Roles of Neuropeptide S in Anesthesia, Analgesia, and Sleep
Abstract
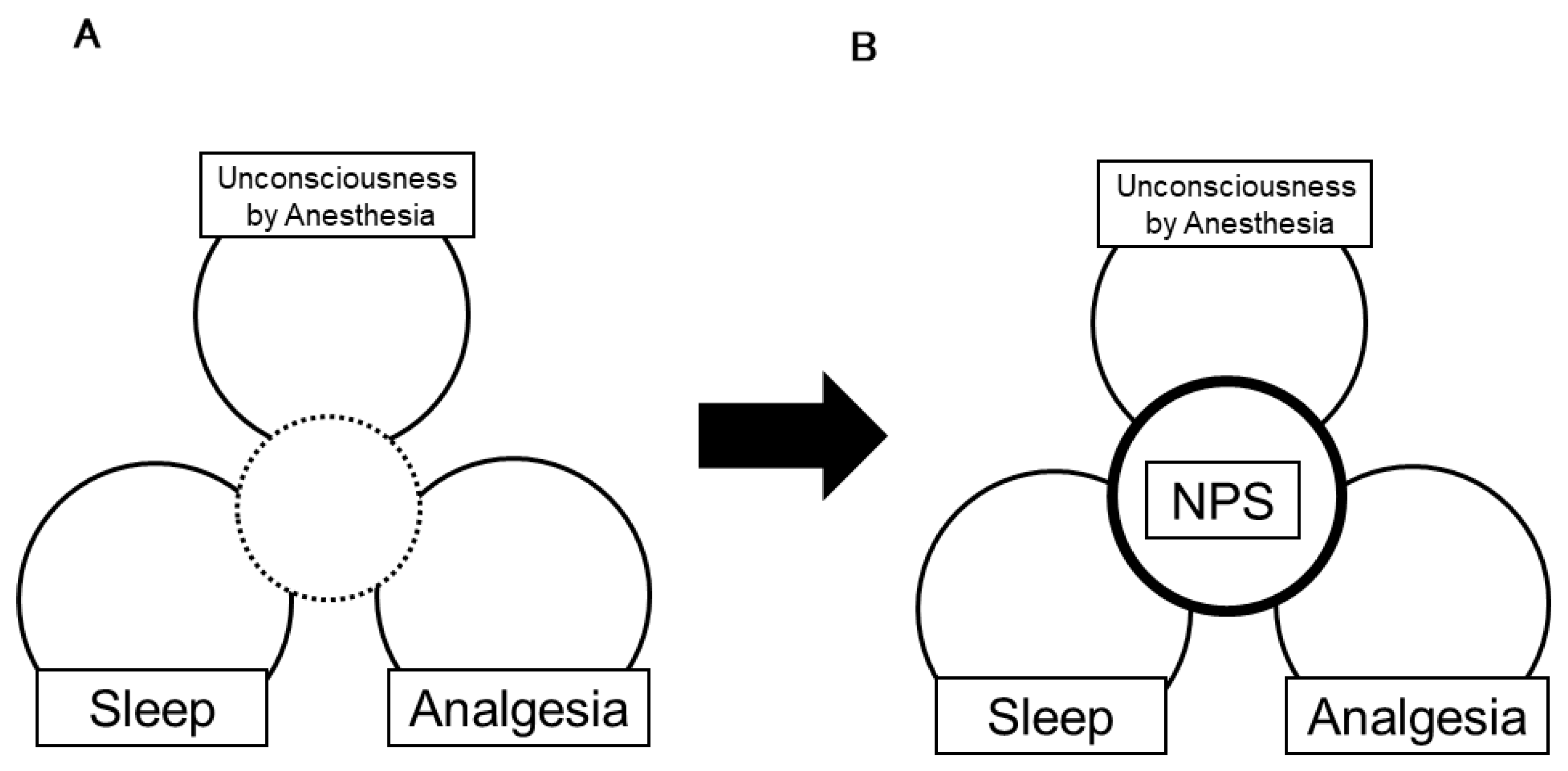
1. Introduction
2. NPS in Anesthesia
2.1. Anesthesia Involves Endogenous Sleep Circuit
2.2. Anesthesia Is a Heterogenic Phenomenon
2.3. NPS Modulates General Anesthesia
2.4. Induction and Emergence from Anesthesia Are Not a Simple Mirror
2.5. Perspective: NPS in Anesthesia
3. NPS in Analgesia
3.1. Noradrenergic Neuronal Activity in the LC Interacts with NPS in Analgesia
3.2. Dopaminergic Neurons in NPS Analgesia
3.3. Other Neuromodulators in NPS Analgesia
3.4. Perspective: NPS in Pain
4. NPS in Sleep
4.1. Overview of Sleep
4.2. NPS and NPSR Roles in Sleep Regulation
4.3. NPS and NPSR Roles in Sleep Disturbance
4.4. NPS–NPSR System: A Possible Research Target to Improve Surgical Stress-Induced Sleep Disturbances
4.5. Perspective: NPS in Sleep
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, Y.L.; Reinscheid, R.K.; Huitron-Resendiz, S.; Clark, S.D.; Wang, Z.; Lin, S.H.; Brucher, F.A.; Zeng, J.; Ly, N.K.; Henriksen, S.J.; et al. Neuropeptide S: A neuropeptide promoting arousal and anxiolytic-like effects. Neuron 2004, 43, 487–497. [Google Scholar] [CrossRef]
- Vendelin, J.; Bruce, S.; Holopainen, P.; Pulkkinen, V.; Rytilä, P.; Pirskanen, A.; Rehn, M.; Laitinen, T.; Laitinen, L.A.; Haahtela, T.; et al. Downstream target genes of the neuropeptide S-NPSR1 pathway. Hum. Mol. Genet. 2006, 15, 2923–2935. [Google Scholar] [CrossRef]
- Reinscheid, R.K.; Ruzza, C. Pharmacology, physiology and genetics of the neuropeptide s system. Pharmaceuticals 2021, 14, 401. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Tao, Y.X. Physiology, pharmacology, and pathophysiology of neuropeptide S receptor. Prog. Mol. Biol. Transl. Sci. 2019, 161, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Hendy, G.N.; Percy, M.E.; Bichet, D.G.; Cole, D.E. G protein-coupled receptor mutations and human genetic disease. Methods Mol. Biol. 2014, 1175, 153–187. [Google Scholar] [CrossRef] [PubMed]
- Reinscheid, R.K.; Mafessoni, F.; Lüttjohann, A.; Jüngling, K.; Pape, H.C.; Schulz, S. Neandertal introgression and accumulation of hypomorphic mutations in the neuropeptide S (NPS) system promote attenuated functionality. Peptides 2021, 138, 170506. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, S.; Skibdal, H.E.; Christiansen, S.H.; Gøtzsche, C.R.; Hassan, M.; Mathé, A.A.; Wegener, G.; Woldbye, D.P.D. Sustained overexpression of neuropeptide S in the amygdala reduces anxiety-like behavior in rats. Behav. Brain Res. 2019, 367, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Qian, X.; Nie, Y.; Sun, N.; Wang, Z.; Wu, J.; Wei, C.; Ma, R.; Wang, Z.; Chai, G.; et al. Neuropeptide S ameliorates cognitive impairment of APP/PS1 transgenic mice by promoting synaptic plasticity and reducing aβ deposition. Front. Behav. Neurosci. 2019, 13, 138. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, Y.; Zhou, L.; Zhao, L.; Wang, J.; Ma, W.; Su, X.; Hui, P.; Guo, B.; Liu, Y.; et al. Gene polymorphisms (rs324957, rs324981) in NPSR1 are associated with increased risk of primary insomnia: A cross-sectional study. Medicine 2020, 99, e21598. [Google Scholar] [CrossRef]
