Abstract
The Neuropeptide S (NPS) system is a rather ‘young’ transmitter system that was discovered and functionally described less than 20 years ago. This review highlights the progress that has been made in elucidating its pharmacology, anatomical distribution, and functional involvement in a variety of physiological effects, including behavior and immune functions. Early on, genetic variations of the human NPS receptor (NPSR1) have attracted attention and we summarize current hypotheses of genetic linkage with disease and human behaviors. Finally, we review the therapeutic potential of future drugs modulating NPS signaling. This review serves as an introduction to the broad collection of original research papers and reviews from experts in the field that are presented in this Special Issue.
1. NPS and the Orphan Receptor Strategy
Neuropeptide S (NPS) was discovered as a ligand of a previously orphan G protein-coupled receptor (GPCR) by using the “orphan receptor strategy”, also known as “reverse pharmacology” [1]. The receptor (previously known as GPR154 or GPRA) was stably expressed in cells that served as a bait to purify the endogenous ligand from brain extracts [2]. The ligand turned out to be a peptide of 20 amino acids that contains a perfectly conserved serine (single amino acid code “S”) at the N-terminus in all species analyzed, and was thus termed accordingly [3]. NPS is encoded as a single copy peptide by a rather small precursor protein (<90 amino acids) that occurs in the genome of all tetrapods but is absent from fish [4]. The seven N-terminal residues are identical in all tetrapods, as well as the overall 20 amino acid length, while the C-terminal half shows more variation (Figure 1).
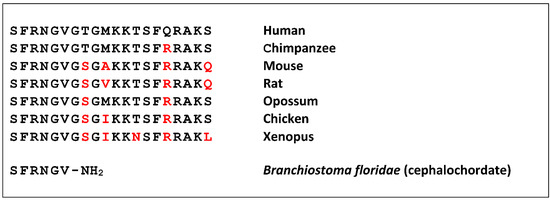
Figure 1.
Neuropeptide S (NPS) or NPS-like peptide sequences. Residues divergent from the human NPS sequence are highlighted in red. The C-terminal amide (NH2) in the Branchiostoma peptide is encoded by glycine in the precursor protein and therefore identical to the consensus.
A shortened peptide has been identified in a cephalochordate that contains the conserved amino terminal residues [5]. Together with the identification of a distantly-related GPCR from the same class of animals, this suggests a rather complex evolution of the NPS system in bilaterians, where teleost fish may have lost both genes [6].
NPSR1 is also a single-copy gene with moderate similarity to other peptide GPCRs, the closest being vasopressin 1A and oxytocin receptors. NPSR1 is found in the genomes of all tetrapods and no convincing orthologues have been identified in fish genomes [7].
2. General Pharmacology
NPSR1 couples via Gαs and Gαq to elevate intracellular cAMP and Ca2+, thus it is an excitatory GPCR [8]. Activation of mitogen-activated protein kinase (MAPK) pathways and opening of neuronal Ca2+ channels have also been described [8,9,10]. NPS activates its receptor at low nanomolar concentrations and structure-activity relationship (SAR) studies have confirmed the importance of the amino terminus for agonist activity, overlapping with the high evolutionary conservation of this part of the peptide structure [11,12,13]. Focused SAR studies performed on Gly5 lead to the identification of peptidergic NPSR1 antagonists, characterized by a D-amino acid with a short branched aliphatic side chain [14,15]. Some well-characterized NPSR1 antagonists identified in the frame of these studies are [D-Cys(t-Bu)5]NPS, [D-Val5]NPS, and [t-Bu-D-Gly5]NPS [15,16,17].
The prototype synthetic NPSR1 antagonist is SHA 68, belonging to the class of diphenyltetrahydro-1H-oxazolo [3,4-α]pyrazines [18] (Figure 2). SHA 68, and the closely related SHA 66, display nanomolar affinity for NPSR1 in vitro but only limited bioavailability in vivo, due to its high lipophilicity. Using the core structure of SHA 68, several refinements have been made to improve in vivo potency. It appears that slight increases in polarity can indeed cause an increased in vivo potency, albeit with a net loss in receptor affinity [19,20,21,22]. Additional high-throughput drug screening programs have yielded further structures with NPSR1 antagonistic profiles (Figure 2), although with lower in vitro and in vivo potency compared to SHA 68 [23,24,25]. None of these compounds has progressed beyond the preclinical stage yet.
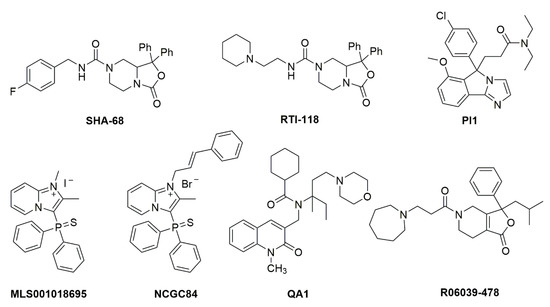
Figure 2.
Chemical structures of NPSR1 antagonists. Details about synthesis and pharmacological activities can be found in the original literature. SHA 68 [18], RTI-118 [68], PI1 [118], MLS001018695 [119], NCGC84 [23], QA1 [120], R06039-478 [121].
3. Anatomical Distribution of the NPS System
Expression of NPS precursor and receptor mRNA have been studied in detail in brain. In rats and mice, the NPS precursor mRNA is expressed in only a few brainstem nuclei, most notably the pericoerulear region and the lateral parabrachial area including the Kölliker–Fuse nucleus [3,26,27]. In rat brain, additional NPS expression is detected in the principal sensory trigeminal nucleus and a few scattered cells in hypothalamus and amygdala. Widespread co-localization with excitatory neurotransmitters—including glutamate, acetylcholine, CRF or galanin—in the brainstem nuclei supports the view that NPS is part of an excitatory signaling system. Notably, in rat or mouse locus coeruleus (LC) NPS is not colocalized with noradrenaline in the LC proper, but found in immediately adjacent neurons. Using transgenic NPS-EGFP mice, the total number of NPS-expressing neurons in the mouse brain was determined to be ~500 [28], which makes it one of the rarest neuropeptides in the brain. Immunohistochemical analyses, however, revealed widespread projections of NPS-immunopositive fibers throughout the mouse brain, with highest densities found in hypothalamus, thalamus, and structures of the extended amygdala [27,28]. It is currently unkown if these fiber projections also represent synaptic terminals, but a few studies have investigated in vivo NPS release in certain brain areas. For example, increased NPS release upon stress exposure has been detected in the rat amygdala by microdialysis, demonstrating that it is a functional neurotransmitter [29]. Regulation of NPS transcripts has been observed in rat brain after sleep deprivation [30] or treatment with neuroleptics [31,32].
NPSR1 mRNA expression is found in many areas of the rodent brain, with highest densities in several cortical structures, thalamic nuclei, hypothalamus, subiculum, and amygdala [3,26,27]. Neuroleptics and chronic morphine also appear to regulate NPSR1 mRNA in certain areas of the rat brain [31,32,33].
Expression of NPS precursor or receptor mRNA in peripheral tissues has not been studied by in-situ hybridization but bulk mRNA has been quantified from whole organs. In rats, precursor and receptor mRNA was detected by RT-PCR in endocrine tissues, including mammary and salivary glands as well as thyroid [3]. Endogenous expression of NPSR1 was also observed in neuroendocrine tumors [9] and established colon cancer cell lines [8].
