Dual Anti-Malarial and GSK3β-Mediated Cytokine-Modulating Activities of Quercetin Are Requisite of Its Potential as a Plant-Derived Therapeutic in Malaria
Abstract
1. Introduction
2. Results
2.1. Quercetin Exhibits Moderate Anti-Plasmodial Activity
2.2. Quercetin Displayed No Adverse Effect in Non-Infected Mice
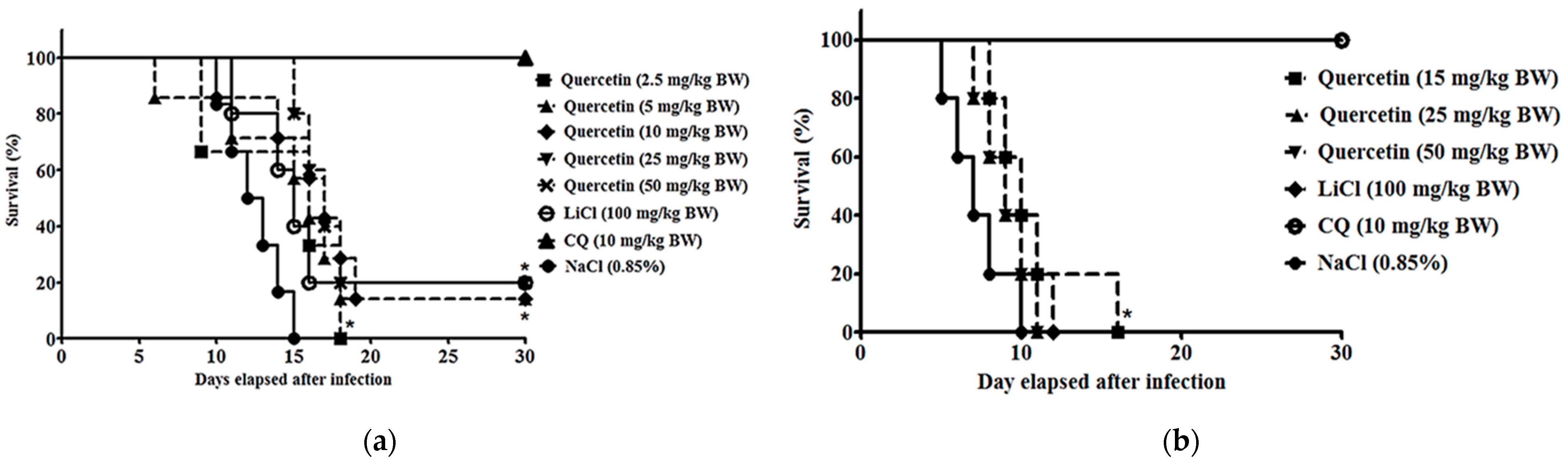
2.3. Quercetin Suppressed Parasitaemia Development and Prolonged Median Survival Time in P. berghei NK65- and ANKA-Infected Animals
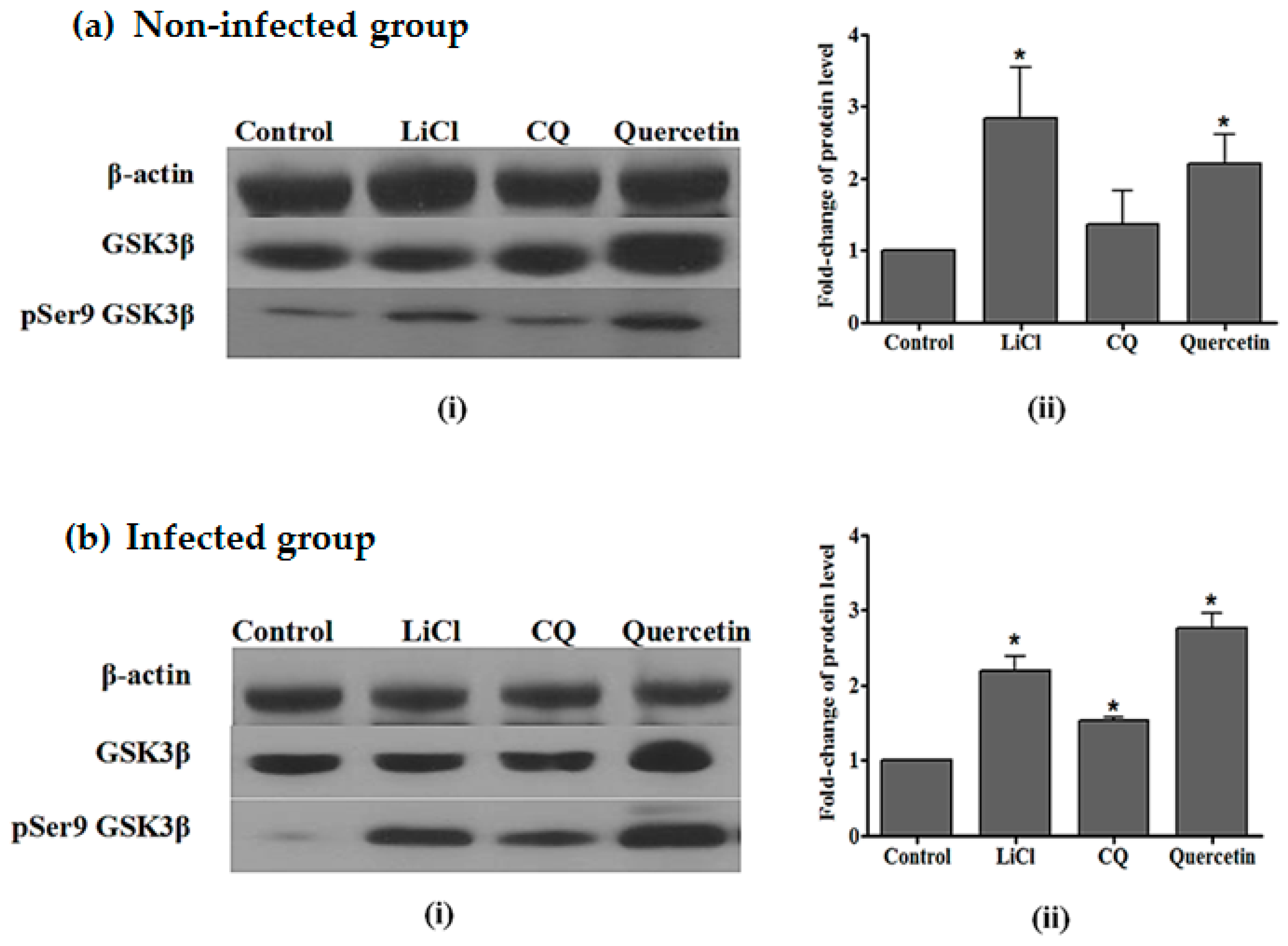
2.4. Quercetin Resulted in Increased GSK3β (Ser9) Phosphorylation in the Liver of P. berghei NK65-Infected Mice
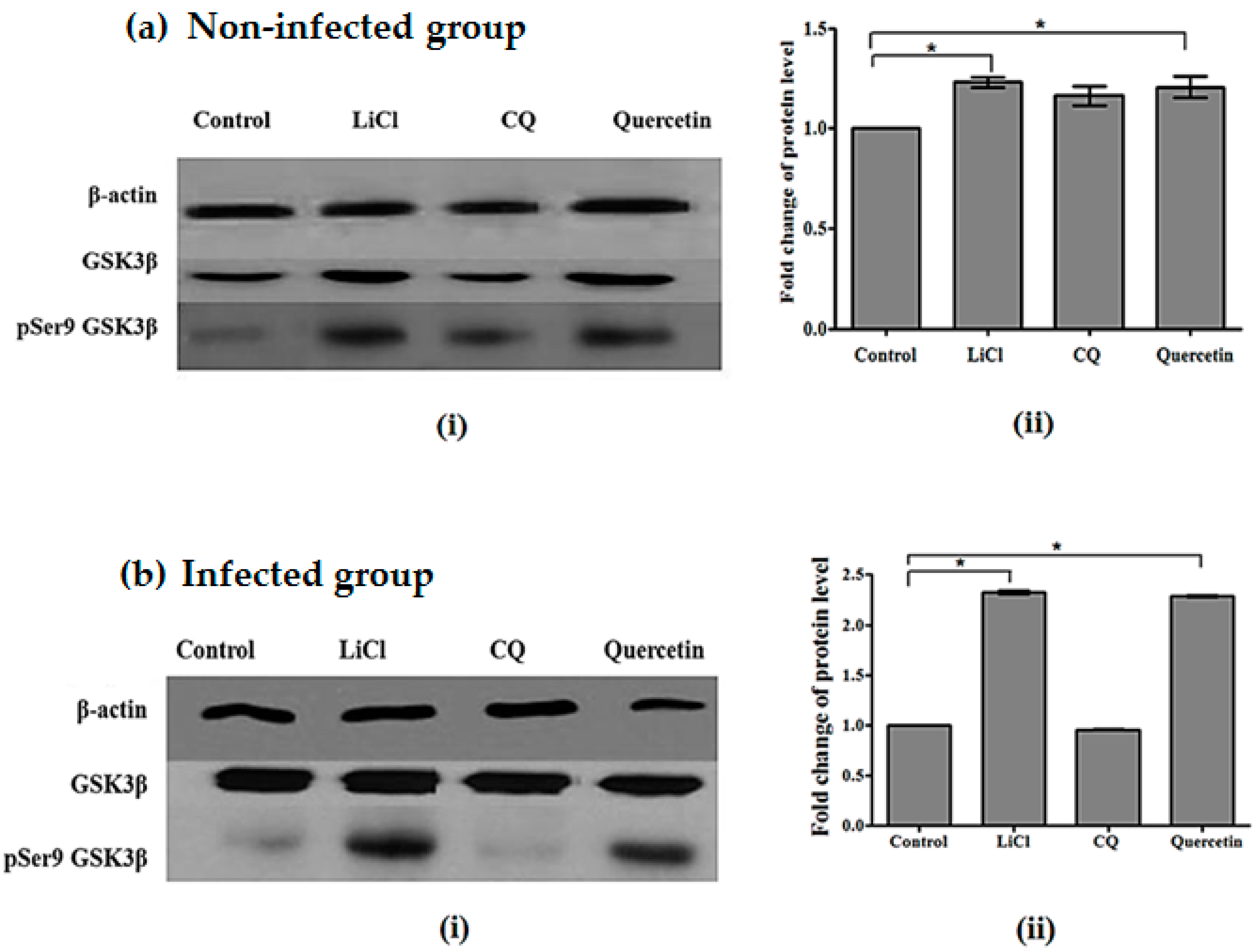
2.5. Quercetin Resulted in Increased GSK3β (Ser9) Phosphorylation in Brains of P. berghei ANKA-Infected Mice
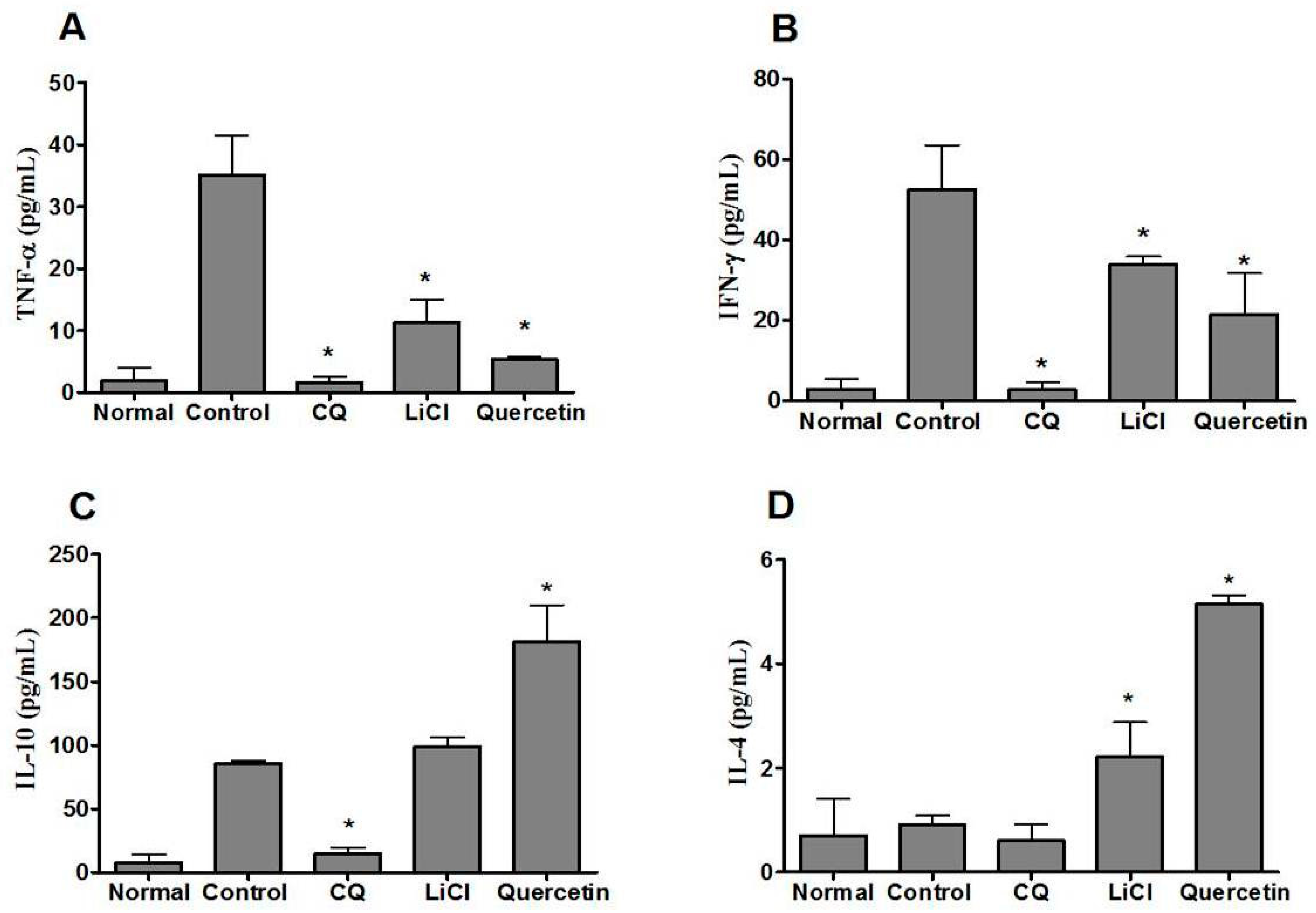
2.6. Quercetin Modulated Pro- and Anti-Inflammatory Cytokine Levels in P. berghei NK65-Infected Animals
3. Discussion
4. Materials and Methods
4.1. Parasites
4.2. In Vitro Anti-Plasmodial Assessment
4.3. In Vitro Cytotoxicity Assessment
4.4. Experimental Animals
4.5. Survivability Test
4.6. Four-Day Suppressive Test
4.7. Western-Blotting Analysis
4.8. Serum Cytokine Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef]
- Zumla, A.; Rao, M.; Wallis, R.S.; Kaufmann, S.H.E.; Rustomjee, R.; Mwaba, P.; Vilaplana, C.; Yeboah-Manu, D.; Chakaya, J.; Ippolito, G.; et al. Host-directed therapies for infectious diseases: Current status, recent progress, and future prospects. Lancet Infect. Dis. 2016, 16, e47–e63. [Google Scholar] [CrossRef]
- Deroost, K.; Pham, T.T.; Opdenakker, G.; Van den Steen, P.E. The immunological balance between host and parasite in malaria. FEMS Microbiol. Rev. 2016, 40, 208–257. [Google Scholar] [CrossRef] [PubMed]
- Grau, G.E.; Fajardo, L.F.; Piguet, P.F.; Allet, B.; Lambert, P.H.; Vassalli, P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 1987, 237, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Haldar, K.; Mohandas, N. Malaria, erythrocytic infection, and anemia. Hematology 2009, 2009, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Adderley, J.D.; von Freyend, S.J.; Jackson, S.A.; Bird, M.J.; Burns, A.L.; Anar, B.; Metcalf, T.; Semblat, J.P.; Billker, O.; Wilson, D.W.; et al. Analysis of erythrocyte signalling pathways during Plasmodium falciparum infection identifies targets for host-directed antimalarial intervention. