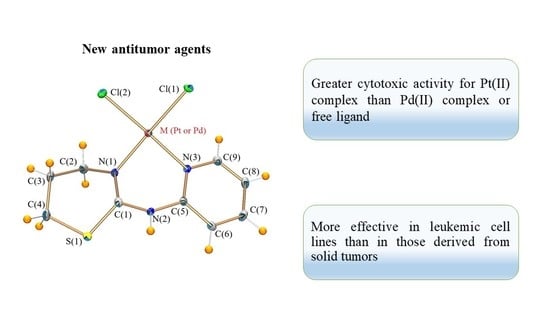
Synthesis, Characterization and Antiproliferative Evaluation of Pt(II) and Pd(II) Complexes with a Thiazine-Pyridine Derivative Ligand †
Abstract
1. Introduction
2. Results and Discussion
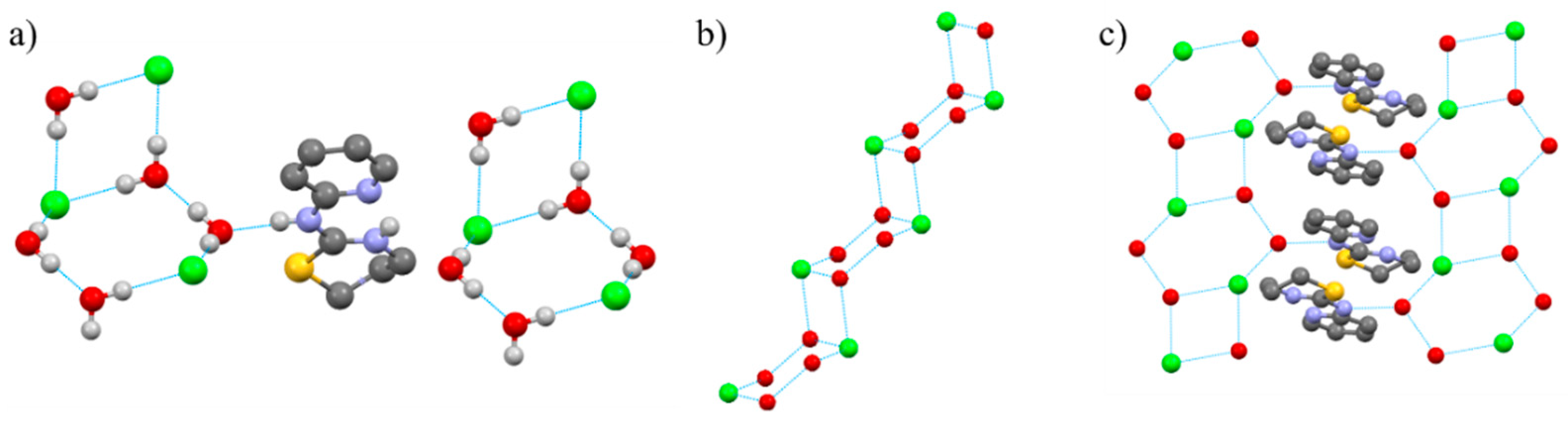
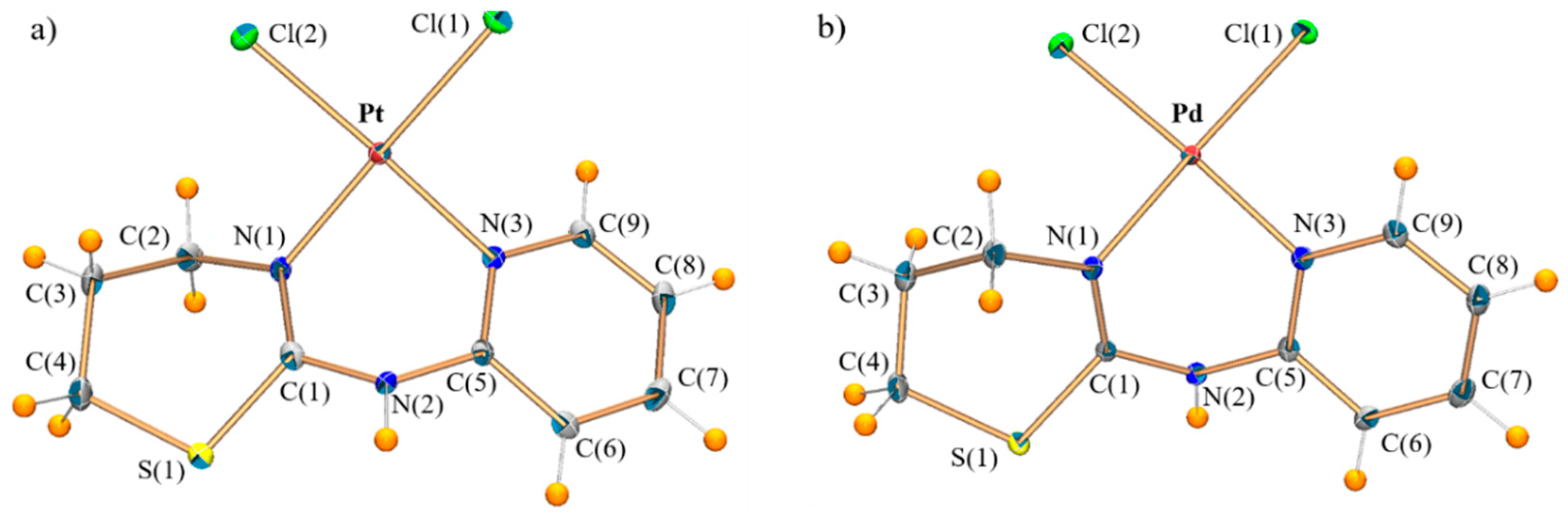
2.1. Crystal Structures
2.2. Spectroscopic Studies
2.3. In Silico ADME Prediction
2.4. Biological Activity
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis of [PtCl2(PyTz)]·C2H6O (PtPyTz)
3.3. Synthesis of [PdCl2(PyTz)]·C2H6O (PdPyTz)
3.4. Isolation of Single Crystals of PyTzHCl·2H2O
3.5. X-ray Diffraction
3.6. Cell Culture and Treatments
3.7. In Vitro Cytotoxicity Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arenaza-Corona, A.; Couce-Fortúnez, M.D.; de Blas, A.; Morales-Morales, D.; Santillan, R.; Höpfl, H.; Rodríguez-Blas, T.; Barba, V. Further Approaches in the Design of Antitumor Agents with Response to Cell Resistance: Looking toward Aza Crown Ether-dtc Complexes. Inorg. Chem. 2020, 59, 15120–15134. [Google Scholar] [CrossRef]
- Fernández-Delgado, E.; de la Cruz-Martínez, F.; Galán, C.; Franco, L.; Espino, J.; Viñuelas-Zahínos, E.; Luna-Giles, F.; Bejarano, I. Pt(II) and Pd(II) complexes with a thiazoline derivative ligand: Synthesis, structural characterization, antiproliferative activity and evaluation of pro-apoptotic ability in tumor cell lines HT-29 and U-937. J. Inorg. Biochem. 2020, 202, 110870. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, T.; Rilak, A.; Bugarčić, Ž.D. Platinum, palladium, gold and ruthenium complexes as anticancer agents: Current clinical uses, cytotoxicity studies and future perspectives. Eur. J. Med. Chem. 2017, 142, 8–31. [Google Scholar] [CrossRef]
- Pasetto, L.M.; D’Andrea, M.R.; Brandes, A.A.; Rossi, E.; Monfardini, S. The development of platinum compounds and their possible combination. Crit. Rev. Oncol. Hematol. 2006, 60, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G. Clinical perspectives on platinum resistance. Drugs 2000, 59, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Pariente, R.; Pariente, J.A.; Rodríguez, A.B.; Espino, J. Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: Effects on oxidative stress and DNA fragmentation. J. Pineal Res. 2016, 60, 55–64. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef]
