N-3 PUFA Prevent Oxidative Stress in a Rat Model of Beta-Amyloid-Induced Toxicity
Abstract
1. Introduction
2. Results
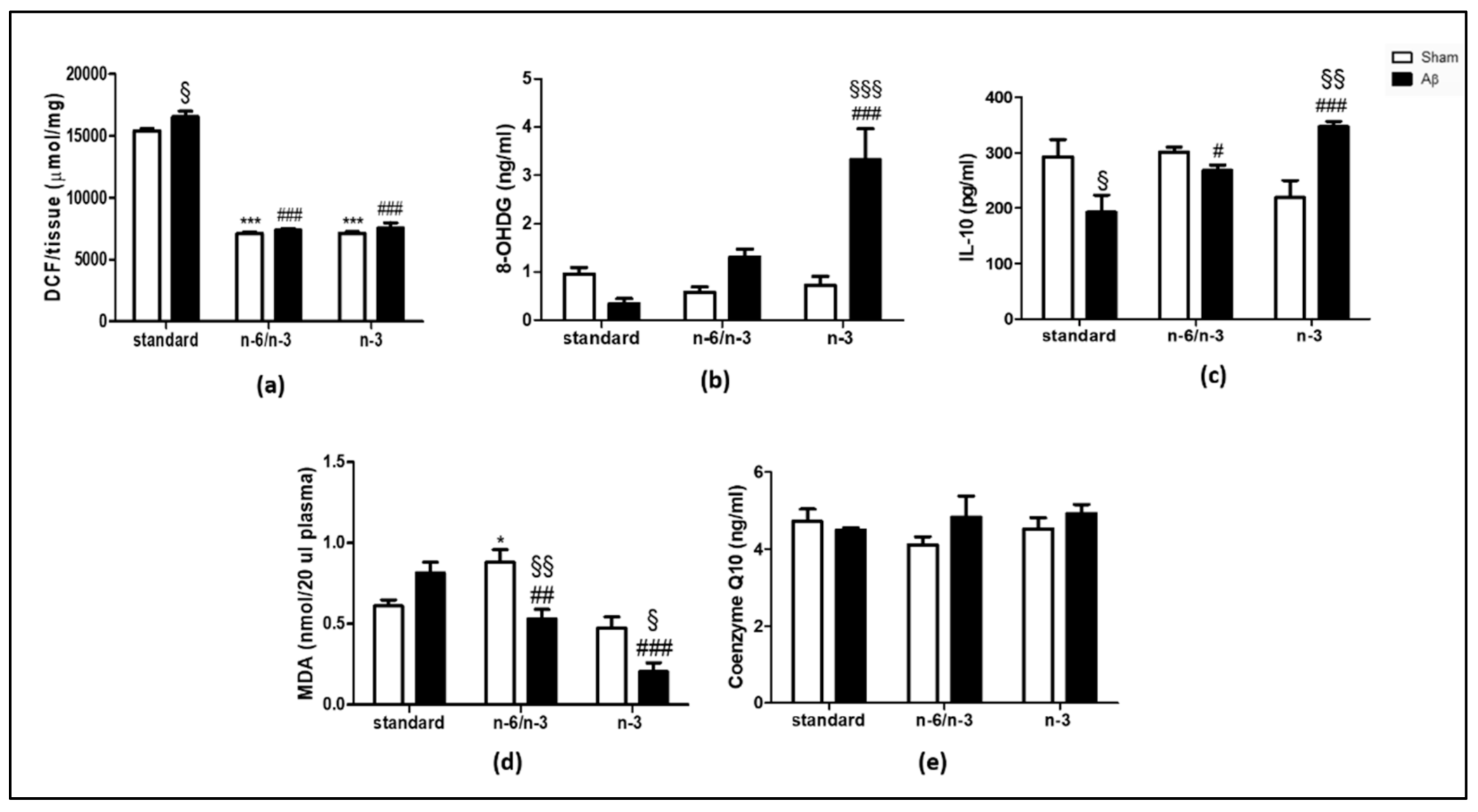
2.1. ROS Production, Levels of 8-Hydroxy-2′-Deoxyguanosine (8-OHdG) and IL-10, Lipid Peroxidation and Coenzyme Q10 (CoQ10)
2.2. NOX1 and NOX2 Expression
2.3. Effects on Antioxidant Enzymes
2.4. Effects on Hippocampal Neurochemistry
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Diets
4.3. Aβ Administration
4.4. Post-Mortem Tissue Analysis
4.5. High-Performance Liquid Chromatography (HPLC) Quantifications
4.6. Enzyme-Linked Immunosorbent Assays (ELISA)
4.7. GSH + GSSG/GSH Assay
4.8. Western Blotting
4.9. ROS Measurement
4.10. MDA Assay Measurement
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luchtman, D.W.; Song, C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: Findings from animal and clinical studies. Neuropharmacology 2013, 64, 550–565. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Effects of dietary polyunsaturated fatty acids on neuronal function. Lipids 1999, 34, 161–169. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Strawn, J.R. Role of Long-Chain Omega-3 Fatty Acids in Psychiatric Practice. PharmaNutrition 2013, 1, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Martin, A.; Joseph, J.A. Essential fatty acids and the brain: Possible health implications. Int. J. Dev. Neurosci. 2000, 18, 383–399. [Google Scholar] [CrossRef]
- Young, G.; Conquer, J. Omega-3 fatty acids and neuropsychiatric disorders. Reprod. Nutr. Dev. 2005, 45, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Liu, Z.; Ouellet, M.; Calon, F.; Bazinet, R.P. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: An in situ study. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Liu, Z.; Bazinet, R.P. Rapid de-esterification and loss of eicosapentaenoic acid from rat brain phospholipids: An intracerebroventricular study. J. Neurochem. 2011, 116, 363–373. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Laye, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Assal, F.; Cummings, J.L. Neuropsychiatric symptoms in the dementias. Curr. Opin. Neurol. 2002, 15, 445–450. [Google Scholar] [CrossRef]
- Morgese, M.G.; Schiavone, S.; Mhillaj, E.; Bove, M.; Tucci, P.; Trabace, L. N-3 PUFA diet enrichment prevents amyloid beta-induced depressive-like phenotype. Pharmacol. Res. 2018, 129, 526–534. [Google Scholar] [CrossRef]
- Sundaram, T.S.; Giromini, C.; Rebucci, R.; Baldi, A. Omega-3 Polyunsaturated Fatty Acids Counteract Inflammatory and Oxidative Damage of Non-Transformed Porcine Enterocytes. Animals 2020, 10, 965. [Google Scholar] [CrossRef]
- Sakai, C.; Ishida, M.; Ohba, H.; Yamashita, H.; Uchida, H.; Yoshizumi, M.; Ishida, T. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS ONE 2017, 12, e0187934. [Google Scholar] [CrossRef]
