Abstract
Identifying disease-modifying therapies for neurological diseases remains one of the greatest gaps in modern medicine. Herein, we present the rationale for intranasal (IN) delivery of deferoxamine (DFO), a high-affinity iron chelator, as a treatment for neurodegenerative and neurovascular disease with a focus on its novel mechanisms. Brain iron dyshomeostasis with iron accumulation is a known feature of brain aging and is implicated in the pathogenesis of a number of neurological diseases. A substantial body of preclinical evidence and early clinical data has demonstrated that IN DFO and other iron chelators have strong disease-modifying impacts in Alzheimer’s disease (AD), Parkinson’s disease (PD), ischemic stroke, and intracranial hemorrhage (ICH). Acting by the disease-nonspecific pathway of iron chelation, DFO targets each of these complex diseases via multifactorial mechanisms. Accumulating lines of evidence suggest further mechanisms by which IN DFO may also be beneficial in cognitive aging, multiple sclerosis, traumatic brain injury, other neurodegenerative diseases, and vascular dementia. Considering its known safety profile, targeted delivery method, robust preclinical efficacy, multiple mechanisms, and potential applicability across many neurological diseases, the case for further development of IN DFO is considerable.
1. Introduction
As the leading cause of global disability and 2nd leading cause of death, the burden of neurological disease is tremendous [1]. Treatment options remain disappointingly limited for the majority of these conditions, especially aging-related diseases such as Alzheimer’s disease (AD) [2,3], other dementias [4], and stroke [5]. Many challenges have contributed to this therapeutic gap including the need to deliver therapeutics in meaningful concentrations across the blood-brain barrier (BBB) [6], elusive disease etiologies [7,8,9], and complex disease mechanisms [3,8,10,11,12], among others. Although the past decade has yielded incredible insight into disease pathogenesis, these discoveries have largely failed to translate into clinical benefit [13,14,15,16]. It is of paramount importance to fill this therapeutic gap in our aging population.
In this review, we present the evidence for intranasal (IN) delivery of deferoxamine (DFO), a metal chelator that has shown promise in both preclinical and early clinical studies in AD, Parkinson’s disease (PD), ischemic stroke, and intracranial hemorrhage. Iron chelation counters several disease-specific processes via disease-nonspecific mechanisms, and brain iron accumulation represents an untapped and highly accessible therapeutic target across many neurological diseases.
2. The Development of Deferoxamine and Intranasal Delivery
DFO, developed over half a century ago, is the most potent and widely used of several FDA-approved iron chelators [17,18]. These therapeutics were originally developed to address systemic iron overload states such as transfusion-dependent thalassemia major but have since seen significant preclinical and clinical development for use across cancer [19,20,21], imaging [22,23], and neurological disease [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. DFO has a short plasma half-life and is typically challenging to deliver systemically, with most delivery methods involving long courses of intravenous (IV) infusion or intramuscular (IM) injection [43]. Such routes struggle to achieve meaningful therapeutic concentrations of drug in the brain due to the blood-brain barrier (BBB) [24], a common problem in neuropharmacology [6]. Furthermore, at the higher doses required to achieve such concentrations, DFO can cause systemic toxicity [44].
In 1989, William H. Frey II discovered the non-invasive intranasal method of bypassing the BBB to deliver and target neurological therapeutic agents to the brain and filed the first patent [45,46]. The IN route bypasses the BBB by rapidly delivering therapeutics extracellularly along the olfactory and trigeminal nerve pathways to the CNS [24,47,48,49,50,51,52]. Over the past three decades, IN delivery has been shown to provide a safe and efficacious means to overcome these challenges and has remarkably transformed the problem of delivering therapeutics to the central nervous system (CNS) [47,48]. IN delivery has been successfully and robustly applied to a wide array of pharmacologic agents, from small molecules to peptides [50], oligonucleotides [53], stem cells, and immune cells [54,55,56,57]. IN delivery minimizes systemic drug exposure while resulting in comparable or higher CNS concentrations compared to alternate methods of delivery [24,47,48,58]. IN delivery is increasingly considered the future of CNS pharmacotherapy, and its emergence is perhaps best attested by the use and efficacy of IN insulin in several clinical trials for AD [59,60,61,62].
The IN delivery of DFO achieves micromolar concentrations in the brain within minutes and offers up to 200-fold greater targeting compared to IV delivery in rodents [24,58]. In a recent review, Farr and Xiong discuss the formulation and delivery of DFO in detail, tabulating studies performed to date across AD, PD, and intracerebral hemorrhage [28]. In this review, we cast the spotlight on IN DFO and its therapeutic mechanisms for human neurological disease. In addition to evidence of IN DFO as a treatment for AD [63], PD [64], and stroke [65], decades of work across a number of research groups investigating systemically administered DFO and other iron chelators support this treatment approach.
3. Iron, Chelation, and the Brain
Iron is essential to a number of physiologic processes within the brain including oxygen transport, neurotransmission, and myelin homeostasis. Elaborate mechanisms transport, distribute, and store iron for use by intracellular iron-binding proteins [66]. However, a fraction of cellular iron that is not protein-bound, labile iron, is highly pro-oxidant. This intracellular labile iron pool is capable of catalyzing the production of hydroxyl radicals, contributing to oxidative stress and cellular damage [42,67,68,69]. Furthermore, labile iron catalyzes the cascade of lipid peroxidation that drives ferroptosis, a recently discovered mechanism of degenerative cell death [68,69]. Brain iron homeostasis is a delicate balance of the need for iron and its toxicity.
In its simplest mechanism, iron chelation therapy sequesters and clears the labile iron pool, thus counteracting oxidative damage resulting from pathologic iron accumulation [18,43,67,70]. As discussed in further detail below and elsewhere, this mechanism has direct relevance to a number of neurological disorders including AD, PD, progressive supranuclear palsy (PSP) [71], ischemic stroke, and intracranial hemorrhage, where iron dyshomeostasis—disruption of the iron balance leading to iron accumulation and consequent toxicity—is a known potential contributor to disease pathogenesis and neuronal injury [12,41,42,68,72,73,74]. Although current evidence strongly suggests that iron overload is associated with oxidative stress and injury in these states, it is unlikely that reversal of iron accumulation alone will reverse the underlying disease processes.
Instead, we suggest that the major mechanism of iron chelation treatment of neurological disease is the disease-nonspecific depletion of brain iron causing engagement of several biochemical pathways that intersect with and potentially slow disease-specific pathogenesis. The depletion of iron activates hypoxia-inducible factor 1α (HIF-1α) by destabilizing its regulatory prolyl-hydroxylase, an iron-dependent protein [75,76]. HIF-1α activates an elaborate transcriptional program designed as an organized cellular response to hypoxia, mediated principally by vascular endothelial growth factor (VEGF), erythropoietin (EPO), inducible nitric oxide synthase (iNOS), and insulin-like growth factor (IGF) [40,75,76]. By these pathways, iron chelators including IN DFO have been found to reverse the accumulation of protein aggregates [28,30,33,34,77,78,79], suppress neuroinflammation [31,38,77,80,81,82,83,84], protect against oxidative stress and neuronal injury [30,31,32,36,81,83,85], improve cerebrovascular function [25,37,40,76], activate pro-survival signaling pathways [30,31,32,33,34,35,36,76], bolster cerebral glucose metabolism [30,31,35], and strengthen synaptic function [35,86,87] (Figure 1). In the following paragraphs, citing the considerable body of preclinical and early clinical research for IN DFO and other iron chelators, we argue multipronged mechanisms by which engagement of these pathways helps to counter pathogenesis across neurological disease.
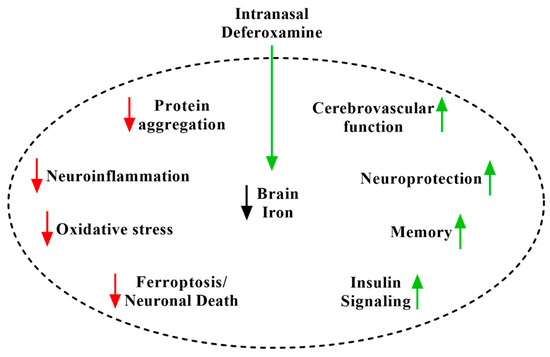
Figure 1.
