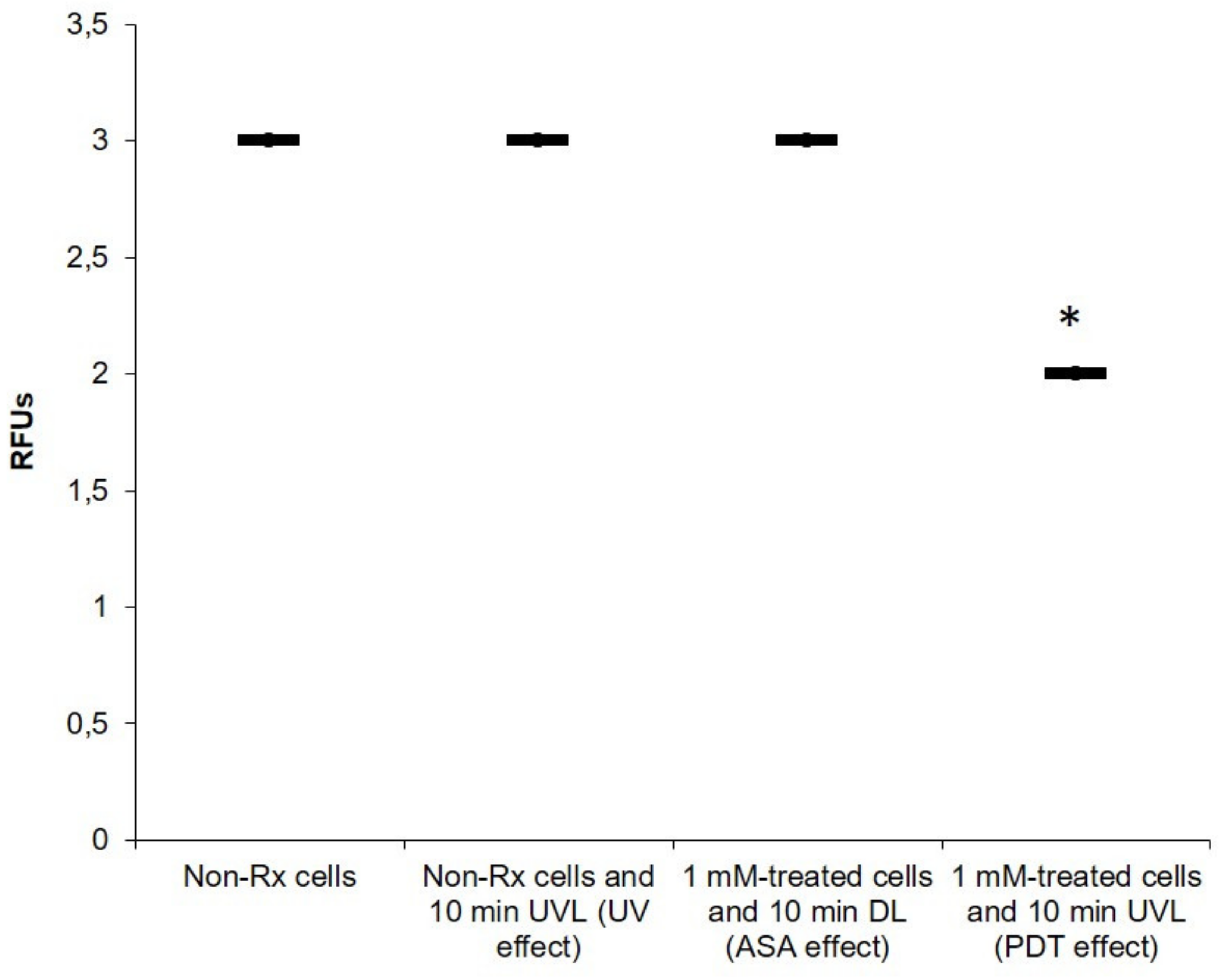
2.1. Cryptococcal Cells Are Susceptible to the Photodynamic Action of ASA
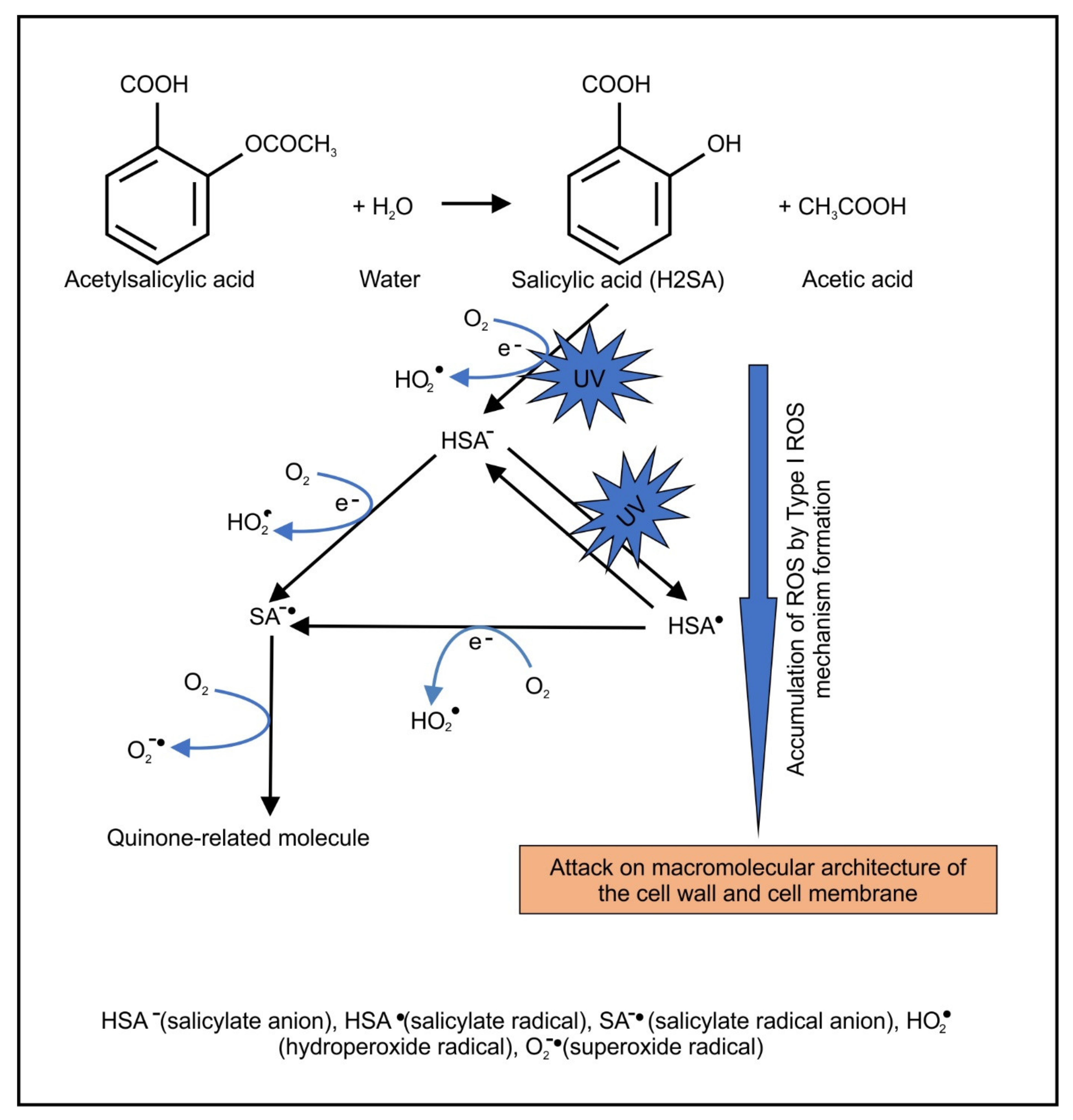
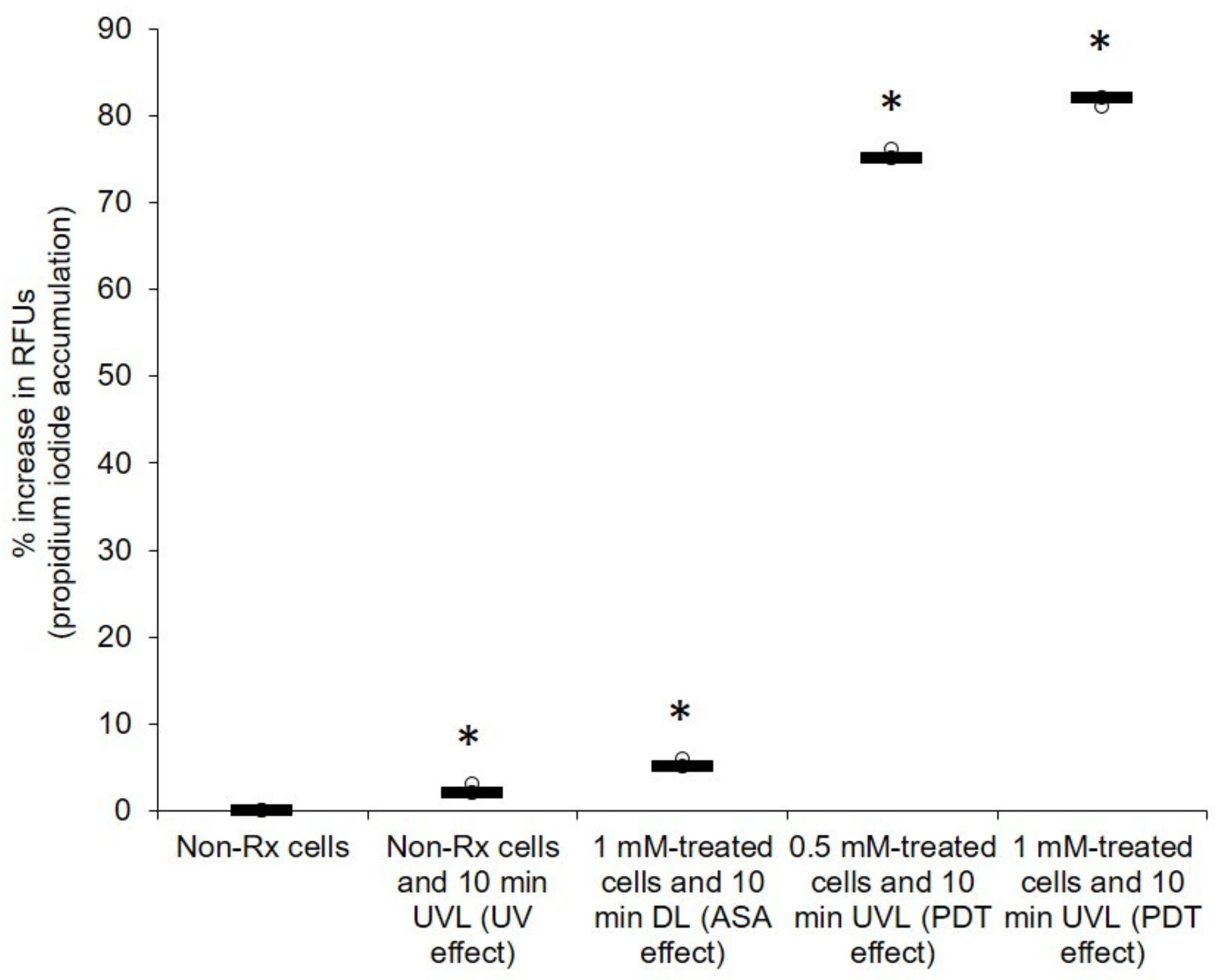
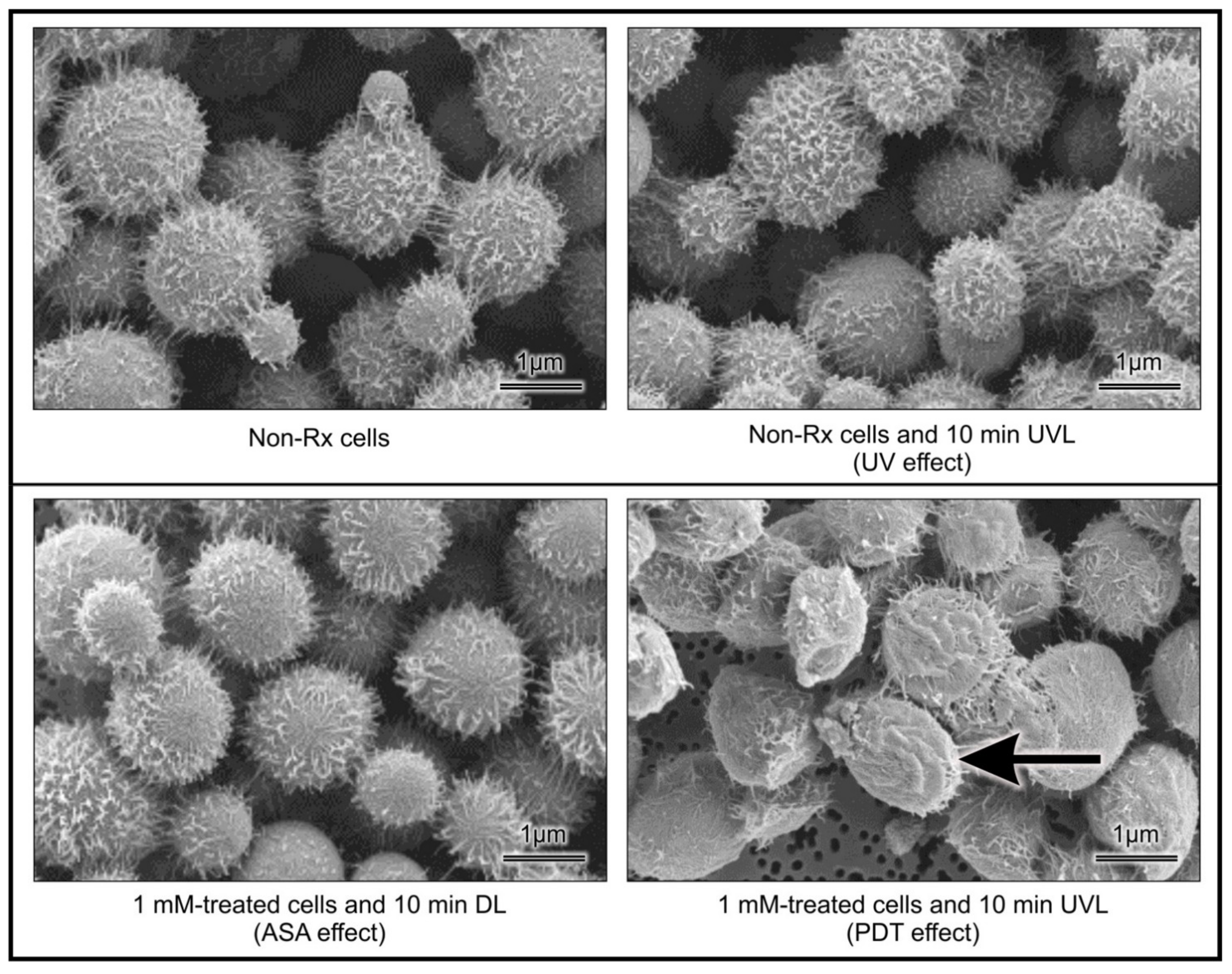
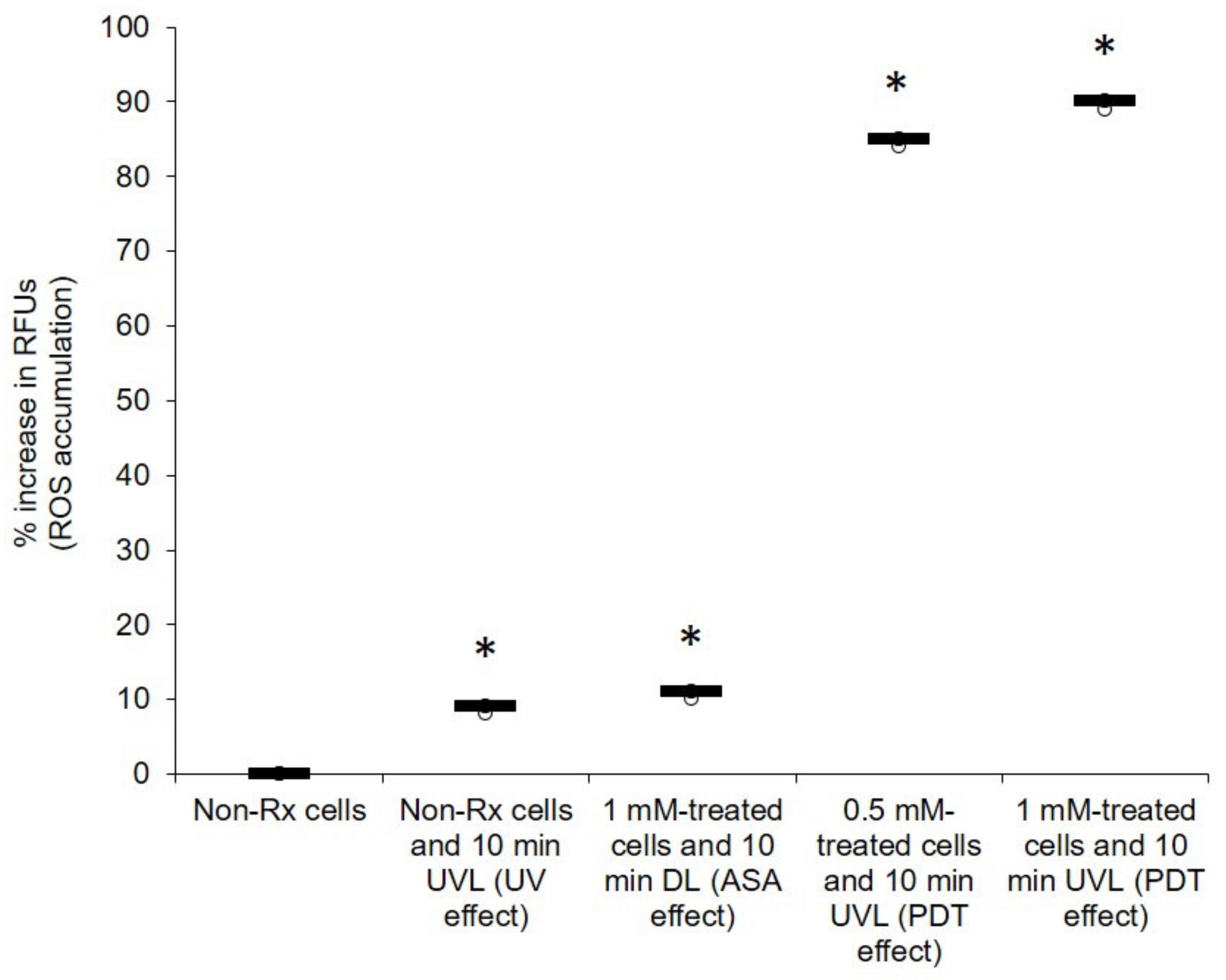
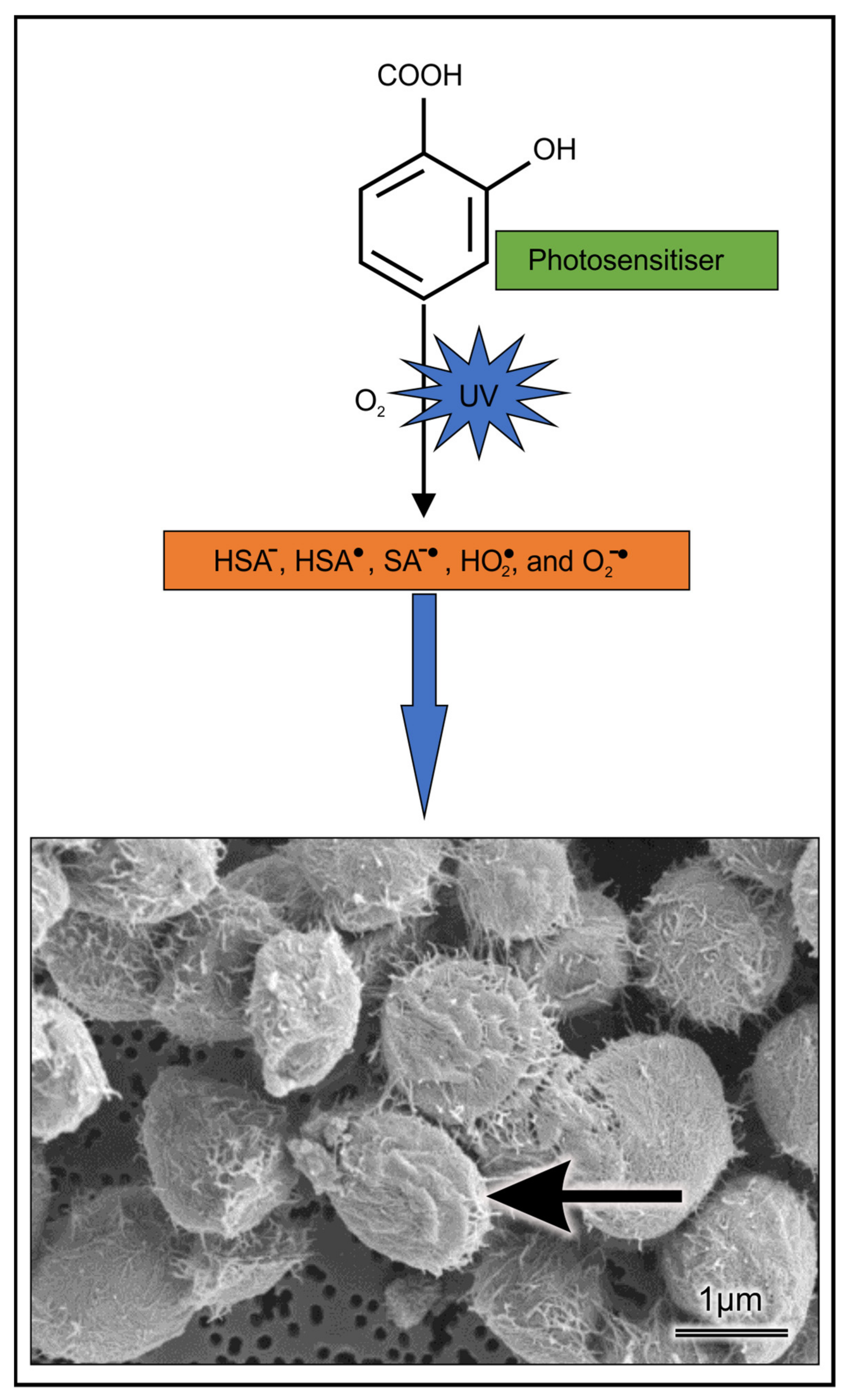
2.2. Light-Activated ASA Kills Cryptococcal Cells via the Production of Harmful Radical Species That Target Cell Walls
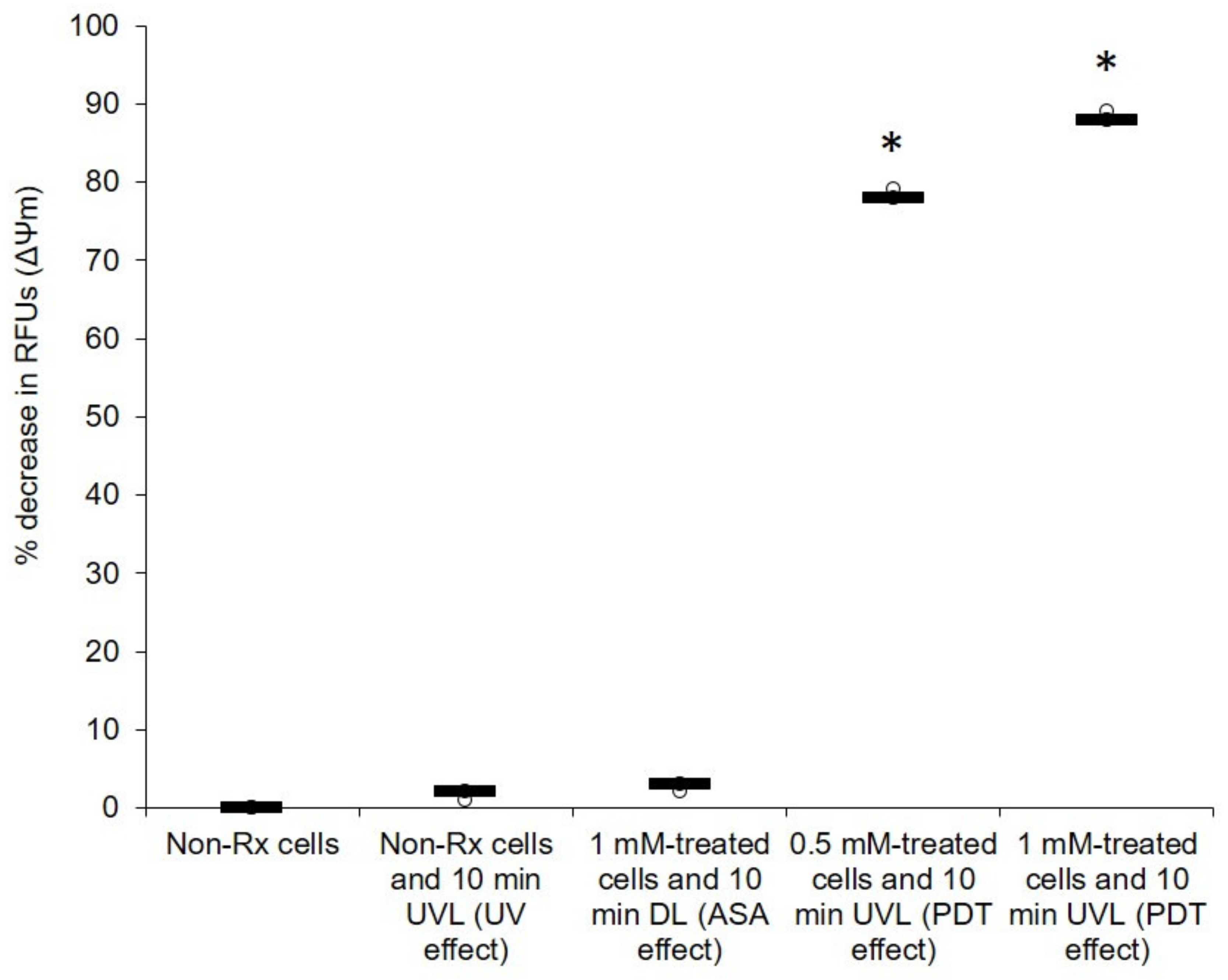
2.3. The Photodynamic Action of ASA Impairs Cryptococcal Mitochondrial Function
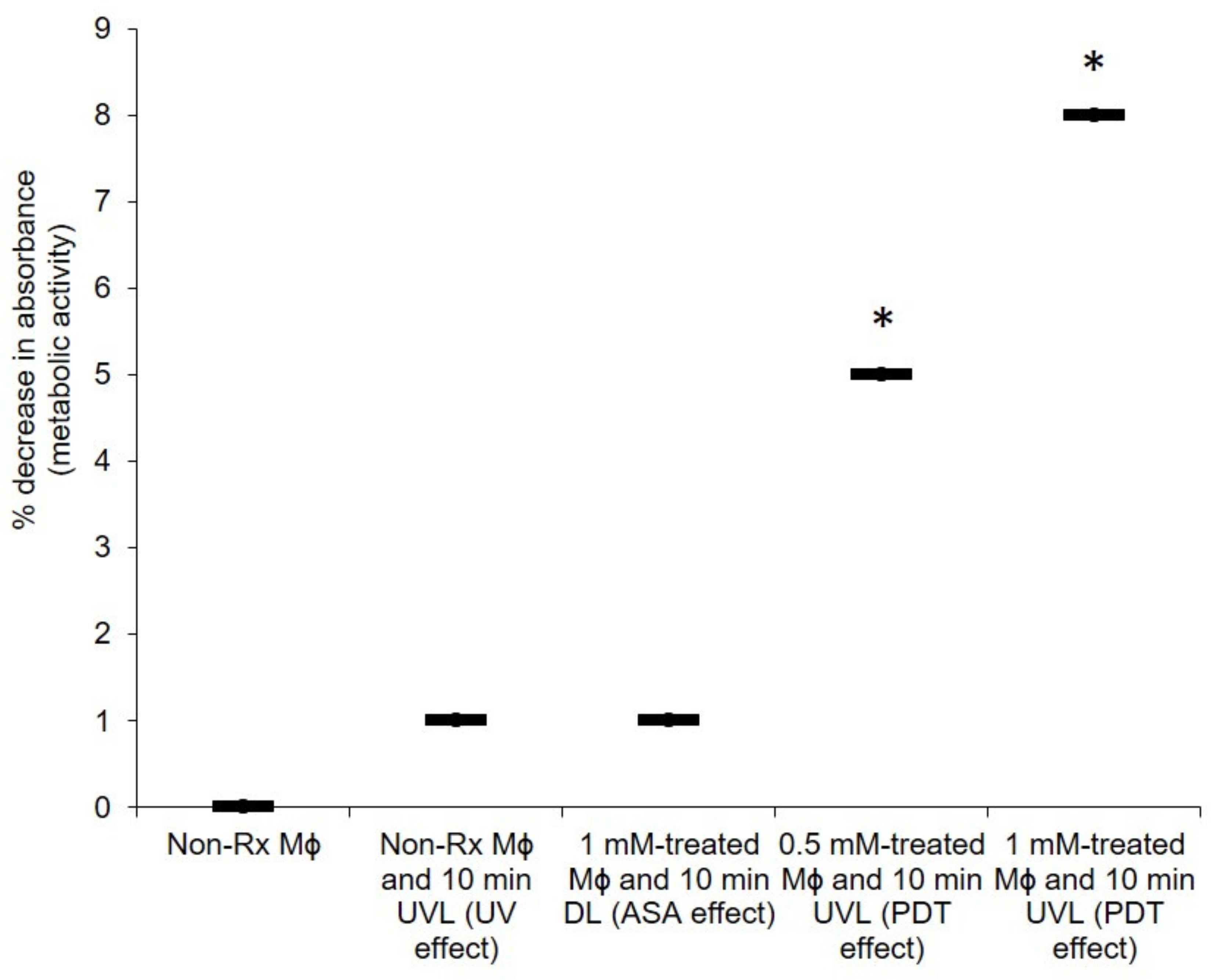
2.4. The PDT Action of ASA Does Not Adversely Affect the Health of Macrophages
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cells, Cultivation and Standardisation
4.3. UV Radiation
4.4. Preparation of Cells for Experimental Assays
4.5. Survival Assay of Cryptococcal Cells
4.6. The Effects of PDT with ASA on the Cell Membrane and Cell Wall
4.6.1. Effect of PDT on Cellular Outer Ultrastructure
4.6.2. Effect of PDT on the Expression of CAP64 Gene
4.7. Effect of PDT with ASA on Cryptococcal Mitochondrial Membrane Potential (ΔΨM) and ROS Accumulation
4.8. Effect of PDT Using ASA on the Health of Macrophages
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Oladele, R.O.; Bongomin, F.; Gago, S.D.; Denning, W. HIV-associated cryptococcal disease in resource-limited settings: A case for “prevention is better than cure”? J. Fungi 2017, 3, e67. [Google Scholar] [CrossRef]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Ford, N.; Shubber, Z.; Jarvis, J.N.; Chiller, T.; Greene, G.; Migone, C.; Vitoria, M.; Doherty, M.; Meintjes, G. CD4 cell count threshold for cryptococcal antigen screening of HIV-infected individuals: A systematic review and meta-analysis. Clin. Infect. Dis. 2018, 66, S152–S159. [Google Scholar] [CrossRef]