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2015, 6, 655. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Cappello, A.R.; Frattaruolo, L.; Badolato, M.; Armentano, B.; Aiello, F. Quercetin and derivatives: Useful tools in inflammation and pain management. Future Med. Chem. 2017, 9, 79–93. [Google Scholar] [CrossRef] [PubMed]
- De Feo, M.; Paladini, A.; Ferri, C.; Carducci, A.; Del Pinto, R.; Varrassi, G.; Grassi, D. Anti-Inflammatory and Anti-Nociceptive Effects of Cocoa: A Review on Future Perspectives in Treatment of Pain. Pain Ther. 2020, 9, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol—A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. Foods 2017, 30, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of flavonoids against coronavirus infection. Chem. Biol. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar]
- Yardim, A.; Kandemir, F.M.; Ozdemir, S.; Kucukler, S.; Comakli, S.; Gur, C.; Celik, H. Quercetin provides protection against the peripheral nerve damage caused by vincristine in rats by suppressing caspase 3, NF-κB, ATF-6 pathways and activating Nrf2, Akt pathways. Neurotoxicology 2020, 81, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-induced RAW264.7 cells: In vitro assessment and a theoretical model. BioMed Res. Int. 2019, 2019, 7039802. [Google Scholar] [CrossRef]
- Heimfarth, L.; Serafini, M.R.; Martins-Filho, P.R.S.; Quintans, J.S.S.; Júnior, L.J.Q. Drug repurposing and cytokine management in response to COVID-19: A review. Int. Immunopharmacol. 2020, 88, 106947. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the Cytokine Storm’in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Rudd, C.E. GSK-3 inhibition as a therapeutic approach against SARs CoV2: Dual benefit of inhibiting viral replication while potentiating the immune response. Front. Immunol. 2020, 11, 1638–1648. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Kinker, B.; Comstock, A.T.; Sajjan, U.S. Quercetin: A promising treatment for the common cold. J. Anc. Dis. Prev. Remedies 2014, 2, 1–3. [Google Scholar] [CrossRef]
