Drug Repurposing in Medical Mycology: Identification of Compounds as Potential Antifungals to Overcome the Emergence of Multidrug-Resistant Fungi
Abstract
1. Introduction
2. Current Antifungal Agents and Their Mechanisms of Resistance
3. Non-Antifungal Drugs Identified as Having a Potential Antifungal Activity against Invasive Fungal Strains
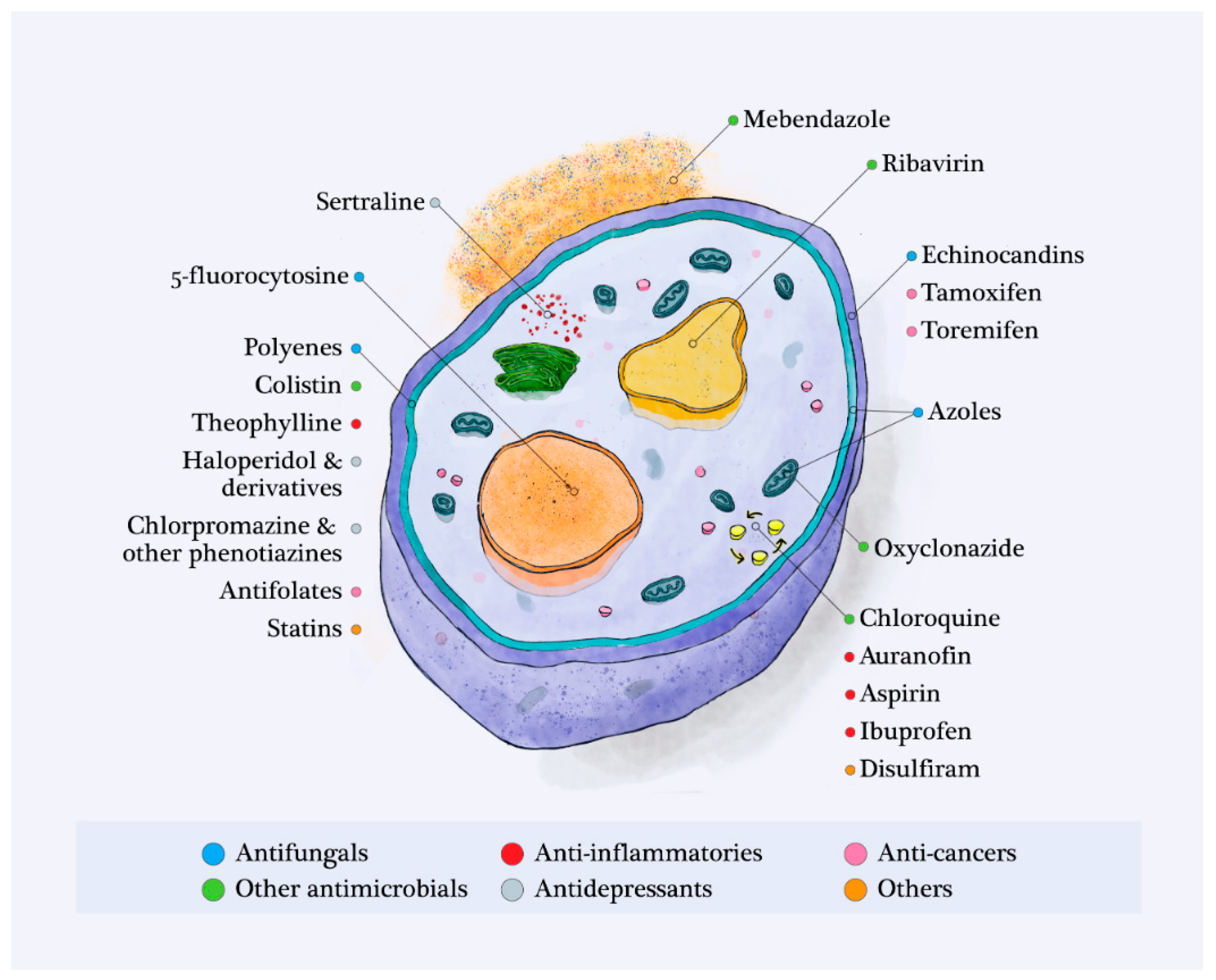
3.1. Antimicrobials Apart from Antifungals
| Drug | First Indication | Antifungal Activity | Activity Range | Antifungal Mechanism of Action | References | |
|---|---|---|---|---|---|---|
| Antimicrobials | Polymyxins Colistin Polymyxin B | Gram-negative bacterial infections | C. albicans | 16–128 µg/mL | Membrane damages on Candida albicans | [41] |
| C. neoformans | ||||||
| R. mucilaginosa | ||||||
| S. apiospermum | ||||||
| L. prolificans | ||||||
| F. oxysporum | ||||||
| F. solani | ||||||
| R. oryzae | ||||||
| Ribavirin | Hepatitis C | C. albicans | 0.37–3.02 µg/mL | Disruption of vacuoles function of C. albicans strains | [41,43] | |
| C. tropicalis | ||||||
| C. parapsilosis | ||||||
| Oxyclozanide | Animal parasitosis | C. albicans | 16–32 µg/mL | Uncoupling the mitochondrial electron transport from phosphorylation and changing the mitochondrial membrane potential | [44] | |
| Chloroquine | Malaria | C. neoformans | 3.19 µg/mL | Iron deprivation | [45] | |
| (10 µM) | ||||||
| C. albicans | 31.2–250 µg/mL | Inhibition of ergosterol biosynthesis | [46] | |||
| S. cerevisiae | NR | Growth inhibition via blocking thiamine transportation | [47] | |||
| Mebendazole | Helminthiasis | C. neoformans | 92.5 ng/mL | Morphological alterations by reducing capsular dimension | [48] | |
| C. gatti | (0.3125 µM) | |||||
| Anti-inflammatory | Auranofin | Rheumatoid arthritis | C. albicans | 0.25–16 µg/mL | Action on reactive-oxygen-mediated cell death | [49,50] |
| A. fumigatus | ||||||
| S. apiospermum | ||||||
| L. prolificans | ||||||
| C. neoformans | ||||||
| Aspirin | Inflammation | Cryptococcus spp. | 1–10 mg/mL | Stress induction via ROS-mediated damage | [51,52] | |
| Ibuprofen | Candida spp. | |||||
| Theophylline | Asthma, COPD | Candida spp. | 1.4–1.8 mg/mL | Membrane damages by ionic and ergosterol modifications | [53] | |
| Antipsychotics | Haloperidol | Psychosis | C. albicans | <4 µg/mL | Possible action on GPCRs, mediators of signals across the cell membrane | [54,55] |
| Trifluperidol | C. neoformans | |||||
| Sertraline | Depression | C. neoformans | 2–6 µg/mL | Inhibition of protein synthesis | [56,57] | |
| Lomentospora prolificans | ||||||
| Scedosporium spp., Fusarium spp. | 8–32 μg/mL | |||||
| Paecilomyces spp., Alternaria spp. and Curvularia spp. | ||||||
| Chlorpromazine | Schizophrenia | Candida spp. | 1–16 µg/mL | Possible modifications of membrane | [58,59] | |
| C. neoformans | ||||||
| Filamentous fungi: Aspergillus spp., Scedosporium spp., Pseudallescheria spp. and | ||||||
| Zygomycetes | ||||||
| Anticancers | Tamoxifen | Breast cancer | Candida spp. C. neoformans | 8–64 µg/mL | Prevention of proteins calmodulin from binding to calcineurin, cell lysis and alteration of fungal development | [60,61,62] |
| Toremifene | Disturb the cell wall integrity via interaction with Ccr1 | |||||
| Others | Disulfiram | Alcoholism | Candida spp. | 1–16 µg/mL | Chelating metals Inhibition of multidrug transporter implicated in drug resistance | [63,64] |
| C. neoformans | ||||||
| Aspergillus spp. |
3.2. Anti-Inflammatory Drugs
3.3. Antipsychotic Drugs
3.4. Anticancer Drugs
3.5. Other Approved Drugs
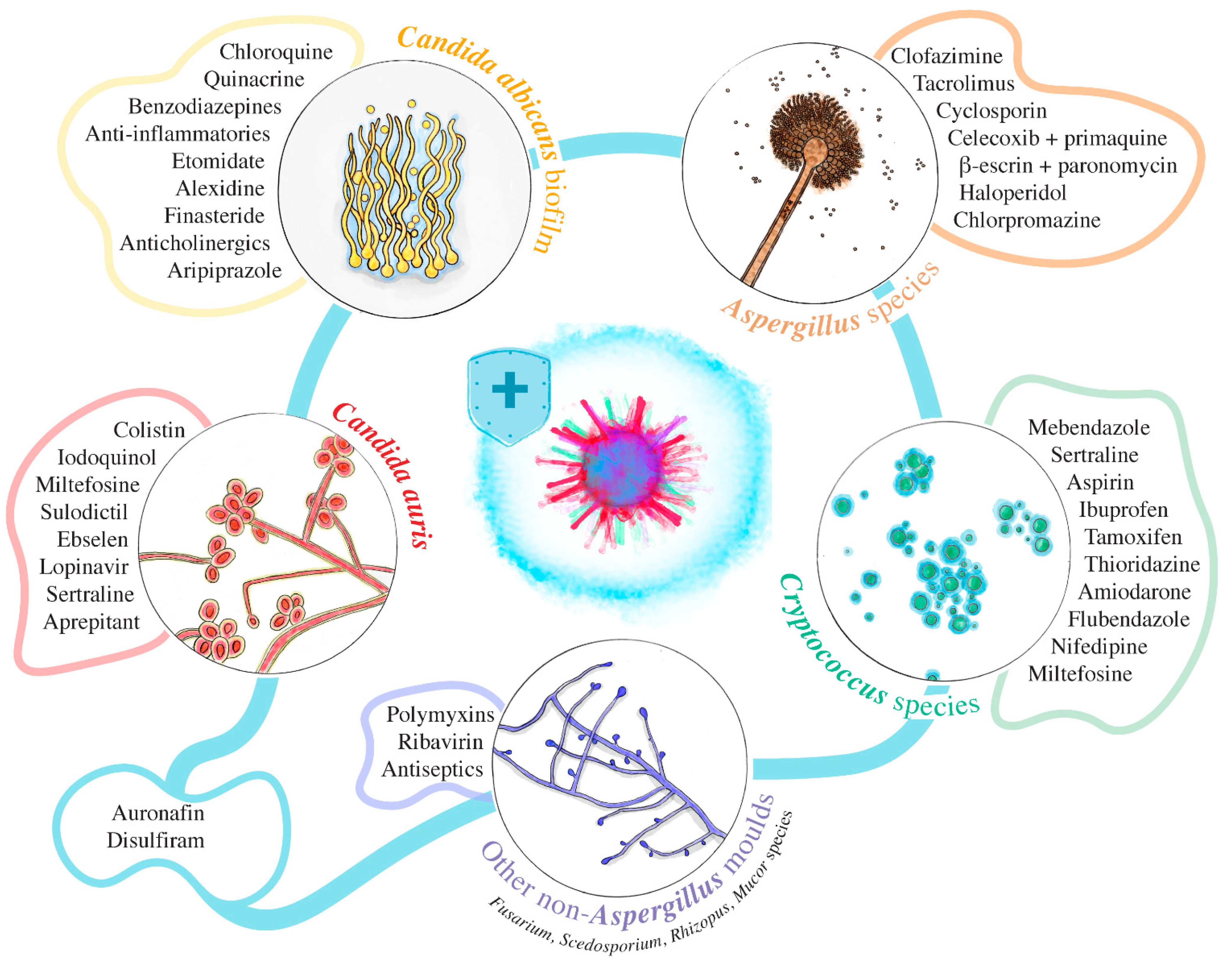
4. Some Emerging Multidrug-Resistant Fungi and Their Compounds with Repurpose Potential Identified through Phenotypic Screening
4.1. C. albicans Biofilms
4.2. C. auris
4.3. Aspergillus Species
4.4. Cryptococcus Species
4.5. Other Non-Aspergillus Molds
5. Further Assessment and Prioritization of Repurpose Potential
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Denning, D.W. Calling upon all public health mycologists: To accompany the country burden papers from 14 countries. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 923–924. [Google Scholar] [CrossRef]
- Livio, P.; Morena, C.; Anna, C.; Massimo, O.; Luana, F.; Bruno, M.; Domenico, P.; Marco, P.; Alessandro, B.; Anna, C.; et al. The epidemiology of fungal infections in patients with hematologic malignancies: The SEIFEM-2004 study. Haematologica 2006, 91, 1068–1075. [Google Scholar]
- Armstrong-James, D.; Meintjes, G.; Brown, G.D. A neglected epidemic: Fungal infections in HIV/AIDS. Trends Microbiol. 2014, 22, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Geddes-McAlister, J.; Shapiro, R.S. New pathogens, new tricks: Emerging, drug-resistant fungal pathogens and future prospects for antifungal therapeutics. Ann. N. Y. Acad. Sci. 2019, 1435, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Goel, N.; Gupta, A.; Gupta, K.B.; Chaudhary, U.; Sood, S. A rare fungal infiltration of lungs in a healthy young girl. Case Rep. Pulmonol. 2011, 2011, 1–3. [Google Scholar] [CrossRef]
- Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K. Overcoming antifungal resistance. Drug Discov. Today Technol. 2014, 11, 65–71. [Google Scholar] [CrossRef]
- Alcazar-Fuoli, L.; Mellado, E. Current status of antifungal resistance and its impact on clinical practice. Br. J. Haematol. 2014, 166, 471–484. [Google Scholar] [CrossRef]
- Meurman, J.H.; Hämäläinen, P.; Janket, S.J. Oral infections in older adults. Aging Health 2006, 2, 1013–1023. [Google Scholar] [CrossRef]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 58, 2–13. [Google Scholar] [CrossRef]
- Barber, A.E.; Riedel, J.; Sae-Ong, T.; Kang, K.; Brabetz, W.; Panagiotou, G.; Deising, H.B.; Kurzai, O. Effects of agricultural fungicide use on Aspergillus fumigatus: Abundance, antifungal susceptibility, and population structure. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Demers, E.G.; Biermann, A.R.; Masonjones, S.; Crocker, A.W.; Ashare, A.; Stajich, J.E.; Hogan, D.A. Evolution of drug resistance in an antifungal-naive chronic Candida lusitaniae infection. Proc. Natl. Acad. Sci. USA 2018, 115, 12040–12045. [Google Scholar] [CrossRef]
- Hall, R.A.; Noverr, M.C. Fungal interactions with the human host: Exploring the spectrum of symbiosis. Curr. Opin. Microbiol. 2017, 40, 58–64. [Google Scholar] [CrossRef]
- Peyclit, L.; Baron, S.A.; Rolain, J.-M. Drug repurposing to fight colistin and carbapenem-resistant bacteria. Front. Cell. Infect. Microbiol. 2019, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Dimasi, J.A.; Feldman, L.; Seckler, A.; Wilson, A. Trends in risks associated with new drug development: Success rates for investigational drugs. Clin. Pharmacol. Ther. 2010, 87, 272–277. [Google Scholar] [CrossRef]
- Houšt’, J.; Spížek, J.; Havlícek, V. Antifungal Drugs. Metabolites 2020, 10. [Google Scholar] [CrossRef]
- Wiederhold, N.P. The antifungal arsenal: Alternative drugs and future targets. Int. J. Antimicrob. Agents 2018, 51, 333–339. [Google Scholar] [CrossRef]
- Rauseo, A.M.; Coler-Reilly, A.; Larson, L.; Spec, A. Hope on the horizon: Novel fungal treatments in development. Open Forum Infect. Dis. 2020, 7, 1–19. [Google Scholar] [CrossRef]
- Su, H.; Han, L.; Huang, X. Potential targets for the development of new antifungal drugs. J. Antibiot. 2018, 71, 978–991. [Google Scholar] [CrossRef]
- Mahboubi, M. Artemisia sieberi Besser essential oil and treatment of fungal infections. Biomed. Pharmacother. 2017, 89, 1422–1430. [Google Scholar] [CrossRef]
- D′agostino, M.; Tesse, N.; Frippiat, J.P.; Machouart, M.; Debourgogne, A. Essential oils and their natural active compounds presenting antifungal properties. Molecules 2019, 24, 3713. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, S.; Vivot, W.; Szusz, W.; Albo, G. Antifungal activity of essential oils against Candida species isolated from clinical samples. Mycopathologia 2019, 184, 615–623. [Google Scholar] [CrossRef]
- Barac, A.; Donadu, M.; Usai, D.; Spiric, V.T.; Mazzarello, V.; Zanetti, S.; Aleksic, E.; Stevanovic, G.; Nikolic, N.; Rubino, S. Antifungal activity of Myrtus communis against Malassezia sp. isolated from the skin of patients with pityriasis versicolor. Infection 2018, 46, 253–257. [Google Scholar] [CrossRef]
- González-Burgos, E.; Gómez-Serranillos, M.P. Natural Products for Vulvovaginal Candidiasis Treatment: Evidence from Clinical Trials. Curr. Top. Med. Chem. 2018, 18, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef]
- Gill, R.; Amberkar Mohan Babu, V.; Meena Kumari, K. Assets and liabilities of drug repositioning. Int. J. Pharma Bio Sci. 2016, 7, 47–53. [Google Scholar] [CrossRef]
- Nett, J.E.; Andes, D.R. Antifungal Agents: Spectrum of activity, pharmacology, and clinical indications. Infect. Dis. Clin. N. Am. 2016, 30, 51–83. [Google Scholar] [CrossRef]
- Kuriyama, T.; Karasawa, T.; Williams, D.W. Antimicrobial Chemotherapy: Significance to Healthcare. In Biofilms in Infection Prevention and Control: A Healthcare Handbook; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 209–244. ISBN 9780123970435. [Google Scholar]
- Scorzoni, L.; de Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.M.A.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal therapy: New advances in the understanding and treatment of mycosis. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, S.M.; Paul, R.A.; Chakrabarti, A.; Mouton, J.W.; Meis, J.F. Invasive aspergillosis by Aspergillus flavus: Epidemiology, diagnosis, antifungal resistance, and management. J. Fungi 2019, 5, 55. [Google Scholar] [CrossRef]