- Xing, L.; Shi, G.; Mostovoy, Y.; Gentry, N.W.; Fan, Z.; McMahon, T.B.; Kwok, P.Y.; Jones, C.R.; Ptáček, L.J.; Fu, Y.H. Mutant neuropeptide S receptor reduces sleep duration with preserved memory consolidation. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Botticelli, L.; Micioni Di Bonaventura, E.; Ubaldi, M.; Ciccocioppo, R.; Cifani, C.; Micioni Di Bonaventura, M.V. The Neural Network of Neuropeptide S (NPS): Implications in food intake and gastrointestinal functions. Pharmaceuticals 2021, 14, 293. [Google Scholar] [CrossRef]
- Acevedo, N.; Ezer, S.; Kebede Merid, S.; Gaertner, V.D.; Söderhäll, C.; D’Amato, M.; Kabesch, M.; Melén, E.; Kere, J.; Pulkkinen, V. Neuropeptide S (NPS) variants modify the signaling and risk effects of NPS Receptor 1 (NPSR1) variants in asthma. PLoS ONE 2017, 12, e0176568. [Google Scholar] [CrossRef]
- Albanese, V.; Ruzza, C.; Marzola, E.; Bernardi, T.; Fabbri, M.; Fantinati, A.; Trapella, C.; Reinscheid, R.K.; Ferrari, F.; Sturaro, C.; et al. Structure-activity relationship studies on oxazolo[3,4-a]pyrazine derivatives leading to the discovery of a novel neuropeptide S receptor antagonist with potent In Vivo activity. J. Med. Chem. 2021, 64, 4089–4108. [Google Scholar] [CrossRef]
- Batran, R.Z.; Gugnani, K.S.; Maher, T.J.; Khedr, M.A. New quinolone derivatives as neuropeptide S receptor antagonists: Design, synthesis, homology modeling, dynamic simulations and modulation of Gq/Gs signaling pathways. Bioorg. Chem. 2021, 111, 104817. [Google Scholar] [CrossRef] [PubMed]
- Bengoetxea, X.; Goedecke, L.; Remmes, J.; Blaesse, P.; Grosch, T.; Lesting, J.; Pape, H.C.; Jüngling, K. Human-specific neuropeptide S receptor variants regulate fear extinction in the basal amygdala of male and female mice depending on threat salience. Biol. Psychiatry 2021. [Google Scholar] [CrossRef] [PubMed]
- Sobanski, T.; Wagner, G. Functional neuroanatomy in panic disorder: Status quo of the research. World J. Psychiatry 2017, 7, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Anedda, F.; Zucchelli, M.; Schepis, D.; Hellquist, A.; Corrado, L.; D’Alfonso, S.; Achour, A.; McInerney, G.; Bertorello, A.; Lördal, M.; et al. Multiple polymorphisms affect expression and function of the neuropeptide S receptor (NPSR1). PLoS ONE 2011, 6, e29523. [Google Scholar] [CrossRef] [PubMed]