- Bongomin, F.; Atikoro, L. “Recurrence of cryptococcal meningitis and the hidden role of patient education and social support,” case reports in Neurological Medicine. Hindawi 2018, 1–4. [Google Scholar] [CrossRef]
- Merry, M.; Boulware, D.R. Cryptococcal meningitis treatment strategies affected by the explosive cost of Flucytosine in the United States: A cost-effectiveness analysis. Clin. Infect. Dis. 2016, 62, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, S.; Abassi, M.; Rhein, J.; Boulware, D.R. Recent advances in AIDS-related cryptococcal meningitis treatment with an emphasis on resource limited settings. Expert Rev. Anti. Infect. Ther. 2017, 15, 331–340. [Google Scholar] [CrossRef]
- Neuville, S.; Dromer, F.; Morin, O.; Dupont, B.; Ronin, O.; Lortholary, O. French cryptococcosis study group, primary cutaneous cryptococcosis: A distinct clinical entity. Clin. Infect. Dis. 2003, 36, 337–347. [Google Scholar] [CrossRef]
- Leopold, W.C.M.; Hole, C.R.; Wozniak, K.L.; Wormley, J.F.L. Cryptococcus and phagocytes: Complex interactions that influence disease outcome. Front. Microbiol. 2016, 7, 105. [Google Scholar] [CrossRef]
- Nyazika, T.K.; Tatuene, J.K.; Kenfak-Foguena, A.; Verweij, P.E.; Meis, J.F.; Robertson, V.J.; Hagen, F. Epidemiology and aetiologies of cryptococcal meningitis in Africa, 1950–2017: Protocol for a systematic review. BMJ Open. 2018, 8, e020654. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.N.; Tilak, R.; Yadav, J.; Bansal, M. Cutaneous Cryptococcus: Marker for disseminated infection. BMJ Case Rep. 2015, 2015, bcr2015210898. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bartelt, L.; Yu, D.; Joshi, A.; Weinbaum, B.; Pierson, T.; Patrizio, M.; Warren, C.A.; Hughes, M.A.; Donowitz, G. Primary cutaneous cryptococcosis treated with debridement and fluconazole monotherapy in an immunosuppressed patient: A case report and review of the literature. Case Rep. Infect. Dis. 2015, 2015, 131356. [Google Scholar] [CrossRef] [PubMed]