The disease-nonspecific mechanisms of iron chelation using intranasal deferoxamine (IN DFO). Depletion of brain iron engages a number of disease-relevant pathways.
4. IN DFO for Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common dementia, afflicting a staggering 50 million individuals worldwide [2]. A seminal two-year single-blind clinical trial found that intramuscular administration of DFO significantly reduced the rate of decline in living skills for AD patients by approximately twofold [39]. In animal models of AD, evidence for the therapeutic efficacy of DFO, IN DFO, and other iron chelators is very strong and has been reviewed extensively elsewhere [28,88]. From the work of multiple research groups, IN DFO reduces cognitive decline and pathological hallmarks in the APP/PS1, P301L, and ICV STZ rodent models of AD [30,31,33,34,35,36,77,89]. Likewise, the preclinical evidence for other iron and metal chelators is also strong across these models [42,70,72].
For many years, the amyloid hypothesis has dominated research on and understanding of AD pathoetiology [90], but the repeated failure of therapeutics targeting amyloid-β (Aβ) has undermined testimony of its accuracy [7,91]. Rather, converging lines of evidence suggest an alternative paradigm defining AD as a spectrum of decline caused by the interaction of aging-driven Aβ and tau neuropathology (Figure 2). In this framework, aging drives abnormal tau processing driving neurodegeneration [92,93,94] with Aβ pathology accelerating [91,93,94,95] and contributing to [90,91,95,96] this process, reconciling spatiotemporal pathological data across the aging population [8,94,97] with the causes of familial forms of AD [90]. The process of consequent cognitive decline appears to involve a dynamic interplay of progressive synaptic dysfunction, neurodegeneration [69,91,95], neuroinflammation [92,98], neurovascular breakdown [99], and the collapse of large-scale brain networks [100]. IN DFO can act on multiple processes in this cascade to slow disease progression. DFO has been shown to reduce Aβ aggregation [31,33,35,77] and hyperphosphorylation of tau [34,101], and involvement of transition metals including iron is thought to contribute to the toxicity of these pathological hallmarks [70,73]. DFO is a robust inhibitor of glycogen synthase kinase-3β (GSK-3β) [30,31,32,34], a well-established therapeutic target in tauopathy, and DFO was found to reverse tau-mediated neurodegeneration in rabbits treated with aluminum [101]. GSK-3β is also hypothesized as a major link between accumulation of Aβ and consequent hyperphosphorylation of tau in AD [91,93,96], placing DFO in position to suppress this acceleratory process. Additionally, iron accumulation and iron-mediated neuronal ferroptosis have been increasingly implicated as contributing to AD neurodegeneration [69,72,73], and IN DFO directly suppresses this process including iron-associated oxidative stress [42,78]. DFO likewise ameliorates neuroinflammation [30,38,81,82,83] which is hypothesized to contribute to Aβ toxicity and damage in AD.
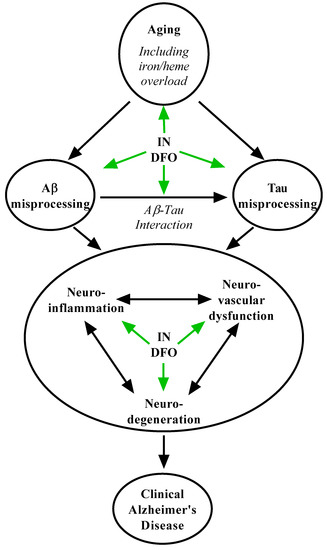
Figure 2.
Putative mechanisms of IN DFO in Alzheimer’s disease. IN DFO engages Alzheimer’s pathogenesis at multiple levels, including a number of contributory aging-related processes, amyloid misprocessing, tau misprocessing, amyloid-tau interaction, neuroinflammation, neurovascular dysfunction, and neurodegeneration.
Acting by its disease-nonspecific mechanism of HIF-1α activation, DFO appears to operate at a number of further levels countering the AD process. In two clinical trials, systemic administration of DFO strongly improved cerebral vasoreactivity and autoregulation especially in older individuals [37,40]. Decline in neurovascular function is thought to contribute to and be a part of AD pathogenesis [99]. DFO also activates glucose transporters including glucose transporter 1 (GLUT1) via HIF-1α [35,75,76], countering cerebral hypometabolism that is an early hallmark of AD. Furthermore, DFO has been robustly associated with activation of the insulin signaling pathway [30,31,32,34,38]. Activation of this pathway is known to act on astrocytes, microglia, and neurons to suppress neuroinflammation and promote neuroplasticity [102], and indeed, intranasal administration of insulin has shown benefit in several Phase 2 AD trials [60,61,62]. Finally, there is also precedent for DFO to improve memory in the absence of disease with IN DFO improving memory in healthy mice [32], possibly via GSK-3β mediated neuroplasticity [102].
Additionally, accumulating free iron may inactivate the human brain muscarinic cholinergic receptor (mAChR), contributing to impaired memory in AD [103]. Further, free heme, which contains iron, increases in the brains of AD patients, and like free iron inactivates the mAChR. A 2.5-fold increase in heme was also reported in the temporal lobe of deceased individuals with AD [104]. Consistent with this finding, the level of ferrochelatase in AD temporal lobe was 4.2 times that in nondemented controls, suggesting up-regulated heme synthesis [104]. In vitro, DFO protects the mAChR from inactivation by both iron and heme [Frey WH 2nd, Bordayo EZ and Hanson LR unpublished results]. Relatively little attention has been paid to the fact that Aβ binds both iron and heme. Heme binding to Aβ prevents Aβ aggregation by forming an Aβ–heme complex, and this Aβ–heme complex has peroxidase activity [105]. Phylogenic variations in the amino acid sequence of Aβ explain tight heme-binding to human Aβ and have been proposed to contribute to the increased human susceptibility to AD [106].
Thus, the positive early systemic clinical trial [39], the overwhelming body of preclinical evidence, and multimodal mechanisms of action strengthen the argument for further development of IN DFO for AD.
5. IN DFO for Parkinson’s Disease
Parkinson’s disease (PD), one of the most common movement disorders, continues to challenge those developing disease-modifying therapies. DFO has been shown to be strongly neuroprotective in several induced rodent models of PD [28], reversing motor deficits and improving survival of dopaminergic neurons following administration of 6-hydroxydopmaine (6-OHDA) [107,108,109], rotenone, methyl-phenyl-tetrahydropyridine (MPTP) [110], and α-synuclein [29]. Other iron chelators have extensively paralleled these findings [70,108,111]. Notably, the iron chelator deferiprone has shown promise in early clinical trials [111], although systemic administration and lower affinity for iron potentially limit this therapy’s efficacy compared to IN DFO.
DFO may act by multiple mechanisms to slow the progression of PD, many overlapping with its role in AD (Figure 3). Although complete detail remains elusive, PD is thought to be driven by the age-related pathological accumulation of α-synuclein aggregates into Lewy bodies [10]. This process results in progressive oxidative stress, lysosomal and mitochondrial dysfunction, and neurodegeneration, with possible roles for prion-like pathological spread and autoimmunity. Dopaminergic neurons within the substantia nigra pars compacta (SNpc) are particularly susceptible to this process [10,13], causing motor deficits in PD. DFO has been shown to directly reduce the expression and aggregation of α-synuclein [29,110]. Furthermore, it is well established that iron selectively accumulates within the SNpc and is associated with its exquisite vulnerability to oxidative stress and ferroptosis [42,69,72,73], and administration of DFO reverses this process [72,78]. DFO administration activates insulin signaling and glucose metabolism [32,34,89,107], and other modulators of these processes, including IN insulin, have similarly shown substantial preclinical efficacy in PD [112]. Finally, via its activation of the HIF-1 response and neurotrophic growth factors [35,86,87], DFO strongly promotes neuronal survival, mechanisms which may in themselves check the progression of degenerative processes in the PD brain. Thus, there is overwhelming evidence and rationale for further development of IN DFO and other iron chelators in PD.
