Ameliorative Effects of Loganin on Arthritis in Chondrocytes and Destabilization of the Medial Meniscus-Induced Animal Model
Abstract
1. Introduction
2. Results
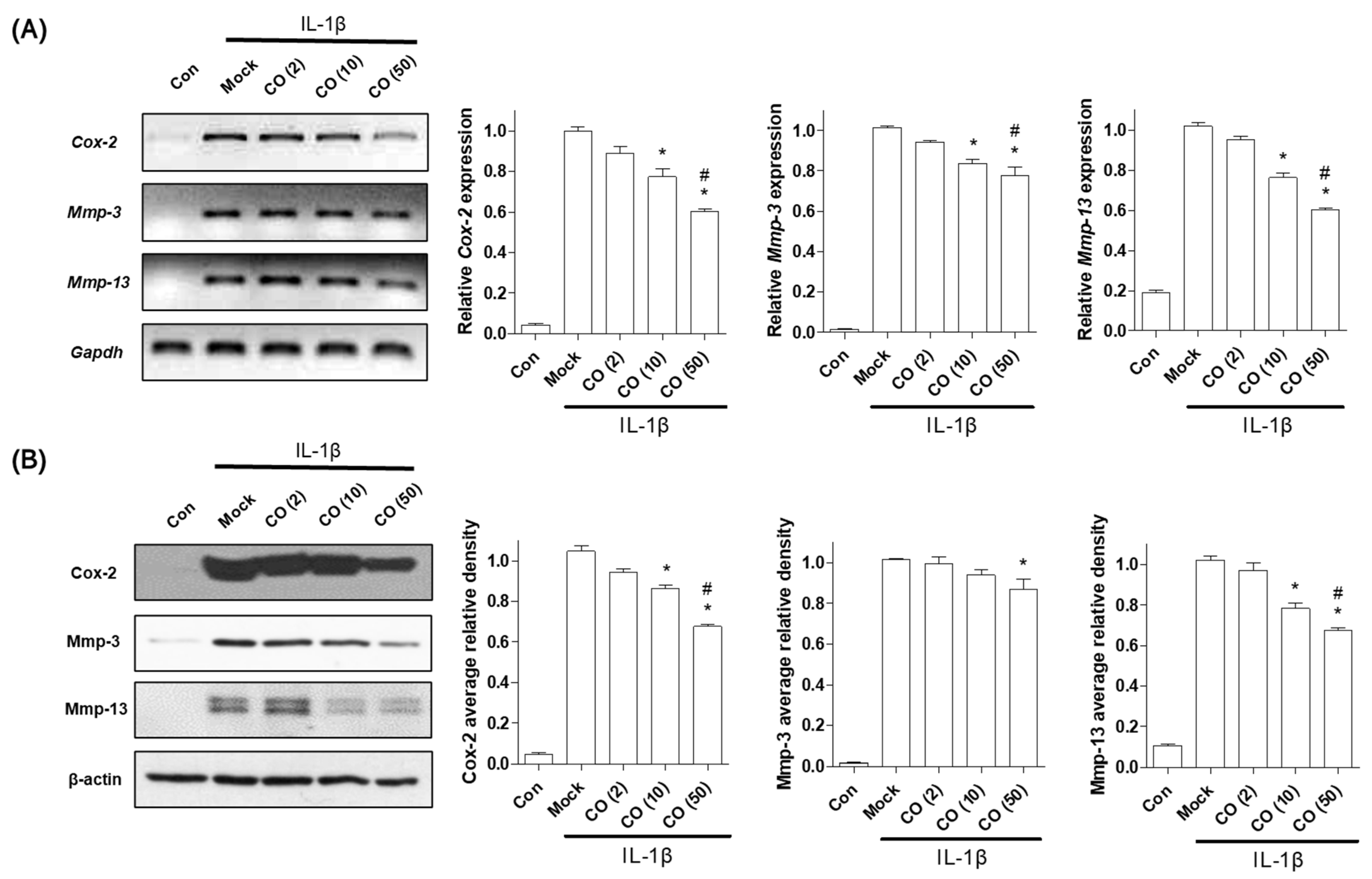
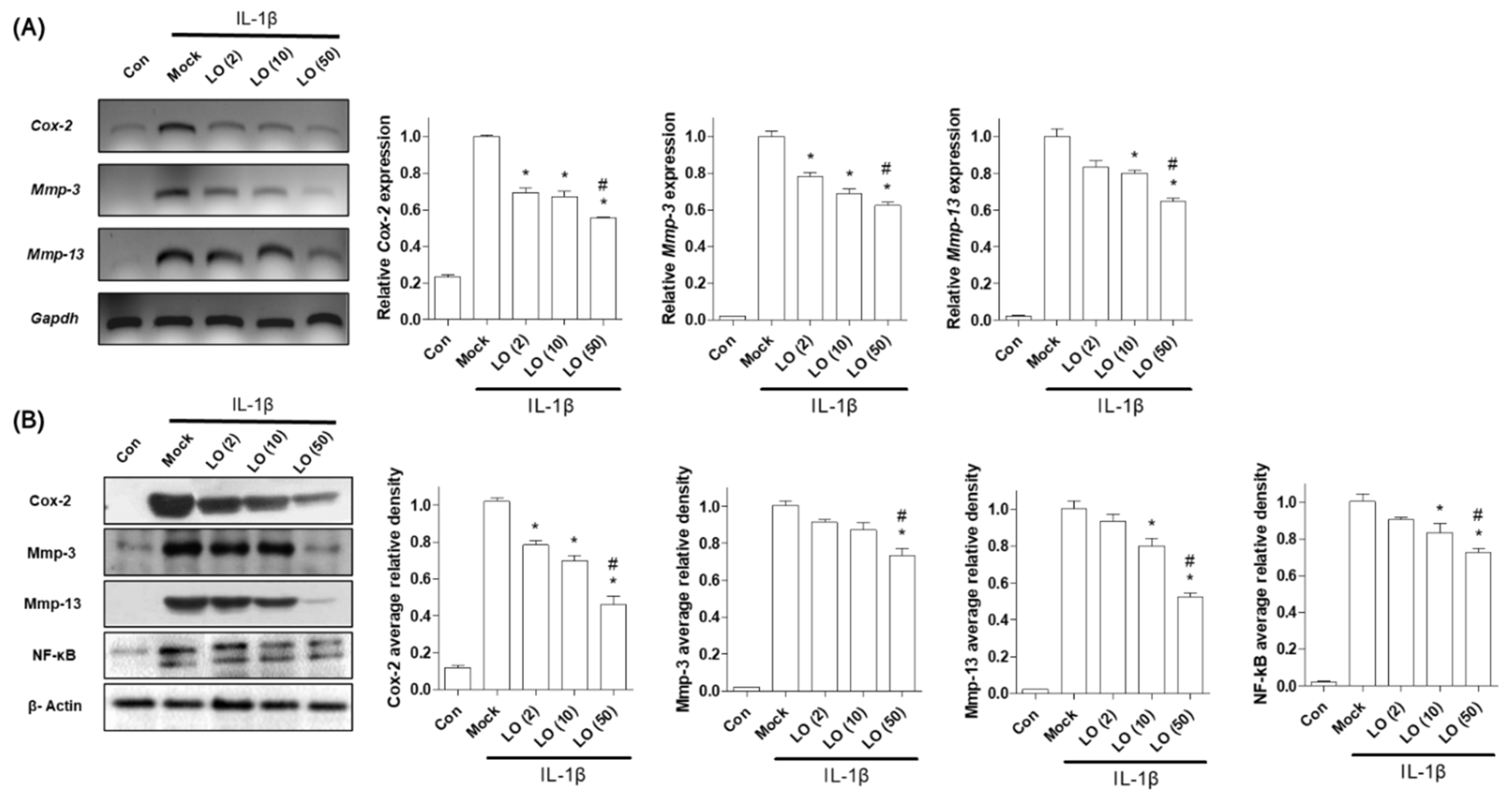
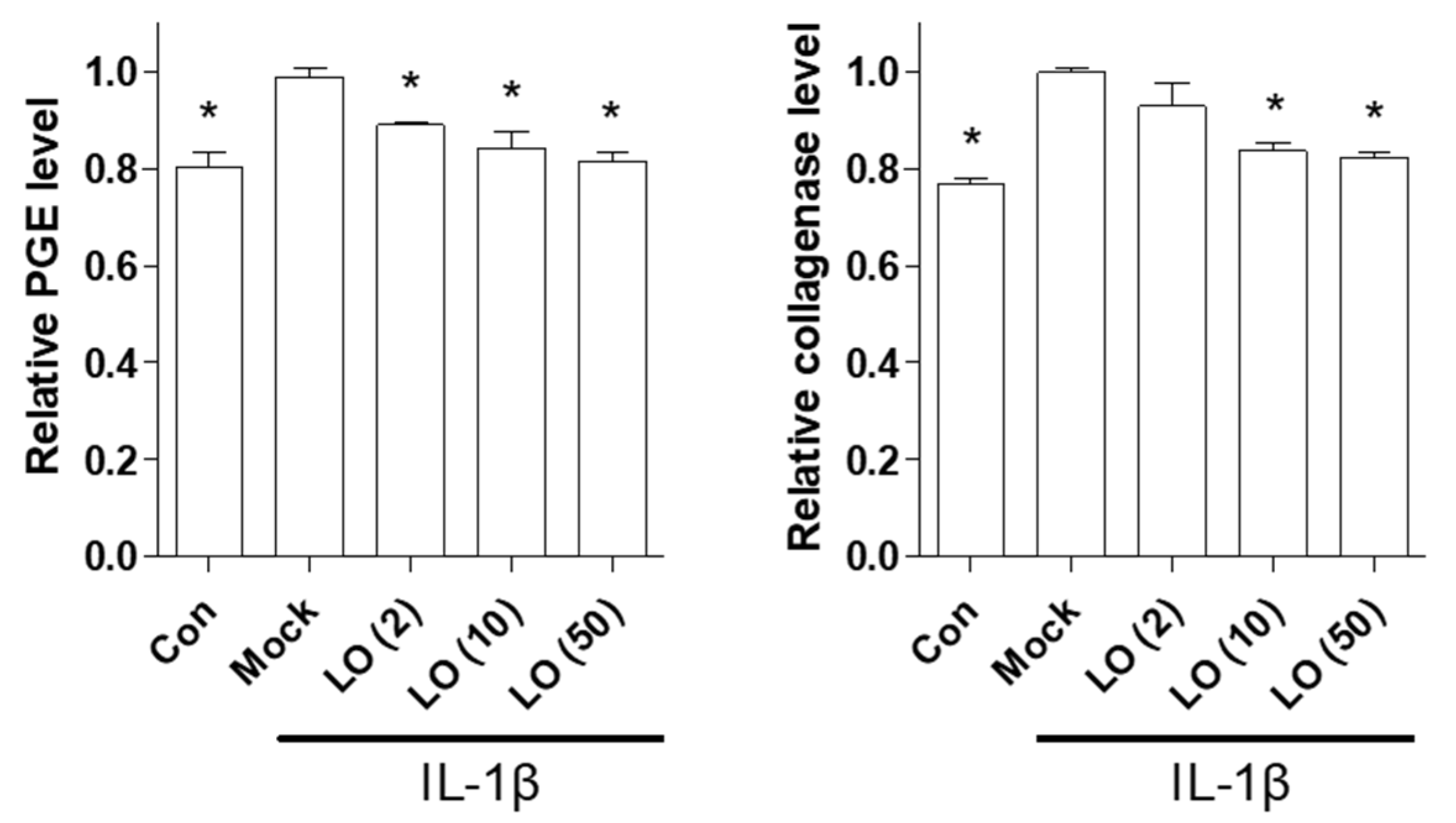
2.1. CO Extract and Loganin Inhibit IL-1β-Mediated Inflammation in Mouse Primary Chondrocytes
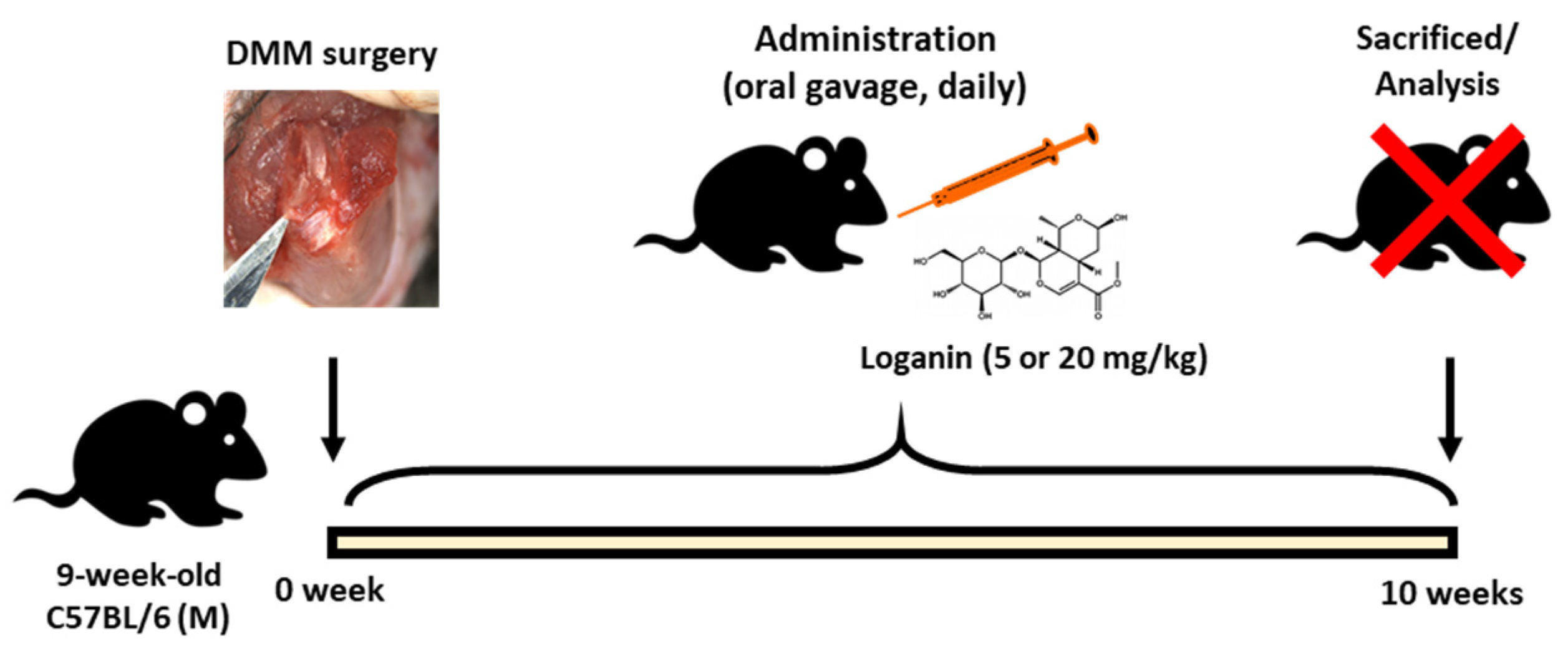
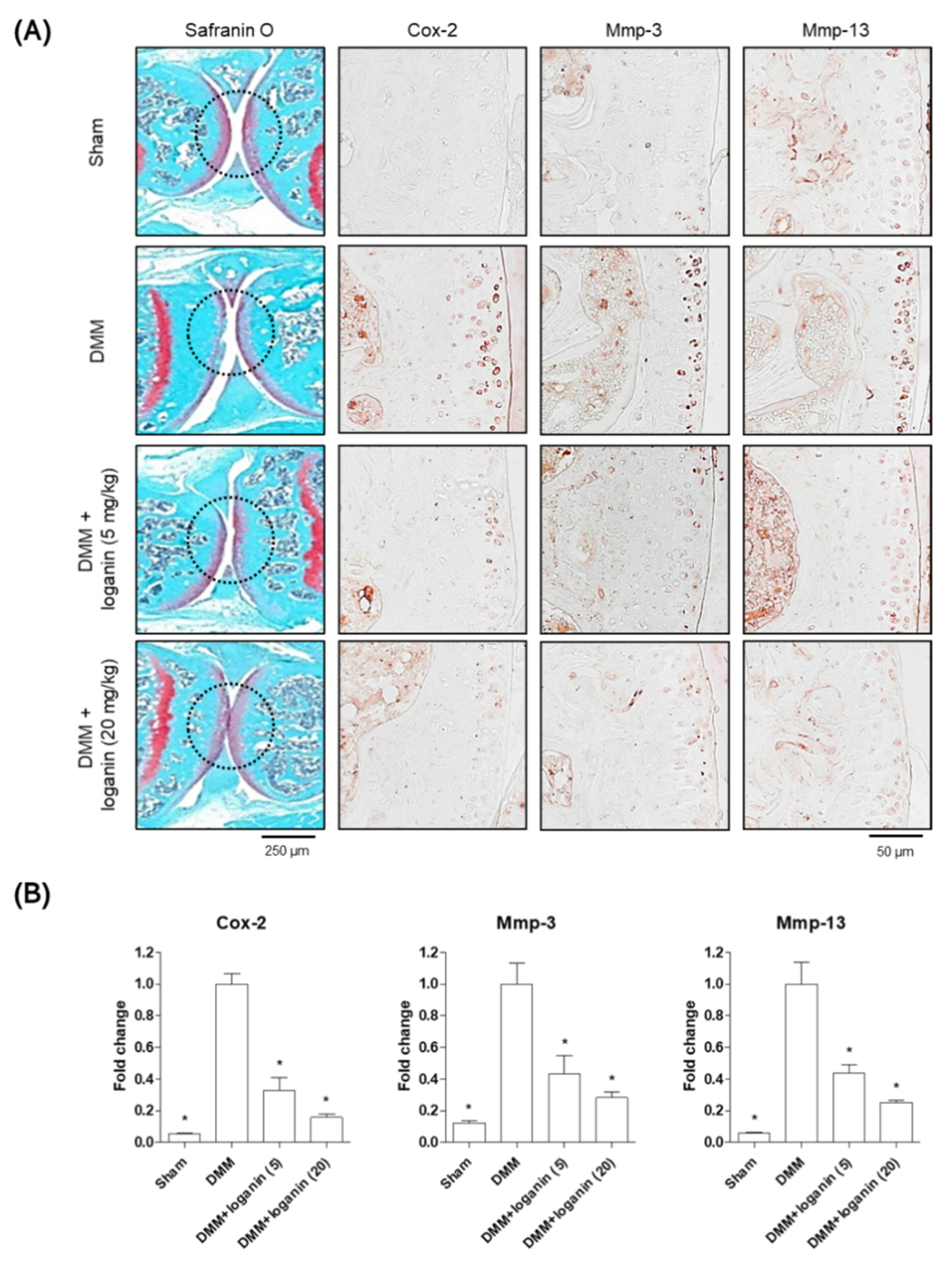
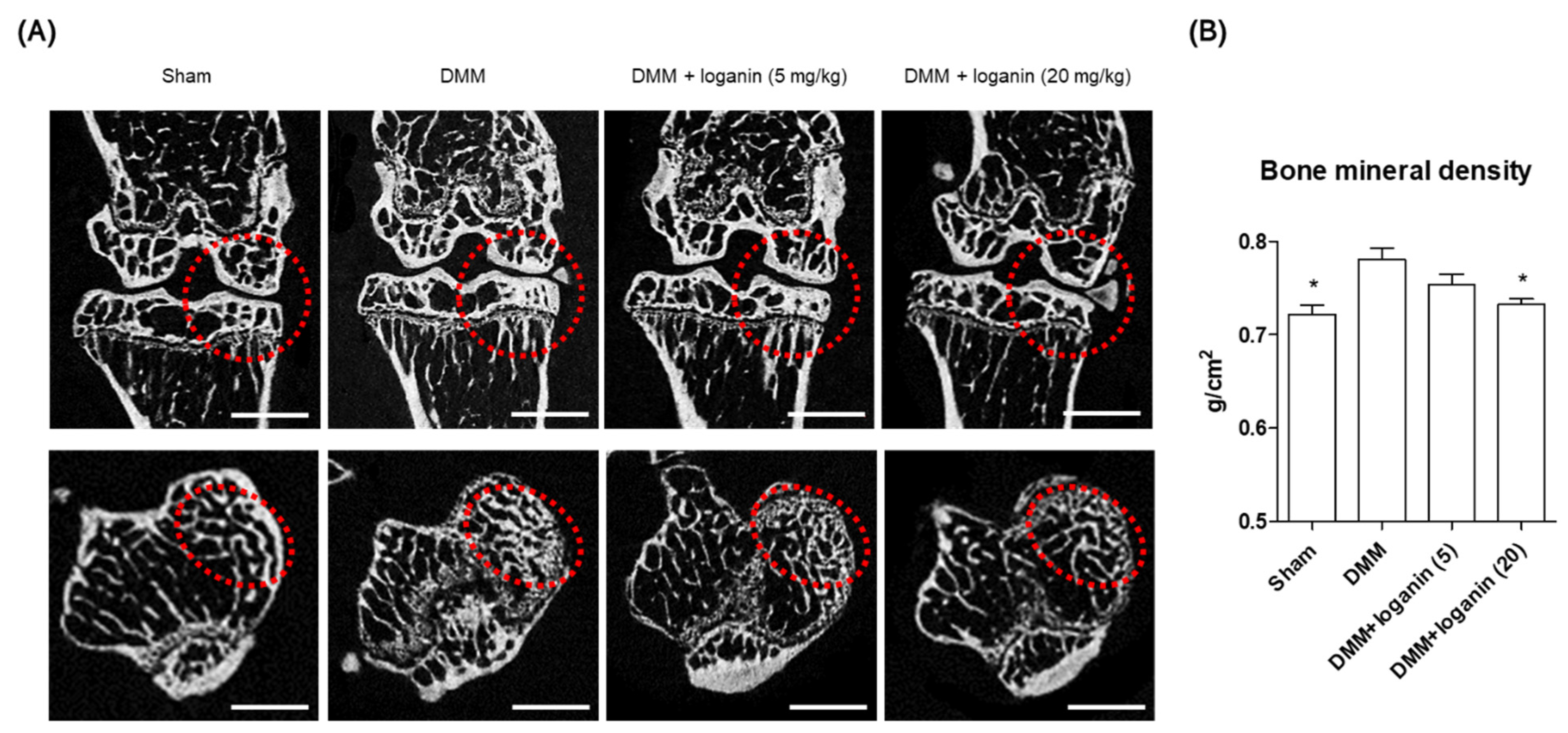
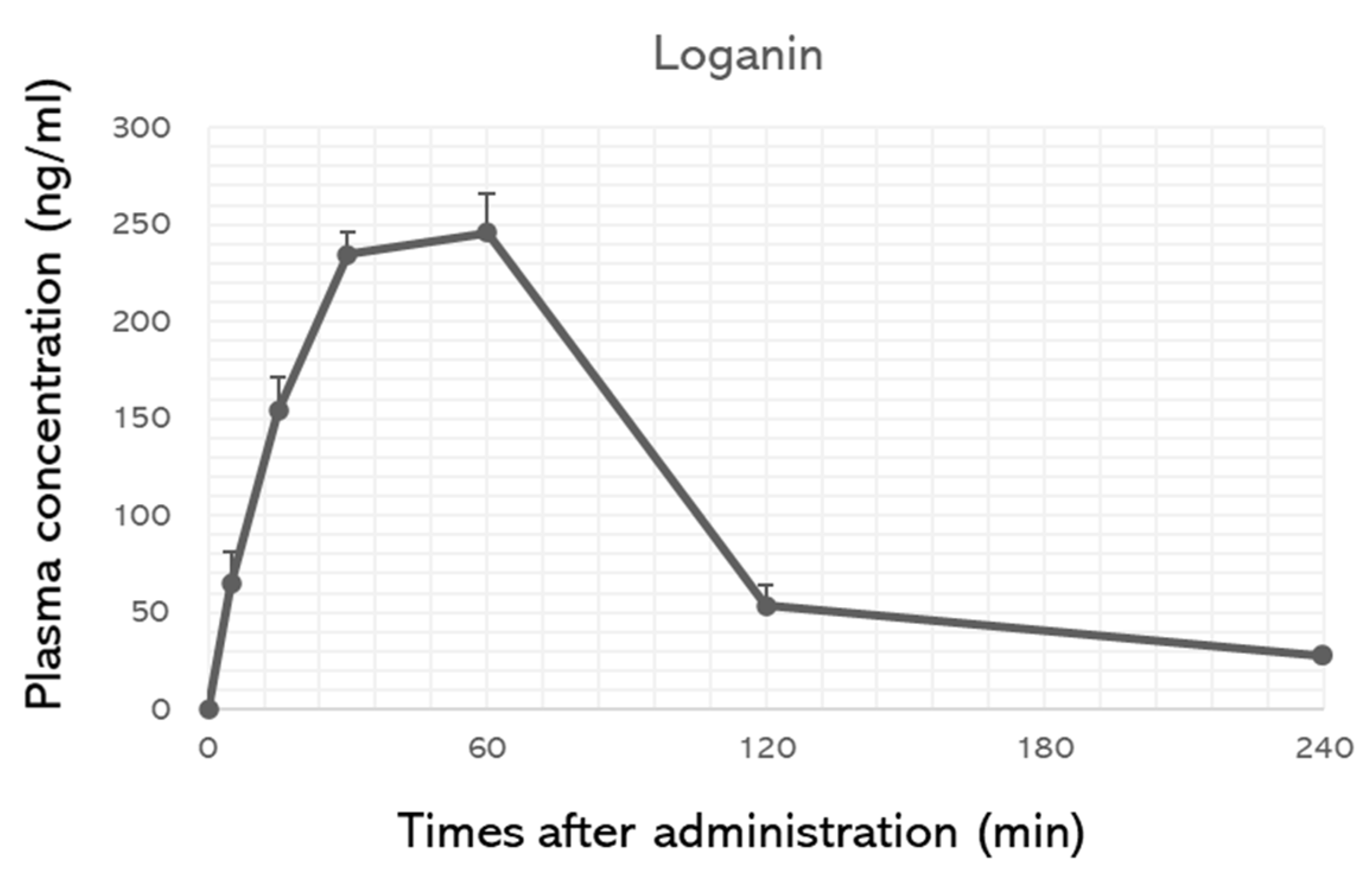
2.2. Oral Administration of Loganin Ameliorates DMM-Induced Arthritis In Vivo
3. Discussion
4. Materials and Methods
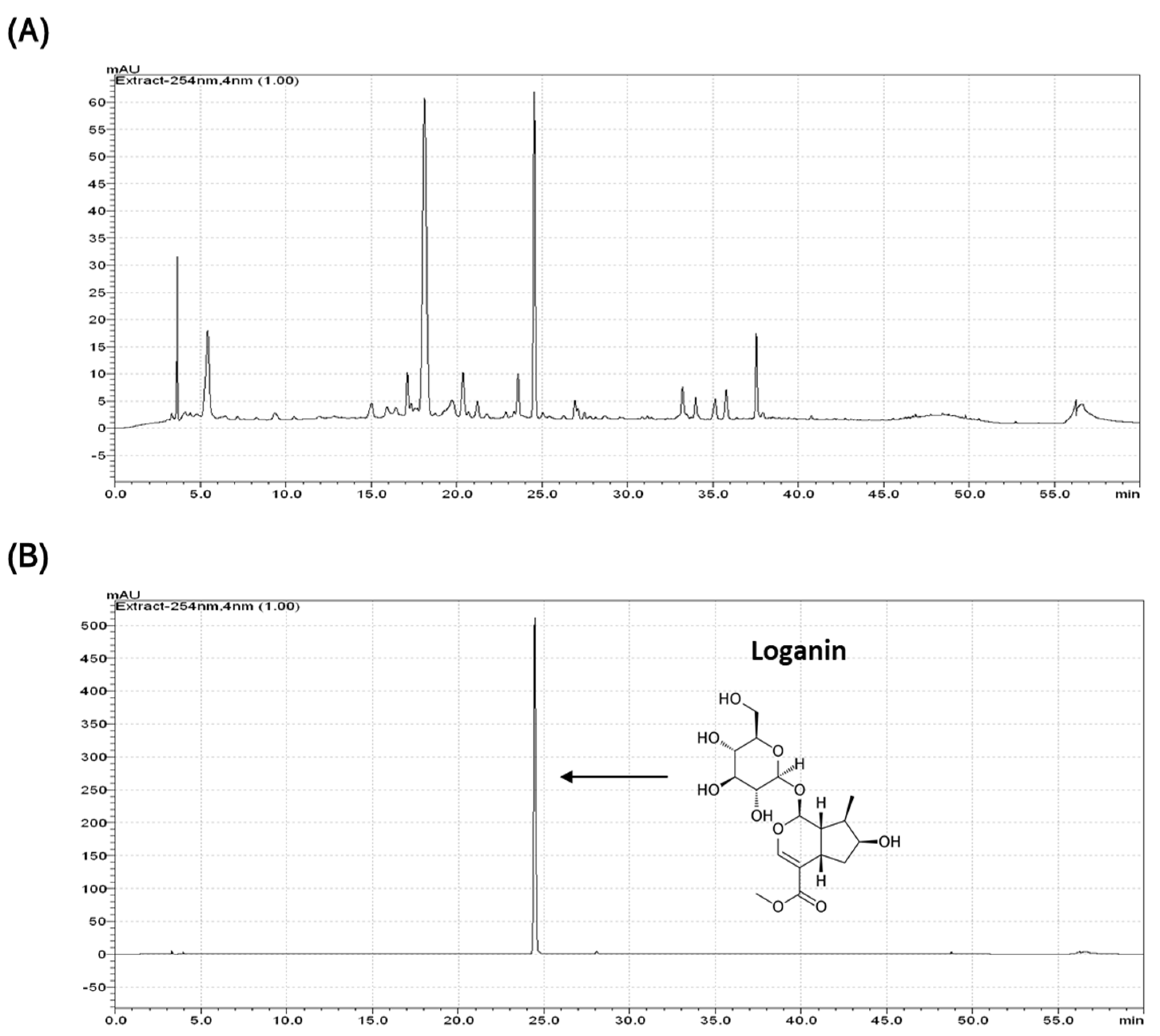
4.1. Extraction and Identification of Loganin from CO Extract
4.2. Mouse Primary Chondrocyte Experiment and Cell Supernatant Analysis
4.3. Western Blot Analysis and Immunohistochemistry
4.4. Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR) and Quantitative RT-PCR
4.5. Animal Studies
4.6. Micro-CT Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mandl, L.A. Osteoarthritis year in review 2018: Clinical. Osteoarthr. Cartil. 2019, 27, 359–364. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Casper-Taylor, M.E.; Barr, A.J.; Williams, S.; Wilcox, R.K.; Conaghan, P.G. Initiating factors for the onset of OA: A systematic review of animal bone and cartilage pathology in OA. J. Orthop. Res. 2020, 38, 1810–1818. [Google Scholar] [CrossRef]
