Screening of Benzimidazole-Based Anthelmintics and Their Enantiomers as Repurposed Drug Candidates in Cancer Therapy
Abstract
1. Introduction
2. Results and Discussion
In Silico Pharmacokinetic Parameters and Target Prediction
3. Materials and Methods
3.1. Cell Cultures and Treatments
3.2. Cell Viability Assay
3.3. IC50 Calculation and Statistical Analysis
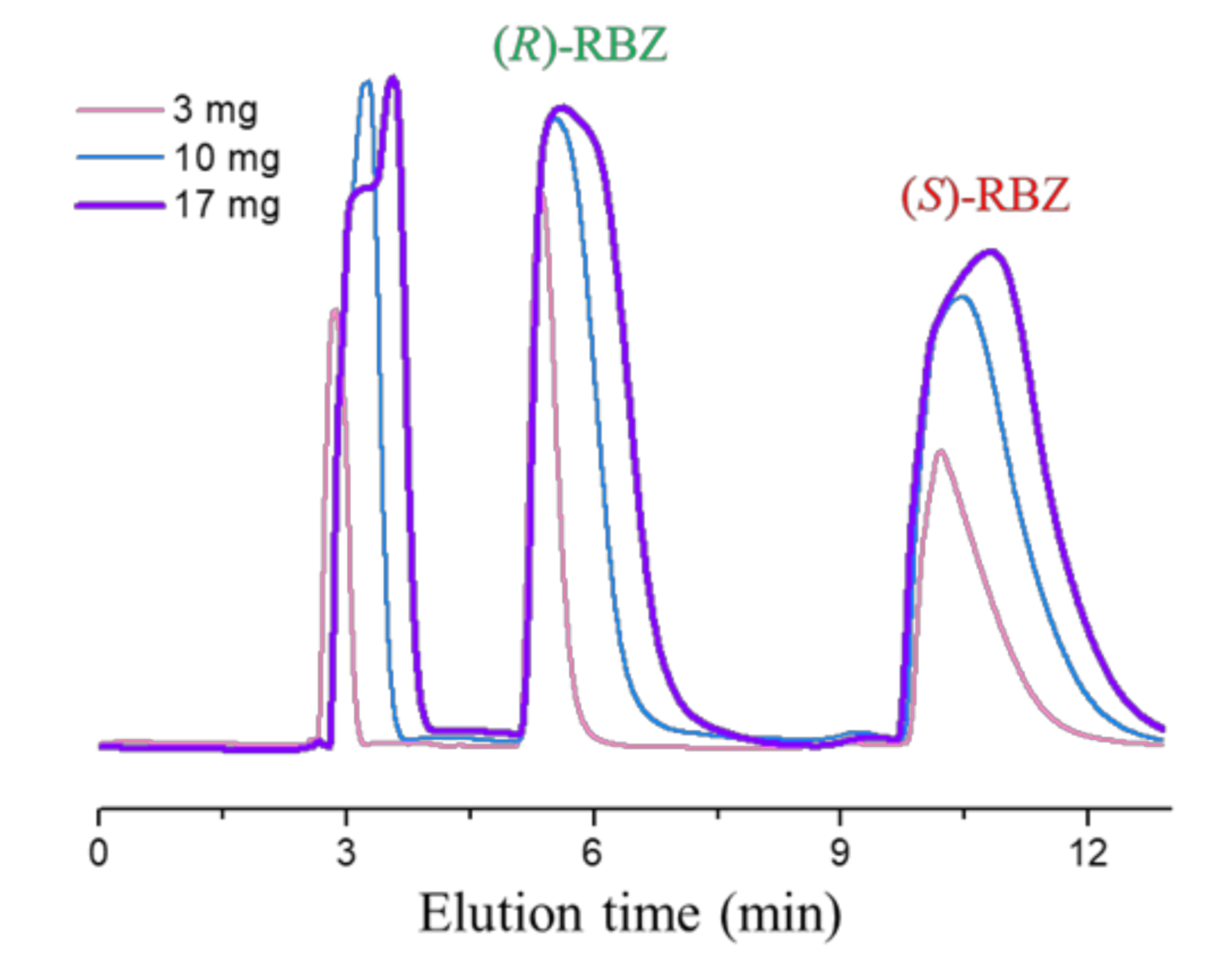
3.4. Enantioselective HPLC
3.5. In silico Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, F.; Sukhatme, V.P.; Bouche, G. Drug repurposing in oncology--patient and health systems opportunities. Nat. Rev. Clin. Oncol. 2015, 12, 732–742. [Google Scholar] [CrossRef]
- Armando, R.G.; Mengual Gómez, D.L.; Gomez, D.E. New drugs are not enough—Drug repositioning in oncology: An update. Int. J. Oncol. 2020, 56, 651–684. [Google Scholar] [CrossRef]
- Hanusova, V.; Skalova, L.; Kralova, V.; Matouskova, P. Potential anti-cancer drugs commonly used for other indications. Curr. Cancer Drug Targets 2015, 15, 35–52. [Google Scholar] [CrossRef]
- Son, D.S.; Lee, E.S.; Adunyah, S.E. The antitumor potentials of benzimidazole anthelmintics as repurposing drugs. Immune Netw. 2020, 20, e29. [Google Scholar] [CrossRef]
- Laudisi, F.; Marônek, M.; Di Grazia, A.; Monteleone, G.; Stolfi, C. Repositioning of anthelmintic drugs for the treatment of cancers of the digestive system. Int. J. Mol. Sci. 2020, 21, 4957. [Google Scholar] [CrossRef]
- Nath, J.; Paul, R.; Ghosh, S.K.; Paul, J.; Singha, B.; Debnath, N. Drug repurposing and relabeling for cancer therapy: Emerging benzimidazole antihelminthics with potent anticancer effects. Life Sci. 2020, 258, 118189. [Google Scholar] [CrossRef]
- Čáňová, K.; Rozkydalová, L.; Vokurková, D.; Rudolf, E. Flubendazole induces mitotic catastrophe and apoptosis in melanoma cells. Toxicol. Vitr. 2018, 46, 313–322. [Google Scholar] [CrossRef]
