1,2,4-Triazoles as Important Antibacterial Agents
Abstract
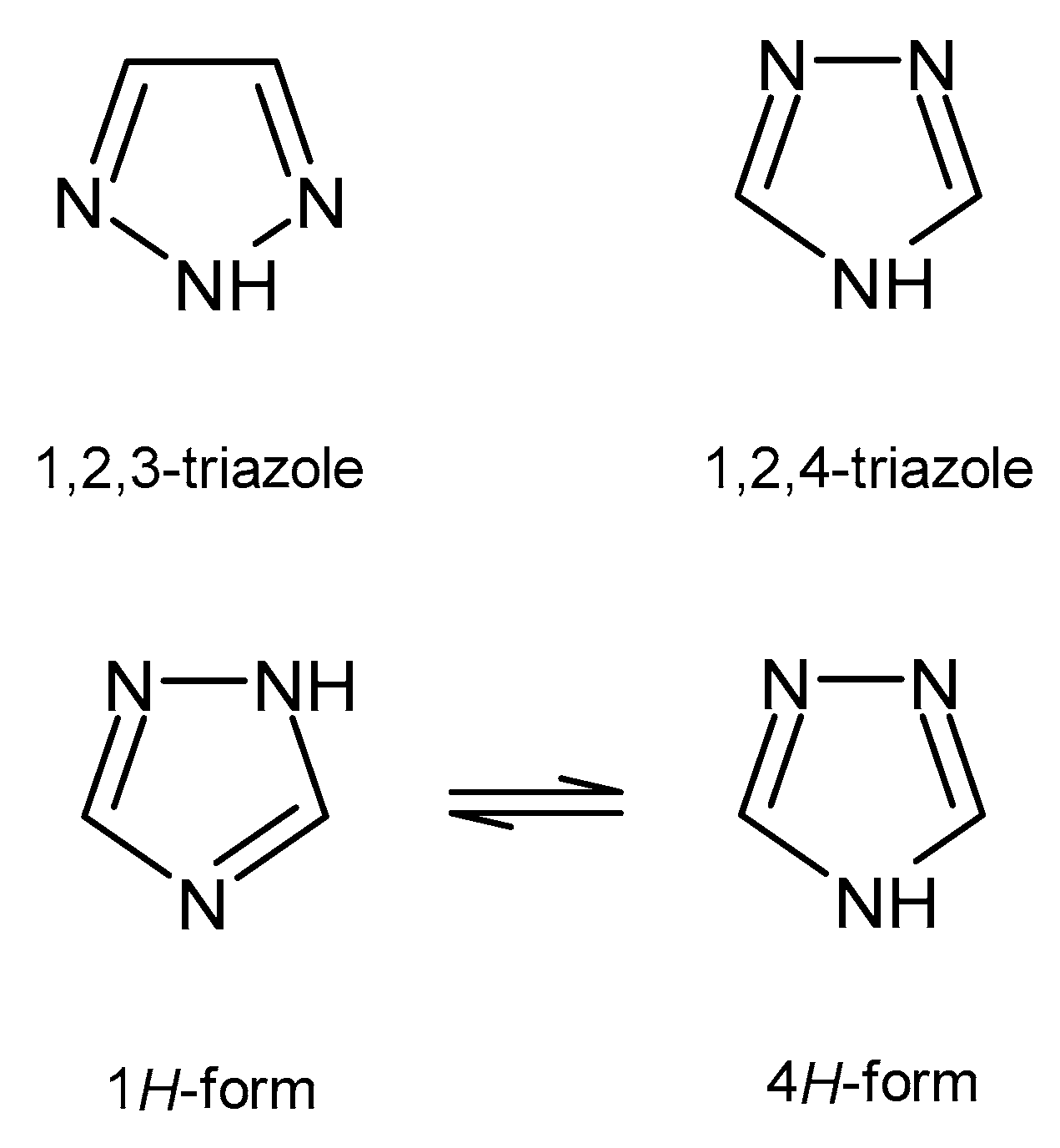
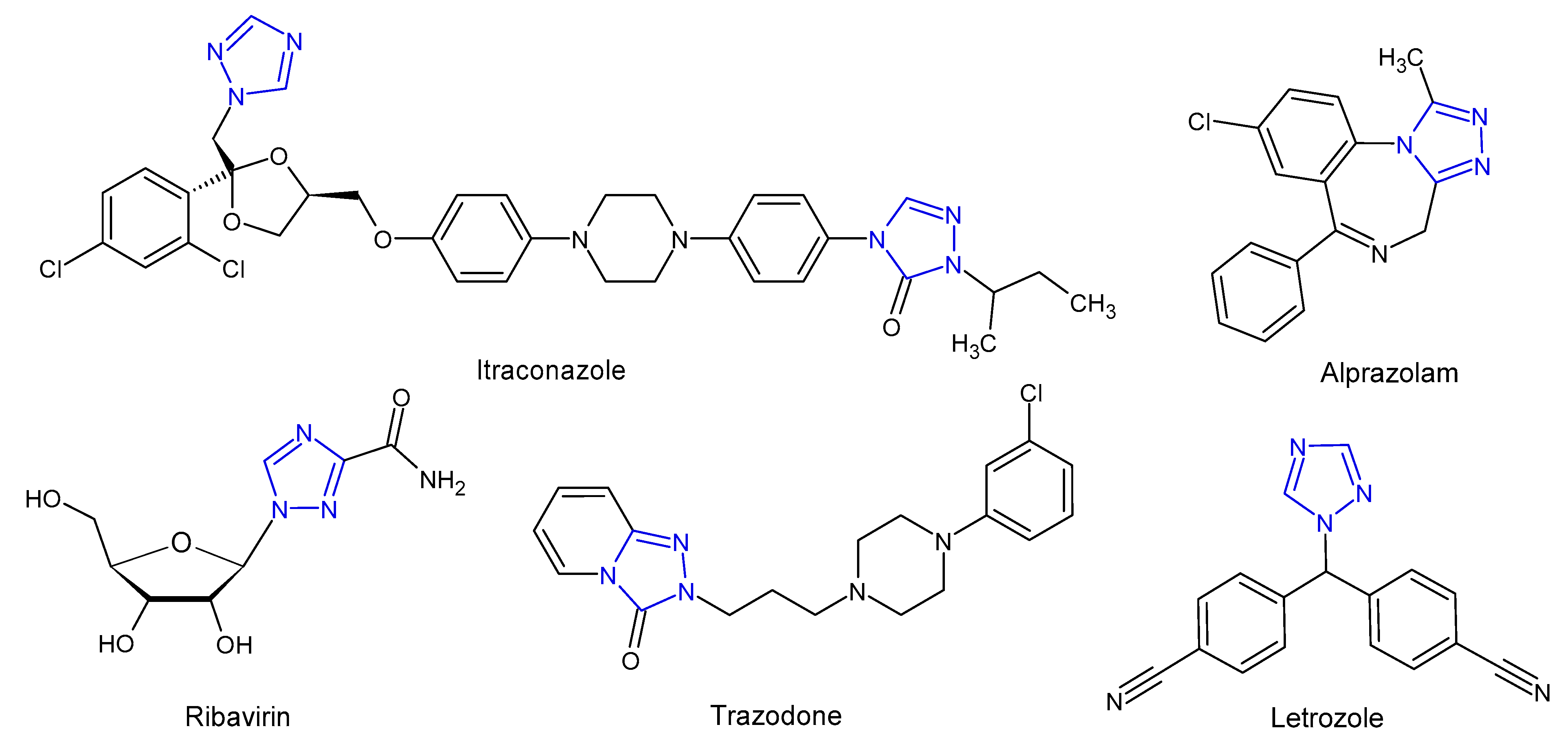
1. Introduction
2. Antibacterial Activity of Derivatives of 1,2,4-Triazole
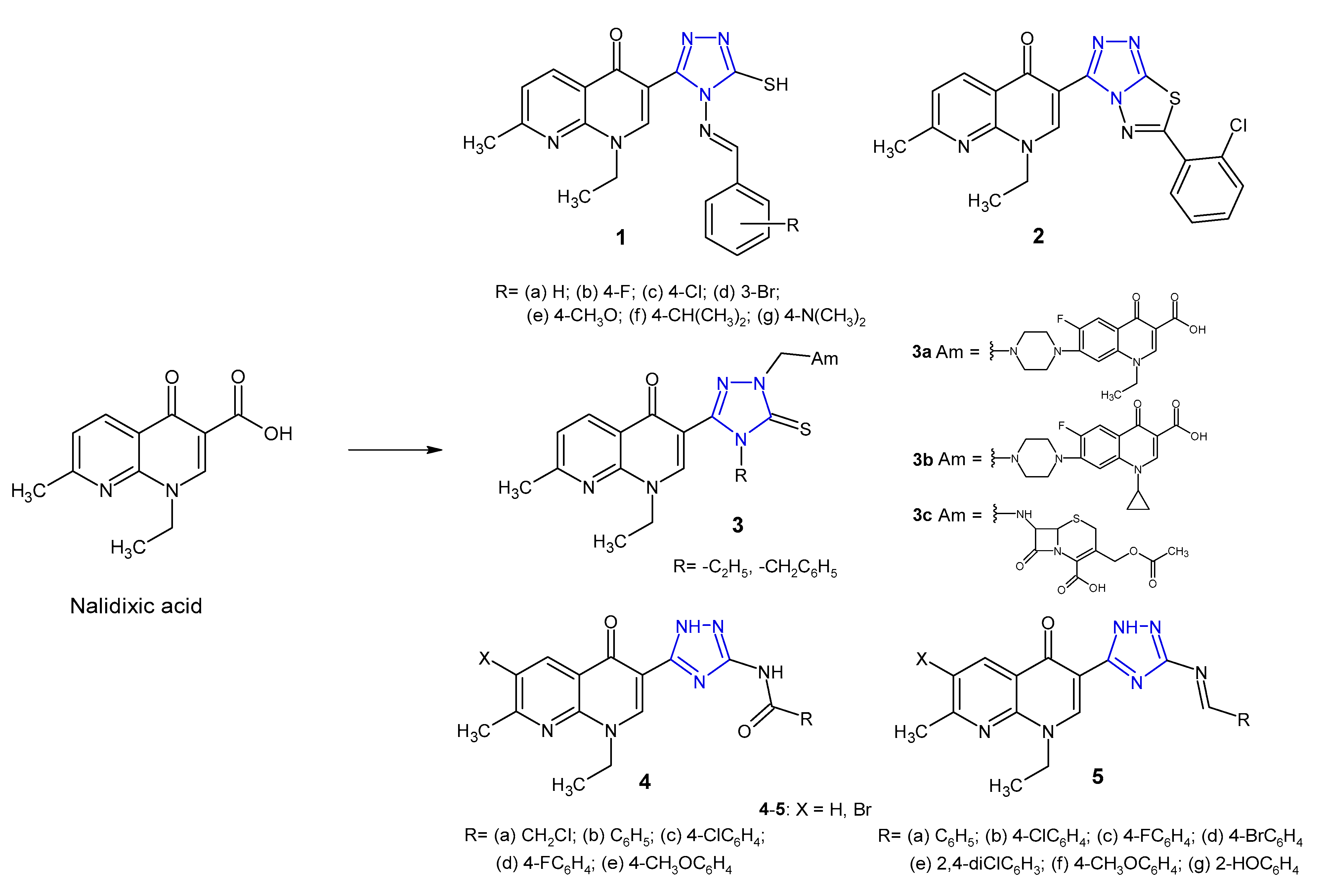
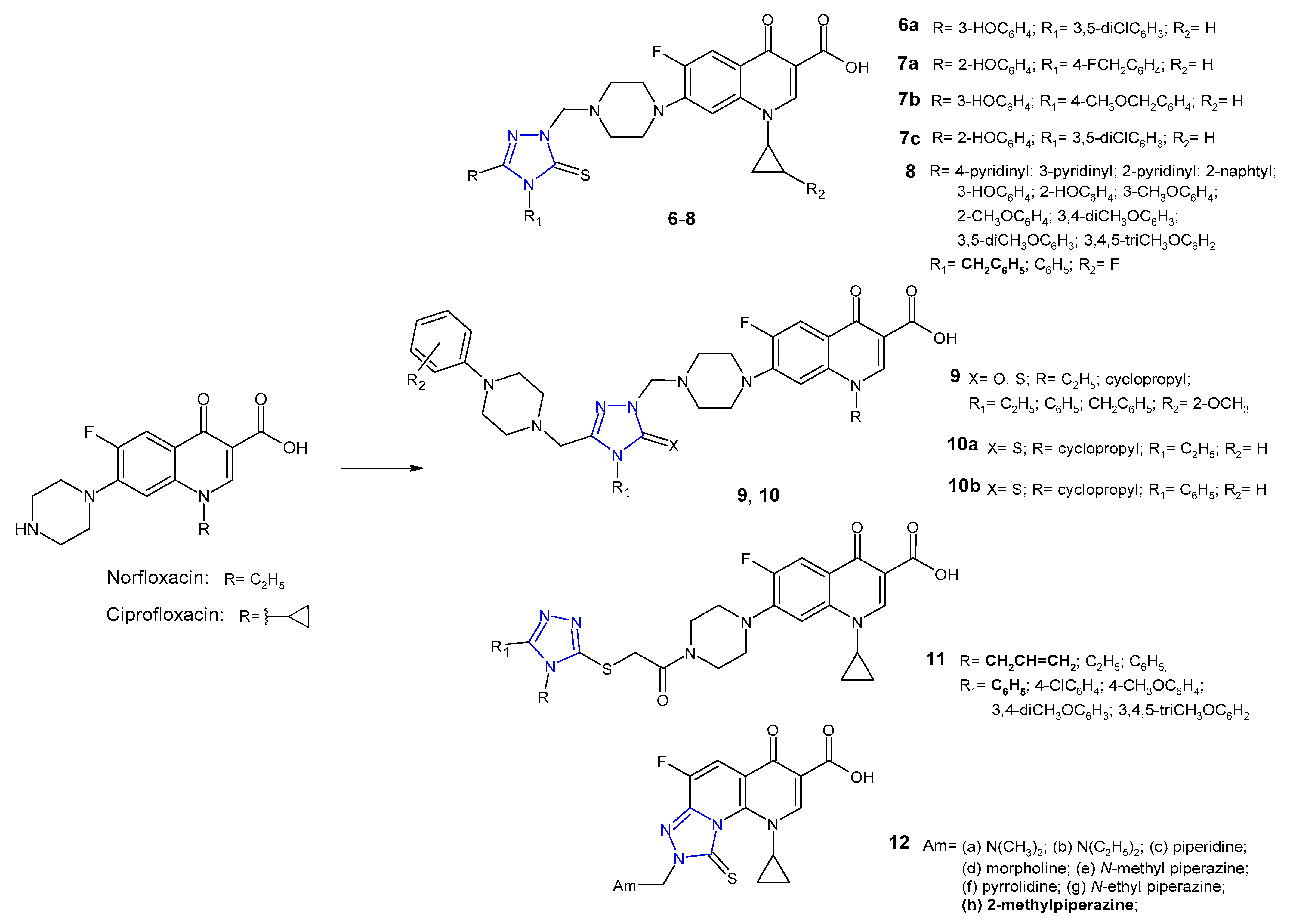
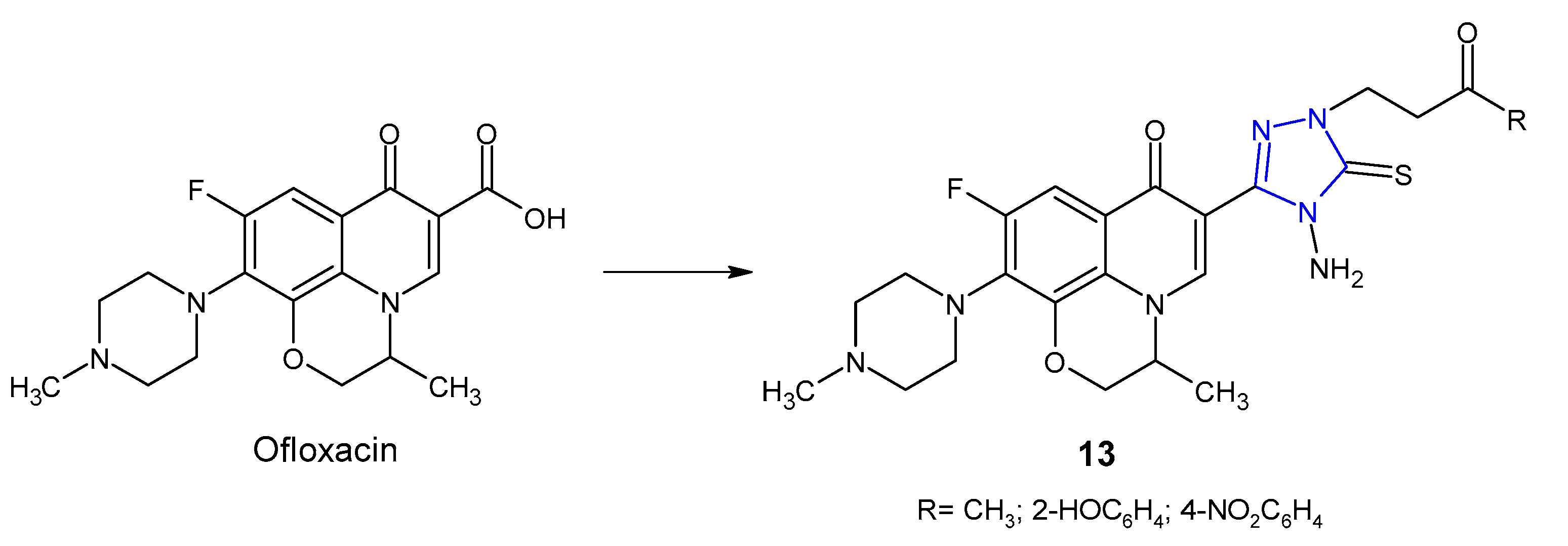
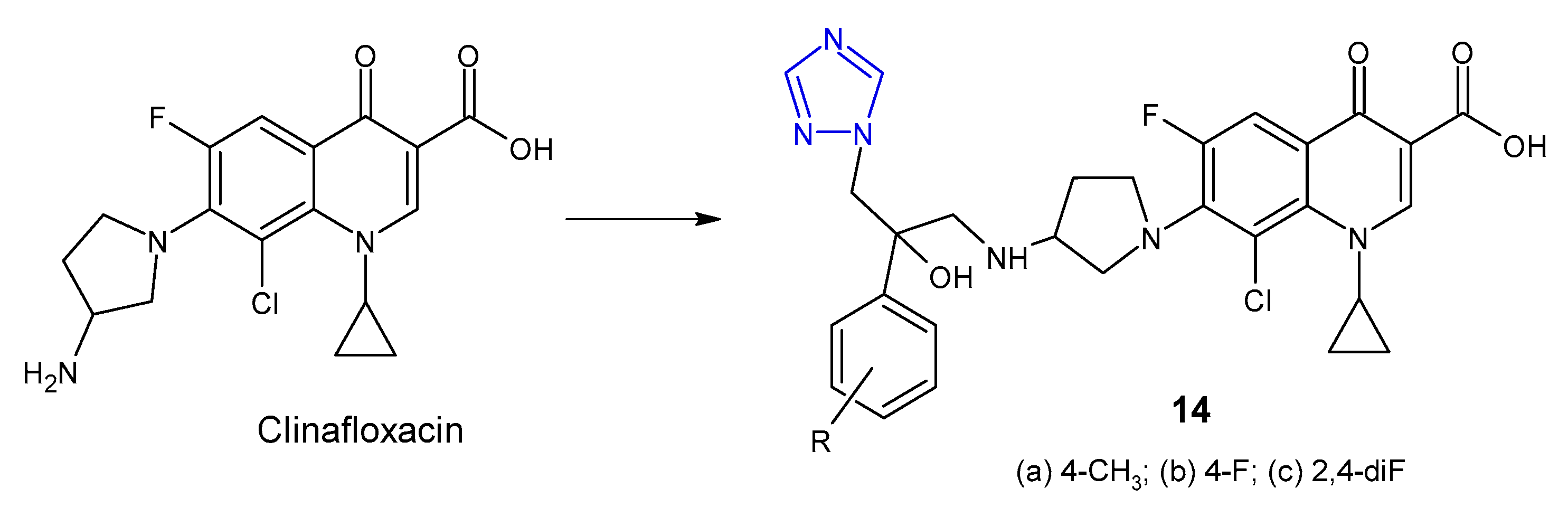
2.1. Triazole Hybrides of Quinolone Antibacterial Agents
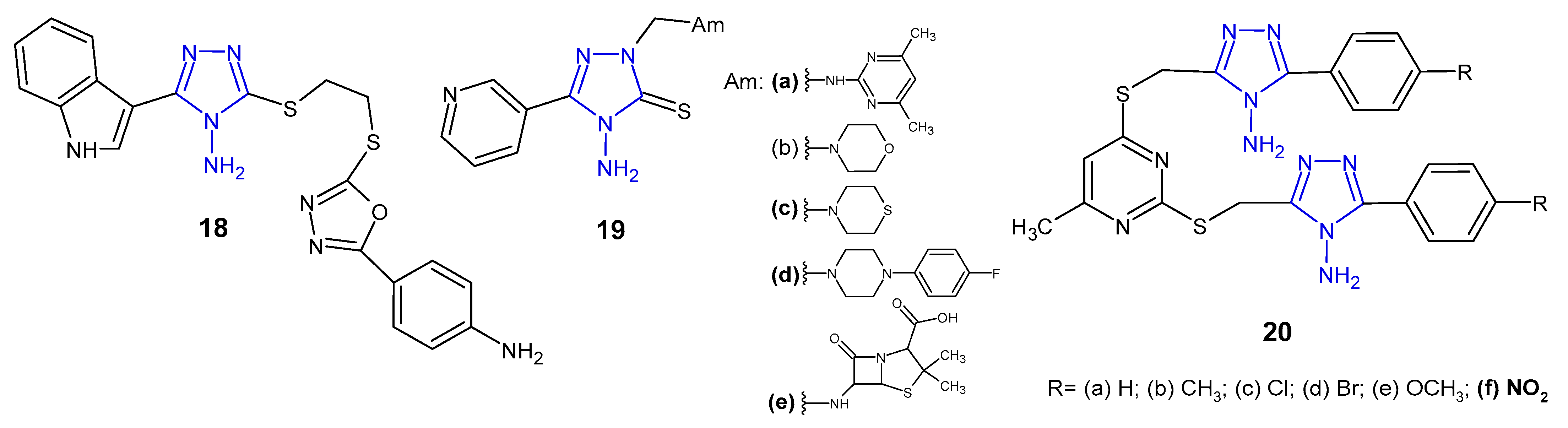
2.2. Antibacterial Activity of 4-Amino-1,2,4-Triazole Derivatives
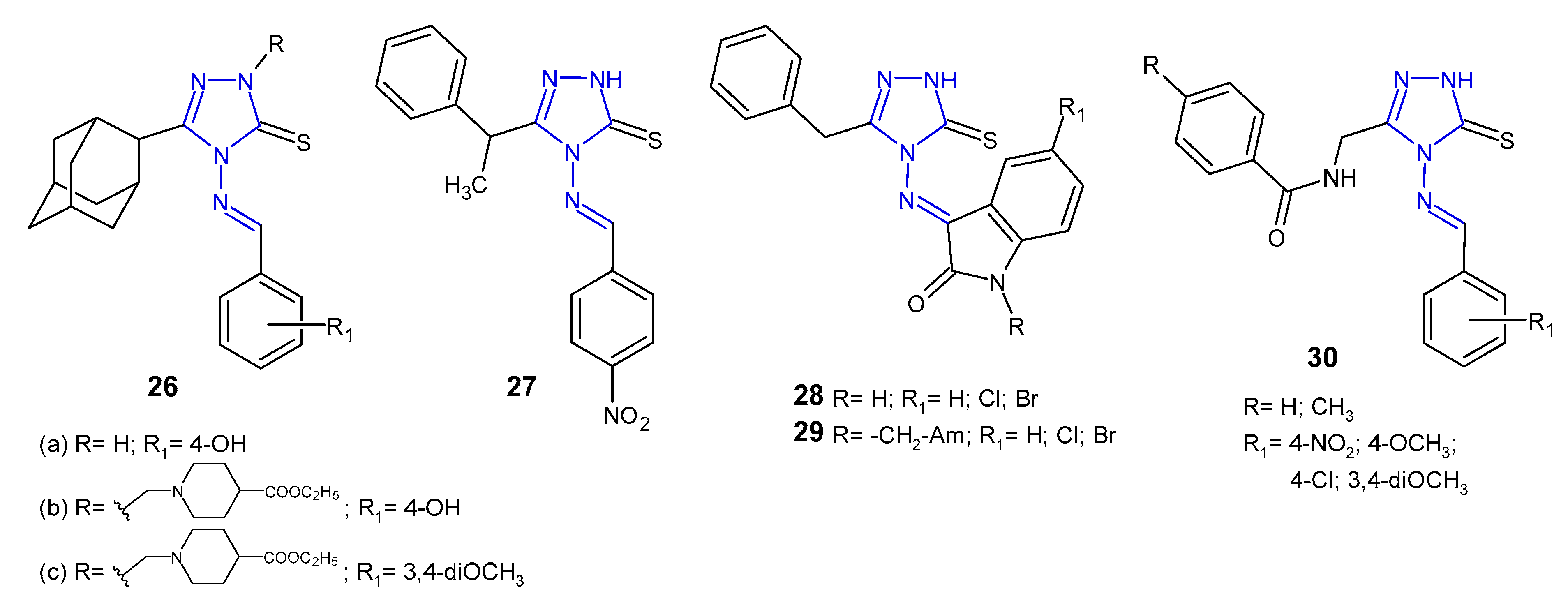
2.3. Antibacterial Activity of Schiff Bases of 4-Amino-1,2,4-Triazole Derivatives
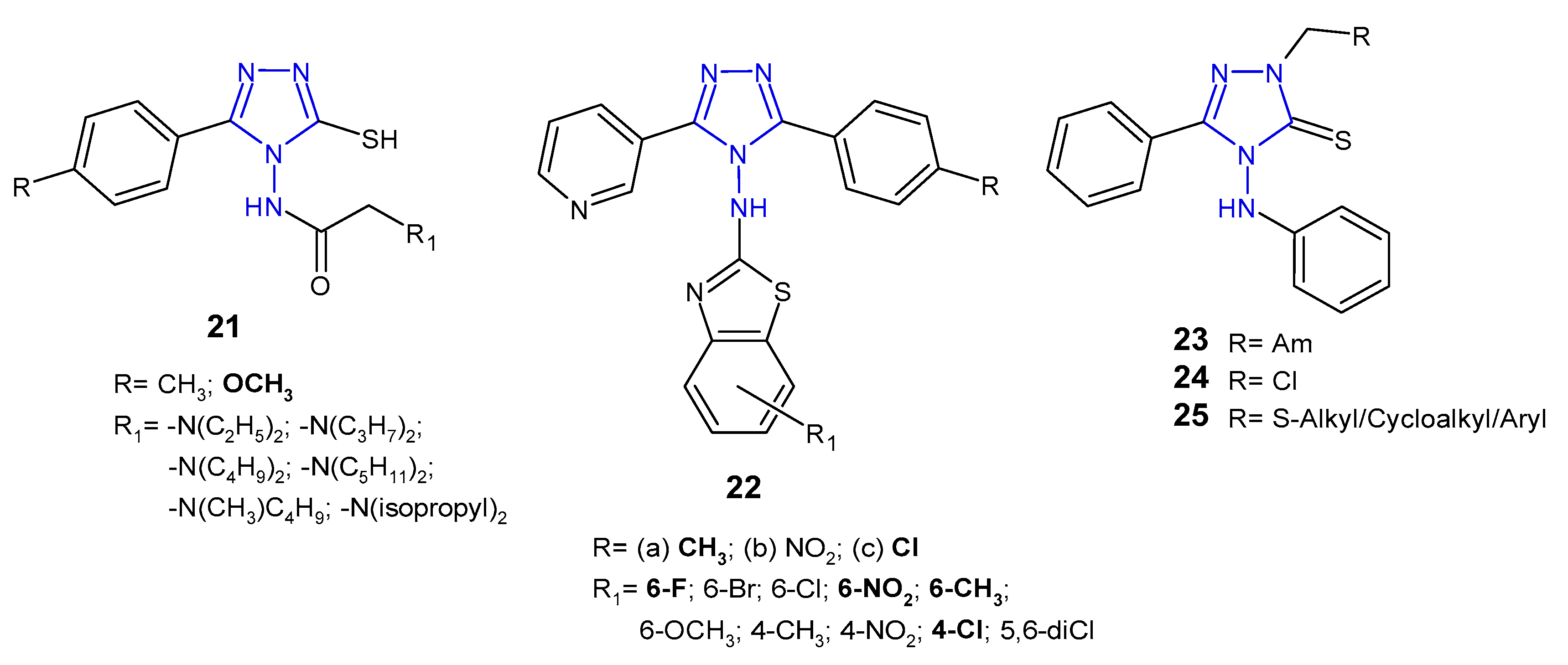
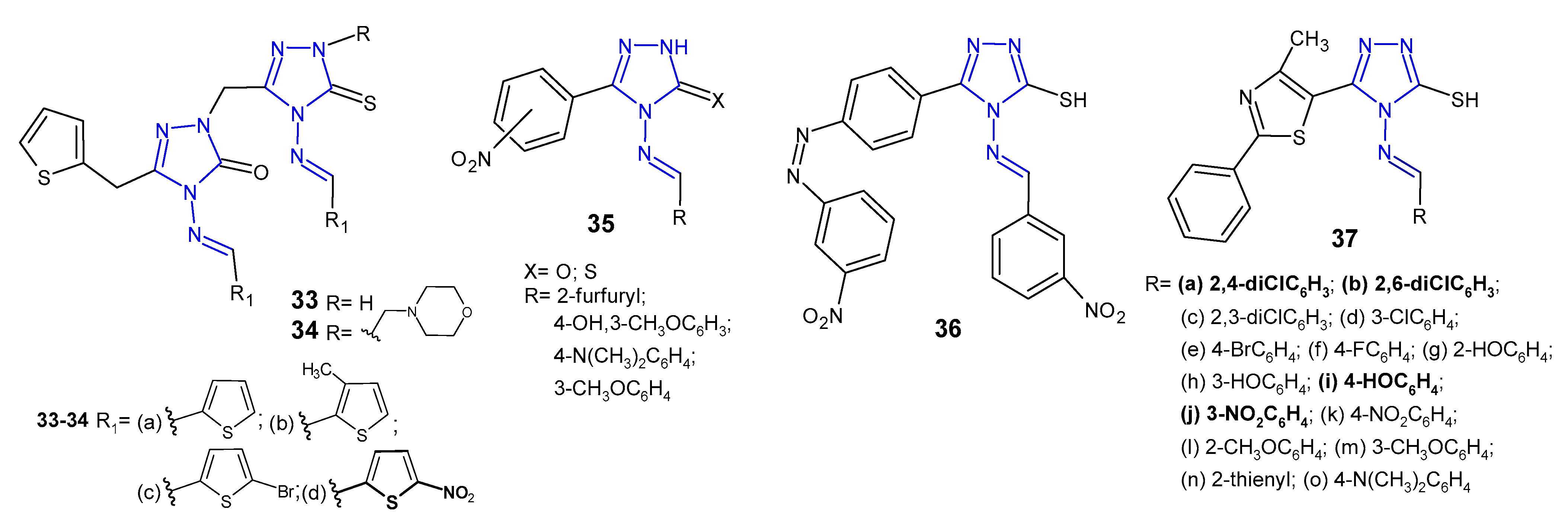
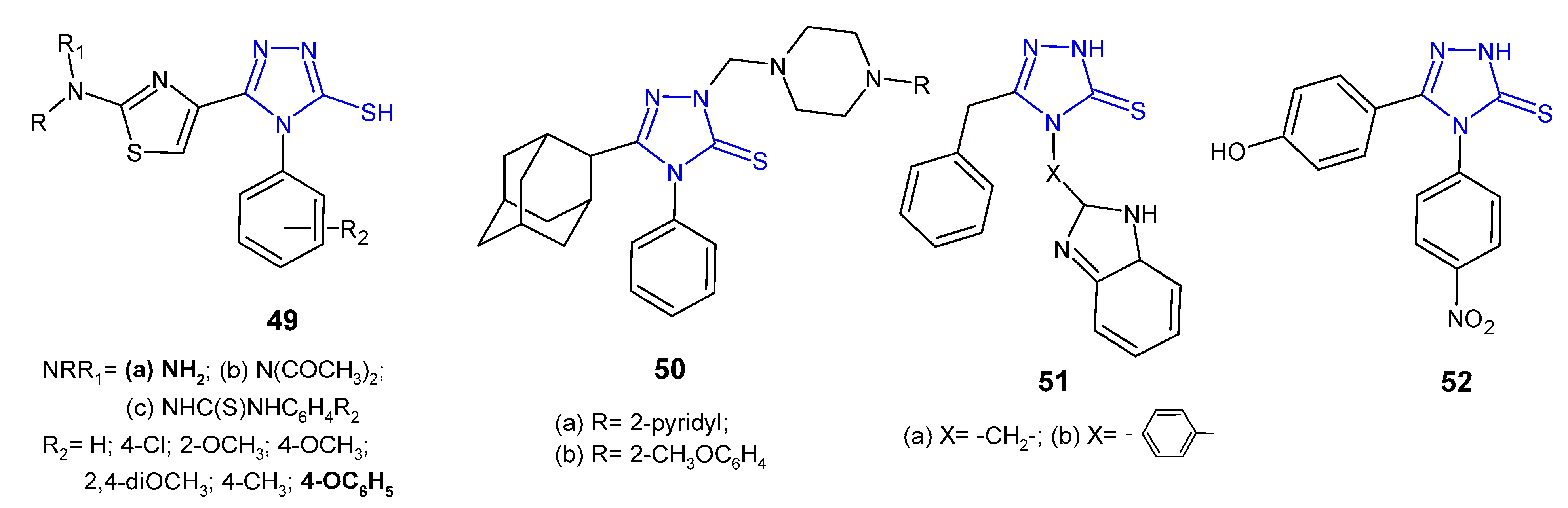
2.4. Antibacterial Activity of 1,2,4-Triazole-3-Thione Derivatives
2.5. Antibacterial Activity of S-Substituted 1,2,4-Triazole-3-Thione Derivatives
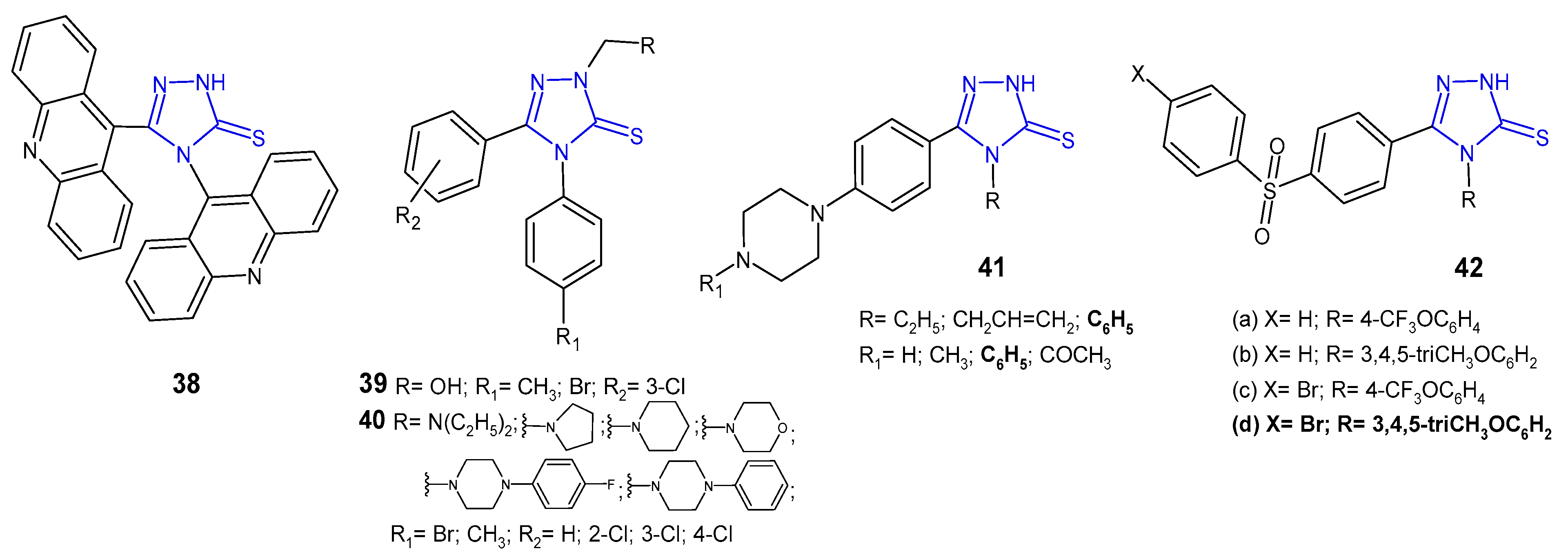
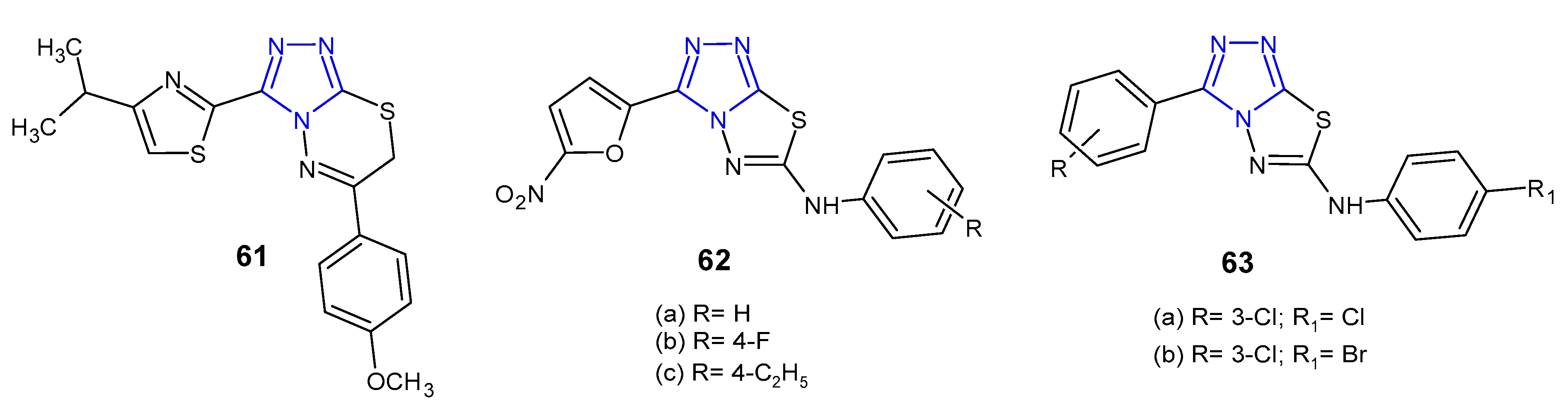
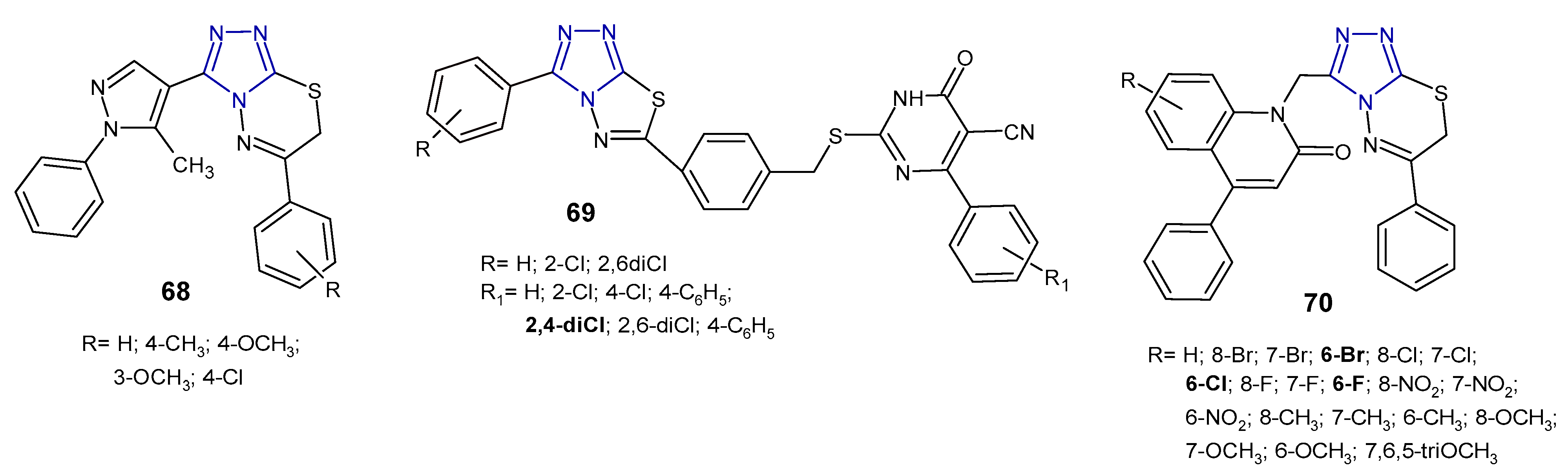
2.6. Antibacterial Activity of Fused 1,2,4-Triazole Derivatives
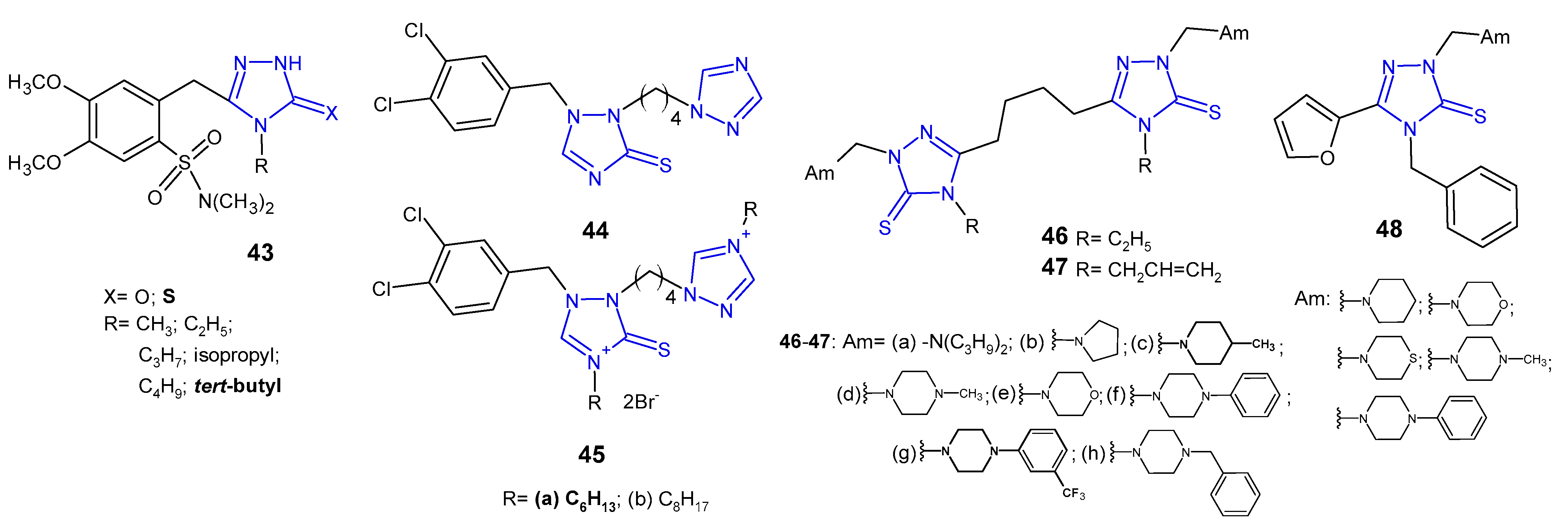
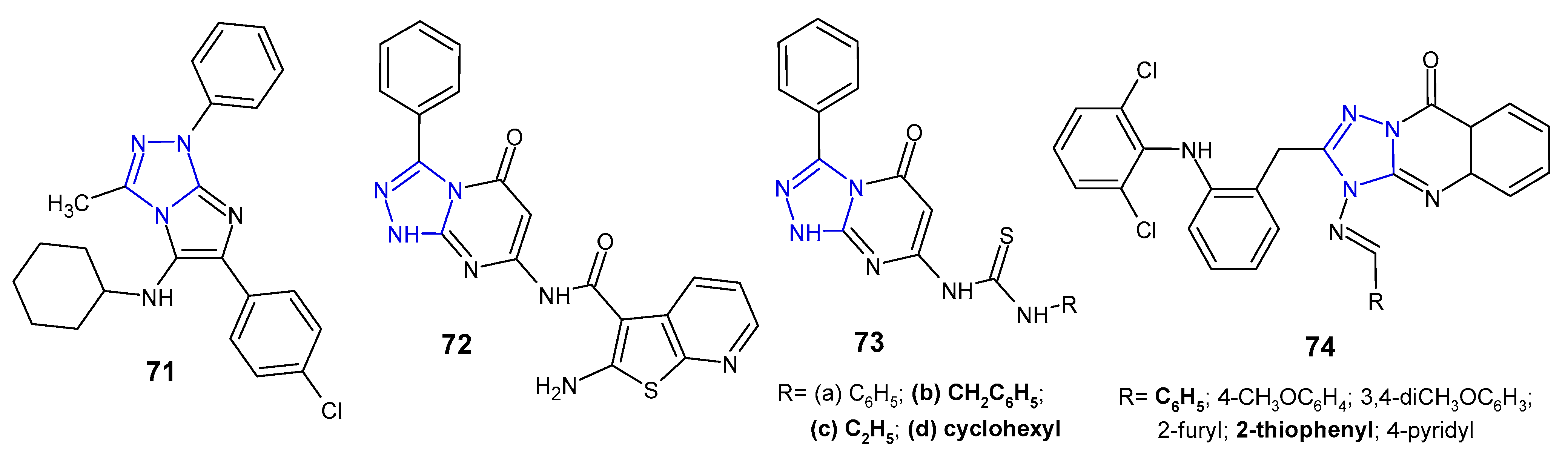
2.7. Miscellaneous 1,2,4-Triazoles with Antibacterial Activity
2.8. Structure-Activity Observations
3. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Duval, R.E.; Grare, M.; Demoré, B. Fight against Antimicrobial Resistance: We Always Need New Antibacterials but for Right Bacteria. Molecules 2019, 24, 3152. [Google Scholar] [CrossRef]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial Resistance in Veterinary Medicine: An Overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet 2016, 357, 176–187. [Google Scholar] [CrossRef]
- Donadu, M.G.; Le, N.T.; Ho, D.V.; Doan, T.Q.; Le, A.T.; Raal, A.; Usai, M.; Marchetti, M.; Sanna, G.; Madeddu, S.; et al. Phytochemical Compositions and Biological Activities of Essential Oils from the Leaves, Rhizomes and Whole Plant of Hornstedtia Bella Škorničk. Antibiotics 2020, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Sahu, J.K.; Kaushik, A. A Review on “Triazoles”: Their Chemistry and Pharmacological Potentials. Curr. Res. Pharm. Sci. 2013, 3, 108–113. [Google Scholar]
- Karaca Gençer, H.; Acar Çevik, U.; Levent, S.; Sağlık, B.N.; Korkut, B.; Özkay, Y.; Ilgın, S.; Öztürk, Y. New Benzimidazole-1,2,4-Triazole Hybrid Compounds: Synthesis, Anticandidal Activity and Cytotoxicity Evaluation. Molecules 2017, 22, 507. [Google Scholar] [CrossRef]
- Appna, N.R.; Nagiri, R.K.; Korupolu, R.B.; Kanugala, S.; Chityal, G.K.; Thipparapu, G.; Banda, N. Design and Synthesis of Novel 4-Hydrazone Functionalized/1,2,4-Triazole Fused Pyrido[2,3-d]Pyrimidine Derivatives, Their Evaluation for Antifungal Activity and Docking Studies. Med. Chem. Res. 2019, 28, 1509–1528. [Google Scholar] [CrossRef]
- Rode, N.D.; Sonawane, A.D.; Nawale, L.; Khedkar, V.M.; Joshi, R.A.; Likhite, A.P.; Sarkar, D.; Joshi, R.R. Synthesis, Biological Evaluation, and Molecular Docking Studies of Novel 3-Aryl-5-(Alkyl-Thio)-1H-1,2,4-Triazoles Derivatives Targeting Mycobacterium Tuberculosis. Chem. Biol. Drug Des. 2017, 90, 1206–1214. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, G.; Zeng, Q.H.; Li, Y.; Wu, Y.; Liu, H.; Wang, J.J.; Zhao, Y. Synthesis, Antioxidant and Anti-Tyrosinase Activity of 1,2,4-Triazole Hydrazones as Antibrowning Agents. Food Chem. 2021, 341, 128265. [Google Scholar] [CrossRef]