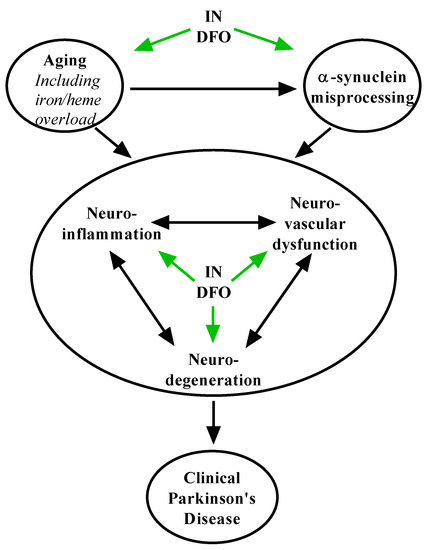
Figure 3.
Putative mechanisms of IN DFO in Parkinson’s disease. IN DFO engages pathways that limit age-related processes such as iron accumulation, α-synuclein misprocessing, neuroinflammation, neurovascular dysfunction, and neurodegeneration to counter the Parkinson’s pathological process and loss of dopaminergic neurons.
6. IN DFO for Ischemic Stroke
Ischemic stroke is one of the leading causes of mortality and disability. Therapeutic use of DFO has shown considerable preclinical efficacy in animal models of stroke. IN DFO was found to significantly reduce infarct volume with middle cerebral artery occlusion (MCAO) in rats with either pre- or post-treatment [24]. These results are supported by efficacy of systemically administered DFO in the same model [113], as well as in rat ischemia induced by carotid ligation [114]. Furthermore, systemic DFO has been shown to cause robust ischemic preconditioning in the rodent brain, a strategy which is thought to ameliorate further damage due to multiple occlusive events, a common complication of stroke [76,115,116,117]. These results are similarly supported by a considerable body of similarly positive results from other iron chelators [70], supporting a real therapeutic role for this strategy in stroke treatment.
Occlusive stroke causes a complex pathophysiologic cascade of ischemic injury. Neurons are exquisitely sensitive to hypoxia, which causes cell death by a number of intersecting mechanisms [11]. By depleting cellular iron, DFO acts as a hypoxia-mimetic [115], bolstering the HIF-1α pathway and thus multiple aspects of the brain’s inherent neuroprotective defense against ischemia [37,40,75]. Additionally, by preconditioning the hypoxic response, DFO may ameliorate damage due to further ischemic events [113,116]. It is increasingly established that cerebral edema and hemorrhagic conversion also participate in damage following ischemic stroke [11], and DFO strongly reduces toxicity associated with these processes due to chelation of redox-active iron. Thus, as in AD and PD, DFO holds considerable promise to modify multiple aspects of the pathogenesis of ischemic stroke (Figure 4) to rescue functional impairment, a feature lacking in many putative strategies developed to date [41]. The speed, CNS targeting, and safety of IN delivery further strengthens this approach.
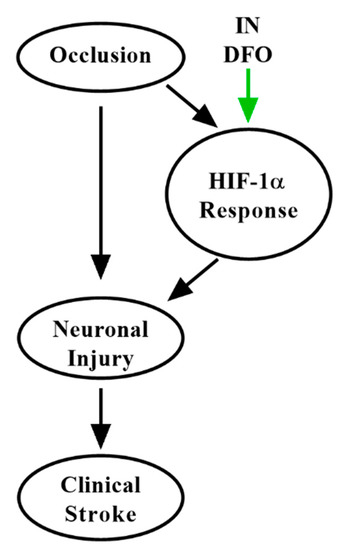
Figure 4.
Putative mechanisms of IN DFO in ischemic stroke. IN DFO bolsters the brain’s intrinsic hypoxic response, contributing to immediate neuroprotection, improved cerebrovascular function, and ischemic preconditioning to minimize the impact of future occlusive events.
7. IN DFO for Intracranial Hemorrhage
DFO has been perhaps best studied in the context of intracranial hemorrhage (ICH), hemorrhagic events constituting around 15% of all strokes. Its substantial preclinical evidence in intracerebral hemorrhage and subarachnoid hemorrhage as well as mechanisms have been extensively reviewed elsewhere [27,85,118]. Succinctly, DFO is thought to chelate redox-active iron released from hemoglobin and myoglobin following ICH [12], minimizing secondary brain damage (Figure 5). Notably, the i-DEF trial evaluating IV DFO after intracerebral hemorrhage did not suggest therapeutic benefit [118]. However, this study was limited by systemic toxicity associated with higher doses of IV DFO, and the low doses studied may not have been sufficient to generate adequate CNS coverage following ICH [119]. IN administration may overcome this hurdle by targeting DFO to the CNS (up to 200-fold) as compared to systemic modes of delivery [24]. Another trial found that administration of DFO positively impacted several endpoints following ICH, although the investigators concluded more conclusive trials were required [120]. Given its substantial preclinical efficacy and the advantages of IN delivery, further investigation of IN DFO in ICH is still warranted.
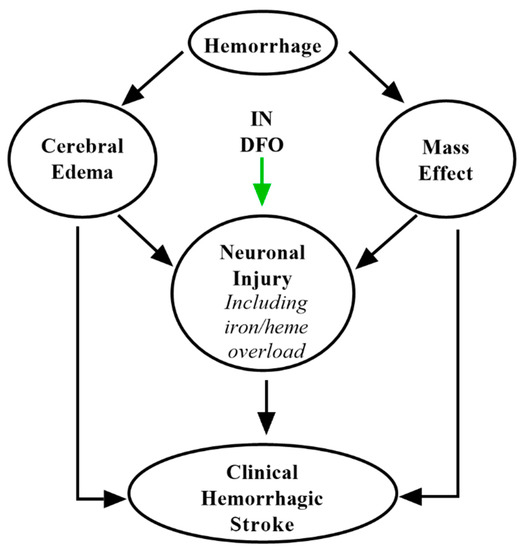
Figure 5.
Putative mechanisms of IN DFO in intracranial hemorrhage. IN DFO minimizes the impact of heme- and iron-mediated neurotoxicity following hematoma.
8. IN DFO for Other Neurological Diseases
The wide-ranging efficacy of IN DFO in neurologic disease largely stems from its disease-nonspecific mechanism intersecting disease processes at multiple pathogenic nodes. Targeting multiple pathologies is an advantage for the treatment complex disorders such as in AD and expands the applicability of IN DFO to other neurological disorders. For example, DFO shows promise as an agent to improve memory in aging and other dementias. DFO was shown to reduce cognitive decline with aging [121], and IN DFO improves baseline performance on a memory task in young, healthy mice [32]. In these settings, it is possible that DFO may simply promote neuronal functioning through chelation of age-associated redox-active iron. Alternatively, its mechanisms promoting synaptic function, glucose metabolism, or cerebrovascular function may play a role and merit further investigation. In multiple sclerosis, brain iron is found to accumulate independently of inflammation and is increasingly thought to contribute to pathogenesis [74]. DFO was found to suppress inflammatory damage in animal models [80,122] and was tolerated in early clinical studies of this disease [123,124]. Insomnia-associated cognitive decline is associated iron accumulation, oxidative stress, and inflammation [73], and may likewise be ameliorated with IN DFO treatment. Furthermore, accumulation of iron is thought to be one of the many pathophysiological sequelae of traumatic brain injury (TBI) [125]. DFO has been shown to suppress this post-TBI iron overload as well as reverse hydrocephalus and cognitive deficits following TBI in animal models [79,126,127,128]. The sequelae of TBI combine neurodegenerative, neuroinflammatory, and both ischemic and hemorrhagic neurovascular components [125], a perfect model wherein DFO may uniquely enact beneficial impact through its multiple intersecting disease-modifying mechanisms.
