Abstract
Background: CCL-11 (eotaxin) is a chemokine with an important role in allergic conditions. Recent evidence indicates that CCL-11 plays a role in brain disorders as well. This paper reviews the associations between CCL-11 and aging, neurodegenerative, neuroinflammatory and neuropsychiatric disorders. Methods: Electronic databases were searched for original articles examining CCL-11 in neuropsychiatric disorders. Results: CCL-11 is rapidly transported from the blood to the brain through the blood-brain barrier. Age-related increases in CCL-11 are associated with cognitive impairments in executive functions and episodic and semantic memory, and therefore, this chemokine has been described as an “Endogenous Cognition Deteriorating Chemokine” (ECDC) or “Accelerated Brain-Aging Chemokine” (ABAC). In schizophrenia, increased CCL-11 is not only associated with impairments in cognitive functions, but also with key symptoms including formal thought disorders. Some patients with mood disorders and premenstrual syndrome show increased plasma CCL-11 levels. In diseases of old age, CCL-11 is associated with lowered neurogenesis and neurodegenerative processes, and as a consequence, increased CCL-11 increases risk towards Alzheimer’s disease. Polymorphisms in the CCL-11 gene are associated with stroke. Increased CCL-11 also plays a role in neuroinflammatory disease including multiple sclerosis. In animal models, neutralization of CCL-11 may protect against nigrostriatal neurodegeneration. Increased production of CCL-11 may be attenuated by glucocorticoids, minocycline, resveratrol and anti-CCL11 antibodies. Conclusions: Increased CCL-11 production during inflammatory conditions may play a role in human disease including age-related cognitive decline, schizophrenia, mood disorders and neurodegenerative disorders. Increased CCL-11 production is a new drug target in the treatment and prevention of those disorders.
Keywords:
brain; behaviour; cytokines; CCL-11; eotaxin; Alzheimer’s disease; aging; stroke; schizophrenia; biomarkers; prevention 1. Introduction
Chemokines consist of a family of small cytokines (7–12 kDa), which prompt directed chemotaxis in nearby responsive cells. Chemokines play a pivotal role in immune functions and inflammatory responses and facilitate leukocyte migration and trafficking [1,2,3]. The discovery of chemokines goes back to 1977 [4,5], but their role in altering the neuroimmune and neurobiological processes did not gain notice until the mid-1990s [6]. Recent studies show a direct role of chemokines in neuroendocrine function, neurotransmission and neurodegeneration within the CNS (Central Nervous System) [7]. Chemokines comprise four families characterized according to the relative position of their cysteine residues and their functions, with CCL (C–C motif chemokine) and CXCL (C-X-C motif Ligand) being the largest [7]. They act by binding to seven transmembrane G protein-coupled receptors, which in turn activate signaling cascades and initiate shape rearrangement and cell movement [7].
One of those chemokines, namely CCL-11 or eosinophil chemotactic protein (eotaxin), is involved in the selective recruitment of eosinophils into inflammatory sites during allergic reactions, and this chemokine is extensively examined in asthma, allergic rhinitis and other eosinophil-related conditions [8].
CCL-11 production is induced by T helper (Th)-2 cytokines, like IL-13 (Interleukin-13), IL-10 (Interleukin-10) and IL-4 (Interleukin-4). It is a product of eosinophils, B-cells, fibroblasts, endothelial cells, macrophages, chondrocytes and other cells [9,10] (Table 1 and Table 2).

Table 1.
Cells producing CCL-11.

Table 2.
Cytokines and other molecules inducing CCL-11.
CCL-11 is transported from the blood to the brain through the Blood-Brain Barrier (BBB) and also synthesized by microglia [11]. Furthermore, there is some evidence that CCL-11 is associated with aging and reduced neurogenesis [12]. Increased levels of CCL-11 have been detected in numerous neuro-inflammatory disorders such as multiple sclerosis [13], as well as neurodegenerative and neuroprogressive disorders including Alzheimer’s disease [8] and psychiatric illnesses including major depression, bipolar disorder and schizophrenia [8,11,13,14,15]. Moreover, increased CCL-11 levels are also associated with neurocognitive deficits in aging, neurodegenerative disorders and major psychiatric disorders such as schizophrenia [11]. This is important, because the association between CCL-11 and hippocampal damage in aging may be important to understand the pathophysiology of Alzheimer’s disease and old-age depression [12,16]. This paper aims to review the associations between CCL-11 and psychiatric disorders and its possible role as an immune biomarker in those disorders.
2. Results and Discussion
2.1. CCL-11 and CCR3 in Allergic Inflammation
Chemokine Receptors (CCRs) can bind to different ligands (CCLs), and chemokines can interact with more than one receptor [17]. The MCP (Monocyte Chemoattractant Protein) family of chemokines binds most often to CCR2, but MCP-2, MCP-3 and MCP-4 can also interact with CCR1 and CCR3 [17]. CCL-11 shows very high homology with the MCP family [18] and CCL-11 signals via the chemokine receptor CCR3 [19]. This receptor is expressed on eosinophils, basophils and Th-2-type lymphocytes, making it an attractive target for allergic disease therapies [19,20]. CCL-11, CCL-24 (eotaxin-2) and CCL-26 (eotaxin-3) all bind to CCR3 [21]. There is some evidence that high concentrations of CCL-11 are sufficient to activate CCR2 in chemotaxis assays and that substimulatory concentrations of CCL-11 can antagonize MCP-1 activity at CCR2, indicating that CCL-11 behaves as a partial agonist at CCR2 [22]. This is in contrast with Ogilvie et al. (2001) who described CCL-11 as a natural antagonist of CCR2 and an agonist of CCR5 [23]. CCL-11 shows a low affinity for binding with CXCR3 (C-X-C chemokine Receptor 3) expressed on Th-1 cells, but it is postulated that this binding can play a role in impaired Th-1 response in pathological conditions [24]. CCL-11 production is stimulated by IL-4, IL-13, IL-10, IL-1β and TNF-α in epithelial cells of the lung and the gastrointestinal tract or fibroblasts [25,26]. In 1994, CCL-11 was identified as a highly specific eosinophil chemokine that can be produced by lymphocytes, macrophages, bronchial smooth muscle cells, endothelial cells and eosinophils and that this chemokine is responsible for the regulation of chemotaxis through binding to the CCR3 [27].
Allergic diseases can be caused by complex interactions between Th-2 cells, mast cells, basophils and eosinophils, which all express CCR3 [28,29]. Romagnani (2002) showed that Th-2 cytokines contribute to the pathogenesis of allergic inflammation, as well as to the manifestation of allergy and asthma and that this proceeds at least in part through the expression of CCR3, which interacts with CCL-11, allowing the recruitment of basophils, eosinophils and mast cells [30]. CCL-11 plays a role in the pathogenesis of allergic airway diseases, inflammatory bowel disorder disease and gastro-intestinal allergic hypersensitivity [30]. Garcia et al. (2005) confirmed the role of CCR3 and CCL-11 (as well as CCR4, CCR8) in allergic inflammation using in vitro/in vivo experimental studies and clinical studies in patients with asthma [31]. The binding of CCL-11 (but also CCL24 and CCL26) to CCR3 is involved in the development of asthma symptoms [21].
Due to the significant role of CCR3 in allergic diseases, research has focused on treatments with chemokine receptor antagonists [32]. For example, inhibition of CCR3 to selectively inhibit eosinophil recruitment into tissue sites can have beneficial effects and be used as an effective therapy for allergic diseases [28].
2.2. CCL-11, the Blood-Brain Barrier and the CNS
CCL-11 is transferred from the blood to brain tissues with a slow phase of influx prior to the rapid phase [33]. The striatum shows an early rapid uptake phase, in contrast to other regions, which present with a delayed uptake phase [33]. CCL-11 may have biphasic effects with neuroprotective and neurotoxic effects, which are detected at physiological and pathological levels of this chemokine, respectively [33]. The same authors also concluded that CCL-11 does not cause a disturbance in the BBB [33]. Nevertheless, CCL-11 may downregulate, in a concentration-dependent manner, the tight junction proteins occludin, zona occludens-1 and claudin-1 in human coronary artery endothelial cells [34], suggesting that CCL-11 may also affect the BBB. In a study that examined patients with schizophrenia, significant associations between increased CCL-11 plasma concentrations and IgA levels directed to claudin-5 (an indicant of BBB breakdown) were found, suggesting that CCL-11 or associated mechanisms may affect the BBB [35].