- He, L.; Shi, L.; Du, Z.; Huang, H.; Gong, R.; Ma, L.; Chen, L.; Gao, S.; Lyu, J.; Gu, H. Mebendazole exhibits potent anti-leukemia activity on acute myeloid leukemia. Exp. Cell Res. 2018, 369, 61–68. [Google Scholar] [CrossRef]
- Spagnuolo, P.A.; Hu, J.; Hurren, R.; Wang, X.; Gronda, M.; Sukhai, M.A.; Di Meo, A.; Boss, J.; Ashali, I.; Beheshti Zavareh, R.; et al. The antihelmintic flubendazole inhibits microtubule function through a mechanism distinct from Vinca alkaloids and displays preclinical activity in leukemia and myeloma. Blood 2010, 115, 4824–4833. [Google Scholar] [CrossRef]
- Hou, Z.J.; Luo, X.; Zhang, W.; Peng, F.; Cui, B.; Wu, S.J.; Zheng, F.M.; Xu, J.; Xu, L.Z.; Long, Z.J.; et al. Flubendazole, FDA-approved anthelmintic, targets breast cancer stem-like cells. Oncotarget 2015, 6, 6326–6340. [Google Scholar] [CrossRef]
- Larsen, A.R.; Bai, R.Y.; Chung, J.H.; Borodovsky, A.; Rudin, C.M.; Riggins, G.J.; Bunz, F. Repurposing the antihelmintic mebendazole as a hedgehog inhibitor. Mol. Cancer Ther. 2015, 14, 3–13. [Google Scholar] [CrossRef]
- Castro, L.S.; Kviecinski, M.R.; Ourique, F.; Parisotto, E.B.; Grinevicius, V.M.; Correia, J.F.; Wilhelm Filho, D.; Pedrosa, R.C. Albendazole as a promising molecule for tumor control. Redox Biol. 2016, 10, 90–99. [Google Scholar] [CrossRef]
- Králová, V.; Hanušová, V.; Rudolf, E.; Cánová, K.; Skálová, L. Flubendazole induces mitotic catastrophe and senescence in colon cancer cells in vitro. J. Pharm. Pharmacol. 2016, 68, 208–218. [Google Scholar] [CrossRef]
- Florio, R.; Veschi, S.; di Giacomo, V.; Pagotto, S.; Carradori, S.; Verginelli, F.; Cirilli, R.; Casulli, A.; Grassadonia, A.; Tinari, N.; et al. The Benzimidazole-Based Anthelmintic Parbendazole: A Repurposed Drug Candidate That Synergizes with Gemcitabine in Pancreatic Cancer. Cancers 2019, 11, 2042. [Google Scholar] [CrossRef]