- Dyall, S.C.; Michael-Titus, A.T. Neurological benefits of omega-3 fatty acids. Neuromol. Med. 2008, 10, 219–235. [Google Scholar] [CrossRef]
- Garrel, C.; Alessandri, J.M.; Guesnet, P.; Al-Gubory, K.H. Omega-3 fatty acids enhance mitochondrial superoxide dismutase activity in rat organs during post-natal development. Int. J. Biochem. Cell Biol. 2012, 44, 123–131. [Google Scholar] [CrossRef]
- Sorce, S.; Krause, K.H. NOX enzymes in the central nervous system: From signaling to disease. Antioxid. Redox Signal. 2009, 11, 2481–2504. [Google Scholar] [CrossRef]
- Chay, K.O.; Nam Koong, K.Y.; Hwang, S.; Kim, J.K.; Bae, C.S. NADPH Oxidase Mediates beta-Amyloid Peptide-Induced Neuronal Death in Mouse Cortical Cultures. Chonnam Med. J. 2017, 53, 196–202. [Google Scholar] [CrossRef]
- Barua, S.; Kim, J.Y.; Yenari, M.A.; Lee, J.E. The role of NOX inhibitors in neurodegenerative diseases. IBRO Rep. 2019, 7, 59–69. [Google Scholar] [CrossRef]
- Wyssenbach, A.; Quintela, T.; Llavero, F.; Zugaza, J.L.; Matute, C.; Alberdi, E. Amyloid beta-induced astrogliosis is mediated by beta1-integrin via NADPH oxidase 2 in Alzheimer’s disease. Aging Cell 2016, 15, 1140–1152. [Google Scholar] [CrossRef]
- Rossary, A.; Arab, K.; Goudable, J.; Steghens, J.P. Fatty acids regulate NOX activity. Ann. Biol. Clin. 2007, 65, 33–40. [Google Scholar]
- Celsi, F.; Ferri, A.; Casciati, A.; D’Ambrosi, N.; Rotilio, G.; Costa, A.; Volonte, C.; Carri, M.T. Overexpression of superoxide dismutase 1 protects against beta-amyloid peptide toxicity: Effect of estrogen and copper chelators. Neurochem. Int. 2004, 44, 25–33. [Google Scholar] [CrossRef]
- Barber, V.S.; Griffiths, H.R. Is glutathione an important neuroprotective effector molecule against amyloid beta toxicity? BioFactors 2003, 17, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, S.; Arsic, A.; Ristic-Medic, D.; Cvetkovic, Z.; Vucic, V. Lipid Peroxidation and Antioxidant Supplementation in Neurodegenerative Diseases: A Review of Human Studies. Antioxidants 2020, 9, 1128. [Google Scholar] [CrossRef] [PubMed]
- Hernando, S.; Requejo, C.; Herran, E.; Ruiz-Ortega, J.A.; Morera-Herreras, T.; Lafuente, J.V.; Ugedo, L.; Gainza, E.; Pedraz, J.L.; Igartua, M.; et al. Beneficial effects of n-3 polyunsaturated fatty acids administration in a partial lesion model of Parkinson’s disease: The role of glia and NRf2 regulation. Neurobiol. Dis. 2019, 121, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.E. Inflammation, depression and dementia: Are they connected? Neurochem. Res. 2007, 32, 1749–1756. [Google Scholar] [CrossRef]
- Joffre, C.; Dinel, A.L.; Chataigner, M.; Pallet, V.; Laye, S. n-3 Polyunsaturated Fatty Acids and Their Derivates Reduce Neuroinflammation during Aging. Nutrients 2020, 12, 647. [Google Scholar] [CrossRef]
