The Relationship between Pulmonary Damage and Peripheral Vascular Manifestations in Systemic Sclerosis Patients
Abstract
1. Introduction
2. Pulmonary Manifestations
3. Interstitial Lung Disease
4. Imaging
5. Pulmonary Function Tests
6. Pulmonary Arterial Hypertension
7. Screening
8. Right Heart Catheterization
9. Peripheral Vascular Manifestations
10. Nailfold Videocapillaroscopy
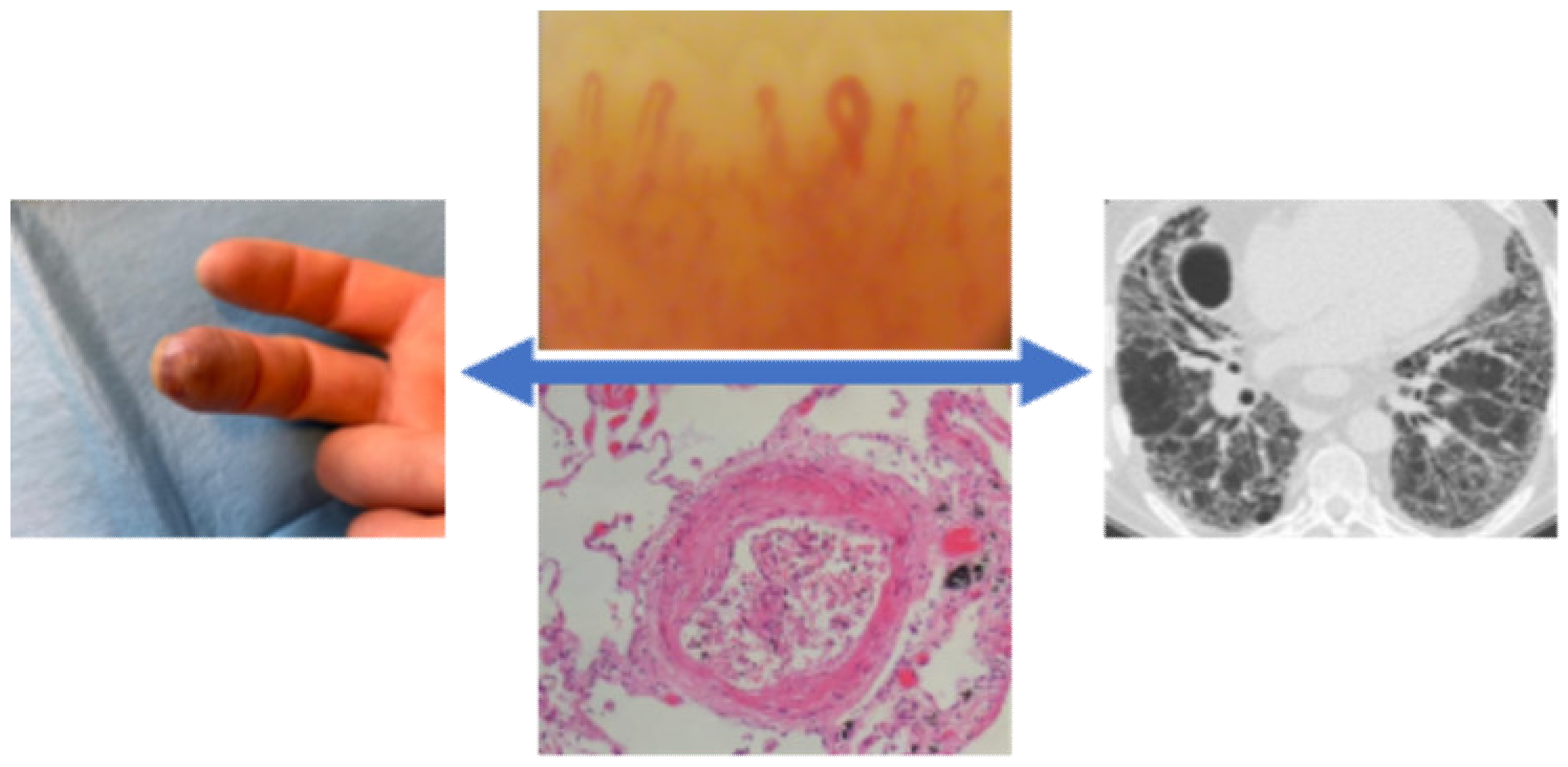
11. The Correlation Between Peripheral Vascular and Pulmonary Involvement
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varga, J.; Trojanowska, M.; Kuwana, M. Pathogenesis of systemic sclerosis: Recent insights of molecular and cellular mechanisms and therapeutic opportunities. J. Scleroderma Relat. Disord. 2017, 2, 137–152. [Google Scholar] [CrossRef]
- Smith, V.; Scirè, C.A.; Talarico, R.; Airo, P.; Alexander, T.; Allanore, Y.; Bruni, C.; Codullo, V.; Dalm, V.; De Vries-Bouwstra, J.; et al. Systemic sclerosis: State of the art on clinical practice guidelines. RMD Open 2018, 4 (Suppl. 1), e000782. [Google Scholar] [CrossRef]
- Hinchcliff, M.; O’Reilly, S. Current and Potential New Targets in Systemic Sclerosis Therapy: A New Hope. Curr. Rheumatol. Rep 2020, 22, 42. [Google Scholar] [CrossRef]
- Ruaro, B.; Soldano, S.; Smith, V.; Paolino, S.; Contini, P.; Montagna, P.; Pizzorni, C.; Casabella, A.; Tardito, S.; Sulli, A.; et al. Correlation between circulating fibrocytes and dermal thickness in limited cutaneous systemic sclerosis patients: A pilot study. Rheumatol. Int. 2019, 39, 1369–1376. [Google Scholar] [CrossRef]
- Bruni, C.; Frech, T.; Manetti, M.; Rossi, F.W.; Furst, D.E.; De Paulis, A.; Rivellese, F.; Guiducci, S.; Matucci-Cerinic, M.; Bellando-Randone, S. Vascular leaking, a pivotal and early pathogenetic event in systemic sclerosis: Should the door be closed? Front. Immunol. 2018, 9, 2045. [Google Scholar] [CrossRef]
- Ruaro, B.; Smith, V.; Sulli, A.; Pizzorni, C.; Tardito, S.; Patané, M.; Paolino, S.; Cutolo, M. Innovations in the Assessment of Primary and Secondary Raynaud's Phenomenon. Front. Pharmacol. 2019, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Altorok, N.; Nada, S.; Kahaleh, B. The isolation and characterization of systemic sclerosis vascular smooth muscle cells: Enhanced proliferation and apoptosis resistance. J. Scleroderma Relat. Disord. 2016, 1, 307–315. [Google Scholar] [CrossRef]
- Ruaro, B.; Nallino, M.G.; Casabella, A.; Salton, F.; Confalonieri, P.; De Tanti, A.; Bruni, C. Monitoring the microcirculation in the diagnosis and follow-up of systemic sclerosis patients: Focus on pulmonary and peripheral vascular manifestations. Microcirculation 2020, 27, e12647. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Sulli, A.; Smith, V.; Pizzorni, C.; Paolino, S.; Alessandri, E.; Trombetta, A.C.; Cutolo, M. Advances in nailfold capillaroscopic analysis in systemic sclerosis. J. Scleroderma Relat. Disord. 2018, 3, 122–131. [Google Scholar] [CrossRef]
- Hoffmann-Vold, A.M.; Maher, T.M.; Philpot, E.E.; Ashrafzadeh, A.; Barake, R.; Barsotti, S.; Bruni, C.; Carducci, P.; Carreira, P.E.; Castellví, I.; et al. The identification and management of interstitial lung disease in systemic sclerosis: Evidence-based European consensus statements. Lancet Rheumatol. 2020, 2, e71–e83. [Google Scholar] [CrossRef]
- Hoffmann-Vold, A.M.; Allanore, Y.; Bendstrup, E.; Bruni, C.; Distler, O.; Maher, T.M.; Wijsenbeek, M.; Kreuter, M. The need for a holistic approach for SSc-ILD—Achievements and ambiguity in a devastating disease. Respir. Res. 2020, 21, 197. [Google Scholar] [CrossRef]
- Bruni, C.; Guignabert, C.; Manetti, M.; Cerinic, M.M.; Humbert, M. The multifaceted problem of pulmonary arterial hypertension in systemic sclerosis. Lancet Rheumatol. 2021, 3, E149–E159. [Google Scholar] [CrossRef]
- Bruni, C.; De Luca, G.; Lazzaroni, M.G.; Zanatta, E.; Lepri, G.; Airò, P.; Dagna, L.; Doria, A.; Matucci-Cerinic, M. Screening for pulmonary arterial hypertension in systemic sclerosis: A systematic literature review. Eur. J. Intern. Med. 2020, 78, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.; Koh, D.; Kelly, D.; Beattie, K.A.; Larché, M.J. Pulmonary arterial hypertension screening practices in scleroderma patients among Canadian rheumatologists. J. Scleroderma Relat. Disord. 2020, 5, 237–241. [Google Scholar] [CrossRef]
- Vandecasteele, E.; Melsens, K.; Thevissen, K.; De Pauw, M.; Deschepper, E.; Decuman, S.; Piette, Y.; De Keyser, F.; Brusselle, G.; Smith, V. Prevalence and incidence of pulmonary arterial hypertension: 10-year follow-up of an unselected systemic sclerosis cohort. J. Scleroderma Relat. Disord. 2017, 2, 196–202. [Google Scholar] [CrossRef]