- Aida, Y.; Maeno, M.; Suzuki, N.; Shiratsuchi, H.; Motohashi, M.; Matsumura, H. The effect of IL-1β on the expression of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human chondrocytes. Life Sci. 2005, 77, 3210–3221. [Google Scholar] [CrossRef] [PubMed]
- Philipot, D.; Guerit, D.; Platano, D.; Chuchana, P.; Olivotto, E.; Espinoza, F.; Dorandeu, A.; Pers, Y.M.; Piette, J.; Borzi, R.M.; et al. p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis. Arthritis Res. 2014, 16, R58. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Khan, N.M.; Ashruf, O.S.; Haqqi, T.M. Inhibition of cartilage degradation and suppression of PGE2 and MMPs expression by pomegranate fruit extract in a model of posttraumatic osteoarthritis. Nutrition 2017, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Carpintero-Fernandez, P.; Varela-Eirin, M.; Lacetera, A.; Gago-Fuentes, R.; Fonseca, E.; Martin-Santamaria, S.; Mayan, M.D. New Therapeutic Strategies for Osteoarthritis by Targeting Sialic Acid Receptors. Biomolecules 2020, 10, 637. [Google Scholar] [CrossRef]
- Cho, H.; Walker, A.; Williams, J.; Hasty, K.A. Study of osteoarthritis treatment with anti-inflammatory drugs: Cyclooxygenase-2 inhibitor and steroids. Biomed. Res. Int. 2015, 2015, 595273. [Google Scholar] [CrossRef]
- Flood, J. The role of acetaminophen in the treatment of osteoarthritis. Am. J. Manag. Care. 2010, 16, S48–S54. [Google Scholar]