- Saag, M.S.; Graybill, R.J.; Larsen, R.A.; Pappas, P.G.; Perfect, J.R.; Powderly, W.G.; Sobel, J.D.; Dismukes, W.E. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin. Infect. Dis. 2000, 30, 710–718. [Google Scholar] [CrossRef]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.H.; et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef]
- Ross, A.J.; Ndayishimiye, E. A review of the management and outcome of patients admitted with cryptococcal meningitis at a regional hospital in KwaZulu-Natal province. S. Afr. Fam. Pract. 2019, 61, 159–164. [Google Scholar] [CrossRef]
- Govender, N.P.; Dlamini, S. Management of HIV-associated cryptococcal disease in South Africa. S. Afr. Med. J. 2014, 104, 869. [Google Scholar] [CrossRef][Green Version]
- Govender, N.P.; Meintjes, G.; Bicanic, T.; Dawood, H.; Harrison, T.S.; Jarvis, J.N.; Karstaedt, A.S.; Maartens, G.; McCarthy, K.M.; Rabie, H.; et al. Guideline for the prevention, diagnosis and management of cryptococcal meningitis among HIV-infected persons: 2013 update. S. Afr. J. HIV Med. 2013, 14, 76–86. [Google Scholar] [CrossRef]
- Abassi, M.; Boulware, D.R.; Rhein, J. Cryptococcal meningitis: Diagnosis and management update. Curr. Trop. Med. Rep. 2015, 2, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Wake, R.; Leeme, T.; Jarvis, J.N. HIV-Associated Cryptococcal meningitis: Bridging the gap between developed and resource-limited settings. Curr. Clin. Microbiol Rep. 2016, 3, 92–102. [Google Scholar] [CrossRef]
- Ogundeji, A.O.; Pohl, C.H.; Sebolai, O.M. The repurposing of anti-psychotic drugs, Quetiapine and Olanzapine, as anti-Cryptococcus drugs. Front. Microbiol. 2017, 8, 815. [Google Scholar] [CrossRef]
