Safety and Tolerability of Mass Diethylcarbamazine and Albendazole Administration for the Elimination of Lymphatic Filariasis in Kenya: An Active Surveillance Study
Abstract
1. Introduction
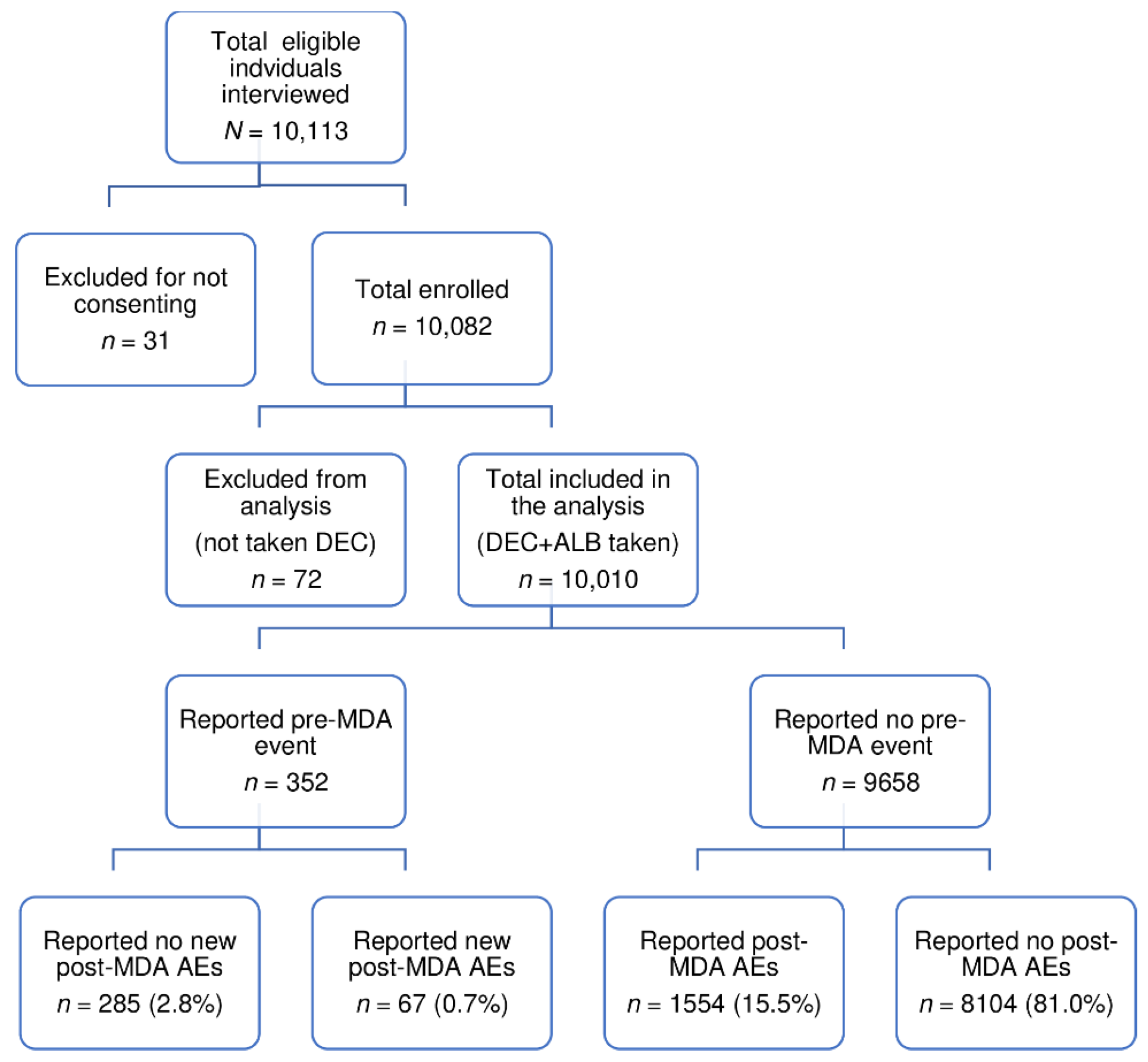
2. Results
2.1. Baseline Characteristics of the Study Population
2.2. Incidence of Post-MDA Adverse Events
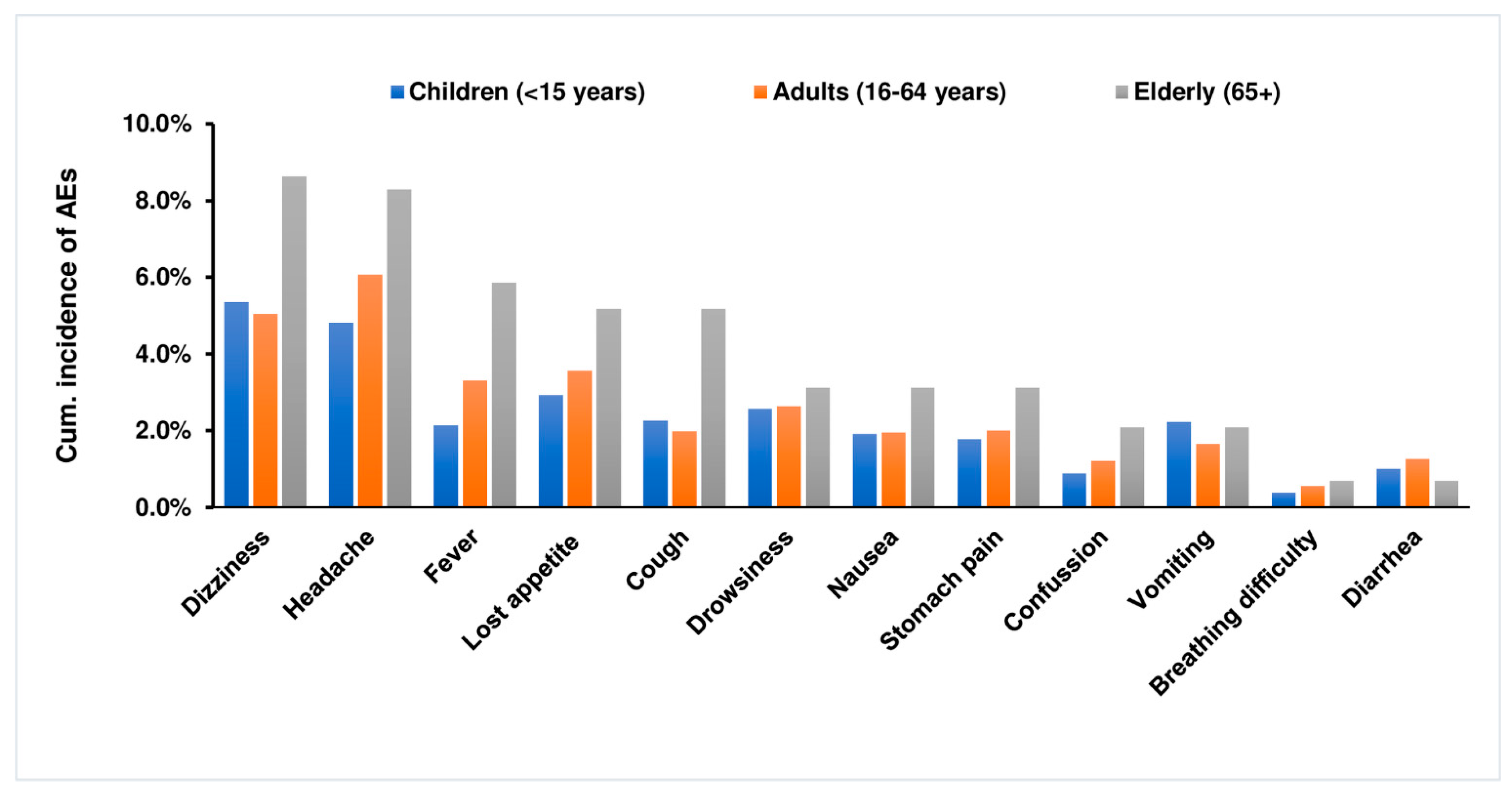
2.3. Incidence and Types of Post-MDA Adverse Events
2.4. Severity Grading of Adverse Events
2.5. Factors Correlated with Post-MDA Adverse Events
2.6. Risk Factors Associated with Post-MDA Adverse Events
3. Discussion
4. Materials and Methods
4.1. Study Design, Area, and Population
4.2. Inclusion Criteria
4.3. Study Sample Size
4.4. Mass Drug Administration (Preventive Chemotherapy)
4.5. Data Collection Procedures and Adverse Events Monitoring
4.6. Data Management
- Grade 1 Mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated.
