The Neural Network of Neuropeptide S (NPS): Implications in Food Intake and Gastrointestinal Functions
Abstract
1. Introduction
2. The NPS System
2.1. The Localization of the NPS System in the CNS
2.2. Summary of the In Vivo Functions of NPS
3. NPS and Food Intake: Preclinical Studies
3.1. NPS in Rodents
3.2. NPS in Avian Species
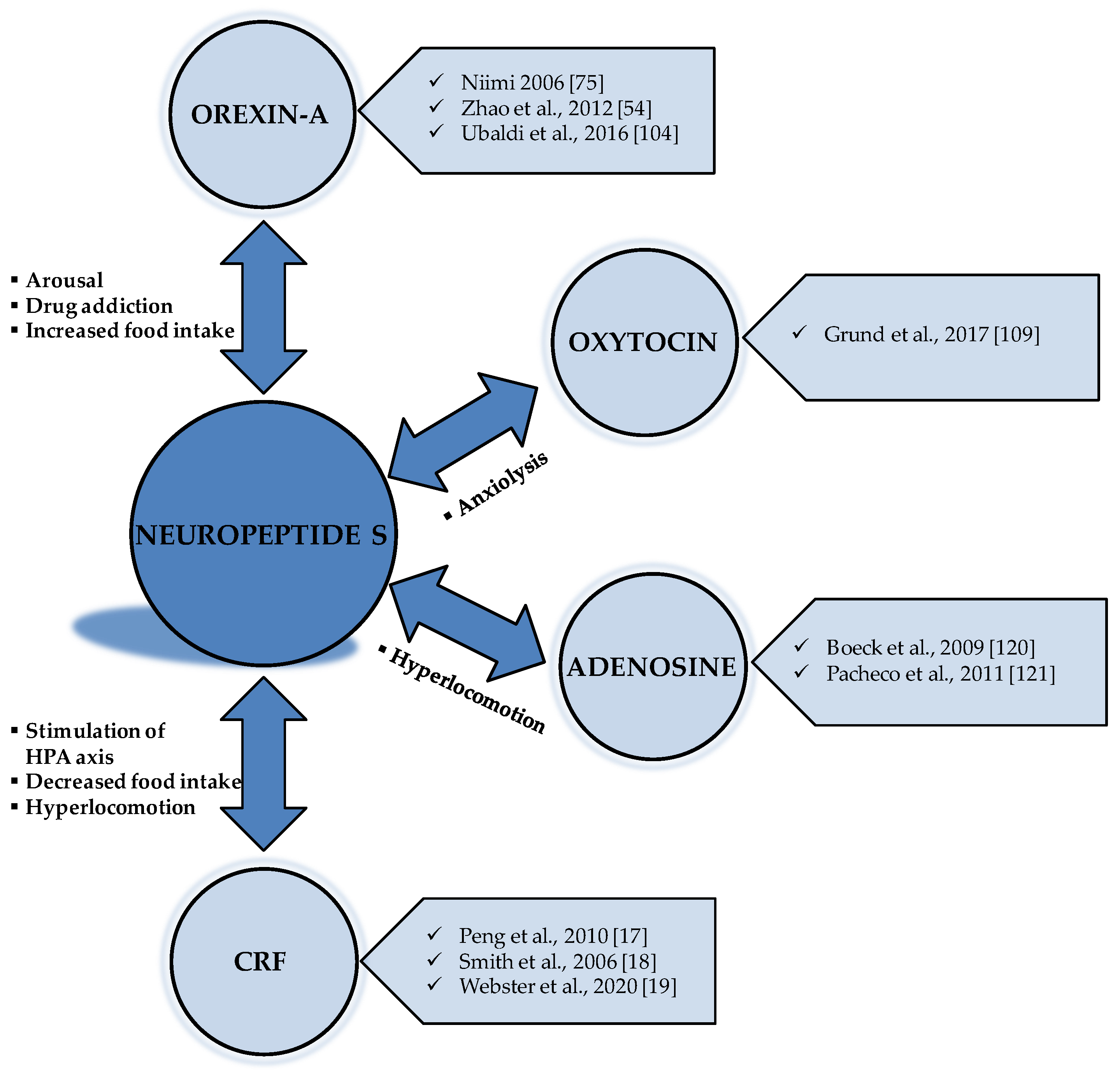
4. The Interaction of NPS with Other Neurotransmitters Implicated in Food Intake
4.1. The Anorexigenic Effect of NPS and CRF Neurotransmission
4.2. The Interaction between NPS and Orexin Neurotransmission
4.3. The Interaction of NPS with Other Neurotransmitters and Future Perspectives
5. The Gastrointestinal Functions Influenced by NPS
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jackson, V.M.; Breen, D.M.; Fortin, J.P.; Liou, A.; Kuzmiski, J.B.; Loomis, A.K.; Rives, M.L.; Shah, B.; Carpino, P.A. Latest approaches for the treatment of obesity. Expert Opin. Drug Discov. 2015, 10, 825–839. [Google Scholar] [CrossRef]
- Martin, K.A.; Mani, M.V.; Mani, A. New targets to treat obesity and the metabolic syndrome. Eur. J. Pharmacol. 2015, 763, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Beck, B. Neuropeptides and obesity. Nutrition 2000, 16, 916–923. [Google Scholar] [CrossRef]
- Hillebrand, J.J.; de Wied, D.; Adan, R.A. Neuropeptides, food intake and body weight regulation: A hypothalamic focus. Peptides 2002, 23, 2283–2306. [Google Scholar] [CrossRef]
- van der Klaauw, A.A. Neuropeptides in Obesity and Metabolic Disease. Clin. Chem. 2018, 64, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Beck, B.; Stricker-Krongrad, A.; Nicolas, J.P.; Burlet, C. Chronic and continuous intracerebroventricular infusion of neuropeptide Y in Long-Evans rats mimics the feeding behaviour of obese Zucker rats. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1992, 16, 295–302. [Google Scholar]
- Kim, M.S.; Rossi, M.; Abusnana, S.; Sunter, D.; Morgan, D.G.; Small, C.J.; Edwards, C.M.; Heath, M.M.; Stanley, S.A.; Seal, L.J.; et al. Hypothalamic localization of the feeding effect of agouti-related peptide and alpha-melanocyte-stimulating hormone. Diabetes 2000, 49, 177–182. [Google Scholar] [CrossRef]
- Qu, D.; Ludwig, D.S.; Gammeltoft, S.; Piper, M.; Pelleymounter, M.A.; Cullen, M.J.; Mathes, W.F.; Przypek, R.; Kanarek, R.; Maratos-Flier, E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 1996, 380, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef]
- Mastorakos, G.; Zapanti, E. The hypothalamic-pituitary-adrenal axis in the neuroendocrine regulation of food intake and obesity: The role of corticotropin releasing hormone. Nutr. Neurosci. 2004, 7, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Micioni Di Bonaventura, E.; Botticelli, L.; Tomassoni, D.; Tayebati, S.K.; Micioni Di Bonaventura, M.V.; Cifani, C. The Melanocortin System behind the Dysfunctional Eating Behaviors. Nutrients 2020, 12, 3502. [Google Scholar] [CrossRef]
- Morton, G.J.; Thatcher, B.S.; Reidelberger, R.D.; Ogimoto, K.; Wolden-Hanson, T.; Baskin, D.G.; Schwartz, M.W.; Blevins, J.E. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E134–E144. [Google Scholar] [CrossRef]
- Vicentic, A.; Jones, D.C. The CART (cocaine- and amphetamine-regulated transcript) system in appetite and drug addiction. J. Pharmacol. Exp. Ther. 2007, 320, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Reinscheid, R.K.; Huitron-Resendiz, S.; Clark, S.D.; Wang, Z.; Lin, S.H.; Brucher, F.A.; Zeng, J.; Ly, N.K.; Henriksen, S.J.; et al. Neuropeptide S: A neuropeptide promoting arousal and anxiolytic-like effects. Neuron 2004, 43, 487–497. [Google Scholar] [CrossRef]
- Cline, M.A.; Prall, B.C.; Smith, M.L.; Calchary, W.A.; Siegel, P.B. Differential appetite-related responses to central neuropeptide S in lines of chickens divergently selected for low or high body weight. J. Neuroendocrinol. 2008, 20, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.L.; Han, R.W.; Chang, M.; Zhang, L.; Zhang, R.S.; Li, W.; Han, Y.F.; Wang, R. Central Neuropeptide S inhibits food intake in mice through activation of Neuropeptide S receptor. Peptides 2010, 31, 2259–2263. [Google Scholar] [CrossRef]
- Smith, K.L.; Patterson, M.; Dhillo, W.S.; Patel, S.R.; Semjonous, N.M.; Gardiner, J.V.; Ghatei, M.A.; Bloom, S.R. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology 2006, 147, 3510–3518. [Google Scholar] [CrossRef]
- Webster, A.N.; Cao, C.; Chowdhury, V.S.; Gilbert, E.R.; Cline, M.A. The hypothalamic mechanism of neuropeptide S-induced satiety in Japanese quail (Coturnix japonica) involves the paraventricular nucleus and corticotropin-releasing factor. Gen. Comp. Endocrinol. 2020, 299, 113558. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Almsned, F.; Ghazal, P.; Ahmed, M.W.; Jafri, M.S.; Bokhari, H. Neuropeptide S receptor gene Asn107 polymorphism in obese male individuals in Pakistan. PLoS ONE 2020, 15, e0243205. [Google Scholar] [CrossRef]
