Old Drug, New Trick: Tilorone, a Broad-Spectrum Antiviral Drug as a Potential Anti-Fibrotic Therapeutic for the Diseased Heart
Abstract
1. Introduction
2. Results
2.1. Tilorone Attenuates Transforming Growth Factor β (TGFβ) Induced Collagen Synthesis In Vitro
2.2. Tilorone Attenuates Cardiac Fibrosis in a Mouse Model with Pre-Existing Pathological Cardiac Remodelling and Cardiac Dysfunction due to Pressure Overload
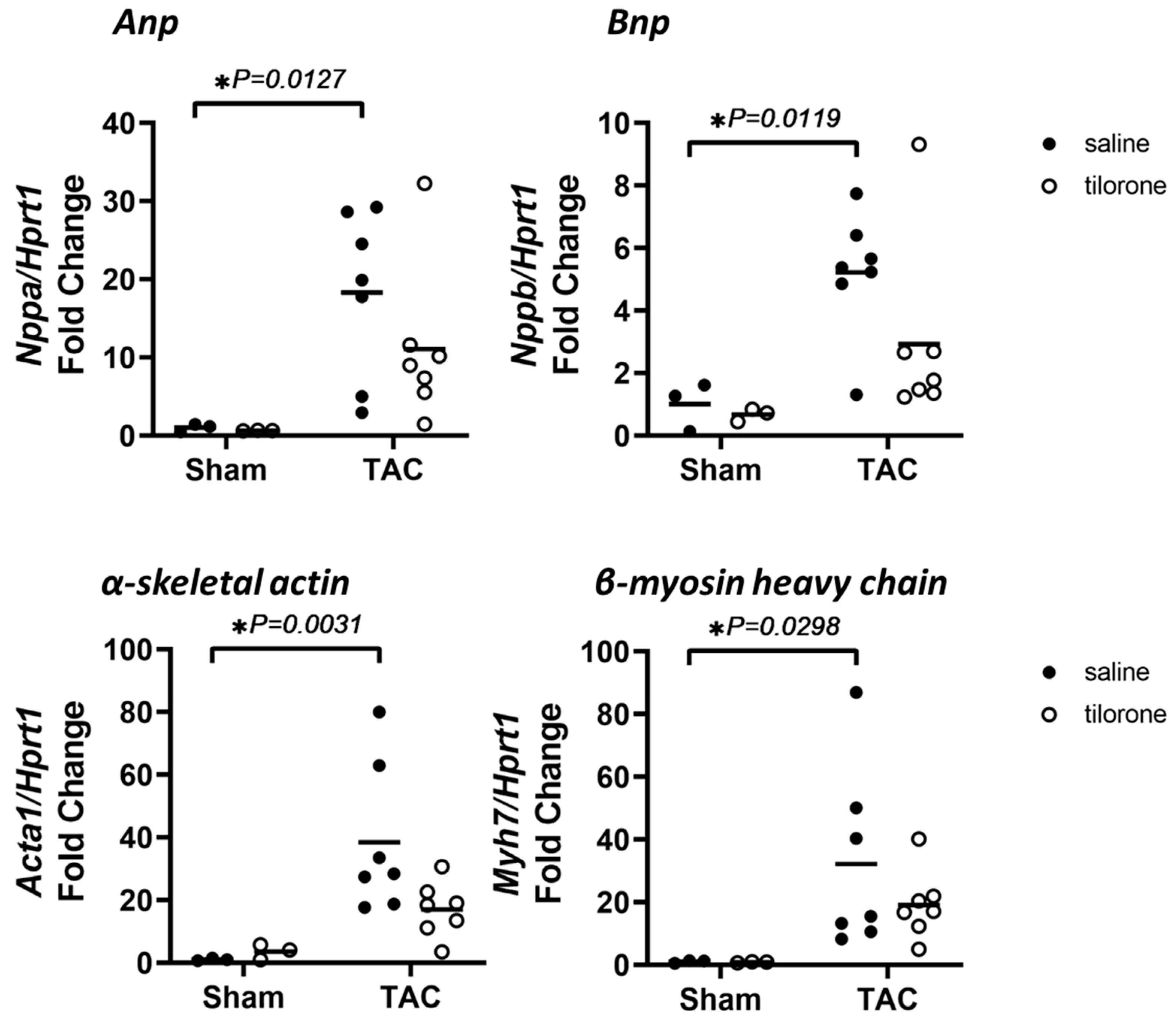
2.3. Treatment with Tilorone Is Associated with a Favourable Cardiac Stress Gene Expression Profile
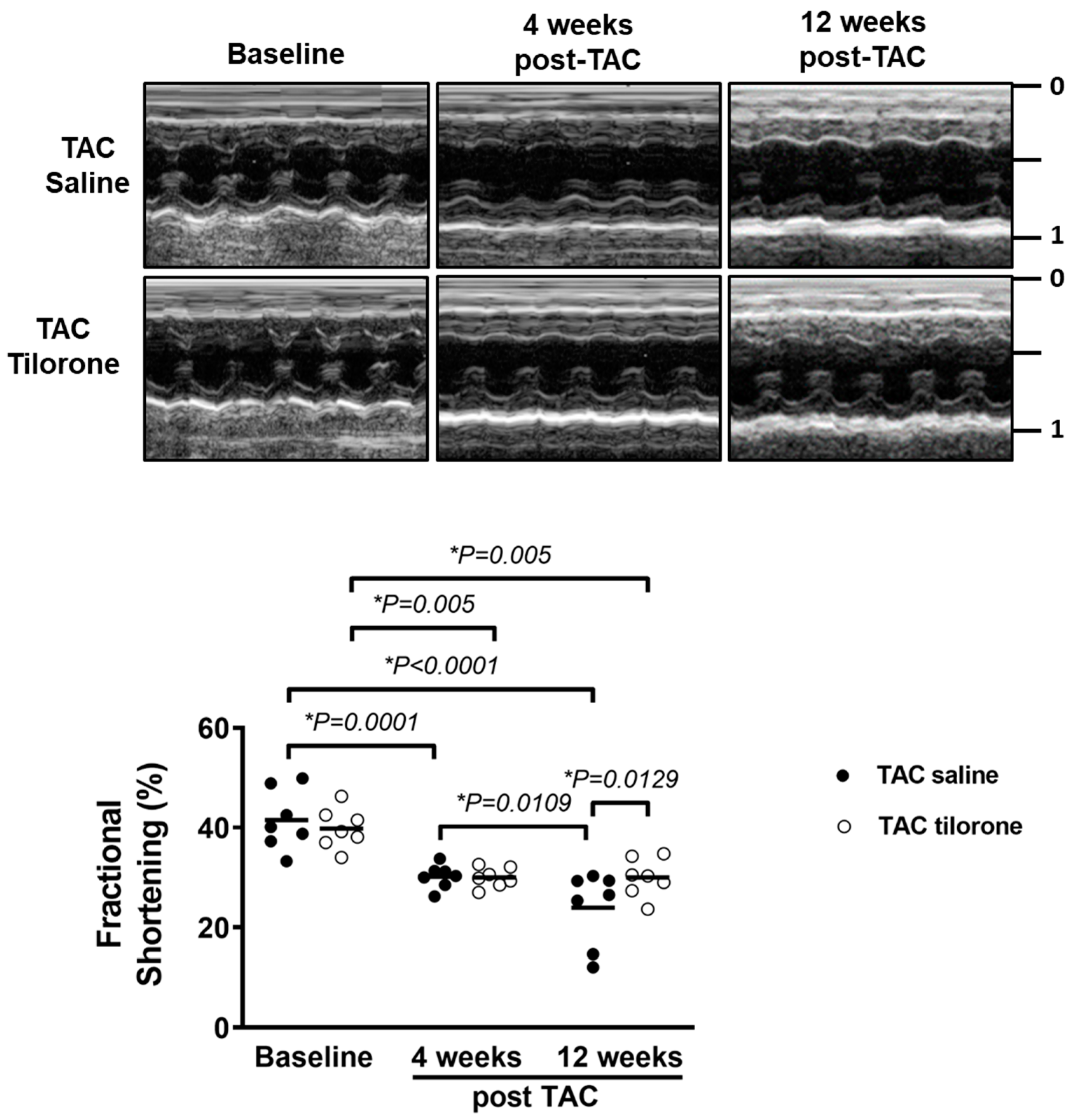
2.4. Treatment with Tilorone Prevented a Further Decline in Cardiac Function
3. Discussion
4. Materials and Methods
4.1. Animal Experimental Procedures
4.2. Study Design
4.3. Pressure Overload Induced by Transverse Aortic Constriction (TAC)
4.4. Left Ventricular Structure and Function
4.5. Tissue Collection
4.6. Proline Assay
4.7. RNA Isolation
4.8. Reverse Transcription
4.9. Quantitative PCR (qPCR)
4.10. Histological Analysis
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponikowski, P.; Anker, S.D.; AlHabib, K.F.; Cowie, M.R.; Force, T.L.; Hu, S.; Jaarsma, T.; Krum, H.; Rastogi, V.; Rohde, L.E.; et al. Heart failure: Preventing disease and death worldwide. ESC Heart Fail. 2014, 1, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Eisen, H.J. Epidemiology of Heart Failure and Scope of the Problem. Cardiol. Clin. 2014, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Frishman, W.H. Beta-Adrenergic blockers. Circulation 2003, 107, e117–e119. [Google Scholar] [CrossRef]
- Sweitzer, N.K. What is an angiotensin converting enzyme inhibitor? Circulation 2003, 108, e16–e18. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 128, 1810–1852. [Google Scholar] [CrossRef]
- Krum, H.; Driscoll, A. Management of heart failure. Med. J. Aust. 2013, 199, 334–339. [Google Scholar] [CrossRef]