Previous research [36] showed that CCL-11 is released by activated astrocytes while the CCR3 receptor is expressed by microglia, causing microglial production of reactive oxygen species (ROS) by upregulating NOX1, leading to excitotoxic neuronal death while inhibition of NOX1 can reverse these effects. The same authors showed that the release of CCL-11 by activated astrocytes causes oxidative stress due to microglial NOX1 activation, thereby inducing increased neurotoxicity (an event linked to the pathogenesis of various neurological disorders) [36]. Zhu et al. (2017) concluded that CCR3 is expressed by hippocampal neurons and that treatment of primary hippocampal neuronal cultures with CCL-11 (in vitro) causes activation of cyclin-dependent kinase 5 (Cdk5) and glycogen synthase kinase-3β (GSK3) [37] and that these effects could be blocked using CCR3 specific antagonists. CCR3 and CCR5 are present on microglia of both control and Alzheimer’s disease (AD) brains [38].
2.3. CCL-11: An Endogenous Cognitive Deteriorating Chemokine
Villeda et al. (2011) established that, in animal models, age-associated rises in CCL-11 are associated with deficits in cognitive functions due to decreased neurogenesis and diminished hippocampal-related learning and memory. Young mice administered CCL-11 developed decreased adult neurogenesis in addition to diminished memory and learning, hence identifying CCL-11 as a chemokine that decreases hippocampal functions with increasing age [12]. However, another study could not find a direct effect of CCL-11 on neuronal cells, but established that CCL-11 promotes microglial migration and activation with subsequent production of ROS, which leads to glutamate-induced neuronal cell death [36]. Baruch et al. (2013) showed that a local (choroid plexus epithelium) shift toward Th-2 (T-helper 2) activation initiates IL-4 and subsequently CCL-11 production in association with cognitive deficits [16]. Thus, based on these findings and those of Villeda and Baruch, it may be concluded that age-related increases in CCL-11 may have detrimental effects on central neuronal functions [39]. The latter authors also confirmed that with age, CCL-11 levels rise in both plasma and Cerebral Spinal Fluid (CSF) and also in different neurodegenerative diseases [40].
Peripheral CCL-11 levels increase with age, and people with cognitive impairments tend to present with higher plasma CCL-11 levels than those without [39]. This suggests that CCL-11 could be a means of predicting cognitive impairments in older individuals [41]. In normal healthy volunteers, CCL-11 is significantly associated with age and the results of different neurocognitive probes as assessed with the neuropsychological tests of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) [11]. More specifically, higher serum levels of CCL-11 are significantly correlated with lower scores on assessments of semantic and episodic memory, including the Verbal Fluency Test, Word List Memory, and Word List True Recall [11]. Moreover, CCL-11 was also associated with lowered scores on the Mini-Mental State Examination (MMSE) and diverse executive tests as measured with the Cambridge Neuropsychological Test Automated Battery (CANTAB), including Spatial Working Memory, which probes the task strategy employed by the central executive and executive working memory ability, and One-Touch Stockings of Cambridge (OTS), which probes spatial planning [11]. Moreover, age and CCL-11 have similar effects on all those neuro-cognitive tests, while CCL-11 is a partial mediator of the effects of age on these tests [11]. Furthermore, a “super-variable” comprising both age and CCL-11 exerted much stronger effects on these different tests. For example, this super-variable explained 75% of the variance in executive functions and 44.3% of the variance in an index of semantic memory. Therefore, these authors concluded that CCL-11 is an Endogenous Cognition Deteriorating Chemokine (ECDC) or “Accelerated Brain-Aging Chemokine” (ABAC) [11].
2.4. CCL-11 in Schizophrenia
Schizophrenia (SCZ) is a chronic psychiatric disorder characterized by neuroprogression, and its aetiology is multifactorial, with genetic and environmental components [42,43]. There is evidence that acute psychotic episodes, chronic schizophrenia and first-episode psychosis are associated with activated macrophage M1, Th-1, Th-2, Th-17 and T regulatory (Treg) responses [42,43,44,45].
CCL-11, as well as other cytokines/chemokines (including CCL-2, CCL-17, CCL-22) are significantly higher in schizophrenic patients as compared with controls [46]. Increased CCL-11 levels show a negative correlation with telomere length and grey matter volume [47]. Combining CCL-11 with four other biomarkers (namely sTNF-R1, sTNF-R2, IL-10 and IL-4) allows predicting the diagnosis of schizophrenia with a sensitivity of 70.0% and a specificity of 89.4% [48]. Frydecka et al. (2018) observed that schizophrenia is accompanied by simultaneous increases in CCL-11 and CCL-2, while increases in both chemokines are known to cause more severe age-related deficiencies in cognitive functions [49]. Recently, it was shown that a combination of CCL-11 with IL-1, IL-1RA, TNF-α, sTNF-R1, sTNFR2 and CCL-2 predicts deficit schizophrenia with a bootstrapped (2000 bootstraps) area under the receiver operating curve of 0.985 [50]. Increased levels of CCL-11 coupled with increased IL-6 and Dickkoph-1-related protein (DKK1) also predict a non-response to treatment with antipsychotics [51].
Most importantly, in schizophrenia, increased levels of CCL-11 strongly impact many neurocognitive tests [9]. Sirivichayakul et al. (2018) established that CCL-11 was highly significantly associated with impairments in many CERAD and CANTAB tests including probes of semantic and episodic memory, as well as executive functions [9]. For example, CCL-11 alone explained 16.0% of the variance in the Verbal Fluency Test (VFT) results and 11.0% of the variance in an index of semantic memory [9]. Interestingly, also formal thought disorders, a key symptom of schizophrenia, were significantly associated with increased levels of CCL-11 [9]. Another study observed highly significant associations between increased CCL-11 levels and cognitive impairments in attention, working memory, episodic and semantic memory and executive functions [50].
Moreover, increased CCL-11 plasma levels are also associated with increased severity scores on different symptom domains of schizophrenia [8,9,50,51,52]. First, in schizophrenia, positive correlations were established between increased CCL-11 levels and negative symptoms [8,9,50,52], but also with psychosis, hostility, excitation, mannerism and psychomotor retardation [9,50,51]. The impact of CCL-11 on these symptoms may be increased by combining CCL-11 levels with other neurotoxic compounds including tryptophan catabolites such as picolinic and xanthurenic acid [11].
Therefore, it was concluded that CCL-11 alone or together with other immune products including TRYCATs, IL-1β, IL-6 and TNF-α, exerts neurotoxic effects on neuronal cells, thereby causing neurocognitive impairments and the symptom domains of schizophrenia [45]. Moreover, such effects may be aggravated by impairments in the Compensatory Immune-Regulatory System (CIRS), including lowered levels of natural IgM directed against oxidative specific epitopes [45].
2.5. CCL-11 in Mood Disorders
After the first study reporting increased levels of CCL-11 in patients with major depression, especially associated with suicidal ideation in 2012, Magalhaes et al. (2014) and Barbosa et al. (2013) also reported increased levels of CCL-11 in patients with bipolar disorder [53,54]. Not only in bipolar disorder, also in persistent depressive disorder or dysthymia, CCL-11 is found to be increased [55]. Simon et al. (2008) assessed serum levels of 22 cytokines/chemokines, including CCL-11, in 49 patients with major depression and 49 matched controls, and reported increased levels of CCL-11 in the context of a “generalized chronic inflammatory state” [56]. Texeira et al. (2018) reported similar results in an independent cohort of patients with major depression, indicating that increased serum levels of CCL-11 were associated with suicidal ideation [52]. Nevertheless, a recent meta-analysis of studies evaluating CCL-11 in depression including 454 participants failed to identify significant differences in CCL-11 between depressed and control subjects [57]. It is possible, however, that this meta-analysis also included patients with milder depression symptoms or different comorbidities explaining the differences between studies [57]. It should be added that a recent paper was unable to detect significant alterations in serum CCL-11 in children with major depression as compared with controls while immune-inflammatory markers were clearly elevated [50]. In cocaine use disorder, CCL-11 combined with TNF-α, IL-1β, CXCL12, CCL-2 and CX3CL1 (C-X3-C motif chemokine Ligand 1) can be used to distinguish primary major depression from substance-induced major depression, indicating that plasma concentrations of CCL-11 may be used as a potential biological marker to differentiate between primary and substance-induced depression [58].