- Ilmarinen, P.; James, A.; Moilanen, E.; Pulkkinen, V.; Daham, K.; Saarelainen, S.; Laitinen, T.; Dahlén, S.E.; Kere, J.; Dahlén, B.; et al. Enhanced expression of neuropeptide S (NPS) receptor in eosinophils from severe asthmatics and subjects with total IgE above 100 IU/mL. Peptides 2014, 51, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.H.; Toledo, A.H. Historical development of modern anesthesia. J. Investig. Surg. J. Acad. Surg. Res. 2012, 25, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.E.; Guo, T.Z.; Lu, J.; Saper, C.B.; Franks, N.P.; Maze, M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat. Neurosci. 2002, 5, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.E.; Lu, J.; Guo, T.; Saper, C.B.; Franks, N.P.; Maze, M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 2003, 98, 428–436. [Google Scholar] [CrossRef]
- Brown, E.N.; Lydic, R.; Schiff, N.D. General anesthesia, sleep, and coma. N. Engl. J. Med. 2010, 363, 2638–2650. [Google Scholar] [CrossRef]
- Franks, N.P.; Zecharia, A.Y. Sleep and general anesthesia. Can. J. Anaesth. J. Can. D’anesthesie 2011, 58, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, H.C., Jr.; Riegelhaupt, P.M.; Kelz, M.B.; Solt, K.; Eckenhoff, R.G.; Orser, B.A.; Goldstein, P.A. Towards a comprehensive understanding of anesthetic mechanisms of action: A decade of discovery. Trends Pharmacol. Sci. 2019, 40, 464–481. [Google Scholar] [CrossRef]
- Reitz, S.L.; Wasilczuk, A.Z.; Beh, G.H.; Proekt, A.; Kelz, M.B. Activation of preoptic tachykinin 1 neurons promotes wakefulness over sleep and volatile anesthetic-induced unconsciousness. Curr. Biol. 2021, 31, 394–405. [Google Scholar] [CrossRef]
- Eikermann, M.; Akeju, O.; Chamberlin, N.L. Sleep and anesthesia: The shared circuit hypothesis has been put to bed. Curr. Biol. 2020, 30, R219–R221. [Google Scholar] [CrossRef] [PubMed]
- Forman, S.A.; Miller, K.W. Mapping general anesthetic sites in heteromeric γ-aminobutyric acid type a receptors reveals a potential for targeting receptor subtypes. Anesth. Analg. 2016, 123, 1263–1273. [Google Scholar] [CrossRef]
- Kalmoe, M.C.; Janski, A.M.; Zorumski, C.F.; Nagele, P.; Palanca, B.J.; Conway, C.R. Ketamine and nitrous oxide: The evolution of NMDA receptor antagonists as antidepressant agents. J. Neurol. Sci. 2020, 412, 116778. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Tiemann, D.; Park, E.; Salehi, A. Alpha-2 Agonists. Anesthesiol. Clin. 2017, 35, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Kushikata, T.; Hirota, K.; Yoshida, H.; Kudo, M.; Lambert, D.G.; Smart, D.; Jerman, J.C.; Matsuki, A. Orexinergic neurons and barbiturate anesthesia. Neuroscience 2003, 121, 855–863. [Google Scholar] [CrossRef]
- Tose, R.; Kushikata, T.; Yoshida, H.; Kudo, M.; Furukawa, K.; Ueno, S.; Hirota, K. Orexin A decreases ketamine-induced anesthesia time in the rat: The relevance to brain noradrenergic neuronal activity. Anesth. Analg. 2009, 108, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guo, Y.; Li, H.; Li, J.; Ran, M.; Guo, J.; Yin, L.; Zhao, S.; Yang, Q.; Dong, H. Selective optogenetic activation of orexinergic terminals in the basal forebrain and locus coeruleus promotes emergence from isoflurane anaesthesia in rats. Br. J. Anaesth. 