- Veschi, S.; Ronci, M.; Lanuti, P.; De Lellis, L.; Florio, R.; Bologna, G.; Scotti, L.; Carletti, E.; Brugnoli, F.; Di Bella, M.C.; et al. Integrative proteomic and functional analyses provide novel insights into the action of the repurposed drug candidate nitroxoline in AsPC-1 cells. Sci. Rep. 2020, 10, 2574. [Google Scholar] [CrossRef]
- Shim, J.S.; Liu, J.O. Recent advances in drug repositioning for the discovery of new anticancer drugs. Int. J. Biol. Sci. 2014, 10, 654–663. [Google Scholar] [CrossRef]
- Guerini, A.E.; Triggiani, L.; Maddalo, M.; Bonù, M.L.; Frassine, F.; Baiguini, A.; Alghisi, A.; Tomasini, D.; Borghetti, P.; Pasinetti, N.; et al. Mebendazole as a candidate for drug repurposing in oncology: An extensive review of current literature. Cancers 2019, 11, 1284. [Google Scholar] [CrossRef]
- Dogra, N.; Kumar, A.; Mukhopadhyay, T. Fenbendazole acts as a moderate microtubule destabilizing agent and causes cancer cell death by modulating multiple cellular pathways. Sci. Rep. 2018, 8, e11926. [Google Scholar] [CrossRef] [PubMed]
- Cama, A.; Verginelli, F.; Lotti, L.V.; Napolitano, F.; Morgano, A.; D’Orazio, A.; Vacca, M.; Perconti, S.; Pepe, F.; Romani, F.; et al. Integrative genetic, epigenetic and pathological analysis of paraganglioma reveals complex dysregulation of NOTCH signaling. Acta Neuropathol. 2013, 126, 575–594. [Google Scholar] [CrossRef] [PubMed]
- Florio, R.; De Lellis, L.; di Giacomo, V.; Di Marcantonio, M.C.; Cristiano, L.; Basile, M.; Verginelli, F.; Verzilli, D.; Ammazzalorso, A.; Prasad, S.C.; et al. Effects of PPARα inhibition in head and neck paraganglioma cells. PLoS ONE 2017, 12, e0178995. [Google Scholar] [CrossRef] [PubMed]
- Vural, G.; Yardimci, M.; Kocak, M.; Yasar, T.