- Johnson, J.L.; Rupasinghe, S.G.; Stefani, F.; Schuler, M.A.; Gonzalez de Mejia, E. Citrus flavonoids luteolin, apigenin, and quercetin inhibit glycogen synthase kinase-3β enzymatic activity by lowering the interaction energy within the binding cavity. J. Med. Food 2011, 14, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, D.; Panneerselvam, P. Screening of potential glycogen synthase kinase-3β inhibitors from herbal Lead by in silico docking technique. Int. J. Chem. Tech. Res. 2015, 8, 834–842. [Google Scholar]
- Jung, Y.; Shin, S.Y.; Lee, Y.H.; Lim, Y. Flavones with inhibitory effects on glycogen synthase kinase 3β. Appl. Biol. Chem. 2017, 60, 227–232. [Google Scholar] [CrossRef]
- Friday, A.J.; Ikpeazu, V.O.; Otuokere, I.; Igwe, K.K. Targeting Glycogen Synthase Kinase-3 (GSK3β) With Naturally Occurring Phytochemicals (Quercetin and its Modelled Analogue): A Pharmacophore Modelling and Molecular Docking Approach. Commun. Phys. Sci. 2020, 5, 497–508. [Google Scholar]
- Kim, Y.J.; Park, W. Anti-inflammatory effect of quercetin on RAW 264.7 mouse macrophages induced with polyinosinic-polycytidylic acid. Molecules 2016, 21, 450. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef] [PubMed]
- Kayano, A.C.A.; Lopes, S.C.; Bueno, F.G.; Cabral, E.C.; Souza-Neiras, W.C.; Yamauchi, L.M.; Foglio, M.A.; Eberlin, M.N.; Mello, J.C.P.; Costa, F.T. In vitro and in vivo assessment of the anti-malarial activity of Caesalpinia pluviosa. Malar. J. 2011, 10, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, D.; Fuehrer, H.P.; Starzengruber, P.; Swoboda, P.; Khan, W.A.; Reismann, J.A.; Mueller, M.S.; Chiba, P.; Noedl, H. Anti-plasmodial activity of flavonol quercetin and its analogues in Plasmodium falciparum: Evidence from clinical isolates in Bangladesh and standardized parasite clones. Parasitol. Res. 2012, 110, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Penna-Coutinho, J.; Aguiar, A.C. Commercial drugs containing flavonoids are active in mice with malaria and in vitro against chloroquine-resistant Plasmodium falciparum. Mem. Inst. Oswaldo Cruz 2018, 113, e180279. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Singh, P.; Rath, S.K. Protective effect of quercetin on chloroquine-induced oxidative stress and hepatotoxicity in mice. Malar. Res. Treat. 2013, 2013, 141734. [Google Scholar]
- Embi, N.; Rylatt, D.B.; Cohen, P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur. J. Biochem. 1980, 107, 519–527. [Google Scholar] [CrossRef]
- Wang, H.; Brown, J.; Martin, M. Glycogen synthase kinase 3: A point of convergence for the host inflammatory response. Cytokine 2011, 53, 130–140. [Google Scholar] [CrossRef]
- Hoffmeister, L.; Diekmann, M.; Brand, K.; Huber, R. GSK3: A Kinase Balancing Promotion and Resolution of Inflammation. Cells 2020, 9, 820. [Google Scholar] [CrossRef]
- Saraswati, A.P.; Ali-Hussaini, S.M.; Krishna, N.H.; Babu, B.N.; Kamal, A. Glycogen synthase kinase-3 and its inhibitors: Potential target for various therapeutic conditions. Eur. J. Med. Chem. 2018, 144, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Eldar-Finkelman, H. Glycogen synthase kinase 3: An emerging therapeutic target. Trends Mol. Med. 2002, 8, 126–132. [Google Scholar] [CrossRef]
- Takahashi-Yanaga, F. Activator or inhibitor? GSK-3 as a new drug target. Biochem. Pharmacol. 2013, 86, 191–199. [Google Scholar] [CrossRef]
- Haque, A.; Best, S.E.; Amante, F.H.; Ammerdorffer, A.; de Labastida, F.; Pereira, T.; Ramm, G.A.; Engwerda, C.R. High parasite burdens cause liver damage in mice following Plasmodium berghei ANKA infection independently of CD8(+) T cell-mediated immune pathology. Infect. Immun. 2011, 79, 1882–1888. [Google Scholar] [CrossRef]
- Mizobuchi, H.; Fujii, W.; Isokawa, S.; Ishizuka, K.; Wang, Y.; Watanabe, S.; Sanjoba, C.; Matsumoto, Y.; Goto, Y. Exacerbation of hepatic injury during rodent malaria by myeloid-related protein 14. PLoS ONE 2018, 13, e0199111. [Google Scholar] [CrossRef]
- Dolabela, M.F.; Oliveira, S.G.; Nascimento, J.M.; Peres, J.M.; Wagner, H.; Póvoa, M.M.; de Oliveira, A.B. In vitro anti-plasmodial activity of extract and constituents from Esenbeckia febrifuga, a plant traditionally used to treat malaria in the Brazilian Amazon. Phytomedicine 2008, 15, 367–372. [Google Scholar] [CrossRef]