- Osani, M.C.; Bannuru, R.R. Efficacy and safety of duloxetine in osteoarthritis: A systematic review and meta-analysis. Korean J. Intern. Med. 2019, 34, 966–973. [Google Scholar] [CrossRef]
- Steinmeyer, J.; Bock, F.; Stove, J.; Jerosch, J.; Flechtenmacher, J. Pharmacological treatment of knee osteoarthritis: Special considerations of the new German guideline. Orthop. Rev. 2018, 10, 7782. [Google Scholar] [CrossRef]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer Plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’ Amico, R.; Cordaro, M.; Crupi, R.; Mandalari, G.; Impellizzeri, D.; et al. The role of Cashew (Anacardium occidentale L.) nuts on an experimental model of painful degenerative joint disease. Antioxidants 2020, 9, 511. [Google Scholar] [CrossRef]
- Moon, S.M.; Lee, S.A.; Han, S.H.; Park, B.R.; Choi, M.S.; Kim, J.S.; Kim, S.G.; Kim, H.J.; Chun, H.S.; Kim, D.K.; et al. Aqueous extract of Codium fragile alleviates osteoarthritis through the MAPK/NF-κB pathways in IL-1β-induced rat primary chondrocytes and a rat osteoarthritis model. Biomed. Pharm. 2018, 97, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Mobasheri, A. Natural products for promoting joint health and managing osteoarthritis. Curr. Rheumatol. Rep. 2018, 20, 72. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Czerwinska, M.E.; Melzig, M.F. Cornus mas and Cornus officinalis—Analogies and differences of two medicinal plants traditionally used. Front. Pharm. 2018, 9, 894. [Google Scholar] [CrossRef]