- Grade 2 Moderate; minimal, local, or non-invasive intervention indicated; limiting age-appropriate Instrumental Activities of Daily Living (ADL).
- Grade 3 Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL.
- Grade 4 Life-threatening consequences; urgent intervention indicated.
- Grade 5 Death related to AE.
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babu, S.; Nutman, T.B. Immunology of Lymphatic Filariasis. Parasite Immunol. 2014, 36, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Nutman, T.B. Immunopathogenesis of Lymphatic Filarial Disease. Semin. Immunopathol. 2012, 34, 847–861. [Google Scholar] [CrossRef]
- Bockarie, M.J.; Taylor, M.J.; Gyapong, J.O. Current Practices in the Management of Lymphatic Filariasis. Expert Rev. Anti Infect. Ther. 2009, 7, 595–605. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2019 Lymphatic Filariasis. Available online: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis (accessed on 9 December 2020).
- Ichimori, K.; King, J.D.; Engels, D.; Yajima, A.; Mikhailov, A.; Lammie, P.; Ottesen, E.A. Global Programme to Eliminate Lymphatic Filariasis: The Processes Underlying Programme Success. PLoS Negl. Trop. Dis. 2014, 8, e3328. [Google Scholar] [CrossRef] [PubMed]
- World Health Assembly. WHA50.29—Elimination of Lymphatic Filariasis as A Public Health Problem; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Ottesen, E.A. Editorial: The Global Programme to Eliminate Lymphatic Filariasis. Trop. Med. Int. Health 2000, 5, 591–594. [Google Scholar] [CrossRef] [PubMed]
- World Health organization. Progress Report 2000–2009 and Strategic Plan 2010–2020 of the Global Programme to Eliminate Lymphatic Felariasis; World Health Organization: Geneva, Switzerland, 2010; ISBN 978-92-4-150072-2. [Google Scholar]
- World Health Organization. Guideline: Alternative Mass Drug Administration Regimens to Eliminate Lymphatic Filariasis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Babu, B.V.; Mishra, S. Mass Drug Administration under the Programme to Eliminate Lymphatic Filariasis in Orissa, India: A Mixed-Methods Study to Identify Factors Associated with Compliance and Non-Compliance. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Cantey, P.T.; Rao, G.; Rout, J.; Fox, L.M. Predictors of Compliance with a Mass Drug Administration Programme for Lymphatic Filariasis in Orissa State, India 2008. Trop. Med. Int. Health 2010, 15, 224–231. [Google Scholar] [CrossRef]
- Adhikari, R.K.; Sherchand, J.B.; Mishra, S.R.; Ranabhat, K.; Devkota, P.; Mishra, D.; Ghimire, Y.C.; Gelal, K.; Mishra, R.; Paudel, R. Factors Determining Non-Compliance to Mass Drug Administration for Lymphatic Filariasis Elimination in Endemic Districts of Nepal. J. Nepal Health Res. Counc. 2014, 12, 124–129. [Google Scholar] [PubMed]
- Krentel, A.; Fischer, P.U.; Weil, G.J. A Review of Factors That Influence Individual Compliance with Mass Drug Administration for Elimination of Lymphatic Filariasis. PLoS Negl. Trop. Dis. 2013, 7, e2447. [Google Scholar] [CrossRef]