- Hoffmann-Vold, A.-M.; Maher, T.M.; Philpot, E.E.; Ashrafzadeh, A.; Distler, O. Evidence based consensus recommendations for the identification and management of interstitial lung disease in systemic sclerosis. Ann. Rheum. Dis. 2019, 78 (Suppl. 2), 104. [Google Scholar]
- Roofeh, D.; Jaafar, S.; Vummidi, D.; Khanna, D. Management of systemic sclerosis-associated interstitial lung disease. Curr. Opin. Rheumatol. 2019, 31, 241–249. [Google Scholar] [CrossRef]
- Khanna, D.; Strek, M.; Southern, B.; Saggar, R.; Hsu, V.; Mayes, M.D.; Silver, R.; Steen, V.D.; Zoz, D.; Rahaghi, F. Expert consensus on the screening, treatment, and management of patients with systemic sclerosis interstitial lung disease, and the potential role of anti-Fibrotics in a treatment paradigm for systemic sclerosis-interstitial lung disease: A Delphi Consensus Study. Arthritis Rheum. 2018, 70, 12–15. [Google Scholar]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef]
- Humbert, M.; Yaici, A.; de Groote, P.; Montani, D.; Sitbon, O.; Launay, D.; Gressin, V.; Guillevin, L.; Clerson, P.; Simonneau, G.; et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: Clinical characteristics at diagnosis and long-term survival. Arthritis Rheumatol. 2011, 63, 3522–3530. [Google Scholar] [CrossRef] [PubMed]
- Hachulla, E.; Launay, D.; Yaici, A.; Berezne, A.; de Groote, P.; Sitbon, O.; Mouthon, L.; Guillevin, L.; Hatron, P.Y.; Simonneau, G.; et al. Pulmonary arterial hypertension associated with systemic sclerosis in patients with functional class II dyspnoea: Mild symptoms but severe outcome. Rheumatology 2010, 49, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Phung, S.; Strange, G.; Chung, L.P.; Leong, J.; Dalton, B.; Roddy, J.; Deague, J.; Playford, D.; Musk, M.; Gabbay, E. Prevalence of pulmonary arterial hypertension in an Australian scleroderma population: Screening allows for earlier diagnosis. Intern. Med. J. 2009, 39, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Vandecasteele, E.; Drieghe, B.; Melsens, K.; Thevissen, K.; De Pauw, M.; Deschepper, E.; Decuman, S.; Bonroy, C.; Piette, Y.; De Keyser, F.; et al. Screening for pulmonary arterial hypertension in an unselected prospective systemic sclerosis cohort. Eur. Respir. J. 2017, 49, pii1602275. [Google Scholar] [CrossRef]
- Coghlan, J.G.; Denton, C.P.; Grunig, E.; Bonderman, D.; Distler, O.; Khanna, D.; Müller-Ladner, U.; Pope, J.E.; Vonk, M.C.; Doelberg, M.; et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: The DETECT study. Ann. Rheum. Dis. 2014, 73, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmuller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019, 53, pii 1801887. [Google Scholar] [CrossRef]
- Lambova, S.N.; Müller-Ladner, U. Nailfold capillaroscopy in systemic sclerosis—State of the art: The evolving knowledge about capillaroscopic abnormalities in systemic sclerosis. J. Scleroderma Relat. Disord. 2019, 4, 200–211. [Google Scholar] [CrossRef]
- Smith, V.; Beeckman, S.; Herrick, A.L.; Decuman, S.; Deschepper, E.; De Keyser, F.; Distler, O.; Foeldvari, I.; Ingegnoli, F.; Müller-Ladner, U.; et al. An EULAR study group pilot study on reliability of simple capillaroscopic definitions to describe capillary morphology in rheumatic diseases. Rheumatology 2016, 55, 883–890. [Google Scholar] [CrossRef]
- Smith, V.; Vanhaecke, A.; Guerra, M.; De Angelis, R.; Deschepper, E.; Denton, C.; De Angelis, R.; Deschepper, E.; Denton, C.; Distler, O.; et al. Fast track algorithm: How to differentiate a scleroderma pattern from a non-scleroderma pattern. Ann. Rheum. Dis. 2019, 78, 1224–1225. [Google Scholar]
- Smith, V.; Riccieri, V.; Pizzorni, C.; Decuman, S.; Deschepper, E.; Bonroy, C.; Sulli, A.; Piette, Y.; De Keyser, F.; Cutolo, M. Nailfold capillaroscopy for prediction of novel future severe organ involvement in systemic sclerosis. J. Rheumatol. 2013, 40, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Herrick, A.L.; Distler, O.; Becker, M.O.; Beltran, E.; Carpentier, P.; Ferri, C.; Inanç, M.; Vlachoyiannopoulos, P.; Chadha-Boreham, H.; et al. Nailfold videocapillaroscopic features and other clinical risk factors for digital ulcers in systemic sclerosis: A multicenter, prospective cohort study. Arthritis Rheum. 2016, 68, 2527–2539. [Google Scholar] [CrossRef]
- Smith, V.; De Keyser, F.; Pizzorni, C.; Van Praet, J.T.; Decuman, S.; Sulli, A.; Deschepper, E.; Cutolo, M. Nailfold capillaroscopy for day-to-day clinical use: Construction of a simple scoring modality as a clinical prognostic index for digital trophic lesions. Ann. Rheum. Dis. 2011, 70, 180–183. [Google Scholar] [CrossRef]