- Allen, D.; Wilson, D.; Drew, R.; Perfect, J. Azole antifungals: 35 years of invasive fungal infection management. Expert Rev. Anti Infect. Ther. 2015, 13, 787–798. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef]
- Kanafani, Z.A.; Perfect, J.R. Resistance to antifungal agents: Mechanisms and clinical impact. Clin. Infect. Dis. 2008, 46, 120–128. [Google Scholar] [CrossRef]
- Yu, D.T.; Peterson, J.F.; Seger, D.L.; Gerth, W.C.; Bates, D.W. Frequency of potential azole drug-drug interactions and consequences of potential fluconazole drug interactions. Pharmacoepidemiol. Drug Saf. 2005, 14, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Canuto, M.M.; Rodero, F.G. Antifungal drug resistance to azoles and polyenes. Lancet Infect. Dis. 2002, 2, 550–563. [Google Scholar] [CrossRef]
- Aguilar-Zapata, D.; Petraitiene, R.; Petraitis, V. Echinocandins: The expanding antifungal armamentarium. Clin. Infect. Dis. 2015, 61, S604–S611. [Google Scholar] [CrossRef]
- Chassot, F.; Venturini, T.P.; Piasentin, F.B.; Santurio, J.M.; Svidzinski, T.I.E.; Alves, S.H. Activity of antifungal agents alone and in combination against echinocandin-susceptible and -resistant Candida parapsilosis strains. Rev. Iberoam. Micol. 2019, 36, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Ciliberto, G.; Mancini, R.; Paggi, M.G. Drug repurposing against COVID-19: Focus on anticancer agents. J. Exp. Clin. Cancer Res. 2020, 39. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.; Hadjadj, L.; Rolain, J.-M.; Olaitan, A.O. Molecular mechanisms of polymyxin resistance: Knowns and unknowns. Int. J. Antimicrob. Agents 2016, 48, 583–591. [Google Scholar] [CrossRef]
- Yousfi, H.; Ranque, S.; Rolain, J.M.; Bittar, F. In vitro polymyxin activity against clinical multidrug-resistant fungi. Antimicrob. Resist. Infect. Control 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Bibi, M.; Murphy, S.; Benhamou, R.I.; Rosenberg, A.; Ulman, A.; Bicanic, T.; Fridman, M.; Berman, J. Combining Colistin and Fluconazole Synergistically Increases Fungal Membrane Permeability and Antifungal Cidality. ACS Infect. Dis. 2021, 7. [Google Scholar] [CrossRef]
- Tournu, H.; Carroll, J.; Latimer, B.; Dragoi, A.M.; Dykes, S.; Cardelli, J.; Peters, T.L.; Eberle, K.E.; Palmer, G.E. Identification of small molecules that disrupt vacuolar function in the pathogen Candida albicans. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Pic, E.; Burgain, A.; Sellam, A. Repurposing the anthelminthic salicylanilide oxyclozanide against susceptible and clinical resistant Candida albicans strains. Med. Mycol. 2019, 57, 387–390. [Google Scholar] [CrossRef]
- Weber, S.M.; Levitz, S.M.; Harrison, T.S. Chloroquine and the fungal phagosome. Curr. Opin. Microbiol. 2000, 3, 349–353. [Google Scholar] [CrossRef]
- Shinde, R.B.; Raut, J.S.; Chauhan, N.M.; Karuppayil, S.M. Chloroquine sensitizes biofilms of Candida albicans to antifungal azoles. Braz. J. Infect. Dis. 2013, 17, 395–400. [Google Scholar] [CrossRef]
- Huang, Z.; Srinivasan, S.; Zhang, J.; Chen, K.; Li, Y.; Li, W.; Quiocho, F.A.; Pan, X. Discovering thiamine transporters as targets of chloroquine using a novel functional genomics strategy. PLoS Genet. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Joffe, L.S.; Schneider, R.; Lopes, W.; Azevedo, R.; Staats, C.C.; Kmetzsch, L.; Schrank, A.; Del Poeta, M.; Vainstein, M.H.; Rodrigues, M.L. The anti-helminthic compound mebendazole has multiple antifungal effects against Cryptococcus neoformans. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Wall, G.; Chaturvedi, A.K.; Wormley, F.L.; Wiederhold, N.P.; Patterson, H.P.; Patterson, T.F.; Lopez-Ribot, J.L. Screening a repurposing library for inhibitors of multidrug-resistant Candida auris identifies ebselen as a repositionable candidate for antifungal drug development. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Patterson, T.F.; Srinivasan, A.; Chaturvedi, A.K.; Fothergill, A.W.; Wormley, F.L.; Ramasubramanian, A.K.; Lopez-Ribot, J.L. Repurposing auranofin as an antifungal: In vitro activity against a variety of medically important fungi. Virulence 2017, 8, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Ogundeji, A.O.; Pohl, C.H.; Sebolai, O.M. Repurposing of aspirin and ibuprofen as candidate anti-Cryptococcus drugs. Antimicrob. Agents Chemother. 2016, 60, 4799–4808. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Sansonetty, F.; Rodrigues, A.G.; Martinez-De-Oliveira, J.; Fonseca, A.F.; Mårdh, P.A. Antifungal activity of ibuprofen alone and in combination with fluconazole against Candida species. J. Med. Microbiol. 2000, 49, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Fatima, Z.; Ahmad, K.; Hameed, S. Repurposing of respiratory drug theophylline against Candida albicans: Mechanistic insights unveil alterations in membrane properties and metabolic fitness. J. Appl. Microbiol. 2020, 129, 860–875. [Google Scholar] [CrossRef]