- Hodges, R.E.; Minich, D.M. Modulation of metabolic detoxification pathways using foods and food-derived components: A scientific review with clinical application. J. Nutr. Metab. 2015, 2015, 760689. [Google Scholar] [CrossRef] [PubMed]
- Baptista, F.G.; Pamplona, A.; Pena, A.C.; Mota, M.M.; Pied, S.; Vigário, A.M. Accumulation of Plasmodium berghei-infected red blood cells in the brain is crucial for the development of cerebral malaria in mice. Infect. Immun. 2010, 78, 4033–4039. [Google Scholar] [CrossRef] [PubMed]
- Ngo-Thanh, H.; Sasaki, T.; Suzue, K.; Yokoo, H.; Isoda, K.; Kamitani, W.; Shimokawa, C.; Hisaeda, H.; Imai, T. Blood–cerebrospinal fluid barrier: Another site disrupted during experimental cerebral malaria caused by Plasmodium berghei ANKA. Int. J. Parasitol. 2020, 50, 1167–1175. [Google Scholar] [CrossRef]
- Plantone, D.; Koudriavtseva, T. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: A mini-review. Clin. Drug Investig. 2018, 38, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Thomé, R.; Lopes, S.C.P.; Costa, F.T.M.; Verinaud, L. Chloroquine: Modes of action of an undervalued drug. Immunol. Lett. 2013, 153, 50–57. [Google Scholar] [CrossRef]
- Helgren, T.R.; Sciotti, R.J.; Lee, P.; Duffy, S.; Avery, V.M.; Igbinoba, O.; Akoto, M.; Hagen, T.J. The synthesis, antimalarial activity and CoMFA analysis of novel aminoalkylated quercetin analogs. Bioorg. Med. Chem. Lett. 2015, 25, 327–332. [Google Scholar] [CrossRef]
- Hassan, W.R.M.; Basir, R.; Ali, A.H.; Embi, N.; Sidek, H.M. Anti-malarial and cytokine-modulating effects of andrographolide in a murine model of malarial infection. Trop. Biomed. 2019, 36, 776–791. [Google Scholar]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Valamehr, B.; Bjordahl, R.; Zhang, B.; Rezner, B.; Rogers, P.; Gaidarova, S.; Moreno, S.; Tuininga, K.; Dougherty, P.; et al. GSK3 inhibition drives maturation of NK cells and enhances their antitumor activity. Cancer Res. 2017, 77, 5664–5675. [Google Scholar] [CrossRef]