- Price, K.A.; Varghese, M.; Sowa, A.; Yuk, F.; Brautigam, H.; Ehrlich, M.E.; Dickstein, D.L. Altered synaptic structure in the hippocampus in a mouse model of Alzheimer’s disease with soluble amyloid-beta oligomers and no plaque pathology. Mol. Neurodegener. 2014, 9, 41. [Google Scholar] [CrossRef]
- Hu, N.W.; Smith, I.M.; Walsh, D.M.; Rowan, M.J. Soluble amyloid-beta peptides potently disrupt hippocampal synaptic plasticity in the absence of cerebrovascular dysfunction in vivo. Brain 2008, 131, 2414–2424. [Google Scholar] [CrossRef]
- Colaianna, M.; Tucci, P.; Zotti, M.; Morgese, M.G.; Schiavone, S.; Govoni, S.; Cuomo, V.; Trabace, L. Soluble beta amyloid(1-42): A critical player in producing behavioural and biochemical changes evoking depressive-related state? Br. J. Pharmacol. 2010, 159, 1704–1715. [Google Scholar] [CrossRef]
- Schiavone, S.; Tucci, P.; Mhillaj, E.; Bove, M.; Trabace, L.; Morgese, M.G. Antidepressant drugs for beta amyloid-induced depression: A new standpoint? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 78, 114–122. [Google Scholar] [CrossRef]
- Morgese, M.G.; Schiavone, S.; Bove, M.; Mhillaj, E.; Tucci, P.; Trabace, L. Sub-chronic celecoxib prevents soluble beta amyloid-induced depressive-like behaviour in rats. J. Affect. Disord. 2018, 238, 118–121. [Google Scholar] [CrossRef]
- Morgese, M.G.; Schiavone, S.; Maffione, A.B.; Tucci, P.; Trabace, L. Depressive-like phenotype evoked by lifelong nutritional omega-3 deficiency in female rats: Crosstalk among kynurenine, Toll-like receptors and amyloid beta oligomers. Brain Behav. Immun. 2020, 87, 444–454. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Hyman, B.T.; van Hoesen, G.W.; Damasio, A.R.; Barnes, C.L. Alzheimer’s disease: Cell-specific pathology isolates the hippocampal formation. Science 1984, 225, 1168–1170. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Hillen, H. The Beta Amyloid Dysfunction (BAD) Hypothesis for Alzheimer’s Disease. Front. Neurosci. 2019, 13, 1154. [Google Scholar] [CrossRef]
- Mhillaj, E.; Morgese, M.G.; Tucci, P.; Furiano, A.; Luongo, L.; Bove, M.; Maione, S.; Cuomo, V.; Schiavone, S.; Trabace, L. Celecoxib Prevents Cognitive Impairment and Neuroinflammation in Soluble Amyloid beta-treated Rats. Neuroscience 2018, 372, 58–73. [Google Scholar] [CrossRef]
- Serini, S.; Calviello, G. Reduction of Oxidative/Nitrosative Stress in Brain and its Involvement in the Neuroprotective Effect of n-3 PUFA in Alzheimer’s Disease. Curr. Alzheimer Res. 2016, 13, 123–134. [Google Scholar] [CrossRef]
- Park, L.; Zhou, P.; Pitstick, R.; Capone, C.; Anrather, J.; Norris, E.H.; Younkin, L.; Younkin, S.; Carlson, G.; McEwen, B.S.; et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. USA 2008, 105, 1347–1352. [Google Scholar] [CrossRef]