- Haarbrink, M.; Abadi, G.K.; Buurman, W.A.; Dentener, M.A.; Terhell, A.J.; Yazdanbakhsh, M. Strong Association of Interleukin-6 and Lipopolysaccharide-Binding Protein with Severity of Adverse Reactions after Diethylcarbamazine Treatment of Microfilaremic Patients. J. Infect. Dis. 2000, 182, 564–569. [Google Scholar] [CrossRef]
- Maizels, R.M.; Denham, D.A. Diethylcarbamazine (DEC): Immunopharmacological Interactions of an Anti-Filarial Drug. Parasitology 1992, 105, S49–S60. [Google Scholar] [CrossRef] [PubMed]
- Haarbrink, M.; Terhell, A.J.; Abadi, G.K.; Mitsui, Y.; Yazdanbakhsh, M. Adverse Reactions Following Diethylcarbamazine (DEC) Intake in ‘Endemic Normals’, Microfilaraemics and Elephantiasis Patients. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 91–96. [Google Scholar] [CrossRef]
- Nsubuga, P.; White, M.E.; Thacker, S.B.; Anderson, M.A.; Blount, S.B.; Broome, C.V.; Chiller, T.M.; Espitia, V.; Imtiaz, R.; Sosin, D. Public Health Surveillance: A Tool for Targeting and Monitoring Interventions. Dis. Control Priorities Dev. Ctries. 2006, 2, 997–1018. [Google Scholar]
- Njenga, S.M.; Kanyi, H.M.; Mutungi, F.M.; Okoyo, C.; Matendechero, H.S.; Pullan, R.L.; Halliday, K.E.; Brooker, S.J.; Wamae, C.N.; Onsongo, J.K.; et al. Assessment of Lymphatic Filariasis Prior to Re-Starting Mass Drug Administration Campaigns in Coastal Kenya. Parasites Vectors 2017, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, W. Report on Active Surveillance for Adverse Events Following the Use of Drug Co-Administrations in the Global Programme to Eliminate Lymphatic Filariasis. Wkly. Epidemiol. Rec. Relev. Épidémiologique Hebd. 2003, 78, 315–317. [Google Scholar]
- McLaughlin, S.I.; Radday, J.; Michel, M.C.; Addiss, D.G.; Beach, M.J.; Lammie, P.J.; Lammie, J.; Rheingans, R.; Lafontant, J. Frequency, Severity, and Costs of Adverse Reactions Following Mass Treatment for Lymphatic Filariasis Using Diethylcarbamazine and Albendazole in Leogane, Haiti, 2000. Am. J. Trop. Med. Hyg. 2003, 68, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Pani, S.P.; Reddy, G.S.; Das, L.K.; Vanamail, P.; Hoti, S.L.; Ramesh, J.; Das, P.K. Tolerability and Efficacy of Single Dose Albendazole, Diethylcarbamazine Citrate (DEC) or Co-Administration of Albendazole with DEC in the Clearance of Wuchereria Bancrofti in Asymptomatic Microfilaraemic Volunteers in Pondicherry, South India: A Hospital-Based Study. Filaria J. 2002, 1, 1. [Google Scholar] [PubMed]
- Gunawardena, S.; Ranganathan, S.S.; Fernandopulle, R. Pharmacovigilance through Consumer Feedback (Reporting) in the Mass Treatment of Lymphatic Filariasis Using Diethylcarbamazine and Albendazole in Two Districts of Sri Lanka. Trop. Med. Int. Health 2008, 13, 1153–1158. [Google Scholar] [CrossRef]