- Pavan, T.R.; Bredemeier, M.; Hax, V.; Capobianco, K.G.; da Silva Mendonça Chakr, R. Capillary loss on nailfold capillary microscopy is associated with mortality in systemic sclerosis. Clin. Rheumatol. 2018, 37, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Ruaro, B.; Smith, V. Macrocirculation versus microcirculation and digital ulcers in systemic sclerosis patients. Rheumatology 2017, 56, 1834–1836. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trombetta, A.C.; Pizzorni, C.; Ruaro, B.; Paolino, S.; Sulli, A.; Smith, V.; Cutolo, M. Effects of Longterm Treatment with Bosentan and Iloprost on Nailfold Absolute Capillary Number, Fingertip Blood Perfusion, and Clinical Status in Systemic Sclerosis. J. Rheumatol. 2016, 43, 2033–2041. [Google Scholar] [CrossRef]
- Ruaro, B.; Casabella, A.; Paolino, S.; Pizzorni, C.; Ghio, M.; Seriolo, C.; Molfetta, L.; Odetti, P.; Smith, V.; Cutolo, M. Dickkopf-1 (Dkk-1) serum levels in systemic sclerosis and rheumatoid arthritis patients: Correlation with the Trabecular Bone Score (TBS). Clinic. Rheumatol. 2018, 37, 3057–3062. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Fischer, A. Update on morbidity and mortality in systemic sclerosis–related interstitial lung disease. J. Scleroderma Relat. Disord. 2021, 6, 11–20. [Google Scholar] [CrossRef]
- Moore, D.F.; Steen, V.D. Overall mortality. J. Scleroderma Relat. Disord. 2021, 6, 3–10. [Google Scholar] [CrossRef]
- Elhai, M.; Meune, C.; Boubaya, M.; Avouac, J.; Hachulla, E.; Balbir-Gurman, A.; Riemekasten, G.; Airò, P.; Joven, B.; Vettori, S.; et al. Mapping and predicting mortality from systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1897–1905. [Google Scholar] [CrossRef]
- Walker, U.A.; Tyndall, A.; Czirják, L.; Denton, C.; Farge-Bancel, D.; Kowal-Bielecka, O.; Müller-Ladner, U.; Bocelli-Tyndall, C.; Matucci-Cerinic, M. Clinical risk assessment of organ manifestations in systemic sclerosis: A report from the EULAR Scleroderma Trials and Research group database. Ann. Rheum. Dis. 2007, 66, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Elhai, M.; Meune, C.; Avouac, J.; Kahan, A.; Allanore, Y. Trends in mortality in patients with systemic sclerosis over 40 years: A systematic review and meta-analysis of cohort studies. Rheumatology 2012, 51, 1017–1026. [Google Scholar] [CrossRef]
- Rubio-Rivas, M.; Royo, C.; Simeón, C.P.; Corbella, X.; Fonollosa, V. Mortality and survival in systemic sclerosis: Systematic review and meta-analysis. Semin. Arthritis Rheum. 2014, 44, 208–219. [Google Scholar] [CrossRef]
- Clements, P.J.; Roth, M.D.; Elashoff, R.; Tashkin, D.P.; Goldin, J.; Silver, R.M.; Sterz, M.; Seibold, J.R.; Schraufnagel, D.; Simms, R.W.; et al. Scleroderma Lung Study (SLS): Differences in the presentation and course of patients with limited versus diffuse systemic sclerosis. Ann. Rheum. Dis. 2007, 66, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Lescoat, A.; Roofeh, D.; Townsend, W.; Hughes, M.; Sandler, R.D.; Zimmermann, F.; Pauling, J.D.; Buch, M.H.; Khanna, D. Domains and outcome measures for the assessment of limited cutaneous systemic sclerosis: A scoping review protocol. BMJ Open 2021, 11, e044765. [Google Scholar] [CrossRef]
- Mouthon, L.; Berezné, A.; Brauner, M.; Kambouchner, M.; Guillevin, L.; Valeyre, D. Pneumopathie infiltrante diffuse de la sclerodermie systemique [Interstitial lung disease in systemic sclerosis]. Rev. Mal. Respir. 2007, 24, 1035–1046. [Google Scholar] [CrossRef]
- Steen, V.D.; Conte, C.; Owens, G.R.; Medsger, T.A., Jr. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheumatol. 1994, 37, 1283–1289. [Google Scholar] [CrossRef]
- Frauenfelder, T.; Winklehner, A.; Nguyen, T.D.; Dobrota, R.; Baumueller, S.; Maurer, B.; Distler, O. Screening for interstitial lung disease in systemic sclerosis: Performance of high-resolution CT with limited number of slices: A prospective study. Ann. Rheum. Dis. 2014, 73, 2069–2073. [Google Scholar] [CrossRef]
- Hoffmann-Vold, A.M.; Aaløkken, T.M.; Lund, M.B.; Garen, T.; Midtvedt, Ø.; Brunborg, C.; Gran, J.T.; Molberg, Ø. Predictive value of serial high-resolution computed tomography analyses and concurrent lung function tests in systemic sclerosis. Arthritis Rheumatol. 2015, 67, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Vold, A.M.; Allanore, Y.; Alves, M.; Brunborg, C.; Airó, P.; Ananieva, L.P.; Alves, M.; Brunborg, C.; Airó, P.; Ananieva, L.P.; et al. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann. Rheum. Dis. 2021, 80, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Confalonieri, M.; Matucci-Cerinic, M.; Salton, F.; Confalonieri, P.; Santagiuliana, M.; Citton, G.M.; Baratella, E.; Bruni, C. The treatment of lung involvement in systemic sclerosis. Pharmaceuticals 2021, 14, 154. [Google Scholar] [CrossRef]