- Dusing, R. Mega clinical trials which have shaped the RAS intervention clinical practice. Ther. Adv. Cardiovasc. Dis. 2016, 10, 133–150. [Google Scholar] [CrossRef]
- Tham, Y.K.; Bernardo, B.C.; Ooi, J.Y.; Weeks, K.L.; McMullen, J.R. Pathophysiology of cardiac hypertrophy and heart failure: Signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015, 89, 1401–1438. [Google Scholar] [CrossRef]
- Bernardo, B.C.; Weeks, K.L.; Pretorius, L.; McMullen, J.R. Molecular distinction between physiological and pathological cardiac hypertrophy: Experimental findings and therapeutic strategies. Pharmacol. Ther. 2010, 128, 191–227. [Google Scholar] [CrossRef]
- Hinderer, S.; Schenke-Layland, K. Cardiac fibrosis-A short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 77–82. [Google Scholar] [CrossRef]
- Ekins, S.; Lane, T.R.; Madrid, P.B. Tilorone: A broad-spectrum antiviral invented in the USA and commercialized in Russia and beyond. Pharm. Res. 2020, 37, 71. [Google Scholar] [CrossRef] [PubMed]
- Ekins, S.; Madrid, P.B. Tilorone, a Broad-Spectrum Antiviral for Emerging Viruses. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Leppäranta, O.; Tikkanen, J.M.; Bespalov, M.M.; Koli, K.; Myllärniemi, M. Bone morphogenetic protein–inducer tilorone identified by high-throughput screening is antifibrotic in vivo. Am. J. Respir. Cell Mol. Biol. 2012, 48, 448–455. [Google Scholar] [CrossRef]
- Merino, D.; Villar, A.V.; García, R.; Tramullas, M.; Ruiz, L.; Ribas, C.; Cabezudo, S.; Nistal, J.F.; Hurlé, M.A. BMP-7 attenuates left ventricular remodelling under pressure overload and facilitates reverse remodelling and functional recovery. Cardiovasc. Res. 2016, 110, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Bacmeister, L.; Schwarzl, M.; Warnke, S.; Stoffers, B.; Blankenberg, S.; Westermann, D.; Lindner, D. Inflammation and fibrosis in murine models of heart failure. Basic Res. Cardiol. 2019, 114, 19. [Google Scholar] [CrossRef]