- Shen, D.W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 2012, 64, 706–721. [Google Scholar] [CrossRef]
- Cvitkovic, E. Cumulative toxicities from cisplatin therapy and current cytoprotective measures. Cancer Treat. Rev. 1998, 24, 265–281. [Google Scholar] [CrossRef]
- Screnci, D.; McKeage, M.J. Platinum neurotoxicity: Clinical profiles, experimental models and neuroprotective approaches. J. Inorg. Biochem. 1999, 77, 105–110. [Google Scholar] [CrossRef]
- Kasturi, J.; Palla, P.R.; Bakshi, V.; Boggula, N. Journal of Drug Delivery and Therapeutics Non-steroidal anti-inflammatory drugs: An overview. J. Drug Deliv. Ther. 2019, 9, 442–448. [Google Scholar]
- Ali, I.; Wani, W.A.; Saleem, K.; Haque, A. Platinum Compounds: A Hope for Future Cancer Chemotherapy. Anticancer. Agents Med. Chem. 2013, 13, 296–306. [Google Scholar] [CrossRef]
- Kapdi, A.R.; Fairlamb, I.J.S. Anti-cancer palladium complexes: A focus on PdX2L2, palladacycles and related complexes. Chem. Soc. Rev. 2014, 43, 4751–4777. [Google Scholar] [CrossRef]
- Coskun, M.D.; Ari, F.; Oral, A.Y.; Sarimahmut, M.; Kutlu, H.M.; Yilmaz, V.T.; Ulukaya, E. Promising anti-growth effects of palladium(II) saccharinate complex of terpyridine by inducing apoptosis on transformed fibroblasts in vitro. Bioorg. Med. Chem. 2013, 21, 4698–4705. [Google Scholar] [CrossRef]
- Bugarčić, Ž.D.; Bogojeski, J.; van Eldik, R. Kinetics, mechanism and equilibrium studies on the substitution reactions of Pd(II) in reference to Pt(II) complexes with bio-molecules. Coord. Chem. Rev. 2015, 292, 91–106. [Google Scholar] [CrossRef]
- Qin, Q.P.; Zou, B.Q.; Tan, M.X.; Luo, D.M.; Wang, Z.F.; Wang, S.L.; Liu, Y.C. High in vitro anticancer activity of a dinuclear palladium(II) complex with a 2-phenylpyridine ligand. Inorg. Chem. Commun. 2018, 96, 106–110. [Google Scholar] [CrossRef]
- Ćoćić, D.; Jovanović, S.; Nišavić, M.; Baskić, D.; Todorović, D.; Popović, S.; Bugarčić, Ž.D.; Petrović, B. New dinuclear palladium(II) complexes: Studies of the nucleophilic substitution reactions, DNA/BSA interactions and cytotoxic activity. J. Inorg. Biochem. 2017, 175, 67–79. [Google Scholar] [CrossRef]