- Morawski, B.; Boulware, D.; Nalintya, E.; Kiragga, A.; Kazooza, F.; Rajasingham, R.; Benjamin, J.; Park, B.J.; Manabe, Y.C.; Kaplan, J.E.; et al. Pre-ART cryptococcal antigen titer associated with preemptive fluconazole failure. In Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), Boston, MA, USA, 22–25 February 2016; Volume 24. abstract 159. [Google Scholar]
- Miró-Canturri, A.; Ayerbe-Algaba, R.; Smani, Y. Drug repurposing for the treatment of bacterial and fungal infections. Front. Microbiol. 2019, 10, 41. [Google Scholar] [CrossRef]
- Truong, M.; Monahan, L.G.; Carter, D.A.; Charles, I.G. Repurposing drugs to fast-track therapeutic agents for the treatment of cryptococcosis. PeerJ 2018, 6, e4761. [Google Scholar] [CrossRef]
- Sebolai, O.M.; Ogundeji, A.O. New antifungal discovery from existing chemical compound collections. In Antifungals: From Genomics to Resistance and the Development of Novel Agents; Coste, A.T., Vandeputte, P., Eds.; Caister Academic Press: Norfolk, UK, 2015; pp. 143–158. [Google Scholar] [CrossRef]
- Ogundeji, A.O.; Pohl, C.H.; Sebolai, O.M. Repurposing of aspirin and ibuprofen as candidate anti-Cryptococcus drugs. Antimicrob. Agents Chemother. 2016, 60, 4799–4808. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, N.D. Aspirin: Old drug, new uses and challenges. J. Gastroenterol. Hepatol. 2011, 26, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Bubna, A.K. Aspirin in dermatology: Revisited. Indian Dermatol. Online J. 2015, 6, 428–435. [Google Scholar] [CrossRef]
- World Health Organization. Model List of Essential Medicines; 21st List; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Management Sciences for Health. International Drug Price Indicator Guide, 2014th ed.; Management Sciences for Health: Medford, MA, USA, 2015. [Google Scholar]