- Milton, R.H.; Abeti, R.; Averaimo, S.; de Biasi, S.; Vitellaro, L.; Jiang, L.; Curmi, P.M.; Breit, S.N.; Duchen, M.R.; Mazzanti, M. CLIC1 function is required for beta-amyloid-induced generation of reactive oxygen species by microglia. J. Neurosci. 2008, 28, 11488–11499. [Google Scholar] [CrossRef]
- Surace, M.J.; Block, M.L. Targeting microglia-mediated neurotoxicity: The potential of NOX2 inhibitors. Cell. Mol. Life Sci. 2012, 69, 2409–2427. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Sorce, S.; Krause, K.H.; Jaquet, V. Targeting NOX enzymes in the central nervous system: Therapeutic opportunities. Cell. Mol. Life Sci. 2012, 69, 2387–2407. [Google Scholar] [CrossRef] [PubMed]
- Basili, S.; Raparelli, V.; Napoleone, L.; del Ben, M.; Merli, M.; Riggio, O.; Nocella, C.; Carnevale, R.; Pignatelli, P.; Violi, F.; et al. Polyunsaturated fatty acids balance affects platelet NOX2 activity in patients with liver cirrhosis. Digest. Liver Dis. 2014, 46, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, K.H.; Mayyas, F.; Abu Zamzam, H.I. Omega-3 fatty acids protects against chronic sleep-deprivation induced memory impairment. Life Sci. 2019, 227, 1–7. [Google Scholar] [CrossRef]
- Acosta, M.J.; Vazquez Fonseca, L.; Desbats, M.A.; Cerqua, C.; Zordan, R.; Trevisson, E.; Salviati, L. Coenzyme Q biosynthesis in health and disease. Biochim. Biophys. Acta 2016, 1857, 1079–1085. [Google Scholar] [CrossRef]
- Giavarotti, L.; Simon, K.A.; Azzalis, L.A.; Fonseca, F.L.; Lima, A.F.; Freitas, M.C.; Brunialti, M.K.; Salomao, R.; Moscardi, A.A.; Montano, M.B.; et al. Mild systemic oxidative stress in the subclinical stage of Alzheimer’s disease. Oxidative Med. Cell. Longev. 2013, 2013, 609019. [Google Scholar] [CrossRef]
- de Bustos, F.; Molina, J.A.; Jimenez-Jimenez, F.J.; Garcia-Redondo, A.; Gomez-Escalonilla, C.; Porta-Etessam, J.; Berbel, A.; Zurdo, M.; Barcenilla, B.; Parrilla, G.; et al. Serum levels of coenzyme Q10 in patients with Alzheimer’s disease. J. Neural Transm. 2000, 107, 233–239. [Google Scholar] [CrossRef]
- Pagani, L.; Eckert, A. Amyloid-Beta interaction with mitochondria. Int. J. Alzheimer’s Dis. 2011, 2011, 925050. [Google Scholar] [CrossRef]
- Spuch, C.; Ortolano, S.; Navarro, C. New insights in the amyloid-Beta interaction with mitochondria. J. Aging Res. 2012, 2012, 324968. [Google Scholar] [CrossRef]
- Kaminsky, Y.G.; Tikhonova, L.A.; Kosenko, E.A. Critical analysis of Alzheimer’s amyloid-beta toxicity to mitochondria. Front. Biosci. 2015, 20, 173–197. [Google Scholar] [CrossRef]
- Carvalho-Silva, M.; Gomes, L.M.; Gomes, M.L.; Ferreira, B.K.; Schuck, P.F.; Ferreira, G.C.; Dal-Pizzol, F.; de Oliveira, J.; Scaini, G.; Streck, E.L. Omega-3 fatty acid supplementation can prevent changes in mitochondrial energy metabolism and oxidative stress caused by chronic administration of L-tyrosine in the brain of rats. Metab. Brain Dis. 2019, 34, 1207–1219. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid beta-peptide (1-42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antiox. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef]