Information about NPS expression in the human brain is limited as only the pons has been investigated in detail. Similar to rodents, clusters of NPS-expressing neurons were found in the human lateral parabrachial nucleus as well as the pericoerulear region, although with a different quantitative distribution pattern compared to rodents [34]. An additional cluster of NPS mRNA-positive neurons was identified in the pontine central gray matter in both human and rat brain. The overall estimate for the human pons predicts a number of ~22,000 NPS-producing neurons. NPSR1 expression was detected by in-situ hybridization in various human tegmental nuclei and the periaqueductal gray, however, a comprehensive brain map for human or primate NPSR1 has yet to be produced.
Anatomical studies using anti-NPSR1 antibodies have so far not been verified by using knockout controls to validate the specificity of the antisera [9,35,36,37,38]. Therefore, the precise subcellular location of the receptor proteins is currently unknown.
4. Physiological and Behavioral Effects of NPS
4.1. Modulation of Animal Behavior
In experimental animals, NPS was found to induce arousal and wakefulness [3,39], reduce fear and anxiety [3,39,40,41,42,43,44], promote learning and memory consolidation [45,46,47], accelerate fear extinction [48,49], attenuate pharmacologically-induced psychotic behaviors [50], stimulate release of stress hormones [51,52] and prefrontal dopamine [53], produce analgesia [54,55,56,57,58,59], attenuate food intake [51,60,61], counteract motor deficits in a model of Parkinson’s disease [62], and promote drug-seeking behaviors including relapse of drug seeking [63,64,65,66,67]. An overview about animal models, routes and location of drug administration, and behavioral paradigms can be found in the review by Ruzza et al. [25]. Importantly, NPS-dependent stimulation of, or relapse to, either alcohol or cocaine seeking behavior can be blocked by NPSR1 antagonists [67,68]. Hypothalamic NPSR1 appear to be critical for modulating drug seeking behaviors and may interact with orexin/hypocretin and corticotropin-releasing factor neurotransmission [63,69,70]. Notably, both central (intracerebroventricular) and intra-amygdala administration of NPS elicited acute anxiolytic-like effects [3,48], while intra-amygdala administration of SHA 68 produced increased anxiety-like behavior [48], suggesting endogenous NPS release upon stress exposure. It appears that NPS may have a unique bifurcated effect on anxiety: while acutely attenuating anxiety-like responses, it later appears to facilitate extinction of fear memories. This combination of activities could be desirable therapeutic effects in the treatment of generalized anxiety disorders, phobias, panic disorder, or post-traumatic stress disorder.
There is limited evidence for a role of NPS in mood regulation. In the high-anxiety Flinders rat model, NPS normalized anxiety-like behaviors without producing anti-depressant effects in the forced swim test [71]. However, localized NPS administrations into the nucleus accumbens (NAc) shell, but not the NAc core or the bed nucleus of the stria terminalis (BNST), were reported to induce anti-depressant effects in a learned helplessness model in rats [72]. Unexpectedly, infusions of the NPSR1 antagonist SHA 68 into the BNST, another important part of the extended amygdala network regulating mood and anxiety, also produced anti-depressant-like effects. However, knockout mouse models did not exhibit depression-like phenotypes. Further studies may be required to clarify possible functions of endogenous NPS in mood regulation.
Prominent phenotypes in knockout mouse models for NPSR1 and NPS include attenuated arousal, deficits in learning and memory including disrupted fear learning, and mildly increased anxiety [45,52,73,74,75]. While the anxiogenic and memory impairment phenotype of NPSR1 knockout mice has not been replicated in all laboratories [76], all studies showed that the stimulant, arousal-promoting, and anxiolytic effects of NPS completely disappeared in NPSR1 knockout animals, demonstrating that NPSR1 is the unique protein by which NPS exerts its biological actions [52,73,74,76].
No respiratory phenotypes were detected in NPSR1 knockout mice, including models of induced asthma [77]. However, central NPS administration can stimulate respiratory frequency in an NPSR1-dependent manner [78].
4.2. NPS and Immune Functions
A few in vitro studies have described effects of NPS on lymphocytes or macrophages [79,80,81]. In general, NPS was found to induce lymphocyte proliferation and promote macrophage phagocytosis. These effects were accompanied by upregulated expression of pro-inflammatory cytokines. Whether any immune-modulatory deficits exist in NPSR1 or NPS knockout mice has not yet been established. Increased expression of NPSR1 in eosinophils from patients with asthma or severe inflammation has been reported [82].
5. Genetics of the Human NPS System and Associations with Disease and Behavior
The region on human chromosome 7 harboring NPSR1 was first identified in a positional cloning approach to contain an asthma-susceptibility gene. NPSR1 was one of the candidate genes and was termed GPRA (G protein-coupled receptor for asthma susceptibility) [35]. Further studies have reported genetic linkage of the NPSR1 locus on human Chr7 with inflammatory or allergic disorders, including asthma, airway hyper-responsiveness, atopic dermatitis, inflammatory bowel syndrome, and rheumatoid arthritis [83,84,85,86,87,88,89,90,91,92,93,94], although some studies failed to replicate such associations [95,96,97,98]. Complex haplotypes in and surrounding the NPSR1 gene, rather than individual single nucleotide polymorphisms (SNPs), were identified in these association studies and a detailed physiological mechanism has not yet been described to explain NPSR1-related functions in allergic or inflammatory diseases. Furthermore, NPSR1 knockout mice did not display asthma-related phenotypes or deficits in respiratory functions [77]. Instead, a neurogenic mechanism involving brainstem NPSR1 has been suggested to regulate NPS-dependent respiratory effects in mice [78]. Despite strong human genetic data, the physiological role of NPSR1 in asthma, allergies, or inflammation remains unclear.
Phenotypes of a missense mutation in NPSR1 received considerable attention because the Ile107Asn variants (rs324981, T/A) display 5–10 fold differences in agonist potency while binding affinity is not affected [8,99]. The rs324981 SNP is human-specific and the derived A-allele produces NPSR1-Asn107 with significantly reduced receptor expression and attenuated functionality. The NPRS1-Asn107 variant is also part of several haplotypes that have been associated with allergic and inflammatory diseases and was postulated to provide some protective effect, despite not showing any significant association on its own.
Due to its pharmacological phenotype, rs324981 was studied most intensely in humans and has been associated with panic disorder, anxiety, fear evaluation, stress responsiveness, maladaptive personality traits, early-onset bipolar disorder, and sleep [100,101,102,103,104,105,106,107,108,109,110,111]. Paradoxically, the ancestral T-allele, coding for the high-sensitivity variant NPSR1-Ile107, was often associated with disease phenotypes, except for a schizophrenia study where T-carriers displayed improved cognitive performance [112]. Tests in healthy human volunteers revealed T-carrier-specific associations with over-interpretation of fear or fearful stimuli, and functional brain scans showed rs324981 allele-dependent changes in neuronal activity in discrete brain areas in controls vs. panic or anxiety disorder patients, in particular involving specific subdivisions of the prefrontal and orbitofrontal cortex [104,106,107,109,113].
Surprisingly, the derived A-allele of rs324981 (encoding the low-functionality variant NPSR1-Asn107) has become the major allele in European and Asian populations, suggesting some form of selection. Analysis of worldwide rs324981 allele frequencies suggests an African origin of the mutation before the onset of migrations by anatomically-modern humans, consistent with the out-of-Africa hypothesis [114].