- Bernardo, B.C.; Gao, X.M.; Winbanks, C.E.; Boey, E.J.; Tham, Y.K.; Kiriazis, H.; Gregorevic, P.; Obad, S.; Kauppinen, S.; Du, X.J.; et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc. Natl. Acad. Sci. USA 2012, 109, 17615–17620. [Google Scholar] [CrossRef]
- Bernardo, B.C.; Weeks, K.L.; Pongsukwechkul, T.; Gao, X.; Kiriazis, H.; Cemerlang, N.; Boey, E.J.; Tham, Y.K.; Johnson, C.J.; Qian, H.; et al. Gene delivery of medium chain acyl-coenzyme A dehydrogenase (MCAD) induces physiological cardiac hypertrophy and protects against pathological remodelling. Clin. Sci. 2018, 132, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.A.; Aronovitz, M.J.; Calamaras, T.D.; Tam, K.; Martin, G.L.; Liu, P.; Bowditch, H.K.; Zhang, P.; Huggins, G.S.; Blanton, R.M. Distinct phenotypes induced by three degrees of transverse aortic constriction in mice. Sci. Rep. 2019, 9, 5844. [Google Scholar] [CrossRef]
- Grotendorst, G.R.; Duncan, M.R. Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J. 2005, 19, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenes. Tissue Repair 2012, 5, S24. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Abdel-Aty, H.; Taylor, A.; Schulz-Menger, J.; Messroghli, D.; Dietz, R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2008, 51, 1581–1587. [Google Scholar] [CrossRef]
- Roubille, F.; Busseuil, D.; Merlet, N.; Kritikou, E.A.; Rheaume, E.; Tardif, J.C. Investigational drugs targeting cardiac fibrosis. Expert Rev. Cardiovasc. Ther. 2014, 12, 111–125. [Google Scholar] [CrossRef]
- Wang, S.; Sun, A.; Li, L.; Zhao, G.; Jia, J.; Wang, K.; Ge, J.; Zou, Y. Up-regulation of BMP-2 antagonizes TGF-β1/ROCK-enhanced cardiac fibrotic signalling through activation of Smurf1/Smad6 complex. J. Cell. Mol. Med. 2012, 16, 2301–2310. [Google Scholar] [CrossRef]
- Dahabreh, Z.; Calori, G.M.; Kanakaris, N.K.; Nikolaou, V.S.; Giannoudis, P.V. A cost analysis of treatment of tibial fracture nonunion by bone grafting or bone morphogenetic protein-7. Int. Orthop. 2009, 33, 1407–1414. [Google Scholar] [CrossRef]