- Grytsai, O.; Valiashko, O.; Penco-Campillo, M.; Dufies, M.; Hagege, A.; Demange, L.; Martial, S.; Pagès, G.; Ronco, C.; Benhida, R. Synthesis and Biological Evaluation of 3-Amino-1,2,4-Triazole Derivatives as Potential Anticancer Compounds. Bioorg. Chem. 2020, 104, 104271. [Google Scholar] [CrossRef]
- Li, S.M.; Tsai, S.E.; Chiang, C.Y.; Chung, C.Y.; Chuang, T.J.; Tseng, C.C.; Jiang, W.P.; Huang, G.J.; Lin, C.Y.; Yang, Y.C.; et al. New Methyl 5-(Halomethyl)-1-Aryl-1H-1,2,4-Triazole-3-Carboxylates as Selective COX-2 Inhibitors and Anti-Inflammatory Agents: Design, Synthesis, Biological Evaluation, and Docking Study. Bioorg. Chem. 2020, 104, 104333. [Google Scholar] [CrossRef]
- Khanage, S.G.; Raju, A.; Mohite, P.B.; Pandhare, R.B. Analgesic Activity of Some 1,2,4-Triazole Heterocycles Clubbed with Pyrazole, Tetrazole, Isoxazole and Pyrimidine. Adv. Pharm. Bull. 2013, 3, 13–18. [Google Scholar] [CrossRef]
- Hichri, F.; Omri, A.; Hossan, A.S.M.; Ben Jannet, H. Alpha-Glucosidase and Amylase Inhibitory Effects of Eruca Vesicaria Subsp. Longirostris Essential Oils: Synthesis of New 1,2,4-Triazole-Thiol Derivatives and 1,3,4-Thiadiazole with Potential Inhibitory Activity. Pharm. Biol. 2019, 57, 564–570. [Google Scholar] [CrossRef]
- Kaproń, B.; Łuszczki, J.J.; Siwek, A.; Karcz, T.; Nowak, G.; Zagaja, M.; Andres-Mach, M.; Stasiłowicz, A.; Cielecka-Piontek, J.; Kocki, J.; et al. Preclinical Evaluation of 1,2,4-Triazole-Based Compounds Targeting Voltage-Gated Sodium Channels (VGSCs) as Promising Anticonvulsant Drug Candidates. Bioorg. Chem. 2020, 94, 103355. [Google Scholar] [CrossRef] [PubMed]
- Navidpour, L.; Shabani, S.; Heidari, A.; Bashiri, M.; Ebrahim-Habibi, A.; Shahhosseini, S.; Shafaroodi, H.; Abbas Tabatabai, S.; Toolabi, M. 5-[Aryloxypyridyl (or Nitrophenyl)]-4H-1,2,4-Triazoles as Novel Flexible Benzodiazepine Analogues: Synthesis, Receptor Binding Affinity and Lipophilicity-Dependent Anti-Seizure Onset of Action. Bioorg. Chem. 2021, 106, 104504. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Zeichner, L.; Casadevall, A.; Galgiani, J.N.; Odds, F.C.; Rex, J.H. An Insight into the Antifungal Pipeline: Selected New Molecules and Beyond. Nat. Rev. Drug Discov. 2010, 9, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Ezelarab, H.A.A.; Abbas, S.H.; Hassan, H.A.; Abuo-Rahma, G.E.D.A. Recent Updates of Fluoroquinolones as Antibacterial Agents. Arch. Pharm. Chem. Life Sci. 2018, 351, 1800141. [Google Scholar] [CrossRef]
- Aggarwal, N.; Kumar, R.; Dureja, P.; Khurana, J.M. Synthesis, Antimicrobial Evaluation and QSAR Analysis of Novel Nalidixic Acid Based 1,2,4-Triazole Derivatives. Eur. J. Med. Chem. 2011, 46, 4089–4099. [Google Scholar] [CrossRef]
- Ceylan, S.; Bayrak, H.; Basoglu Ozdemir, S.; Uygun, Y.; Mermer, A.; Demirbas, N.; Ulker, S. Microwave-Assisted and Conventional Synthesis of Novel Antimicrobial 1,2,4-Triazole Derivatives Containing Nalidixic Acid Skeleton. Heterocycl. Commun. 2016, 22, 229–237. [Google Scholar] [CrossRef]