- Shah, R.M.; Jimenez, S.; Wechsler, R. Significance of ground-glass opacity on HRCT in long-term follow-up of patients with systemic sclerosis. J. Thorac. Imaging 2007, 22, 120–124. [Google Scholar] [CrossRef]
- Occhipinti, M.; Bruni, C.; Camiciottoli, G.; Bartolucci, M.; Bellando-Randone, S.; Bassetto, A.; Cuomo, G.; Giuggioli, D.; Ciardi, G.; Fabbrizzi, A.; et al. Quantitative analysis of pulmonary vasculature in systemic sclerosis at spirometry-gated chest CT. Ann. Rheum. Dis. 2020, 79, 1210–1217. [Google Scholar] [CrossRef]
- Launay, D.; Remy-Jardin, M.; Michon-Pasturel, U.; Mastora, I.; Hachulla, E.; Lambert, M.; Delannoy, V.; Queyrel, V.; Duhamel, A.; Matran, R.; et al. High resolution computed tomography in fibrosing alveolitis associated with systemic sclerosis. J. Rheumatol. 2006, 33, 1789–1801. [Google Scholar] [PubMed]
- Goldin, J.; Elashoff, R.; Kim, H.J.; Yan, X.; Lynch, D.; Strollo, D.; Roth, M.D.; Clements, P.; Furst, D.E.; Khanna, D.; et al. Treatment of scleroderma-interstitial lung disease with cyclophosphamide is associated with less progressive fibrosis on serial thoracic high-resolution CT scan than placebo: Findings from the Scleroderma Lung Study. Chest 2009, 136, 1333–1340. [Google Scholar] [CrossRef]
- Bouros, D.; Wells, A.U.; Nicholson, A.G.; Colby, T.V.; Polychronopoulos, V.; Pantelidis, P.; Haslam, P.L.; Vassilakis, D.A.; Black, C.M.; du Bois, R.M. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am. J. Respir. Crit. Care Med. 2002, 165, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Zamora, F.D.; Kim, H.J.; Wang, Q. Prevalence of pulmonary function test abnormalities and their correlation to high resolution computer tomography in a large scleroderma population. Am. J. Respir. Crit. Care Med. 2013, 187, A2920. [Google Scholar]
- Suliman, Y.A.; Dobrota, R.; Huscher, D.; Nguyen-Kim, T.D.; Maurer, B.; Jordan, S.; Speich, R.; Frauenfelder, T.; Distler, O. Brief Report: Pulmonary Function Tests: High Rate of False-Negative Results in the Early Detection and Screening of Scleroderma-Related Interstitial Lung Disease. Arthritis Rheumatol. 2015, 67, 3256–3261. [Google Scholar] [CrossRef] [PubMed]
- Baratella, E.; Marrocchio, C.; Cifaldi, R.; Santagiuliana, M.; Bozzato, A.M.; Crivelli, P.; Ruaro, B.; Salton, F.; Confalonieri, M.; Cova, M.A. Interstitial lung disease in patients with antisynthetase syndrome: A retrospective case series study. Jpn. J. Radiol. 2021, 39, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Mulkoju, R.; Saka, V.K.; Rajaram, M.; Kumari, R.; Negi, V.S.; Mohanty Mohapatra, M.; Govindaraj, V.; Dwivedi, D.P.; Mahesh Babu, V. Pulmonary Manifestations in Systemic Sclerosis: Hospital-Based Descriptive Study. Cureus 2020, 12, e8649. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.H.; Song, Y.W. Pharmacological Interventions for Pulmonary Involvement in Rheumatic Diseases. Pharmaceuticals 2021, 14, 251. [Google Scholar] [CrossRef]
- Ooi, G.C.; Mok, M.Y.; Tsang, K.W.; Wong, Y.; Khong, P.L.; Fung, P.C.; Chan, S.; Tse, H.F.; Wong, R.W.; Lam, W.K.; et al. Interstitial lung disease in systemic sclerosis. Acta Radiol. 2003, 44, 258–264. [Google Scholar]
- Olson, A.; Hartmann, N.; Patnaik, P.; Wallace, L.; Schlenker-Herceg, R.; Nasser, M.; Richeldi, L.; Hoffmann-Vold, A.M.; Cottin, V. Estimation of the prevalence of progressive fibrosing interstitial lung diseases: Systematic literature review and data from a physician survey. Adv. Ther. 2021, 38, 854–867. [Google Scholar] [CrossRef]
- Spagnolo, P.; Distler, O.; Ryerson, C.J.; Tzouvelekis, A.; Lee, J.S.; Bonella, F.; Bouros, D.; Hoffmann-Vold, A.M.; Crestani, B.; Matteson, E.L. Mechanisms of progressive fibrosis in connective tissue disease (CTD)-associated interstitial lung diseases (ILDs). Ann. Rheum. Dis. 2021, 80, 143–150. [Google Scholar] [CrossRef]
- Ruaro, B.; Salton, F.; Braga, L.; Wade, B.; Confalonieri, P.; Volpe, M.C.; Baratella, E.; Maiocchi, S.; Confalonieri, M. The History and Mystery of Alveolar Epithelial Type II Cells: Focus on Their Physiologic and Pathologic Role in Lung. Int. J. Mol. Sci. 2021, 22, 2566. [Google Scholar] [CrossRef] [PubMed]
- Goh, N.S.; Desai, S.R.; Veeraraghavan, S.; Hansell, D.M.; Copley, S.J.; Maher, T.M.; Corte, T.J.; Sander, C.R.; Ratoff, J.; Devaraj, A.; et al. Interstitial lung disease in systemic sclerosis: A simple staging system. Am. J. Respir. Crit. Care Med. 2008, 177, 1248–1254. [Google Scholar]