Increased CCL-11 levels may also play a role in Premenstrual Syndrome (PMS), re-labelled as a Menstrual Cycle-Associated Syndrome (MCAS) [45]. Thus, a recent paper shows that CCL-11 is (along with CCL-2 and CCL-5) significantly increased in MCAS as compared with women without MCAS. The increased levels of CCL-11 (and CCL-2, CCL-5, CXCL10 and CXCL8) are highly significantly associated with the severity of MCAS symptoms as measured with the Daily Record of Severity of Problems (DRSP) score [45]. Moreover, the sum of three neurotoxic chemokines (namely CCL-2 + CCL-11 + CCL-5) is significantly associated with the depressive and anxiety subdomains of the MCAS [45]. This is important as MCAS/PMS is one of the predictors of mood disorders including major depression, and therefore, these findings further suggest that CCL-11 may be associated with the pathophysiology of depression (Table 3).

Table 3.
Studies on CCL-11 regarding different psychiatric disorders.
2.6. CCL-11 in Other Psychiatric Disorders
2.6.1. CCL-11 in Obsessive-Compulsive Disorder
In the study of Fontenelle (2012), forty patients with OCD and 40 healthy controls had their plasma assessed for chemokines including CCL-11 (and CCL-2, CCL-3, CCL-24, CXCL8, CXCL9, CXCL10) and other immune mediators like TNF-α, sTNFR1, sTNFR2 and interleukin-1 receptor antagonist [59]. However, there were no significant differences in the blood levels of CCL-11 between patients with OCD and controls, whereas CCL-3, CXCL8, sTNFR1 and sTNFR2 were significantly increased [59] (Table 3).
2.6.2. CCL-11 in Autism Spectrum Disorder
There are few studies that show high levels of CCL-11 in ASD [60,61,62]. A meta-analysis reviewed the results of 17 ASD studies with a total sample size of 743 patients with ASD versus 592 healthy controls [62]. This study examined 19 cytokines and reported significantly higher plasma/serum levels of 12 of the cytokines/chemokines in ASD versus controls including CCL-11 (p = 0.01), IL-1β (p < 0.001), IL-6 (p = 0.03), IL-8 (p = 0.04), IFN-γ (p = 0.02) and CCL-2 (p < 0.05), whereas TGF-β1 levels were significantly lower in ASD (p < 0.001) [62]. Importantly, increased CCL-11 (and IL-6, IL-10 and MCP-3) were found in the anterior cingulate gyrus and increased CCL-2 and TGF-β1 in the CSF, anterior cingulate gyrus and cerebellum of ASD brain tissues [63] (Table 3).
2.6.3. CCL-11 in Substance Abuse Disorders
Kuo et al. (2018) included 344 heroin-dependent Taiwanese patients under methadone maintenance treatment as compared with 87 normal control subjects in order to investigate plasma CCL-11 and a SNP (Single-Nucleotide Polymorphism) of the CCL-11 gene and Fibroblast Growth Factor-2 (FGF-2). In patients, but not normal controls, CCL-11 showed an adequate sensitivity and specificity in association with age using a cut-off at 45 years whilst increased plasma FGF-2 levels were correlated with the high CCL-11 level [64]. Decreased plasma levels of CCL-11 were observed in 87 abstinent patients with alcohol use disorder as compared with 55 controls [65]. Moreover, subjects with mood disorders and/or anxiety had lower CCL-11 concentrations than non-comorbid patients, and this effect was pronounced in women [65]. Preclinical models of alcohol use in male Wistar rats showed alcohol-induced circulating chemokine alterations in CCL-11, CXCL12 and CX3CL1 [65], indicating an important contribution of CCL-11 to alcohol use disorder (Table 3).
2.7. CCL-11 in Neuro-Inflammatory Disorders
2.7.1. CCL-11 and Parkinson’s Disease
Parkinson’s disease is a neuro-inflammatory disorder in which immune-inflammatory processes are involved in the degeneration of dopaminergic neurons via, amongst other mediators, chemokines [66]. Scalzo et al. (2011) found no significant differences in chemokine levels between patients with Parkinson’s disease and controls [66]. Lindqvist et al. (2013) measured immune-inflammatory biomarkers in CSF samples from Parkinson’s disease patients to determine the relationships with non-motor Parkinson’s disease symptoms, and they reported increased levels of CSF CCL-11, C-reactive protein, IL-6 and TNF-α in association with depression, fatigue and cognitive impairments [67]. On the other hand, Moghadam-Ahmadi et al. (2018) concluded that CCL-11 is not involved in the diagnosis or treatment of Parkinson’s disease [68]. In animal models, Chandra et al. (2017) reported that neutralization of CCL-11 and CCL-5 may protect against nigrostriatal neurodegeneration and therefore the progression of Parkinson’s disease [69].
2.7.2. CCL-11 and Alzheimer’s Disease
As mentioned above, CCL-11 is known to cause cognitive decline with age, but less is known regarding its involvement in the pathogenesis of Alzheimer’s diseases. CCL-11 is considered to contribute a probable risk factor for the development of Alzheimer’s disease [37]. CCR3 and CCR5, which are present on microglia, are more expressed by reactive microglia of patients with Alzheimer’s disease than controls [38]. Lalli et al. (2015) identified a haplotype of SNPs on chromosome 17 within a chemokine gene cluster that modifies the age of onset of Alzheimer’s disease and additionally confers a strong protective effect. Importantly, this haplotype disrupts the associations between increasing age and increasing CCL-11 levels, suggesting CCL-11 may be a novel modifier of Alzheimer’s disease [70]. Guerreiro et al. (2015) reported that the association between the age of onset of Alzheimer’s disease and CCL-11 could lead to the development of immunomodulating therapies, which could be used to delay the onset of the disease [71]. Nevertheless, another study showed that CCL-2 is a better biomarker than CCL-11 for the progression of Alzheimer’s disease, although both chemokines employ the same CCR2 signaling pathway for the accumulation of microglia at sites of neuroinflammation [72]. Deleting CCR3 in mice induces a decrease in synaptic loss, as well as a decrease in spatial learning and memory deficits, further suggesting that age-related increments in CCL-11 confer risk to Alzheimer’s disease [37]. Similar findings were reported by Baruch et al. (2013) who showed that increased production of CCL-11 due to destructive Th-2 inflammation is associated with cognitive dysfunctions [16]. Overall, it appears that age-related increases in CCL-11 confer risk for Alzheimer’s disease while antagonizing CCR3 may lead to therapeutic benefits [37].
2.7.3. CCL-11 and Multiple Sclerosis
Multiple Sclerosis (MS) is a chronic autoimmune and neuro-inflammatory disease of the central nervous system characterized by damage to myelinated axons with varying degrees of destruction of myelin and axons [73,74]. In a meta-analysis, which reviewed 226 research papers and 13,526 patients, Bai et al. (2019) found that 13 CSF cytokines (from a list of 26) and 21 blood cytokines (from a list of 37) were elevated in MS as compared with controls and that CSF CCL-11 was significantly increased with a large effect size in patients with MS [75]. Huber et al. (2018) reported that during the relapse phase, CCL-11 may be downregulated, while during the secondary progressive phase, CCL-11 is upregulated to achieve plateau levels [40]. In other studies, it was detected that CCL-11 (and CCL-5) may discriminate clinical subtypes of multiple sclerosis [76,77,78]. Thus, both CCL-11 and CCL-5 were lowered during the inflammatory phase as compared with progressive multiple sclerosis [77]. Michael et al. (2013) identified important chemokine profile markers including CCL-11 in patients with Neuromyelitis Optica (NMO), which were additionally different from those with MS [76]. These authors examined 29 aquaporin antibody-positive NMO patients and found CCL-11 (and CCL-4, G-CSF and myeloperoxidase) to be an important marker in NMO [76]. In NMO Spectrum Disorders (NMOSD), increasing production of TNF-α and interleukin-1β can stimulate CCL-11 binding to CCR3, which may lead to eosinophil hypersensitivity during remission, but not in MS [77]. Moreover, increased CCL-11 levels may be a critical step in NMOSD eosinophil restoration during remission while both elevated levels of CCL-11 and CCL-13 may be important in eosinophil recruitment during NMOSD remission [77,78].