- Han, Y.; Jung, H.W.; Park, Y.K. Selective therapeutic effect of Cornus officinalis fruits on the damage of different organs in STZ-induced diabetic rats. Am. J. Chin. Med. 2014, 42, 1169–1182. [Google Scholar] [CrossRef]
- Sohn, D.W.; Kim, H.Y.; Kim, S.D.; Lee, E.J.; Kim, H.S.; Kim, J.K.; Hwang, S.Y.; Cho, Y.H.; Kim, S.W. Elevation of intracavernous pressure and NO-cGMP activity by a new herbal formula in penile tissues of spontaneous hypertensive male rats. J. Ethnopharmacol. 2008, 120, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.J.; Feng, J.F.; Lu, X.M.; Lv, M.P.; Cao, S.S.; Li, R.F.; Li, Y.; Li, X.M. Effect of Cornus officinalis fruit core extract on the cardiac hypertrophy induced by two kidney two clip. Zhong Yao Cai 2012, 35, 1985–1989. [Google Scholar]
- Hwang, K.A.; Hwang, Y.J.; Song, J. Antioxidant activities and oxidative stress inhibitory effects of ethanol extracts from Cornus officinalis on raw 264.7 cells. BMC Complement. Altern. Med. 2016, 16, 196. [Google Scholar] [CrossRef]
- Ma, W.; Wang, K.-J.; Cheng, C.-S.; Yan, G.-q.; Lu, W.-L.; Ge, J.-F.; Cheng, Y.-X.; Li, N. Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J. Ethnopharmacol. 2014, 153, 840–845. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Chu, L.W.; Chen, J.Y.; Hsieh, S.L.; Chang, Y.C.; Dai, Z.K.; Wu, B.N. Loganin attenuates high glucose-induced schwann cells pyroptosis by inhibiting ROS generation and NLRP3 inflammasome activation. Cells 2020, 9, 1948. [Google Scholar] [CrossRef]