- Saldana, D.C.; Hague, C.J.; Murphy, D.; Coxson, H.O.; Tschirren, J.; Peterson, S.; Sieren, J.P.; Kirby, M.; Ryerson, C.J. Association of Computed Tomography Densitometry with Disease Severity, Functional Decline, and Survival in Systemic Sclerosis-associated Interstitial Lung Disease. Ann. Am. Thorac. Soc. 2020, 17, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Colaci, M.; Giuggioli, D.; Sebastiani, M.; Manfredi, A.; Lumetti, F.; Luppi, F.; Cerri, S.; Ferri, C. Predictive value of isolated DLCO reduction in systemic sclerosis patients without cardio-pulmonary involvement at baseline. Reumatismo 2015, 85, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Volkmann, E.R.; Tseng, C.H.; Kim, H.J.; Goldin, J.; Clements, P.; Furst, D.; Khanna, D.; Kleerup, E.; Roth, M.D.; et al. Relationship between quantitative radiographic assessments of interstitial lung disease and physiological and clinical features of systemic sclerosis. Ann. Rheum. Dis. 2016, 75, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Gargani, L.; Bruni, C.; De Marchi, D.; Romei, C.; Guiducci, S.; Bellando-Randone, S.; Aquaro, G.D.; Pepe, A.; Neri, E.; Colagrande, S.; et al. Lung magnetic resonance imaging in systemic sclerosis: A new promising approach to evaluate pulmonary involvement and progression. Clin. Rheumatol. 2020, 7. [Google Scholar] [CrossRef]
- Barskova, T.; Gargani, L.; Guiducci, S.; Randone, S.B.; Bruni, C.; Carnesecchi, G.; Conforti, M.L.; Porta, F.; Pignone, A.; Caramella, D.; et al. Lung ultrasound for the screening of interstitial lung disease in very early systemic sclerosis. Ann. Rheum. Dis. 2013, 72, 390–395. [Google Scholar] [CrossRef]
- Hughes, M.; Bruni, C.; Cuomo, G.; Delle Sedie, A.; Gargani, L.; Gutierrez, M.; Lepri, G.; Ruaro, B.; Santiago, T.; Suliman, Y.; et al. The role of ultrasound in systemic sclerosis: On the cutting edge to foster clinical and research advancement. J. Scleroderma Relat. Disord. 2020. [Google Scholar] [CrossRef]
- Gargani, L.; Bruni, C.; Romei, C.; Frumento, P.; Moreo, A.; Agoston, G.; Guiducci, S.; Bellando-Randone, S.; Lepri, G.; Belloli, L.; et al. Prognostic Value of Lung Ultrasound B-Lines in Systemic Sclerosis. Chest 2020, 158, 1515–1525. [Google Scholar] [CrossRef]
- Behr, J.; Furst, D.E. Pulmonary function tests. Rheumatology 2008, 47 (Suppl. 5), v65–v67. [Google Scholar] [CrossRef]
- Roofeh, D.; Lin, C.J.F.; Goldin, J.; Kim, G.H.; Furst, D.E.; Denton, C.P.; Huang, S.; Khanna, D. focuSSced investigators. Tocilizumab Prevents Progression of Early Systemic Sclerosis Associated Interstitial Lung Disease. Arthritis Rheumatol. 2021, 3. [Google Scholar] [CrossRef]
- Rubio-Rivas, M.; Corbella, X.; Pestaña-Fernández, M.; Tolosa-Vilella, C.; Guillen-Del Castillo, A.; Colunga-Argüelles, D.; Trapiella-Martínez, L.; Iniesta-Arandia, N.; Castillo-Palma, M.J.; Sáez-Comet, L.; et al. First clinical symptom as a prognostic factor in systemic sclerosis: Results of a retrospective nationwide cohort study. Clin. Rheumatol. 2018, 37, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.; Branagan, P.; Moloney, F.; Haroon, M.; O’Connell, O.J.; O’Connor, T.M.; O’Regan, K.; Harney, S.; Henry, M.T. Biomarkers to identify ILD and predict lung function decline in scleroderma lung disease or idiopathic pulmonary fibrosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2015, 32, 228–236. [Google Scholar]
- Bernstein, E.J.; Jaafar, S.; Assassi, S.; Domsic, R.T.; Frech, T.M.; Gordon, J.K.; Broderick, R.J.; Hant, F.N.; Hinchcliff, M.E.; Shah, A.A.; et al. Performance Characteristics of Pulmonary Function Tests for the Detection of Interstitial Lung Disease in Adults With Early Diffuse Cutaneous Systemic Sclerosis. Arthritis Rheum. 2020, 72, 1892–1896. [Google Scholar] [CrossRef] [PubMed]
- Steen, V.D.; Graham, G.; Conte, C.; Owens, G.; Medsger, T.A., Jr. Isolated diffusing capacity reduction in systemic sclerosis. Arthritis Rheum. 1992, 35, 765–770. [Google Scholar] [CrossRef]
- Galiè, N.; McLaughlin, V.V.; Rubin, L.J.; Simonneau, G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1802148. [Google Scholar] [CrossRef]
- Thakkar, V.; Stevens, W.; Prior, D.; Youssef, P.; Liew, D.; Gabbay, E.; Roddy, J.; Walker, J.G.; Zochling, J.; Sahhar, J.; et al. The inclusion of N-terminal pro-brain natriuretic peptide in a sensitive screening strategy for systemic sclerosis-related pulmonary arterial hypertension: A cohort study. Arthritis Res. Ther. 2013, 15, R193. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Cailes, J.B.; Phillips, G.D.; Wells, A.U.; Black, C.M.; Bois, R.M. Comparison of Doppler echocardiography and right heart catheterization to assess pulmonary hypertension in systemic sclerosis. Br. J. Rheumatol. 1997, 36, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Voilliot, D.; Magne, J.; Dulgheru, R.; Kou, S.; Henri, C.; Caballero, L.; De Sousa, C.; Sprynger, M.; Andre, B.; Pierard, L.A.; et al. Cardiovascular outcome in systemic sclerosis. Acta Cardiol. 2015, 70, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Markusse, I.M.; Meijs, J.; de Boer, B.; Bakker, J.A.; Schippers, H.P.C.; Schouffoer, A.A.; Bakker, J.A.; Schippers, H.; Schouffoer, A.A.; Ajmone Marsan, N.; et al. Predicting cardiopulmonary involvement in patients with systemic sclerosis: Complementary value of nailfold videocapillaroscopy patterns and disease-specific autoantibodies. Rheumatology 2017, 56, 1081–1088. [Google Scholar] [CrossRef][Green Version]