- Xu, Y.L.; Gall, C.M.; Jackson, V.R.; Civelli, O.; Reinscheid, R.K. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J. Comp. Neurol. 2007, 500, 84–102. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Shintani, Y.; Miyajima, N. G Protein-Coupled Receptor Protein and DNA Thereof. U.S. Patent No. 7,250,272, 31 July 2007. [Google Scholar]
- Reinscheid, R.K. Phylogenetic appearance of neuropeptide S precursor proteins in tetrapods. Peptides 2007, 28, 830–837. [Google Scholar] [CrossRef]
- Reinscheid, R.K. Neuropeptide S: Anatomy, pharmacology, genetics and physiological functions. Results Probl. Cell Differ. 2008, 46, 145–158. [Google Scholar] [CrossRef]
- Reinscheid, R.K.; Xu, Y.L. Neuropeptide S and its receptor: A newly deorphanized G protein-coupled receptor system. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2005, 11, 532–538. [Google Scholar] [CrossRef]
- Thompson, M.D.; Hendy, G.N.; Percy, M.E.; Bichet, D.G.; Cole, D.E. G protein-coupled receptor mutations and human genetic disease. Methods Mol. Biol. 2014, 1175, 153–187. [Google Scholar] [CrossRef] [PubMed]
- Dal Ben, D.; Antonini, I.; Buccioni, M.; Lambertucci, C.; Marucci, G.; Vittori, S.; Volpini, R.; Cristalli, G. Molecular modeling studies on the human neuropeptide S receptor and its antagonists. ChemMedChem 2010, 5, 371–383. [Google Scholar] [CrossRef]
- Reinscheid, R.K.; Xu, Y.L. Neuropeptide S as a novel arousal promoting peptide transmitter. FEBS J. 2005, 272, 5689–5693. [Google Scholar] [CrossRef]
- Reinscheid, R.K.; Xu, Y.L.; Okamura, N.; Zeng, J.; Chung, S.; Pai, R.; Wang, Z.; Civelli, O. Pharmacological characterization of human and murine neuropeptide s receptor variants. J. Pharmacol. Exp. Ther. 2005, 315, 1338–1345. [Google Scholar] [CrossRef]
- Ruzza, C.; Ferrari, F.; Guerrini, R.; Marzola, E.; Preti, D.; Reinscheid, R.K.; Calo, G. Pharmacological profile of the neuropeptide S receptor: Dynamic mass redistribution studies. Pharmacol. Res. Perspect. 2018, 6, e00445. [Google Scholar] [CrossRef]
- Laitinen, T.; Polvi, A.; Rydman, P.; Vendelin, J.; Pulkkinen, V.; Salmikangas, P.; Makela, S.; Rehn, M.; Pirskanen, A.; Rautanen, A.; et al. Characterization of a common susceptibility locus for asthma-related traits. Science 2004, 304, 300–304. [Google Scholar] [CrossRef]
- Okamura, N.; Hashimoto, K.; Iyo, M.; Shimizu, E.; Dempfle, A.; Friedel, S.; Reinscheid, R.K. Gender-specific association of a functional coding polymorphism in the Neuropeptide S receptor gene with panic disorder but not with schizophrenia or attention-deficit/hyperactivity disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.K.; Ring, R.H. Immunohistochemical localization of the neuropeptide S receptor in the rat central nervous system. Neuroscience 2011, 172, 153–163. [Google Scholar] [CrossRef]
- Clark, S.D.; Duangdao, D.M.; Schulz, S.; Zhang, L.; Liu, X.; Xu, Y.L.; Reinscheid, R.K. Anatomical characterization of the neuropeptide S system in the mouse brain by in situ hybridization and immunohistochemistry. J. Comp. Neurol. 2011, 519, 1867–1893. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, J.; Zhou, A.; Theodorsson, E.; Fahrenkrug, J.; Reinscheid, R.K. Molecular fingerprint of neuropeptide S-producing neurons in the mouse brain. J. Comp. Neurol. 2011, 519, 1847–1866. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates–The New Coronal Set, 5th ed; Elsevier Elsevier Academic Press: Boston, MA, USA; Amsterdam, The Netherlands, 2004. [Google Scholar]
- Berretta, S. Cortico-amygdala circuits: Role in the conditioned stress response. Stress 2005, 8, 221–232. [Google Scholar] [CrossRef]
- Phelps, E.A.; LeDoux, J.E. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 2005, 48, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [PubMed]
- Duangdao, D.M.; Clark, S.D.; Okamura, N.; Reinscheid, R.K. Behavioral phenotyping of neuropeptide S receptor knockout mice. Behav. Brain Res. 2009, 205, 1–9. [Google Scholar] [CrossRef][Green Version]
- Leonard, S.K.; Dwyer, J.M.; Sukoff Rizzo, S.J.; Platt, B.; Logue, S.F.; Neal, S.J.; Malberg, J.E.; Beyer, C.E.; Schechter, L.E.; Rosenzweig-Lipson, S.; et al. Pharmacology of neuropeptide S in mice: Therapeutic relevance to anxiety disorders. Psychopharmacology 2008, 197, 601–611. [Google Scholar] [CrossRef]
- Rizzi, A.; Vergura, R.; Marzola, G.; Ruzza, C.; Guerrini, R.; Salvadori, S.; Regoli, D.; Calo, G. Neuropeptide S is a stimulatory anxiolytic agent: A behavioural study in mice. Br. J. Pharmacol. 2008, 154, 471–479. [Google Scholar] [CrossRef]