- Skvrce, N.M.; Šarinić, V.M.; Mucalo, I.; Krnić, D.; Božina, N.; Tomić, S. Adverse Drug Reactions Caused by Drug-Drug Interactions Reported to Croatian Agency for Medicinal Products and Medical Devices: A Retrospective Observational Study. Croat. Med. J. 2011, 52, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Yimer, G.; Gry, M.; Amogne, W.; Makonnen, E.; Habtewold, A.; Petros, Z.; Aderaye, G.; Schuppe-Koistinen, I.; Lindquist, L.; Aklillu, E. Evaluation of Patterns of Liver Toxicity in Patients on Antiretroviral and Anti-Tuberculosis Drugs: A Prospective Four Arm Observational Study in Ethiopian Patients. PLoS ONE 2014, 9, e94271. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.W.; Medeiros, Z.; dos Santos, Z.C.; da Costa, G.M.; Braga, C. Adverse Reactions Following Mass Drug Administration with Diethylcarbamazine in Lymphatic Filariasis Endemic Areas in the Northeast of Brazil. Rev. Da Soc. Bras. De Med. Trop. 2012, 45, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Budge, P.J.; Herbert, C.; Andersen, B.J.; Weil, G.J. Adverse Events Following Single Dose Treatment of Lymphatic Filariasis: Observations from a Review of the Literature. PLoS Negl. Trop. Dis. 2018, 12, e0006454. [Google Scholar] [CrossRef] [PubMed]
- Colombo, D.; Zagni, E.; Nica, M.; Rizzoli, S.; Ori, A.; Bellia, G. Gender Differences in the Adverse Events’ Profile Registered in Seven Observational Studies of a Wide Gender-Medicine (MetaGeM) Project: The MetaGeM Safety Analysis. Drug Des. Dev. Ther. 2016, 10, 2917–2927. [Google Scholar] [CrossRef]
- Lavan, A.H.; Gallagher, P. Predicting Risk of Adverse Drug Reactions in Older Adults. Ther. Adv. Drug Saf. 2016, 7, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Davies, E.A.; O’Mahony, M.S. Adverse Drug Reactions in Special Populations–the Elderly. Br. J. Clin. Pharmacol. 2015, 80, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.V.; Rath, K.; Kerketta, A.S.; Swain, B.K.; Mishra, S.; Kar, S.K. Adverse Reactions Following Mass Drug Administration during the Programme to Eliminate Lymphatic Filariasis in Orissa State, India. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Fimbo, A.M.; Minzi, O.M.S.; Mmbando, B.P.; Barry, A.; Nkayamba, A.F.; Mwamwitwa, K.W.; Malishee, A.; Seth, M.D.; Makunde, W.H.; Gurumurthy, P.; et al. Prevalence and Correlates of Lymphatic Filariasis Infection and Its Morbidity Following Mass Ivermectin and Albendazole Administration in Mkinga District, North-Eastern Tanzania. J. Clin. Med. 2020, 9, 1550. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, E.K.; Sanuku, N.; Baea, M.; Satofan, S.; Maki, E.; Lombore, B.; Schmidt, M.S.; Siba, P.M.; Weil, G.J.; Kazura, J.W. Efficacy, Safety, and Pharmacokinetics of Coadministered Diethylcarbamazine, Albendazole, and Ivermectin for Treatment of Bancroftian Filariasis. Clin. Infect. Dis. 2016, 62, 334–341. [Google Scholar] [CrossRef]
- Fox, L.M.; Furness, B.W.; Haser, J.K.; Desire, D.; Brissau, J.; Milord, M.; Lafontant, J.; Lammie, P.J.; Beach, M.J. Tolerance and Efficacy of Combined Diethylcarbamazine and Albendazole for Treatment of Wuchereria Bancrofti and Intestinal Helminth Infections in Haitian Children. Am. J. Trop. Med. Hyg. 2005, 73, 115–121. [Google Scholar] [CrossRef]
- Glaxosmithkline. Zentel-400-Mg-Tablets-GDS23. Available online: https://gskpro.com/content/dam/global/hcpportal/en_BW/PI/Zentel-400-mg-Tablets-GDS23.pdf15432 (accessed on 14 November 2020).