- Stylianou, M.; Kulesskiy, E.; Lopes, J.P.; Granlund, M.; Wennerberg, K.; Urban, C.F. Antifungal application of nonantifungal drugs. Antimicrob. Agents Chemother. 2014, 58, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Liu, N.; Tu, J.; Li, Z.; Han, G.; Li, J.; Sheng, C. Drug Repurposing of Haloperidol: Discovery of New Benzocyclane Derivatives as Potent Antifungal Agents against Cryptococcosis and Candidiasis. ACS Infect. Dis. 2019, 6. [Google Scholar] [CrossRef]
- Zhai, B.; Wu, C.; Wang, L.; Sachs, M.S.; Lin, X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob. Agents Chemother. 2012, 56, 3758–3766. [Google Scholar] [CrossRef]
- Villanueva-Lozano, H.; González, G.M.; Espinosa-Mora, J.E.; Bodden-Mendoza, B.A.; Andrade, A.; Martínez-Reséndez, M.F.; Treviño-Rangel, R.D.J. Evaluation of the expanding spectrum of sertraline against uncommon fungal pathogens. J. Infect. Chemother. 2020, 26, 309–311. [Google Scholar] [CrossRef]
- Vitale, R.G.; Afeltra, J.; Meis, J.F.G.M.; Verweij, P.E. Activity and post antifungal effect of chlorpromazine and trifluopherazine against Aspergillus, Scedosporium and zygomycetes. Mycoses 2007, 50, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Galgóczy, L.; Bácsi, A.; Homa, M.; Virágh, M.; Papp, T.; Vágvölgyi, C. In vitro antifungal activity of phenothiazines and their combination with amphotericin B against different Candida species. Mycoses 2011, 54. [Google Scholar] [CrossRef] [PubMed]
- Butts, A.; Koselny, K.; Chabrier-Roselló, Y.; Semighini, C.P.; Brown, J.C.S.; Wang, X.; Annadurai, S.; DiDone, L.; Tabroff, J.; Childers, W.E.; et al. Estrogen receptor antagonists are anti-cryptococcal agents that directly bind EF hand proteins and synergize with fluconazole in vivo. mBio 2014, 5. [Google Scholar] [CrossRef]
- Dolan, K.; Montgomery, S.; Buchheit, B.; DiDone, L.; Wellington, M.; Krysan, D.J. Antifungal activity of tamoxifen: In vitro and in vivo activities and mechanistic characterization. Antimicrob. Agents Chemother. 2009, 53, 3337–3346. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guo, X.; Jiang, G.; Wu, G.; Miao, H.; Liu, K.; Chen, S.; Sakamoto, N.; Kuno, T.; Yao, F.; et al. NADPH-cytochrome P450 reductase ccr1 is a target of tamoxifen and participates in its antifungal activity via regulating cell wall integrity in fission yeast. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Shukla, S.; Sauna, Z.E.; Prasad, R.; Ambudkar, S.V. Disulfiram is a potent modulator of multidrug transporter Cdr1p of Candida albicans. Biochem. Biophys. Res. Commun. 2004, 322, 520–525. [Google Scholar] [CrossRef]
- Sauna, Z.E.; Shukla, S.; Ambudkar, S.V. Disulfiram, an old drug with new potential therapeutic uses for human cancers and fungal infections. Mol. Biosyst. 2005, 1, 127–134. [Google Scholar] [CrossRef]
- Rossato, L.; Camargo dos Santos, M.; Vitale, R.G.; de Hoog, S.; Ishida, K. Alternative treatment of fungal infections: Synergy with non-antifungal agents. Mycoses 2020. [Google Scholar] [CrossRef]
- Rossi, S.A.; De Oliveira, H.C.; Agreda-Mellon, D.; Lucio, J.; Soares Mendes-Giannini, M.J.; García-Cambero, J.P.; Zaragoza, O. Identification of off-patent drugs that show synergism with amphotericin B or that present antifungal action against Cryptococcus neoformans and Candida spp. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Te, H.S.; Randall, G.; Jensen, D.M. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol. Hepatol. 2007, 3, 218–225. [Google Scholar]
- Yousfi, H.; Cassagne, C.; Ranque, S.; Rolain, J.M.; Bittar, F. Repurposing of ribavirin as an adjunct therapy against invasive Candida strains in an in vitro study. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Swan, G.E. The pharmacology of halogenated salicylanilides and their anthelmintic use in animals. J. S. Afr. Vet. Assoc. 1999, 70, 61–70. [Google Scholar] [CrossRef]
- Ayerbe-Algaba, R.; Gil-Marqués, M.L.; Miró-Canturri, A.; Parra-Millán, R.; Pachón-Ibáñez, M.E.; Jiménez-Mejías, M.E.; Pachón, J.; Smani, Y. The anthelmintic oxyclozanide restores the activity of colistin against colistin-resistant Gram-negative bacilli. Int. J. Antimicrob. Agents 2019, 54, 507–512. [Google Scholar] [CrossRef]
- ANSES RCP—DOUVISTOME. Available online: http://www.ircp.anmv.anses.fr/rcp.aspx?NomMedicament=DOUVISTOME (accessed on 17 February 2021).
- Rolain, J.M.; Colson, P.; Raoult, D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents 2007, 30, 297–308. [Google Scholar] [CrossRef]
- Plantone, D.; Koudriavtseva, T. Current and Future Use of Chloroquine and Hydroxychloroquine in Infectious, Immune, Neoplastic, and Neurological Diseases: A Mini-Review. Clin. Drug Investig. 2018, 38, 653–671. [Google Scholar] [CrossRef]