Finally, there is evidence for the development of IN DFO in vascular dementia, the second leading cause of dementia worldwide. DFO has been shown to reverse cognitive deficits following hemorrhagic and ischemic vascular events in animal models, including events induced by diabetes [25]. Vascular dementia is a highly heterogenous entity involving generally stepwise cognitive decline secondary to progressive ischemic brain injury, and is at the crossroads of neurovascular dysfunction, neurodegeneration, and metabolic syndrome [129]. As in TBI, DFO may uniquely operate at the center of this pathological process modifying each of its components. DFO may prevent cognitive dysfunction secondary to ischemia via activation of the HIF-1α response, providing neuroprotection following occlusive events akin to its role following ischemic stroke [24,76]. Furthermore, DFO may provide ischemic preconditioning within the brain, preventing further decline caused by progressive neurovascular dysfunction [86,113,115,116,117]. Moreover, by improving cerebrovascular function, DFO may prevent much of this neurovascular decline with aging [37,40]. Additionally, neurodegenerative changes are often associated with vascular dementia [129], and thus DFO’s pro-survival mechanisms in PD and AD are relevant to progression in this condition. Lastly, metabolic syndrome and brain insulin resistance have been increasingly associated with the spectrum of neurodegeneration and neurovascular decline [102,129]. DFO activates insulin signaling and may improve memory, glucose metabolism, and cognitive functioning by this mechanism as well [30,35,89,112]. As a major cause of dementia and disability with relatively few therapeutic options on the horizon, IN DFO represents a promising yet relatively underdeveloped treatment for vascular dementia. Likewise, iron chelation has emerged as a neuroprotective strategy in a number of other neurological diseases including amyotrophic lateral sclerosis (ALS) [42] and PSP [71].
9. Conclusions
The complex and often progressive nature of neurological disease has undoubtedly contributed to the repeated failure of potential therapeutics. It is increasingly recognized that “silver bullets”—therapies that operate on one target within a cascade—are unlikely to be of major benefit when multiple mechanisms contribute to pathogenesis, as is the case in neurodegenerative and neurovascular disease. In the absence of measures for complete prevention or regeneration of lost brain function, so-called “dirty drugs” that operate on multiple events in these pathogenic cascades may be the only realistic future for meaningful benefit in these diseases, such as Alzheimer’s disease [88]. Herein, the evidence has been presented for IN DFO as one such therapy. Brain iron chelation may act as a general disease-nonspecific mechanism that engages multiple pathways, including downstream effectors of HIF-1α and insulin signaling, to intersect with and possibly slow the progression of multiple human neurological diseases including AD, PD, ischemic stroke, and ICH. Citing preclinical and early clinical work across decades, multiple research groups, alternative delivery mechanisms, and other iron chelators, we have argued that these putative mechanisms represent significant promise for translational efficacy. Intranasal delivery is poised to transform and mitigate the challenge of delivering pharmaceuticals to the CNS including DFO. By exerting its effects on multiple pathways including neurodegeneration, neuroinflammation, neurovascular dysfunction, and metabolic syndrome, IN DFO is positioned to have widespread benefit across neurodegenerative and neurovascular disease.
Author Contributions
Conceptualization: J.K., J.M.F., W.H.F.II, and L.R.H. Outline and format: J.K., J.M.F., and L.R.H. Writing—original draft: J.K. Writing—review and editing: J.M.F., W.H.F.II, L.R.H. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was provided for this contribution.
Acknowledgments
Thanks to Tate Bowe for helping with early literature searches and outlines for the manuscript.
Conflicts of Interest
W.H.F.II and L.R.H. are inventors on a patent owned by HealthPartners Institute related to intranasal deferoxamine. J.K. and J.M.F. have no competing interests.
References
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, Regional, and National Burden of Neurological Disorders, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Nichols, E.; Szoeke, C.E.I.; Vollset, S.E.; Abbasi, N.; Abd-Allah, F.; Abdela, J.; Aichour, M.T.E.; Akinyemi, R.O.; Alahdab, F.; Asgedom, S.W.; et al. Global, Regional, and National Burden of Alzheimer’s Disease and Other Dementias, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Spina, S.; Miller, B.L. Frontotemporal Dementia. Lancet 2015, 386, 1672–1682. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nguyen, G.; Cercy, K.; Johnson, C.O.; Alam, T.; Parmar, P.G.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abejie, A.N.; et al. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N. Engl. J. Med. 2018, 379, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.-P.; Fenart, L. Modelling of the Blood–Brain Barrier in Drug Discovery and Development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Trojanowski, J.Q. Tauists, Baptists, Syners, Apostates, and New Data. Ann. Neurol. 2002, 52, 263–265. [Google Scholar] [CrossRef]
- Jagust, W. Imaging the Evolution and Pathophysiology of Alzheimer Disease. Nat. Rev. Neurosci. 2018, 19, 687–700. [Google Scholar] [CrossRef]
- Weiner, W.J. There Is No Parkinson Disease. Arch. Neurol. 2008, 65, 705–708. [Google Scholar] [CrossRef]
- Olanow, C.W.; Tatton, W.G. Etiology and pathogenesis of Parkinson’s disease. Annu. Rev. Neurosci. 1999, 22, 123–144. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef]
- Keep, R.F.; Hua, Y.; Xi, G. Intracerebral Haemorrhage: Mechanisms of Injury and Therapeutic Targets. Lancet Neurol. 2012, 11, 720–731. [Google Scholar] [CrossRef]
- Olanow, C.W.; Kieburtz, K.; Schapira, A.H.V. Why Have We Failed to Achieve Neuroprotection in Parkinson’s Disease? Ann. Neurol. 2008, 64, S101–S110. [Google Scholar] [CrossRef] [PubMed]
- Mitsumoto, H.; Brooks, B.R.; Silani, V. Clinical Trials in Amyotrophic Lateral Sclerosis: Why so Many Negative Trials and How Can Trials Be Improved? Lancet Neurol. 2014, 13, 1127–1138. [Google Scholar] [CrossRef]
- Pfeuffer, S.; Ruck, T.; Kleinschnitz, C.; Wiendl, H.; Meuth, S.G. Failed, Interrupted and Inconclusive Trials on Relapsing Multiple Sclerosis Treatment: Update 2010–2015. Expert Rev. Neurother. 2016, 16, 689–700. [Google Scholar] [CrossRef]