Recently, a gain-of-function variant (NPSR1-His206) has been described in a single family that dramatically reduces total sleep time [115]. This observation confirms earlier genetic association reports of the high-activity variant NPSR1-Ile107 with delayed bedtime [101] and supports a significant role for NPS in arousal.
A mutation reducing agonist potency 10–20 fold has been identified in the NPS peptide itself, producing Leu6-NPS (instead of Val6; rs4751440, G/C) [94,116]. Thus far, no phenotypes have been associated with this variant, which might be due to the relatively low allele frequency and geographically restricted occurrence in European and Indian populations. Interestingly, genetic analysis showed that the rs4751440 mutation may have originated in Neandertals, since it is found in all currently available Neandertal genomes. Most likely, the mutant allele was introduced into the anatomically-modern human gene pool by interbreeding with Neandertals in Southern Europe after the main migrations to East Asia had already occurred [114].
6. Therapeutic Potential
The prospect of a non-sedative anxiolytic stimulated a lot of interest in the pharmaceutical industry to search for small-molecule NPSR1 agonists. However, so far, no such molecules have been discovered. In contrast, various independent structures of small-molecule NPSR1 antagonists have been described (Figure 2), but thus far none of them have entered clinical trials. Based on preclinical data, such compounds may have therapeutic potential as sleep medications or in the treatment of drug addiction, especially to prevent relapse of drug abuse [23,25,68,117].
7. Concluding Remarks
An impressive number of physiological functions have been identified for the NPS system in the relatively short time since its discovery. Together with the plethora of genetic association data for NPSR1 variants with human disease and behaviors, increased efforts to identify therapeutic applications for this interesting transmitter system appear to be promising and warranted. The articles in this Special Issue reflect the continuing progress in our knowledge about the NPS system and its therapeutic potential.
Author Contributions
Conceptualization, R.K.R., C.R.; writing—original draft preparation, R.K.R., C.R.; writing—review and editing, R.K.R., C.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Civelli, O.; Saito, Y.; Wang, Z.; Nothacker, H.-P.; Reinscheid, R.K. Orphan GPCRs and their ligands. Pharmacol. Ther. 2006, 110, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Shintani, Y.; Miyajima, N.; Yoshimura, K. Novel G protein-coupled receptor protein and DNA thereof. World Patent WO 02/31145 A1, 18 April 2002. [Google Scholar]
- Xu, Y.-L.; Reinscheid, R.K.; Huitron-Resendiz, S.; Clark, S.D.; Wang, Z.; Lin, S.H.; Brucher, F.A.; Zeng, J.; Ly, N.K.; Henriksen, S.J.; et al. Neuropeptide S: A neuropeptide promoting arousal and anxiolytic-like effects. Neuron 2004, 43, 487–497. [Google Scholar] [CrossRef]
- Reinscheid, R.K. Phylogenetic appearance of neuropeptide S precursor proteins in tetrapods. Peptides 2007, 28, 830–837. [Google Scholar] [CrossRef]
- Elphick, M.R. NG peptides: A novel family of neurophysin-associated neuropeptides. Gene 2010, 458, 20–26. [Google Scholar] [CrossRef][Green Version]
- Mirabeau, O.; Joly, J.S. Molecular evolution of peptidergic signaling systems in bilaterians. Proc. Natl. Acad. Sci. USA 2013, 110, E2028–E2037. [Google Scholar] [CrossRef]
- Pitti, T.; Manoj, N. Molecular evolution of the neuropeptide S receptor. PLoS ONE 2012, 7, e34046. [Google Scholar] [CrossRef]
- Reinscheid, R.K.; Xu, Y.-L.; Okamura, N.; Zeng, J.; Chung, S.; Pai, R.; Wang, Z.; Civelli, O. Pharmacological characterization of human and murine neuropeptide S receptor variants. J. Pharmacol. Exp. Ther. 2005, 315, 1338–1345. [Google Scholar] [CrossRef]
- Pulkkinen, V.; Ezer, S.; Sundman, L.; Hagström, J.; Remes, S.; Söderhäll, C.; Dario, G.; Haglund, C.; Kere, J.; Arola, J. Neuropeptide S receptor 1 (NPSR1) activates cancer-related pathways and is widely expressed in neuroendocrine tumors. Virchows Arch. 2014, 465, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, F.; Kügler, S.; Blaesse, P.; Lange, M.D.; Skryabin, B.V.; Pape, H.C.; Jüngling, K. Neuronal expression of the human neuropeptide S receptor NPSR1 identifies NPS-induced calcium signaling pathways. PLoS ONE 2015, 10, e0117319. [Google Scholar] [CrossRef]
- Roth, A.L.; Marzola, E.; Rizzi, A.; Arduin, M.; Trapella, C.; Corti, C.; Vergura, R.; Martinelli, P.; Salvadori, S.; Regoli, D.; et al. Structure-activity studies on neuropeptide S: Identification of the amino acid residues crucial for receptor activation. J. Biol. Chem. 2006, 281, 20809–20816. [Google Scholar] [CrossRef]
- Camarda, V.; Trapella, C.; Caló, G.; Guerrini, R.; Rizzi, A.; Ruzza, C.; Fiorini, S.; Marzola, E.; Reinscheid, R.K.; Regoli, D.; et al. Synthesis and biological activity of human neuropeptide S analogues modified in position 2. J. Med. Chem. 2008, 51, 655–658. [Google Scholar] [CrossRef]
- Camarda, V.; Trapella, C.; Calo’, G.; Guerrini, R.; Rizzi, A.; Ruzza, C.; Fiorini, S.; Marzola, E.; Reinscheid, R.K.; Regoli, D.; et al. Structure-activity study at positions 3 and 4 of human neuropeptide S. Bioorganic Med. Chem. 2008, 16, 8841–8845. [Google Scholar] [CrossRef]
- Guerrini, R.; Camarda, V.; Trapella, C.; Calò, G.; Rizzi, A.; Ruzza, C.; Fiorini, S.; Marzola, E.; Reinscheid, R.K.; Regoli, D.; et al. Synthesis and biological activity of human neuropeptide S analogues modified in position 5: Identification of potent and pure neuropeptide S receptor antagonists. J. Med. Chem. 2009, 52, 524–529. [Google Scholar] [CrossRef]
- Guerrini, R.; Camarda, V.; Trapella, C.; Caló, G.; Rizzi, A.; Ruzza, C.; Fiorini, S.; Marzola, E.; Reinscheid, R.K.; Regoli, D.; et al. Further studies at neuropeptide S position 5: Discovery of novel neuropeptide S receptor antagonists. J. Med. Chem. 2009, 52, 4068–4071. [Google Scholar] [CrossRef][Green Version]
