Contribution of Pro-Inflammatory Molecules Induced by Respiratory Virus Infections to Neurological Disorders
Abstract
1. Introduction
2. Neuroinflammation Induced by Respiratory Viruses
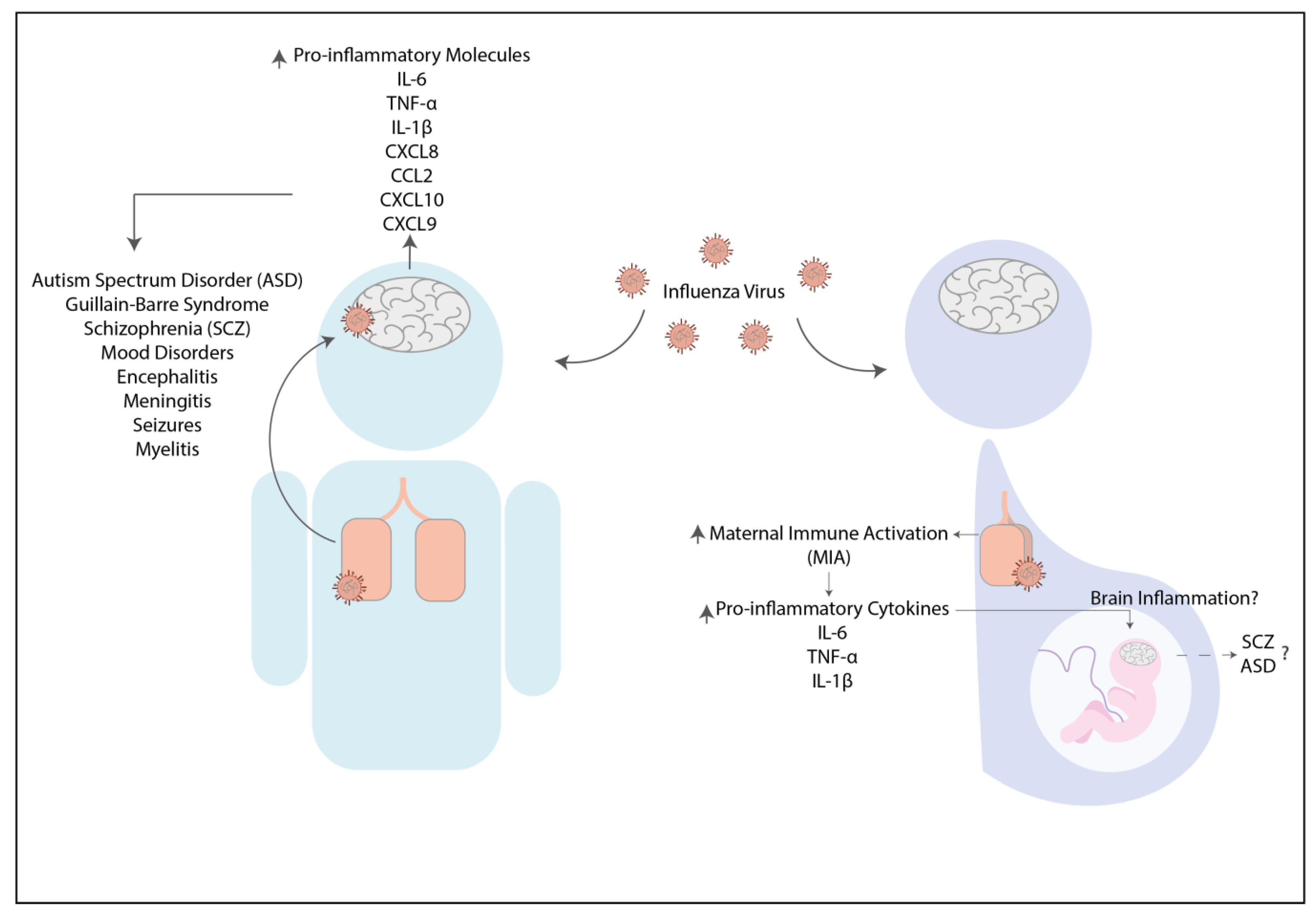
2.1. Influenza Virus
2.2. Human Respiratory Syncytial Virus
2.3. Severe Acute Respiratory Syndrome Coronavirus 2

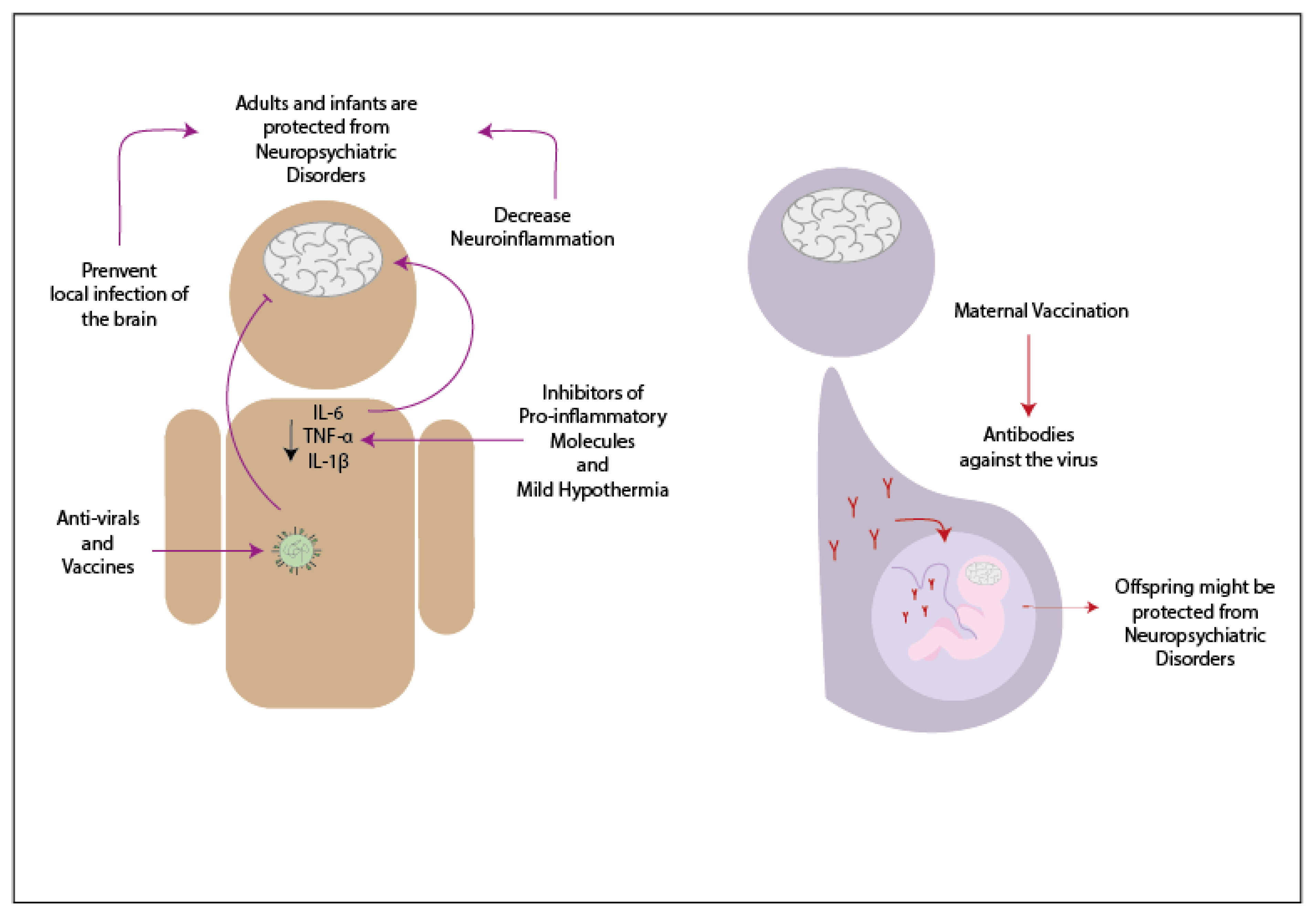
3. Potential Treatments to Decrease Neurologic Symptoms Caused by Infections
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cavaillon, J.M. Pro- versus anti-inflammatory cytokines: Myth or reality. Cell. Mol. Biol. 2001, 47, 695–702. [Google Scholar] [PubMed]
- Chi, H.; Barry, S.P.; Roth, R.J.; Wu, J.J.; Jones, E.A.; Bennett, A.M.; Flavell, R.A. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 2274–2279. [Google Scholar] [CrossRef]
- Galic, M.A.; Riazi, K.; Pittman, Q.J. Cytokines and brain excitability. Front. Neuroendocrinol. 2012, 33, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Kronfol, Z.; Remick, D.G. Cytokines and the brain: Implications for clinical psychiatry. Am. J. Psychiatry 2000, 157, 683–694. [Google Scholar] [CrossRef]
- Donzis, E.J.; Tronson, N.C. Modulation of learning and memory by cytokines: Signaling mechanisms and long term consequences. Neurobiol. Learn. Mem. 2014, 115, 68–77. [Google Scholar] [CrossRef] [PubMed]
- McAfoose, J.; Baune, B.T. Evidence for a cytokine model of cognitive function. Neurosci. Biobehav. Rev. 2009, 33, 355–366. [Google Scholar] [CrossRef]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain. Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Beattie, E.C.; Stellwagen, D.; Morishita, W.; Bresnahan, J.C.; Byeong, K.H.; Von Zastrow, M.; Beattie, M.S.; Malenka, R.C. Control of synaptic strength by glial TNFα. Science 2002, 295, 2282–2285. [Google Scholar] [CrossRef] [PubMed]
- Balschun, D.; Wetzel, W.; Rey, A.; Pitossi, F.; Schneider, H.; Zuschratter, W.; Besedovsky, H.O. Interleukin-6: A cytokine to forget. FASEB J. 2004, 18, 1788–1790. [Google Scholar] [CrossRef]
- Goshen, I.; Kreisel, T.; Ounallah-Saad, H.; Renbaum, P.; Zalzstein, Y.; Ben-Hur, T.; Levy-Lahad, E.; Yirmiya, R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 2007, 32, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.M.; Walsh, C.; Delaney, A.; Nolan, Y.; Campbell, V.A.; Lynch, M.A. Lipopolysaccharide-induced increase in signalling in hippocampus is abrogated by IL-10—A role for IL-1β? J. Neurochem. 2004, 88, 635–646. [Google Scholar] [CrossRef]
- Wilson, C.J.; Finch, C.E.; Cohen, H.J. Cytokines and cognition—The case for a head-to-toe inflammatory paradigm. J. Am. Geriatr. Soc. 2002, 50, 2041–2056. [Google Scholar] [CrossRef] [PubMed]
- Stellwagen, D.; Malenka, R.C. Synaptic scaling mediated by glial TNF-α. Nature 2006, 440, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.; Sauder, C.; Czub, S.; Bachmann, E.; Schimmer, S.; Hegyi, A.; Czub, M. Activation of microglia cells is dispensable for the induction of rat retroviral spongiform encephalopathy. J. Neurovirol. 2001, 7, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.E.; Hao, C.; Manos, J.; Ransohoff, R.M.; Gruol, D.L. Altered hippocampal synaptic transmission in transgenic mice with astrocyte-targeted enhanced CCL2 expression. Brain Behav. Immun. 2011, 25, S106–S119. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Morganti, J.M.; Bachstetter, A.D.; Hudson, C.E.; Peters, M.M.; Grimmig, B.A.; Weeber, E.J.; Bickford, P.C.; Gemma, C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. 2011, 31, 16241–16250. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, G.K.; Wdowicz, A.; Pickering, M.; Watters, O.; Halley, P.; O’Sullivan, N.C.; Mooney, C.; O’Connell, D.J.; O’Connor, J.J.; Murphy, K.J. CX3CL1 is up-regulated in the rat hippocampus during memory-associated synaptic plasticity. Front. Cell. Neurosci. 2014, 8, 233. [Google Scholar] [CrossRef]
- Rostène, W.; Dansereau, M.A.; Godefroy, D.; Van Steenwinckel, J.; Goazigo, A.R.L.; Mélik-Parsadaniantz, S.; Apartis, E.; Hunot, S.; Beaudet, N.; Sarret, P. Neurochemokines: A menage a trois providing new insights on the functions of chemokines in the central nervous system. J. Neurochem. 2011, 118, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, H.; Liu, J.; Dong, J.; Xiong, H. Chemokine CCL2 modulation of neuronal excitability and synaptic transmission in rat hippocampal slices. J. Neurochem. 2011, 116, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Lauro, C.; Catalano, M.; Trettel, F.; Limatola, C. Fractalkine in the nervous system: Neuroprotective or neurotoxic molecule? Ann. N. Y. Acad. Sci. 2015, 1351, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Homberg, J.R.; Kyzar, E.J.; Nguyen, M.; Norton, W.H.; Pittman, J.; Poudel, M.K.; Gaikwad, S.; Nakamura, S.; Koshiba, M.; Yamanouchi, H.; et al. Understanding autism and other neurodevelopmental disorders through experimental translational neurobehavioral models. Neurosci. Biobehav. Rev. 2016, 65, 292–312. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F.; Posthuma, D. Biological pathways and networks implicated in psychiatric disorders. Curr. Opin. Behav. Sci. 2015. [Google Scholar] [CrossRef]