Ö.; Kurt, A.; Harem, I.S.; Carradori, S.; Sciamanna, I.; Siles-Lucas, M.; Fabiani, M.; et al. Efficacy of novel albendazole salt formulations against secondary cystic echinococcosis in experimentally infected mice. Parasitology 2020, 147, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Priotti, J.; Baglioni, M.V.; García, A.; Rico, M.J.; Leonardi, D.; Lamas, M.C.; Menacho Márquez, M. Repositioning of anti-parasitic drugs in cyclodextrin inclusion complexes for treatment of triple-negative breast cancer. AAPS PharmSciTech. 2018, 19, 3734–3741. [Google Scholar] [CrossRef]
- Casulli, A.; Morales, M.A.; Gallinella, B.; Turchetto, L.; Pozio, E. 2-Hydroxypropyl-beta-cyclodextrin improves the effectiveness of albendazole against encapsulated larvae of Trichinella spiralis in a murine model. J. Antimicrob. Chemother. 2006, 58, 886–890. [Google Scholar] [CrossRef]
- Zimmermann, S.C.; Tichý, T.; Vávra, J.; Dash, R.P.; Slusher, C.E.; Gadiano, A.J.; Wu, Y.; Jančařík, A.; Tenora, L.; Monincová, L.; et al. N-substituted prodrugs of mebendazole provide improved aqueous solubility and oral bioavailability in mice and dogs. J. Med. Chem. 2018, 61, 3918–3929. [Google Scholar] [CrossRef]
- Tabanez, A.M.M.; Nogueira, A.B.; Milani, A.; Eusébio, S.M.E.; Paixão, J.A.; Kabuk, N.H.; Jajuga, M.; Ildiz, O.G.; Fausto, R. Thiabendazole and thiabendazole-formic acid solvate: A computational, crystallographic, spectroscopic and thermal study. Molecules 2020, 25, 3083. [Google Scholar] [CrossRef]
- Mena-Hernández, J.; Jung-Cook, H.; Llaguno-Munive, M.; García-López, P.; Ganem-Rondero, A.; López-Ramírez, S.; Barragán-Aroche, F.; Rivera-Huerta, M.; Mayet-Cruz, L. Preparation and evaluation of mebendazole microemulsion for intranasal delivery: An alternative approach for glioblastoma treatment. AAPS PharmSciTech. 2020, 21, 264. [Google Scholar] [CrossRef]
- Pawluk, S.A.; Roels, C.A.; Wilby, K.J.; Ensom, M.H. A review of pharmacokinetic drug-drug interactions with the anthelmintic medications albendazole and mebendazole. Clin. Pharmacokinet. 2015, 54, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Takayanagui, O.M. Therapy for neurocysticercosis. Expert. Rev. Neurother. 2004, 4, 129–139. [Google Scholar] [CrossRef]