Autoimmune encephalomyelitis (EAE), an experimental model of multiple sclerosis, is accompanied by elevated levels of CCL-11 in the CSF, a tighter blood-brain barrier, reduced antigen-specific responses and a predominant anti-inflammatory Th-2 phenotype, suggesting that CCL-11 may protect against neuroinflammation in this model [79].
2.7.4. CCL-11 and Stroke
CCL-11 is not only an immune marker, which has great importance in aging, neuroinflammation and neurodegeneration, but this chemokine is also associated with stroke [80,81]. Khavinson et al. (2016) reviewed that cardiovascular disorders are accompanied by increased CCL-11 concentrations, and accordingly, these authors proposed to use the term “protein of senility” to describe the detrimental effects of CCL-11, this in contrast to lowered levels of plasma Growth Differentiation Factor (GDF)11, which increases the risk of myocardial infarction and stroke and, therefore, should be considered as a “protein of juvenility” [80]. Bone GDF11 or Morphogenetic Protein 11 (BMP 11) is a Transforming Growth Factor-β (TGF-β) family member protein that is produced by humans, rats and mice [80]. Interestingly, GDF11 has paradoxical effects starting from embryonic development, and the role of GDF11 in aging and medical disease varies throughout the lifespan [81]. A survey performed by Sharma et al. (2014) indicated that CCL-11 may be employed as one of the most important biomarkers for acute stroke even in patients with stroke-like symptoms [81]. Selected from a list of 262 potential biomarkers, the latter authors selected five biomarkers, which were combined in a model built with stepwise selection, validated by bootstrapping and included CCL-11, epidermal growth factor receptor, S100A12, metalloproteinase inhibitor-4 and prolactin. This model could not only be used as an external validating criterion for the diagnosis stroke, but also as a biomarker for the management of stroke and stroke-like symptoms in patients [81]. Roy-O’Reilly et al. (2017) showed lower CCL-11 levels in stroke patients as compared with controls and, additionally, that lower post-stroke CCL-11 levels predict increased stroke severity and poorer functional outcomes 12 months after ischemic stroke [82]. Moreover, a CCL-11 gene polymorphism is associated with different types of ischaemic stroke [83]. A study on various CCL-11 gene variants in Chinese people suggested a strong association with ischaemic stroke, although there were no associations with ischaemic stroke subtypes [84]. Six tag SNPs in the CCL-11 gene (rs1129844, rs17809012, rs1860183, rs1860184, rs4795898 and rs4795895) were studied in 620 patients with stroke and in a control group of 425 Han population patients in China [83]. These authors reported that all polymorphisms of the CCL-11 gene had different effects on the pathogenesis of lacunar stroke [83]. Another large-n study stratified 1500 ischaemic stroke patients into TOAST (Trial of ORG 10172 in Acute Stroke Treatment) subtypes and reported significant associations among the -1382A>G variant of the CCL-11 gene with intracranial large artery atherosclerosis and small vessel occlusion [85].
2.8. Possible Novel Treatments Targeting CCL-11
Resveratrol and its metabolites modulate the expression of CCL-11 [86]. In 2011, Yang et al. investigated the effects of resveratrol in modulating inflammation by determining the expression and release of CCL-11 in cultured Human Pulmonary Artery Endothelial Cells (HPAEC) treated with the proinflammatory cytokines IL-13 and TNF-α [86]. Exposure to resveratrol suppressed IL-13- and TNF-α-induced CCL-11 gene expression, as well as attenuated CCL-11 promoter activity, in association with inhibition of Janus Kinase-1 (JAK-1) expression, reduction in phosphorylated-STAT6 and decrements in the p65 subunit of NF-κB [86]. Not only does resveratrol have the ability to modulate CCL-11, but also piceatannol, one of resveratrol’s metabolites, had a similar potency as resveratrol [86]. This in vitro model can be used for further screening and discovering polyphenols with anti-inflammatory activities [86].
A human single-chain fragment variable antibody that neutralizes human CCL-11 (CAT-212) was produced using antibody phage display and converted to whole antibody IgG4 format (CAT-213) [87]. Further optimization entailed a reduction of the length of the variable heavy chain complementarity-determining region 3 by one amino acid resulting in a 1000-fold increase in potency compared with the parent anti-CCL11 antibody [87]. CAT-213 neutralizes the ability of CCL-11 to increase intracellular calcium signaling (with an IC(50) value of 2.86 nM), migration of CCR3-expressing L1.2 cells (with an IC(50) value of 0.48 nM) and inhibition of the CCL-11-evoked shape change of human eosinophils in vitro [87]. CAT-213 and CAT-212 do not bind or neutralize MCP-1, a chemokine with a similar structure [87]. In vivo and in vitro probes conducted by those authors with recombinant human CCL-11 (Cambridge Bioscience and R&D Systems), mouse CCL-11 (R&D Systems) and synthetic human, rat and monkey CCL-11 (Albachem Limited) showed that CAT-212 and CAT- 213 are potent and may be used as a therapy targeting high CCL-11 levels [87]. New clinical trials with anti-CCL11 neutralizing antibodies are expected to have encouraging results in inflammatory diseases [87].
Minocycline treatment can significantly reduce the amounts of several inflammatory factors, including CCL-11 (and CCL-2, MCP-5, IL-6 and IL-10), and therefore, this drug has potent anti-inflammatory and neuroprotective properties in rodent models of Huntington’s, Parkinson’s, Alzheimer’s and motor neuron disease [88].
The glycosaminoglycan heparin has anti-inflammatory activity and is exclusively found in mast cells, which are localized within Airway Smooth Muscle (ASM) bundles of asthmatic airways [89]. IL-13 induces the production of multiple inflammatory mediators from ASM including CCL-11 [89]. Inhibition of IL-13-dependent CCL-11 release by heparin involves, but does not depend on sulphation, though the loss of N-sulphation reduced the attenuating activity, which could be restored by N-acetylation [89].
In vitro studies have shown an inhibitory effect of glucocorticosteroids on CCL-11 production, although there are few in vivo studies [90,91,92]. The use of glucocorticosteroids to inhibit CCL-11 mRNA expression was studied by Jahnsen et al. (1999) who examined transcript levels and chemotactic activity of CCR3-binding chemokines in nasal polyps. Treatments with glucocorticosteroids reduce mRNA levels in polyps to levels that are found in turbinate mucosa for all chemokines [90]. Whether this result was caused indirectly via extracellular mediators that regulate chemokine production or by a direct effect of glucocorticoids could not be determined. Nevertheless, glucocorticoids may directly inhibit cytokine-induced production of CCL-11 (and MCP-4 and CCL5) in epithelial cells in vitro [91,92].
Interestingly, the detrimental effects of CCL-11 suppressing hippocampal neurogenesis can be attenuated by supra-lactate threshold exercise, hence supporting the benefits of exercise on the aging brain [93].
3. Materials and Methods
We searched online libraries, including PubMed/MEDLINE, Google Scholar and Scopus. The main search terms were “Chemokine CCL11” [MeSH] (Medical Subject Headings) or “biomarkers” [MeSH] and “stress” [MeSH]” and “Chemokine CCL11” [MeSH], “schizophrenia” and “CCL11” [MeSH] and “brain” [MeSH] with filters activated, namely publication date from 01/01/1990 to 31/12/2019 and papers written in English. Thus, our references were 94 (ninety-four).
4. Conclusions
Increased CCL-11 production in the course of immune-mediated inflammatory conditions may play a role in age-related cognitive decline, schizophrenia, mood disorders, Alzheimer’s disease, OCD and ASD. Plasma levels of CCL-11 are increased not only in schizophrenia and age-related cognitive impairments, but also in some patients with mood disorders and premenstrual syndrome. Increased CCL-11 levels including in old age are associated with neurodegeneration, reduced neurogenesis and an increased risk of Alzheimer’s disease. Increased CCL-11 also plays a role in neuroinflammatory disease including multiple sclerosis. Therefore, increased CCL-11 is a new drug target for the treatment and prevention of those disorders. The production of CCL-11 may be attenuated by glucocorticoids, minocycline, resveratrol, CCR3 antagonists (R321) and anti-CCL11 antibodies including CAT-212 and CAT-213. In sum, these findings show real encouraging results for future treatment of all conditions characterized by increased CCL-11 levels (Figure 1).