- Ruzza, C.; Rizzi, A.; Camarda, V.; Pulga, A.; Marzola, G.; Filaferro, M.; Novi, C.; Ruggieri, V.; Marzola, E.; Vitale, G.; et al. [tBu-D-Gly5]NPS, a pure and potent antagonist of the neuropeptide S receptor: In vitro and in vivo studies. Peptides 2012, 34, 404–411. [Google Scholar] [CrossRef]
- Camarda, V.; Rizzi, A.; Ruzza, C.; Zucchini, S.; Marzola, G.; Marzola, E.; Guerrini, R.; Salvadori, S.; Reinscheid, R.K.; Regoli, D.; et al. In vitro and in vivo pharmacological characterization of the neuropeptide S receptor antagonist [D-Cys(tBu)5]neuropeptide S. J. Pharmacol. Exp. Ther. 2009, 328, 549–555. [Google Scholar] [CrossRef]
- Okamura, N.; Habay, S.A.; Zeng, J.; Chamberlin, A.R.; Reinscheid, R.K. Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor. J. Pharmacol. Exp. Ther. 2008, 325, 893–901. [Google Scholar] [CrossRef]
- Ruzza, C.; Rizzi, A.; Trapella, C.; Pela’, M.; Camarda, V.; Ruggieri, V.; Filaferro, M.; Cifani, C.; Reinscheid, R.K.; Vitale, G.; et al. Further studies on the pharmacological profile of the neuropeptide S receptor antagonist SHA 68. Peptides 2010, 31, 915–925. [Google Scholar] [CrossRef]
- Trapella, C.; Pela, M.; Del Zoppo, L.; Calo, G.; Camarda, V.; Ruzza, C.; Cavazzini, A.; Costa, V.; Bertolasi, V.; Reinscheid, R.K.; et al. Synthesis and separation of the enantiomers of the neuropeptide S receptor antagonist (9 R/S)-3-Oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic Acid 4-fluoro-benzylamide (SHA 68). J. Med. Chem. 2011, 54, 2738–2744. [Google Scholar] [CrossRef]
- Hassler, C.; Zhang, Y.; Gilmour, B.; Graf, T.; Fennell, T.; Snyder, R.; Deschamps, J.R.; Reinscheid, R.K.; Garau, C.; Runyon, S.P. Identification of neuropeptide s antagonists: Structure-Activity relationship studies, x-ray crystallography, and in vivo evaluation. ACS Chem. Neurosci. 2014, 5, 731–744. [Google Scholar] [CrossRef]
- Albanese, V.; Ruzza, C.; Marzola, E.; Bernadi, T.; Fabbri, M.; Fantinati, A.; Trapella, C.; Reinscheid, R.K.; Ferrari, F.; Sturaro, C.; et al. Structure-Activity Relationship Studies on Oxazolo[3,4-a]pyrazine Derivatives Leading to the Discovery of a Novel Neuropeptide S Receptor Antagonist with Potent In Vivo Activity. J. Med. Chem. 2021. [Google Scholar] [CrossRef]
- Thorsell, A.; Tapocik, J.D.; Liu, K.; Zook, M.; Bell, L.; Flanigan, M.; Patnaik, S.; Marugan, J.; Damadzic, R.; Dehdashti, S.J.; et al. A novel brain penetrant NPS receptor antagonist, NCGC00185684, blocks alcohol-induced ERK-phosphorylation in the central amygdala and decreases operant alcohol self-administration in rats. J. Neurosci. 2013, 33, 10132–10142. [Google Scholar] [CrossRef]
- Batran, R.Z.; Dawood, D.H.; El-Seginy, S.A.; Maher, T.J.; Gugnani, K.S.; Rondon-Ortiz, A.N. Coumarinyl pyranopyrimidines as new neuropeptide S receptor antagonists; design, synthesis, homology and molecular docking. Bioorg. Chem. 2017, 75, 274–290. [Google Scholar] [CrossRef]
- Ruzza, C.; Calò, G.; Di Maro, S.; Pacifico, S.; Trapella, C.; Salvadori, S.; Preti, D.; Guerrini, R. Neuropeptide S receptor ligands: A patent review (2005–2016). Expert Opin. Ther. Pat. 2017, 27, 347–362. [Google Scholar] [CrossRef]
- Xu, Y.-L.; Gall, C.M.; Jackson, V.R.; Civelli, O.; Reinscheid, R.K. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J. Comp. Neurol. 2007, 500, 84–102. [Google Scholar] [CrossRef]
- Clark, S.D.; Duangdao, D.M.; Schulz, S.; Zhang, L.; Liu, X.; Xu, Y.L.; Reinscheid, R.K. Anatomical characterization of the neuropeptide S system in the mouse brain by in situ hybridization and immunohistochemistry. J. Comp. Neurol. 2011, 519, 1867–1893. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, J.; Zhou, A.; Theodorsson, E.; Fahrenkrug, J.; Reinscheid, R.K. Molecular fingerprint of neuropeptide s-producing neurons in the mouse brain. J. Comp. Neurol. 2011, 519, 1847–1866. [Google Scholar] [CrossRef]
- Ebner, K.; Rjabokon, A.; Pape, H.C.; Singewald, N. Increased in vivo release of neuropeptide S in the amygdala of freely moving rats after local depolarisation and emotional stress. Amino Acids 2011, 41, 991–996. [Google Scholar] [CrossRef][Green Version]
- Adori, C.; Barde, S.; Vas, S.; Ebner, K.; Su, J.; Svensson, C.; Mathé, A.A.; Singewald, N.; Reinscheid, R.R.; Uhlén, M.; et al. Exploring the role of neuropeptide S in the regulation of arousal: A functional anatomical study. Brain Struct. Funct. 2016, 221, 3521–3546. [Google Scholar] [CrossRef]
- Palasz, A.; Rojczyk, E.; Golyszny, M.; Filipczyk, L.; Worthington, J.J.; Wiaderkiewicz, R. Long-term treatment with haloperidol affects neuropeptide S and NPSR mRNA levels in the rat brain. Acta Neuropsychiatr. 2016, 28, 110–116. [Google Scholar] [CrossRef]
- Pałasz, A.; Rojczyk, E. Neuroleptics Affect Neuropeptide S and NPSR mRNA Levels in the Rat Brain. J. Mol. Neurosci. 2015, 57, 352–357. [Google Scholar] [CrossRef]
- Ghazal, P.; Ciccocioppo, R.; Ubaldi, M. Morphine dependence is associated with changes in neuropeptide S receptorexpression and function in rat brain. Peptides 2013, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Adori, C.; Barde, S.; Bogdanovic, N.; Uhlén, M.; Reinscheid, R.K.; Kovacs, G.G.; Hökfelt, T. Neuropeptide S-and Neuropeptide S receptor-expressing neuron populations in the human pons. Front. Neuroanat. 2015, 9, e126. [Google Scholar] [CrossRef]
- Laitinen, T.; Polvi, A.; Rydman, P.; Vendelin, J.; Pulkkinen, V.; Salmikangas, P.; Mäkelä, S.; Rehn, M.; Pirskanen, A.; Rautanen, A.; et al. Characterization of a Common Susceptibility Locus for Asthma-Related Traits. Science 2004, 304, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Vendelin, J.; Pulkkinen, V.; Rehn, M.; Pirskanen, A.; Räisänen-Sokolowski, A.; Laitinen, A.; Laitinen, L.A.; Kere, J.; Laitinen, T. Characterization of GPRA, a novel G protein-coupled receptor related to asthma. Am. J. Respir. Cell Mol. Biol. 2005, 33, 262–270. [Google Scholar] [CrossRef]