- Elmaleh, D.R.; Farlow, M.R.; Conti, P.S.; Tompkins, R.G.; Kundakovic, L.; Tanzi, R.E. Developing Effective Alzheimer’s Disease Therapies: Clinical Experience and Future Directions. J. Alzheimers Dis. 2019, 71, 715–732. [Google Scholar] [CrossRef]
- Ballas, S.K.; Zeidan, A.M.; Duong, V.H.; DeVeaux, M.; Heeney, M.M. The Effect of Iron Chelation Therapy on Overall Survival in Sickle Cell Disease and β-Thalassemia: A Systematic Review. Am. J. Hematol. 2018, 93, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, N.F.; Brittenham, G.M. Iron-Chelating Therapy and the Treatment of Thalassemia. Blood 1997, 89, 739–761. [Google Scholar] [CrossRef] [PubMed]
- Donfrancesco, A.; Deb, G.; De Sio, L.; Cozza, R.; Castellano, A. Role of Deferoxamine in Tumor Therapy. Acta Haematol. 1996, 95, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Terai, S.; Sakaida, I. Deferoxamine for Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2011, 365, 576–578. [Google Scholar] [CrossRef]
- Kalinowski, D.S.; Richardson, D.R. The Evolution of Iron Chelators for the Treatment of Iron Overload Disease and Cancer. Pharmacol. Rev. 2005, 57, 547. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Lyashchenko, S.K.; Riedl, C.; Ruan, S.; Zanzonico, P.B.; Lake, D.; Jhaveri, K.; Zeglis, B.; Lewis, J.S.; O’Donoghue, J.A. First-in-Human Human Epidermal Growth Factor Receptor 2–Targeted Imaging Using 89Zr-Pertuzumab PET/CT: Dosimetry and Clinical Application in Patients with Breast Cancer. J. Nucl. Med. 2018, 59, 900–906. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, J.A.; Lewis, J.S.; Pandit-Taskar, N.; Fleming, S.E.; Schöder, H.; Larson, S.M.; Beylergil, V.; Ruan, S.; Lyashchenko, S.K.; Zanzonico, P.B.; et al. Pharmacokinetics, Biodistribution, and Radiation Dosimetry for 89Zr-Trastuzumab in Patients with Esophagogastric Cancer. J. Nucl. Med. 2018, 59, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.R.; Roeytenberg, A.; Martinez, P.M.; Coppes, V.G.; Sweet, D.C.; Rao, R.J.; Marti, D.L.; Hoekman, J.D.; Matthews, R.B.; Frey, W.H.; et al. Intranasal Deferoxamine Provides Increased Brain Exposure and Significant Protection in Rat Ischemic Stroke. J. Pharmacol. Exp. Ther. 2009, 330, 679. [Google Scholar] [CrossRef]
- Abdul, Y.; Li, W.; Ward, R.; Abdelsaid, M.; Hafez, S.; Dong, G.; Jamil, S.; Wolf, V.; Johnson, M.H.; Fagan, S.C.; et al. Deferoxamine Treatment Prevents Post-Stroke Vasoregression and Neurovascular Unit Remodeling Leading to Improved Functional Outcomes in Type 2 Male Diabetic Rats: Role of Endothelial Ferroptosis. Transl. Stroke Res. 2020. [Google Scholar] [CrossRef]
- Chen, J.; Marks, E.; Lai, B.; Zhang, Z.; Duce, J.A.; Lam, L.Q.; Volitakis, I.; Bush, A.I.; Hersch, S.; Fox, J.H. Iron Accumulates in Huntington’s Disease Neurons: Protection by Deferoxamine. PLoS ONE 2013, 8, e77023. [Google Scholar] [CrossRef]
- Cui, H.-J.; He, H.; Yang, A.-L.; Zhou, H.-J.; Wang, C.; Luo, J.-K.; Lin, Y.; Tang, T. Efficacy of Deferoxamine in Animal Models of Intracerebral Hemorrhage: A Systematic Review and Stratified Meta-Analysis. PLoS ONE 2015, 10, e0127256. [Google Scholar] [CrossRef]
- Farr, A.C.; Xiong, M.P. Challenges and Opportunities of Deferoxamine Delivery for Treatment of Alzheimer’s Disease, Parkinson’s Disease, and Intracerebral Hemorrhage. Mol. Pharm. 2020. [Google Scholar] [CrossRef]
- Febbraro, F.; Andersen, K.J.; Sanchez-Guajardo, V.; Tentillier, N.; Romero-Ramos, M. Chronic Intranasal Deferoxamine Ameliorates Motor Defects and Pathology in the α-Synuclein RAAV Parkinson’s Model. Exp. Neurol. 2013, 247, 45–58. [Google Scholar] [CrossRef]
- Fine, J.M.; Renner, D.B.; Forsberg, A.C.; Cameron, R.A.; Galick, B.T.; Le, C.; Conway, P.M.; Stroebel, B.M.; Frey, W.H.; Hanson, L.R. Intranasal Deferoxamine Engages Multiple Pathways to Decrease Memory Loss in the APP/PS1 Model of Amyloid Accumulation. Neurosci. Lett. 2015, 584, 362–367. [Google Scholar] [CrossRef]
- Fine, J.M.; Baillargeon, A.M.; Renner, D.B.; Hoerster, N.S.; Tokarev, J.; Colton, S.; Pelleg, A.; Andrews, A.; Sparley, K.A.; Krogh, K.M.; et al. Intranasal Deferoxamine Improves Performance in Radial Arm Water Maze, Stabilizes HIF-1α, and Phosphorylates GSK3β in P301L Tau Transgenic Mice. Exp. Brain Res. 2012, 219, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.M.; Kosyakovsky, J.; Baillargeon, A.M.; Tokarev, J.V.; Cooner, J.M.; Svitak, A.L.; Faltesek, K.A.; Frey, W.H., II; Hanson, L.R. Intranasal Deferoxamine Can Improve Memory in Healthy C57 Mice, Suggesting a Partially Non-Disease-Specific Pathway of Functional Neurologic Improvement. Brain Behav. 2020, 10, e01536. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, T.; Zheng, W.; Shan, Z.-Y.; Teng, W.-P.; Wang, Z.-Y. Intranasal Deferoxamine Reverses Iron-Induced Memory Deficits and Inhibits Amyloidogenic APP Processing in a Transgenic Mouse Model of Alzheimer’s Disease. Neurobiol. Aging 2013, 34, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, P.; Zhong, M.-L.; Wang, T.; Huang, X.-S.; Li, J.-Y.; Wang, Z.-Y. Deferoxamine Inhibits Iron Induced Hippocampal Tau Phosphorylation in the Alzheimer Transgenic Mouse Brain. Neurochem. Int. 2013, 62, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, Y.-X.; Wang, T.; Zhong, M.-L.; Yang, Z.-H.; Hao, L.-J.; Chai, R.; Zhang, S. Intranasal Deferoxamine Attenuates Synapse Loss via Up-Regulating the P38/HIF-1α Pathway on the Brain of APP/PS1 Transgenic Mice. Front. Aging Neurosci. 2015, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.R.; Fine, J.M.; Renner, D.B.; Svitak, A.L.; Burns, R.B.; Nguyen, T.M.; Tuttle, N.J.; Marti, D.L.; Panter, S.S.; Frey, W.H. Intranasal Delivery of Deferoxamine Reduces Spatial Memory Loss in APP/PS1 Mice. Drug Deliv. Transl. Res. 2012, 2, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Sorond, F.A.; Tan, C.O.; LaRose, S.; Monk, A.D.; Fichorova, R.; Ryan, S.; Lipsitz, L.A. Deferoxamine, Cerebrovascular Hemodynamics, and Vascular Aging. Stroke 2015, 46, 2576–2583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, X.-Y.; Cao, J.-B.; Zhang, L.-M.; Li, Y.-F.; Mi, W.-D. Deferoxamine Attenuates Lipopolysaccharide-Induced Neuroinflammation and Memory Impairment in Mice. J. Neuroinflamm. 2015, 12, 20. [Google Scholar] [CrossRef]
- McLachlan, D.R.C.; Kruck, T.P.A.; Kalow, W.; Andrews, D.F.; Dalton, A.J.; Bell, M.Y.; Smith, W.L. Intramuscular Desferrioxamine in Patients with Alzheimer’s Disease. Lancet 1991, 337, 1304–1308. [Google Scholar] [CrossRef]
- Sorond, F.A.; Shaffer, M.L.; Kung, A.L.; Lipsitz, L.A. Desferroxamine Infusion Increases Cerebral Blood Flow: A Potential Association with Hypoxia-Inducible Factor-1. Clin. Sci. 2009, 116, 771–779. [Google Scholar] [CrossRef]