- Mohamed, N.G.; Sheha, M.M.; Hassan, H.Y.; Abdel-Hafez, L.J.M.; Omar, F.A. Synthesis, Antimicrobial Activity and Molecular Modeling Study of 3-(5-Amino-(2H)-1,2,4-Triazol-3-Yl]-Naphthyridinones as Potential DNA-Gyrase Inhibitors. Bioorg. Chem. 2018, 81, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Wujec, M.; Kosikowska, U.; Malm, A.; Rajtar, B.; Polz-Dacewicz, M. Synthesis and in Vitro Activity of 1,2,4-Triazole-Ciprofloxacin Hybrids against Drug-Susceptible and Drug-Resistant Bacteria. Eur. J. Med. Chem. 2013, 60, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kosikowska, U.; Andrzejczuk, S.; Plech, T.; Malm, A. Inhibitory Effect of 1,2,4-Triazole-Ciprofloxacin Hybrids on Haemophilus Parainfluenzae and Haemophilus Influenzae Biofilm Formation in Vitro under Stationary Conditions. Res. Microbiol. 2016, 167, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Kapron, B.; Paneth, A.; Kosikowska, U.; Malm, A.; Strzelczyk, A.; Staczek, P.; Swiatek, L.; Rajtar, B.; Polz-Dacewicz, M. Search for Factors Affecting Antibacterial Activity and Toxicity of 1,2,4-Triazole-Ciprofloxacin Hybrids. Eur. J. Med. Chem. 2015, 97, 94–103. [Google Scholar] [CrossRef]
- Plech, T.; Kaproń, B.; Paneth, A.; Kosikowska, U.; Malm, A.; Strzelczyk, A.; Stączek, P.; Świątek, Ł.; Rajtar, B.; Polz-Dacewicz, M. Determination of the Primary Molecular Target of 1,2,4-Triazole-Ciprofloxacin Hybrids. Molecules 2015, 20, 6254–6272. [Google Scholar] [CrossRef]
- Gao, Y.; Na, L.X.; Xu, Z.; Zhang, S.; Wang, A.P.; Kai, L.; Guo, H.Y.; Liu, M.L. Design, Synthesis and Antibacterial Evaluation of 1-[(1R,2S)-2-Fluorocyclopropyl]Ciprofloxacin-1,2,4-Triazole-5(4H)-Thione Hybrids. Chem. Biodivers. 2018, 15, e1800261. [Google Scholar] [CrossRef]
- Mermer, A.; Demirci, S.; Ozdemir, S.B.; Demirbas, A.; Ulker, S.; Ayaz, F.A.; Aksakal, F.; Demirbas, N. Conventional and Microwave Irradiated Synthesis, Biological Activity Evaluation and Molecular Docking Studies of Highly Substituted Piperazine-Azole Hybrids. Chin. Chem. Lett. 2017, 28, 995–1005. [Google Scholar] [CrossRef]
- Mermer, A.; Faiz, O.; Demirbas, A.; Demirbas, N.; Alagumuthu, M.; Arumugam, S. Piperazine-Azole-Fluoroquinolone Hybrids: Conventional and Microwave Irradiated Synthesis, Biological Activity Screening and Molecular Docking Studies. Bioorg. Chem. 2019, 85, 308–318. [Google Scholar] [CrossRef]
- Mohammed, H.H.H.; Abdelhafez, E.S.M.N.; Abbas, S.H.; Moustafa, G.A.I.; Hauk, G.; Berger, J.M.; Mitarai, S.; Arai, M.; Abd El-Baky, R.M.; Abuo-Rahma, G.E.D.A. Design, Synthesis and Molecular Docking of New N-4-Piperazinyl Ciprofloxacin-Triazole Hybrids with Potential Antimicrobial Activity. Bioorg. Chem. 2019, 88, 102952. [Google Scholar] [CrossRef]
- Gao, L.Z.; Xie, Y.S.; Li, T.; Huang, W.L.; Hu, G.Q. Synthesis and Antibacterial Activity of Novel [1,2,4]Triazolo[3,4-h][1,8]Naphthyridine-7-Carboxylic Acid Derivatives. Chin. Chem. Lett. 2015, 26, 149–151. [Google Scholar] [CrossRef]
- Jubie, S.; Prabitha, P.; Rajesh Kumar, R.; Kalirajan, R.; Gayathri, R.; Sankar, S.; Elango, K. Design, Synthesis, and Docking Studies of Novel Ofloxacin Analogues as Antimicrobial Agents. Med. Chem. Res. 2012, 21, 1403–1410. [Google Scholar] [CrossRef]
- Wang, Y.; Damu, G.L.V.; Lv, J.S.; Geng, R.X.; Yang, D.C.; Zhou, C.H. Design, Synthesis and Evaluation of Clinafloxacin Triazole Hybrids as a New Type of Antibacterial and Antifungal Agents. Bioorg. Med. Chem. Lett. 2012, 22, 5363–5366. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Sharma, R.; Pattanayak, P. Synthesis and Evaluation of 4-Amino-5-Phenyl-4H-[1,2,4]-Triazole-3-Thiol Derivatives as Antimicrobial Agents. Med. Chem. Res. 2010, 19, 127–135. [Google Scholar] [CrossRef]
- Muthal, N.; Ahirwar, J.; Ahriwar, D.; Masih, P.; Mahmdapure, T.; Sivakumar, T. Synthesis, Antimicrobial and Anti-Inflammatory Activity of Some 5-Substituted-3-Pyridine-1, 2, 4-Triazoles. Int. J. Pharm Tech Res. 2010, 2, 2450–2455. [Google Scholar]