2021, 126, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Kelz, M.B.; Sun, Y.; Chen, J.; Cheng Meng, Q.; Moore, J.T.; Veasey, S.C.; Dixon, S.; Thornton, M.; Funato, H.; Yanagisawa, M. An essential role for orexins in emergence from general anesthesia. Proc. Natl. Acad. Sci. USA 2008, 105, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Shirasaka, T.; Yonaha, T.; Onizuka, S.; Tsuneyoshi, I. Effects of orexin-A on propofol anesthesia in rats. J. Anesth. 2011, 25, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.P.; Wang, C.; Xie, J.F.; Zhao, P.; Dai, L.R.; Shao, Y.F.; Lin, J.S.; Hou, Y.P. Neuropeptide S reduces propofol-or ketamine-induced slow wave states through activation of cognate receptors in the rat. Neuropeptides 2017, 63, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kushikata, T.; Sawada, M.; Niwa, H.; Kudo, T.; Kudo, M.; Tonosaki, M.; Hirota, K. Ketamine and propofol have opposite effects on postanesthetic sleep architecture in rats: Relevance to the endogenous sleep-wakefulness substances orexin and melanin-concentrating hormone. J. Anesth. 2016, 30, 437–443. [Google Scholar] [CrossRef]
- Kushikata, T.; Yoshida, H.; Kudo, M.; Salvadori, S.; Calo, G.; Hirota, K. The effects of neuropeptide S on general anesthesia in rats. Anesth. Analg. 2011, 112, 845–849. [Google Scholar] [CrossRef]
- Sato, T.; Araki, I.; Kushikata, T.; Ohkawa, H.; Ishihara, H.; Matsuki, A. Decreased hypothalamic prostaglandin D2 and prostaglandin E2 contents during isoflurane anaesthesia in rats. Can. J. Anaesth. J. Can. D’anesthesie 1995, 42, 1031–1034. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, W.; Chang, M.; Peng, Y.L.; Gao, Y.H.; Zhang, J.N.; Han, R.W.; Wang, R. Neuropeptide S produces antinociceptive effects at the supraspinal level in mice. Regul. Pept. 2009, 156, 90–95. [Google Scholar] [CrossRef]
- Peng, Y.L.; Zhang, J.N.; Chang, M.; Li, W.; Han, R.W.; Wang, R. Effects of central neuropeptide S in the mouse formalin test. Peptides 2010, 31, 1878–1883. [Google Scholar] [CrossRef]
- Yaksh, T.L.; Rudy, T.A. Analgesia mediated by a direct spinal action of narcotics. Science 1976, 192, 1357–1358. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.Y.; Wang, P.X.; Wei, S.Q.; Traub, R.J.; Li, J.F.; Cao, D.Y. The Role of descending pain modulation in chronic primary pain: Potential application of drugs targeting serotonergic system. Neural Plast. 2019, 2019, 1389296. [Google Scholar] [CrossRef] [PubMed]
- Llorca-Torralba, M.; Borges, G.; Neto, F.; Mico, J.A.; Berrocoso, E. Noradrenergic Locus Coeruleus pathways in pain modulation. Neuroscience 2016, 338, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L.; Llorca-Torralba, M.; Berrocoso, E.; Micó, J.A. Monoamines as drug targets in chronic pain: Focusing on neuropathic pain. Front. Neurosci. 2019, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Jinushi, K.; Kushikata, T.; Kudo, T.; Calo, G.; Guerrini, R.; Hirota, K. Central noradrenergic activity affects analgesic effect of Neuropeptide S. J. Anesth. 2018, 32, 48–53. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Kovelowski, C.J.; Nichols, M.L.; Hruby, V.J.; Porreca, F. Characterization of supraspinal antinociceptive actions of opioid delta agonists in the rat. Pain 1995, 62, 287–293. [Google Scholar] [CrossRef]