- Espino, J.; Fernández-Delgado, E.; Estirado, S.; de la Cruz-Martinez, F.; Villa-Carballar, S.; Viñuelas-Zahínos, E.; Luna-Giles, F.; Pariente, J.A. Synthesis and structure of a new thiazoline-based palladium(II) complex that promotes cytotoxicity and apoptosis of human promyelocytic leukemia HL-60 cells. Sci. Rep. 2020, 10, 1–16. [Google Scholar]
- Orlova, M.A.; Trofimova, T.P.; Filimonova, M.V.; Proshin, A.N.; Zaitsev, D.A. Effect of the thiazine and thiourea derivatives as NO-synthase effectors on the survival of leukemic cells. Russ. Chem. Bull. 2013, 62, 1111–1114. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 389–397. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Li, K.; Cong, Y.W.; Pu, S.P.; Zhu, H.Y.; Xie, X.G.; Jin, Y.; Lin, J. Preparation, characterisation and bioactivity evaluation of the inclusion complex formed between picoplatin and γ-cyclodextrin. Carbohydr. Res. 2014, 396, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Barros-García, F.J.; Bernalte-García, A.; Higes-Rolando, F.J.; Luna-Giles, F.; Pedrero-Marín, R. X-ray and spectroscopic characterisation of cobalt(III) and nickel(II) complexes with 2-(2-pyridyl)iminotetrahydro-1,3-thiazine hydrochloride·water (1/2) (PyTzHCl·2H2O) in the solid state and study of its interaction with cobalt(II) and nickel(II) in aqu. Polyhedron 2004, 23, 1453–1460. [Google Scholar] [CrossRef]
- Bernalte-Garcia, A.; García-Barros, F.J.; Higes-Rolando, F.J.; Luna-Giles, F.; Pedrero-Marín, R. Synthesis and physic-chemical properties of a copper(II) complex with 2-(2-pyridyl)iminotetrahydro-1,3-thiazine hydrochloride-water (1/2) (PyTzHCl·2H2O). Crystal structure of PyTz and [{CuCl(PyTz)}2(μ-Cl)2]. J. Inorg. Biochem. 2004, 98, 15–23. [Google Scholar] [CrossRef]
- García-Cuesta, M.C.; Lozano, A.M.; Meléndez-Martínez, J.J.; Luna-Giles, F.; Ortiz, A.L.; González-Méndez, L.M.; Cumbrera, F.L. Structure determination of nitrato-κO-bis[2-(2-pyridyl-κN)amino-5,6-dihydro-4H-1,3-thiazine-κN]copper(II) nitrate via molecular modelling coupled with X-ray powder diffractometry. J. Appl. Crystallogr. 2004, 37, 993–999. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 1987, 2, S1–S19. [Google Scholar] [CrossRef]