- Sundman, L.; Saarialho-Kere, U.; Vendelin, J.; Lindfors, K.; Assadi, G.; Kaukinen, K.; Westerholm-Ormio, M.; Savilahti, E.; Mäki, M.; Alenius, H.; et al. Neuropeptide S receptor 1 expression in the intestine and skin—Putative role in peptide hormone secretion. Neurogastroenterol. Motil. 2010, 22, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.K.; Ring, R.H. Immunohistochemical localization of the neuropeptide S receptor in the rat central nervous system. Neuroscience 2011, 172, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, A.; Vergura, R.; Marzola, G.; Ruzza, C.; Guerrini, R.; Salvadori, S.; Regoli, D.; Calo, G. Neuropeptide S is a stimulatory anxiolytic agent: A behavioural study in mice. Br. J. Pharmacol. 2008, 154, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.K.; Dwyer, J.M.; Sukoff Rizzo, S.J.; Platt, B.; Logue, S.F.; Neal, S.J.; Malberg, J.E.; Beyer, C.E.; Schechter, L.E.; Rosenzweig-Lipson, S.; et al. Pharmacology of neuropeptide S in mice: Therapeutic relevance to anxiety disorders. Psychopharmacology 2008, 197, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, I.A.; Dine, J.; Yen, Y.C.; Buell, D.R.; Herrmann, L.; Holsboer, F.; Eder, M.; Landgraf, R.; Schmidt, U. Intranasally administered neuropeptide S (NPS) exerts anxiolytic effects following internalization into NPS receptor-expressing neurons. Neuropsychopharmacology 2012, 37, 1323–1337. [Google Scholar] [CrossRef][Green Version]
- Dine, J.; Ionescu, I.A.; Avrabos, C.; Yen, Y.C.; Holsboer, F.; Landgraf, R.; Schmidt, U.; Eder, M. Intranasally applied neuropeptide S shifts a high-anxiety electrophysiological endophenotype in the ventral hippocampus towards a “normal”-anxiety one. PLoS ONE 2015, 10, e0120272. [Google Scholar] [CrossRef]
- Slattery, D.A.; Naik, R.R.; Grund, T.; Yen, Y.C.; Sartori, S.B.; Füchsl, A.; Finger, B.C.; Elfving, B.; Nordemann, U.; Guerrini, R.; et al. Selective breeding for high anxiety introduces a synonymous SNP that increases Neuropeptide S receptor activity. J. Neurosci. 2015, 35, 4599–4613. [Google Scholar] [CrossRef] [PubMed]
- Zoicas, I.; Menon, R.; Neumann, I.D. Neuropeptide S reduces fear and avoidance of con-specifics induced by social fear conditioning and social defeat, respectively. Neuropharmacology 2016, 108, 284–291. [Google Scholar] [CrossRef]
- Okamura, N.; Garau, C.; Duangdao, D.M.; Clark, S.D.; Jüngling, K.; Pape, H.-C.; Reinscheid, R.K. Neuropeptide S enhances memory during the consolidation phase and interacts with noradrenergic systems in the brain. Neuropsychopharmacology 2011, 36, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Lukas, M.; Neumann, I.D. Nasal application of neuropeptide S reduces anxiety and prolongs memory in rats: Social versus non-social effects. Neuropharmacology 2012, 62, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Han, R.W.; Zhang, R.S.; Xu, H.J.; Chang, M.; Peng, Y.L.; Wang, R. Neuropeptide S enhances memory and mitigates memory impairment induced by MK801, scopolamine or Aβ1-42 in mice novel object and object location recognition tasks. Neuropharmacology 2013, 70, 261–267. [Google Scholar] [CrossRef]
- Jüngling, K.; Seidenbecher, T.; Sosulina, L.; Lesting, J.; Sangha, S.; Clark, S.D.; Okamura, N.; Duangdao, D.M.; Xu, Y.-L.; Reinscheid, R.K.; et al. Neuropeptide S-Mediated Control of Fear Expression and Extinction: Role of Intercalated GABAergic Neurons in the Amygdala. Neuron 2008, 59, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.B.; Maurer, V.; Murphy, C.; Schmuckermair, C.; Muigg, P.; Neumann, I.D.; Whittle, N.; Singewald, N. Combined neuropeptide S and D-cycloserine augmentation prevents the return of fear in extinction-impaired rodents: Advantage of dual versus single drug approaches. Int. J. Neuropsychopharmacol. 2016, 19, 1–11. [Google Scholar] [CrossRef]
- Okamura, N.; Reinscheid, R.K.; Ohgake, S.; Iyo, M.; Hashimoto, K. Neuropeptide S attenuates neuropathological, neurochemical and behavioral changes induced by the NMDA receptor antagonist MK-801. Neuropharmacology 2010, 58, 166–172. [Google Scholar] [CrossRef]
- Smith, K.L.; Patterson, M.; Dhillo, W.S.; Patel, S.R.; Semjonous, N.M.; Gardiner, J.V.; Ghatei, M.A.; Bloom, S.R. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology 2006, 147, 3510–3518. [Google Scholar] [CrossRef]
- Zhu, H.; Mingler, M.K.; McBride, M.L.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Williams, M.T.; Vorhees, C.V.; Rothenberg, M.E. Abnormal response to stress and impaired NPS-induced hyperlocomotion, anxiolytic effect and corticosterone increase in mice lacking NPSR1. Psychoneuroendocrinology 2010, 35, 1119–1132. [Google Scholar] [CrossRef]
- Si, W.; Aluisio, L.; Okamura, N.; Clark, S.D.; Fraser, I.; Sutton, S.W.; Bonaventure, P.; Reinscheid, R.K. Neuropeptide S stimulates dopaminergic neurotransmission in the medial prefrontal cortex. J. Neurochem. 2010, 115, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chang, M.; Peng, Y.L.; Gao, Y.H.; Zhang, J.N.; Han, R.W.; Wang, R. Neuropeptide S produces antinociceptive effects at the supraspinal level in mice. Regul. Pept. 2009, 156, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.L.; Zhang, J.N.; Chang, M.; Li, W.; Han, R.W.; Wang, R. Effects of central neuropeptide S in the mouse formalin test. Peptides 2010, 31, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Victor Holanda, A.D.; Asth, L.; Santos, A.R.; Guerrini, R.; Soares-Rachetti, V.D.P.; Calo’, G.; André, E.; Gavioli, E.C. Central adenosine A1 and A2A receptors mediate the antinociceptive effects of neuropeptide S in the mouse formalin test. Life Sci. 2015, 120, 8–12. [Google Scholar] [CrossRef]
- Holanda, V.A.D.; Oliveira, M.C.; Souza, L.S.; Lobão-Soares, B.; André, E.; Da Silva Junior, E.D.; Guerrini, R.; Calo, G.; Ruzza, C.; Gavioli, E.C. Dopamine D1 and D2 receptors mediate neuropeptide S-induced antinociception in the mouse formalin test. Eur. J. Pharmacol. 2019, 859, 172557. [Google Scholar] [CrossRef]
- Lee, M.T.; Chiu, Y.T.; Chiu, Y.C.; Hor, C.C.; Lee, H.J.; Guerrini, R.; Calo, G.; Chiou, L.C. Neuropeptide S-initiated sequential cascade mediated by OX1, NK1, mGlu5 and CB1 receptors: A pivotal role in stress-induced analgesia. J. Biomed. Sci. 2020, 27, 7. [Google Scholar] [CrossRef]
- Jinushi, K.; Kushikata, T.; Kudo, T.; Calo, G.; Guerrini, R.; Hirota, K. Central noradrenergic activity affects analgesic effect of Neuropeptide S. J. Anesth. 2018, 32, 48–53. [Google Scholar] [CrossRef]