- Materazzo, S.; Carradori, S.; Ferretti, R.; Gallinella, B.; Secci, D.; Cirilli, R. Effect of the water content on the retention and enantioselectivity of albendazole and fenbendazole sulfoxides using amylose-based chiral stationary phases in organic–aqueous conditions. J. Chromatogr. A 2014, 1327, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, R.; Guglielmi, P.; Formica, F.R.; Casulli, A.; Carradori, S. The sodium salt of the enantiomers of ricobendazole: Preparation, solubility and chiroptical properties. J. Pharm. Biomed. Anal. 2017, 139, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, R.; Carradori, S.; Casulli, A.; Pierini, M. A chromatographic study on the retention behavior of the amylose tris(3-chloro-5-methylphenylcarbamate) chiral stationary phase under aqueous conditions. J. Sep. Sci. 2018, 1–8. [Google Scholar] [CrossRef]
- Palmeira, A.; Sousa, E.; Vasconcelos, M.H.; Pinto, M.M. Three decades of P-gp inhibitors: Skimming through several generations and scaffolds. Curr. Med. Chem. 2012, 19, 1946–2025. [Google Scholar] [CrossRef]
- Koul, H.K.; Pall, M.; Koul, S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer 2013, 4, 342–359. [Google Scholar] [CrossRef]
- Yang, K.; Wang, F.; Zhang, H.; Wang, X.; Chen, L.; Su, X.; Wu, X.; Han, Q.; Chen, Z.; Chen, Z.S.; et al. Target inhibition of CBP induced cell senescence in BCR-ABL-T315I mutant chronic myeloid leukemia. Front. Oncol. 2021, 10, 588641. [Google Scholar] [CrossRef]
- Ozkan, E.; Bakar-Ates, F. The trinity of matrix metalloproteinases, inflammation, and cancer: A literature review of recent updates. Antiinflamm. Antiallergy Agents Med. Chem. 2020, 19, 206–221. [Google Scholar] [CrossRef]
