Temperate Zone Plant Natural Products—A Novel Resource for Activity against Tropical Parasitic Diseases
Abstract
1. Introduction
2. Results and Discussion
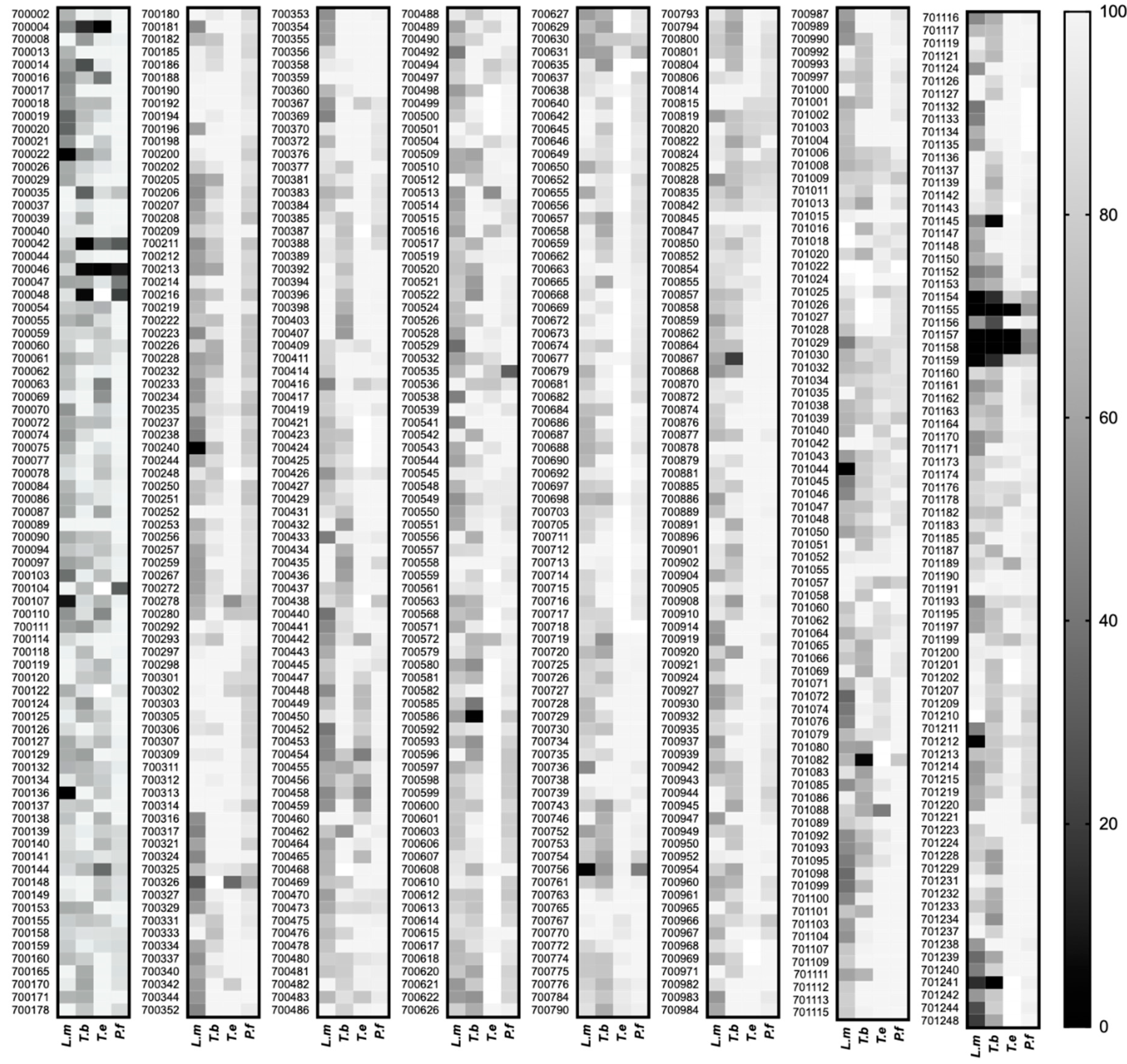
2.1. Screening the PhytoQuest Phytopure Library for Growth Inhibition Identifies Multiple Hits across Different Parasites
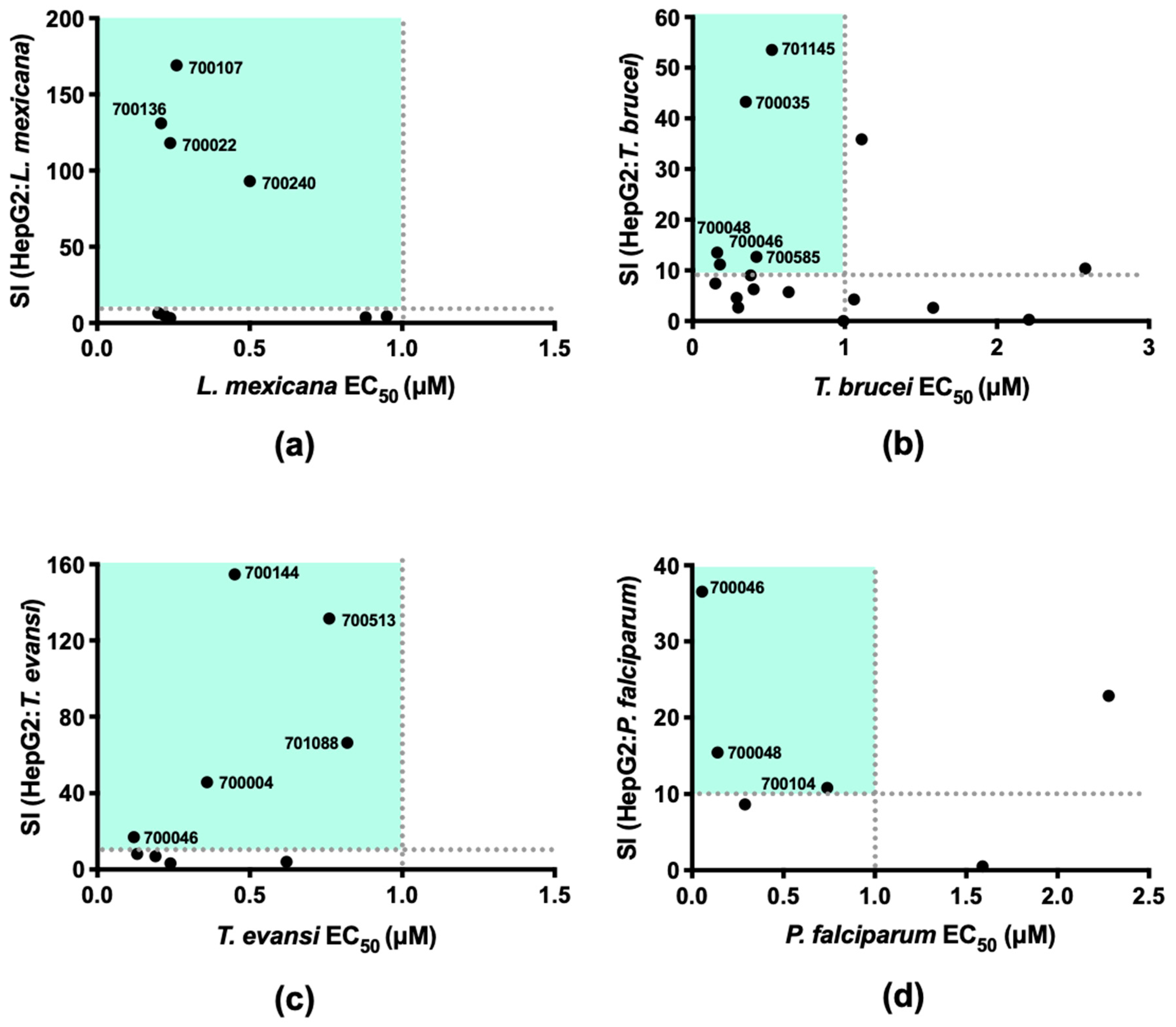
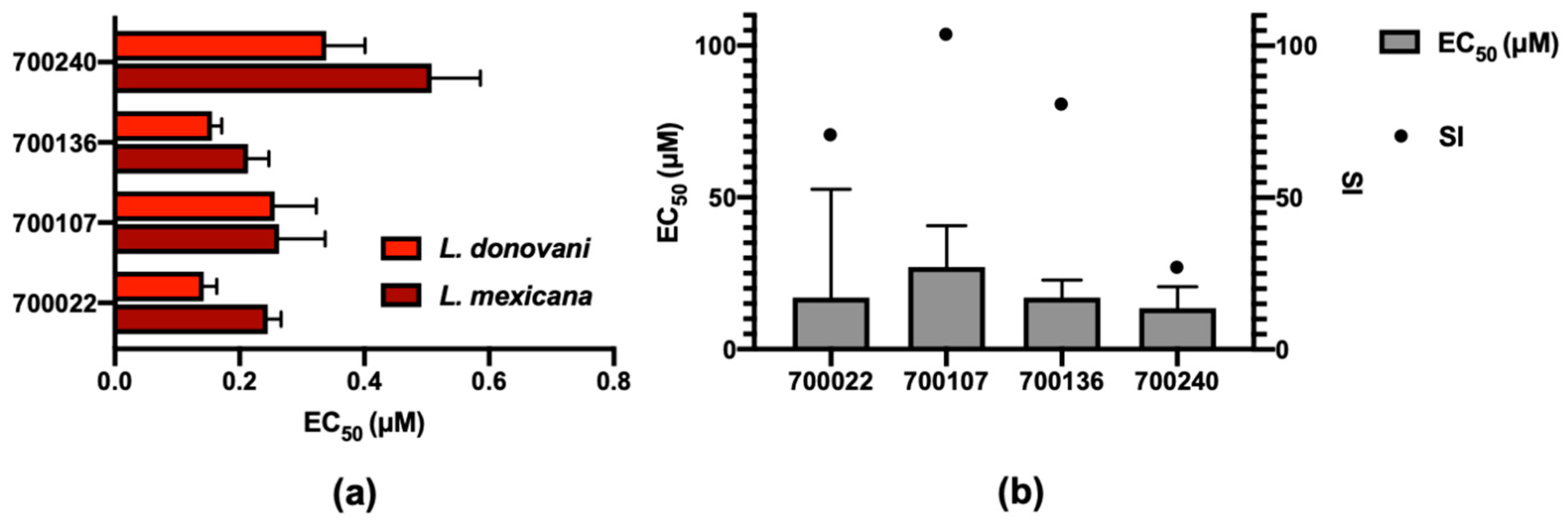
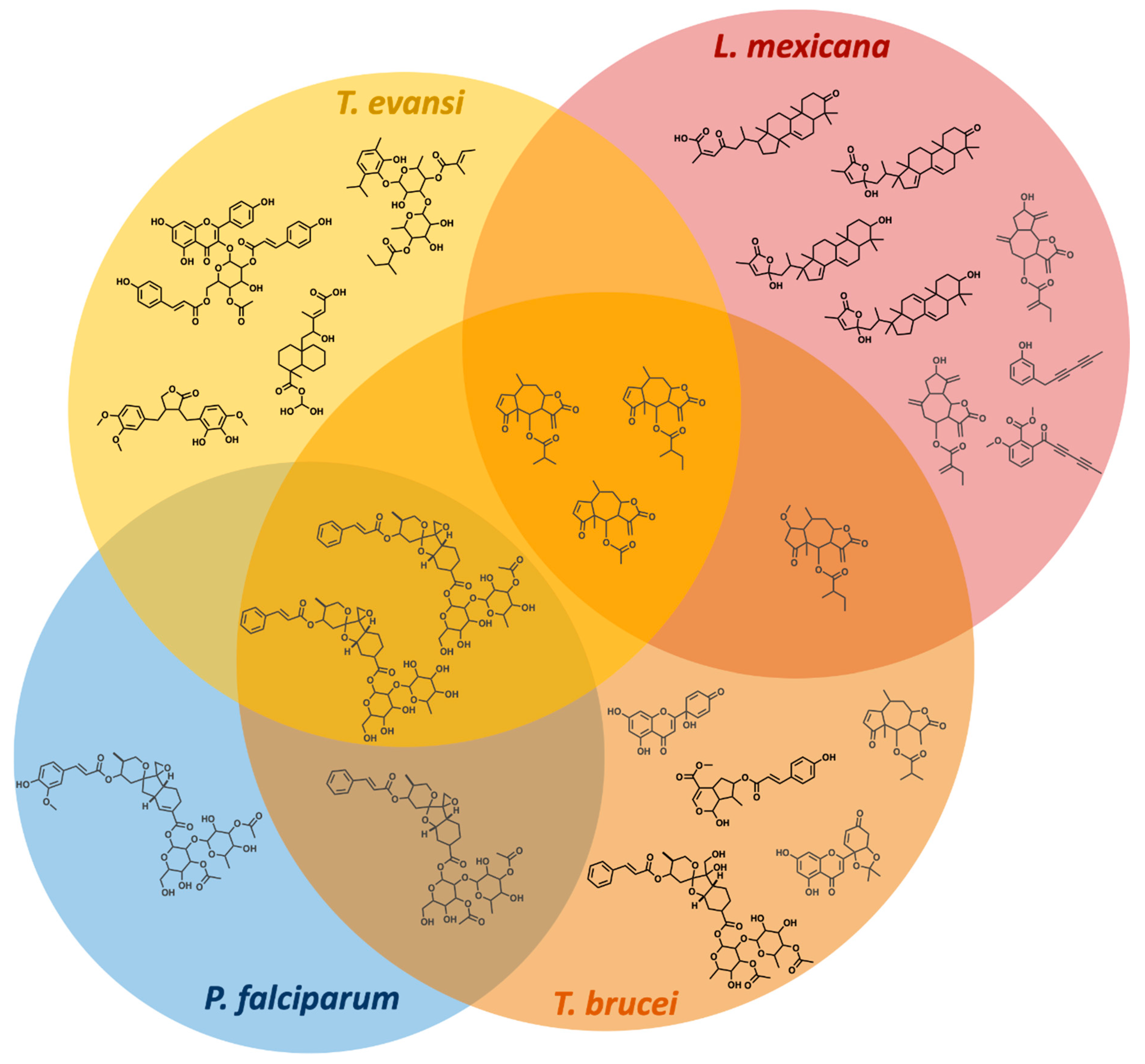
2.2. Demonstrating Selectivity of These Anti-Parasitic Hits
2.3. L. mexicana Parasites with Resistance to 700022 Have Cross-Resistance to Miltefosine but Not to Amphotericin B
3. Materials and Methods
3.1. Culture of Parasites and Human Cell Lines
