Potential of Fatty Acid Amide Hydrolase (FAAH), Monoacylglycerol Lipase (MAGL), and Diacylglycerol Lipase (DAGL) Enzymes as Targets for Obesity Treatment: A Narrative Review
Abstract
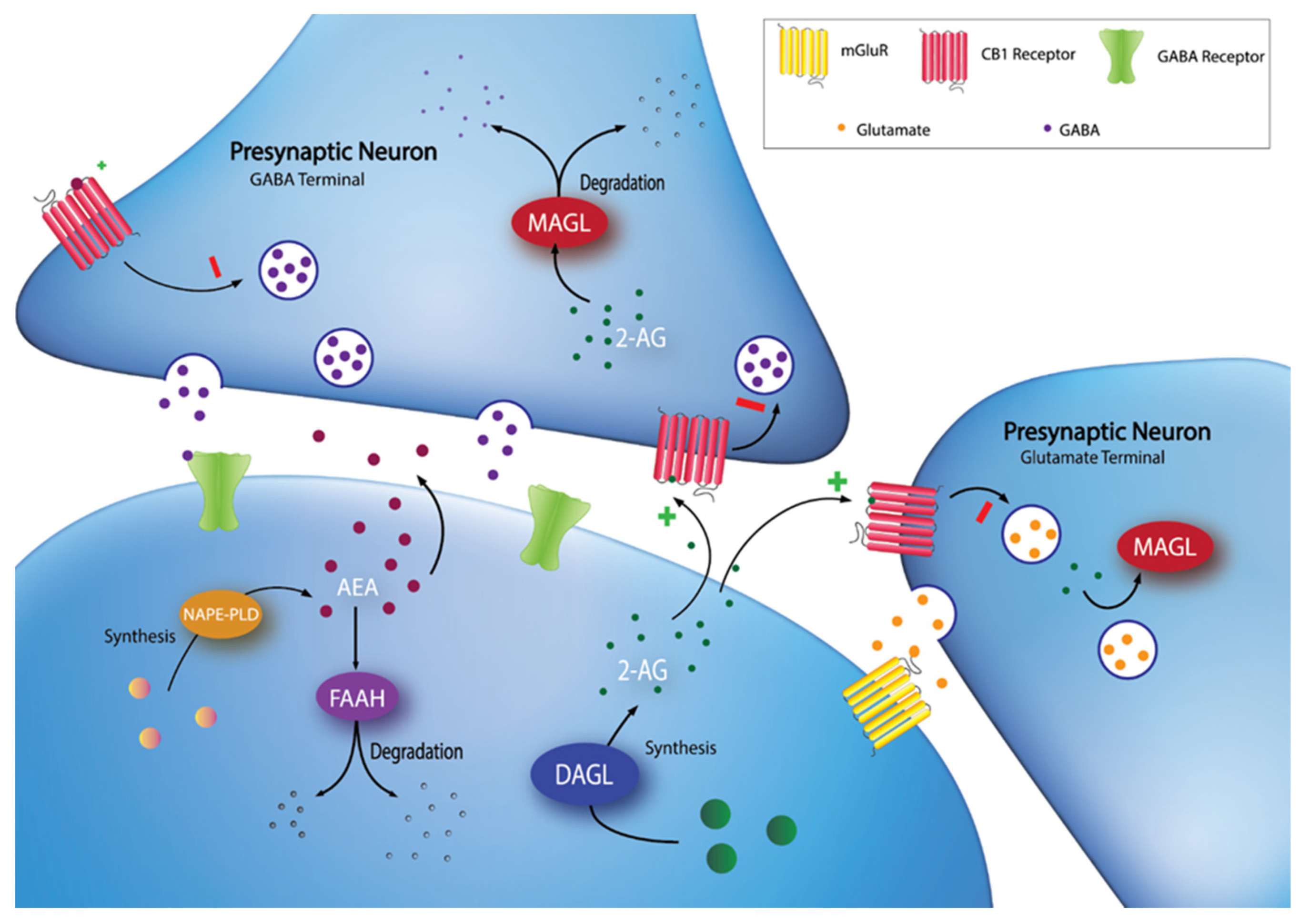
:1. Introduction
2. Search Strategy
3. Current Evidence for a Role of Modulating FAAH, MAGL, and DAGL Activity in Obesity-Related Outcomes
3.1. Animal Studies Involving Pharmacological Manipulation
3.2. Animal Studies Involving Genetic Manipulation
3.3. Human Studies Involving Genetic Association
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef] [Green Version]
- NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128,9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [Green Version]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Spieker, E.A.; Pyzocha, N. Economic Impact of Obesity. Prim. Care 2016, 43, 83–95. [Google Scholar] [CrossRef]
- Richey, J.M.; Woolcott, O. Re-visiting the Endocannabinoid System and Its Therapeutic Potential in Obesity and Associated Diseases. Curr. Diab. Rep. 2017, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [Green Version]
- Pamplona, F.A.; Takahashi, R.N. Psychopharmacology of the endocannabinoids: Far beyond anandamide. J. Psychopharmacol. 2012, 26, 7–22. [Google Scholar] [CrossRef]
- Gregus, A.M.; Buczynski, M.W. Druggable Targets in Endocannabinoid Signaling. Adv. Exp. Med. Biol. 2020, 1274, 177–201. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Hryhorowicz, S.; Rychter, A.M.; Zawada, A.; Słomski, R.; Dobrowolska, A.; Krela-Kaźmierczak, I. What Role Does the Endocannabinoid System Play in the Pathogenesis of Obesity? Nutrients 2021, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, M.; Lotfi Yaghin, N.; Alizadeh, M. Blood Level of 2-arachidonoyl glycerol (2-AG), Neuropeptide Y and Omentin and Their Correlation with Food Habits in Obese Women. Galen. Med. J. 2020, 9, e1721. [Google Scholar] [CrossRef]
- Côté, M.; Matias, I.; Lemieux, I.; Petrosino, S.; Alméras, N.; Després, J.P.; Di Marzo, V. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int. J. Obes. 2007, 31, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Matias, I.; Gatta-Cherifi, B.; Tabarin, A.; Clark, S.; Leste-Lasserre, T.; Marsicano, G.; Piazza, P.V.; Cota, D. Endocannabinoids measurement in human saliva as potential biomarker of obesity. PLoS ONE 2012, 7, e42399. [Google Scholar] [CrossRef] [Green Version]