- Fateley, W.G.; Dollish, F.R.; McDevitt, N.T.; Bentley, F.F. Infrared and Raman Selection Rules for Molecular and Lattice Vibrations: The Correlation Method; Wiley-Interscience: New York, NY, USA, 1972. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed.; John Wiley & Sons: New York, NY, USA, 1997; ISBN 9780470027325. [Google Scholar]
- Ferraro, J.R. Low-Frequency Vibrations of Inorganic and Coordination Compounds; Springer Plenum Press: New York, NY, USA, 1971; ISBN 978-1-4684-1811-8. [Google Scholar]
- Nakamoto, K.; Mccarthy, P.J.; Fujita, J.; Condrate, R.A.; Behnke, G.T. Infrared Studies of Ligand-Ligand Interaction in Dihalogenodiammineplatinum(II) Complexes. Inorg. Chem. 1965, 4, 36–43. [Google Scholar] [CrossRef]
- Dehand, J.; Jordanov, J. Complexes of Pt(II), Pd(II), Rh(I) and Rh(III) with nitrogen and sulfur-containing heterocyclic ligands of biological interest. Synthesis, characterization and influence of pH. Inorg. Chim. Acta 1976, 17, 37–44. [Google Scholar] [CrossRef]
- Belluco, U.; Benetollo, F.; Bertani, R.; Bombieri, G.; Michelin, R.A.; Mozzon, M.; Pombeiro, A.J.L.; Guedes da Silva, F.C. Stereochemical investigation of the addition of primary and secondary aliphatic amines to the nitrile complexes cis- and trans-[PtCl2(NCMe)2]. X-ray structures of the amidine complexes trans-[Pt(NH2Pri)2{Z-N(H)=C(NHPr i)Me}]Cl2·4H2O and trans-[PtCl2(NCMe). Inorg. Chim. Acta 2002, 330, 229–239. [Google Scholar] [CrossRef]
- Durig, J.R.; Layton, R.; Sink, D.W.; Mitchell, B.R. Far infrared spectra of palladium compounds-I. The influence of ligands upon the palladium chloride stretching frequency. Spectrochim. Acta 1965, 21, 1367–1378. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New Substructure Filters for Removal of Pan Assay Interference Compounds (PAINS) from Screening Libraries and for Their Exclusion in Bioassays. J. Med. Chem. 2010, 53, 2719. [Google Scholar] [CrossRef] [PubMed]