- PubChem. Diethylcarbamazine Citrate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/15432 (accessed on 14 November 2020).
- Ayo, J.A.; Agu, H.; Madaki, I. Food and Drug Interactions: Its Side Effects. Nutr. Food Sci. 2005, 35, 243–252. [Google Scholar] [CrossRef]
- Silumbwe, A.; Zulu, J.M.; Halwindi, H.; Jacobs, C.; Zgambo, J.; Dambe, R.; Chola, M.; Chongwe, G.; Michelo, C. A Systematic Review of Factors That Shape Implementation of Mass Drug Administration for Lymphatic Filariasis in Sub-Saharan Africa. BMC Public Health 2017, 17, 484. [Google Scholar] [CrossRef] [PubMed]
- Nujum, Z.T. Coverage and Compliance to Mass Drug Administration for Lymphatic Filariasis Elimination in a District of Kerala, India. Int. Health 2011, 3, 22–26. [Google Scholar] [CrossRef] [PubMed]
- County Government of Kilifi. Kilifi County Intergrated Development Plan 2018–2022, towards Realizing People Focused Transformation for Wealth Creation’ Kilifi; County Government of Kilifi: Kilifi, Kenya, 2018. [Google Scholar]
- World Health Organization. Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control. Interventions: A Manual for Health Professionals and Programme Managers; World Health Organization: Geneva, Switzerland, 2006; ISBN 978-92-4-154710-9. [Google Scholar]
- Kenya Department of Preventive and Promotive Health, Ministry of Health (MOH). Training Manual for Lymphatic Filariasis Mass Drug Administration; Kenya Department of Preventive and Promotive Health, Ministry of Health (MOH): Nairobi, Kenya, 2018.
- World Health Organization A Practical Handbook on the Pharmacovigilance of Antiretroviral Medicines; World Health Organization: Geneva, Switzerland, 2009; ISBN 978-92-4-154794-9.
- Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 14 November 2020).

| Variable | Frequency n (%) | |
|---|---|---|
| Sex | Female | 5343 (53.4) |
| Male | 4667 (46.6) | |
| Age in Years | 2 to 15 | 4456 (44.5) |
| 16–20 | 1315 (13.1) | |
| 21–64 | 3949 (39.5) | |
| 65–99 | 290 (2.9) | |
| Body Mass Index | Underweight | 1898 (19.0) |
| Normal | 3145 (31.5) | |
| Overweight | 2239 (22.5) | |
| Obese | 2693 (27.0) | |
| Concomitant Medication | Yes | 436 (4.4) |
| No | 9574 (95.7) | |
| Received DA during MDA in the Previous Year (2017) | Yes | 5875 (62.3) |
| No | 3572 (37.8) | |
| Slept Under a Bed net the Previous Night | Yes | 9296 (93.5) |
| No | 642 (6.5) | |
| House Has Mosquito Mesh Screen on Windows | Yes | 4800 (48.4) |
| No | 5127 (51.7) | |
| Indoor Spraying to Prevent Mosquitoes | Yes | 3184 (32.2) |
| No | 6703 (67.8) | |
| Number of DEC Tablets Given | 1 | 1721 (17.2) |
| 2 | 2383 (23.8) | |