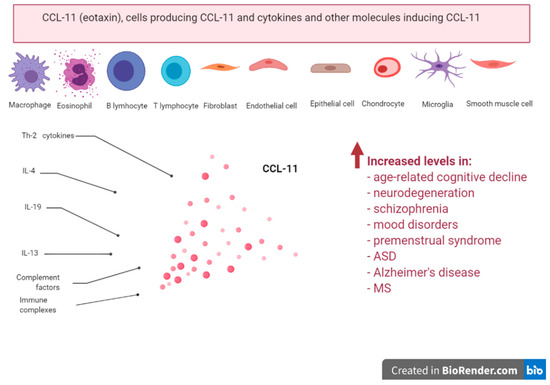
Figure 1.
Take-home message; producers and inducers of CCL-11; increased levels in age-related cognitive decline, neurodegeneration, schizophrenia, mood disorders, premenstrual syndrome, ASD, Alzheimer’s disease and multiple sclerosis (MS).
Author Contributions
Conceptualization, M.I. and M.M. (Michael Maes); methodology, M.I., M.M. (Michael Maes); software, M.I.; validation, M.I., Z.A., M.M. (Michael Maes), M.M. (Marianna Murdjeva); formal analysis, M.I.; investigation, M.I., Z.A., A.M., D.M.; resources, M.I., Z.A.; data curation, M.I.; writing—original draft preparation, M.I., M.M. (Michael Maes); writing—review and editing, M.I., A.M., D.M., M.M. (Michael Maes), M.M. (Marianna Murdjeva); visualization, M.I.; supervision, M.M. (Michael Maes), M.M. (Mariana Murdjeva); project administration, M.I.; funding acquisition, M.I. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledged support (financial, computational, logistic) from the project BG05M2OP001-1.002-0005 /29.03.2018 (2018–2023)—Center for Competence “Personalized Innovative Medicine (PERIMED)”, funded by the Science and Education for Smart Growth Operational Programme, co-funded by the European Union through the European Structural and Investment Funds.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ABAC | Accelerated Brain-Ageing Chemokine |
| AD | Alzheimer’s Disease |
| Anti-CCL11 | Eotaxin-1 Antibody |
| ASD | Autism Spectrum Disorder |
| ASM | Airway Smooth Muscle |
| BBB | Blood-Brain Barrier |
| BMP | Bone Morphogenetic Proteins |
| CANTAB | Cambridge Neuropsychological Test Automated Battery |
| CCL | C-C motif chemokine Ligand |
| CCL-11 | Eotaxin-1, eosinophil chemotactic protein |
| CCL-2 | C-C motif chemokine Ligand 2 |
| CCL-5 | C-C motif chemokine Ligand 5 |
| CCL-24 | Eotaxin-2 |
| CCL-26 | Eotaxin-3 |
| CCR | Chemokine Receptors |
| Cdk5 | Cyclin-dependent kinase 5 |
| CERAD | Consortium to Establish a Registry for Alzheimer’s Disease |
| CIRS | Compensatory Immune-Regulatory System |
| CNS | Central Nervous System |
| CSF | Cerebrospinal Fluid |
| CX3CL1 | C-X3-C motif chemokine Ligand 1 |
| CXCL | C-X-C motif Ligand |
| CXCR3 | C-X-C chemokine receptor 3 |
| CXCL9 | C-C motif chemokine Ligand 9 |
| CXCL8 | C-C motif chemokine Ligand 8 |
| CXCL12 | C-C motif chemokine Ligand 12 |
| DKK1 | Dickkoph-1-related protein |
| DRSP | Daily Record of Severity of Problems |
| EAE | Autoimmune Encephalomyelitis |
| ECDC | Endogenous Cognition Deteriorating Chemokine |
| FGF-2 | Fibroblast Growth Factor- 2 |
| GDF | Growth Differentiation Factor |
| GSK3 | Glycogen Synthase Kinase-3β |
| HPAEC | Human Pulmonary Artery Endothelial Cells |
| IFN-γ | Interferon-gamma |
| IgA | Immunoglobulin A |
| IgG4 | Immunoglobulin G4 |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-13 | Interleukin-13 |
| IL-1β | Interleukin-1 beta |
| IL-1RA | Interleukin-1 Receptor Antagonist |
| MCAS | Menstrual Cycle-Associated Syndrome |
| MCP | Monocyte Chemoattractant Protein |
| MMSE | Mini-Mental State Examination |
| MS | Multiple Sclerosis |
| NF-κB | Nuclear Factor-kappa B |
| NMO | Neuromyelitis Optica |
| NMOSD | NMO Spectrum Disorders |
| NOX1 | NADPH Oxidase 1 |
| OCD | Obsessive-Compulsive Disorder |
| OTS | One-Touch Stockings of Cambridge |
| PMS | Premenstrual Syndrome |
| ROS | Reactive Oxygen Species |
| SCZ | Schizophrenia |
| SNPs | Single-Nucleotide Polymorphisms |
| sTNF-R1 | Soluble Tumour Necrosis Factor-Receptor 1 |
| sTNF-R2 | Soluble Tumour Necrosis Factor-Receptor 2 |
| TGF-β1 | Transforming Growth Factor-beta 1 |
| Th-1 | T helper-1 |
| Th-2 | T helper-2 |
| Th-17 | T helper-17 |
| TNF-α | Tumour Necrosis Factor-alpha |
| TOAST | Trial of ORG 10172 in Acute Stroke Treatment |
| Treg | T regulatory |
| TRYCAT | Tryptophan Catabolites |
| VFT | Verbal Fluency Test |
References
- Foxman, E.F.; Campbell, J.J.; Butcher, E.C. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J. Cell Biol. 1997, 139, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.M.; Baggiolini, M.; Charo, I.F.; Hébert, C.A.; Horuk, R.; Matsushima, K.; Miller, L.H.; Oppenheim, J.J.; Power, C.A. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000, 52, 145–176. [Google Scholar] [PubMed]
- Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 1994, 76, 301–314. [Google Scholar] [CrossRef]
- Wu, V.Y.; Walz, D.A.; McCoy, L.E. Purification and characterization of human and bovine platelet factor 4. Prep. Biochem. 1977, 7, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Callewaere, C.; Banisadr, G.; Rostène, W.; Parsadaniantz, S.M. Chemokines and chemokine receptors in the brain: Implication in neuroendocrine regulation. J. Mol. Endocrinol. 2007, 38, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Tani, M.; Ransohoff, R.M. Do chemokines mediate inflammatory cell invasion of the central nervous system parenchyma? Brain Pathol. 1994, 4, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Reaux-Le Goazigo, A.; Van Steenwinckel, J.; Rostene, W.; Melik Parsadaniantz, S. Current status of chemokines in the adult CNS. Prog. Neurobiol. 2013, 104, 67–92. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Gama, C.S.; Rocha, N.P.; Teixeira, M.M. Revisiting the Role of Eotaxin-1/CCL11 in Psychiatric Disorders. Front. Psychiatr. 2018, 9, 241. [Google Scholar] [CrossRef]
- Sirivichayakul, S.; Kanchanatawan, B.; Thika, S.; Carvalho, A.F.; Maes, M. A new schizophrenia model: Immune activation is associated with induction of different neurotoxic products which together determine memory impairments and schizophrenia symptom dimensions. CNS Neurol. Disord. Drug Targets 2019, 18, 124–140. [Google Scholar] [CrossRef]
- Kindstedt, E.; Holm, C.K.; Sulniute, R.; Martinez-Carrasco, I.; Lundmark, R.; Lundberg, P. CCL11, a novel mediator of inflammatory bone resorption. Sci. Rep. 2017, 7, 5334. [Google Scholar] [CrossRef]
- Sirivichayakul, S.; Kanchanatawan, B.; Thika, S.; Carvalho, A.F.; Maes, M. Eotaxin, an Endogenous Cognitive Deteriorating Chemokine (ECDC), Is a Major Contributor to Cognitive Decline in Normal People and to Executive, Memory, and Sustained Attention Deficits, Formal Thought Disorders, and Psychopathology in Schizophrenia Patients. Neurotox. Res. 2019, 35, 122–138. [Google Scholar] [CrossRef]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef]