- De Scordilli, M.; Pinamonti, B.; Albani, S.; Gregorio, C.; Barbati, G.; Daneluzzi, C.; Korcova, R.; Perkan, A.; Fabris, E.; Geri, P.; et al. Reliability of noninvasive hemodynamic assessment with Doppler echocardiography: Comparison with the invasive evaluation. J. Cardiovasc. Med. 2019, 20, 682–690. [Google Scholar] [CrossRef]
- Oudiz, R.; Langleben, D. Cardiac catheterization in pulmonary arterial hypertension: An updated guide to proper use. Adv. Pulm. Hypertens. J. 2005, 4, 15–25. [Google Scholar] [CrossRef]
- Herrick, A.L. Raynaud’s phenomenon. J. Scleroderma Relat. Disord. 2019, 4, 89–101. [Google Scholar] [CrossRef]
- Pauling, J.D.; Frech, T.M.; Hughes, M.; Gordon, J.K.; Domsic, R.T.; Anderson, M.E.; Ingegnoli, F.; McHugh, N.J.; Johnson, S.R.; Hudson, M.; et al. Patient-reported outcome instruments for assessing Raynaud’s phenomenon in systemic sclerosis: A SCTC vascular working group report. J. Scleroderma Relat. Disord. 2018, 3, 249–252. [Google Scholar] [CrossRef]
- Ruaro, B.; Pizzorni, C.; Paolino, S.; Alessandri, E.; Sulli, A. Aminaphtone Efficacy in Primary and Secondary Raynaud's Phenomenon: A Feasibility Study. Front. Pharmacol. 2019, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Thevissen, K.; Trombetta, A.C.; Pizzorni, C.; Ruaro, B.; Piette, Y.; Paolino, S.; De Keyser, F.; Sulli, A.; Melsens, K.; et al. Nailfold Capillaroscopy and clinical applications in Systemic Sclerosis. Microcirculation 2016, 105, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Bruni, C.; Ruaro, B.; Confalonieri, M.; Matucci-Cerinic, M.; Randone, S.B. Digital Ulcers in Systemic Sclerosis. Presse Med. 2021, 3, 104064. [Google Scholar] [CrossRef]
- Amanzi, L.; Braschi, F.; Fiori, G.; Galluccio, F.; Miniati, I.; Guiducci, S.; Conforti, M.L.; Kaloudi, O.; Nacci, F.; Sacu, O.; et al. Digital ulcers in scleroderma: Staging, characteristics and sub-setting through observation of 1614 digital lesions. Rheumatology 2010, 49, 1374–1382. [Google Scholar] [CrossRef]
- Minier, T.; Guiducci, S.; Bellando-Randone, S.; Bruni, C.; Lepri, G.; Czirják, L.; Distler, O.; Walker, U.A.; Fransen, J.; Allanore, Y.; et al. Preliminary analysis of the very early diagnosis of systemic sclerosis (VEDOSS) EUSTAR multicentre study: Evidence for puffy fingers as a pivotal sign for suspicion of systemic sclerosis. Ann. Rheum. Dis. 2014, 73, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Vasile, M.; Avouac, J.; Sciarra, I.; Stefanantoni, K.; Iannace, N.; Cravotto, E.; Valesini, G.; Allanore, Y.; Riccieri, V. From VEDOSS to established systemic sclerosis diagnosis according to ACR/EULAR 2013 classification criteria: A French-Italian capillaroscopic survey. Clin. Exp. Rheumatol. 2018, 36, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Bernero, E.; Sulli, A.; Ferrari, G.; Ravera, F.; Pizzorni, C.; Ruaro, B.; Zampogna, G.; Alessandri, E.; Cutolo, M. Prospective capillaroscopy-based study on transition from primary to secondary Raynaud’s phenomenon: Preliminary results. Reumatismo 2013, 65, 186–191. [Google Scholar] [CrossRef]
- Cutolo, M.; Grassi, W.; Matucci Cerinic, M. Raynaud’s phenomenon and the role of capillaroscopy. Arthr. Rheum. 2003, 48, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Pizzorni, C.; Sulli, A.; Smith, V.; Ruaro, B.; Trombetta, A.C.; Cutolo, M.; Paolino, S. Primary Raynaud’s phenomenon and nailfold videocapillaroscopy: Age-related changes in capillary morphology. Clin. Rheumatol. 2017, 36, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Herrick, A.L.; Murray, A. The role of capillaroscopy and thermography in the assessment and management of Raynaud’s phenomenon. Autoimmun. Rev. 2018, 17, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Trombetta, A.C.; Melsens, K.; Pizzorni, C.; Sulli, A.; Ruaro, B.; Paolino, S.; Deschepper, E.; Smith, V. Automated assessment of absolute nailfold capillary number on videocapillaroscopic images: Proof of principle and validation in systemic sclerosis. Microcirculation 2018, 25, e12447. [Google Scholar] [CrossRef]
- Cutolo, M.; Melsens, K.; Herrick, A.L.; Foeldvari, I.; Deschepper, E.; De Keyser, F.; Distler, O.; Ingegnoli, F.; Mostmans, Y.; Müller-Ladner, U.; et al. EULAR Study Group on Microcirculation in Rheumatic Diseases. Reliability of simple capillaroscopic definitions in describing capillary morphology in rheumatic diseases. Rheumatology 2018, 57, 757–759. [Google Scholar] [CrossRef]