- Veschi, S.; De Lellis, L.; Florio, R.; Lanuti, P.; Massucci, A.; Tinari, N.; De Tursi, M.; di Sebastiano, P.; Marchisio, M.; Natoli, C.; et al. Effects of repurposed drug candidates nitroxoline and nelfinavir as single agents or in combination with erlotinib in pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2018, 37, 236. [Google Scholar] [CrossRef]
- Ammazzalorso, A.; De Lellis, L.; Florio, R.; Bruno, I.; De Filippis, B.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Perconti, S.; Verginelli, F.; et al. Cytotoxic effect of a family of peroxisome proliferator-activated receptor antagonists in colorectal and pancreatic cancer cell lines. Chem. Biol. Drug Des. 2017, 90, 1029–1035. [Google Scholar] [CrossRef]
- Ammazzalorso, A.; De Lellis, L.; Florio, R.; Laghezza, A.; De Filippis, B.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Tortorella, P.; Veschi, S.; et al. Synthesis of novel benzothiazole amides: Evaluation of PPAR activity and anti-proliferative effects in paraganglioma, pancreatic and colorectal cancer cell lines. Bioorg. Med. Chem. Lett. 2019, 29, 2302–2306. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, R.; Carradori, S.; Casulli, A.; De Monte, C. WO2017021992–Salts of Compounds Having a Benzimidazolic Structure, Uses and Process for the Preparation Thereof. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017021992 (accessed on 21 March 2021).
- Veschi, S.; Carradori, S.; De Lellis, L.; Florio, R.; Brocco, D.; Secci, D.; Guglielmi, P.; Spano, M.; Sobolev, A.P.; Cama, A. Synthesis and evaluation of a large library of nitroxoline derivatives as pancreatic cancer antiproliferative agents. J. Enzym. Inhib. Med. Chem. 2020, 35, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
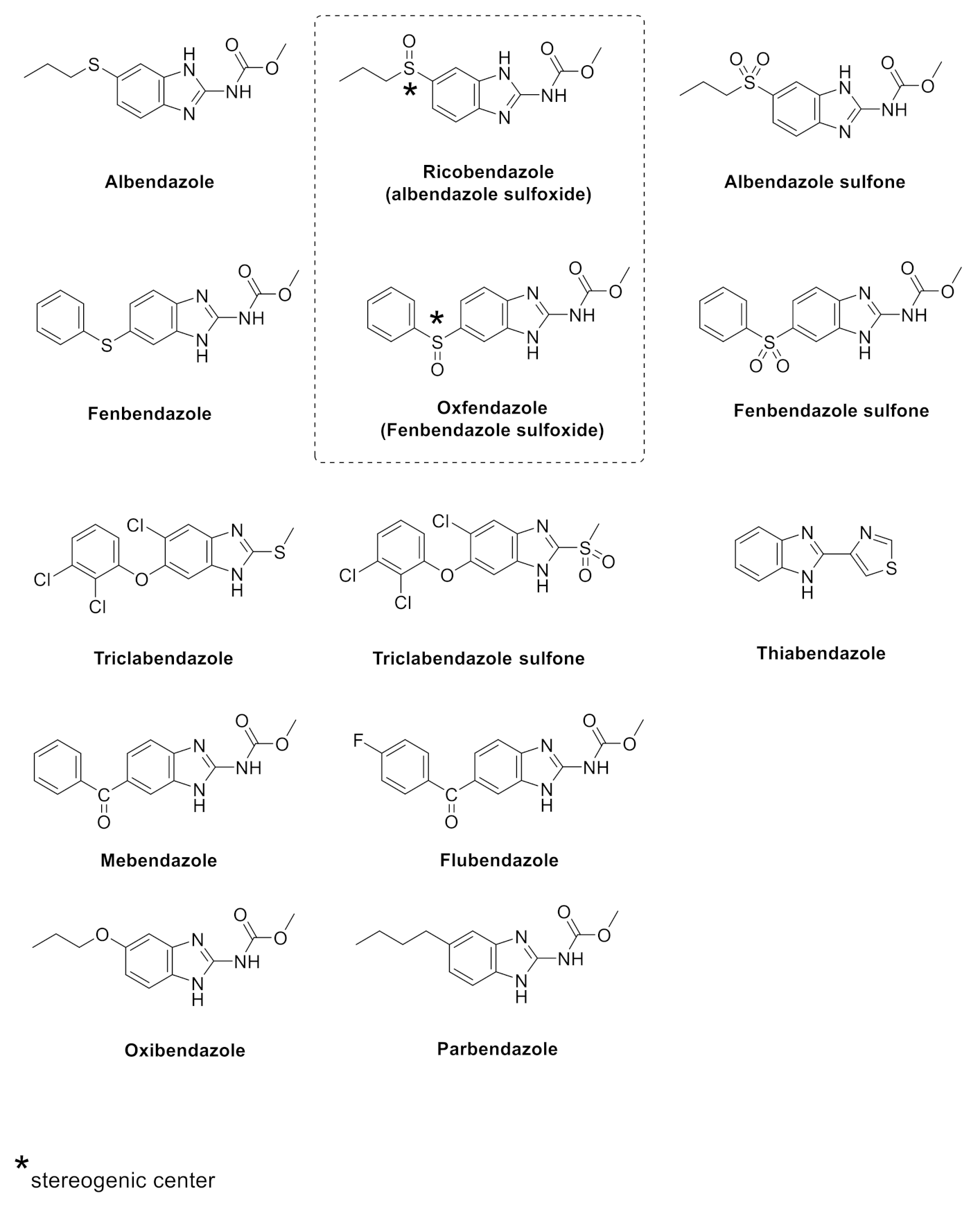
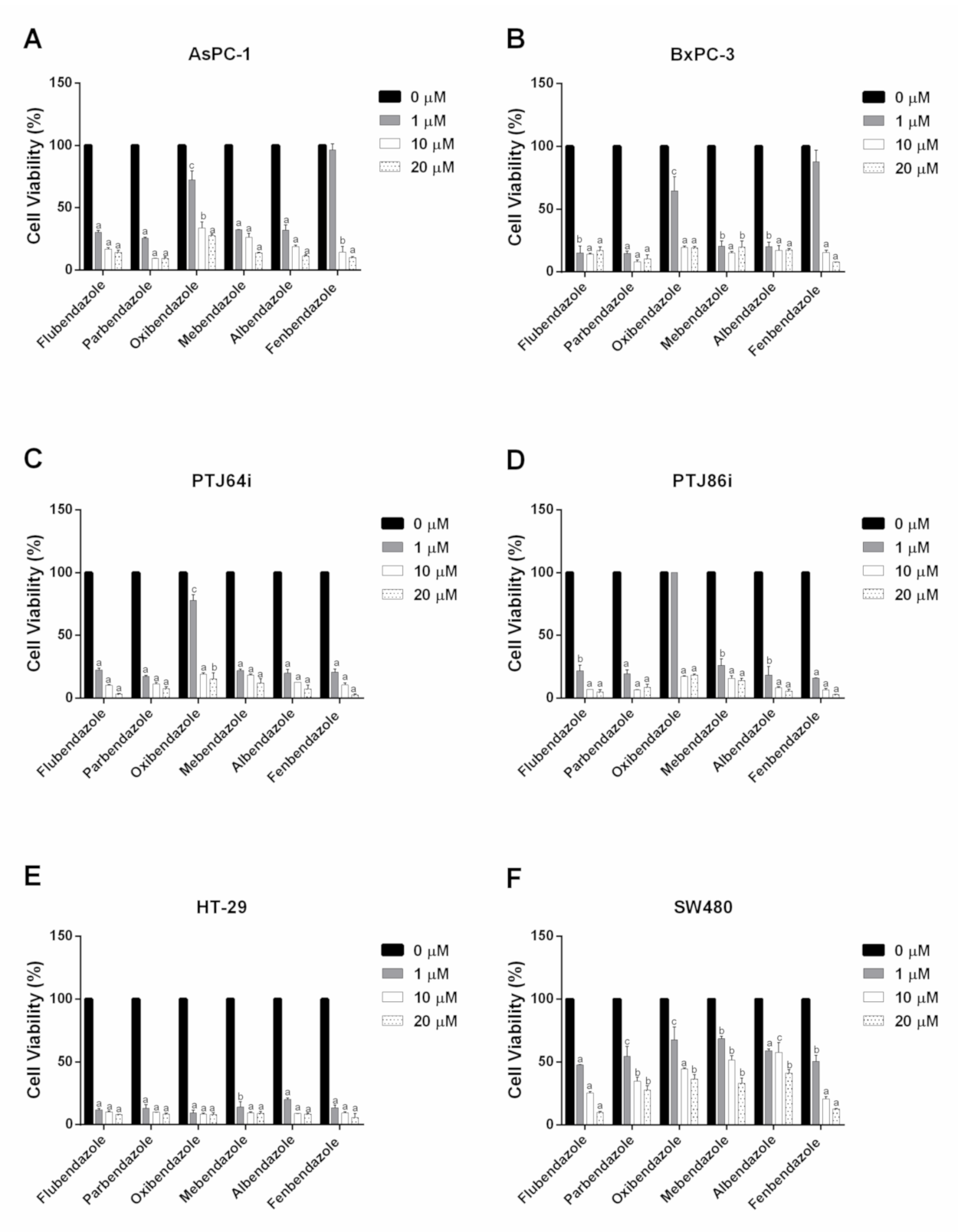

| Compound | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| Pancreatic Cancer | Paraganglioma | Colorectal Cancer | ||||
| AsPC-1 | BxPC-3 | PTJ64i | PTJ86i | HT-29 | SW480 | |
| Flubendazole | 0.23 | 0.01 | 0.14 | 0.25 | 0.01 | 0.67 |