- Sørensen, T.L.; Tani, M.; Jensen, J.; Pierce, V.; Lucchinetti, C.; Folcik, V.A.; Qin, S.; Rottman, J.; Sellebjerg, F.; Strieter, R.M.; et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Investig. 1999, 103, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Eyre, H.; Baune, B.T. Neuroplastic changes in depression: A role for the immune system. Psychoneuroendocrinology 2012, 37, 1397–1416. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.J.; Corrigan, F.; Baune, B.T. Knockout of CXCR5 increases the population of immature neural cells and decreases proliferation in the hippocampal dentate gyrus. J. Neuroinflamm. 2014, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Baruch, K.; Ron-Harel, N.; Gal, H.; Deczkowska, A.; Shifrut, E.; Ndifon, W.; Mirlas-Neisberg, N.; Cardon, M.; Vaknin, I.; Cahalon, L.; et al. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc. Natl. Acad. Sci. USA 2013, 110, 2264–2269. [Google Scholar] [CrossRef]
- Yamagami, S.; Tanaka, H.; Endo, N. Monocyte chemoattractant protein-2 can exert its effects through the MCP-1 receptor (CC CKR2B). FEBS Lett. 1997, 400, 329–332. [Google Scholar] [CrossRef]
- Jose, P.J.; Griffiths-Johnson, D.A.; Collins, P.D.; Walsh, D.T.; Moqbel, R.; Totty, N.F.; Truong, O.; Hsuan, J.J.; Williams, T.J. Eotaxin: A potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J. Exp. Med. 1994, 179, 881–887. [Google Scholar] [CrossRef]
- Ponath, P.D.; Qin, S.; Post, T.W.; Wang, J.; Wu, L.; Gerard, N.P.; Newman, W.; Gerard, C.; Mackay, C.R. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J. Exp. Med. 1996, 183, 2437–2448. [Google Scholar] [CrossRef]
- Sallusto, F.; Mackay, C.R.; Lanzavecchia, A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science 1997, 277, 2005–2007. [Google Scholar] [CrossRef]
- Pease, J.E. Asthma, allergy and chemokines. Curr. Drug Targets 2006, 7, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, R.; Sabroe, I.; LaRosa, G.; Williams, T.J.; Pease, J.E. The CC chemokine eotaxin (CCL11) is a partial agonist of CC chemokine receptor 2b. J. Biol. Chem. 2001, 276, 42957–42964. [Google Scholar] [CrossRef]
- Ogilvie, P.; Bardi, G.; Clark-Lewis, I.; Baggiolini, M.; Uguccioni, M. Eotaxin is a natural antagonist for CCR2 and an agonist for CCR5. Blood 2001, 97, 1920–1924. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Siciliano, S.J.; Waldburger, K.E.; Sirotina-Meisher, A.; Staruch, M.J.; Daugherty, B.L.; Gould, S.L.; Springer, M.S.; DeMartino, J.A. Binding and functional properties of recombinant and endogenous CXCR3 chemokine receptors. J. Biol. Chem. 1998, 273, 18288–18291. [Google Scholar] [CrossRef] [PubMed]
- Paplińska, M.; Grubek-Jaworska, H.; Chazan, R. Role of eotaxin in the pathophysiology of asthma. Pneumonol. Alergol. Pol. 2007, 75, 180–185. [Google Scholar] [PubMed]
- Lv, J.; Xiong, Y.; Li, W.; Cui, X.; Cheng, X.; Leng, Q.; He, R. IL-37 inhibitsIL-4/IL-13-induced CCL11 production and lung eosinophilia in murine allergic asthma. Allergy 2018, 73, 1642–1652. [Google Scholar] [CrossRef]
- Amerio, P.; Frezzolini, A.; Feliciani, C.; Verdolini, R.; Teofoli, P.; De Pità, O.; Puddu, P. Eotaxins and CCR3 receptor in inflammatory and allergic skin diseases: Therapeutical implications. Curr. Drug Targets Inflamm. Allergy 2003, 2, 81–94. [Google Scholar] [CrossRef]
- Erin, E.M.; Williams, T.J.; Barnes, P.J.; Hansel, T.T. Eotaxin receptor (CCR3) antagonism in asthma and allergic disease. Curr. Drug Targets Inflamm. Allergy 2002, 1, 201–214. [Google Scholar] [CrossRef]
- Lacy, P. Chapter-2 Eosinophil Cytokines in Allergy. Cytokine Eff. Funct. Tissues 2017, 173–218. [Google Scholar] [CrossRef]
- Romagnani, S. Cytokines and chemoattractants in allergic inflammation. Mol. Immunol. 2002, 38, 881–885. [Google Scholar] [CrossRef]
- Garcia, G.; Godot, V.; Humbert, M. New chemokine targets for asthma therapy. Curr. Allergy Asthma Rep. 2005, 5, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Elsner, J.; Escher, S.E.; Forssmann, U. Chemokine receptor antagonists: A novel therapeutic approach in allergic diseases. Allergy 2004, 59, 1243–1258. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.A.; Morofuji, Y.; Owen, J.B.; Banks, W.A. Rapid transport of CCL11 across the blood-brain barrier: Regional variation and importance of blood cells. J. Pharmacol. Exp. 2014, 349, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, M.S.; Wang, X.; Wang, H.; Rafael, C.; Yao, Q.; Chen, C. Eotaxin increases monolayer permeability of human coronary artery endothelial cells. Arter. Thromb. Vasc. Biol. 2009, 29, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Sirivichayakul, S.; Kanchanatawan, B.; Vojdani, A. Breakdown of the paracellular tight and adherens junctions in the gut and blood brain barrier and damage to the vascular barrier in patients with deficit schizophrenia. Neurotox. Res. 2019, 36, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, B.; Horiuchi, H.; Mizuno, T.; Takeuchi, H.; Suzumura, A. CCL11 enhances excitotoxic neuronal death by producing reactive oxygen species in microglia. Glia 2015, 63, 2274–2284. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, B.; Sun, X.; Zhu, Q.; Sui, Y. Targeting CCR3 to Reduce Amyloid-β Production, Tau Hyperphosphorylation, and Synaptic Loss in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 7964–7978. [Google Scholar] [CrossRef]
- Xia, M.Q.; Qin, S.X.; Wu, L.J.; Mackay, C.R.; Hyman, B.T. Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer’s disease brains. Am. J. Pathol. 1998, 153, 31–37. [Google Scholar] [CrossRef]
- Hoefer, J.; Luger, M.; Dal-Pont, C.; Culig, Z.; Schennach, H.; Jochberger, S. The "aging factor" eotaxin-1 (ccl11) is detectable in transfusion blood products and increases with the donor’s age. Front. Aging Neurosci. 2017, 9, 402. [Google Scholar] [CrossRef]
- Huber, A.K.; Giles, D.A.; Segal, B.M.; Irani, D.N. An emerging role for eotaxins in neurodegenerative disease. Clin. Immunol. 2018, 189, 29–33. [Google Scholar] [CrossRef]
- Butcher, L.; Pérès, K.; André, P.; Morris, R.H.; Walter, S.; Dartigues, J.-F.; Rodriguez-Mañas, L.; Féart, C.; Erusalimsky, J.D. Association between plasma CCL11 (eotaxin-1) and cognitive status in older adults: Differences between rural and urban dwellers. Exp. Gerontol. 2018, 113, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Meltzer, H.Y.; Buckley, P.; Bosmans, E. Plasma-soluble interleukin-2 and transferrin receptor in schizophrenia and major depression. Eur. Arch. Psychiatry Clin. Neurosci. 1995, 244, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Noto, C.; Ota, V.K.; Gouvea, E.S.; Rizzo, L.B.; Spindola, L.M.; Honda, P.H.; Cordeiro, Q.; Belangero, S.I.; Bressan, R.A.; Gadelha, A.; et al. Effects of risperidone on cytokine profile in drug-naïve first-episode psychosis. Int. J. Neuropsychopharmacol. 2014, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Noto, C.; Ota, V.K.; Santoro, M.L.; Gouvea, E.S.; Silva, P.N.; Spindola, L.M.; Cordeiro, Q.; Bressan, R.A.; Gadelha, A.; Brietzke, E.; et al. Depression, cytokine, and cytokine by treatment interactions modulate gene expression in antipsychotic naïve first episode psychosis. Mol. Neurobiol. 2016, 53, 5701–5709. [Google Scholar] [CrossRef]