- Peng, Y.L.; Han, R.W.; Chang, M.; Zhang, L.; Zhang, R.S.; Li, W.; Han, Y.F.; Wang, R. Central Neuropeptide S inhibits food intake in mice through activation of Neuropeptide S receptor. Peptides 2010, 31, 2259–2263. [Google Scholar] [CrossRef]
- Cifani, C.; Di Bonaventura, M.V.M.; Cannella, N.; Fedeli, A.; Guerrini, R.; Calo, G.; Ciccocioppo, R.; Ubaldi, M. Effect of neuropeptide S receptor antagonists and partial agonists on palatable food consumption in the rat. Peptides 2011, 32, 44–50. [Google Scholar] [CrossRef]
- Didonet, J.J.; Cavalcante, J.C.; Souza, L.D.; Costa, M.S.; André, E.; Soares-Rachetti, V.D.; Guerrini, R.; Gavioli, E.C. Neuropeptide S counteracts 6-OHDA-induced motor deficits in mice. Behav. Brain Res. 2014, 266, 29–36. [Google Scholar] [CrossRef]
- Cannella, N.; Economidou, D.; Kallupi, M.; Stopponi, S.; Heilig, M.; Massi, M.; Ciccocioppo, R. Persistent Increase of Alcohol-Seeking Evoked by Neuropeptide S: An Effect Mediated by the Hypothalamic Hypocretin System. Neuropsychopharmacology 2009, 34, 2125–2134. [Google Scholar] [CrossRef]
- Cannella, N.; Kallupi, M.; Li, H.W.; Stopponi, S.; Cifani, C.; Ciccocioppo, R.; Ubaldi, M. Neuropeptide S differently modulates alcohol-related behaviors in alcohol-preferring and non-preferring rats. Psychopharmacology 2016, 233, 2915–2924. [Google Scholar] [CrossRef]
- Pañeda, C.; Huitron-Resendiz, S.; Frago, L.M.; Chowen, J.A.; Picetti, R.; De Lecea, L.; Roberts, A.J. Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice. J. Neurosci. 2009, 29, 4155–4161. [Google Scholar] [CrossRef][Green Version]
- Kallupi, M.; Cannella, N.; Economidou, D.; Ubaldi, M.; Ruggeri, B.; Weiss, F.; Massi, M.; Marugan, J.; Heilig, M.; Bonnavion, P.; et al. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc. Natl. Acad. Sci. USA 2010, 107, 19567–19572. [Google Scholar] [CrossRef] [PubMed]
- Kallupi, M.; De Guglielmo, G.; Cannella, N.; Li, H.W.; Caló, G.; Guerrini, R.; Ubaldi, M.; Renger, J.J.; Uebele, V.N.; Ciccocioppo, R. Hypothalamic Neuropeptide S receptor blockade decreases discriminative cue-induced reinstatement of cocaine seeking in the rat. Psychopharmacology 2013, 226, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Schmoutz, C.D.; Zhang, Y.; Runyon, S.P.; Goeders, N.E. Antagonism of the neuropeptide S receptor with RTI-118 decreases cocaine self-administration and cocaine-seeking behavior in rats. Pharmacol. Biochem. Behav. 2012, 103, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Cannella, N.; Kallupi, M.; Ruggeri, B.; Ciccocioppo, R.; Ubaldi, M. The role of the neuropeptide S system in addiction: Focus on its interaction with the CRF and hypocretin/orexin neurotransmission. Prog. Neurobiol. 2013, 100, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Ubaldi, M.; Giordano, A.; Severi, I.; Li, H.; Kallupi, M.; De Guglielmo, G.; Ruggeri, B.; Stopponi, S.; Ciccocioppo, R.; Cannella, N. Activation of hypocretin-1/orexin-a neurons projecting to the bed nucleus of the stria terminalis and paraventricular nucleus is critical for reinstatement of alcohol seeking by neuropeptide S. Biol. Psychiatry 2016, 79, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Wegener, G.; Finger, B.C.; Elfving, B.; Keller, K.; Liebenberg, N.; Fischer, C.W.; Singewald, N.; Slattery, D.A.; Neumann, I.D.; Mathé, A.A. Neuropeptide S alters anxiety, but not depression-like behaviour in Flinders Sensitive Line rats: A genetic animal model of depression. Int. J. Neuropsychopharmacol. 2012, 15, 375–387. [Google Scholar] [CrossRef]
- Shirayama, Y.; Ishima, T.; Oda, Y.; Okamura, N.; Iyo, M.; Hashimoto, K. Opposite roles for neuropeptide S in the nucleus accumbens and bed nucleus of the stria terminalis in learned helplessness rats. Behav. Brain Res. 2015, 291, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Duangdao, D.M.; Clark, S.D.; Okamura, N.; Reinscheid, R.K. Behavioral phenotyping of Neuropeptide S receptor knockout mice. Behav. Brain Res. 2009, 205, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fendt, M.; Buchi, M.; Bürki, H.; Imobersteg, S.; Ricoux, B.; Suply, T.; Sailer, A.W. Neuropeptide S receptor deficiency modulates spontaneous locomotor activity and the acoustic startle response. Behav. Brain Res. 2011, 217, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Si, W.; Garau, C.; Jüngling, K.; Pape, H.-C.; Schulz, S.; Reinscheid, R.K. Neuropeptide S precursor knockout mice display memory and arousal deficits. Eur. J. Neurosci. 2017, 46, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Ruzza, C.; Pulga, A.; Rizzi, A.; Marzola, G.; Guerrini, R.; Calo’, G. Behavioural phenotypic characterization of CD-1 mice lacking the neuropeptide S receptor. Neuropharmacology 2012, 62, 1999–2009. [Google Scholar] [CrossRef]
- Allen, I.C.; Pace, A.J.; Jania, L.A.; Ledford, J.G.; Latour, A.M.; Snouwaert, J.N.; Bernier, V.; Stocco, R.; Therien, A.G.; Koller, B.H. Expression and function of NPSR1/GPRA in the lung before and after induction of asthma-like disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L1005–L1017. [Google Scholar] [CrossRef]
- Zhu, H.; Perkins, C.; Mingler, M.K.; Finkelman, F.D.; Rothenberg, M.E. The role of neuropeptide S and neuropeptide S receptor 1 in regulation of respiratory function in mice. Peptides 2011, 32, 818–825. [Google Scholar] [CrossRef][Green Version]
- Pulkkinen, V.; Majuri, M.L.; Wang, G.; Holopainen, P.; Obase, Y.; Vendelin, J.; Wolff, H.; Rytilä, P.; Laitinen, L.A.; Haahtela, T.; et al. Neuropeptide S and G protein-coupled receptor 154 modulate macrophage immune responses. Hum. Mol. Genet. 2006, 15, 1667–1679. [Google Scholar] [CrossRef]
- Yao, Y.; Su, J.; Yang, G.; Zhang, G.; Lei, Z.; Zhang, F.; Li, X.; Kou, R.; Liu, Y.; Liu, J. Effects of neuropeptide S on the proliferation of splenic lymphocytes, phagocytosis, and proinflammatory cytokine production of pulmonary alveolar macrophages in the pig. Peptides 2011, 32, 118–124. [Google Scholar] [CrossRef]