- Hanafy, K.A.; Gomes, J.A.; Selim, M. Rationale and Current Evidence for Testing Iron Chelators for Treating Stroke. Curr. Cardiol. Rep. 2019, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Masaldan, S.; Bush, A.I.; Devos, D.; Rolland, A.S.; Moreau, C. Striking While the Iron Is Hot: Iron Metabolism and Ferroptosis in Neurodegeneration. Iron Soul Life Earth Revisit. Chem. React. Ferroptosis Ther. 2019, 133, 221–233. [Google Scholar] [CrossRef]
- Porter, J.B. Deferoxamine Pharmacokinetics. Transfus.-Relat. Iron Overload Sick. Cell Anemia 2001, 38, 63–68. [Google Scholar] [CrossRef]
- Howland, M.A. Risks of Parenteral Deferoxamine for Acute Iron Poisoning. J. Toxicol. Clin. Toxicol. 1996, 34, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Frey, W.H., II. Method of Administering Neurologic Agents to the Brain. U.S. Patent 5,624,898, 29 April 1997. [Google Scholar]
- Frey, W.H., II. Neurologic Agents for Nasal Administration to the Brain. World Intellectual Property Organization No. PCT/US1990/007099, 13 June 1991. [Google Scholar]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal Delivery to the Central Nervous System: Mechanisms and Experimental Considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-Brain Drug Delivery: An Update on Clinical Challenges and Progress towards Approval of Anti-Alzheimer Drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef]
- Thorne, R.G.; Frey, W.H. Delivery of Neurotrophic Factors to the Central Nervous System. Clin. Pharmacokinet. 2001, 40, 907–946. [Google Scholar] [CrossRef]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H. Delivery of Insulin-like Growth Factor-I to the Rat Brain and Spinal Cord along Olfactory and Trigeminal Pathways Following Intranasal Administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal Delivery of Biologics to the Central Nervous System. Deliv. Ther. Cent. Nerv. Syst. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Wolak, D.J.; Pizzo, M.E.; Thorne, R.G. Rapid Transport within Cerebral Perivascular Spaces Underlies Widespread Tracer Distribution in the Brain after Intranasal Administration. J. Cereb. Blood Flow Metab. 2015, 35, 371–381. [Google Scholar] [CrossRef]
- Hashizume, R.; Ozawa, T.; Gryaznov, S.M.; Bollen, A.W.; Lamborn, K.R.; Frey, W.H., II; Deen, D.F. New Therapeutic Approach for Brain Tumors: Intranasal Delivery of Telomerase Inhibitor GRN163. Neuro-Oncology 2008, 10, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Danielyan, L.; Schäfer, R.; von Ameln-Mayerhofer, A.; Buadze, M.; Geisler, J.; Klopfer, T.; Burkhardt, U.; Proksch, B.; Verleysdonk, S.; Ayturan, M.; et al. Intranasal Delivery of Cells to the Brain. Eur. J. Cell Biol. 2009, 88, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Danielyan, L.; Schäfer, R.; von Ameln-Mayerhofer, A.; Bernhard, F.; Verleysdonk, S.; Buadze, M.; Lourhmati, A.; Klopfer, T.; Schaumann, F.; Schmid, B.; et al. Therapeutic Efficacy of Intranasally Delivered Mesenchymal Stem Cells in a Rat Model of Parkinson Disease. Rejuvenation Res. 2011, 14, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Danielyan, L.; Beer-Hammer, S.; Stolzing, A.; Schäfer, R.; Siegel, G.; Fabian, C.; Kahle, P.; Biedermann, T.; Lourhmati, A.; Buadze, M.; et al. Intranasal Delivery of Bone Marrow-Derived Mesenchymal Stem Cells, Macrophages, and Microglia to the Brain in Mouse Models of Alzheimer’s and Parkinson’s Disease. Cell Transplant. 2014, 23, 123–139. [Google Scholar] [CrossRef] [PubMed]
- van Velthoven, C.T.J.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Nasal Administration of Stem Cells: A Promising Novel Route to Treat Neonatal Ischemic Brain Damage. Pediatr. Res. 2010, 68, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Kosyakovsky, J.; Witthuhn, B.A.; Svitak, A.L.; Frey, W.H.; Hanson, L.R.; Fine, J.M. Quantifying Intranasally Administered Deferoxamine in Rat Brain Tissue with Mass Spectrometry. ACS Chem. Neurosci. 2019, 10, 4571–4578. [Google Scholar] [CrossRef] [PubMed]
- Reger, M.A.; Watson, G.S.; Frey, W.H.; Baker, L.D.; Cholerton, B.; Keeling, M.L.; Belongia, D.A.; Fishel, M.A.; Plymate, S.R.; Schellenberg, G.D.; et al. Effects of Intranasal Insulin on Cognition in Memory-Impaired Older Adults: Modulation by APOE Genotype. Neurobiol. Aging 2006, 27, 451–458. [Google Scholar] [CrossRef]
- Claxton, A.; Baker, L.D.; Hanson, A.; Trittschuh, E.H.; Cholerton, B.; Morgan, A.; Callaghan, M.; Arbuckle, M.; Behl, C.; Craft, S. Long-Acting Intranasal Insulin Detemir Improves Cognition for Adults with Mild Cognitive Impairment or Early-Stage Alzheimer’s Disease Dementia. J. Alzheimers Dis. 2015, 44, 897–906. [Google Scholar] [CrossRef]
- Craft, S.; Claxton, A.; Baker, L.D.; Hanson, A.J.; Cholerton, B.; Trittschuh, E.H.; Dahl, D.; Caulder, E.; Neth, B.; Montine, T.J.; et al. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer’s Disease Biomarkers: A Pilot Clinical Trial. J. Alzheimers Dis. 2017, 57, 1325–1334. [Google Scholar] [CrossRef]
- Reger, M.A.; Watson, G.S.; Green, P.S.; Wilkinson, C.W.; Baker, L.D.; Cholerton, B.; Fishel, M.A.; Plymate, S.R.; Breitner, J.C.S.; DeGroodt, W.; et al. Intranasal Insulin Improves Cognition and Modulates β-Amyloid in Early AD. Neurology 2008, 70, 440. [Google Scholar] [CrossRef]
- Frey, W.H., II; Panter, S.S.; Hanson, L.R. Method of Treating Alzheimer’s Disease Comprising Administering Deferoxamine (DFO) to the Upper One-Third of the Nasal Cavity. U.S. Patent 7,776,312, 17 August 2010. [Google Scholar]
- Hanson, L.R.; Panter, S.S.; Frey, W.H., II. Method of Treating Parkinson’s Disease Comprising Administering Deferoxamine (DFO) to the Upper One-Third of the Nasal Cavity. U.S. Patent 9,205,066, 8 December 2015. [Google Scholar]
- Frey, W.H., II; Panter, S.S.; Hanson, L.R. Method of Treating Stroke Comprising Administering Metal Chelators to the Upper One-Third of the Nasal Cavity. U.S. Patent 9,345,676, 24 May 2016. [Google Scholar]
- Rouault, T.A.; Cooperman, S. Brain Iron Metabolism. Semin. Pediatric Neurol. 2006, 13, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kakhlon, O.; Cabantchik, Z.I. The Labile Iron Pool: Characterization, Measurement, and Participation in Cellular Processes. Free Radic. Biol. Med. 2002, 33, 1037–1046. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Singh, Y.P.; Pandey, A.; Vishwakarma, S.; Modi, G. A Review on Iron Chelators as Potential Therapeutic Agents for the Treatment of Alzheimer’s and Parkinson’s Diseases. Mol. Divers. 2019, 23, 509–526. [Google Scholar] [CrossRef]