- Roomruangwong, C.; Sirivichayakul, S.; Carvalho, A.F.; Maes, M. The uterine-chemokine-brain axis: Menstrual cycle-associated symptoms (mcas) are in part mediated by CCL2, CCL5, CCL11, CXCL8 and CXCL10. Preprints 2019, 2019090329. [Google Scholar] [CrossRef]
- Hong, S.; Lee, E.; Martin, S.; Soontornniyomkij, B.; Soontornniyomkij, V.; Achim, C.L.; Reuter, C.; Irwin, M.R.; Eyler, L.T.; Jeste, D.V. Abnormalities in chemokine levels in schizophrenia and their clinical correlates hhs public access. Schizophr. Res. 2017, 181, 63–69. [Google Scholar] [CrossRef]
- Czepielewski, L.S.; Massuda, R.; Panizzutti, B.; Grun, L.K.; Barbe-Tuana, F.M.; Teixeira, A.L.; Barch, D.M.; Gama, C.S. Telomere length and CCL11 levels are associated with gray matter volume and episodic memory performance in schizophrenia: Evidence of pathological accelerated aging. Schizophr. Bull. 2018, 44, 158–167. [Google Scholar] [CrossRef]
- Noto, C.; Maes, M.; Ota, V.K.; Teixeira, A.L.; Bressan, R.A.; Gadelha, A.; Brietzke, E. High predictive value of immune-inflammatory biomarkers for schizophrenia diagnosis and association with treatment resistance. World J. Biol. Psychiatry 2015, 27, 422–429. [Google Scholar] [CrossRef]
- Frydecka, D.; Krzystek-Korpacka, M.; Lubeiro, A.; Stramecki, F.; Stańczykiewicz, B.; Beszłej, J.; Piotrowski, P.; Kotowicz, K.; Szewczuk-Bogusławska, M.; Pawlak-Adamska, E.; et al. Profiling inflammatory signatures of schizophrenia: A cross-sectional and meta-analysis study. Brain Behav. Immun. 2018, 71, 28–36. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Almulla, A.F.; Maes, M. The neuroimmune and neurotoxic fingerprint of major neurocognitive psychosis or deficit schizophrenia: A supervised machine learning study. Neurotox. Res. 2020, 37, 753–771. [Google Scholar] [CrossRef]
- Al-Dujaili, A.H.; Mousa, R.F.; Al-hakeim, H.K.; Maes, M. High mobility group protein 1 and dickkopf-related protein 1 in schizophrenia and treatment-resistant schizophrenia: Associations with interleukin-6, symptom domains, and neurocognitive impairments. Preprints 2019, 2019120100. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Reis, H.J.; Nicolato, R.; Brito-Melo, G.; Correa, H.; Teixeira, M.M.; Romano-Silva, M.A. Increased serum levels of CCL11/eotaxin in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, P.V.; Jansen, K.; Stertz, L.; Ferrari, P.; Pinheiro, R.T.; da Silva, R.A.; Kapczinski, F. Peripheral eotaxin-1 (CCL11) levels and mood disorder diagnosis in a population-based sample of young adults. J Psychiatr. Res. 2014, 48, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, I.G.; Rocha, N.P.; Bauer, M.E.; De Miranda, A.S.; Huguet, R.B.; Reis, H.J.; Zunszain, P.A.; Horowitz, M.A.; Pariante, C.M.; Teixeira, A.L. Chemokines in bipolar disorder: Trait or state? Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 159–165. [Google Scholar] [CrossRef]
- Ho, P.S.; Yen, C.H.; Chen, C.Y.; Huang, S.Y.; Liang, C.S. Changes in cytokine and chemokine expression distinguish dysthymic disorder from major depression and healthy controls. Psychiatry Res. 2017, 248, 20–27. [Google Scholar] [CrossRef]
- Simon, N.M.; McNamara, K.; Chow, C.W.; Maser, R.S.; Papakostas, G.I.; Pollack, M.H.; Nierenberg, A.A.; Fava, M.; Wong, K.K. A detailed examination of cytokine abnormalities in Major Depressive Disorder. Eur. Neuropsychopharmacol. 2008, 18, 230–233. [Google Scholar] [CrossRef]
- Leighton, S.P.; Nerurkar, L.; Krishnadas, R.; Johnman, C.; Graham, G.J.; Cavanagh, J. Chemokines in depression in health and in inflammatory illness: A systematic review and meta-analysis. Mol. Psychiatry 2018, 23, 48–58. [Google Scholar] [CrossRef]
- Garcıa-Marchena, N.; Barrera, M.; MestrePinto´, J.I.; Araos, P.; Serrano, A.; Pe´rez-Maña´, C.; Papaseit, E.; Alías-Ferri, M.; Ruiz, J.J.; De Fonseca, F.R.; et al. Inflammatory mediators and dual depression: Potential biomarkers in plasma of primary and substance-induced major depression in cocaine and alcohol use disorders. PLoS ONE 2019, 14, e0213791. [Google Scholar] [CrossRef]
- Fontenelle, L.F.; Barbosa, I.G.; Luna, J.V.; De Sousa, L.P.; Abreu, M.N.; Teixeira, A.L. A cytokine study of adult patients with obsessive-compulsive disorder. Compr. Psychiatry 2012, 53, 797–804. [Google Scholar] [CrossRef]
- Ashwood, P.; Wills, S.; Van De Water, J. The immune response in autism: A new frontier for autism research. J. Leukoc. Biol. 2006, 80, 1–5. [Google Scholar] [CrossRef]
- Cunha, G.R.; Asevedo, E.; Mansur, R.B.; Zugman, A.; Pan, P.M.; Gadelha, A.; Belangero, S.I.; Rizzo, L.B.; Coelho, R.; Stertz, L.; et al. Inflammation, neurotrophism and oxidative stress and childhood psychopathology in a large community sample. Acta Psychiatr. Scand. 2015, 134, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; Quintana, D.S.; Glozier, N.; Lloyd, A.R.; Hickie, I.B.; Guastella, A.J. Cytokine aberrations in autism spectrum disorder: A systematic review and meta-analysis. Mol. Psychiatry 2015, 20, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.W.; Jyonouchi, H.; Comi, A.M.; Connors, S.L.; Milstien, S.; Varsou, A.; Heyes, M.P. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr. Neurol. 2005, 33, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.; Liu, T.; Tsou, H.; Hsu, Y.; Wang, S.; Fang, C.-P.; Liu, C.-C.; Chen, A.C.; Liu, Y.-L. Inflammatory chemokine eotaxin-1 is correlated with age in heroin dependent patients under methadone maintenance therapy. Drug Alcohol Depend. 2018, 183, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marchena, N.; Araos, P.F.; Barrios, V.; Sanchez-Marin, L.; Chowen, J.A.; Pedraz, M.; Castilla-Ortega, E.; Romero-Sanchiz, P.; Ponce, G.; Gavito, A.L.; et al. Plasma Chemokines in patients with alcohol use disorders: Association of CCL11 (Eotaxin-1) with psychiatric comorbidity. Front. Psychiatry 2016, 7, 214. [Google Scholar] [CrossRef]
- Scalzo, P.; De Miranda, A.S.; Guerra Amaral, D.C.; De Carvalho Vilela, M.; Cardoso, F.; Teixeira, A.L. Serum levels of chemokines in Parkinson’s disease. NeuroImmunoModulation 2011, 18, 240–244. [Google Scholar] [CrossRef]
- Lindqvist, D.; Hall, S.; Surova, Y.; Nielsen, H.M.; Janelidze, S.; Brundin, L.; Hansson, O. Cerebrospinal fluid inflammatory markers in Parkinson’s disease—Associations with depression, fatigue, and cognitive impairment. Brain Behav. Immun. 2013, 33, 183–189. [Google Scholar] [CrossRef]