- Okusaga, O.; Yolken, R.H.; Langenberg, P.; Lapidus, M.; Arling, T.A.; Dickerson, F.B.; Scrandis, D.A.; Severance, E.; Cabassa, J.A.; Balis, T.; et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J. Affect. Disord. 2011, 130, 220–225. [Google Scholar] [CrossRef]
- Kieling, C.; Baker-Henningham, H.; Belfer, M.; Conti, G.; Ertem, I.; Omigbodun, O.; Rohde, L.A.; Srinath, S.; Ulkuer, N.; Rahman, A. Child and adolescent mental health worldwide: Evidence for action. Lancet 2011, 378, 1515–1525. [Google Scholar] [CrossRef]
- Tomonaga, K. Virus-induced neurobehavioral disorders: Mechanisms and implications. Trends Mol. Med. 2004, 10, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Fineberg, A.M.; Ellman, L.M. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol. Psychiatry 2013, 73, 951–966. [Google Scholar] [CrossRef]
- Rizzo, F.R.; Musella, A.; De Vito, F.; Fresegna, D.; Bullitta, S.; Vanni, V.; Guadalupi, L.; Stampanoni Bassi, M.; Buttari, F.; Mandolesi, G.; et al. Tumor Necrosis Factor and Interleukin-1β Modulate Synaptic Plasticity during Neuroinflammation. Neural Plast. 2018, 2018, 8430123. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the etiological links behind neurodegenerative diseases: Inflammatory cytokines and bioactive kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef]
- Wu, Y.; Kang, R.; Yan, Y.; Gao, K.; Li, Z.; Jiang, J.; Chi, X.; Xia, L. Epidemiology of schizophrenia and risk factors of schizophrenia-associated aggression from 2011 to 2015. J. Int. Med. Res. 2018, 46, 4039–4049. [Google Scholar] [CrossRef]
- Cannon, T.D. How Schizophrenia Develops: Cognitive and Brain Mechanisms Underlying Onset of Psychosis. Trends Cogn. Sci. 2015, 19, 744–756. [Google Scholar] [CrossRef]
- Vita, A.; De Peri, L.; Deste, G.; Sacchetti, E. Progressive loss of cortical gray matter in schizophrenia: A meta-analysis and meta-regression of longitudinal MRI studies. Transl. Psychiatry 2012, 2, e190. [Google Scholar] [CrossRef] [PubMed]
- Gur, R.E.; Turetsky, B.I.; Bilker, W.B.; Gur, R.C. Reduced gray matter volume in schizophrenia. Arch. Gen. Psychiatry 1999, 56, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Nadira, K. First episode schizophrenia: Review. South Afr. Fam. Pract. 2015, 57, 29–33. [Google Scholar] [CrossRef]
- Fond, G.; Lançon, C.; Korchia, T.; Auquier, P.; Boyer, L. The Role of Inflammation in the Treatment of Schizophrenia. Front. Psychiatry 2020, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhou, D.F.; Cao, L.Y.; Zhang, P.Y.; Wu, G.Y.; Shen, Y.C. Changes in serum interleukin-2, -6, and -8 levels before and during treatment with risperidone and haloperidol: Relationship to outcome in schizophrenia. J. Clin. Psychiatry 2004, 65, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Kenis, G.; Bignotti, S.; Tura, G.J.B.; De Jong, R.; Bosmans, E.; Pioli, R.; Altamura, C.; Scharpé, S.; Maes, M. The inflammatory response system in treatment-resistant schizophrenia: Increased serum interleukin-6. Schizophr. Res. 1998, 32, 9–15. [Google Scholar] [CrossRef]
- Allswede, D.M.; Cannon, T.D. Prenatal inflammation and risk for schizophrenia: A role for immune proteins in neurodevelopment. Dev. Psychopathol. 2018, 30, 1157–1178. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Zare-Shahabadi, A.; Rezaei, N.; Garcia-Gutierrez, M.S.; Schiavone, S.; Arsenijevic, N.N. Cytokine Alterations in Schizophrenia: An Updated Review. Front. Psychiatry 2019, 10, 892. [Google Scholar] [CrossRef]
- Won, H.; Mah, W.; Kim, E. Autism spectrum disorder causes, mechanisms, and treatments: Focus on neuronal synapses. Front. Mol. Neurosci. 2013, 6, 19. [Google Scholar] [CrossRef]
- Libbey, J.E.; Sweeten, T.L.; McMahon, W.M.; Fujinami, R.S. Autistic disorder and viral infections. J. Neurovirol. 2005, 11, 1–10. [Google Scholar] [CrossRef]
- Jones, K.L.; Croen, L.A.; Yoshida, C.K.; Heuer, L.; Hansen, R.; Zerbo, O.; Delorenze, G.N.; Kharrazi, M.; Yolken, R.; Ashwood, P.; et al. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol. Psychiatry 2017, 22, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Paraschivescu, C.; Barbosa, S.; Lorivel, T.; Glaichenhaus, N.; Davidovicid, L.; Davidovic, L. Cytokine changes associated with the maternal immune activation (mia) model of autism: A penalized regression approach. PLoS ONE 2020, 15, e0231609. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; Breen, E.J.; Alvares, G.A.; Glozier, N.; Hickie, I.B.; Hunt, A.; Hui, J.; Beilby, J.; Ravine, D.; Wray, J.; et al. Cytokine levels and associations with symptom severity in male and female children with autism spectrum disorder. Mol. Autism 2017, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; Glozier, N.; Dale, R.; Guastella, A.J. The Immune System, Cytokines, and Biomarkers in Autism Spectrum Disorder. Neurosci. Bull. 2017, 33, 194–204. [Google Scholar] [CrossRef]