- Heras-Sandoval, D.; Pedraza-Chaverri, J.; Perez-Rojas, J.M. Role of docosahexaenoic acid in the modulation of glial cells in Alzheimer’s disease. J. Neuroinflamm. 2016, 13, 61. [Google Scholar] [CrossRef]
- Lucena, C.F.; Roma, L.P.; Graciano, M.F.; Veras, K.; Simoes, D.; Curi, R.; Carpinelli, A.R. Omega-3 supplementation improves pancreatic islet redox status: In vivo and in vitro studies. Pancreas 2015, 44, 287–295. [Google Scholar] [CrossRef]
- Nur, E.; Verwijs, M.; de Waart, D.R.; Schnog, J.J.; Otten, H.M.; Brandjes, D.P.; Biemond, B.J.; Elferink, R.P.; Group, C.S. Increased efflux of oxidized glutathione (GSSG) causes glutathione depletion and potentially diminishes antioxidant defense in sickle erythrocytes. Biochim. Biophys. Acta 2011, 1812, 1412–1417. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Kim, H.S.; Ye, S.K.; Cho, I.H.; Jung, J.E.; Kim, D.H.; Choi, S.; Kim, Y.S.; Park, C.G.; Kim, T.Y.; Lee, J.W.; et al. 8-hydroxydeoxyguanosine suppresses NO production and COX-2 activity via Rac1/STATs signaling in LPS-induced brain microglia. Free Radic. Biol. Med. 2006, 41, 1392–1403. [Google Scholar] [CrossRef]
- Choi, S.; Choi, H.H.; Lee, S.H.; Ko, S.H.; You, H.J.; Ye, S.K.; Chung, M.H. Anti-inflammatory effects of 8-hydroxy-2′-deoxyguanosine on lipopolysaccharide-induced inflammation via Rac suppression in Balb/c mice. Free Radic. Biol. Med. 2007, 43, 1594–1603. [Google Scholar] [CrossRef]
- Haddad, J.J.; Fahlman, C.S. Redox- and oxidant-mediated regulation of interleukin-10: An anti-inflammatory, antioxidant cytokine? Biochem. Biophys. Res. Commun. 2002, 297, 163–176. [Google Scholar] [CrossRef]
- Bogdan, C.; Vodovotz, Y.; Nathan, C. Macrophage deactivation by interleukin 10. J. Exp. Med. 1991, 174, 1549–1555. [Google Scholar] [CrossRef]
- Ferrucci, L.; Cherubini, A.; Bandinelli, S.; Bartali, B.; Corsi, A.; Lauretani, F.; Martin, A.; Andres-Lacueva, C.; Senin, U.; Guralnik, J.M. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J. Clin. Endocrinol. Metab. 2006, 91, 439–446. [Google Scholar] [CrossRef]
- O’Farrell, K.; Harkin, A. Stress-related regulation of the kynurenine pathway: Relevance to neuropsychiatric and degenerative disorders. Neuropharmacology 2017, 112, 307–323. [Google Scholar] [CrossRef]
- Oxenkrug, G. Serotonin-kynurenine hypothesis of depression: Historical overview and recent developments. Curr. Drug Targets 2013, 14, 514–521. [Google Scholar] [CrossRef]