- Holanda, V.A.D.; Oliveira, M.C.; Souza, L.S.; Lobão-Soares, B.; André, E.; Da Silva Junior, E.D.; Guerrini, R.; Calo, G.; Ruzza, C.; Gavioli, E.C. Dopamine D(1) and D(2) receptors mediate neuropeptide S-induced antinociception in the mouse formalin test. Eur. J. Pharmacol. 2019, 859, 172557. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, X.; You, Z.; Wang, S.; Lim, G.; Yang, J.; McCabe, M.; Li, N.; Marota, J.; Chen, L.; et al. Persistent nociception induces anxiety-like behavior in rodents: Role of endogenous neuropeptide S. Pain 2014, 155, 1504–1515. [Google Scholar] [CrossRef]
- Du, J.; Fang, J.; Xu, Z.; Xiang, X.; Wang, S.; Sun, H.; Shao, X.; Jiang, Y.; Liu, B.; Fang, J. Electroacupuncture suppresses the pain and pain-related anxiety of chronic inflammation in rats by increasing the expression of the NPS/NPSR system in the ACC. Brain Res. 2020, 1733, 146719. [Google Scholar] [CrossRef]
- Yang, F.; Peng, L.; Luo, J.; Yi, H.; Hu, X. Intra-amygdala microinfusion of neuropeptide S attenuates neuropathic pain and suppresses the response of spinal microglia and astrocytes after spinal nerve ligation in rats. Peptides 2016, 82, 26–34. [Google Scholar] [CrossRef]
- Lee, M.T.; Chiu, Y.T.; Chiu, Y.C.; Hor, C.C.; Lee, H.J.; Guerrini, R.; Calo, G.; Chiou, L.C. Neuropeptide S-initiated sequential cascade mediated by OX(1), NK(1), mGlu(5) and CB(1) receptors: A pivotal role in stress-induced analgesia. J. Biomed. Sci. 2020, 27, 7. [Google Scholar] [CrossRef] [PubMed]
- Toyama, S.; Shimoyama, N.; Tagaito, Y.; Nagase, H.; Saitoh, T.; Yanagisawa, M.; Shimoyama, M. Nonpeptide orexin-2 receptor agonist attenuates morphine-induced sedative effects in rats. Anesthesiology 2018, 128, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Vanini, G.; Torterolo, P. Sleep-wake neurobiology. Adv. Exp. Med. Biol. 2021, 1297, 65–82. [Google Scholar] [CrossRef]
- Krueger, J.M.; Nguyen, J.T.; Dykstra-Aiello, C.J.; Taishi, P. Local sleep. Sleep Med. Rev. 2019, 43, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Aserinsky, E.; Kleitman, N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 1953, 118, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Aserinsky, E. The discovery of REM sleep. J. Hist. Neurosci. 1996, 5, 213–227. [Google Scholar] [CrossRef]
- Deibel, S.H.; Rota, R.; Steenland, H.W.; Ali, K.; McNaughton, B.L.; Tatsuno, M.; McDonald, R.J. Assessment of sleep, K-complexes, and sleep spindles in a T21 light-dark cycle. Front. Neurosci. 2020, 14, 551843. [Google Scholar] [CrossRef]