- Kordulewska, N.K.; Kostyra, E.; Piskorz-Ogórek, K.; Moszyńska, M.; Cieślińska, A.; Fiedorowicz, E.; Jarmołowska, B. Serum cytokine levels in children with spectrum autism disorder: Differences in pro- and anti-inflammatory balance. J. Neuroimmunol. 2019, 337, 577066. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Le, H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J. Neuroimmunol. 2001, 120, 170–179. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I.; Patel, A.B.; Doyle, R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl. Psychiatry 2016, 6, 844. [Google Scholar] [CrossRef] [PubMed]
- Atluri, V.S.R.; Hidalgo, M.; Samikkannu, T.; Kurapati, K.R.V.; Nair, M. Synaptic Plasticity and Neurological Disorders in Neurotropic Viral Infections. Neural Plast. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Benros, M.E.; Sørensen, H.J.; Nielsen, P.R.; Nordentoft, M.; Mortensen, P.B.; Petersen, L. The association between infections and general cognitive ability in young men—A nationwide study. PLoS ONE 2015, 10, e0124005. [Google Scholar] [CrossRef]
- Koyuncu, O.O.; Hogue, I.B.; Enquist, L.W. Virus infections in the nervous system. Cell Host Microbe 2013, 13, 379–393. [Google Scholar] [CrossRef]
- Mori, I.; Nishiyama, Y.; Yokochi, T.; Kimura, Y. Olfactory transmission of neurotropic viruses. J. Neurovirol. 2005, 11, 129–137. [Google Scholar] [CrossRef]
- Obermeier, B.; Verma, A.; Ransohoff, R.M. The blood-brain barrier. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 133, pp. 39–59. ISBN 9780444634320. [Google Scholar]
- Rhea, E.M.; Banks, W.A. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front. Neurosci. 2019, 13, 521. [Google Scholar] [CrossRef]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood–brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217, 217. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Russo, M.V.; McGavern, D.B. Immune Surveillance of the CNS following Infection and Injury. Trends Immunol. 2015, 36, 637–650. [Google Scholar] [CrossRef]
- Negi, N.; Das, B.K. CNS: Not an immunoprivilaged site anymore but a virtual secondary lymphoid organ. Int. Rev. Immunol. 2018, 37, 57–68. [Google Scholar] [CrossRef]
- Ousman, S.S.; Kubes, P. Immune surveillance in the central nervous system. Nat. Neurosci. 2012, 15, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Kivisäkk, P.; Mahad, D.J.; Callahan, M.K.; Trebst, C.; Tucky, B.; Wei, T.; Wu, L.; Baekkevold, E.S.; Lassmann, H.; Staugaitis, S.M.; et al. Human cerebrospinal fluid central memory CD4+ T cells: Evidence for trafficking through choroid plexus and meninges via P-selectin. Proc. Natl. Acad. Sci. USA 2003, 100, 8389–8394. [Google Scholar] [CrossRef] [PubMed]
- Hatterer, E.; Davoust, N.; Didier-Bazes, M.; Vuaillat, C.; Malcus, C.; Belin, M.F.; Nataf, S. How to drain without lymphatics? Dendritic cells migrate from the cerebrospinal fluid to the B-cell follicles of cervical lymph nodes. Blood 2006, 107, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Nakajima, K. Role of the Immune System in the Development of the Central Nervous System. Front. Neurosci. 2019, 13, 916. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Zu, H. bing Microglial polarization: Novel therapeutic mechanism against Alzheimer’s disease. Inflammopharmacology 2020, 28, 95–110. [Google Scholar] [CrossRef]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.A.; Bohmwald, K.; Cespedes, P.F.; Gomez, R.S.; Riquelme, S.A.; Cortes, C.M.; Valenzuela, J.A.; Sandoval, R.A.; Pancetti, F.C.; Bueno, S.M.; et al. Impaired learning resulting from Respiratory Syncytial Virus infection. Proc. Natl. Acad. Sci. USA 2013, 110, 9112–9117. [Google Scholar] [CrossRef]
- Bohmwald, K.; Soto, J.A.; Andrade-Parra, C.; Fernández-Fierro, A.; Espinoza, J.A.; Ríos, M.; Eugenin, E.A.; González, P.A.; Opazo, M.C.; Riedel, C.A.; et al. Lung pathology due to hRSV infection impairs blood–brain barrier permeability enabling astrocyte infection and a long-lasting inflammation in the CNS. Brain Behav. Immun. 2021, 91, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Bohmwald, K.; Gálvez, N.M.S.; Ríos, M.; Kalergis, A.M. Neurologic Alterations Due to Respiratory Virus Infections. Front. Cell. Neurosci. 2018, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Baselga-Moreno, V.; Trushakova, S.; McNeil, S.; Sominina, A.; Nunes, M.C.; Draganescu, A.; Unal, S.; Koul, P.; Kyncl, J.; Zhang, T.; et al. Influenza epidemiology and influenza vaccine effectiveness during the 2016-2017 season in the Global Influenza Hospital Surveillance Network (GIHSN). BMC Public Health 2019, 19, 487. [Google Scholar] [CrossRef]