- Stapleton, M.; Rhodes, L. Photosensitizers for photodynamic therapy of cutaneous disease. J. Dermatol. Treat. 2003, 14, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakov, I.P.; Sosedova, Y.A.; Plyusnin, V.F.; Grivin, V.P.; Bazhin, N.M. Photochemistry of salicylate anion in aqueous solution. Russ. Chem. Bull. 2007, 5, 1318–1324. [Google Scholar] [CrossRef]
- Pozdnyakov, I.P.; Plyusnin, V.F.; Grivin, V.P.; Vorobyev, D.Y.; Kruppa, A.I.; Lemmetyinen, H. Photochemistry of sulfosalicylic acid in aqueous solutions. J. Photochem. Photobiol. A Chem. 2004, 162, 153–162. [Google Scholar] [CrossRef]
- Pozdnyakov, I.P.; Plyusnin, V.F.; Grivin, V.P.; Vorobyev, D.Y.; Bazhin, N.M.; Vauthey, E. Photochemistry of Fe(III) and sulfosalicylic acid aqueous solutions. J. Photochem. Photobiol. A Chem. 2006, 182, 75–81. [Google Scholar] [CrossRef]
- Norman, C.; Howell, K.A.; Millar, A.H.; Whelan, J.M.; Day, D.A. Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant. Physiol. 2004, 134, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Levy, G. Pharmacokinetics of aspirin in man. J. Investig. Dermatol. 1976, 67, 667–668. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J. Filobasidiella Kwon-Chung (1975). In The Yeast, a Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier Sciences BV: Amsterdam, The Netherlands, 2011; pp. 1443–1456. [Google Scholar]
- Chang, Y.C.; Penoyer, L.A.; Kwon-Chung, K.J. The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect. Immun. 1996, 64, 1977–1983. [Google Scholar] [CrossRef]
- Okabayashi, K.; Hasegawa, A.; Watanabe, T. Microreview: Capsule-associated genes of Cryptococcus neoformans. Mycopathologia 2007, 163, 1–8. [Google Scholar] [CrossRef]
- Casadevall, A.; Coelho, C.; Cordero, R.J.B.; Dragotakes, Q.; Jung, E.; Vij, R. The capsule of Cryptococcus neoformans. Virulence 2019, 10, 822–831. [Google Scholar] [CrossRef]
- Voelz, K.; May, R.C. Cryptococcal interactions with the host immune system. Eukaryot. Cell 2010, 9, 835–846. [Google Scholar] [CrossRef]