- Roth, A.L.; Marzola, E.; Rizzi, A.; Arduin, M.; Trapella, C.; Corti, C.; Vergura, R.; Martinelli, P.; Salvadori, S.; Regoli, D.; et al. Structure-activity studies on neuropeptide S: Identification of the amino acid residues crucial for receptor activation. J. Biol. Chem. 2006, 281, 20809–20816. [Google Scholar] [CrossRef] [PubMed]
- Ruzza, C.; Rizzi, A.; Trapella, C.; Pela, M.; Camarda, V.; Ruggieri, V.; Filaferro, M.; Cifani, C.; Reinscheid, R.K.; Vitale, G.; et al. Further studies on the pharmacological profile of the neuropeptide S receptor antagonist SHA 68. Peptides 2010, 31, 915–925. [Google Scholar] [CrossRef]
- Vitale, G.; Filaferro, M.; Ruggieri, V.; Pennella, S.; Frigeri, C.; Rizzi, A.; Guerrini, R.; Calo, G. Anxiolytic-like effect of neuropeptide S in the rat defensive burying. Peptides 2008, 29, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Greenwell, T.N. Neuropeptide S: A novel activating anxiolytic? Neuron 2004, 43, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Reinscheid, R.K. Neuropeptide S: A novel modulator of stress and arousal. Stress 2007, 10, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.A.; Casagrande, T.S.; Moretti, M.; Constantino, L.; Petronilho, F.; Guerra, G.C.; Calo, G.; Guerrini, R.; Dal-Pizzol, F.; Quevedo, J.; et al. Lithium attenuates behavioral and biochemical effects of neuropeptide S in mice. Peptides 2009, 30, 1914–1920. [Google Scholar] [CrossRef]
- Castro, A.A.; Moretti, M.; Casagrande, T.S.; Martinello, C.; Petronilho, F.; Steckert, A.V.; Guerrini, R.; Calo, G.; Dal Pizzol, F.; Quevedo, J.; et al. Neuropeptide S produces hyperlocomotion and prevents oxidative stress damage in the mouse brain: A comparative study with amphetamine and diazepam. Pharmacol. Biochem. Behav. 2009, 91, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chang, M.; Peng, Y.L.; Gao, Y.H.; Zhang, J.N.; Han, R.W.; Wang, R. Neuropeptide S produces antinociceptive effects at the supraspinal level in mice. Regul. Pept. 2009, 156, 90–95. [Google Scholar] [CrossRef]
- Peng, Y.L.; Zhang, J.N.; Chang, M.; Li, W.; Han, R.W.; Wang, R. Effects of central neuropeptide S in the mouse formalin test. Peptides 2010, 31, 1878–1883. [Google Scholar] [CrossRef]
- Han, R.W.; Yin, X.Q.; Chang, M.; Peng, Y.L.; Li, W.; Wang, R. Neuropeptide S facilitates spatial memory and mitigates spatial memory impairment induced by N-methyl-D-aspartate receptor antagonist in mice. Neurosci. Lett. 2009, 455, 74–77. [Google Scholar] [CrossRef]
- Okamura, N.; Garau, C.; Duangdao, D.M.; Clark, S.D.; Jungling, K.; Pape, H.C.; Reinscheid, R.K. Neuropeptide S enhances memory during the consolidation phase and interacts with noradrenergic systems in the brain. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011, 36, 744–752. [Google Scholar] [CrossRef]
- Zhao, P.; Shao, Y.F.; Zhang, M.; Fan, K.; Kong, X.P.; Wang, R.; Hou, Y.P. Neuropeptide S promotes wakefulness through activation of the posterior hypothalamic histaminergic and orexinergic neurons. Neuroscience 2012, 207, 218–226. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.H.; Chang, M.; Peng, Y.L.; Yao, J.; Han, R.W.; Wang, R. Neuropeptide S inhibits the acquisition and the expression of conditioned place preference to morphine in mice. Peptides 2009, 30, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Kallupi, M.; de Guglielmo, G.; Cannella, N.; Li, H.W.; Calo, G.; Guerrini, R.; Ubaldi, M.; Renger, J.J.; Uebele, V.N.; Ciccocioppo, R. Hypothalamic neuropeptide S receptor blockade decreases discriminative cue-induced reinstatement of cocaine seeking in the rat. Psychopharmacology 2013, 226, 347–355. [Google Scholar] [CrossRef]
- Paneda, C.; Huitron-Resendiz, S.; Frago, L.M.; Chowen, J.A.; Picetti, R.; de Lecea, L.; Roberts, A.J. Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 4155–4161. [Google Scholar] [CrossRef][Green Version]
- Badia-Elder, N.E.; Henderson, A.N.; Bertholomey, M.L.; Dodge, N.C.; Stewart, R.B. The effects of neuropeptide S on ethanol drinking and other related behaviors in alcohol-preferring and -nonpreferring rats. Alcohol. Clin. Exp. Res. 2008, 32, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Cannella, N.; Kallupi, M.; Li, H.W.; Stopponi, S.; Cifani, C.; Ciccocioppo, R.; Ubaldi, M. Neuropeptide S differently modulates alcohol-related behaviors in alcohol-preferring and non-preferring rats. Psychopharmacology 2016, 233, 2915–2924. [Google Scholar] [CrossRef]
- Cannella, N.; Kallupi, M.; Ruggeri, B.; Ciccocioppo, R.; Ubaldi, M. The role of the neuropeptide S system in addiction: Focus on its interaction with the CRF and hypocretin/orexin neurotransmission. Prog. Neurobiol. 2013, 100, 48–59. [Google Scholar] [CrossRef]
- Beck, B.; Fernette, B.; Stricker-Krongrad, A. Peptide S is a novel potent inhibitor of voluntary and fast-induced food intake in rats. Biochem. Biophys. Res. Commun. 2005, 332, 859–865. [Google Scholar] [CrossRef]