- Keter, F.K.; Kanyanda, S.; Lyantagaye, S.S.L.; Darkwa, J.; Rees, D.J.G.; Meyer, M. In vitro evaluation of dichloro-bis(pyrazole)palladium(II) and dichloro-bis(pyrazole)platinum(II) complexes as anticancer agents. Cancer Chemother. Pharmacol. 2008, 63, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Miklášová, N.; Fischer-Fodor, E.; Lönnecke, P.; Tomuleasa, C.I.; Virag, P.; Perde Schrepler, M.; Mikláš, R.; Silaghi Dumitrescu, L.; Hey-Hawkins, E. Antiproliferative effect of novel platinum(II) and palladium(II) complexes on hepatic tumor stem cells in vitro. Eur. J. Med. Chem. 2012, 49, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ćoćić, D.; Jovanović, S.; Radisavljević, S.; Korzekwa, J.; Scheurer, A.; Puchta, R.; Baskić, D.; Todorović, D.; Popović, S.; Matić, S.; et al. New monofunctional platinum(II) and palladium(II) complexes: Studies of the nucleophilic substitution reactions, DNA/BSA interaction, and cytotoxic activity. J. Inorg. Biochem. 2018, 189, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Icsel, C.; Yilmaz, V.T.; Aygun, M.; Cevatemre, B.; Alper, P.; Ulukaya, E. Palladium(ii) and platinum(ii) saccharinate complexes with bis(diphenylphosphino)methane/ethane: Synthesis, S-phase arrest and ROS-mediated apoptosis in human colon cancer cells. Dalt. Trans. 2018, 47, 11397–11410. [Google Scholar] [CrossRef] [PubMed]
- Price, J.H.; Williamson, A.N.; Schramm, R.F.; Wayland, B.B. Palladium(II) and Platinum(II) Alkyl Sulfoxide Complexes. Examples of Sulfur-Bonded, Mixed Sulfur- and Oxygen-Bonded, and Totally Oxygen-Bonded Complexes. Inorg. Chem. 1972, 11, 1280–1284. [Google Scholar] [CrossRef]
- Bruker, AXS Inc. SADABS; Bruker: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, M. SHELXS-14, Program for Crystal Structures Solution; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]


| S(1)-C(1) | 1.744(2) | S(1A)-C(1A) | 1.873(6) |
| C(1)-N(1) | 1.302(2) | C(1A)-N(1A) | 1.229(8) |
| C(1)-N(2) | 1.355(2) | C(1A)-N(2A) | 1.408(5) |
| N(1)-C(2) | 1.396(7) | N(1A)-C(2A) | 1.558(7) |
| N(2)-C(5) | 1.401(2) | N(2A)-C(5A) | 1.415(9) |
| N(3)-C(5) | 1.328(2) | N(3A)-C(5A) | 1.312(12) |
| N(3)-C(9) | 1.322(2) | C(9)-C(8) | 1.382(2) |
| S(1)-C(1)-N(1) | 125.5(1) | S(1A)-C(1A)-N(1A) | 128.4(3) |
| N(1)-C(1)-N(2) | 122.0(1) | N(1A)-C(1A)-N(2A) | 124.7(4) |
| N(2)-C(5)-N(3) | 118.4(1) | N(2A)-C(5A)-N(3A) | 121.1(7) |
| N(3)-C(5)-C(6) | 122.9(1) | C(5A)-N(3A)-C(6) | 119.5 (7) |
| D-H···A | A position | D···A (Å) | D-H···A (°) |
| N(2)-H(2)···O(2W) | x, y, z | 2.701(2) | 168.7(1) |