- Shinde, R.B.; Rajput, S.B.; Raut, J.S.; Mohan Karuppayil, S. An in vitro repositioning study reveals antifungal potential of chloroquine to inhibit growth and morphogenesis in Candida albicans. J. Gen. Appl. Microbiol. 2013, 59, 167–170. [Google Scholar] [CrossRef]
- Bai, R.Y.; Staedtke, V.; Wanjiku, T.; Rudek, M.A.; Joshi, A.; Gallia, G.L.; Riggins, G.J. Brain penetration and efficacy of different mebendazole polymorphs in a mouse brain tumor model. Clin. Cancer Res. 2015, 21, 3462–3470. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Tariq, V.N.; McCrory, R.M. Demonstration of synergy with fluconazole and either ibuprofen, sodium salicylate, or propylparaben against Candida albicans in vitro. Antimicrob. Agents Chemother. 1995, 39, 2610–2614. [Google Scholar] [CrossRef]
- Torres, N.S.; Abercrombie, J.J.; Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K.; Leung, K.P. Screening a commercial library of pharmacologically active small molecules against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2016, 60, 5663–5672. [Google Scholar] [CrossRef]
- She, P.; Liu, Y.; Wang, Y.; Tan, F.; Luo, Z.; Wu, Y. Antibiofilm efficacy of the gold compound auranofin on dual species biofilms of Staphylococcus aureus and Candida spp. J. Appl. Microbiol. 2020, 128, 88–101. [Google Scholar] [CrossRef]
- Holbrook, S.Y.L.; Garzan, A.; Dennis, E.K.; Shrestha, S.K.; Garneau-Tsodikova, S. Repurposing antipsychotic drugs into antifungal agents: Synergistic combinations of azoles and bromperidol derivatives in the treatment of various fungal infections. Eur. J. Med. Chem. 2017, 139, 12–21. [Google Scholar] [CrossRef]
- Rhein, J.; Nielsen, K.; Boulware, D.R.; Meya, D.B. Sertraline for HIV-associated cryptococcal meningitis—Authors’ reply. Lancet Infect. Dis. 2016, 16, 1111–1112. [Google Scholar] [CrossRef]
- Moraes, D.C.; Ferreira-Pereira, A. Insights on the anticandidal activity of non-antifungal drugs. J. Mycol. Med. 2019, 29, 253–259. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, T.F.; Machado, C.; de, S.; Serafin, M.B.; Bottega, A.; Foletto, V.S.; Coelho, S.S.; Hörner, R. Repositioning or Redirection of Antidepressant Drugs in the Treatment of Bacterial and Fungal Infections. Am. J. Ther. 2020, 27, e528–e532. [Google Scholar] [CrossRef]
- Nayak, R.; Xu, J. Effects of sertraline hydrochloride and fluconazole combinations on Cryptococcus neoformans and Cryptococcus gattii. Mycology 2010, 1, 99–105. [Google Scholar] [CrossRef][Green Version]
- Rossato, L.; Loreto, É.S.; Zanette, R.A.; Chassot, F.; Santurio, J.M.; Alves, S.H. In vitro synergistic effects of chlorpromazine and sertraline in combination with amphotericin B against Cryptococcus neoformans var. grubii. Folia Microbiol. (Praha) 2016, 61, 399–403. [Google Scholar] [CrossRef]
- Rhein, J.; Huppler Hullsiek, K.; Tugume, L.; Nuwagira, E.; Mpoza, E.; Evans, E.E.; Kiggundu, R.; Pastick, K.A.; Ssebambulidde, K.; Akampurira, A.; et al. Adjunctive sertraline for HIV-associated cryptococcal meningitis: A randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect. Dis. 2019, 19, 843–851. [Google Scholar] [CrossRef]
- Boulware, D.R.; Nalintya, E.; Rajasingham, R.; Kirumira, P.; Naluyima, R.; Turya, F.; Namanda, S.; Rutakingirwa, M.K.; Skipper, C.P.; Nikweri, Y.; et al. Adjunctive sertraline for asymptomatic cryptococcal antigenemia: A randomized clinical trial. Med. Mycol. 2020, 58, 1037–1043. [Google Scholar] [CrossRef]
- Homa, M.; Galgóczy, L.; Tóth, E.; Tóth, L.; Papp, T.; Chandrasekaran, M.; Kadaikunnan, S.; Alharbi, N.S.; Vágvölgyi, C. In vitro antifungal activity of antipsychotic drugs and their combinations with conventional antifungals against Scedosporium and Pseudallescheria isolates. Med. Mycol. 2015, 53, 890–895. [Google Scholar] [CrossRef]
- Montoya, M.C.; Krysan, D.J. Repurposing estrogen receptor antagonists for the treatment of infectious disease. mBio 2018, 9. [Google Scholar] [CrossRef]
- Butts, A.; Martin, J.A.; DiDone, L.; Bradley, E.K.; Mutz, M.; Krysan, D.J. Structure-Activity Relationships for the Antifungal Activity of Selective Estrogen Receptor Antagonists Related to Tamoxifen. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Quezada, H.; Martínez-Vázquez, M.; López-Jácome, E.; González-Pedrajo, B.; Andrade, Á.; Fernández-Presas, A.M.; Tovar-García, A.; García-Contreras, R. Repurposed anti-cancer drugs: The future for anti-infective therapy? Expert Rev. Anti Infect. Ther. 2020, 18, 609–612. [Google Scholar] [CrossRef]
- Ngan, N.T.T.; Mai, N.T.H.; Tung, N.L.N.; Lan, N.P.H.; Tai, L.T.H.; Phu, N.H.; Chau, N.V.V.; Binh, T.Q.; Hung, L.Q.; Beardsley, J.; et al. A randomized open label trial of tamoxifen combined with amphotericin B and fluconazole for cryptococcal meningitis. Wellcome Open Res. 2019, 4. [Google Scholar] [CrossRef]