- Gadegoni, H.; Manda, S. Synthesis and Screening of Some Novel Substituted Indoles Contained 1,3,4-Oxadiazole and 1,2,4-Triazole Moiety. Chin. Chem. Lett. 2013, 24, 127–130. [Google Scholar] [CrossRef]
- Shi, Z.; Zhao, Z.; Huang, M.; Fu, X. Ultrasound-Assisted, One-Pot, Three-Component Synthesis and Antibacterial Activities of Novel Indole Derivatives Containing 1,3,4-Oxadiazole and 1,2,4-Triazole Moieties. Comptes Rendus Chim. 2015, 18, 1320–1327. [Google Scholar] [CrossRef]
- Ceylan, S. Synthesis and Biological Evaluation of New Mannich and Schiff Bases Containing 1,2,4-Triazole and 1,3,4-Oxadiazole Nucleus. Med. Chem. Res. 2016, 25, 1958–1970. [Google Scholar] [CrossRef]
- Madhu Sekhar, M.; Nagarjuna, U.; Padmavathi, V.; Padmaja, A.; Reddy, N.V.; Vijaya, T. Synthesis and Antimicrobial Activity of Pyrimidinyl 1,3,4-Oxadiazoles, 1,3,4-Thiadiazoles and 1,2,4-Triazoles. Eur. J. Med. Chem. 2018, 145, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Upmanyu, N.; Kumar, S.; Porwal, P.; Shah, K.; Mishra, P. Synthesis and Evaluation of 4-(Substituted)-Acetylamino-3-Mercapto-5-(4- Substituted) Phenyl-1,2,4-Triazole Derivatives as Antimicrobial Agents. Med. Chem. Res. 2012, 21, 1967–1976. [Google Scholar] [CrossRef]
- Patel, N.B.; Khan, I.H.; Rajani, S.D. Pharmacological Evaluation and Characterizations of Newly Synthesized 1,2,4-Triazoles. Eur. J. Med. Chem. 2010, 45, 4293–4299. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.B.; Khan, I.H.; Pannecouque, C.; De Clercq, E. Anti-HIV, Antimycobacterial and Antimicrobial Studies of Newly Synthesized 1,2,4-Triazole Clubbed Benzothiazoles. Med. Chem. Res. 2013, 22, 1320–1329. [Google Scholar] [CrossRef]
- Ghattas, A.E.B.A.G.; Moustafa, H.M.; Hassanein, E.A.A.; Hussein, B.R.M. Synthesis and Antibacterial Activity of Some New 4-Anilino-5-Phenyl-4H-1,2,4-Triazole-3-Thiol Derivatives. Arab. J. Chem. 2016, 9, S1654–S1659. [Google Scholar] [CrossRef]
- Al-Omar, M.A.; Al-Abdullah, E.S.; Shehata, I.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, Antimicrobial, and Anti-Inflammatory Activities of Novel 5-(1-Adamantyl)-4-Arylideneamino-3-Mercapto-1,2,4-Triazoles and Related Derivatives. Molecules 2010, 15, 2526–2550. [Google Scholar] [CrossRef] [PubMed]
- Çalisir, M.M.; Kocyigit-Kaymakcioglu, B.; Özbek, B.; Ötük, G. Synthesis and Antimicrobial Activity of Some Novel Schiff Bases Containing 1,2,4-Triazole-3-Thione. E-J. Chem. 2010, 7, 458–464. [Google Scholar] [CrossRef]
- Murthy, Y.L.N.; Govindh, B.; Diwakar, B.S.; Nagalakshmi, K.; Rao, K.V.R. Synthesis and Bioevaluation of Schiff and Mannich Bases of Isatin Derivatives with 4-Amino-5-Benzyl-2,4-Dihydro-3H-1,2,4-Triazole-3-Thione. Med. Chem. Res. 2012, 21, 3104–3110. [Google Scholar] [CrossRef]
- Mange, Y.J.; Isloor, A.M.; Malladi, S.; Isloor, S.; Fun, H.K. Synthesis and Antimicrobial Activities of Some Novel 1,2,4-Triazole Derivatives. Arab. J. Chem. 2013, 6, 177–181. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, Z.; Liu, X.; Li, G. Microwave-Assisted Synthesis and Biological Activity of New Schiff Bases Derived from Dimers of 4-Amino-3-[3-(1-Benzyl)Indole]-5-Thiomethyl-1,2,4- Triazole. Res. Chem. Intermed. 2013, 39, 1897–1905. [Google Scholar] [CrossRef]
- Peng, Y.L.; Liu, X.L.; Wang, X.H.; Zhao, Z.G. Microwave-Assisted Synthesis and Antibacterial Activity of Derivatives of 3-[1-(4-Fluorobenzyl)-1H-Indol-3-Yl]-5-(4-Fluorobenzylthio)-4H-1,2, 4-Triazol-4-Amine. Chem. Pap. 2014, 68, 401–408. [Google Scholar] [CrossRef]