| 3 | 5880 (58.7) | |
| 4 | 26 (0.3) | |
| Chronic Illness | Yes | 82 (0.8) |
| No | 9928 (99.2) | |
| Adverse Events | Total Number of Events | Severity Grading | ||
|---|---|---|---|---|
| Grade 1 (Mild) | Grade 2 (Moderate) | Grade 3 (Severe) | ||
| Dizziness | 550 | 489 (88.9% | 60 (10.9%) | 1 (0.2%) |
| Headache | 530 | 426 (80.4%) | 97 (18.3%) | 7 (1.3%) |
| Loss of App | 312 | 288 (92.3%) | 24 (7.7%) | |
| Drowsiness | 245 | 222 (90.6%) | 23 (9.4%) | |
| Fever | 239 | 218 (91.2%) | 21 (8.8%) | |
| Cough | 208 | 187 (89.9%) | 21 (10.1%) | |
| Nausea | 185 | 163 (88.1%) | 21 (11.4%) | 1 (0.5%) |
| Stomach Pain | 183 | 156 (85.2 %) | 27 (14.8%) | |
| Vomiting | 175 | 144 (82.3 %) | 31 (17.7%) | |
| Confusion | 98 | 93 (94.9 %) | 5 (5.1%) | |
| Diarrhoea | 103 | 86 (83.5 %) | 17 (16.5%) | |
| Difficulty in Breathing | 37 | 30 (81.1 %) | 7 (18.9%) | |
| Total | 2865 | 2502 (87.3%) | 354 (12.4%) | 9 (0.3%) |
| Variables | Adverse Event | X2 | p-Value | ||
|---|---|---|---|---|---|
| No (n = 8389) | Yes (n = 1621) | ||||
| Sex | Male | 3927 (84.14) | 740 (15.86) | 0.74 | 0.39 |
| Female | 4462 (83.51) | 881 (16.49) | |||
| Age in Years | 2–15 years | 3795 (85.17) | 661 (14.83) | 24.50 | <0.001 |
| 16 to 20 | 1056 (80.30) | 259 (19.70) | |||
| 21–64 | 3311 (83.84) | 638 (16.16) | |||
| 65–99 | 227 (78.28) | 63 (21.72) | |||
| Taking Concomitant Medication | Yes | 323 (74.08) | 113 (25.92) | 31.76 | <0.001 |
| No | 8066 (84.25) | 1508 (15.75) | |||
| Received DA During MDA in the Previous Year (2017) | Yes | 4911 (83.59) | 964 (16.41) | 0.57 | 0.45 |
| No | 3007 (84.18) | 565 (15.82) | |||
| Slept Under a Bed Net the Previous Night | Yes | 7818 (84.1) | 1478 (15.9) | 11.62 | 0.001 |
| No | 507 (78.97) | 135 (21.03) | |||
| Availability of Screen on the Windows | Yes | 4093 (85.27) | 707 (14.73) | 15.17 | <0.001 |
| No | 4224 (82.39) | 903 (17.61) | |||
| Indoor Spraying to Prevent Mosquitoes | Yes | 2659 (83.51) | 525 (16.49) | 0.14 | 0.70 |
| No | 5618 (83.81) | 1085 (16.19) | |||
| Number of DEC Tablets Given | 1 | 1488 (86.46) | 233 (13.54) | 18.52 | <0.001 |
| 2 | 2026 (85.02) | 357 (14.98) | |||
| 3 | 4854 (82.55) | 1026 (17.45) | |||
| 4 | 21 (80.77) | 5 (19.23) | |||
| Chronic illness | Yes | 62 (75.61) | 20 (24.39) | 4.09 | 0.04 |
| No | 8327(83.87) | 1601(16.13) | |||
| Type of Meal | Carbohydrate | 4436(85.79) | 735 (14.21) | 20.60 | <0.001 |
| Fatty | 751(82.44) | 160(17.56) | |||
| High protein | 604 (80.11) | 150(19.89) | |||
| Variables | Crude Risk Ratios | p-Value | 95% CI | Adjusted Risk Ratios | p-Value | 95% CI | |
|---|---|---|---|---|---|---|---|