- Yao, Y.; Su, J.; Zhang, F.; Lei, Z. Effects of central and peripheral administration of neuropeptide S on the level of serum proinflammatory cytokines in pigs. Neuroimmunomodulation 2014, 21, 45–51. [Google Scholar] [CrossRef]
- Ilmarinen, P.; James, A.; Moilanen, E.; Pulkkinen, V.; Daham, K.; Saarelainen, S.; Laitinen, T.; Dahlén, S.E.; Kere, J.; Dahlén, B.; et al. Enhanced expression of neuropeptide S (NPS) receptor in eosinophils from severe asthmatics and subjects with total IgE above 100 IU/ml. Peptides 2014, 51, 100–109. [Google Scholar] [CrossRef]
- Melén, E.; Bruce, S.; Doekes, G.; Kabesch, M.; Laitinen, T.; Lauener, R.; Lindgren, C.M.; Riedler, J.; Scheynius, A.; Van Hage-Hamsten, M.; et al. Haplotypes of G protein-coupled receptor 154 are associated with childhood allergy and asthma. Am. J. Respir. Crit. Care Med. 2005, 171, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Kormann, M.S.D.; Carr, D.; Klopp, N.; Illig, T.; Leupold, W.; Fritzsch, C.; Weiland, S.K.; Von Mutius, E.; Kabesch, M. G-Protein-coupled receptor polymorphisms are associated with asthma in a large German population. Am. J. Respir. Crit. Care Med. 2005, 171, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
- Hersh, C.P.; Raby, B.A.; Soto-Quirós, M.E.; Murphy, A.J.; Avila, L.; Lasky-Su, J.; Sylvia, J.S.; Klanderman, B.J.; Lange, C.; Weiss, S.T.; et al. Comprehensive testing of positionally cloned asthma genes in two populations. Am. J. Respir. Crit. Care Med. 2007, 176, 849–857. [Google Scholar] [CrossRef]
- Bruce, S.; Nyberg, F.; Melén, E.; James, A.; Pulkkinen, V.; Orsmark-Pietras, C.; Bergström, A.; Dahlén, B.; Wickman, M.; Von Mutius, E.; et al. The protective effect of farm animal exposure on childhood allergy is modified by NPSR1 polymorphisms. J. Med. Genet. 2009, 46, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Pulkkinen, V.; Haataja, R.; Hannelius, U.; Helve, O.; Pitkänen, O.M.; Karikoski, R.; Rehn, M.; Marttila, R.; Lindgren, C.M.; Hästbacka, J.; et al. G protein-coupled receptor for asthma susceptibility associates with respiratory distress syndrome. Ann. Med. 2006, 38, 357–366. [Google Scholar] [CrossRef]
- Malerba, G.; Lindgren, C.M.; Xumerle, L.; Kiviluoma, P.; Trabetti, E.; Laitinen, T.; Galavotti, R.; Pescollderungg, L.; Boner, A.L.; Kere, J.; et al. Chromosome 7p linkage and GPR154 gene association in Italian families with allergic asthma. Clin. Exp. Allergy 2007, 37, 83–89. [Google Scholar] [CrossRef]
- D’Amato, M.; Zucchelli, M.; Seddighzadeh, M.; Anedda, F.; Lindblad, S.; Kere, J.; Alfredsson, L.; Klareskog, L.; Padyukov, L. Analysis of neuropeptide S receptor gene (NPSR1) polymorphism in rheumatoid arthritis. PLoS ONE 2010, 5, e9315. [Google Scholar] [CrossRef]
- Vergara, C.; Jiménez, S.; Acevedo, N.; Martínez, B.; Mercado, D.; Gusmão, L.; Rafaels, N.; Hand, T.; Barnes, K.C.; Caraballo, L. Association of G-protein-coupled receptor 154 with asthma and total IgE in a population of the Caribbean coast of Colombia. Clin. Exp. Allergy 2009, 39, 1558–1568. [Google Scholar] [CrossRef]
- D’Amato, M.; Bruce, S.; Bresso, F.; Zucchelli, M.; Ezer, S.; Pulkkinen, V.; Lindgren, C.; Astegiano, M.; Rizzetto, M.; Gionchetti, P.; et al. Neuropeptide S Receptor 1 Gene Polymorphism Is Associated With Susceptibility to Inflammatory Bowel Disease. Gastroenterology 2007, 133, 808–817. [Google Scholar] [CrossRef]
- Castro-Giner, F.; De Cid, R.; Gonzalez, J.R.; Jarvis, D.; Heinrich, J.; Janson, C.; Omenaas, E.R.; Matheson, M.C.; Pin, I.; Antó, J.M.; et al. Positionally cloned genes and age-specific effects in asthma and atopy: An international population-based cohort study (ECRHS). Thorax 2010, 65, 124–131. [Google Scholar] [CrossRef][Green Version]
- Robledo, G.; González-Gay, M.A.; Fernández-Gutiérrez, B.; Lamas, J.R.; Balsa, A.; Pascual-Salcedo, D.; Castañeda, S.; Blanco, R.; González-Alvaro, I.; García, A.; et al. NPSR1 gene is associated with reduced risk of rheumatoid arthritis. J. Rheumatol. 2012, 39, 1166–1170. [Google Scholar] [CrossRef]
- Acevedo, N.; Ezer, S.; Merid, S.K.; Gaertner, V.D.; Söderhäll, C.; D’Amato, M.; Kabesch, M.; Melén, E.; Kere, J.; Pulkkinen, V. Neuropeptide S (NPS) variants modify the signaling and risk effects of NPS Receptor 1 (NPSR1) variants in asthma. PLoS ONE 2017, 12, e0176568. [Google Scholar] [CrossRef]
- Shin, H.D.; Park, K.S.; Park, C.S. Lack of association of GPRA (G protein-coupled receptor for asthma susceptibility) haplotypes with high serum IgE or asthma in a Korean population. J. Allergy Clin. Immunol. 2004, 114, 1226–1227. [Google Scholar] [CrossRef] [PubMed]
- Veal, C.D.; Reynolds, N.J.; Meggitt, S.J.; Allen, M.H.; Lindgren, C.M.; Kere, J.; Trembath, R.C.; Barker, J.N. Absence of association between asthma and high serum immunoglobulin E associated GPRA haplotypes and adult atopic dermatitis. J. Investig. Dermatol. 2005, 125, 399–401. [Google Scholar] [CrossRef]
- Wu, H.; Romieu, I.; Sienra-Monge, J.J.; del Rio-Navarro, B.E.; Burdett, L.; Yuenger, J.; Li, H.; Chanock, S.J.; London, S.J. Lack of association between genetic variation in G-protein-coupled receptor for asthma susceptibility and childhood asthma and atopy. Genes Immun. 2008, 9, 224–230. [Google Scholar] [CrossRef]
- Blakey, J.D.; Sayers, I.; Ring, S.M.; Strachan, D.P.; Hall, I.P. Positionally cloned asthma susceptibility gene polymorphisms and disease risk in the British 1958 Birth Cohort. Thorax 2009, 64, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Bernier, V.; Stocco, R.; Bogusky, M.J.; Joyce, J.G.; Parachoniak, C.; Grenier, K.; Arget, M.; Mathieu, M.C.; O’Neill, G.P.; Slipetz, D.; et al. Structure-function relationships in the neuropeptide S receptor: Molecular consequences of the asthma-associated mutation N107I. J. Biol. Chem. 2006, 281, 24704–24712. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Hashimoto, K.; Iyo, M.; Shimizu, E.; Dempfle, A.; Friedel, S.; Reinscheid, R.K. Gender-specific association of a functional coding polymorphism in the Neuropeptide S receptor gene with panic disorder but not with schizophrenia or attention-deficit/hyperactivity disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; O’Connor, G.T.; Wilk, J.B. Genome-wide association of sleep and circadian phenotypes. BMC Med. Genet. 2007, 8 (Suppl. 1), S9. [Google Scholar] [CrossRef] [PubMed]