- Ünver, Y.; Deniz, S.; Çelik, F.; Akar, Z.; Küçük, M.; Sancak, K. Synthesis of New 1,2,4-Triazole Compounds Containing Schiff and Mannich Bases (Morpholine) with Antioxidant and Antimicrobial Activities. J. Enzym. Inhib. Med. Chem. 2016, 31, 89–95. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Sivakumar, K.K.; Sureshkumar, K.; Manjushree, M. Design, Synthesis, Characterisation and in-Vitro Antimicrobial Activity of Some Hybridized Triazole Scaffolds. Future J. Pharm. Sci. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Thakkar, S.S.; Thakor, P.; Doshi, H.; Ray, A. 1,2,4-Triazole and 1,3,4-Oxadiazole Analogues: Synthesis, MO Studies, in Silico Molecular Docking Studies, Antimalarial as DHFR Inhibitor and Antimicrobial Activities. Bioorg. Med. Chem. 2017, 25, 4064–4075. [Google Scholar] [CrossRef]
- Nastasă, C.; Vodnar, D.C.; Ionuţ, I.; Stana, A.; Benedec, D.; Tamaian, R.; Oniga, O.; Tiperciuc, B. Antibacterial Evaluation and Virtual Screening of New Thiazolyl-Triazole Schiff Bases as Potential DNA-Gyrase Inhibitors. Int. J. Mol. Sci. 2018, 19, 222. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.E.-S.; El-Kazak, A.M. Synthesis and Antimicrobial Activity of Some New 1,3-Thiazoles, 1,3,4-Thiadiazoles, 1,2,4-Triazoles and 1,3-Thiazines Incorporating Acridine and 1,2,3,4-Tetrahydroacridine Moieties. Eur. J. Chem. 2010, 1, 6–11. [Google Scholar] [CrossRef]
- Plech, T.; Wujec, M.; Kaproń, B.; Kosikowska, U.; Malm, A. Synthesis and Antibacterial Activity of Some Novel N2-Hydroxymethyl and N2-Aminomethyl Derivatives of 4-Aryl-5-(3-Chlorophenyl)-2,4-Dihydro-3H-1,2,4-Triazole-3-Thione. Heteroat. Chem. 2011, 22, 737–743. [Google Scholar] [CrossRef]
- Plech, T.; Wujec, M.; Majewska, M.; Kosikowska, U.; Malm, A. Microbiologically Active Mannich Bases Derived from 1,2,4-Triazoles. the Effect of C-5 Substituent on Antibacterial Activity. Med. Chem. Res. 2013, 22, 2531–2537. [Google Scholar] [CrossRef]
- Rajak, H.; Thakur, B.S.; Parmar, P.; Kumar, P.; Gupta, A.K.; Agrawal, N.; Chander Sharma, P.; Mahila Vishwavidyalaya, B. Antimicrobial Activity of Some Novel Triazole-3-Thione Containing Substituted Piperazine Moiety. Pharma Chem. 2011, 3, 422–426. [Google Scholar]
- Barbuceanu, S.F.; Saramet, G.; Almajan, G.L.; Draghici, C.; Barbuceanu, F.; Bancescu, G. New Heterocyclic Compounds from 1,2,4-Triazole and 1,3,4-Thiadiazole Class Bearing Diphenylsulfone Moieties. Synthesis, Characterization and Antimicrobial Activity Evaluation. Eur. J. Med. Chem. 2012, 49, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Zoumpoulakis, P.; Camoutsis, C.; Pairas, G.; Soković, M.; Glamočlija, J.; Potamitis, C.; Pitsas, A. Synthesis of Novel Sulfonamide-1,2,4-Triazoles, 1,3,4-Thiadiazoles and 1,3,4-Oxadiazoles, as Potential Antibacterial and Antifungal Agents. Biological Evaluation and Conformational Analysis Studies. Bioorg. Med. Chem. 2012, 20, 1569–1583. [Google Scholar] [CrossRef]
- Wang, Q.P.; Zhang, J.Q.; Damu, G.L.V.; Wan, K.; Zhang, H.Z.; Zhou, C.H. Synthesis and Biological Activities of Thio-Triazole Derivatives as Novel Potential Antibacterial and Antifungal Agents. Sci. Chin. Chem. 2012, 55, 2134–2153. [Google Scholar] [CrossRef]
- Koparir, M. Synthesis and Biological Activities of Some New Mannich Bases of 5,5′-Butane-1,4-Diylbis[4-Ethyl-2,4-Dihydro-3H-1,2,4-Triazole-3-Thiones. Chem. Sci. Trans. 2013, 2, 701–710. [Google Scholar] [CrossRef]