3.2. Screening
3.3. Log Concentration v Normalised Response Curves to Estimate EC50
3.4. Generation of 700022-Resistant L. mexicana
3.5. Morphological Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Control of Neglected Tropical Diseases. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases (accessed on 2 December 2020).
- Cheuka, P.M.; Mayoka, G.; Mutai, P.; Chibale, K. The role of natural products in drug discovery and development against neglected tropical diseases. Molecules 2017, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- Zinsstag, J.; Crump, L.; Schelling, E.; Hattendorf, J.; Maidane, Y.O.; Ali, K.O.; Muhummed, A.; Umer, A.A.; Aliyi, F.; Nooh, F.; et al. Climate change and One Health. FEMS Microbiol. Lett. 2018, 365, 1–9. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- Booth, M. Climate Change and the Neglected Tropical Diseases. Adv. Parasitol. 2018, 100, 39–126. [Google Scholar] [PubMed]
- Ganesan, A. The impact of natural products upon modern drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 306–317. [Google Scholar] [CrossRef]
- Clark, A.M. Natural products as a resource for new drugs. Pharm. Res. 1996, 13, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.; Williams, R.M. Rabe rest in peace: Confirmation of the rabe-kindler conversion of d-quinotoxine into quinine: Experimental affirmation of the woodward-doering formal total synthesis of quinine. Angew. Chem. Int. Ed. 2008, 47, 1736–1740. [Google Scholar] [CrossRef]
- Woodward, R.B.; Doering, W.E. The Total Synthesis of Quinine1. J. Am. Chem. Soc. 1944, 66, 849. [Google Scholar] [CrossRef]
- PhytoQuest Phytoquest—Unlocking Iminosugars. Available online: http://www.phytoquest.co.uk/services.php (accessed on 2 December 2020).
- World Health Organization Global leishmaniasis surveillance, 2017–2018, and first report on 5 additional indicators. Wkly. Epidemiol. Rec. 2020, 95, 265–280.
- WHO Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 4 December 2020).