- Gandhi, M.H.; Emmady, P.D. Physiology, K Complex. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- McCarley, R.W. Neurobiology of REM and NREM sleep. Sleep Med. 2007, 8, 302–330. [Google Scholar] [CrossRef]
- Moruzzi, G.; Magoun, H.W. Brain stem reticular formation and activation of the EEG. Electroencephalogr. Clin. Neurophysiol. 1949, 1, 455–473. [Google Scholar] [CrossRef]
- Saper, C.B.; Fuller, P.M. Wake-sleep circuitry: An overview. Curr. Opin. Neurobiol. 2017, 44, 186–192. [Google Scholar] [CrossRef]
- Saper, C.B.; Fuller, P.M.; Pedersen, N.P.; Lu, J.; Scammell, T.E. Sleep state switching. Neuron 2010, 68, 1023–1042. [Google Scholar] [CrossRef]
- Datta, S. Cellular and chemical neuroscience of mammalian sleep. Sleep Med. 2010, 11, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Spada, J.; Sander, C.; Burkhardt, R.; Häntzsch, M.; Mergl, R.; Scholz, M.; Hegerl, U.; Hensch, T. Genetic association of objective sleep phenotypes with a functional polymorphism in the neuropeptide S receptor gene. PLoS ONE 2014, 9, e98789. [Google Scholar] [CrossRef]
- Chauveau, F.; Claverie, D.; Lardant, E.; Varin, C.; Hardy, E.; Walter, A.; Canini, F.; Rouach, N.; Rancillac, A. Neuropeptide S promotes wakefulness through the inhibition of sleep-promoting ventrolateral preoptic nucleus neurons. Sleep 2020, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Shao, Y.F.; Zhang, M.; Fan, K.; Kong, X.P.; Wang, R.; Hou, Y.P. Neuropeptide S promotes wakefulness through activation of the posterior hypothalamic histaminergic and orexinergic neurons. Neuroscience 2012, 207, 218–226. [Google Scholar] [CrossRef]
- Peever, J.; Fuller, P.M. The Biology of REM Sleep. Curr. Biol. 2017, 27, R1237–R1248. [Google Scholar] [CrossRef] [PubMed]
- Oishi, M.; Kushikata, T.; Niwa, H.; Yakoshi, C.; Ogasawara, C.; Calo, G.; Guerrini, R.; Hirota, K. Endogenous neuropeptide S tone influences sleep-wake rhythm in rats. Neurosci. Lett. 2014, 581, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.F.; Shao, Y.F.; Wang, H.L.; Wang, C.; Cui, G.F.; Kong, X.P.; Wang, L.X.; Chen, Y.N.; Cong, C.Y.; Chen, H.L.; et al. Neuropeptide S counteracts paradoxical sleep deprivation-induced anxiety-like behavior and sleep disturbances. Front. Cell. Neurosci. 2018, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Adori, C.; Barde, S.; Vas, S.; Ebner, K.; Su, J.; Svensson, C.; Mathé, A.A.; Singewald, N.; Reinscheid, R.R.; Uhlén, M.; et al. Exploring the role of neuropeptide S in the regulation of arousal: A functional anatomical study. Brain Struct. Funct. 2016, 221, 3521–3546. [Google Scholar] [CrossRef] [PubMed]