- Koparir, M.; Orek, C. Synthesis and Biological Activities of Some Novel Aminomethyl Derivatives of 5,5′-Butane-1,4-Diyl-Bis[4-Allyl-2,4-Dihydro-3H-1,2,4-Triazole-3-Thiones. Chem. Sci. Trans. 2013, 2, 181–191. [Google Scholar] [CrossRef]
- Basoglu, S.; Yolal, M.; Demirci, S.; Demirbas, N.; Bektas, H.; Karaoglu, S.A. Design, Synthesis and Antimicrobial Activities of Some Azole Derivatives. Acta Pol. Pharm. Drug Res. 2013, 70, 229–236. [Google Scholar]
- Hassan, G.S.; El-Messery, S.M.; Al-Omary, F.A.M.; Al-Rashood, S.T.; Shabayek, M.I.; Abulfadl, Y.S.; Habib, E.S.E.; El-Hallouty, S.M.; Fayad, W.; Mohamed, K.M.; et al. Nonclassical Antifolates, Part 4. 5-(2-Aminothiazol-4-Yl)-4-Phenyl-4H-1,2, 4-Triazole-3-Thiols as a New Class of DHFR Inhibitors: Synthesis, Biological Evaluation and Molecular Modeling Study. Eur. J. Med. Chem. 2013, 66, 135–145. [Google Scholar] [CrossRef]
- Al-Aabdullah, E.S.; Asiri, H.H.; Lahsasni, S.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, Antimicrobial, and Anti-Inflammatory Activity, of Novel s-Substituted and n-Substituted 5-(1-Adamantyl)-1,2,4-Triazole-3-Thiols. Drug Des. Dev. Ther. 2014, 8, 505–517. [Google Scholar] [CrossRef]
- Barot, K.P.; Manna, K.S.; Ghate, M.D. Design, Synthesis and Antimicrobial Activities of Some Novel 1,3,4-Thiadiazole, 1,2,4-Triazole-5-Thione and 1,3-Thiazolan-4-One Derivatives of Benzimidazole. J. Saudi Chem. Soc. 2017, 21, S35–S43. [Google Scholar] [CrossRef]
- Beyzaei, H.; Ghanbari Kudeyani, M.; Samareh Delarami, H.; Aryan, R. Synthesis, Antimicrobial and Antioxidant Evaluation, and Molecular Docking Study of 4,5-Disubstituted 1,2,4-Triazole-3-Thiones. J. Mol. Struct. 2020, 1215, 128273. [Google Scholar] [CrossRef]
- El-Feky, S.; Abou-zeid, L.; Massoud, M.; Shokralla, S.; Eisa, H. Synthesis, Molecular Modeling of Novel 1,2,4-Triazole Derivatives with Potential Antimicrobial and Antiviral Activities. Acta Pharm. Sci. 2010, 52, 353–364. [Google Scholar]
- Orek, C.; Koparir, P.; Koparir, M. N-Cyclohexyl-2-[5-(4-Pyridyl)-4-(p-Tolyl)-4H-1,2,4-Triazol-3-Ylsulfanyl]-Acetamide Dihydrate: Synthesis, Experimental, Theoretical Characterization and Biological Activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 923–934. [Google Scholar] [CrossRef]
- Singh, R.; Pujar, G.V.; Purohit, M.N.; Chandrashekar, V.M. Synthesis, in Vitro Cytotoxicity, and Antibacterial Studies of New Asymmetric Bis-1,2,4-Triazoles. Med. Chem. Res. 2013, 22, 2163–2173. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; El Tamany, E.S.H.; Abd El Fattah, M.E.D.; Boraei, A.T.A.; Abd El-Nabi, H.M. Regioselective Synthesis, Characterization and Antimicrobial Evaluation of S-Glycosides and S,N-Diglycosides of 1,2-Dihydro-5-(1H-Indol-2-Yl)-1,2,4- Triazole-3-Thione. Eur. J. Med. Chem. 2013, 66, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Park, S.W. Access to a New Class of Biologically Active Quinoline Based 1,2,4-Triazoles. Eur. J. Med. Chem. 2014, 71, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Jin, J.; Chaudhary, A.S.; Hsieh, Y.H.; Zhang, H.; Dai, C.; Damera, K.; Chen, W.; Tai, P.C.; Wang, B. Design, Synthesis and Evaluation of Triazole-Pyrimidine Analogues as SecA Inhibitors. Chem. Med. Chem. 2016, 11, 43–56. [Google Scholar] [CrossRef]
- Suresh Kumar, G.V.; Rajendra Prasad, Y.; Mallikarjuna, B.P.; Chandrashekar, S.M. Synthesis and Pharmacological Evaluation of Clubbed Isopropylthiazole Derived Triazolothiadiazoles, Triazolothiadiazines and Mannich Bases as Potential Antimicrobial and Antitubercular Agents. Eur. J. Med. Chem. 2010, 45, 5120–5129. [Google Scholar] [CrossRef]