- Wong, S.K.; Jann, M.L.S.; Sudi, S.; Hasan, M.; Chin, L.P.; Embi, N.; Sidek, H.M. Anti-malarial and anti-inflammatory effects of Gynura procumbens are mediated by kaempferol via inhibition of glycogen synthase kinase-3β (GSK3β). Sains Malays. 2015, 44, 1489–1500. [Google Scholar]
- Somsak, V.; Nakinchat, S. Anti-malarial and anti-hypoglycemic effects of Moringa oleifera leaf Extraction Plasmodium berghei infection in mice. Asian J. Pharm. 2017, 1, 12–17. [Google Scholar]
- Ounjaijean, S.; Benjasak, N.; Sae-Lao, S.; Somsak, V. Kaempferol Addition Increases the Anti-malarial Activity of Artesunate in Experimental Mice. J. Trop. Med. 2020, 2020, 6165928. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxid. Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef]
- Yang, Z.H.; Sun, X.; Mei, C.; Sun, X.B.; Liu, X.D.; Chang, Q. An in vitro transport model for rapid screening and predicting the permeability of candidate compounds at blood-brain barrier. J. Asian Nat. Prod. Res. 2011, 13, 1087–1097. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer’s disease. Alzheimer Res. Ther. 2014, 6, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, W.; Zhou, A.L.; Zhao, M.; Jiang, D.R. Inhibitory effect of oxymatrine on hepatocyte apoptosis via TLR4/PI3K/Akt/GSK-3β signaling pathway. World J. Gastroenterol. 2017, 23, 3839–3849. [Google Scholar] [CrossRef]
- Zhang, F.; Phiel, C.J.; Spece, L.; Gurvich, N.; Klein, P.S. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium: Evidence for autoregulation of GSK-3. J. Biol. Chem. 2003, 278, 33067–33077. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.A.; Noor, E.; Hasidah, M.S. Suppression of Plasmodium berghei parasitemia by LiCl in an animal infection model. Trop Biomed. 2010, 27, 624–631. [Google Scholar]
- Dai, M.; Freeman, B.; Shikani, H.J.; Bruno, F.P.; Collado, J.E.; Macias, R.; Reznik, S.E.; Davies, P.; Spray, D.C.; Tanowitz, H.B.; et al. Altered Regulation of Akt Signaling with Murine Cerebral Malaria, Effects on Long-Term Neuro-Cognitive Function, Restoration with Lithium Treatment. PLoS ONE 2012, 7, e44117. [Google Scholar] [CrossRef] [PubMed]
- Coban, C. The host targeting effect of chloroquine in malaria. Curr. Opin. Immunol. 2020, 66, 98–107. [Google Scholar] [CrossRef]