- Moreo, A.; Ambrosio, G.; De Chiara, B.; Pu, M.; Tran, T.; Mauri, F.; Raman, S.V. Influence of myocardial fibrosis on left ventricular diastolic function: Noninvasive assessment by cardiac magnetic resonance and echo. Circ. Cardiovasc. Imaging 2009, 2, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Ekins, S.; Lingerfelt, M.A.; Comer, J.E.; Freiberg, A.N.; Mirsalis, J.C.; O’Loughlin, K.; Harutyunyan, A.; McFarlane, C.; Green, C.E.; Madrid, P.B. Efficacy of tilorone dihydrochloride against Ebola virus infection. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- Vitiello, A.; Pelliccia, C.; Ferrara, F. COVID-19 Patients with pulmonary fibrotic tissue: Clinical pharmacological rational of antifibrotic therapy. SN Compr. Clin. Med. 2020, 1–4. [Google Scholar] [CrossRef]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and cardiovascular disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, P.; Tang, D.; Zhu, T.; Han, R.; Zhan, C.; Liu, W.; Zeng, H.; Tao, Q.; Xia, L. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc. Imaging 2020, 13, 2330–2339. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Weeks, K.L.; Henstridge, D.C.; Salim, A.; Shaw, J.E.; Marwick, T.H.; McMullen, J.R. CORP: Practical tools for improving experimental design and reporting of laboratory studies of cardiovascular physiology and metabolism. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H627–H639. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.C.; Nguyen, S.S.; Gao, X.M.; Tham, Y.K.; Ooi, J.Y.Y.; Patterson, N.L.; Kiriazis, H.; Su, Y.; Thomas, C.J.; Lin, R.C.Y.; et al. Inhibition of miR-154 protects against cardiac dysfunction and fibrosis in a mouse model of pressure overload. Sci. Rep. 2016, 6, 22442. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.C.; Nguyen, S.S.; Winbanks, C.E.; Gao, X.-M.; Boey, E.J.H.; Tham, Y.K.; Kiriazis, H.; Ooi, J.Y.Y.; Porrello, E.R.; Igoor, S.; et al. Therapeutic silencing of miR-652 restores heart function and attenuates adverse remodeling in a setting of established pathological hypertrophy. FASEB J. 2014, 28, 5097–5110. [Google Scholar] [CrossRef]