- Quah, Y.; Lee, S.J.; Lee, E.B.; Birhanu, B.T.; Ali, M.S.; Abbas, M.A.; Boby, N.; Im, Z.E.; Park, S.C. Cornus officinalis ethanolic extract with potential anti-allergic, anti-inflammatory, and antioxidant activities. Nutrients 2020, 12, 3317. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, Y.; Zhao, D.; Feng, X.; Zhang, L.; Liu, C. Loganin prevents BV-2 microglia cells from Aβ1-42 -induced inflammation via regulating TLR4/TRAF6/NF-κB axis. Cell Biol. Int. 2018, 42, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Pan, Y.B.; Cao, Y.Q.; Wang, C.; Jiang, W.D.; Zhai, W.F.; Lu, J.G. Loganin alleviates LPS-activated intestinal epithelial inflammation by regulating TLR4/NF-κB and JAK/STAT3 signaling pathways. Kaohsiung J. Med. Sci. 2020, 36, 257–264. [Google Scholar] [CrossRef]
- Jung, Y.-A.; Jung, Y.-S.; Hwang, G.-S. 1 H NMR-based metabolomic study of Cornus officinalis from different geographical origin. J. Kor. Magn. Reson. Soc. 2011, 15, 90–103. [Google Scholar] [CrossRef]
- Adotey, J.P.; Adukpo, G.E.; Opoku Boahen, Y.; Armah, F.A. A review of the ethnobotany and pharmacological importance of Alstonia boonei De Wild (Apocynaceae). ISRN Pharm. 2012, 2012, 587160. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Mo, E.J.; Jo, Y.H.; Kim, S.B.; Hwang, B.Y.; Lee, M.K. Variation of loganin content in Cornus officinalis fruits at different extraction conditions and maturation stages. Biosci. Biotechnol. Biochem. 2017, 81, 1973–1977. [Google Scholar] [CrossRef]