- Ruaro, B.; Sulli, A.; Pizzorni, C.; Smith, V.; Gotelli, E.; Alsheyyab, J.; Trombetta, A.C.; Cutolo, M. Longitudinal assessment of nailfold capillary number, peripheral blood perfusion and dermal thickness in systemic sclerosispatients over a period of 5 years. Ann. Rheum. Dis. 2018, 77, 1508. [Google Scholar]
- Ruaro, B.; Pizzorni, C.; Paolino, S.; Smith, V.; Ghio, M.; Casabella, A.; Alessandri, E.; Patané, M.; Sulli, A.; Cutolo, M. Correlations between nailfold microvascular damage and skin involvement in systemic sclerosis patients. Microvasc. Res. 2019, 125, 103874. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Casabella, A.; Paolino, S.; Pizzorni, C.; Alessandri, E.; Seriolo, C.; Botticella, G.; Molfetta, L.; Odetti, P.; Smith, V.; et al. Correlation between bone quality and microvascular damage in systemic sclerosis patients. Rheumatology 2018, 57, 1548–1554. [Google Scholar] [CrossRef]
- Bruni, C.; Guiducci, S.; Bellando-Randone, S.; Lepri, G.; Braschi, F.; Fiori, G.; Bartoli, F.; Peruzzi, F.; Blagojevic, J.; Matucci-Cerinic, M. Digital ulcers as a sentinel sign for early internal organ involvement in very early systemic sclerosis. Rheumatology 2015, 54, 72–76. [Google Scholar] [CrossRef]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Ghizzoni, C.; Sebastiani, M.; Manfredi, A.; Campomori, F.; Colaci, M.; Giuggioli, D.; Ferri, C. Prevalence and evolution of scleroderma pattern at nailfold videocapillaroscopy in sistemic sclerosis patients: Clinical and prognostic implications. Microvasc. Res. 2015, 99, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Sulli, A.; Secchi, M.E.; Pizzorni, C.; Cutolo, M. Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients. Arthritis Rheum. 2012, 64, 821–825. [Google Scholar] [CrossRef]
- Medsger, T.A., Jr.; Silman, A.J.; Steen, V.D.; Black, C.M.; Akesson, A.; Bacon, P.A.; Harris, C.A.; Jablonska, S.; Jayson, M.I.; Jimenez, S.A.; et al. A disease severity scale for systemic sclerosis: Development and testing. J. Rheumatol. 1999, 26, 2159–2167. [Google Scholar] [PubMed]
- Smith, V.; Vanhaecke, A.; Guerra, M.; Ruaro, B.; Sulli, A.; Vandecasteele, E. Capillaroscopy in systemic sclerosis related pulmonary arterial hypertension. Ann. Rheum. Dis. 2019, 78, 854–855. [Google Scholar]
- Jehangir, M.; Qayoom, S.; Jeelani, S.; Yousuf, R. Nailfold capillaroscopy in patients of systemic sclerosis and its association with disease severity as evidenced by high resolution computed tomography lung: A hospital based cross sectional study. Int. J. Res. Med. Sci. 2017, 3, 5. [Google Scholar]
- Pizzorni, C.; Ruaro, B.; Paolino, S.; Camellino, D.; Cimmino, M.A.; Cutolo, M.; Sulli, A. Twelve year follow-up on progression of nailfold microangiopathy detected through transition between different capillaroscopic patterns of microvascular damage in systemic sclerosis. Ann. Rheum. Dis. 2016, 75, 746. [Google Scholar] [CrossRef]
- Guillen-Del-Castillo, A.; Simeon-Aznar, C.P.; Callejas-Moraga, E.L.; Tolosa-Vilella, C.; Alonso-Vila, S.; Fonollosa-Pla, V.; Selva-O’Callaghan, A. Quantitative videocapillaroscopy correlates with functional respiratory parameters: A clue for vasculopathy as a pathogenic mechanism for lung injury in systemic sclerosis. Arthritis Res. Ther. 2018, 20, 281. [Google Scholar] [CrossRef] [PubMed]
- Caetano, J.; Paula, F.S.; Amaral, M.; Oliveira, S.; Alves, J.D. Nailfold videocapillaroscopy changes are associated with the presence and severity of systemic sclerosis-related interstitial lung disease. J. Clin. Rheumatol. 2019, 25, e12–e15. [Google Scholar] [CrossRef]
- Xia, Z.; Wang, G.; Xiao, H.; Guo, S.; Liu, Y.; Meng, F.; Liu, D.; Li, G.; Zong, L. Diagnostic value of nailfold videocapillaroscopy in systemic sclerosis secondary pulmonary arterial hypertension: A meta-analysis. Intern. Med. J. 2018, 48, 1355–1359. [Google Scholar] [CrossRef]
- Meier, F.; Geyer, M.; Tiede, H.; Rieth, A.; Ghofrani, H.A.; Müller-Ladner, U.; Zong, L. Is nailfold videocapillaroscopy a valuable diagnostic tool in pulmonary hypertension? Eur. Respir. J. 2012, 40, 972. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, M.K.; Kim, H.Y.; Park, S.H. Capillary dimension measured by computer-based digitalized image correlated with plasma endothelin-1 levels in patients with systemic sclerosis. Clin. Rheumatol. 2010, 29, 247–254. [Google Scholar] [CrossRef]