- Singh, O.P.; Singh, B.; Chakravarty, J.; Sundar, S. Current challenges in treatment options for visceral leishmaniasis in India: A public health perspective. Infect. Dis. Poverty 2016, 5. [Google Scholar] [CrossRef]
- Elmahallawy, E.; Agil, A. Treatment of Leishmaniasis: A Review and Assessment of Recent Research. Curr. Pharm. Des. 2015, 21, 2259–2275. [Google Scholar] [CrossRef] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sunyoto, T.; Potet, J.; Boelaert, M. Why miltefosine—A life-saving drug for leishmaniasis-is unavailable to people who need it the most. BMJ Glob. Health 2018, 3, 1–10. [Google Scholar] [CrossRef]
- WHO | Trypanosomiasis, African. Available online: http://www.who.int/topics/trypanosomiasis_african/en/ (accessed on 27 January 2014).
- Mesu, V.K.B.K.; Kalonji, W.M.; Bardonneau, C.; Mordt, O.V.; Blesson, S.; Simon, F.; Delhomme, S.; Bernhard, S.; Kuziena, W.; Lubaki, J.-P.F.; et al. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: A pivotal multicentre, randomised, non-inferiority trial. Lancet 2018, 391, 144–154. [Google Scholar] [CrossRef]
- Schmid, C.; Richer, M.; Bilenge, C.M.M.; Josenando, T.; Chappuis, F.; Menthelot, C.R.; Nangouma, A.; Doua, F.; Asumu, P.N.; Simarro, P.P.; et al. Effectiveness of a 10-day melarsoprol schedule for the treatment of late-stage human African Trypanosomiasis: Confirmation from a multinational study (IMPAMEL II). J. Infect. Dis. 2005, 191, 1922–1931. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ralston, K.S.; Kabututu, Z.P.; Melehani, J.H.; Oberholzer, M.; Hill, K.L. The Trypanosoma brucei Flagellum: Moving Parasites in New Directions. Annu. Rev. Microbiol. 2009, 63, 335–362. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.H.; He, C.Y.; De Graffenried, C.L.; Murrells, L.J.; Warren, G. Ordered assembly of the duplicating Golgi in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 2006, 103, 7676–7681. [Google Scholar] [CrossRef]
- Dean, S.; Gould, M.K.; Dewar, C.E.; Schnaufer, A.C. Single point mutations in ATP synthase compensate for mitochondrial genome loss in trypanosomes. Proc. Natl. Acad. Sci. USA 2013, 110, 14741–14746. [Google Scholar] [CrossRef]
- Desquesnes, M.; Dargantes, A.; Lai, D.-H.; Lun, Z.-R.; Holzmuller, P.; Jittapalapong, S. Trypanosoma evansi and Surra: A Review and Perspectives on Transmission, Epidemiology and Control, Impact, and Zoonotic Aspects. BioMed Res. Int. 2013, 2013, 321237. [Google Scholar] [CrossRef] [PubMed]
- Schnaufer, A.; Domingo, G.J.; Stuart, K. Natural and induced dyskinetoplastic trypanosomatids: How to live without mitochondrial DNA. Int. J. Parasitol. 2002, 32, 1071–1084. [Google Scholar] [CrossRef]
- Lai, D.-H.; Hashimi, H.; Lun, Z.-R.; Ayala, F.J.; Lukes, J. Adaptations of Trypanosoma brucei to gradual loss of kinetoplast DNA: Trypanosoma equiperdum and Trypanosoma evansi are petite mutants of T. brucei. Proc. Natl. Acad. Sci. USA 2008, 105, 1999–2004. [Google Scholar] [CrossRef]
- Hooft van Huijsduijnen, R.; Wells, T.N. The antimalarial pipeline. Curr. Opin. Pharmacol. 2018, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Burrows, J.N.; Burlot, E.; Campo, B.; Cherbuin, S.; Jeanneret, S.; Leroy, D.; Spangenberg, T.; Waterson, D.; Wells, T.N.C.; Willis, P. Antimalarial drug discovery—The path towards eradication. Parasitology 2014, 141, 128–139. [Google Scholar] [CrossRef]
- Fairhurst, R.M.; Dondorp, A.M. Artemisinin-Resistant Plasmodium falciparum Malaria. Emerg. Infect. 10 2016, 4, 409–429. [Google Scholar] [CrossRef]
- Cockram, P.E.; Smith, T.K. Active Natural Product Scaffolds against Trypanosomatid Parasites: A Review. J. Nat. Prod. 2018, 81, 2138–2154. [Google Scholar] [CrossRef]