- de Oliveira Neto, A.S.; Souza, I.L.A.; Amorim, M.E.S.; de Freitas Souza, T.; Rocha, V.N.; do Couto, R.O.; Fabri, R.L.; de Freitas Araújo, M.G. Antifungal efficacy of atorvastatin-containing emulgel in the treatment of oral and vulvovaginal candidiasis. Med. Mycol. 2020. [Google Scholar] [CrossRef]
- Macreadie, I.G.; Johnson, G.; Schlosser, T.; Macreadie, P.I. Growth inhibition of Candida species and Aspergillus fumigatus by statins. FEMS Microbiol. Lett. 2006, 262, 9–13. [Google Scholar] [CrossRef]
- de Queiroz Ribeiro, N.; Costa, M.C.; Magalhães, T.F.F.; Carneiro, H.C.S.; Oliveira, L.V.; Fontes, A.C.L.; Santos, J.R.A.; Ferreira, G.F.; de Sousa Araujo, G.R.; Alves, V.; et al. Atorvastatin as a promising anticryptococcal agent. Int. J. Antimicrob. Agents 2017, 49, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Singhal, S.; Mathur, T.; Upadhyay, D.J.; Rattan, A. Antifungal potential of disulfiram. Jpn. J. Med. Mycol. 2007, 48, 109–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hao, W.; Qiao, D.; Han, Y.; Du, N.; Li, X.; Fan, Y.; Ge, X.; Zhang, H. Identification of disulfiram as a potential antifungal drug by screening small molecular libraries. J. Infect. Chemother. 2020. [Google Scholar] [CrossRef]
- Blanc, M.; Daeppen, J.-B. Does disulfiram still have a role in alcoholism treatment? Rev. Med. Suisse 2005, 1, 1728–1730. [Google Scholar]
- Talbot, G.H.; Bradley, J.; Edwards, J.E.; Gilbert, D.; Scheid, M.; Bartlett, J.G. Bad bugs need drugs: An update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 42, 657–668. [Google Scholar] [CrossRef]
- Osei Sekyere, J. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen 2018, 7. [Google Scholar] [CrossRef]
- Ramirez-Garcia, A.; Pellon, A.; Rementeria, A.; Buldain, I.; Barreto-Bergter, E.; Rollin-Pinheiro, R.; De Meirelles, J.V.; Xisto, M.I.D.S.; Ranque, S.; Havlicek, V.; et al. Scedosporium and Lomentospora: An updated overview of underrated opportunists. Med. Mycol. 2018, 56, S102–S125. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Kathwate, G.H.; Shinde, R.B.; Mohan Karuppayil, S. Non-antifungal drugs inhibit growth, morphogenesis and biofilm formation in Candida albicans. J. Antibiot. 2021, 1–8. [Google Scholar] [CrossRef]
- Kim, J.H.; Cheng, L.W.; Chan, K.L.; Tam, C.C.; Mahoney, N.; Friedman, M.; Shilman, M.M.; Land, K.M. Antifungal Drug Repurposing. Antibiotics 2020, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Ješić, M.; Ranin, L. Influence of acetylsalicylic acid (Aspirin) on biofilm production by Candida species. J. Chemother. 2004, 16, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Kulkarny, V.V.; Chavez-Dozal, A.; Rane, H.S.; Jahng, M.; Bernardo, S.M.; Parra, K.J.; Lee, S.A. Quinacrine inhibits Candida albicans growth and filamentation at neutral pH. Antimicrob. Agents Chemother. 2014, 58, 7501–7509. [Google Scholar] [CrossRef] [PubMed]
- Holanda, M.A.V.; Da Silva, C.R.; De A Neto, J.B.; Do Av Sá, L.G.; Do Nascimento, F.B.S.A.; Barroso, D.D.; Da Silva, L.J.; Cândido, T.M.; Leitão, A.C.; Barbosa, A.D.; et al. Evaluation of the antifungal activity in vitro of midazolam against fluconazole-resistant Candida spp. isolates. Future Microbiol. 2021, 16, 71–81. [Google Scholar] [CrossRef]
- Juvêncio da Silva, L.; Dias Barroso, F.D.; Vieira, L.S.; Carlos Mota, D.R.; da Silva Firmino, B.K.; Rocha da Silva, C.; de Farias Cabral, V.P.; Cândido, T.M.; Sá, L.G.D.A.V.; Barbosa da Silva, W.M.; et al. Diazepam’s antifungal activity in fluconazole-resistant Candida spp. and biofilm inhibition in C. albicans: Evaluation of the relationship with the proteins ALS3 and SAP5. J. Med. Microbiol. 2021. [Google Scholar] [CrossRef]
- Chavez-Dozal, A.A.; Jahng, M.; Rane, H.S.; Asare, K.; Kulkarny, V.V.; Bernardo, S.M.; Lee, S.A. In vitro analysis of flufenamic acid activity against Candida albicans biofilms. Int. J. Antimicrob. Agents 2014, 43, 86–91. [Google Scholar] [CrossRef]
- Do Amaral Valente Sá, L.G.; Da Silva, C.R.; De Andrade Neto, J.B.; Do Nascimento, F.B.S.A.; Barroso, F.D.D.; Da Silva, L.J.; De Farias Cabral, V.P.; Barbosa, A.D.; Silva, J.; Marinho, E.S.; et al. Antifungal activity of etomidate against growing biofilms of fluconazole-resistant Candida spp. strains, binding to mannoproteins and molecular docking with the ALS3 protein. J. Med. Microbiol. 2020, 69, 1221–1227. [Google Scholar] [CrossRef]
- Chavez-Dozal, A.A.; Lown, L.; Jahng, M.; Walraven, C.J.; Lee, S.A. In vitro analysis of finasteride activity against Candida albicans urinary biofilm formation and filamentation. Antimicrob. Agents Chemother. 2014, 58, 5855–5862. [Google Scholar] [CrossRef]
- Nile, C.; Falleni, M.; Cirasola, D.; Alghamdi, A.; Anderson, O.F.; Delaney, C.; Ramage, G.; Ottaviano, E.; Tosi, D.; Bulfamante, G.; et al. Repurposing Pilocarpine Hydrochloride for Treatment of Candida albicans Infections. mSphere 2019, 4. [Google Scholar] [CrossRef]