- Gao, X.-M.; Kiriazis, H.; Moore, X.-L.; Feng, X.-H.; Sheppard, K.; Dart, A.; Du, X.-J. Regression of pressure overload-induced left ventricular hypertrophy in mice. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2702–H2707. [Google Scholar] [CrossRef]
- Donner, D.G.; Kiriazis, H.; Du, X.J.; Marwick, T.H.; McMullen, J.R. Improving the quality of preclinical research echocardiography: Observations, training and guidelines for measurement. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H58–H70. [Google Scholar] [CrossRef] [PubMed]





| Sham | TAC | |||
|---|---|---|---|---|
| Saline | Tilorone | Saline | Tilorone | |
| No. of Animals | 3 | 3 | 7 | 7 |
| Body Weight (g) | 30.6 ± 0.2 | 29.5 ± 0.6 | 30.2 ± 1.0 | 31.1 ± 0.7 |
| Tibial Length (mm) | 16.1 ± 0.1 | 16.1 ± 0.1 | 16.2 ± 0.1 | 16.3 ± 0.1 |
| Heart Weight (mg) | 140.2 ± 13.1 | 140.0 ± 11.8 | 203.3 ± 20.6 | 199.5 ± 10.5 |
| Atrial Weight (mg) | 6.4 ± 1.3 | 7.3 ± 0.5 | 14.6 ± 2.4 * | 14.3 ± 1.3 * |
| Lung Weight (mg) | 162.6 ± 7.6 | 176.3 ± 7.0 | 244.5 ± 55.0 | 203.4 ± 16.8 |
| HW/BW (mg/g) | 4.6 ± 0.5 | 4.7 ± 0.3 | 6.7 ± 0.7 * | 6.4 ± 0.3 |
| AW/BW (mg/g) | 0.21 ± 0.05 | 0.25 ± 0.02 | 0.49 ± 0.09* | 0.46 ± 0.04 |
| LW/BW (mg/g) | 5.3 ± 0.3 | 6.0 ± 0.1 | 8.3 ± 2.1 | 6.6 ± 0.6 |
| HW/TL (mg/mm) | 8.7 ± 0.8 | 8.7 ± 0.8 | 12.5 ± 1.2 * | 12.3 ± 0.7 * |
| AW/TL (mg/mm) | 0.40 ± 0.08 | 0.45 ± 0.03 | 0.90 ± 0.15 * | 0.88 ± 0.08 * |
| LW/TL (mg/mm) | 10.1 ± 0.5 | 10.9 ± 0.5 | 15.1 ± 3.4 | 12.5 ± 1.0 |
| BASELINE | 4 WEEKS POST-TAC | ENDPOINT | ||||
|---|---|---|---|---|---|---|
| TAC | TAC | TAC | ||||
| Saline | Tilorone | Saline | Tilorone | Saline | Tilorone | |
| No. of Animals | 7 | 7 | 7 | 7 | 7 | 7 |
| Body Weight (g) | 29.1 ± 0.4 | 28.9 ± 0.6 | 29.8 ± 0.6 | 29.8 ± 0.5 | 30.4 ± 1.2 | 30.9 ± 0.8 |
| Heart Rate (bpm) | 521 ± 16 | 559 ± 31 | 562 ± 14 | 546 ± 25 | 566 ± 22 | 574 ± 13 |