- Duarte, N.; Ramalhete, C.; Lourenço, L. Plant Terpenoids as Lead Compounds Against Malaria and Leishmaniasis. Stud. Nat. Prod. Chem. 2019, 62, 243–306. [Google Scholar] [CrossRef]
- Smith, A.B.; Hale, K.J.; Vaccaro, H.A.; Rivero, R.A. Phyllanthoside-Phyllanthostatin Synthetic Studies. 9. Total Syntheses of (−)-Phyllanthostatin 1, (+)-Phyllanthostatin 2, and (+)-Phyllanthostatin 3. J. Am. Chem. Soc. 1991, 113, 2112–2122. [Google Scholar] [CrossRef]
- Pettit, G.R.; Schaufelberger, D.E.; Nieman, R.A.; Dufresne, C.; Saenz-Renauld, J.A. Antineoplastic agents, 177.1 isolation and structure of phyllanthostatin 6. J. Nat. Prod. 1990, 53, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- González, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef]
- Tan, N.; Kaloga, M.; Radtke, O.A.; Kiderlen, A.F.; Öksüz, S.; Ulubelen, A.; Kolodziej, H. Abietane diterpenoids and triterpenoic acids from Salvia cilicica and their antileishmanial activities. Phytochemistry 2002, 61, 881–884. [Google Scholar] [CrossRef]
- Farimani, M.M.; Khodaei, B.; Moradi, H.; Aliabadi, A.; Ebrahimi, S.N.; De Mieri, M.; Kaiser, M.; Hamburger, M. Phytochemical Study of Salvia leriifolia Roots: Rearranged Abietane Diterpenoids with Antiprotozoal Activity. J. Nat. Prod. 2018, 81, 1384–1390. [Google Scholar] [CrossRef]
- Uddin, G.; Rauf, A.; Arfan, M.; Waliullah; Khan, I.; Ali, M.; Taimur, M.; Ur-Rehman, I. Samiullah Pistagremic acid a new leishmanicidal triterpene isolated from Pistacia integerrima Stewart. J. Enzyme Inhib. Med. Chem. 2012, 27, 646–648. [Google Scholar] [CrossRef][Green Version]
- Handa, M.; Murata, T.; Kobayashi, K.; Selenge, E.; Miyase, T.; Batkhuu, J.; Yoshizaki, F. Lipase inhibitory and LDL anti-oxidative triterpenes from Abies sibirica. Phytochemistry 2013, 86, 168–175. [Google Scholar] [CrossRef]
- Kim, C.S.; Oh, J.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Structural Characterization of Terpenoids from Abies holophylla Using Computational and Statistical Methods and Their Biological Activities. J. Nat. Prod. 2018, 81, 1795–1802. [Google Scholar] [CrossRef]
- Whiteland, H.L.; Chakroborty, A.; Forde-Thomas, J.E.; Crusco, A.; Cookson, A.; Hollinshead, J.; Fenn, C.A.; Bartholomew, B.; Holdsworth, P.A.; Fisher, M.; et al. An Abeis procera-derived tetracyclic triterpene containing a steroid-like nucleus core and a lactone side chain attenuates in vitro survival of both Fasciola hepatica and Schistosoma mansoni. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 465–474. [Google Scholar] [CrossRef]
- He, Y.; Fan, Q.; Cai, T.; Huang, W.; Xie, X.; Wen, Y.; Shi, Z. Molecular mechanisms of the action of Arctigenin in cancer. Biomed. Pharmacother. 2018, 108, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Valsta, L.M.; Kilkkinen, A.; Mazur, W.; Nurmi, T.; Lampi, A.-M.; Ovaskainen, M.-L.; Korhonen, T.; Adlercreutz, H.; Pietinen, P. Phyto-oestrogen database of foods and average intake in Finland. Br. J. Nutr. 2003, 89, S31–S38. [Google Scholar] [CrossRef] [PubMed]
- Bess, E.N.; Bisanz, J.E.; Yarza, F.; Bustion, A.; Rich, B.E.; Li, X.; Kitamura, S.; Waligurski, E.; Ang, Q.Y.; Alba, D.L.; et al. Genetic basis for the cooperative bioactivation of plant lignans by Eggerthella lenta and other human gut bacteria. Nat. Microbiol. 2020, 5, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhou, H.; Zhang, S.; Dai, W.; Zhang, Y.; Hong, L.; Chen, F.; Cao, J. Activation of reactive oxygen species-mediated mitogen-activated protein kinases pathway regulates both extrinsic and intrinsic apoptosis induced by arctigenin in Hep G2. J. Pharm. Pharmacol. 