- Machado, C.B.; da Silva, C.R.; Barroso, F.D.; Campos, R.D.S.; Sá, L.V.; do Nascimento, F.A.; Cavalcanti, B.C.; Júnior, H.V.N.; Neto, J.A. In vitro evaluation of anti-fungal activity of tropicamide against strains of Candida spp. resistant to fluconazole in planktonic and biofilm form. J. Mycol. Med. 2021, 31. [Google Scholar] [CrossRef]
- Rajasekharan, S.K.; Lee, J.H.; Lee, J. Aripiprazole repurposed as an inhibitor of biofilm formation and sterol biosynthesis in multidrug-resistant Candida albicans. Int. J. Antimicrob. Agents 2019, 54, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.A.; Ahmad, A. Candida auris—the growing menace to global health. Mycoses 2019, 62, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R. Candida auris and multidrug resistance: Defining the new normal. Fungal Genet. Biol. 2019, 131. [Google Scholar] [CrossRef]
- Kean, R.; Ramage, G. Combined Antifungal Resistance and Biofilm Tolerance: The Global Threat of Candida auris. mSphere 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Allaw, F.; Kara Zahreddine, N.; Ibrahim, A.; Tannous, J.; Taleb, H.; Bizri, A.R.; Dbaibo, G.; Kanj, S.S. First Candida auris Outbreak during a COVID-19 Pandemic in a Tertiary-Care Center in Lebanon. Pathogens 2021, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-S.; Roma, J.S.; Shen, M.; Fernandes, C.M.; Tsang, P.S.; Forbes, H.E.; Boshoff, H.; Lazzarini, C.; Del Poeta, M.; Zheng, W.; et al. Identification of Antifungal Compounds against Multidrug Resistant Candida auris Utilizing a High Throughput Drug Repurposing Screen. Antimicrob. Agents Chemother. 2021. [Google Scholar] [CrossRef]
- Eldesouky, H.E.; Lanman, N.A.; Hazbun, T.R.; Seleem, M.N. Aprepitant, an antiemetic agent, interferes with metal ion homeostasis of Candida auris and displays potent synergistic interactions with azole drugs. Virulence 2020, 11, 1466–1481. [Google Scholar] [CrossRef]
- Eldesouky, H.E.; Salama, E.A.; Lanman, N.A.; Hazbun, T.R.; Seleem, M.N. Potent synergistic interactions between lopinavir and azole antifungal drugs against emerging multidrug-resistant Candida auris. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef]
- Gowri, M.; Jayashree, B.; Jeyakanthan, J.; Girija, E.K. Sertraline as a promising antifungal agent: Inhibition of growth and biofilm of Candida auris with special focus on the mechanism of action in vitro. J. Appl. Microbiol. 2020, 128, 426–437. [Google Scholar] [CrossRef]
- Kim, J.H.; Chan, K.L.; Cheng, L.W.; Tell, L.A.; Byrne, B.A.; Clothier, K.; Land, K.M. High efficiency drug repurposing design for new antifungal agents. Methods Protoc. 2019, 2, 31. [Google Scholar] [CrossRef]
- Vermorel-Faure, O.; Lebeau, B.; Mallaret, M.R.; Michallet, M.; Brut, A.; Ambroise-Thomas, P.; Grillot, R. Food-related fungal infection risk in agranulocytosis. Mycological control of 273 food items offered to patients hospitalized in sterile units. Press. Med. 1993, 22, 157–160. [Google Scholar]
- Meis, J.F.; Chowdhary, A.; Rhodes, J.L.; Fisher, M.C.; Verweij, P.E. Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [PubMed]
- De Lucca, A.J. Harmful fungi in both agriculture and medicine. Rev. Iberoam. Micol. 2007, 24, 3–13. [Google Scholar] [CrossRef]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Osherov, N.; Kontoyiannis, D.P. The anti- Aspergillus drug pipeline: Is the glass half full or empty? Med. Mycol. 2017, 55, 118–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vallières, C.; Singh, N.; Alexander, C.; Avery, S.V. Repurposing Nonantifungal Approved Drugs for Synergistic Targeting of Fungal Pathogens. ACS Infect. Dis. 2020, 6, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- May, R.C.; Stone, N.R.H.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117. [Google Scholar] [CrossRef]
- Spadari, C.D.C.; Wirth, F.; Lopes, L.B.; Ishida, K. New approaches for cryptococcosis treatment. Microorganisms 2020, 8, 613. [Google Scholar] [CrossRef]
- Truong, M.; Monahan, L.G.; Carter, D.A.; Charles, I.G. Repurposing drugs to fast-track therapeutic agents for the treatment of cryptococcosis. PeerJ 2018, 2018. [Google Scholar] [CrossRef]
- Tortorano, A.M.; Richardson, M.; Roilides, E.; van Diepeningen, A.; Caira, M.; Munoz, P.; Johnson, E.; Meletiadis, J.; Pana, Z.D.; Lackner, M.; et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin. Microbiol. Infect. 2014, 20, 27–46. [Google Scholar] [CrossRef]