- Aslam, S.; Lan, X.R.; Zhang, B.W.; Chen, Z.L.; Wang, L.; Niu, D.K. Aerobic prokaryotes do not have higher GC contents than anaerobic prokaryotes, but obligate aerobic prokaryotes have. BMC Evol. Biol. 2019, 19, 35. [Google Scholar] [CrossRef]
- Ingavale, S.S.; Chang, Y.C.; Lee, H.; McClelland, C.M.; Leong, M.L.; Kwon-Chung, K.J. Importance of mitochondria in survival of Cryptococcus neoformans under low oxygen conditions and tolerance to cobalt chloride. PLoS Pathog. 2008, 4, e1000155. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 1–26. [Google Scholar] [CrossRef]
- Houstek, J.; Pícková, A.; Vojtísková, A.; Mrácek, T.; Pecina, P.; Jesina, P. Mitochondrial diseases and genetic defects of ATP synthase. Biochim. Biophys. Acta 2006, 1757, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Croudace, J.E.; Lammas, D.A.; May, R.C. Expulsion of live pathogenic yeast by macrophages. Curr. Biol. 2006, 16, 2156–2160. [Google Scholar] [CrossRef] [PubMed]
- Kock, J.L.F.; Sebolai, O.M.; Pohl, C.H.; van Wyk, P.W.J.; Lodolo, E.J. Oxylipin studies expose aspirin as antifungal. FEMS Yeast Res. 2007, 7, 1207–1217. [Google Scholar] [CrossRef]
- Mang, T.S.; Mikulski, L.; Hall, R.E. Photodynamic inactivation of normal and antifungal resistant Candida species. Photodiagnosis Photodyn. Ther. 2010, 7, 98–105. [Google Scholar] [CrossRef]
- Eliopoulos, G.M.; Perea, S.; Patterson, T.F. Antifungal resistance in pathogenic fungi. Clin. Infect. Dis. 2002, 35, 1073–1080. [Google Scholar] [CrossRef]
- Katiraee, F.; Teifoori, F.; Soltani, M. Emergence of azole-resistant Candida species in AIDS patients with oropharyngeal candidiasis in Iran. Curr. Med. Mycol. 2015, 1, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.; Szemán-Nagy, G.; Kiss, A.; Nagy, Z.L.; Tálas, L.; Rácz, D.; Majoros, L.; Tóth, Z.; Szigeti, Z.M.; Pócsi, I.; et al. Antifungal activity of an original amino-isocyanonaphthalene (ICAN) compound family: Promising broad spectrum antifungals. Molecules 2020, 25, 903. [Google Scholar] [CrossRef]