- Barbati, F.; Moriondo, M.; Pisano, L.; Calistri, E.; Lodi, L.; Ricci, S.; Giovannini, M.; Canessa, C.; Indolfi, G.; Azzari, C. Epidemiology of respiratory syncytial virus-related hospitalization over a 5-year period in Italy: Evaluation of seasonality and age distribution before vaccine introduction. Vaccines 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Earle, J.; Kanodia, R.; Kist, D.; Emamian, E.S.; Patterson, P.H.; Shi, L.; Sidwell, R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: Implications for genesis of autism and schizophrenia. Cell. Mol. Neurobiol. 2002, 22, 25–33. [Google Scholar] [CrossRef]
- Moreno, J.L.; Kurita, M.; Holloway, T.; López, J.; Cadagan, R.; Martínez-Sobrido, L.; García-Sastre, A.; González-Maeso, J. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT2Aand mGlu2 receptors in the adult offspring. J. Neurosci. 2011, 31, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Peña, M.; Jara, C.; Flores, J.C.; Hoyos-Bachiloglu, R.; Iturriaga, C.; Medina, M.; Carcey, J.; Espinoza, J.; Bohmwald, K.; Kalergis, A.M.; et al. Severe respiratory disease caused by human respiratory syncytial virus impairs language learning during early infancy. Sci. Rep. 2020, 10, 22356. [Google Scholar] [CrossRef]
- Guadarrama-Ortiz, P.; Choreño-Parra, J.A.; Sánchez-Martínez, C.M.; Pacheco-Sánchez, F.J.; Rodríguez-Nava, A.I.; García-Quintero, G. Neurological Aspects of SARS-CoV-2 Infection: Mechanisms and Manifestations. Front. Neurol. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, D.; van de Pasch, S.; Doets, A.Y.; Jacobs, B.C.; van Vliet, J.; Garssen, M.P.J. Guillain–Barré syndrome after SARS-CoV-2 infection. Eur. J. Neurol. 2020, 27, 1757–1758. [Google Scholar] [CrossRef] [PubMed]
- Szcześniak, D.; Gładka, A.; Misiak, B.; Cyran, A.; Rymaszewska, J. The SARS-CoV-2 and mental health: From biological mechanisms to social consequences. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 28, 110046. [Google Scholar] [CrossRef] [PubMed]
- El Ramahi, R.; Freifeld, A. Epidemiology, diagnosis, treatment, and prevention of influenza infection in oncology patients. J. Oncol. Pract. 2019, 15, 177–184. [Google Scholar] [CrossRef]
- Resa-Infante, P.; Recuero-Checa, M.Á.; Zamarreño, N.; Llorca, Ó.; Ortín, J. Structural and Functional Characterization of an Influenza Virus RNA Polymerase-Genomic RNA Complex. J. Virol. 2010, 84, 10477–10487. [Google Scholar] [CrossRef] [PubMed]
- Asha, K.; Kumar, B. Emerging Influenza D Virus Threat: What We Know so Far! J. Clin. Med. 2019, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26, D49. [Google Scholar] [CrossRef] [PubMed]
- Louten, J. Influenza Viruses. In Essential Human Virology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 171–191. [Google Scholar]
- Uyeki, T.M.; Peiris, M. Novel Avian Influenza A Virus Infections of Humans. Infect. Dis. Clin. N. A. 2019, 33, 907–932. [Google Scholar] [CrossRef] [PubMed]
- Gounder, A.P.; Boon, A.C.M. Influenza Pathogenesis: The Effect of Host Factors on Severity of Disease. J. Immunol. 2019, 202, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care 2019, 23, 258. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.P.; Teixeira, M.M.; Garcia, C.C. The inflammatory response triggered by Influenza virus: A two edged sword. Inflamm. Res. 2017, 66, 283–302. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Goraya, M.U.; Maarouf, M.; Huang, S.; Chen, J.L. Host immune response to influenza A virus infection. Front. Immunol. 2018, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.E.; Lee, H.K. Host protective immune Responses against influenza a virus infection. Viruses 2020, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, A.; Lee, S.H.; Wang, S.M.; Manikam, R.; Rajarajeswaran, J.; Raju, C.S.; Sekaran, S.D. Immune responses to influenza virus and its correlation to age and inherited factors. Front. Microbiol. 2016, 7, 1841. [Google Scholar] [CrossRef] [PubMed]
- Schmolke, M.; García-Sastre, A. Evasion of innate and adaptive immune responses by influenza A virus. Cell. Microbiol. 2010, 12, 873–880. [Google Scholar] [CrossRef]
- Wang, G.F.; Li, W.; Li, K. Acute encephalopathy and encephalitis caused by influenza virus infection. Curr. Opin. Neurol. 2010, 23, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Goenka, A.; Michael, B.D.; Ledger, E.; Hart, I.J.; Absoud, M.; Chow, G.; Lilleker, J.; Lunn, M.; Mckee, D.; Peake, D.; et al. Neurological Manifestations of Influenza Infection in Children and Adults: Results of a National British Surveillance Study. J. Neurol. Neurosurg. Psychiatry 2013, 84, e2. [Google Scholar] [CrossRef]