- Douglas, A.P.; Chen, C.-A.; Slavin, M.A. Emerging infections caused by non-Aspergillus filamentous fungi. Clin. Microbiol. Infect. 2016, 22, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.M.; George, E. Scedosporium apiospermum: An emerging opportunistic pathogen that must be distinguished from Aspergillus and other hyalohyphomycetes. J. Cutan. Pathol. 2009, 36, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, H.; Ranque, S.; Cassagne, C.; Rolain, J.M.; Bittar, F. Identification of repositionable drugs with novel antimycotic activity by screening the Prestwick Chemical Library against emerging invasive moulds. J. Glob. Antimicrob. Resist. 2020, 21, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.S.; Montelongo-Jauregui, D.; Abercrombie, J.J.; Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K.; Leung, K.P. Antimicrobial and antibiofilm activity of synergistic combinations of a commercially available small compound library with colistin against Pseudomonas aeruginosa. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Dovizio, M.; Bruno, A.; Tacconelli, S.; Patrignani, P. Mode of action of aspirin as a chemopreventive agent. Recent Results Cancer Res. 2013, 191, 39–65. [Google Scholar] [CrossRef] [PubMed]
- Bjorkman, S.; Ove Elisson, L.; Gabrielsson, J. Pharmacokinetics of Quinacrine after Intrapleural Instillation in Rabbits and Man. J. Pharm. Pharmacol. 1989, 41, 160–163. [Google Scholar] [CrossRef]
- Steinfort, D.P.; Steinfort, C. Effect of long-term nebulized colistin on lung function and quality of life in patients with chronic bronchial sepsis. Intern. Med. J. 2007, 37, 495–498. [Google Scholar] [CrossRef]
- Rolain, J.M.; Baquero, F. The refusal of the Society to accept antibiotic toxicity: Missing opportunities for therapy of severe infections. Clin. Microbiol. Infect. 2016, 22, 423–427. [Google Scholar] [CrossRef]
| Antifungal Classes | Mechanisms of Action | Clinical Indications | Side Effects | Mechanisms of Resistance | Common Resistant Species |
|---|---|---|---|---|---|
| Polyenes Amphotericin B Nystatin | Ergosterol binding (membrane) permeabilization by ion channel formation Cell content leakage | Invasive fungal infection Topical Candida infections | Renal toxicity Hypokalemia Phlebitis Immunoallergic reaction | Deficiencies in ERG2 and ERG3 genes Ergosterol synthesis alteration Modifications in membrane sterols Changes of enzymatic activity or signaling pathways | Scedosporium spp., Candida lusitaniae, Aspergillus terreus |
| Azoles Fluconazole Itraconazole Voriconazole Posaconazole Efinaconazole Isavuconazole | Inhibition of lanosterol Ergosterol synthesis inhibition Alteration of fungal membrane fluidity and agility | All invasive candidiasis Cryptococcal meningitis Aspergillus spp. infections | Digestive disturbancesCephalgias Hepatotoxicity Drug interactions (CYPP450) | Over expression of efflux pump’s function ERG11 gene mutations inducing blockage in azoles binding Up-regulation of enzyme target Bypass pathway development by ERG3 gene mutation | FCZ: Candida krusei, Aspergillus spp., Scedosporium spp., Fusarium spp., Mucorales ITZ: Fusarium spp. VRZ: Mucorales |
| Echinocandins Micafungin Caspofungin Anidulafungin | Inhibition of β-1,3-glucan synthase (β-GS) Formation of a defective cell wall | Invasive candidiasis Invasive aspergillosis (2nd intention) | Good overall tolerance | Mutations on FKS1 gene (encoding for a subunit of β-GS) Decrease of affinity between drug and target | Cryptococcus spp., Fusarium spp., Scedosporium spp., Mucorales |
| 5-fluorocytosine | Nucleoside analogue Disruption of protein synthesis Inhibition of DNA synthesis | Cryptococcosis Invasive candidiasis if treatment failure Always in association | Gastrointestinal troubles Hepatotoxicity Hematotoxicity | Mutations on FUR1 gene (encoding uracil phosphoribosyl transferase) Mutations on FCY1 gene (encoding cytosine deaminase enzyme) | Ineffective against many filamentous fungi |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peyclit, L.; Yousfi, H.; Rolain, J.-M.; Bittar, F. Drug Repurposing in Medical Mycology: Identification of Compounds as Potential Antifungals to Overcome the Emergence of Multidrug-Resistant Fungi. Pharmaceuticals 2021, 14, 488. https://doi.org/10.3390/ph14050488
Peyclit L, Yousfi H, Rolain J-M, Bittar F. Drug Repurposing in Medical Mycology: Identification of Compounds as Potential Antifungals to Overcome the Emergence of Multidrug-Resistant Fungi. Pharmaceuticals. 2021; 14(5):488. https://doi.org/10.3390/ph14050488
Chicago/Turabian StylePeyclit, Lucie, Hanane Yousfi, Jean-Marc Rolain, and Fadi Bittar. 2021. "Drug Repurposing in Medical Mycology: Identification of Compounds as Potential Antifungals to Overcome the Emergence of Multidrug-Resistant Fungi" Pharmaceuticals 14, no. 5: 488. https://doi.org/10.3390/ph14050488
APA StylePeyclit, L., Yousfi, H., Rolain, J.-M., & Bittar, F. (2021). Drug Repurposing in Medical Mycology: Identification of Compounds as Potential Antifungals to Overcome the Emergence of Multidrug-Resistant Fungi. Pharmaceuticals, 14(5), 488. https://doi.org/10.3390/ph14050488