| N(1)-H(1)··· N(3) | x, y, z | 2.647(2) | 133.7(1) |
| O(2W)-H(4W)···O(1W) | x, y, z | 2.761(2) | 173.6(2) |
| O(2W)-H(3W)···Cl(1) | −x, −y + 1, −z + 1 | 3.177(1) | 173.9(2) |
| O(1W)-H(2W)···Cl(1) | x, −y−1, +z−1 | 3.162(1) | 173.5(1) |
| O(1W)-H(1W)···Cl(1) | −x+1, −y + 1, −z + 1 | 3.209(1) | 171.8(2) |
| PtPyTz | Pt-Cl(1) | 2.306(1) | Pt-Cl(2) | 2.306 |
| Pt-N(1) | 2.013(2) | Pt-N(3) | 2.017(2) | |
| Cl(1)-Pt-Cl(2) | 90.1(1) | Cl(1)-Pt-N(1) | 177.9(1) | |
| Cl(1)-Pt-N(3) | 91.5(1) | Cl(2)-Pt-N(1) | 91.0(1) | |
| Cl(2)-Pt-N(3) | 177.5(1) | N(1)-Pt-N(3) | 87.4(1) | |
| D-H···A | A position | D···A (Å) | D-H···A(°) | |
| N(2)-H(2)···O | x, y, z | 2.736(3) | 162.5(2) | |
| O-H(0)··· Cl(2) | x, y +1, z+1/2 | 3.136(2) | 135.3(2) | |
| PdPyTz | Pd-Cl(1) | 2.302(1) | Pd-Cl(2) | 2.303(1) |
| Pd-N(1) | 2.016(1) | Pd-N(3) | 2.021(1) | |
| Cl(1)-Pd-Cl(2) | 90.6(2) | Cl(1)-Pd-N(1) | 177.7(2) | |
| Cl(1)-Pd-N(3) | 91.5(1) | Cl(2)-Pd-N(1) | 90.8(1) | |
| Cl(2)-Pd-N(3) | 176.2(1) | N(1)-Pd-N(3) | 87.1(1) | |
| D-H···A | A position | D···A (Å) | D-H···A(°) | |
| N(2)-H(2)···O | x, y, z | 2.744(2) | 175.8(2) | |
| O-H(0)···Cl(2) | x, y−1, z | 3.180(3) | 162.1(1) |
| U-937 | HL-60 | HeLa | SK-OV-3 | MCF10A | |
|---|---|---|---|---|---|
| PtPyTz | 26.36 ± 2.56 b | 39.25 ± 3.86 b | 63.38 ± 5.63 b | 77.97 ± 6.36 b | 75.94 ± 7.74 b |
| PdPyTz | 90.96 ± 17.66 c | 115.20 ± 17.01 c | 78.62 ± 8.21 c | 117.20 ± 11.69 c | 81.54 ± 8.29 c |
| PyTz | 79.42 ± 14.64 c | 107.10 ± 15.78 c | 82.31 ± 9.41 c | 97.32 ± 10.05 c | 101.70 ± 2.00 d |
| Cisplatin | 7.89 ± 0.54 a | 11.32 ± 1.04 a | 16.08 ± 1.01 a | 55.41 ± 3.04 a | 50.34 ± 5.16 a |
| PyTzHCl·2H2O | PtPyTz | PdPyTz | |
|---|---|---|---|
| Crystal shape | Plate | Plate | Prism |
| Colour | Colourless | Yellow | Orange |
| Size (mm) | 0.59 × 0.30 × 0.11 | 0.17 × 0.13 × 0.07 | 0.36 × 0.33 × 0.14 |
| Chemical formula | C9H16N3OS | C11H17Cl2N3OPtS | C11H17Cl2N3OPdS |
| Formula weight | 265.76 | 505.32 | 416.63 |
| Crystal system | Triclinic | Monoclinic | Monoclinic |
| Space group | P-1 | P21/c | P21/c |
| Unit cell dimensions | |||
| a (Å) | 6.9676(2) | 12.6205(4) | 12.6048(4) |
| b (Å) | 9.5773(3) | 9.0616(2) | 9.0445(3) |
| c (Å) | 9.5927(3) | 13.8145(4) | 13.8167(5) |
| α (°) | 96.001(2) | ||