| LVPW (mm) | 0.80 ± 0.01 | 0.78 ± 0.02 | 1.11 ± 0.01 * | 1.16 ± 0.01 * | 1.07 ± 0.03 * | 1.08 ± 0.02 *‡ |
| IVS (mm) | 0.79 ± 0.01 | 0.79 ± 0.01 | 1.15 ± 0.01 * | 1.19 ± 0.01 * | 1.08 ± 0.03 * | 1.05 ± 0.04 *‡ |
| LVEDD (mm) | 3.89 ± 0.11 | 4.04 ± 0.06 | 4.24 ± 0.08 * | 4.21 ± 0.10 | 4.32 ± 0.20 * | 4.39 ± 0.12 * |
| LVESD (mm) | 2.28 ± 0.14 | 2.43 ± 0.06 | 2.96 ± 0.07 * | 2.95 ± 0.09 * | 3.31 ± 0.28 *# | 3.08 ± 0.14 * |
| Fractional Shortening (%) | 42 ± 2 | 40 ± 2 | 30 ± 1 * | 30 ± 1 * | 24 ± 3 *‡ | 30 ± 1 *† |
| Ejection Fraction (%) | 79 ± 2 | 78 ± 2 | 66 ± 1 * | 66 ± 1 * | 55 ± 5 *‡ | 65 ± 2 *† |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horlock, D.; Kaye, D.M.; Winbanks, C.E.; Gao, X.-M.; Kiriazis, H.; Donner, D.G.; Gregorevic, P.; McMullen, J.R.; Bernardo, B.C. Old Drug, New Trick: Tilorone, a Broad-Spectrum Antiviral Drug as a Potential Anti-Fibrotic Therapeutic for the Diseased Heart. Pharmaceuticals 2021, 14, 263. https://doi.org/10.3390/ph14030263
Horlock D, Kaye DM, Winbanks CE, Gao X-M, Kiriazis H, Donner DG, Gregorevic P, McMullen JR, Bernardo BC. Old Drug, New Trick: Tilorone, a Broad-Spectrum Antiviral Drug as a Potential Anti-Fibrotic Therapeutic for the Diseased Heart. Pharmaceuticals. 2021; 14(3):263. https://doi.org/10.3390/ph14030263
Chicago/Turabian StyleHorlock, Duncan, David M. Kaye, Catherine E. Winbanks, Xiao-Ming Gao, Helen Kiriazis, Daniel G. Donner, Paul Gregorevic, Julie R. McMullen, and Bianca C. Bernardo. 2021. "Old Drug, New Trick: Tilorone, a Broad-Spectrum Antiviral Drug as a Potential Anti-Fibrotic Therapeutic for the Diseased Heart" Pharmaceuticals 14, no. 3: 263. https://doi.org/10.3390/ph14030263
APA StyleHorlock, D., Kaye, D. M., Winbanks, C. E., Gao, X.-M., Kiriazis, H., Donner, D. G., Gregorevic, P., McMullen, J. R., & Bernardo, B. C. (2021). Old Drug, New Trick: Tilorone, a Broad-Spectrum Antiviral Drug as a Potential Anti-Fibrotic Therapeutic for the Diseased Heart. Pharmaceuticals, 14(3), 263. https://doi.org/10.3390/ph14030263