- Kullmann, S.; Kleinridders, A.; Small, D.M.; Fritsche, A.; Haring, H.U.; Preissl, H.; Heni, M. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet. Diabetes Endocrinol. 2020, 8, 524–534. [Google Scholar] [CrossRef]
- Pradhan, G.; Samson, S.L.; Sun, Y. Ghrelin: Much more than a hunger hormone. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 619–624. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S., Jr. Leptin Function and Regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.; Jhanwar-Uniyal, M.; Burlet, A.; Chapleur-Chateau, M.; Leibowitz, S.F.; Burlet, C. Rapid and localized alterations of neuropeptide Y in discrete hypothalamic nuclei with feeding status. Brain Res. 1990, 528, 245–249. [Google Scholar] [CrossRef]
- Sahu, A.; Kalra, P.S.; Kalra, S.P. Food deprivation and ingestion induce reciprocal changes in neuropeptide Y concentrations in the paraventricular nucleus. Peptides 1988, 9, 83–86. [Google Scholar] [CrossRef]
- Ramos, E.J.; Meguid, M.M.; Campos, A.C.; Coelho, J.C. Neuropeptide Y, alpha-melanocyte-stimulating hormone, and monoamines in food intake regulation. Nutrition 2005, 21, 269–279. [Google Scholar] [CrossRef]
- Sohn, J.W. Network of hypothalamic neurons that control appetite. Bmb Rep. 2015, 48, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hernandez-Sanchez, D.; Herzog, H. Regulation of Feeding-Related Behaviors by Arcuate Neuropeptide Y Neurons. Endocrinology 2019, 160, 1411–1420. [Google Scholar] [CrossRef]
- Bellinger, L.L.; Bernardis, L.L. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: Lessons learned from lesioning studies. Physiol. Behav. 2002, 76, 431–442. [Google Scholar] [CrossRef]
- Bernardis, L.L. Hypophagia, hypodipsia, and hypoactivity following electrolytic lesions in the dorsomedial hypothalamic nuclei of mature rats of both sexes. J. Neural Transm. 1972, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Micioni Di Bonaventura, M.V.; Pucci, M.; Giusepponi, M.E.; Romano, A.; Lambertucci, C.; Volpini, R.; Micioni Di Bonaventura, E.; Gaetani, S.; Maccarrone, M.; D’Addario, C.; et al. Regulation of adenosine A2A receptor gene expression in a model of binge eating in the amygdaloid complex of female rats. J. Psychopharmacol. 2019. [Google Scholar] [CrossRef]
- Stuber, G.D.; Wise, R.A. Lateral hypothalamic circuits for feeding and reward. Nat. Neurosci. 2016, 19, 198–205. [Google Scholar] [CrossRef]
- Fedeli, A.; Braconi, S.; Economidou, D.; Cannella, N.; Kallupi, M.; Guerrini, R.; Calo, G.; Cifani, C.; Massi, M.; Ciccocioppo, R. The paraventricular nucleus of the hypothalamus is a neuroanatomical substrate for the inhibition of palatable food intake by neuropeptide S. Eur. J. Neurosci. 2009, 30, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Niimi, M. Centrally administered neuropeptide S activates orexin-containing neurons in the hypothalamus and stimulates feeding in rats. Endocrine 2006, 30, 75–79. [Google Scholar] [CrossRef]
- Cifani, C.; Micioni Di Bonaventura, M.V.; Cannella, N.; Fedeli, A.; Guerrini, R.; Calo, G.; Ciccocioppo, R.; Ubaldi, M. Effect of neuropeptide S receptor antagonists and partial agonists on palatable food consumption in the rat. Peptides 2011, 32, 44–50. [Google Scholar] [CrossRef]
- Cline, M.A.; Godlove, D.C.; Nandar, W.; Bowden, C.N.; Prall, B.C. Anorexigenic effects of central neuropeptide S involve the hypothalamus in chicks (Gallus gallus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 657–663. [Google Scholar] [CrossRef]
- Sutton, A.K.; Myers, M.G., Jr.; Olson, D.P. The Role of PVH Circuits in Leptin Action and Energy Balance. Annu. Rev. Physiol. 2016, 78, 207–221. [Google Scholar] [CrossRef]
- Yi, J.; Gilbert, E.R.; Siegel, P.B.; Cline, M.A. Fed and fasted chicks from lines divergently selected for low or high body weight have differential hypothalamic appetite-associated factor mRNA expression profiles. Behav. Brain Res. 2015, 286, 58–63. [Google Scholar] [CrossRef]
- McConn, B.R.; Gilbert, E.R.; Cline, M.A. Fasting and refeeding induce differential changes in hypothalamic mRNA abundance of appetite-associated factors in 7day-old Japanese quail, Coturnix japonica. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 227, 60–67. [Google Scholar] [CrossRef]
- Hagan, J.J.; Leslie, R.A.; Patel, S.; Evans, M.L.; Wattam, T.A.; Holmes, S.; Benham, C.D.; Taylor, S.G.; Routledge, C.; Hemmati, P.; et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc. Natl. Acad. Sci. USA 1999, 96, 10911–10916. [Google Scholar] [CrossRef]
- Toth, A.; Hajnik, T.; Zaborszky, L.; Detari, L. Effect of basal forebrain neuropeptide Y administration on sleep and spontaneous behavior in freely moving rats. Brain Res. Bull. 2007, 72, 293–301. [Google Scholar] [CrossRef]