| β (°) | 97.830(2) | 109.540(2) | 109.575(2) |
| γ (°) | 101.185(2) | ||
| Cell volume (Å3) | 616.44(4) | 1488.8(7) | 1484.12(9) |
| Z | 2 | 4 | 4 |
| Dcalc (g cm−3) | 1.432 | 2.254 | 1.865 |
| μ (mm−1) | 0.47 | 9.918 | 1.746 |
| F(000) | 280 | 960 | 832 |
| θ range | 2.16–35.57 | 2.74–32.26 | 2.74–30.29 |
| Index ranges | −11≤ h ≤ 11, | −19 ≤ h ≤ 19, | −19 ≤ h ≤ 19, |
| −16 ≤ k ≤ 16, | −13 ≤ k ≤ 13, | −13 ≤ k ≤ 13, | |
| −16 ≤ l ≤ 16 | −21 ≤ l ≤ 21 | −21 ≤ l ≤ 21 | |
| Temperature (K) | 100 | 100 | 100 |
| Independent reflections | 6033 | 5660 | 5649 |
| Observed reflections | 5254 [F > 4.0 σ(F)] | 4407 [F > 4.0 σ(F)] | 4746 [F > 4.0 σ(F)] |
| No. of refined parameters | 195 | 180 | 181 |
| R [F > 4.0 σ(F)] | 0.042 | 0.030 | 0.027 |
| wR [F > 4.0 σ(F)] | 0.125 | 0.044 | 0.051 |
| GOF | 1.052 | 0.993 | 1.021 |
| ρmax, ρmin (e Å−3) | 0.622, −1,210 | 1.604, −1.464 | 0.545, 1.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Tarriño, S.; Espino, J.; Luna-Giles, F.; Rodríguez, A.B.; Pariente, J.A.; Viñuelas-Zahínos, E. Synthesis, Characterization and Antiproliferative Evaluation of Pt(II) and Pd(II) Complexes with a Thiazine-Pyridine Derivative Ligand. Pharmaceuticals 2021, 14, 395. https://doi.org/10.3390/ph14050395
Gutiérrez-Tarriño S, Espino J, Luna-Giles F, Rodríguez AB, Pariente JA, Viñuelas-Zahínos E. Synthesis, Characterization and Antiproliferative Evaluation of Pt(II) and Pd(II) Complexes with a Thiazine-Pyridine Derivative Ligand. Pharmaceuticals. 2021; 14(5):395. https://doi.org/10.3390/ph14050395
Chicago/Turabian StyleGutiérrez-Tarriño, Silvia, Javier Espino, Francisco Luna-Giles, Ana B. Rodríguez, José A. Pariente, and Emilio Viñuelas-Zahínos. 2021. "Synthesis, Characterization and Antiproliferative Evaluation of Pt(II) and Pd(II) Complexes with a Thiazine-Pyridine Derivative Ligand" Pharmaceuticals 14, no. 5: 395. https://doi.org/10.3390/ph14050395
APA StyleGutiérrez-Tarriño, S., Espino, J., Luna-Giles, F., Rodríguez, A. B., Pariente, J. A., & Viñuelas-Zahínos, E. (2021). Synthesis, Characterization and Antiproliferative Evaluation of Pt(II) and Pd(II) Complexes with a Thiazine-Pyridine Derivative Ligand. Pharmaceuticals, 14(5), 395. https://doi.org/10.3390/ph14050395