- De Oca, M.M.; Kumar, R.; de Labastida Rivera, F.; Amante, F.H.; Sheel, M.; Faleiro, R.J.; Bunn, P.T.; Best, S.E.; Beattie, L.; Ng, S.S.; et al. Type I interferons regulate immune responses in humans with blood-stage Plasmodium falciparum infection. Cell Rep. 2016, 17, 399–412. [Google Scholar]
- Varo, R.; Crowley, V.M.; Sitoe, A.; Madrid, L.; Serghides, L.; Kain, K.C.; Bassat, Q. Adjunctive therapy for severe malaria: A review and critical appraisal. Malar. J. 2018, 17, 1–18. [Google Scholar] [CrossRef]
- Worthen, R.J.; Zighelboim, S.S.G.; Jaramillo, C.S.T.; Beurel, E. Anti-inflammatory IL-10 administration rescues depression-associated learning and memory deficits in mice. J. Neuroinflamm. 2020, 17, 1–16. [Google Scholar] [CrossRef]
- Makler, M.T.; Ries, J.M.; Williams, J.A.; Bancroft, J.E.; Piper, R.C.; Gibbins, B.L.; Hinrichs, D.J. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 1993, 48, 739–741. [Google Scholar] [CrossRef]
- Ali, A.H.; Agustar, H.K.; Hassan, N.I.; Latip, J.; Embi, N.; Sidek, H.M. Data on antiplasmodial and stage-specific inhibitory effects of Aromatic (Ar)-Turmerone against Plasmodium falciparum 3D7. Data Brief. 2020, 33, 106592. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Dahari, D.E.; Salleh, R.M.; Mahmud, F.; Chin, L.P.; Embi, N.; Sidek, H.M. Anti-malarial activities of two soil actinomycete isolates from sabah via inhibition of glycogen synthase kinase 3β. Trop. Life Sci. Res. 2016, 27, 53. [Google Scholar] [CrossRef]
- Peters, W.; Portus, J.H.; Robinson, B.L. The chemotherapy of rodent malaria, XXII: The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann. Trop. Med. Parasitol. 1975, 69, 155–171. [Google Scholar] [CrossRef]
- Ali, A.H.; Sudi, S.; Basir, R.; Embi, N.; Sidek, H.M. The Antimalarial Effect of Curcumin Is Mediated by the Inhibition of Glycogen Synthase Kinase-3 β. J. Med. Food 2017, 20, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Schägger, H.; Von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
| P. berghei strain | P. berghei NK65 | P. berghei ANKA | ||||
|---|---|---|---|---|---|---|