- Lee, S.H.; Lyoo, C.H.; Ahn, S.J.; Rinne, J.O.; Lee, M.S. Brain Regional Iron Contents in Progressive Supranuclear Palsy. Parkinsonism Relat. Disord. 2017, 45, 28–32. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Carvalho, A.F.; Maes, M.; Walker, A.J.; Puri, B.K. Why Should Neuroscientists Worry about Iron? The Emerging Role of Ferroptosis in the Pathophysiology of Neuroprogressive Diseases. Behav. Brain Res. 2018, 341, 154–175. [Google Scholar] [CrossRef]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The Role of Iron in Brain Ageing and Neurodegenerative Disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef]
- Stankiewicz, J.M.; Neema, M.; Ceccarelli, A. Iron and Multiple Sclerosis. Int. Conf. Nutr. Brain 2014, 35, S51–S58. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 and Mechanisms of Hypoxia Sensing. Curr. Opin. Cell Biol. 2001, 13, 167–171. [Google Scholar] [CrossRef]
- Siddiq, A.; Aminova, L.R.; Troy, C.M.; Suh, K.; Messer, Z.; Semenza, G.L.; Ratan, R.R. Selective Inhibition of Hypoxia-Inducible Factor (HIF) Prolyl-Hydroxylase 1 Mediates Neuroprotection against Normoxic Oxidative Death via HIF- and CREB-Independent Pathways. J. Neurosci. 2009, 29, 8828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, M. Deferoxamine Enhances Alternative Activation of Microglia and Inhibits Amyloid Beta Deposits in APP/PS1 Mice. Brain Res. 2017, 1677, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.M.; Tang, H.L. Cell Recovery by Reversal of Ferroptosis. Biol. Open 2019, 8, bio043182. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jing, Y.; Xu, C.; Zhao, J.; Gong, Q.; Chen, S. HIF-1α and VEGF Are Involved in Deferoxamine-Ameliorated Traumatic Brain Injury. J. Surg. Res. 2020, 246, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Bowern, N.; Ramshaw, I.A.; Clark, I.A.; Doherty, P.C. Inhibition of Autoimmune Neuropathological Process by Treatment with an Iron-Chelating Agent. J. Exp. Med. 1984, 160, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.; Mohammed, F.; Álvarez-Ciara, A.; Nguyen, M.A.; Dietrich, W.D.; Rajguru, S.M.; Streit, W.J.; Prasad, A. Neuroinflammation, Oxidative Stress, and Blood-Brain Barrier (BBB) Disruption in Acute Utah Electrode Array Implants and the Effect of Deferoxamine as an Iron Chelator on Acute Foreign Body Response. Biomaterials 2019, 188, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, K.; Chen, L.; Ning, J.; Li, X.; Yang, T.; Terrando, N.; Gu, J.; Tao, G. Deferoxamine Regulates Neuroinflammation and Iron Homeostasis in a Mouse Model of Postoperative Cognitive Dysfunction. J. Neuroinflamm. 2016, 13, 268. [Google Scholar] [CrossRef]
- Zeinivand, M.; Nahavandi, A.; Zare, M. Deferoxamine Regulates Neuroinflammation and Oxidative Stress in Rats with Diabetes-Induced Cognitive Dysfunction. Inflammopharmacology 2020, 28, 575–583. [Google Scholar] [CrossRef]
- LeBlanc, R.H.; Chen, R.; Selim, M.H.; Hanafy, K.A. Heme Oxygenase-1-Mediated Neuroprotection in Subarachnoid Hemorrhage via Intracerebroventricular Deferoxamine. J. Neuroinflamm. 2016, 13, 244. [Google Scholar] [CrossRef]
- Zeng, L.; Tan, L.; Li, H.; Zhang, Q.; Li, Y.; Guo, J. Deferoxamine Therapy for Intracerebral Hemorrhage: A Systematic Review. PLoS ONE 2018, 13, e0193615. [Google Scholar] [CrossRef]
- Nouri, F.; Salehinejad, P.; Nematollahi-mahani, S.N.; Kamarul, T.; Zarrindast, M.R.; Sharifi, A.M. Deferoxamine Preconditioning of Neural-Like Cells Derived from Human Wharton’s Jelly Mesenchymal Stem Cells as a Strategy to Promote Their Tolerance and Therapeutic Potential: An In Vitro Study. Cell. Mol. Neurobiol. 2016, 36, 689–700. [Google Scholar] [CrossRef]
- Nowicki, M.; Kosacka, J.; Spanel-Borowski, K.; Borlak, J. Deferoxamine-Induced Neurite Outgrowth and Synapse Formation in Postnatal Rat Dorsal Root Ganglion (DRG) Cell Cultures. Eur. J. Cell Biol. 2009, 88, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Amit, T.; Avramovich-Tirosh, Y.; Youdim, M.B.H.; Mandel, S. Targeting Multiple Alzheimer’s Disease Etiologies with Multimodal Neuroprotective and Neurorestorative Iron Chelators. FASEB J. 2008, 22, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.M.; Forsberg, A.C.; Stroebel, B.M.; Faltesek, K.A.; Verden, D.R.; Hamel, K.A.; Raney, E.B.; Crow, J.M.; Haase, L.R.; Knutzen, K.E.; et al. Intranasal Deferoxamine Affects Memory Loss, Oxidation, and the Insulin Pathway in the Streptozotocin Rat Model of Alzheimer’s Disease. J. Neurol. Sci. 2017, 380, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef]
- Busche, M.A.; Wegmann, S.; Dujardin, S.; Commins, C.; Schiantarelli, J.; Klickstein, N.; Kamath, T.V.; Carlson, G.A.; Nelken, I.; Hyman, B.T. Tau Impairs Neural Circuits, Dominating Amyloid-β Effects, in Alzheimer Models in Vivo. Nat. Neurosci. 2019, 22, 57–64. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-β and Tau: The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the Pathologic Process in Alzheimer Disease: Age Categories From 1 to 100 Years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef]
- Sala Frigerio, C.; De Strooper, B. Alzheimer’s Disease Mechanisms and Emerging Roads to Novel Therapeutics. Annu. Rev. Neurosci. 2016, 39, 57–79. [Google Scholar] [CrossRef]
- Busche, M.A.; Hyman, B.T. Synergy between Amyloid-β and Tau in Alzheimer’s Disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Lowe, V.J.; Wiste, H.J.; Senjem, M.L.; Weigand, S.D.; Therneau, T.M.; Boeve, B.F.; Josephs, K.A.; Fang, P.; Pandey, M.K.; Murray, M.E.; et al. Widespread Brain Tau and It’s Association with Ageing, Braak Stage and Alzheimer’s Dementia. Brain 2018, 141, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Henstridge, C.M.; Hyman, B.T.; Spires-Jones, T.L. Beyond the Neuron–Cellular Interactions Early in Alzheimer Disease Pathogenesis. Nat. Rev. Neurosci. 2019, 20, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Zacchigna, S.; Lambrechts, D.; Carmeliet, P. Neurovascular Signalling Defects in Neurodegeneration. Nat. Rev. Neurosci. 2008, 9, 169–181. [Google Scholar] [CrossRef]
- Jones, D.T.; Graff-Radford, J.; Lowe, V.J.; Wiste, H.J.; Gunter, J.L.; Senjem, M.L.; Botha, H.; Kantarci, K.; Boeve, B.F.; Knopman, D.S.; et al. Tau, Amyloid, and Cascading Network Failure across the Alzheimer’s Disease Spectrum. Cortex 2017, 97, 143–159. [Google Scholar] [CrossRef]