- Badr, S.M.I.; Barwa, R.M. Synthesis of Some New [1,2,4]Triazolo[3,4-b][1,3,4]Thiadiazines and [1,2,4]Triazolo[3,4-b][1,3,4] Thiadiazoles Starting from 5-Nitro-2-Furoic Acid and Evaluation of Their Antimicrobial Activity. Bioorg. Med. Chem. 2011, 19, 4506–4512. [Google Scholar] [CrossRef]
- Plech, T.; Wujec, M.; Kosikowska, U.; Malm, A.; Kaproń, B. Studies on the Synthesis and Antibacterial Activity of 3,6-Disubstituted 1,2,4-Triazolo[3,4-b]1,3,4-Thiadiazoles. Eur. J. Med. Chem. 2012, 47, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Hunashal, R.D.; Satyanarayana, D. One Pot Synthesis of 3-(Substituted Phenoxymethyl)-6-Phenyl/Substituted Phenoxymethyl-1,2,4-Triazolo[3,4-B][1,3,4] Thiadiazole Derivatives as Antimicrobial Agents. Int. J. Pharm. Bio Sci. 2012, 3, 183–192. [Google Scholar]
- Penta, S.; Gadidasu, K.K.; Basavoju, S.; Rajeswar Rao, V. An Efficient One-Pot Synthesis of Pyrazolyl-[1,2,4]Triazolo[3,4-b][1,3,4] Thiadiazin-6-Yl)-2H-Pyran-2-One Derivatives via Multicomponent Approach and Their Potential Antimicrobial and Nematicidal Activities. Tetrahedron Lett. 2013, 54, 5663–5666. [Google Scholar] [CrossRef]
- Behalo, M.S.; Aly, A.A.; Wasfy, A.F.; Rizk, M.M. Synthesis of Some Novel 1,2,4-Triazole Derivatives as Potential Antimicrobial Agents. Eur. J. Chem. 2013, 4, 92–97. [Google Scholar] [CrossRef]
- Sanjeeva Reddy, C.; Sanjeeva Rao, L.; Sunitha, B.; Nagaraj, A. Synthesis and Antibacterial Activity of N-Substituted-[1,2,4]Triazoles and 1,2,4-Triazole[3,4-b][1,3,4]Thiadiazines. Indian J. Chem. 2015, 54, 1283–1289. [Google Scholar]
- Cui, P.; Li, X.; Zhu, M.; Wang, B.; Liu, J.; Chen, H. Design, Synthesis and Antimicrobial Activities of Thiouracil Derivatives Containing Triazolo-Thiadiazole as SecA Inhibitors. Eur. J. Med. Chem. 2017, 127, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Verma, A.; Mukerjee, A.; Mandal, M.K. Synthesis, Characterization and Antimicrobial Evaluation of Some Novel 1,2,4-Triazolo[3,4-b][1,3,4]Thiadiazine Bearing Substituted Phenylquinolin-2-One Moiety. Arab. J. Chem. 2019, 12, 3046–3053. [Google Scholar] [CrossRef]
- Aouali, M.; Mhalla, D.; Allouche, F.; El Kaim, L.; Tounsi, S.; Trigui, M.; Chabchoub, F. Synthesis, Antimicrobial and Antioxidant Activities of Imidazotriazoles and New Multicomponent Reaction toward 5-Amino-1-Phenyl[1,2,4]Triazole Derivatives. Med. Chem. Res. 2015, 24, 2732–2741. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Hussein, H.A.R.; Abu-zied, K.M. Synthesis of Novel 1,2,4-Triazolopyrimidines and Their Evaluation as Antimicrobial Agents. Med. Chem. Res. 2017, 26, 120–130. [Google Scholar] [CrossRef]
- Mohan Krishna, K.; Inturi, B.; Pujar, G.V.; Purohit, M.N.; Vijaykumar, G.S. Design, Synthesis and 3D-QSAR Studies of New Diphenylamine Containing 1,2,4-Triazoles as Potential Antitubercular Agents. Eur. J. Med. Chem. 2014, 84, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Aneja, D.K.; Hussain, K.; Lohan, P.; Ranjan, P.; Arora, S.; Sharma, C.; Aneja, K.R. Synthesis and Biological Evaluation of Dihydroindeno and Indeno [1,2-e] [1,2,4]Triazolo [3,4-b] [1,3,4]Thiadiazines as Antimicrobial Agents. Eur. J. Med. Chem. 2011, 46, 5065–5073. [Google Scholar] [CrossRef] [PubMed]
- Seelam, N.; Shrivastava, S.P.; Prasanthi, S.; Gupta, S. Synthesis and in Vitro Study of Some Fused 1,2,4-Triazole Derivatives as Antimycobacterial Agents. J. Saudi Chem. Soc. 2016, 20, 411–418. [Google Scholar] [CrossRef]