- Shin, S.A.; Joo, B.J.; Lee, J.S.; Ryu, G.; Han, M.; Kim, W.Y.; Park, H.H.; Lee, J.H.; Lee, C.S. Phytochemicals as anti-inflammatory agents in animal models of prevalent inflammatory diseases. Molecules 2020, 25, 5932. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.S.; Rattner, J.B.; Matyas, J.R. Communication between paired chondrocytes in the superficial zone of articular cartilage. J. Anat. 2004, 205, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Skioldebrand, E.; Thorfve, A.; Bjorklund, U.; Johansson, P.; Wickelgren, R.; Lindahl, A.; Hansson, E. Biochemical alterations in inflammatory reactive chondrocytes: Evidence for intercellular network communication. Heliyon 2018, 4, e00525. [Google Scholar] [CrossRef] [PubMed]
- Houard, X.; Goldring, M.B.; Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 2013, 15, 375. [Google Scholar] [CrossRef]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB signaling pathways in osteoarthritic cartilage destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1β signaling in osteoarthritis—Chondrocytes in focus. Cell. Signal. 2019, 53, 212–223. [Google Scholar] [CrossRef]
- Watanabe, Y.; Namba, A.; Honda, K.; Aida, Y.; Matsumura, H.; Shimizu, O.; Suzuki, N.; Tanabe, N.; Maeno, M. IL-1β stimulates the expression of prostaglandin receptor EP4 in human chondrocytes by increasing production of prostaglandin E2. Connect. Tissue Res. 2009, 50, 186–193. [Google Scholar] [CrossRef]