- Savory, J.; Huang, Y.; Wills, M.; Herman, M. Reversal by Desferrioxamine of Tau Protein Aggregates Following Two Days of Treatment in Aluminum-Induced Neurofibrillary Degeneration in Rabbit: Implications for Clinical Trials in Alzheimer’s Disease. Neurotoxicology 1998, 19, 209–214. [Google Scholar]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain Insulin Resistance in Type 2 Diabetes and Alzheimer Disease: Concepts and Conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Fawcett, J.R.; Bordayo, E.Z.; Jackson, K.; Liu, H.; Peterson, J.; Svitak, A.; Frey, W.H., II. Inactivation of the Human Brain Muscarinic Acetylcholine Receptor by Oxidative Damage Catalyzed by a Low Molecular Weight Endogenous Inhibitor from Alzheimer’s Brain Is Prevented by Pyrophosphate Analogs, Bioflavonoids and Other Antioxidants. Brain Res. 2002, 950, 10–20. [Google Scholar] [CrossRef]
- Atamna, H.; Frey, W.H. A Role for Heme in Alzheimer’s Disease: Heme Binds Amyloid β and Has Altered Metabolism. Proc. Natl. Acad. Sci. USA 2004, 101, 11153. [Google Scholar] [CrossRef]
- Atamna, H.; Boyle, K. Amyloid-β Peptide Binds with Heme to Form a Peroxidase: Relationship to the Cytopathologies of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2006, 103, 3381. [Google Scholar] [CrossRef]
- Atamna, H.; Frey, W.H., II; Ko, N. Human and Rodent Amyloid-β Peptides Differentially Bind Heme: Relevance to the Human Susceptibility to Alzheimer’s Disease. Arch. Biochem. Biophys. 2009, 487, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.M.; Forsberg, A.C.; Renner, D.B.; Faltesek, K.A.; Mohan, K.G.; Wong, J.C.; Arneson, L.C.; Crow, J.M.; Frey, W.H.; Hanson, L.R. Intranasally-Administered Deferoxamine Mitigates Toxicity of 6-OHDA in a Rat Model of Parkinson׳s Disease. Brain Res. 2014, 1574, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Siew, C.J.; Ponnusamy, K. Effect of Quercetin and Desferrioxamine on 6-Hydroxydopamine (6-OHDA) Induced Neurotoxicity in Striatum of Rats. J. Toxicol. Sci. 2013, 38, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, J.; Wang, L.; Zhang, H.; Yu, S.; Li, Z.; Jiang, F.; Niu, Y.; Yuan, J.; Cui, X.; et al. Ameliorating Effects of Combined Curcumin and Desferrioxamine on 6-OHDA-Induced Rat Mode of Parkinson’s Disease. Cell Biochem. Biophys. 2014, 70, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Hao, L.-J.; Yang, Z.-H.; Chai, R.; Zhang, S.; Gu, Y.; Gao, H.-L.; Zhong, M.-L.; Wang, T.; Li, J.-Y.; et al. Deferoxamine-Mediated up-Regulation of HIF-1α Prevents Dopaminergic Neuronal Death via the Activation of MAPK Family Proteins in MPTP-Treated Mice. Exp. Neurol. 2016, 280, 13–23. [Google Scholar] [CrossRef]
- Martin-Bastida, A.; Ward, R.J.; Newbould, R.; Piccini, P.; Sharp, D.; Kabba, C.; Patel, M.C.; Spino, M.; Connelly, J.; Tricta, F.; et al. Brain Iron Chelation by Deferiprone in a Phase 2 Randomised Double-Blinded Placebo Controlled Clinical Trial in Parkinson’s Disease. Sci. Rep. 2017, 7, 1398. [Google Scholar] [CrossRef]
- Fine, J.M.; Stroebel, B.M.; Faltesek, K.A.; Terai, K.; Haase, L.; Knutzen, K.E.; Kosyakovsky, J.; Bowe, T.J.; Fuller, A.K.; Frey, W.H.; et al. Intranasal Delivery of Low-Dose Insulin Ameliorates Motor Dysfunction and Dopaminergic Cell Death in a 6-OHDA Rat Model of Parkinson’s Disease. Neurosci. Lett. 2020, 714, 134567. [Google Scholar] [CrossRef]
- Zhao, Y.; Rempe, D.A. Prophylactic Neuroprotection against Stroke: Low-Dose, Prolonged Treatment with Deferoxamine or Deferasirox Establishes Prolonged Neuroprotection Independent of HIF-1 Function. J. Cereb. Blood Flow Metab. 2011, 31, 1412–1423. [Google Scholar] [CrossRef]
- Palmer, C.; Roberts, R.L.; Bero, C. Deferoxamine Posttreatment Reduces Ischemic Brain Injury in Neonatal Rats. Stroke 1994, 25, 1039–1045. [Google Scholar] [CrossRef]
- Bartolome, S.; Dhillon, N.K.; Buch, S.; Casillan, A.J.; Wood, J.G.; O’Brien-Ladner, A.R. Deferoxamine mimics the pattern of hypoxia-related injury at the microvasculature. Shock 2009, 31, 481–485. [Google Scholar] [CrossRef]
- Bergeron, M.; Gidday, J.M.; Yu, A.Y.; Semenza, G.L.; Ferriero, D.M.; Sharp, F.R. Role of Hypoxia-Inducible Factor-1 in Hypoxia-Induced Ischemic Tolerance in Neonatal Rat Brain. Ann. Neurol. 2000, 48, 285–296. [Google Scholar] [CrossRef]
- Mu, D.; Chang, Y.S.; Vexler, Z.S.; Ferriero, D.M. Hypoxia-Inducible Factor 1α and Erythropoietin Upregulation with Deferoxamine Salvage after Neonatal Stroke. Exp. Neurol. 2005, 195, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.; Foster, L.D.; Moy, C.S.; Xi, G.; Hill, M.D.; Morgenstern, L.B.; Greenberg, S.M.; James, M.L.; Singh, V.; Clark, W.M.; et al. Deferoxamine Mesylate in Patients with Intracerebral Haemorrhage (i-DEF): A Multicentre, Randomised, Placebo-Controlled, Double-Blind Phase 2 Trial. Lancet Neurol. 2019, 18, 428–438. [Google Scholar] [CrossRef]
- Yeatts, S.D.; Palesch, Y.Y.; Moy, C.S.; Selim, M. High Dose Deferoxamine in Intracerebral Hemorrhage (Hi-Def) Trial: Rationale, Design, and Methods. Neurocrit. Care 2013, 19, 257–266. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, W.; Zhu, C.; Kong, Z.; Xu, Y.; Liu, G.; Gao, X. The Clinical Effect of Deferoxamine Mesylate on Edema after Intracerebral Hemorrhage. PLoS ONE 2015, 10, e0122371. [Google Scholar] [CrossRef]
- de Lima, M.N.M.; Dias, C.P.; Torres, J.P.; Dornelles, A.; Garcia, V.A.; Scalco, F.S.; Guimarães, M.R.; Petry, R.C.; Bromberg, E.; Constantino, L.; et al. Reversion of Age-Related Recognition Memory Impairment by Iron Chelation in Rats. Neurobiol. Aging 2008, 29, 1052–1059. [Google Scholar] [CrossRef]
- Weigel, K.J.; Lynch, S.G.; LeVine, S.M. Iron Chelation and Multiple Sclerosis. ASN Neuro 2013, 6, AN20130037. [Google Scholar] [CrossRef]
- Lynch, S.; Fonseca, T.; LeVine, S. A Multiple Course Trial of Desferrioxamine in Chronic Progressive Multiple Sclerosis. Cell. Mol. Biol. 2000, 46, 865–869. [Google Scholar]
- Lynch, S.G.; Peters, K.; LeVine, S.M. Desferrioxamine in Chronic Progressive Multiple Sclerosis: A Pilot Study. Mult. Scler. J. 1996, 2, 157–160. [Google Scholar] [CrossRef]
- Delic, V.; Beck, K.D.; Pang, K.C.H.; Citron, B.A. Biological Links between Traumatic Brain Injury and Parkinson’s Disease. Acta Neuropathol. Commun. 2020, 8, 45. [Google Scholar] [CrossRef]
- Long, D.A.; Ghosh, K.; Moore, A.N.; Dixon, C.E.; Dash, P.K. Deferoxamine Improves Spatial Memory Performance Following Experimental Brain Injury in Rats. Brain Res. 1996, 717, 109–117. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, R.; Li, M.; Li, F.; Meng, H.; Zhu, G.; Lin, J.; Feng, H. Deferoxamine Attenuates Iron-Induced Long-Term Neurotoxicity in Rats with Traumatic Brain Injury. Neurol. Sci. 2013, 34, 639–645. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Z.; Xi, G.; Keep, R.F.; Hua, Y. Deferoxamine Attenuates Acute Hydrocephalus After Traumatic Brain Injury in Rats. Transl. Stroke Res. 2014, 5, 586–594. [Google Scholar] [CrossRef]
- Iadecola, C. The Pathobiology of Vascular Dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).