- Moghadam-Ahmadi, A.; Khorramdelazad, H.; Hassanshahi, G.; Shahsavari, S.; Moadab, A.; Vakilian, A. Eotaxins and C-C chemokine receptor type 3 in Parkinson’s disease. Acta Neurol. Belg. 2020, 120, 589–594. [Google Scholar] [CrossRef]
- Chandra, G.; Roy, A.; Rangasamy, S.B.; Pahan, K. Induction of adaptive immunity leads to nigrostriatal disease progression in mptp mouse model of parkinson’s disease. J. Immunol. 2017, 198, 4312–4326. [Google Scholar] [CrossRef]
- Lalli, M.A.; Bettcher, B.M.; Arcila, M.L.; Garcia, G.; Guzman, C.; Madrigal, L.; Ramirez, L.; Acosta-Uribe, J.; Baena, A.; Wojta, K.J.; et al. Whole-genome sequencing suggests a chemokine gene cluster that modifies age atonset in familial Alzheimer’s disease. Mol. Psychiatry 2015, 20, 1294–1300. [Google Scholar] [CrossRef]
- Guerreiro, R.; Bras, J. The age factor in Alzheimer’s disease. Genome Med. 2015, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Westin, K.; Buchhave, P.; Nielsen, H.; Minthon, L.; Janciauskiene, S.; Hansson, O. CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer’s disease. PLoS ONE 2012, 7, e30525. [Google Scholar] [CrossRef]
- Hauser, S.L.; Goodwin, D.S. Multiple sclerosis and other demyelinating diseases. Harrison’s Princ. Intern. Med. 17th Ed. 2008, 2611–2621. [Google Scholar]
- Weinshenker, B.G. Epidemiology of multiple sclerosis. Neurol. Clin. 1996, 14, 291–308. [Google Scholar] [CrossRef]
- Bai, Z.; Chen, D.; Wang, L.; Zhao, Y.; Liu, T.; Yu, Y.; Yan, T.; Cheng, Y. Cerebrospinal fluid and blood cytokines as biomarkers for multiple sclerosis: A systematic review and meta-analysis of 226 studies with 13,526 multiple sclerosis patients. Front. Neurosci. 2019, 13, 1026. [Google Scholar] [CrossRef] [PubMed]
- Michael, B.D.; Elsone, L.; Griffiths, M.J.; Faragher, B.; Borrow, R.; Solomon, T.; Jacob, A. Post-acute serum eosinophil and neutrophil-associated cytokine/chemokine profile can distinguish between patients with neuromyelitis optica and multiple sclerosis; and identifies potential pathophysiological mechanisms—A pilot study. Cytokine 2013, 64, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Yang, T.; Wang, J.; Zhao, T.; Wang, L.; Kang, Y.; Cheng, C.; Fan, Y. Elevated plasma chemokines for eosinophils in neuromyelitis optica spectrum disorders during remission. Front. Neurol. 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Paredes, L.; Casrouge, A.; Decalf, J.; de Andrés, C.; Villar, L.M.; Pérez de Diego, R.; Alonso, B.; Álvarez Cermeño, J.C.; Arroyo, R.; Tejera-Alhambra, M.; et al. Multimarker risk stratification approach at multiple sclerosis onset. Clin. Immunol. 2017, 181, 43–50. [Google Scholar] [CrossRef]
- Adzemovic, M.Z.; Öckinger, J.; Zeitelhofer, M.; Hochmeister, S.; Beyeen, A.D.; Paulson, A.; Gillett, A.; Thessen Hedreul, M.; Covacu, R.; Lassmann, H.; et al. Expression of Ccl11 associates with immune response modulation and protection against neuroinflammation in rats. PLoS ONE 2012, 7, e39794. [Google Scholar] [CrossRef]
- Khavinson, V.K.; Kuznik, B.I.; Ryzhak, G.A.; Linkova, N.S.; Kozina, L.S.; Sall, T.S. Protein of senility CCL11, “protein of juvenility” GDF11 and their role in age-related pathology. Adv. Gerontol. 2016, 29, 722–731. [Google Scholar]
- Sharma, R.; Macy, S.; Richardson, K.; Lokhnygina, Y.; Laskowitz, D.T. A blood-based biomarker panel to detect acute stroke. J. Stroke Cereb. Dis. 2014, 23, 910–918. [Google Scholar] [CrossRef]
- Roy-O’Reilly, M.; Ritzel, R.M.; Conway, S.E.; Staff, I.; Fortunato, G.; McCullough, L.D. CCL11 (Eotaxin-1) levels predict long-term functional outcomes in patients following ischemic stroke. Transl. Stroke Res. 2017, 8, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Ni, G.; Ma, J.; Liu, H.; Mao, Z.; Sun, H.; Zhang, X. Impact of tag single nucleotide polymorphisms (snps) in ccl11 gene on risk of subtypes of ischemic stroke in xinjiang han populations. Med. Sci. Monit. 2017, 23, 4291–4298. [Google Scholar] [CrossRef][Green Version]
- Zhao, N.; Liu, X.; Wang, Y.; Liu, X.; Li, J.; Yu, L.; Ma, L.; Wang, S.; Zhang, H.; Liu, L.; et al. Association of inflammatory gene polymorphisms with ischemic stroke in a Chinese Han population. J. Neuroinflammation 2012, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Munshi, A.; Das, S.; Kaul, S. Genetic determinants in ischaemic stroke subtypes: Seven-year findings and a review. Gene 2015, 555, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Lin, C.Y.; Hsieh, T.C.; Olson, S.C.; Wu, J.M. Control of eotaxin-1 expression and release by resveratrol and its metabolites in culture human pulmonary artery endothelial cells. Am. J. Cardiovasc. Dis. 2011, 1, 16–30. [Google Scholar] [PubMed]
- Main, S.; Handy, R.; Wilton, J.; Smith, S.; Williams, L.; Fou, L.D.; Andrews, J.; Conroy, L.A.; May, R.; Anderson, I.; et al. A potent human anti-eotaxin1 antibody, CAT-213: Isolation by phage display and in vitro and in vivo efficacy. J. Pharmacol. Exp. Ther. 2006, 319, 1395–1404. [Google Scholar] [CrossRef]
- Garwood, C.J.; Cooper, J.D.; Hanger, D.P.; Noble, W. Anti-inflammatory impact of minocycline in a mouse model of tauopathy. Front. Psychiatry 2010, 1, 136. [Google Scholar] [CrossRef]
- Kanabar, V.; Page, C.P.; Simcock, D.E.; Karner, C.; Mahn, K.; O’Connor, B.J.; Hirst, S.J. Heparin and structurally related polymers attenuate eotaxin-1 (CCL11) release from human airway smooth muscle. Br. J. Pharm. 2008, 154, 833–842. [Google Scholar] [CrossRef]
- Jahnsen, F.L.; Haye, R.; Gran, E.; Brandtzaeg, P.; Johansen, F.E. Glucocorticosteroids inhibit mRNA expression for eotaxin, eotaxin-2, and monocyte-chemotactic protein-4 in human airway inflammation with eosinophilia. J. Immunol. 1999, 163, 1545–1551. [Google Scholar]
- Stellato, C.; Collins, P.; Ponath, P.D.; Soler, D.; Newman, W.; La Rosa, G.; Li, H.; White, J.; Schwiebert, L.M.; Bickel, C.; et al. Production of the novel C-C chemokine MCP-4 by airway cells and comparison of its biological activity to other C-C chemokines. J. Clin. Investig. 1997, 99, 926–936. [Google Scholar] [CrossRef][Green Version]
- Lilly, C.M.; Nakamura, H.; Kesselman, H.; Nagler Anderson, C.; Asano, K.; Garcia Zepeda, E.A.; Rothenberg, M.E.; Drazen, J.M.; Luster, A.D. Expression of eotaxin by human lung epithelial cells: Induction by cytokines and inhibition by glucocorticoids. J. Clin. Investig. 1997, 99, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Lezi, E.; Burns, J.M.; Swerdlow, R.H. Effect of high-intensity exercise on aged mouse brain mitochondria, neurogenesis, and inflammation. Neurobiol. Aging 2014, 35, 2574–2583. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).