- Thysen, S.; Luyten, F.P.; Lories, R.J. Targets, models and challenges in osteoarthritis research. Dis. Model. Mech. 2015, 8, 17–30. [Google Scholar] [CrossRef]
- Oike, J.; Okumo, T.; Ikemoto, H.; Kunieda, Y.; Nakai, S.; Takemura, H.; Takagi, H.; Kanzaki, K.; Sunagawa, M. Preventive effect of the Japanese traditional herbal medicine Boiogito on posttraumatic osteoarthritis in rats. Medicines 2020, 7, 74. [Google Scholar] [CrossRef]
- Fang, H.; Huang, L.; Welch, I.; Norley, C.; Holdsworth, D.W.; Beier, F.; Cai, D. Early changes of articular cartilage and subchondral bone in the DMM mouse model of osteoarthritis. Sci. Rep. 2018, 8, 2855. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, J.; Wu, J.; Chen, Q.; Du, W.; Fu, F.; Yu, H.; Yao, S.; Jin, H.; Tong, P.; et al. Loganin ameliorates cartilage degeneration and osteoarthritis development in an osteoarthritis mouse model through inhibition of NF-κB activity and pyroptosis in chondrocytes. J. Ethnopharmacol. 2020, 247, 112261. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, T.P.; Lin, W.L.; Tsai, T.H. Pharmacokinetic interactions of herbal medicines for the treatment of chronic hepatitis. J. Food Drug Anal. 2017, 25, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Kang, L.J.; Jang, D.; Jeon, J.; Lee, H.; Choi, S.; Han, S.J.; Oh, E.; Nam, J.; Kim, C.S.; et al. Cirsium japonicum var. maackii and apigenin block Hif-2α-induced osteoarthritic cartilage destruction. J. Cell. Mol. Med. 2019, 23, 5369–5379. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.; Lee, C.G.; Yun, S.H.; Hwang, S.; Jeon, H.; Kim, J.; Yeo, S.; Jeong, H.; Yun, S.-H.; Jeong, S.-Y. Ameliorative Effects of Loganin on Arthritis in Chondrocytes and Destabilization of the Medial Meniscus-Induced Animal Model. Pharmaceuticals 2021, 14, 135. https://doi.org/10.3390/ph14020135
Park E, Lee CG, Yun SH, Hwang S, Jeon H, Kim J, Yeo S, Jeong H, Yun S-H, Jeong S-Y. Ameliorative Effects of Loganin on Arthritis in Chondrocytes and Destabilization of the Medial Meniscus-Induced Animal Model. Pharmaceuticals. 2021; 14(2):135. https://doi.org/10.3390/ph14020135
Chicago/Turabian StylePark, Eunkuk, Chang Gun Lee, Seung Hee Yun, Seokjin Hwang, Hyoju Jeon, Jeonghyun Kim, Subin Yeo, Hyesoo Jeong, Seong-Hoon Yun, and Seon-Yong Jeong. 2021. "Ameliorative Effects of Loganin on Arthritis in Chondrocytes and Destabilization of the Medial Meniscus-Induced Animal Model" Pharmaceuticals 14, no. 2: 135. https://doi.org/10.3390/ph14020135
APA StylePark, E., Lee, C. G., Yun, S. H., Hwang, S., Jeon, H., Kim, J., Yeo, S., Jeong, H., Yun, S.-H., & Jeong, S.-Y. (2021). Ameliorative Effects of Loganin on Arthritis in Chondrocytes and Destabilization of the Medial Meniscus-Induced Animal Model. Pharmaceuticals, 14(2), 135. https://doi.org/10.3390/ph14020135