| Parbendazole | 0.58 | 0.03 | 0.04 | 0.17 | 0.01 | 0.57 |
| Oxibendazole | 1.45 | 1.07 | 1.79 | 3.29 | 0.01 | 0.96 |
| Mebendazole | 0.08 | 0.40 | 0.01 | 0.19 | 0.08 | 1.26 |
| Albendazole | 0.19 | 0.10 | 0.05 | 0.29 | 0.28 | 0.17 |
| Fenbendazole | 3.26 | 2.66 | 0.10 | 0.15 | 0.02 | 0.78 |
| Compound | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| Pancreatic Cancer | Paraganglioma | Colorectal Cancer | ||||
| AsPC-1 | BxPC-3 | PTJ64i | PTJ86i | HT-29 | SW480 | |
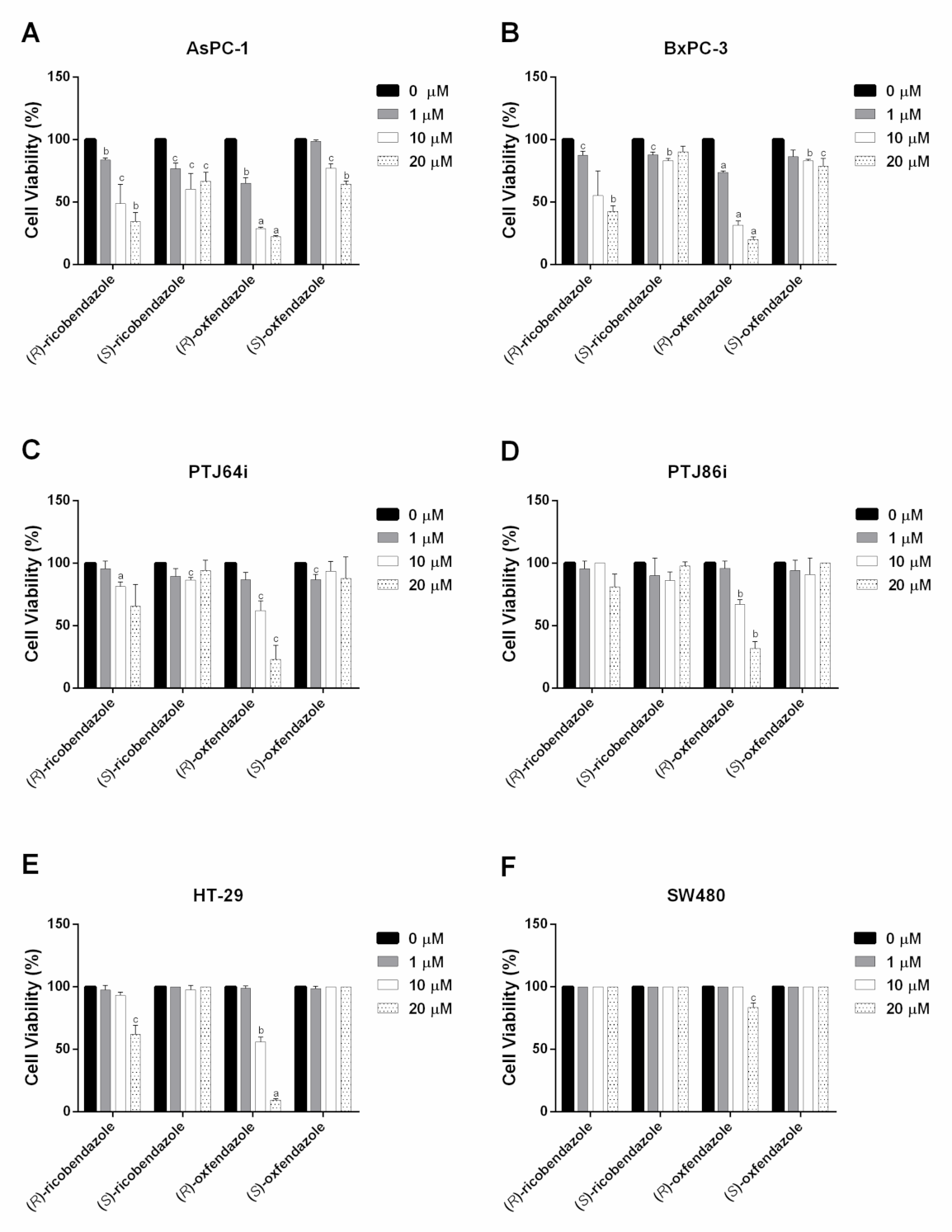
| (R)-ricobendazole | 9.09 | 13.6 | >20 | >20 | >20 | >20 |
| (S)-ricobendazole | >20 | >20 | >20 | >20 | >20 | >20 |
| (R)-oxfendazole | 1.18 | 1.82 | 10.02 | 12.41 | 10.02 | >20 |
| (S)-oxfendazole | >20 | >20 | >20 | >20 | >20 | >20 |
| Physicochemical Properties | FLU | PAR | OXI | MEB | ALB | FEN |
|---|---|---|---|---|---|---|
| Molecular Weight (MW) | 313.28 | 247.29 | 249.27 | 295.29 | 265.33 | 299.35 |
| H-Bond Acceptors (HBA) | 5 | 3 | 4 | 4 | 3 | 3 |
| H-Bond Donators (HBD) | 2 | 2 | 2 | 2 | 2 | 2 |
| Consensus Log P * | 2.56 | 2.52 | 1.85 | 2.26 | 2.29 | 2.91 |
| Lipinski Violations | 0 | 0 | 0 | 0 | 0 | 0 |
| GI Absorption | High | High | High | High | High | High |
| P-gp Substrate | No | No | No | No | No | No |
| PAINS Alerts | 0 | 0 | 0 | 0 | 0 | 0 |
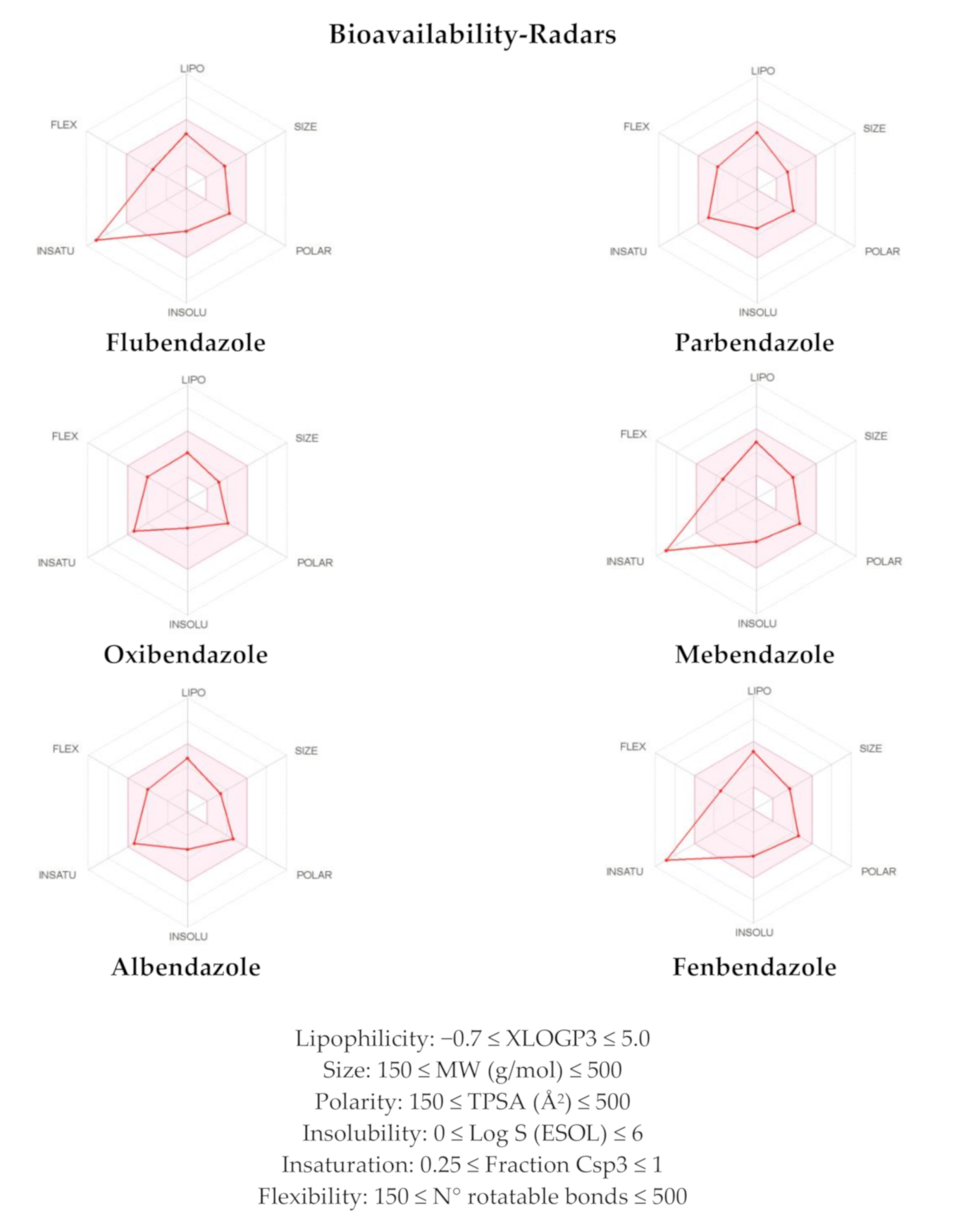
| Cmpd | WLOGP a | TPSA (Å2) a,b | XLOGP3 b | Log S (ESOL) b | MW b | Csp3 b | No. of Rotatable Bonds b |
|---|---|---|---|---|---|---|---|
| FLU | 3.34 | 84.08 | 2.84 | −3.72 | 313.28 | 0.06 | 5 |
| PAR | 2.89 | 67.01 | 3.28 | −3.41 | 247.29 | 0.38 | 6 |
| OXI | 2.34 | 76.24 | 2.27 | −2.79 | 249.27 | 0.33 | 6 |
| MEB | 2.78 | 84.08 | 3.01 | −3.74 | 295.29 | 0.06 | 5 |
| ALB | 3.05 | 92.31 | 2.81 | −3.23 | 265.33 | 0.33 | 6 |
| FEN | 3.70 | 92.31 | 3.47 | −4.08 | 299.35 | 0.07 | 5 |
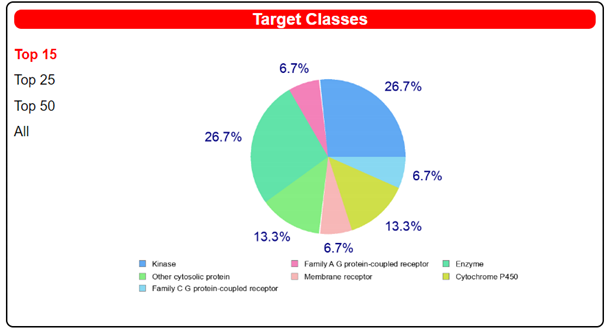 | |||
|---|---|---|---|