- Oxenkrug, G.F.; Turski, W.A.; Zgrajka, W.; Weinstock, J.V.; Summergrad, P. Tryptophan-kynurenine metabolism and insulin resistance in hepatitis C patients. Hepat. Res. Treat. 2013, 2013, 149247. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Meininger, V.; Brew, B.J. Implications for the kynurenine pathway and quinolinic acid in amyotrophic lateral sclerosis. Neuro-Degener. Dis. 2005, 2, 166–176. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Williams, K.R.; Smith, D.G.; Smythe, G.A.; Croitoru-Lamoury, J.; Brew, B.J. Quinolinic acid in the pathogenesis of Alzheimer’s disease. Adv. Exp. Med. Biol. 2003, 527, 167–176. [Google Scholar]
- Heneka, M.T.; O’Banion, M.K.; Terwel, D.; Kummer, M.P. Neuroinflammatory processes in Alzheimer’s disease. J. Neural Transm. 2010, 117, 919–947. [Google Scholar] [CrossRef]
- Tejera, D.; Heneka, M.T. Microglia in Alzheimer’s disease: The good, the bad and the ugly. Curr. Alzheimer Res. 2016, 13, 370–380. [Google Scholar] [CrossRef]
- Hopperton, K.E.; Trepanier, M.O.; Giuliano, V.; Bazinet, R.P. Brain omega-3 polyunsaturated fatty acids modulate microglia cell number and morphology in response to intracerebroventricular amyloid-beta 1-40 in mice. J. Neuroinflamm. 2016, 13, 257. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Morgese, M.G.; Colaianna, M.; Mhillaj, E.; Zotti, M.; Schiavone, S.; D’Antonio, P.; Harkin, A.; Gigliucci, V.; Campolongo, P.; Trezza, V.; et al. Soluble beta amyloid evokes alteration in brain norepinephrine levels: Role of nitric oxide and interleukin-1. Front. Neurosci. 2015, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, D.L.; Heneka, M.T.; Gavrilyuk, V.; Dello Russo, C.; Weinberg, G.; Galea, E. Noradrenergic regulation of inflammatory gene expression in brain. Neurochem. Int. 2002, 41, 357–365. [Google Scholar] [CrossRef]
- Marien, M.R.; Colpaert, F.C.; Rosenquist, A.C. Noradrenergic mechanisms in neurodegenerative diseases: A theory. Brain Res. 2004, 45, 38–78. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Ruan, L.; Qian, L.; Liu, X.; Le, Y. Norepinephrine promotes microglia to uptake and degrade amyloid beta peptide through upregulation of mouse formyl peptide receptor 2 and induction of insulin-degrading enzyme. J. Neurosci. 2010, 30, 11848–11857. [Google Scholar] [CrossRef]
- Mori, K.; Ozaki, E.; Zhang, B.; Yang, L.; Yokoyama, A.; Takeda, I.; Maeda, N.; Sakanaka, M.; Tanaka, J. Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology 2002, 43, 1026–1034. [Google Scholar] [CrossRef]
- Madrigal, J.L.; Leza, J.C.; Polak, P.; Kalinin, S.; Feinstein, D.L. Astrocyte-derived MCP-1 mediates neuroprotective effects of noradrenaline. J. Neurosci. 2009, 29, 263–267. [Google Scholar] [CrossRef]