- Amin, R.; Ford-Jones, E.; Richardson, S.E.; Macgregor, D.; Tellier, R.; Heurter, H.; Fearon, M.; Bitnun, A. Acute childhood encephalitis and encephalopathy associated with influenza: A prospective 11-year review. Pediatric Infect. Dis. J. 2008, 27, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Paksu, M.S.; Aslan, K.; Kendirli, T.; Akyildiz, B.N.; Yener, N.; Yildizdas, R.D.; Davutoglu, M.; Yaman, A.; Isikay, S.; Sensoy, G.; et al. Neuroinfluenza: Evaluation of seasonal influenza associated severe neurological complications in children (a multicenter study). Child’s Nerv. Syst. 2018, 34, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.B.; Zhu, J.; Hu, J.; Wang, L.M.; Zhang, H. H7N9 influenza A-induced pneumonia associated with acute myelitis in an adult. Intern. Med. 2014, 53, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.H.; Vallat, W.; Norton, G.; Chen, D.; Yong, H. Influenza B-related meningoencephalitis in adults Unusual association of diseases/symptoms. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Radzišauskienė, D.; Vitkauskaitė, M.; Žvinytė, K.; Mameniškienė, R. Neurological complications of pandemic A(H1N1)2009pdm, postpandemic A(H1N1)v, and seasonal influenza A. Brain Behav. 2021, 11, e01916. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, K.M.; Wang, C. Post-infectious neurological disorders. Ther. Adv. Neurol. Disord. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, H.C.; Hartung, H.P.; Kieseier, B.C.; Hughes, R.A.C. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect. Dis. 2010, 10, 643–651. [Google Scholar] [CrossRef]
- Flinkkilä, E.; Keski-Rahkonen, A.; Marttunen, M.; Raevuori, A. Prenatal inflammation, infections and mental disorders. Psychopathology 2016, 49, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, N.; Math, S.; Kulkarni, G.; Chaturvedi, S. The neuropsychiatric aspects of influenza/swine flu: A selective review. Ind. Psychiatry J. 2011, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Atladóttir, H.Ó.; Henriksen, T.B.; Schendel, D.E.; Parner, E.T. Autism after infection, febrile episodes, and antibiotic use during pregnancy: An exploratory study. Pediatrics 2012, 130, e1447–e1454. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Wong, C.K.; Chan, P.K.S.; Lindegardh, N.; White, N.J.; Hayden, F.G.; Wong, E.H.C.; Wong, K.S.; Cockram, C.S.; Sung, J.J.Y.; et al. Acute Encephalopathy Associated with Influenza A Infection in Adults. Emerg. Infect. Dis. 2010, 16, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Paiva, T.M.; Theotonio, G.; Paulino, R.S.; Benega, M.A.; Silva, D.B.B.; Borborema, S.E.T.; Ikeda, T.I.; Kisielius, J.J.; Ueda, M.; Oliveira, M.I.; et al. Influenza virus A(H3N2) strain isolated from cerebrospinal fluid from a patient presenting myelopathy post infectious. J. Clin. Virol. 2013, 58, 283–285. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steininger, C.; Popow-Kraupp, T.; Laferl, H.; Seiser, A.; Gödl, I.; Djamshidian, S.; Puchhammer-Stöckl, E. Acute encephalopathy associated with influenza A virus infection. Clin. Infect. Dis. 2003, 36, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Kobayashi, M.; Uemura, O.; Iwasa, M.; Ando, T.; Katoh, T.; Nakamura, C.; Maki, N.; Togari, H.; Wada, Y. PCR on cerebrospinal fluid to show influenza-associated acute encephalopathy or encephalitis. Lancet 1998, 352, 873–875. [Google Scholar] [CrossRef]
- Ito, Y.; Ichiyama, T.; Kimura, H.; Shibata, M.; Ishiwada, N.; Kuroki, H.; Furukawa, S.; Morishima, T. Detection of influenza virus RNA by reverse transcription-PCR and proinflammatory cytokines in influenza-virus-associated encephalopathy. J. Med. Virol. 1999, 58, 420–425. [Google Scholar] [CrossRef]
- Ichiyama, T.; Nishikawa, M.; Yoshitomi, T.; Hayashi, T.; Furukawa, S. Tumor necrosis factor-α, interleukin-1β and interleukin-6 in cerebrospinal fluid from children with prolonged febrile seizures: Comparison with acute encephalitis/encephalopathy. Neurology 1998, 50, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Matsushige, T.; Inoue, H.; Shirabe, K.; Fukano, R.; Ichiyama, T. Serum and cerebrospinal fluid cytokine profile of patients with 2009 pandemic H1N1 influenza virus-associated encephalopathy. Cytokine 2011, 54, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Ota, C.; Kitaoka, S.; Chiba, Y.; Takayanagi, M.; Kitamura, T.; Yamamoto, K.; Fujie, H.; Mikami, H.; Uematsu, M.; et al. Elevated serum levels of neutrophil elastase in patients with influenza virus-associated encephalopathy. J. Neurol. Sci. 2015, 349, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Landreau, F.; Galeano, P.; Caltana, L.R.; Masciotra, L.; Chertcoff, A.; Pontoriero, A.; Baumeister, E.; Amoroso, M.; Brusco, H.A.; Tous, M.I.; et al. Effects of Two Commonly Found Strains of Influenza A Virus on Developing Dopaminergic Neurons, in Relation to the Pathophysiology of Schizophrenia. PLoS ONE 2012, 7, e51068. [Google Scholar] [CrossRef] [PubMed]
- Shinya, K.; Silvano, F.D.; Morita, T.; Shimada, A.; Nakajima, M.; Ito, T.; Otsuki, K.; Umemura, T. Encephalitis in Mice Inoculated Intranasally with an Influenza Virus Strain Originated from a Water Bird. J. Vet. Med. Sci. 1998, 60, 627–629. [Google Scholar] [CrossRef]
- Shinya, K.; Shimada, A.; Ito, T.; Otsuki, K.; Morita, T.; Tanaka, H.; Takada, A.; Kida, H.; Umemura, T. Avian influenza virus intranasally inoculated infects the central nervous system of mice through the general visceral afferent nerve. Arch. Virol. 2000, 145, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Wilk, E.; Michaelsen-Preusse, K.; Gerhauser, I.; Baumgärtner, W.; Geffers, R.; Schughart, K.; Korte, M. Long-term neuroinflammation induced by influenza a virus infection and the impact on hippocampal neuron morphology and function. J. Neurosci. 2018, 38, 3060–3080. [Google Scholar] [CrossRef]