- Al-Barazanji, K.A.; Wilson, S.; Baker, J.; Jessop, D.S.; Harbuz, M.S. Central orexin-A activates hypothalamic-pituitary-adrenal axis and stimulates hypothalamic corticotropin releasing factor and arginine vasopressin neurones in conscious rats. J. Neuroendocrinol. 2001, 13, 421–424. [Google Scholar] [CrossRef]
- Hanson, E.S.; Dallman, M.F. Neuropeptide Y (NPY) may integrate responses of hypothalamic feeding systems and the hypothalamo-pituitary-adrenal axis. J. Neuroendocrinol. 1995, 7, 273–279. [Google Scholar] [CrossRef]
- Kakui, N.; Kitamura, K. Direct evidence that stimulation of neuropeptide Y Y5 receptor activates hypothalamo-pituitary-adrenal axis in conscious rats via both corticotropin-releasing factor- and arginine vasopressin-dependent pathway. Endocrinology 2007, 148, 2854–2862. [Google Scholar] [CrossRef][Green Version]
- Russell, S.H.; Small, C.J.; Dakin, C.L.; Abbott, C.R.; Morgan, D.G.; Ghatei, M.A.; Bloom, S.R. The central effects of orexin-A in the hypothalamic-pituitary-adrenal axis in vivo and in vitro in male rats. J. Neuroendocrinol. 2001, 13, 561–566. [Google Scholar] [CrossRef]
- Bale, T.L.; Vale, W.W. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 525–557. [Google Scholar] [CrossRef] [PubMed]
- Vale, W.; Spiess, J.; Rivier, C.; Rivier, J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981, 213, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Kormos, V.; Gaszner, B. Role of neuropeptides in anxiety, stress, and depression: From animals to humans. Neuropeptides 2013, 47, 401–419. [Google Scholar] [CrossRef]
- Ghitza, U.E.; Gray, S.M.; Epstein, D.H.; Rice, K.C.; Shaham, Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: A role of CRF1 receptors. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2006, 31, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Cottone, P.; Sabino, V.; Roberto, M.; Bajo, M.; Pockros, L.; Frihauf, J.B.; Fekete, E.M.; Steardo, L.; Rice, K.C.; Grigoriadis, D.E.; et al. CRF system recruitment mediates dark side of compulsive eating. Proc. Natl. Acad. Sci. USA 2009, 106, 20016–20020. [Google Scholar] [CrossRef]
- Micioni Di Bonaventura, M.V.; Ciccocioppo, R.; Romano, A.; Bossert, J.M.; Rice, K.C.; Ubaldi, M.; St Laurent, R.; Gaetani, S.; Massi, M.; Shaham, Y.; et al. Role of bed nucleus of the stria terminalis corticotrophin-releasing factor receptors in frustration stress-induced binge-like palatable food consumption in female rats with a history of food restriction. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 11316–11324. [Google Scholar] [CrossRef]
- Micioni Di Bonaventura, M.V.; Ubaldi, M.; Giusepponi, M.E.; Rice, K.C.; Massi, M.; Ciccocioppo, R.; Cifani, C. Hypothalamic CRF1 receptor mechanisms are not sufficient to account for binge-like palatable food consumption in female rats. Int. J. Eat. Disord. 2017, 50, 1194–1204. [Google Scholar] [CrossRef]
- Pucci, M.; Micioni Di Bonaventura, M.V.; Giusepponi, M.E.; Romano, A.; Filaferro, M.; Maccarrone, M.; Ciccocioppo, R.; Cifani, C.; D’Addario, C. Epigenetic regulation of nociceptin/orphanin FQ and corticotropin-releasing factor system genes in frustration stress-induced binge-like palatable food consumption. Addict. Biol. 2016, 21, 1168–1185. [Google Scholar] [CrossRef]
- Micioni Di Bonaventura, M.V.; Vitale, G.; Massi, M.; Cifani, C. Effect of Hypericum perforatum Extract in an Experimental Model of Binge Eating in Female Rats. J. Obes. 2012, 2012, 956137. [Google Scholar] [CrossRef]
- Alboni, S.; Micioni Di Bonaventura, M.V.; Benatti, C.; Giusepponi, M.E.; Brunello, N.; Cifani, C. Hypothalamic expression of inflammatory mediators in an animal model of binge eating. Behav. Brain Res. 2017, 320, 420–430. [Google Scholar] [CrossRef]
- Jungling, K.; Liu, X.; Lesting, J.; Coulon, P.; Sosulina, L.; Reinscheid, R.K.; Pape, H.C. Activation of neuropeptide S-expressing neurons in the locus coeruleus by corticotropin-releasing factor. J. Physiol. 2012, 590, 3701–3717. [Google Scholar] [CrossRef]
- Ebner, K.; Rjabokon, A.; Pape, H.C.; Singewald, N. Increased in vivo release of neuropeptide S in the amygdala of freely moving rats after local depolarisation and emotional stress. Amino Acids 2011, 41, 991–996. [Google Scholar] [CrossRef][Green Version]
- Qin, C.; Li, J.; Tang, K. The Paraventricular Nucleus of the Hypothalamus: Development, Function, and Human Diseases. Endocrinology 2018, 159, 3458–3472. [Google Scholar] [CrossRef] [PubMed]
- Li, M.S.; Peng, Y.L.; Jiang, J.H.; Xue, H.X.; Wang, P.; Zhang, P.J.; Han, R.W.; Chang, M.; Wang, R. Neuropeptide S Increases locomotion activity through corticotropin-releasing factor receptor 1 in substantia nigra of mice. Peptides 2015, 71, 196–201. [Google Scholar] [CrossRef]