- Laas, K.; Eensoo, D.; Paaver, M.; Lesch, K.P.; Reif, A.; Harro, J. Further evidence for the association of the NPSR1 gene A/T polymorphism (Asn107Ile) with impulsivity and hyperactivity. J. Psychopharmacol. 2015, 29, 878–883. [Google Scholar] [CrossRef]
- Laas, K.; Reif, A.; Kiive, E.; Domschke, K.; Lesch, K.P.; Veidebaum, T.; Harro, J. A functional NPSR1 gene variant and environment shape personality and impulsive action: A longitudinal study. J. Psychopharmacol. 2014, 28, 227–236. [Google Scholar] [CrossRef]
- Raczka, K.A.; Gartmann, N.; Mechias, M.L.; Reif, A.; Büchel, C.; Deckert, J.; Kalisch, R. A neuropeptide S receptor variant associated with overinterpretation of fear reactions: A potential neurogenetic basis for catastrophizing. Mol. Psychiatry 2010, 15, 1067–1074. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donner, J.; Haapakoski, R.; Ezer, S.; Meln, E.; Pirkola, S.; Gratacs, M.; Zucchelli, M.; Anedda, F.; Johansson, L.E.; Sderhll, C.; et al. Assessment of the neuropeptide S system in anxiety disorders. Biol. Psychiatry 2010, 68, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Domschke, K.; Reif, A.; Weber, H.; Richter, J.; Hohoff, C.; Ohrmann, P.; Pedersen, A.; Bauer, J.; Suslow, T.; Kugel, H.; et al. Neuropeptide S receptor gene converging evidence for a role in panic disorder. Mol. Psychiatry 2011, 16, 938–948. [Google Scholar] [CrossRef]
- Dannlowski, U.; Kugel, H.; Franke, F.; Stuhrmann, A.; Hohoff, C.; Zwanzger, P.; Lenzen, T.; Grotegerd, D.; Suslow, T.; Arolt, V.; et al. Neuropeptide-S (NPS) receptor genotype modulates basolateral amygdala responsiveness to aversive stimuli. Neuropsychopharmacology 2011, 36, 1879–1885. [Google Scholar] [CrossRef]
- Glotzbach-Schoon, E.; Andreatta, M.; Reif, A.; Ewald, H.; Tröger, C.; Baumann, C.; Deckert, J.; Mühlberger, A.; Pauli, P. Contextual fear conditioning in virtual reality is affected by 5httlpr and npsr1 polymorphisms: Effects on fear-potentiated startle. Front. Behav. Neurosci. 2013, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Tupak, S.V.; Reif, A.; Pauli, P.; Dresler, T.; Herrmann, M.J.; Domschke, K.; Jochum, C.; Haas, E.; Baumann, C.; Weber, H.; et al. Neuropeptide S receptor gene: Fear-specific modulations of prefrontal activation. Neuroimage 2013, 66, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Klauke, B.; Deckert, J.; Zwanzger, P.; Baumann, C.; Arolt, V.; Pauli, P.; Reif, A.; Domschke, K. Neuropeptide S receptor gene (NPSR) and life events: G × e effects on anxiety sensitivity and its subdimensions. World J. Biol. Psychiatry 2014, 15, 17–25. [Google Scholar] [CrossRef]
- Laas, K.; Reif, A.; Akkermann, K.; Kiive, E.; Domschke, K.; Lesch, K.P.; Veidebaum, T.; Harro, J. Interaction of the neuropeptide S receptor gene Asn107Ile variant and environment: Contribution to affective and anxiety disorders, and suicidal behaviour. Int. J. Neuropsychopharmacol. 2014, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Lennertz, L.; Quednow, B.B.; Schuhmacher, A.; Petrovsky, N.; Frommann, I.; Schulze-Rauschenbach, S.; Landsberg, M.W.; Steinbrecher, A.; Höfels, S.; Pukrop, R.; et al. The functional coding variant Asn107Ile of the neuropeptide S receptor gene (NPSR1) is associated with schizophrenia and modulates verbal memory and the acoustic startle response. Int. J. Neuropsychopharmacol. 2012, 15, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Gechter, J.; Liebscher, C.; Geiger, M.J.; Wittmann, A.; Schlagenhauf, F.; Lueken, U.; Wittchen, H.U.; Pfleiderer, B.; Arolt, V.; Kircher, T.; et al. Association of NPSR1 gene variation and neural activity in patients with panic disorder and agoraphobia and healthy controls. NeuroImage Clin. 2019, 24, 102029. [Google Scholar] [CrossRef] [PubMed]
- Reinscheid, R.K.; Mafessoni, F.; Lüttjohann, A.; Jüngling, K.; Pape, H.-C.; Schulz, S. Neandertal introgression and accumulation of hypomorphic mutations in the neuropeptide S (NPS) system promote attenuated functionality. Peptides 2021, 138, 170506. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Shi, G.; Mostovoy, Y.; Gentry, N.W.; Fan, Z.; McMahon, T.B.; Kwok, P.Y.; Jones, C.R.; Ptáček, L.J.; Fu, Y.H. Mutant neuropeptide S receptor reduces sleep duration with preserved memory consolidation. Sci. Transl. Med. 2019, 11, eaax2014. [Google Scholar] [CrossRef]
- Deng, C.; He, X.; Hsueh, A.J.W. A single-nucleotide polymorphism of human neuropeptide S gene originated from Europe shows decreased bioactivity. PLoS ONE 2013, 8, e83009. [Google Scholar] [CrossRef]
- Bonano, J.S.; Runyon, S.P.; Hassler, C.; Glennon, R.A.; Stevens Negus, S. Effects of the neuropeptide S receptor antagonist RTI-118 on abuse-related facilitation of intracranial self-stimulation produced by cocaine and methylenedioxypyrovalerone (MDPV) in rats. Eur. J. Pharmacol. 2014, 743, 98–105. [Google Scholar] [CrossRef]
- Trotter, B.W.; Nanda, K.K.; Manley, P.J.; Uebele, V.N.; Condra, C.L.; Gotter, A.L.; Menzel, K.; Henault, M.; Stocco, R.; Renger, J.J.; et al. Tricyclic imidazole antagonists of the Neuropeptide S Receptor. Bioorganic Med. Chem. Lett. 2010, 20, 4704–4708. [Google Scholar] [CrossRef]
- Patnaik, S.; Marugan, J.J.; Liu, K.; Zheng, W.; Southall, N.; Dehdashti, S.J.; Thorsell, A.; Heilig, M.; Bell, L.; Zook, M.; et al. Structure-activity relationship of imidazopyridinium analogues as antagonists of neuropeptide s receptor. J. Med. Chem. 2013, 56, 9045–9056. [Google Scholar] [CrossRef]
- Melamed, J.Y.; Zartman, A.E.; Kett, N.R.; Gotter, A.L.; Uebele, V.N.; Reiss, D.R.; Condra, C.L.; Fandozzi, C.; Lubbers, L.S.; Rowe, B.A.; et al. Synthesis and evaluation of a new series of Neuropeptide S receptor antagonists. Bioorganic Med. Chem. Lett. 2010, 20, 4700–4703. [Google Scholar] [CrossRef]
- Runyon, S.; Zhang, Y.; Hassler, C.; Gilmour, B. Composition and method for Neuropeptide S receptor (NPSR) antagonists. World Patent WO/2013/086200, 13 June 2013. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).