- Jurgens, H.A.; Amancherla, K.; Johnson, R.W. Influenza Infection Induces Neuroinflammation, Alters Hippocampal Neuron Morphology, and Impairs Cognition in Adult Mice. J. Neurosci. 2012, 32, 3958–3968. [Google Scholar] [CrossRef]
- Jang, H.; Boltz, D.; Sturm-Ramirez, K.; Shepherd, K.R.; Jiao, Y.; Webster, R.; Smeyne, R.J. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc. Natl. Acad. Sci. USA 2009, 106, 14063–14068. [Google Scholar] [CrossRef]
- Jang, H.; Boltz, D.; McClaren, J.; Pani, A.K.; Smeyne, M.; Korff, A.; Webster, R.; Smeyne, R.J. Inflammatory effects of highly pathogenic H5N1 influenza virus infection in the CNS of mice. J. Neurosci. 2012, 32, 1545–1559. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, R.; Jiang, Z.; Gu, L.; Chen, Y.; Dai, J.; Li, K. Influenza Virus Induces Inflammatory Response in Mouse Primary Cortical Neurons with Limited Viral Replication. BioMed Res. Int. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.P.; Lee, S.M.Y.; Cheung, T.K.W.; Nicholls, J.M.; Peiris, J.S.M.; Ip, N.Y. Avian influenza H5N1 virus induces cytopathy and proinflammatory cytokine responses in human astrocytic and neuronal cell lines. Neuroscience 2010, 168, 613–623. [Google Scholar] [CrossRef]
- Ng, Y.P.; Yip, T.F.; Peiris, J.S.M.; Ip, N.Y.; Lee, S.M.Y. Avian influenza A H7N9 virus infects human astrocytes and neuronal cells and induces inflammatory immune responses. J. Neurovirol. 2018, 24, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.E.; Kim, M.; Lee, J.H.; Chang, B.J.; Song, C.S.; Nahm, S.S. Neonatal influenza infection causes pathological changes in the mouse brain. Vet. Res. 2014, 45, 63. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Feldon, J.; Dammann, O. Schizophrenia and autism: Both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatric Res. 2011, 69, 26R–33R. [Google Scholar] [CrossRef] [PubMed]
- Kępińska, A.P.; Iyegbe, C.O.; Vernon, A.C.; Yolken, R.; Murray, R.M.; Pollak, T.A. Schizophrenia and Influenza at the Centenary of the 1918-1919 Spanish Influenza Pandemic: Mechanisms of Psychosis Risk. Front. Psychiatry 2020, 11, 72. [Google Scholar] [CrossRef]
- Brown, A.S. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev. Neurobiol. 2012, 72, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Mednick, S.A.; Machon, R.A.; Huttunen, M.O.; Bonett, D. Adult Schizophrenia Following Prenatal Exposure to an Influenza Epidemic. Arch. Gen. Psychiatry 1988, 45, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Begg, M.D.; Gravenstein, S.; Schaefer, C.A.; Wyatt, R.J.; Bresnahan, M.; Babulas, V.P.; Susser, E.S. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry 2004, 61, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fatemi, S.H.; Sidwell, R.W.; Patterson, P.H. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 2003, 23, 297–302. [Google Scholar] [CrossRef]
- Fang, S.Y.; Wang, S.; Huang, N.; Yeh, H.H.; Chen, C.Y. Prenatal infection and autism spectrum disorders in childhood: A population-based case-control study in Taiwan. Paediatr. Perinat. Epidemiol. 2015, 29, 307–316. [Google Scholar] [CrossRef]
- Atladóttir, H.O.´; Thorsen, P.; Østergaard, L.; Schendel, D.E.; Lemcke, S.; Abdallah, M.; Parner, E.T. Maternal Infection Requiring Hospitalization during Pregnancy and Autism Spectrum Disorders. J. Autism Dev. Disord. 2010, 40, 1423–1430. [Google Scholar] [CrossRef]
- Malkova, N.V.; Yu, C.Z.; Hsiao, E.Y.; Moore, M.J.; Patterson, P.H. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain. Behav. Immun. 2012, 26, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Mahic, M.; Che, X.; Susser, E.; Levin, B.; Reichborn-Kjennerud, T.; Magnus, P.; Stoltenberg, C.; Chauhan, L.; Briese, T.; Bresnahan, M.; et al. Epidemiological and Serological Investigation into the Role of Gestational Maternal Influenza Virus Infection and Autism Spectrum Disorders. mSphere 2017, 2, 2020. [Google Scholar] [CrossRef] [PubMed]
- Calvo, C.; García-García, M.L.; Blanco, C.; Vázquez, M.C.; Frías, M.E.; Pérez-Breña, P.; Casas, I. Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J. Clin. Virol. 2008, 42, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Bohmwald, K.; Espinoza, J.A.; Rey-Jurado, E.; Gómez, R.S.; González, P.A.; Bueno, S.M.; Riedel, C.A.; Kalergis, A.M. Human Respiratory Syncytial Virus: Infection and Pathology. Semin. Respir. Crit. Care Med. 2016, 37, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, L.F.; Palomino, M.A.; Larrañaga, C. Surveillance for respiratory syncytial virus in infants hospitalized for acute lower respiratory infection in Chile (1989 to 2000). J. Clin. Microbiol. 2003, 41, 4879–4882. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.L.; Amarasinghe, G.K.; Bányai, K.; Bào, Y.; Basler, C.F.; Bavari, S.; Bejerman, N.; Blasdell, K.R.; Briand, F.-X.; Briese, T.; et al. Taxonomy of the order Mononegavirales: Update 2016. Arch. Virol. 2016, 161, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, R.; Chiappe, S.; Porcella, A.; Rosatelli, D.; Fanos, V. Bronchiolitis-associated encephalopathy in critically-ill infants: An underestimated complication? J. Matern. Neonatal Med. 2010, 23, 431–436. [Google Scholar] [CrossRef]