- Thompson, M.D.; Sakurai, T.; Rainero, I.; Maj, M.C.; Kukkonen, J.P. Orexin Receptor Multimerization versus Functional Interactions: Neuropharmacological Implications for Opioid and Cannabinoid Signalling and Pharmacogenetics. Pharmaceuticals 2017, 10, 79. [Google Scholar] [CrossRef]
- Sakurai, T. Roles of orexins and orexin receptors in central regulation of feeding behavior and energy homeostasis. CNS Neurol. Disord. Drug Targets 2006, 5, 313–325. [Google Scholar] [CrossRef]
- Kallupi, M.; Cannella, N.; Economidou, D.; Ubaldi, M.; Ruggeri, B.; Weiss, F.; Massi, M.; Marugan, J.; Heilig, M.; Bonnavion, P.; et al. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc. Natl. Acad. Sci. USA 2010, 107, 19567–19572. [Google Scholar] [CrossRef] [PubMed]
- Ubaldi, M.; Giordano, A.; Severi, I.; Li, H.; Kallupi, M.; de Guglielmo, G.; Ruggeri, B.; Stopponi, S.; Ciccocioppo, R.; Cannella, N. Activation of Hypocretin-1/Orexin-A Neurons Projecting to the Bed Nucleus of the Stria Terminalis and Paraventricular Nucleus Is Critical for Reinstatement of Alcohol Seeking by Neuropeptide, S. Biol. Psychiatry 2016, 79, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Cannella, N.; Economidou, D.; Kallupi, M.; Stopponi, S.; Heilig, M.; Massi, M.; Ciccocioppo, R. Persistent increase of alcohol-seeking evoked by neuropeptide S: An effect mediated by the hypothalamic hypocretin system. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2009, 34, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Blundell, J.E.; Halford, J.C.; Upton, N.; Porter, R.; Johns, A.; Jeffrey, P.; Summerfield, S.; Rodgers, R.J. Anorexia and weight loss in male rats 24 h following single dose treatment with orexin-1 receptor antagonist SB-334867. Behav. Brain Res. 2005, 157, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Kay, K.; Parise, E.M.; Lilly, N.; Williams, D.L. Hindbrain orexin 1 receptors influence palatable food intake, operant responding for food, and food-conditioned place preference in rats. Psychopharmacology 2014, 231, 419–427. [Google Scholar] [CrossRef]
- Piccoli, L.; Micioni Di Bonaventura, M.V.; Cifani, C.; Costantini, V.J.; Massagrande, M.; Montanari, D.; Martinelli, P.; Antolini, M.; Ciccocioppo, R.; Massi, M.; et al. Role of orexin-1 receptor mechanisms on compulsive food consumption in a model of binge eating in female rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2012, 37, 1999–2011. [Google Scholar] [CrossRef]
- Grund, T.; Goyon, S.; Li, Y.; Eliava, M.; Liu, H.; Charlet, A.; Grinevich, V.; Neumann, I.D. Neuropeptide S Activates Paraventricular Oxytocin Neurons to Induce Anxiolysis. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 12214–12225. [Google Scholar] [CrossRef]
- Onaka, T.; Takayanagi, Y. Role of oxytocin in the control of stress and food intake. J. Neuroendocrinol. 2019, 31, e12700. [Google Scholar] [CrossRef]
- Dayi, A.; Kiray, M.; Sisman, A.; Ozbal, S.; Baykara, B.; Aksu, I.; Uysal, N. Dose dependent effects of oxytocin on cognitive defects and anxiety disorders in adult rats following acute infantile maternal deprivation stress. Biotech. Histochem. Off. Publ. Biol. Stain Comm. 2019, 94, 469–480. [Google Scholar] [CrossRef]
- van den Burg, E.H.; Stindl, J.; Grund, T.; Neumann, I.D.; Strauss, O. Oxytocin Stimulates Extracellular Ca2+ Influx Through TRPV2 Channels in Hypothalamic Neurons to Exert Its Anxiolytic Effects. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015, 40, 2938–2947. [Google Scholar] [CrossRef]
- Iwasa, T.; Matsuzaki, T.; Mayila, Y.; Yanagihara, R.; Yamamoto, Y.; Kawakita, T.; Kuwahara, A.; Irahara, M. Oxytocin treatment reduced food intake and body fat and ameliorated obesity in ovariectomized female rats. Neuropeptides 2019, 75, 49–57. [Google Scholar] [CrossRef]
- Maejima, Y.; Iwasaki, Y.; Yamahara, Y.; Kodaira, M.; Sedbazar, U.; Yada, T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging 2011, 3, 1169–1177. [Google Scholar] [CrossRef]
- Maejima, Y.; Yokota, S.; Nishimori, K.; Shimomura, K. The Anorexigenic Neural Pathways of Oxytocin and Their Clinical Implication. Neuroendocrinology 2018, 107, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Micioni Di Bonaventura, M.V.; Gallelli, C.A.; Koczwara, J.B.; Smeets, D.; Giusepponi, M.E.; De Ceglia, M.; Friuli, M.; Micioni Di Bonaventura, E.; Scuderi, C.; et al. Oleoylethanolamide decreases frustration stress-induced binge-like eating in female rats: A novel potential treatment for binge eating disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2020. [Google Scholar] [CrossRef]
- Romano, A.; Tempesta, B.; Micioni Di Bonaventura, M.V.; Gaetani, S. From Autism to Eating Disorders and More: The Role of Oxytocin in Neuropsychiatric Disorders. Front. Neurosci. 2015, 9, 497. [Google Scholar] [CrossRef]