- Thomasson, J.; Canini, F.; Poly-Thomasson, B.; Trousselard, M.; Granon, S.; Chauveau, F. Neuropeptide S overcomes short term memory deficit induced by sleep restriction by increasing prefrontal cortex activity. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2017, 27, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, D.X. Improve postoperative sleep: What can we do? Curr. Opin. Anaesthesiol. 2018, 31, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Dette, F.; Cassel, W.; Urban, F.; Zoremba, M.; Koehler, U.; Wulf, H.; Graf, J.; Steinfeldt, T. Occurrence of rapid eye movement sleep deprivation after surgery under regional anesthesia. Anesth. Analg. 2013, 116, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Haxhibeqiri, S.; Haxhibeqiri, V.; Agani, F.; Goci Uka, A.; Hoxha, B.; Dzubur Kulenovic, A.; Kučukalić, A.; Avdibegović, E.; Sinanović, O.; Babic, D.; et al. Association of neuropeptide S receptor 1 and glutamate decarboxylase 1 gene polymorphisms with posttraumatic stress disorder. Psychiatr. Danub. 2019, 31, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Keifer, J.C.; Baghdoyan, H.A.; Lydic, R. Sleep disruption and increased apneas after pontine microinjection of morphine. Anesthesiology 1992, 77, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; van Dam, C.J.; Niesters, M.; van Velzen, M.; Fossler, M.J.; Demitrack, M.A.; Olofsen, E. Benefit and risk evaluation of biased μ-receptor agonist oliceridine versus morphine. Anesthesiology 2020, 133, 559–568. [Google Scholar] [CrossRef]
- Ghazal, P.; Ciccocioppo, R.; Ubaldi, M. Morphine dependence is associated with changes in neuropeptide S receptor expression and function in rat brain. Peptides 2013, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Song, B.; Zhu, J. Sleep disturbances after general anesthesia: Current perspectives. Front. Neurol. 2020, 11, 629. [Google Scholar] [CrossRef]
- Yanagisawa, M. Toward the mysteries of sleep. Keio J. Med. 2019, 68, 27. [Google Scholar] [CrossRef] [PubMed]
- Cutrufello, N.J.; Ianus, V.D.; Rowley, J.A. Opioids and sleep. Curr. Opin. Pulm. Med. 2020, 26, 634–641. [Google Scholar] [CrossRef]
- Tang, N.K.Y.; Stella, M.T.; Banks, P.D.W.; Sandhu, H.K.; Berna, C. The effect of opioid therapy on sleep quality in patients with chronic non-malignant pain: A systematic review and exploratory meta-analysis. Sleep Med. Rev. 2019, 45, 105–126. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushikata, T.; Hirota, K.; Saito, J.; Takekawa, D. Roles of Neuropeptide S in Anesthesia, Analgesia, and Sleep. Pharmaceuticals 2021, 14, 483. https://doi.org/10.3390/ph14050483
Kushikata T, Hirota K, Saito J, Takekawa D. Roles of Neuropeptide S in Anesthesia, Analgesia, and Sleep. Pharmaceuticals. 2021; 14(5):483. https://doi.org/10.3390/ph14050483
Chicago/Turabian StyleKushikata, Tetsuya, Kazuyoshi Hirota, Junichi Saito, and Daiki Takekawa. 2021. "Roles of Neuropeptide S in Anesthesia, Analgesia, and Sleep" Pharmaceuticals 14, no. 5: 483. https://doi.org/10.3390/ph14050483
APA StyleKushikata, T., Hirota, K., Saito, J., & Takekawa, D. (2021). Roles of Neuropeptide S in Anesthesia, Analgesia, and Sleep. Pharmaceuticals, 14(5), 483. https://doi.org/10.3390/ph14050483