| Compound/Drugs | Dosage (mg/kg BW) | Parasitaemia Suppression on Day 4 (%) | Median Survival Time (Days) | Dosage (mg/kg BW) | Parasitaemia Suppression on Day 4 (%) | Median Survival Time (Days) |
| Quercetin | 2.5 | 46.9 ± 3.5 a | 9 b | 15 | 36.1 ± 5.7 a | 10 a |
| 5 | 51.1 ± 5.7 a | 13 | 25 | 24.9 ± 3.8 a, b | 9 b | |
| 10 | 53.5 ± 4.3 a | 14 | 50 | 24.3 ± 1.8 a, b | 9 b | |
| 25 | 60.7 ± 2.4 a | 17 a | ||||
| 50 | 49.3 ± 3.6 a | 17 a | ||||
| CQ (Anti-malarial reference drug) | 10 | 96.0 ± 1.1 a | >30 a | 10 | 94.3 ± 1.8 a | >30 a |
| LiCl (GSK3 inhibitor reference) | 100 | 69.4 ± 3.2 | 15 a | 100 | 85.2 ± 0.9 a | 10 a |
| 0.85% (w/v) NaCl (Negative control) | 0.2 mL | - | 13 | 0.2 mL | - | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.H.; Sudi, S.; Shi-Jing, N.; Hassan, W.R.M.; Basir, R.; Agustar, H.K.; Embi, N.; Sidek, H.M.; Latip, J. Dual Anti-Malarial and GSK3β-Mediated Cytokine-Modulating Activities of Quercetin Are Requisite of Its Potential as a Plant-Derived Therapeutic in Malaria. Pharmaceuticals 2021, 14, 248. https://doi.org/10.3390/ph14030248
Ali AH, Sudi S, Shi-Jing N, Hassan WRM, Basir R, Agustar HK, Embi N, Sidek HM, Latip J. Dual Anti-Malarial and GSK3β-Mediated Cytokine-Modulating Activities of Quercetin Are Requisite of Its Potential as a Plant-Derived Therapeutic in Malaria. Pharmaceuticals. 2021; 14(3):248. https://doi.org/10.3390/ph14030248
Chicago/Turabian StyleAli, Amatul Hamizah, Suhaini Sudi, Ng Shi-Jing, Wan Rozianoor Mohd Hassan, Rusliza Basir, Hani Kartini Agustar, Noor Embi, Hasidah Mohd Sidek, and Jalifah Latip. 2021. "Dual Anti-Malarial and GSK3β-Mediated Cytokine-Modulating Activities of Quercetin Are Requisite of Its Potential as a Plant-Derived Therapeutic in Malaria" Pharmaceuticals 14, no. 3: 248. https://doi.org/10.3390/ph14030248
APA StyleAli, A. H., Sudi, S., Shi-Jing, N., Hassan, W. R. M., Basir, R., Agustar, H. K., Embi, N., Sidek, H. M., & Latip, J. (2021). Dual Anti-Malarial and GSK3β-Mediated Cytokine-Modulating Activities of Quercetin Are Requisite of Its Potential as a Plant-Derived Therapeutic in Malaria. Pharmaceuticals, 14(3), 248. https://doi.org/10.3390/ph14030248