- Lage, R.; Dieguez, C.; Lopez, M. Caffeine treatment regulates neuropeptide S system expression in the rat brain. Neurosci. Lett. 2006, 410, 47–51. [Google Scholar] [CrossRef]
- Wurts, S.W.; Edgar, D.M. Caffeine during sleep deprivation: Sleep tendency and dynamics of recovery sleep in rats. Pharmacol. Biochem. Behav. 2000, 65, 155–162. [Google Scholar] [CrossRef]
- Boeck, C.R.; Martinello, C.; de Castro, A.A.; Moretti, M.; Dos Santos Casagrande, T.; Guerrini, R.; Calo, G.; Gavioli, E.C. Blockade of adenosine A2A receptor counteracts neuropeptide-S-induced hyperlocomotion in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2010, 381, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.; Pescador, B.B.; Mendonca, B.P.; Ramos, S.F.; Guerrini, R.; Calo, G.; de Andrade, V.M.; Gavioli, E.C.; Boeck, C.R. Role of the ecto-nucleotidases in the cooperative effect of adenosine and neuropeptide-S on locomotor activity in mice. Pharmacol. Biochem. Behav. 2011, 99, 726–730. [Google Scholar] [CrossRef][Green Version]
- Micioni Di Bonaventura, M.V.; Cifani, C.; Lambertucci, C.; Volpini, R.; Cristalli, G.; Froldi, R.; Massi, M. Effects of A(2)A adenosine receptor blockade or stimulation on alcohol intake in alcohol-preferring rats. Psychopharmacology 2012, 219, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Micioni Di Bonaventura, M.V.; Cifani, C.; Lambertucci, C.; Volpini, R.; Cristalli, G.; Massi, M. A2A adenosine receptor agonists reduce both high-palatability and low-palatability food intake in female rats. Behav. Pharmacol. 2012, 23, 567–574. [Google Scholar] [CrossRef]
- Gardella, E.; Romei, C.; Cavallero, A.; Trapella, C.; Fedele, E.; Raiteri, L. Neuropeptide S inhibits release of 5-HT and glycine in mouse amygdala and frontal/prefrontal cortex through activation of the neuropeptide S receptor. Neurochem. Int. 2013, 62, 360–366. [Google Scholar] [CrossRef]
- Raiteri, L.; Luccini, E.; Romei, C.; Salvadori, S.; Calo, G. Neuropeptide S selectively inhibits the release of 5-HT and noradrenaline from mouse frontal cortex nerve endings. Br. J. Pharmacol. 2009, 157, 474–481. [Google Scholar] [CrossRef]
- Si, W.; Aluisio, L.; Okamura, N.; Clark, S.D.; Fraser, I.; Sutton, S.W.; Bonaventure, P.; Reinscheid, R.K. Neuropeptide S stimulates dopaminergic neurotransmission in the medial prefrontal cortex. J. Neurochem. 2010, 115, 475–482. [Google Scholar] [CrossRef]
- Botticelli, L.; Micioni Di Bonaventura, E.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Romano, A.; Quaglia, W.; Cifani, C.; Micioni Di Bonaventura, M.V. Underlying Susceptibility to Eating Disorders and Drug Abuse: Genetic and Pharmacological Aspects of Dopamine D4 Receptors. Nutrients 2020, 12, 2288. [Google Scholar] [CrossRef]
- Gluck, M.E.; Viswanath, P.; Stinson, E.J. Obesity, Appetite, and the Prefrontal Cortex. Curr. Obes. Rep. 2017, 6, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.Z.; Volkow, N.D. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011, 12, 652–669. [Google Scholar] [CrossRef] [PubMed]
- Cifani, C.; Koya, E.; Navarre, B.M.; Calu, D.J.; Baumann, M.H.; Marchant, N.J.; Liu, Q.R.; Khuc, T.; Pickel, J.; Lupica, C.R.; et al. Medial prefrontal cortex neuronal activation and synaptic alterations after stress-induced reinstatement of palatable food seeking: A study using c-fos-GFP transgenic female rats. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 8480–8490. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.G.; Navarre, B.M.; Cifani, C.; Pickens, C.L.; Bossert, J.M.; Shaham, Y. Role of dorsal medial prefrontal cortex dopamine D1-family receptors in relapse to high-fat food seeking induced by the anxiogenic drug yohimbine. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011, 36, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Palasz, A.; Rojczyk, E. Neuroleptics Affect Neuropeptide S and NPSR mRNA Levels in the Rat Brain. J. Mol. Neurosci. Mn 2015, 57, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Palasz, A.; Rojczyk, E.; Golyszny, M.; Filipczyk, L.; Worthington, J.J.; Wiaderkiewicz, R. Long-term treatment with haloperidol affects neuropeptide S and NPSR mRNA levels in the rat brain. Acta Neuropsychiatr. 2016, 28, 110–116. [Google Scholar] [CrossRef]
- Han, R.W.; Chang, M.; Peng, Y.L.; Qiao, L.Y.; Yin, X.Q.; Li, W.; Wang, R. Central Neuropeptide S inhibits distal colonic transit through activation of central Neuropeptide S receptor in mice. Peptides 2009, 30, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Barone, F.C.; Deegan, J.F.; Price, W.J.; Fowler, P.J.; Fondacaro, J.D.; Ormsbee, H.S., 3rd. Cold-restraint stress increases rat fecal pellet output and colonic transit. Am. J. Physiol. 1990, 258, G329–G337. [Google Scholar] [CrossRef]