2020, 72, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.B.; Danton, O.; Kaiser, M.; Han, S.; Moreno, A.; Abd Algaffar, S.; Khalid, S.; Oh, W.K.; Hamburger, M.; Mäser, P. Lignans, Amides, and Saponins from Haplophyllum tuberculatum and Their Antiprotozoal Activity. Molecules 2020, 25, 2825. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Tooulakou, G.; Bilia, A.R.; Psaras, G.K.; Karabourniotis, G.; Skaltsa, H. Erinea formation on Quercus ilex leaves: Anatomical, physiological and chemical responses of leaf trichomes against mite attack. Phytochemistry 2011, 72, 230–237. [Google Scholar] [CrossRef]
- Kim, N.; Park, S.J.; Nhiem, N.X.; Song, J.H.; Ko, H.J.; Kim, S.H. Cycloartane-type triterpenoid derivatives and a flavonoid glycoside from the burs of Castanea crenata. Phytochemistry 2019, 158, 135–141. [Google Scholar] [CrossRef]
- Cai, S.; Risinger, A.L.; Nair, S.; Peng, J.; Anderson, T.J.C.; Du, L.; Powell, D.R.; Mooberry, S.L.; Cichewicz, R.H. Identification of Compounds with Efficacy against Malaria Parasites from Common North American Plants. J. Nat. Prod. 2016, 79, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Sharma, R.; Biagini, G.A.; Horrocks, P. A validated bioluminescence-based assay for the rapid determination of the initial rate of kill for discovery antimalarials. J. Antimicrob. Chemother. 2017, 72, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Sharma, R.; Mete, A.; Biagini, G.A.; Wetzel, D.M.; Horrocks, P.D. The relative rate of kill of the MMV Malaria Box compounds provides links to the mode of antimalarial action and highlights scaffolds of medicinal chemistry interest. J. Antimicrob. Chemother. 2020, 75, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Seifert, K.; Matu, S.; Pérez-Victoria, F.J.; Castanys, S.; Gamarro, F.; Croft, S.L. Characterisation of Leishmania donovani promastigotes resistant to hexadecylphosphocholine (miltefosine). Int. J. Antimicrob. Agents 2003, 22, 380–387. [Google Scholar] [CrossRef]
- Beneke, T.; Demay, F.; Hookway, E.; Ashman, N.; Jeffery, H.; Smith, J.; Valli, J.; Becvar, T.; Myskova, J.; Lestinova, T.; et al. Genetic dissection of a Leishmania flagellar proteome demonstrates requirement for directional motility in sand fly infections. PLoS Pathog. 2019, 15, e1007828. [Google Scholar] [CrossRef]
- Zauli, R.C.; Yokoyama-Yasunaka, J.K.U.; Miguel, D.C.; Moura, A.S.; Pereira, L.I.A.; Da Silva, I.A.; Lemes, L.G.N.; Dorta, M.L.; De Oliveira, M.A.P.; Pitaluga, A.N.; et al. A dysflagellar mutant of Leishmania (Viannia) braziliensis isolated from a cutaneous leishmaniasis patient. Parasites Vectors 2012, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.L.; Hameed, H.; Thomason, A.; Maciej-Hulme, M.L.; Saif Abou-Akkada, S.; Horrocks, P.; Price, H.P. Development of NanoLuc-PEST expressing Leishmania mexicana as a new drug discovery tool for axenic- and intramacrophage-based assays. PLoS Negl. Trop. Dis. 2018, 12, 1–20. [Google Scholar] [CrossRef]
- Bates, P.A.; Robertson, C.D.; Coombs, G.H.; Tetley, L. Axenic cultivation and characterization of Leishmania mexicana amastigote-like forms. Parasitology 1992, 105, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Debrabant, A.; Joshi, M.B.; Pimenta, P.F.P.; Dwyer, D.M. Generation of Leishmania donovani axenic amastigotes: Their growth and biological characteristics. Int. J. Parasitol. 2004, 34, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, E.; Leal, S.; Ochatt, C.; Cross, G. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 1999, 99, 89–101. [Google Scholar] [CrossRef]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.H.; Hasenkamp, S.; Horrocks, P. Analysis of the molecular mechanisms governing the stage-specific expression of a prototypical housekeeping gene during intraerythrocytic development of P. falciparum. J. Mol. Biol. 2011, 408, 205–221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trager, W.; Jensen, J. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Hasenkamp, S.; Sidaway, A.; Devine, O.; Roye, R.; Horrocks, P. Evaluation of bioluminescence-based assays of anti-malarial drug activity. Malar. J. 2013, 12, 1–10. [Google Scholar] [CrossRef]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum Erythrocytic Stages in Culture. J. Parasitol. 1979, 65, 418. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, P.; Pickard, M.R.; Parekh, H.H.; Patel, S.P.; Pathak, R.B. Synthesis and biological evaluation of 3-(4-chlorophenyl)-4-substituted pyrazole derivatives. Org. Biomol. Chem. 2013, 11, 4891–4898. [Google Scholar] [CrossRef] [PubMed]
- Aldulaimi, O.; Uche, F.I.; Hameed, H.; Mbye, H.; Ullah, I.; Drijfhout, F.; Claridge, T.D.W.; Horrocks, P.; Li, W.W. A characterization of the antimalarial activity of the bark of Cylicodiscus gabunensis Harms. J. Ethnopharmacol. 2017, 198, 221–225. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]
- Jain, S.K.; Sahu, R.; Walker, L.A.; Tekwani, B.L. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J. Vis. Exp. 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mikus, J.; Steverding, D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue®. Parasitol. Int. 2000, 48, 265–269. [Google Scholar] [CrossRef]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and Inexpensive Fluorescence-Based Technique for High-Throughput Antimalarial Drug Screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef]
- Berry, S.L.; Walker, K.; Hoskins, C.; Telling, N.D.; Price, H.P. Nanoparticle-mediated magnetic hyperthermia is an effective method for killing the human-infective protozoan parasite Leishmania mexicana in vitro. Sci. Rep. 2019, 9, 1059. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Australia, 2020. [Google Scholar]
- Revelle, W. Psych: Procedures for Personality and Psychological Research; R Package Version 2.0.12; Northwestern University: Evanston, IL, USA, 2019; Available online: https://CRAN.R-project.org/package=psych (accessed on 7 March 2021).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hameed, H.; King, E.F.B.; Doleckova, K.; Bartholomew, B.; Hollinshead, J.; Mbye, H.; Ullah, I.; Walker, K.; Van Veelen, M.; Abou-Akkada, S.S.; et al. Temperate Zone Plant Natural Products—A Novel Resource for Activity against Tropical Parasitic Diseases. Pharmaceuticals 2021, 14, 227. https://doi.org/10.3390/ph14030227
Hameed H, King EFB, Doleckova K, Bartholomew B, Hollinshead J, Mbye H, Ullah I, Walker K, Van Veelen M, Abou-Akkada SS, et al. Temperate Zone Plant Natural Products—A Novel Resource for Activity against Tropical Parasitic Diseases. Pharmaceuticals. 2021; 14(3):227. https://doi.org/10.3390/ph14030227
Chicago/Turabian StyleHameed, Hamza, Elizabeth F. B. King, Katerina Doleckova, Barbara Bartholomew, Jackie Hollinshead, Haddijatou Mbye, Imran Ullah, Karen Walker, Maria Van Veelen, Somaia Saif Abou-Akkada, and et al. 2021. "Temperate Zone Plant Natural Products—A Novel Resource for Activity against Tropical Parasitic Diseases" Pharmaceuticals 14, no. 3: 227. https://doi.org/10.3390/ph14030227
APA StyleHameed, H., King, E. F. B., Doleckova, K., Bartholomew, B., Hollinshead, J., Mbye, H., Ullah, I., Walker, K., Van Veelen, M., Abou-Akkada, S. S., Nash, R. J., Horrocks, P. D., & Price, H. P. (2021). Temperate Zone Plant Natural Products—A Novel Resource for Activity against Tropical Parasitic Diseases. Pharmaceuticals, 14(3), 227. https://doi.org/10.3390/ph14030227