| Sex | Female | 1 | 1 | ||||
| Male | 0.96 | 0.39 | 0.88–1.05 | ||||
| Age in Years | 2 to 15 | 0.92 | 0.09 | 0.83–1.01 | 1.19 | 0.07 | 0.99–1.42 |
| 16 to 20 | 1.22 | 0.003 | 1.07–1.39 | 1.22 | 0.02 | 1.03–1.45 | |
| 21 to 64 | 1 | 1 | |||||
| 65 to 99 | 1.34 | 0.01 | 1.07–1.69 | 1.26 | 0.14 | 0.93–1.70 | |
| Taking Concomitant Medication | Yes | 1.65 | <0.001 | 1.39–1.94 | 1 | ||
| No | 1 | 1.82 | <0.001 | 1.48–2.25 | |||
| Received DA During MDA in the Previous Year (2017) | No | 0.96 | 0.45 | 0.88–1.06 | |||
| Yes | 1 | ||||||
| Number of DEC Tablets Given | 1 | 1 | |||||
| 2 | 1.11 | 0.20 | 0.95–1.29 | 1.08 | 0.39 | 0.90–1.30 | |
| ≥3 | 1.29 | <0.001 | 1.13–1.47 | 1.24 | 0.05 | 1.00–1.53 | |
| Chronic Illness | Yes | 1.51 | 0.04 | 1.03–2.22 | 1.18 | 0.46 | 0.76–1.84 |
| No | 1 | 1 | |||||
| Type of Meal before MDA | Carbohydrate | 1 | 1 | ||||
| Fatty | 1.24 | 0.008 | 1.06–1.44 | 1.22 | 0.01 | 1.04–1.42 | |
| High protein | 1.40 | <0.001 | 1.19–1.64 | 1.40 | <0.001 | 1.19–1.63 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaemba, C.; Barry, A.; Omondi, W.P.; Bota, K.; Matendechero, S.; Wandera, C.; Siyoi, F.; Kirui, E.; Oluka, M.; Nambwa, P.; et al. Safety and Tolerability of Mass Diethylcarbamazine and Albendazole Administration for the Elimination of Lymphatic Filariasis in Kenya: An Active Surveillance Study. Pharmaceuticals 2021, 14, 264. https://doi.org/10.3390/ph14030264
Khaemba C, Barry A, Omondi WP, Bota K, Matendechero S, Wandera C, Siyoi F, Kirui E, Oluka M, Nambwa P, et al. Safety and Tolerability of Mass Diethylcarbamazine and Albendazole Administration for the Elimination of Lymphatic Filariasis in Kenya: An Active Surveillance Study. Pharmaceuticals. 2021; 14(3):264. https://doi.org/10.3390/ph14030264
Chicago/Turabian StyleKhaemba, Christabel, Abbie Barry, Wyckliff P. Omondi, Kefa Bota, Sultani Matendechero, Cecilia Wandera, Fred Siyoi, Elvis Kirui, Margaret Oluka, Pamela Nambwa, and et al. 2021. "Safety and Tolerability of Mass Diethylcarbamazine and Albendazole Administration for the Elimination of Lymphatic Filariasis in Kenya: An Active Surveillance Study" Pharmaceuticals 14, no. 3: 264. https://doi.org/10.3390/ph14030264
APA StyleKhaemba, C., Barry, A., Omondi, W. P., Bota, K., Matendechero, S., Wandera, C., Siyoi, F., Kirui, E., Oluka, M., Nambwa, P., Gurumurthy, P., Njenga, S. M., Guantai, A., & Aklillu, E. (2021). Safety and Tolerability of Mass Diethylcarbamazine and Albendazole Administration for the Elimination of Lymphatic Filariasis in Kenya: An Active Surveillance Study. Pharmaceuticals, 14(3), 264. https://doi.org/10.3390/ph14030264