- Camilleri, M.; Linden, D.R. Measurement of Gastrointestinal and Colonic Motor Functions in Humans and Animals. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Monnikes, H.; Schmidt, B.G.; Tache, Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology 1993, 104, 716–723. [Google Scholar] [CrossRef]
- Petrella, C.; Agostini, S.; Guerrini, R.; Calo, G.; Giaquinto, A.; De Nuccio, C.; Improta, G.; Broccardo, M. Neuropeptide S inhibits stress-stimulated faecal output in the rat. Pharmacol. Res. 2011, 64, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, M.; Sinen, O.; Bayramoglu, O. Central neuropeptide-S administration alleviates stress-induced impairment of gastric motor functions through orexin-A. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2020, 31, 65–72. [Google Scholar] [CrossRef]
- Camilleri, M.; Carlson, P.; Zinsmeister, A.R.; McKinzie, S.; Busciglio, I.; Burton, D.; Zucchelli, M.; D’Amato, M. Neuropeptide S receptor induces neuropeptide expression and associates with intermediate phenotypes of functional gastrointestinal disorders. Gastroenterology 2010, 138, 98–107.e4. [Google Scholar] [CrossRef][Green Version]
- D’Amato, M.; Bruce, S.; Bresso, F.; Zucchelli, M.; Ezer, S.; Pulkkinen, V.; Lindgren, C.; Astegiano, M.; Rizzetto, M.; Gionchetti, P.; et al. Neuropeptide s receptor 1 gene polymorphism is associated with susceptibility to inflammatory bowel disease. Gastroenterology 2007, 133, 808–817. [Google Scholar] [CrossRef]
- Wan Saudi, W.S.; Halim, M.A.; Rudholm-Feldreich, T.; Gillberg, L.; Rosenqvist, E.; Tengholm, A.; Sundbom, M.; Karlbom, U.; Naslund, E.; Webb, D.L.; et al. Neuropeptide S inhibits gastrointestinal motility and increases mucosal permeability through nitric oxide. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G625–G634. [Google Scholar] [CrossRef]
- Wan Saudi, W.S.; Sjoblom, M. Neuropeptide S reduces duodenal bicarbonate secretion and ethanol-induced increases in duodenal motility in rats. PLoS ONE 2017, 12, e0175312. [Google Scholar] [CrossRef] [PubMed]

| Rodents | Injection | Result | Reference |
|---|---|---|---|
| Male mice | ICV | ↓ standard chow food intake in 18 h fasted mice. | [17] |
| Male rats | ICV | ↓ standard chow food intake after an overnight fasting. ↓ HPF intake in sated rats. | [61] |
| Male rats | ICV | ↑ 2 h standard chow food intake. | [75] |
| Male rats | ICV and PVN | ↓ Standard chow food intake in 24 h fasted rats. | [18] |
| Male rats | ICV, PVN, LH and CeA | ↓ HPF consumption after ICV, PVN and LH injections. No changes on HPF consumption after CeA injection. | [74] |
| Male rats | ICV | ↓ HPF consumption. | [44] |
| Male rats | ICV | ↓ standard chow food intake in freely feeding and 12 h food restricted rats. ↓ HPF consumption. | [76] |
| Female rats | ICV | ↑ standard chow food intake at doses 0.075 and 0.3 nmol. No influence on sucrose solution intake. | [58] |
| Avian Species | Injection | Result | Reference |
|---|---|---|---|
| Broiler chicks | ICV, LH and PVN | ↓ Food intake in 3 h faster chicks, after ICV, LH and PVN injections. | [77] |
| LWS and HWS chicks | ICV | ↓ Food intake in both lines, but with a more pronounced effect in HWS chicks. | [16] |
| LWS and HWS chicks | - | ↑ NPS mRNA in LWS chicks compared to HWS. ↓ NPS mRNA in LWS and HWS after 3 h of fasting. | [79] |
| Japanese quails | - | ↑ NPS mRNA in 3 h fasted quails. ↑ NPS mRNA in refed quails, after 6 h of fasting. | [80] |
| Japanese quails | ICV | ↓ Food intake in 6 h fasted quails. | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botticelli, L.; Micioni Di Bonaventura, E.; Ubaldi, M.; Ciccocioppo, R.; Cifani, C.; Micioni Di Bonaventura, M.V. The Neural Network of Neuropeptide S (NPS): Implications in Food Intake and Gastrointestinal Functions. Pharmaceuticals 2021, 14, 293. https://doi.org/10.3390/ph14040293
Botticelli L, Micioni Di Bonaventura E, Ubaldi M, Ciccocioppo R, Cifani C, Micioni Di Bonaventura MV. The Neural Network of Neuropeptide S (NPS): Implications in Food Intake and Gastrointestinal Functions. Pharmaceuticals. 2021; 14(4):293. https://doi.org/10.3390/ph14040293
Chicago/Turabian StyleBotticelli, Luca, Emanuela Micioni Di Bonaventura, Massimo Ubaldi, Roberto Ciccocioppo, Carlo Cifani, and Maria Vittoria Micioni Di Bonaventura. 2021. "The Neural Network of Neuropeptide S (NPS): Implications in Food Intake and Gastrointestinal Functions" Pharmaceuticals 14, no. 4: 293. https://doi.org/10.3390/ph14040293
APA StyleBotticelli, L., Micioni Di Bonaventura, E., Ubaldi, M., Ciccocioppo, R., Cifani, C., & Micioni Di Bonaventura, M. V. (2021). The Neural Network of Neuropeptide S (NPS): Implications in Food Intake and Gastrointestinal Functions. Pharmaceuticals, 14(4), 293. https://doi.org/10.3390/ph14040293