- Bueno, S.M.; González, P.A.; Cautivo, K.M.; Mora, J.E.; Leiva, E.D.; Tobar, H.E.; Fennelly, G.J.; Eugenin, E.A.; Jacobs, W.R.; Riedel, C.A.; et al. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc. Natl. Acad. Sci. USA 2008, 105, 20822–20827. [Google Scholar] [CrossRef] [PubMed]
- Lay, M.K.; González, P.A.; León, M.A.; Céspedes, P.F.; Bueno, S.M.; Riedel, C.A.; Kalergis, A.M. Advances in understanding respiratory syncytial virus infection in airway epithelial cells and consequential effects on the immune response. Microbes Infect. 2013, 15, 230–242. [Google Scholar] [CrossRef]
- Varga, S.; Schwarze, J.; De Giovanni, M.; González, P.A.; Tognarelli, E.I.; Bueno, S.M. Immune-Modulation by the Human Respiratory Syncytial Virus: Focus on Dendritic Cells. Front. Immunol. 2019, 1, 810. Available online: www.frontiersin.org (accessed on 19 March 2021). [CrossRef]
- Wan, Y.Y.; Flavell, R.A. How diverse-CD4 effector T cells and their functions. J. Mol. Cell Biol. 2009, 1, 20–36. [Google Scholar] [CrossRef]
- Olson, M.R.; Hartwig, S.M.; Varga, S.M. The Number of Respiratory Syncytial Virus (RSV)-Specific Memory CD8 T Cells in the Lung Is Critical for Their Ability to Inhibit RSV Vaccine-Enhanced Pulmonary Eosinophilia. J. Immunol. 2008, 181, 7958–7968. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.; Carreno, L.; Bueno, S.; Riedel, C.; Kalergis, A. Understanding Respiratory Syncytial Virus Infection to Improve Treatment and Immunity. Curr. Mol. Med. 2013, 13, 1122–1139. [Google Scholar] [CrossRef]
- Vallbracht, S.; Unsöld, H.; Ehl, S. Functional impairment of cytotoxic T cells in the lung airways following respiratory virus infections. Eur. J. Immunol. 2006, 36, 1434–1442. [Google Scholar] [CrossRef]
- Bueno, S.M.; González, P.A.; Pacheco, R.; Leiva, E.D.; Cautivo, K.M.; Tobar, H.E.; Mora, J.E.; Prado, C.E.; Zúñiga, J.P.; Jiménez, J.; et al. Host immunity during RSV pathogenesis. Int. Immunopharmacol. 2008, 8, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, L.L.; Ng, Y.T.Y.T.; Butler, I.J.I.J.; Bodensteiner, J.B.; Wright, R.B.; Pomerantz, W.J.; Luria, J.W.; Church, N.R.; Anas, N.G.; Hall, C.B.; et al. Neurologic complications associated with respiratory syncytial virus. Pediatric Neurol. 2005, 32, 307–310. [Google Scholar] [CrossRef]
- Ng, Y.; Cox, C.; Atkins, J.; Butler, I.J. Encephalopathy Associated With Respiratory Syncytial Virus Bronchiolitis. J. Child Neurol. 2001, 16, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Cappel, R.; Thiry, L.; Clinet, G. Viral Antibodies in the CSF After Acute CNS Infections. Arch. Neurol. 1975, 32, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Ioi, H.; Ushio, M.; Yamanaka, G.; Matsumoto, S.; Nakayama, T. Cerebrospinal fluid analysis in children with seizures from respiratory syncytial virus infection. Scand. J. Infect. Dis. 2009, 41, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Kho, N.; Kerrigan, J.F.; Tong, T.; Browne, R.; Knilans, J. Respiratory Syncytial Virus Infection and Neurologic Abnormalities: Retrospective Cohort Study. J. Child Neurol. 2004, 19, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Zlateva, K.T.; Van Ranst, M. Detection of subgroup B respiratory syncytial virus in the cerebrospinal fluid of a patient with respiratory syncytial virus pneumonia. Pediatric Infect. Dis. J. 2004, 23, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Kashiwagi, Y.; Ioi, H.; Morichi, S.; Oana, S.; Yamanaka, G.; Takekuma, K.; Hoshika, A.; Sawai, J.; Kato, Y. Production of chemokines in respiratory syncytial virus infection with central nervous system manifestations. J. Infect. Chemother. 2012, 18, 827–831. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, T.; He, H.; Qiu, H.; Wu, X.; Chen, J.; Wang, M.; Chen, C.; Huang, S. Respiratory syncytial virus prolifically infects N2a neuronal cells, leading to TLR4 and nucleolin protein modulations and RSV F protein co-localization with TLR4 and nucleolin. J. Biomed. Sci. 2018, 25, 13. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Hasöksüz, M.; Kiliç, S.; Saraç, F. Coronaviruses and sars-cov-2. Turkish J. Med. Sci. 2020, 50, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Burki, T. Understanding Variants of SARS-CoV-2. Lancet 2021, 397, 462. [Google Scholar] [CrossRef]
- Soucheray, S. New COVID-19 Variants Found in New York, California. Available online: https://www.cidrap.umn.edu/news-perspective/2021/02/new-covid-19-variants-found-new-york-california (accessed on 19 March 2021).
- World Health Organization. Weekly Epidemiological Update—2 February 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update---2-february-2021 (accessed on 19 March 2021).