- Madrigal, J.L.; Kalinin, S.; Richardson, J.C.; Feinstein, D.L. Neuroprotective actions of noradrenaline: Effects on glutathione synthesis and activation of peroxisome proliferator activated receptor delta. J. Neurochem. 2007, 103, 2092–2101. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier Academic Press: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Francavilla, M.; Colaianna, M.; Zotti, M.; Morgese, M.G.; Trotta, P.; Tucci, P.; Schiavone, S.; Cuomo, V.; Trabace, L. Extraction, characterization and in vivo neuromodulatory activity of phytosterols from microalga Dunaliella tertiolecta. Curr. Med. Chem. 2012, 19, 3058–3067. [Google Scholar] [CrossRef]
- Schiavone, S.; Tucci, P.; Trabace, L.; Morgese, M.G. Early Celastrol Administration Prevents Ketamine-Induced Psychotic-Like Behavioral Dysfunctions, Oxidative Stress and IL-10 Reduction in The Cerebellum of Adult Mice. Molecules 2019, 24, 3993. [Google Scholar] [CrossRef]
- Bove, M.; Tucci, P.; Dimonte, S.; Trabace, L.; Schiavone, S.; Morgese, M.G. Postnatal Antioxidant and Anti-inflammatory Treatments Prevent Early Ketamine-Induced Cortical Dysfunctions in Adult Mice. Front. Neurosci. 2020, 14, 590088. [Google Scholar] [CrossRef]
- Pirozzi, C.; Lama, A.; Annunziata, C.; Cavaliere, G.; de Caro, C.; Citraro, R.; Russo, E.; Tallarico, M.; Iannone, M.; Ferrante, M.C.; et al. Butyrate prevents valproate-induced liver injury: In vitro and in vivo evidence. FASEB J. 2020, 34, 676–690. [Google Scholar] [CrossRef]
- Kirkland, R.A.; Saavedra, G.M.; Franklin, J.L. Rapid activation of antioxidant defenses by nerve growth factor suppresses reactive oxygen species during neuronal apoptosis: Evidence for a role in cytochrome c redistribution. J. Neurosci. 2007, 27, 11315–11326. [Google Scholar] [CrossRef]
- Fan, L.M.; Geng, L.; Cahill-Smith, S.; Liu, F.; Douglas, G.; McKenzie, C.A.; Smith, C.; Brooks, G.; Channon, K.M.; Li, J.M. Nox2 contributes to age-related oxidative damage to neurons and the cerebral vasculature. J. Clin. Investig. 2019, 129, 3374–3386. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgese, M.G.; Schiavone, S.; Bove, M.; Colia, A.L.; Dimonte, S.; Tucci, P.; Trabace, L. N-3 PUFA Prevent Oxidative Stress in a Rat Model of Beta-Amyloid-Induced Toxicity. Pharmaceuticals 2021, 14, 339. https://doi.org/10.3390/ph14040339
Morgese MG, Schiavone S, Bove M, Colia AL, Dimonte S, Tucci P, Trabace L. N-3 PUFA Prevent Oxidative Stress in a Rat Model of Beta-Amyloid-Induced Toxicity. Pharmaceuticals. 2021; 14(4):339. https://doi.org/10.3390/ph14040339
Chicago/Turabian StyleMorgese, Maria Grazia, Stefania Schiavone, Maria Bove, Anna Laura Colia, Stefania Dimonte, Paolo Tucci, and Luigia Trabace. 2021. "N-3 PUFA Prevent Oxidative Stress in a Rat Model of Beta-Amyloid-Induced Toxicity" Pharmaceuticals 14, no. 4: 339. https://doi.org/10.3390/ph14040339
APA StyleMorgese, M. G., Schiavone, S., Bove, M., Colia, A. L., Dimonte, S., Tucci, P., & Trabace, L. (2021). N-3 PUFA Prevent Oxidative Stress in a Rat Model of Beta-Amyloid-Induced Toxicity. Pharmaceuticals, 14(4), 339. https://doi.org/10.3390/ph14040339