- Thomas, B.; Duval, R.E.; Fontanay, S.; Varbanov, M.; Boisbrun, M. Synthesis and Antibacterial Evaluation of Bis-Thiazolium, Bis-Imidazolium, and Bis-Triazolium Derivatives. Chem. Med. Chem. 2019, 14, 1232–1237. [Google Scholar] [CrossRef]
- Stingaci, E.; Zveaghinteva, M.; Pogrebnoi, S.; Lupascu, L.; Valica, V.; Uncu, L.; Smetanscaia, A.; Drumea, M.; Petrou, A.; Ciric, A.; et al. New Vinyl-1,2,4-Triazole Derivatives as Antimicrobial Agents: Synthesis, Biological Evaluation and Molecular Docking Studies. Bioorg. Med. Chem. Lett. 2020, 30, 127368. [Google Scholar] [CrossRef]
- Al-Sa’doni, H.H.; Delmani, F.-A.; Al Balushi, A.M.; Al-Ahmad, A.H.; Alsawakhneh, S.O.; Al-Soud, Y.A. Synthesis and Antibacterial Activity of Some New 1,2,4-Triazole Derivatives Bearing Carbohydrazide Moiety. Eur. J. Chem. 2020, 11, 113–119. [Google Scholar] [CrossRef]








| Microorganisms and Minimal Inhibition Concentration (µg/mL) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-Positive Bacteria | Gram-Negative Bacteria | ||||||||||||||
| Compd. | Ref. | Drug-Susceptible | Drug-Resistant | Drug-Susceptible | Drug-Resistant | ||||||||||
| S. aureus | S. epidermidis | B. subtilis | M. luteus | Others | MRSA | VRE | E.coli | P. aeruginosa | Others | MDR E.coli | ESBLE | PAR | |||
| 6a CPX VCN | [21] | 0.72 2.96 - | 0.35 1.48 - | < - | 1.44 5.88 - | B. cereus 0.18 0.36 - | 0.088 1.48 0.68 | - | 0.022 0.024 - | 0.088 0.72 - | P. mirabilis 0.044 0.045 - | - | - | - | - |
| 7a | [23] | 0.19 | 0.046 | 0.011 | 0.76 | B. cereus 0.093 | 0.046 | - | 0.011 | 0.046 | P. mirabilis 0.023 | K. pneumoniae 0.093 | |||
| 7b | 0.091 | 0.091 | 0.091 | 1.49 | 0.18 | 0.046 | 0.011 | 0.18 | 0.023 | 0.091 | - | - | - | ||
| 7c CPX VCN | [24] | 0.09 0.72 - | 0.18 1.48 - | 0.09 0.09 - | 0.70 5.88 - | 0.09 0.36 - | 0.045 – 0.68 | 0.024 0.024 - | 0.176 0.72 - | 0.044 0.045 - | 0.088 0.36 - | ||||
| 12h CPX | [29] | ≤0.125 ≤0.25 | - | - | - | - | ≤0.5 ≤4.0 | - | ≤0.125 ≤0.25 | - | - | ≤0.25 ≤8.0 | - | - | |
| 14c CFX CHL | [31] | 0.5 0.5 16 | - | 0.5 0.5 32 | 0.5 0.5 8 | - | 0.25 1 16 | - | 1 0.5 32 | 0.5 0.5 32 | S. dysenteriae 0.5 1 32 | B. proteus 0.25 0.5 32 | - | - | - |
| 18 AMX | [35] | 2 16 | - | < | - | - | - | - | 8 16 | < | - | - | - | - | |
| 22a (R1= 6-NO2)AMP | [39] | ≤ | - | - | - | - | - | - | 12.5 100 | 25 100 | - | - | - | - | |
| 33d AMP STR | [48] | 0.98 35 - | - | - | - | B. cereus 0.98 15 - | - | - | 3.9 10 - | 31.25 >128 - | Y. pseudotuberculosis 7.8 18 - | - | - | - | |
| 46c,g 47c,g CHL | [59] [60] | 1.56 1.56 3.12 | - | - | - | - | - | - | 3.12 3.12 6.25 | 3.12 3.12 6.25 | - | - | - | - | |
| 59e GEN AMP | [63] | 0.5 2 2 | - | 0.5 2 0.5 | 2 2 2 | - | - | - | 2 0.5 2 | < | - | - | - | - | |
| 78a (R= decyl) CHX | [86] | 0.5 1 | - | - | - | E. faecalis 0.5 2 | 0.5 1 | 0.5 2 | 0.5 0.5 | 2 8 | - | - | 1 1 | 2 16 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzelecka, M.; Świątek, P. 1,2,4-Triazoles as Important Antibacterial Agents. Pharmaceuticals 2021, 14, 224. https://doi.org/10.3390/ph14030224
Strzelecka M, Świątek P. 1,2,4-Triazoles as Important Antibacterial Agents. Pharmaceuticals. 2021; 14(3):224. https://doi.org/10.3390/ph14030224
Chicago/Turabian StyleStrzelecka, Małgorzata, and Piotr Świątek. 2021. "1,2,4-Triazoles as Important Antibacterial Agents" Pharmaceuticals 14, no. 3: 224. https://doi.org/10.3390/ph14030224
APA StyleStrzelecka, M., & Świątek, P. (2021). 1,2,4-Triazoles as Important Antibacterial Agents. Pharmaceuticals, 14(3), 224. https://doi.org/10.3390/ph14030224