- Fuchs, B.B.; Tegos, G.P.; Hamblin, M.R.; Mylonakis, E. Susceptibility of Cryptococcus neoformans to photodynamic inactivation is associated with cell wall integrity. Antimicrob. Agents Chemother. 2007, 51, 2929–2936. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.M.; Alves, O.A.; Ferreira, M.V.; Amorim, J.C.; Sousa, G.R.; Silveira, L.B.; Prates, R.A.; Avila, T.V.; Baltazar Lde, M.; de Souza Dda, G.; et al. Cryptococcus gattii: In vitro susceptibility to photodynamic inactivation. Photochem. Photobiol. 2011, 87, 357–364. [Google Scholar] [CrossRef]
- Von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair, a Chemical Perspective; Springer-Verlag: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Guinea, J.; Cuenca-Estrella, M.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Howard, S.J. The Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). EUCAST Definitive Document E. Def 7.3: Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts; EUCAST: Copenhagen, Denmark, 2015; Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7_3_Yeast_testing_definitive.pdf (accessed on 1 December 2015).
- Harrington, B.; Valigosky, M. Monitoring ultraviolet lamps in biological safety cabinets with cultures of standard bacterial strains on TSA blood agar. Lab. Med. 2007, 38, 165–168. [Google Scholar] [CrossRef]
- Madu, U.L.; Ogundeji, A.O.; Pohl, C.H.; Albertyn, J.; Sebolai, O.M. Elucidation of the role of 3-hydroxy fatty acids in Cryptococcus-amoeba interactions. Front. Microbiol. 2017, 8, 765. [Google Scholar] [CrossRef]
- Van Wyk, P.W.J.; Wingfield, M.J. Ascospore ultrastructure and development in Ophiostoma cucullatum. Mycologia 1991, 83, 698–707. [Google Scholar] [CrossRef]