- Avouac, J.; Lepri, G.; Smith, V.; Toniolo, E.; Hurabielle, C.; Vallet, A.; Amrouche, F.; Kahan, A.; Cutolo, M.; Allanore, Y. Sequential nailfold videocapillaroscopy examinations have responsiveness to detect organ progression in systemic sclerosis. Semin. Arthritis Rheum. 2017, 47, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Hofstee, H.M.; Vonk Noordegraaf, A.; Voskuyl, A.E.; Dijkmans, B.A.; Postmus, P.E.; Smulders, Y.M.; Serné, E.H. Nailfold capillary density is associated with the presence and severity of pulmonary arterial hypertension in systemic sclerosis. Ann. Rheum. Dis. 2009, 68, 191–195. [Google Scholar] [CrossRef]
- Pizzorni, C.; Sulli, A.; Smith, V.; Lladó, A.; Paolino, S.; Cutolo, M.; Ruaro, B. Capillaroscopy 2016: New perspectives in systemic sclerosis. Acta Reumatol. Port. 2016, 41, 8–14. [Google Scholar] [PubMed]
- Corrado, A.; Correale, M.; Mansueto, N.; Monaco, I.; Carriero, A.; Mele, A.; Colia, R.; Di Biase, M.; Cantatore, F.P. Nailfold capillaroscopic changes in patients with idiopathic pulmonary arterial hypertension and systemic sclerosis-related pulmonary arterial hypertension. Microvasc. Res. 2017, 114, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E.; Allen, P.D.; Moore, T.; Hillier, V.; Taylor, C.J.; Herrick, A.L. Computerized nailfold video capillaroscopy—A new tool for assessment of Raynaud’s phenomenon. J. Rheumatol. 2005, 32, 841–848. [Google Scholar] [PubMed]
- Smith, V.; Decuman, S.; Sulli, A.; Bonroy, C.; Piettte, Y.; Deschepper, E.; de Keyser, F.; Cutolo, M. Do worsening scleroderma capillaroscopic patterns predict future severe organ involvement? A pilot study. Ann. Rheum. Dis. 2012, 71, 1636–1639. [Google Scholar] [CrossRef] [PubMed]
- Riccieri, V.; Vasile, M.; Iannace, N.; Stefanantoni, K.; Sciarra, I.; Vizza, C.D.; Badagliacca, R.; Poscia, R.; Papa, S.; Mezzapesa, M.; et al. Systemic sclerosis patients with and without pulmonary arterial hypertension: A nailfold capillaroscopy study. Rheumatology 2013, 52, 1525–1528. [Google Scholar] [CrossRef] [PubMed]
- Chaisson, N.F.; Hassoun, P.M. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013, 144, 1346–1356. [Google Scholar] [CrossRef]
- Hax, V.; Bredemeier, M.; Didonet Moro, A.L.; Pavan, T.R.; Vieira, M.V.; Pitrez, E.H.; da Silva Chakr, R.M.; Xavier, R.M. Clinical algorithms for the diagnosis and prognosis of interstitial lung disease in systemic sclerosis. Semin. Arthritis Rheum. 2017, 47, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Karayusuf, L.; Akdoğan, A.; Kılıç, L.; Karadağ, Ö.; Kalyoncu, U.; Bilgen, Ş.A.; Ertenli, I.; Kiraz, S. Evaluation of association between capillaroscopic findings and organ involvements in Turkish systemic sclerosis patients. RAED Derg. 2014, 6, 48–52. [Google Scholar] [CrossRef]
- Ong, Y.Y.; Nikoloutsopoulos, T.; Bond, C.P.; Smith, M.D.; Ahern, M.J.; Roberts-Thomson, P.J. Decreased nailfold capillary density in limited scleroderma with pulmonary hypertension. Asian Pac. J. Allergy Immunol. 1998, 16, 81–86. [Google Scholar]
- Hurabielle, C.; Avouac, J.; Lepri, G.; de Risi, T.; Kahan, A.; Allanore, Y. Skin Telangiectasia and the Identification of a Subset of Systemic Sclerosis Patients with Severe Vascular Disease. Arthritis Care Res. 2016, 68, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Dababneh, L.; Cikach, F.; Alkukhun, L.; Dweik, R.A.; Tonelli, A.R. Sublingual microcirculation in pulmonary arterial hypertension. Ann. Am. Thorac. Soc. 2014, 11, 504–512. [Google Scholar] [CrossRef]
- D’Angelo, W.A.; Fries, J.F.; Masi, A.T.; Shulman, L.E. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am. J. Med. 1969, 46, 428–440. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruaro, B.; Confalonieri, M.; Salton, F.; Wade, B.; Baratella, E.; Geri, P.; Confalonieri, P.; Kodric, M.; Biolo, M.; Bruni, C. The Relationship between Pulmonary Damage and Peripheral Vascular Manifestations in Systemic Sclerosis Patients. Pharmaceuticals 2021, 14, 403. https://doi.org/10.3390/ph14050403
Ruaro B, Confalonieri M, Salton F, Wade B, Baratella E, Geri P, Confalonieri P, Kodric M, Biolo M, Bruni C. The Relationship between Pulmonary Damage and Peripheral Vascular Manifestations in Systemic Sclerosis Patients. Pharmaceuticals. 2021; 14(5):403. https://doi.org/10.3390/ph14050403
Chicago/Turabian StyleRuaro, Barbara, Marco Confalonieri, Francesco Salton, Barbara Wade, Elisa Baratella, Pietro Geri, Paola Confalonieri, Metka Kodric, Marco Biolo, and Cosimo Bruni. 2021. "The Relationship between Pulmonary Damage and Peripheral Vascular Manifestations in Systemic Sclerosis Patients" Pharmaceuticals 14, no. 5: 403. https://doi.org/10.3390/ph14050403
APA StyleRuaro, B., Confalonieri, M., Salton, F., Wade, B., Baratella, E., Geri, P., Confalonieri, P., Kodric, M., Biolo, M., & Bruni, C. (2021). The Relationship between Pulmonary Damage and Peripheral Vascular Manifestations in Systemic Sclerosis Patients. Pharmaceuticals, 14(5), 403. https://doi.org/10.3390/ph14050403