| Target | Common Name | Target Class | Probability |
| MAP kinase p38 alpha | MAPK14 | Kinase | 1 |
| Adenosine A2a receptor | ADORA2A | Family A G protein-coupled receptor | 0.13079 |
| 1-acylglycerol-3-phosphate O-acyltransferase beta | AGPAT2 | Enzyme | 0.09787 |
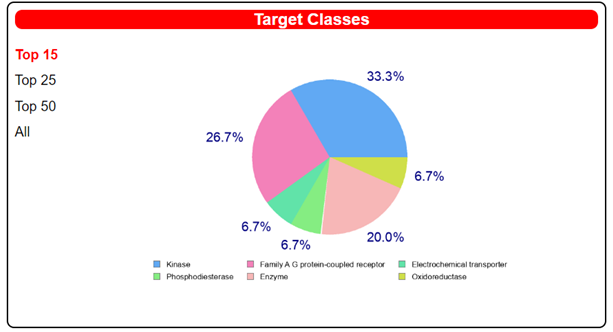 | |||
|---|---|---|---|
| Target | Common Name | Target Class | Probability |
| Tyrosine-protein kinase ABL | ABL1 | Kinase | 1 |
| Vascular endothelial growth factor receptor 2 | KDR | Kinase | 1 |
| Adenosine A2a receptor | ADORA2A | Family A G protein-coupled receptor | 0.1194 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florio, R.; Carradori, S.; Veschi, S.; Brocco, D.; Di Genni, T.; Cirilli, R.; Casulli, A.; Cama, A.; De Lellis, L. Screening of Benzimidazole-Based Anthelmintics and Their Enantiomers as Repurposed Drug Candidates in Cancer Therapy. Pharmaceuticals 2021, 14, 372. https://doi.org/10.3390/ph14040372
Florio R, Carradori S, Veschi S, Brocco D, Di Genni T, Cirilli R, Casulli A, Cama A, De Lellis L. Screening of Benzimidazole-Based Anthelmintics and Their Enantiomers as Repurposed Drug Candidates in Cancer Therapy. Pharmaceuticals. 2021; 14(4):372. https://doi.org/10.3390/ph14040372
Chicago/Turabian StyleFlorio, Rosalba, Simone Carradori, Serena Veschi, Davide Brocco, Teresa Di Genni, Roberto Cirilli, Adriano Casulli, Alessandro Cama, and Laura De Lellis. 2021. "Screening of Benzimidazole-Based Anthelmintics and Their Enantiomers as Repurposed Drug Candidates in Cancer Therapy" Pharmaceuticals 14, no. 4: 372. https://doi.org/10.3390/ph14040372
APA StyleFlorio, R., Carradori, S., Veschi, S., Brocco, D., Di Genni, T., Cirilli, R., Casulli, A., Cama, A., & De Lellis, L. (2021). Screening of Benzimidazole-Based Anthelmintics and Their Enantiomers as Repurposed Drug Candidates in Cancer Therapy. Pharmaceuticals, 14(4), 372. https://doi.org/10.3390/ph14040372