- Canedo-Marroquín, G.; Saavedra, F.; Andrade, C.A.; Berrios, R.V.; Rodríguez-Guilarte, L.; Opazo, M.C.; Riedel, C.A.; Kalergis, A.M. SARS-CoV-2: Immune Response Elicited by Infection and Development of Vaccines and Treatments. Front. Immunol. 2020, 11, 569760. [Google Scholar] [CrossRef]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first Case of Meningitis/Encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Carignan, A.; Valiquette, L.; Grenier, C.; Musonera, J.B.; Nkengurutse, D.; Marcil-Héguy, A.; Vettese, K.; Marcoux, D.; Valiquette, C.; Xiong, W.T.; et al. Anosmia and dysgeusia associated with SARS-CoV-2 infection: An age-matched case−control study. CMAJ 2020, 192, E702–E707. [Google Scholar] [CrossRef] [PubMed]
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A.; Kneen, R.; Defres, S.; Sejvar, J.; Solomon, T. Neurological associations of COVID-19. Lancet Neurol. 2020, 19, 767–783. [Google Scholar] [CrossRef]
- Domingues, R.B.; Mendes-Correa, M.C.; de Moura Leite, F.B.V.; Sabino, E.C.; Salarini, D.Z.; Claro, I.; Santos, D.W.; de Jesus, J.G.; Ferreira, N.E.; Romano, C.M.; et al. First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J. Neurol. 2020, 267, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Reichard, R.R.; Kashani, K.B.; Boire, N.A.; Constantopoulos, E.; Guo, Y.; Lucchinetti, C.F. Neuropathology of COVID-19: A spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020, 140, 1–6. [Google Scholar] [CrossRef]
- Duarte-Neto, A.N.; Monteiro, R.A.A.; da Silva, L.F.F.; Malheiros, D.M.A.C.; de Oliveira, E.P.; Theodoro-Filho, J.; Pinho, J.R.R.; Gomes-Gouvêa, M.S.; Salles, A.P.M.; de Oliveira, I.R.S.; et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology 2020, 77, 186–197. [Google Scholar] [CrossRef]
- Generoso, J.S.; Barichello de Quevedo, J.L.; Cattani, M.; Lodetti, B.F.; Sousa, L.; Collodel, A.; Diaz, A.P.; Felipe, D.-P. Neurobiology of COVID-19: How can the virus affect the brain? Braz. J. Psychiatry 2021. [Google Scholar] [CrossRef] [PubMed]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, M.; Chen, X.; Montaner, L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020, 108, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Alpert, O.; Begun, L.; Garren, P.; Solhkhah, R. Cytokine storm induced new onset depression in patients with COVID-19. A new look into the association between depression and cytokines -two case reports. Brain Behav. Immun. Health 2020, 9, 100173. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; Jones, M.; Doshi, P.; Spencer, E.A.; Onakpoya, I.; Heneghan, C.J. Oseltamivir for influenza in adults and children: Systematic review of clinical study reports and summary of regulatory comments. BMJ 2014, 348, g2545. [Google Scholar] [CrossRef]
- Studahl, M. Influenza virus and CNS manifestations. J. Clin. Virol. 2003, 28, 225–232. [Google Scholar] [CrossRef]
- Munakata, M.; Kato, R.; Yokoyama, H.; Haginoya, K.; Tanaka, Y.; Kayaba, J.; Kato, T.; Takayanagi, R.; Endo, H.; Hasegawa, R.; et al. Combined therapy with hypothermia and anticytokine agents in influenza A encephalopathy. Brain Dev. 2000, 22, 373–377. [Google Scholar] [CrossRef]
- Khandaker, G.; Zurynski, Y.; Buttery, J.; Marshall, H.; Richmond, P.C.; Dale, R.C.; Royle, J.; Gold, M.; Snelling, T.; Whitehead, B.; et al. Neurologic complications of influenza A(H1N1)pdm09 surveillance in 6 pediatric hospitals. Neurology 2012, 79, 1474–1481. [Google Scholar] [CrossRef]
- Lurie, D.I. An integrative approach to neuroinflammation in psychiatric disorders and neuropathic pain. J. Exp. Neurosci. 2018, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bardage, C.; Persson, I.; Ortqvist, A.; Bergman, U.; Ludvigsson, J.F.; Granath, F. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: Population based cohort study in Stockholm, Sweden. BMJ 2011, 343, d5956. [Google Scholar] [CrossRef]
- Chang, K.-H.; Lyu, R.-K.; Lin, W.-T.; Huang, Y.-T.; Lin, H.-S.; Chang, S.-H. Gulllain-Barre Syndrome After Trivalent Influenza Vaccination in Adults. Front. Neurol. 2019, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.; Cutland, C.L.; Jones, S.; Hugo, A.; Madimabe, R.; Simões, E.A.F.; Weinberg, A.; Madhi, S.A. Duration of infant protection against influenza illness conferred by maternal immunization secondary analysis of a randomized clinical trial. JAMA Pediatrics 2016, 170, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Huang, J.; Dai, D.; Feng, Y.; Liu, L.; Nie, S. Acute myelitis after SARS-CoV-2 infection: A case report. medRxiv 2020. [Google Scholar] [CrossRef]

| Strain | H5N1 (Vietnam/1203/04) | H7N7 (rSC35M) | H1N1 (A/PR/8/34 and A/Shantou/169/2006) | H5N1 (A/Hong Kong/483/97) | H7N9 (H7/SH2/13) | H1N1 (A/PR/8/34) | H3N2 (maHK68) |
|---|---|---|---|---|---|---|---|
| Neurotropic effect | Yes | Yes | Yes | Yes | Yes | No | No |
| Infection of cells | Neurons and microglia | No information | Neurons | Astrocytes | Neurons and astrocytes | No information | No information |
| Pro-inflammatory Cytokines | Increase of IL-1β, IL-12(p70), IL-2, IL-13, and TNF-α | Increase of IFN-γ and TNF-α | Increase of IL-6 and TNF-α | Increase of IL-6 and TNF-α | Increase of IL-6, IL-8, TNF-α and IFN-β | Increase of TNF-α, IL-1β, and IL-6 | Increase of TNF-α |
| Chemokines | Increase of CCL2, CCL3, CCL4, CCL11, CXCL10, and CXCL1 | No information | Increase of CXCL-10 | No information | Increase of CCL2 | No information | No information |
| Grown factors | Increase of G-CSF, GM-CSF, M-CSF and VEGF | No information | No information | No information | No information | Decrease of BDNF and NGF | No information |
| Anti-inflammatory Cytokines | Increase of IL-10 | No information | No information | No information | No information | No information | No information |
| Clinical Findings | Pro-Inflammatory Molecules | Consequences |
|---|---|---|
| hRSV genetic material in cerebrospinal fluid | Elevated levels of IL-6, IL-8, CCL2, and CCL4 and low levels of TNF-α in cerebrospinal fluid | Encephalitis |
| Encephalopathies | ||
| Learning difficulties | To be defined | Language difficulties |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohmwald, K.; Andrade, C.A.; Kalergis, A.M. Contribution of Pro-Inflammatory Molecules Induced by Respiratory Virus Infections to Neurological Disorders. Pharmaceuticals 2021, 14, 340. https://doi.org/10.3390/ph14040340
Bohmwald K, Andrade CA, Kalergis AM. Contribution of Pro-Inflammatory Molecules Induced by Respiratory Virus Infections to Neurological Disorders. Pharmaceuticals. 2021; 14(4):340. https://doi.org/10.3390/ph14040340
Chicago/Turabian StyleBohmwald, Karen, Catalina A. Andrade, and Alexis M. Kalergis. 2021. "Contribution of Pro-Inflammatory Molecules Induced by Respiratory Virus Infections to Neurological Disorders" Pharmaceuticals 14, no. 4: 340. https://doi.org/10.3390/ph14040340
APA StyleBohmwald, K., Andrade, C. A., & Kalergis, A. M. (2021). Contribution of Pro-Inflammatory Molecules Induced by Respiratory Virus Infections to Neurological Disorders. Pharmaceuticals, 14(4), 340. https://doi.org/10.3390/ph14040340