- Rotor-Gene SYBR Green Handbook. Rotor-Gene SYBR Green RT-PCR Kit and Additional Protocols, US. 2014. Available online: http://www.qiagen.com (accessed on 1 January 2014).
- Madu, U.L.; Ogundeji, A.O.; Mochochoko, B.M.; Pohl, C.H. Cryptococcal 3-hydroxy fatty acids protect cells against amoebal phagocytosis. Front. Microbiol. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, T.L.; Milic, N.M.; Winham, S.J.; Garovic, V.D. Beyond bar and line graphs: Time for a new data presentation paradigm. PLoS Biol. 2015, 13, e1002128. [Google Scholar] [CrossRef] [PubMed]
| Primer | Accession No. | Sequence (5’–3’) | Band Size |
|---|---|---|---|
| Actin | U10867 | F-TGTACAATGGTATTGCCGACC R-CTGGTCCCTCAATCGTCCAC | 200 bp |
| CAP64 | L40026 | F-GCCACGCCCACATTGACT R-ACTCTTCCTCGATCAATGTC | 200 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogundeji, A.O.; Mjokane, N.; Folorunso, O.S.; Pohl, C.H.; Nyaga, M.M.; Sebolai, O.M. The Repurposing of Acetylsalicylic Acid as a Photosensitiser to Inactivate the Growth of Cryptococcal Cells. Pharmaceuticals 2021, 14, 404. https://doi.org/10.3390/ph14050404
Ogundeji AO, Mjokane N, Folorunso OS, Pohl CH, Nyaga MM, Sebolai OM. The Repurposing of Acetylsalicylic Acid as a Photosensitiser to Inactivate the Growth of Cryptococcal Cells. Pharmaceuticals. 2021; 14(5):404. https://doi.org/10.3390/ph14050404
Chicago/Turabian StyleOgundeji, Adepemi O., Nozethu Mjokane, Olufemi S. Folorunso, Carolina H. Pohl, Martin M. Nyaga, and Olihile M. Sebolai. 2021. "The Repurposing of Acetylsalicylic Acid as a Photosensitiser to Inactivate the Growth of Cryptococcal Cells" Pharmaceuticals 14, no. 5: 404. https://doi.org/10.3390/ph14050404
APA StyleOgundeji, A. O., Mjokane, N., Folorunso, O. S., Pohl, C. H., Nyaga, M. M., & Sebolai, O. M. (2021). The Repurposing of Acetylsalicylic Acid as a Photosensitiser to Inactivate the Growth of Cryptococcal Cells. Pharmaceuticals, 14(5), 404. https://doi.org/10.3390/ph14050404