- Yagin, N.L.; Hajjarzadeh, S.; Aliasgharzadeh, S.; Aliasgari, F.; Mahdavi, R. The association of dietary patterns with endocannabinoids levels in overweight and obese women. Lipids Health Dis. 2020, 19, 161. [Google Scholar] [CrossRef]
- Azar, S.; Sherf-Dagan, S.; Nemirovski, A.; Webb, M.; Raziel, A.; Keidar, A.; Goitein, D.; Sakran, N.; Shibolet, O.; Tam, J.; et al. Circulating Endocannabinoids Are Reduced Following Bariatric Surgery and Associated with Improved Metabolic Homeostasis in Humans. Obes. Surg. 2019, 29, 268–276. [Google Scholar] [CrossRef]
- Murphy, T.; Le Foll, B. Targeting the Endocannabinoid CB1 Receptor to Treat Body Weight Disorders: A Preclinical and Clinical Review of the Therapeutic Potential of Past and Present CB1 Drugs. Biomolecules 2020, 10, 855. [Google Scholar] [CrossRef]
- Christopoulou, F.D.; Kiortsis, D.N. An overview of the metabolic effects of rimonabant in randomized controlled trials: Potential for other cannabinoid 1 receptor blockers in obesity. J. Clin. Pharm. Ther. 2011, 36, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.E.; Mocelin, C.A.; Petersen, G.O.; Leal, M.B.; Thiesen, F.V. Rimonabant: An antagonist drug of the endocannabinoid system for the treatment of obesity. Pharmacol. Rep. 2009, 61, 217–224. [Google Scholar] [CrossRef]
- Christensen, R.; Kristensen, P.K.; Bartels, E.M.; Bliddal, H.; Astrup, A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomised trials. Lancet 2007, 370, 1706–1713. [Google Scholar] [CrossRef]
- Moreira, F.A.; Crippa, J.A. The psychiatric side-effects of rimonabant. Braz. J. Psychiatry 2009, 31, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Le Foll, B.; Gorelick, D.A.; Goldberg, S.R. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology 2009, 205, 171–174. [Google Scholar] [CrossRef] [Green Version]
- Toczek, M.; Malinowska, B. Enhanced endocannabinoid tone as a potential target of pharmacotherapy. Life Sci. 2018, 204, 20–45. [Google Scholar] [CrossRef] [PubMed]
- Laleh, P.; Yaser, K.; Alireza, O. Oleoylethanolamide: A novel pharmaceutical agent in the management of obesity-an updated review. J. Cell Physiol. 2019, 234, 7893–7902. [Google Scholar] [CrossRef]
- Maccarrone, M.; Rossi, S.; Bari, M.; De Chiara, V.; Fezza, F.; Musella, A.; Gasperi, V.; Prosperetti, C.; Bernardi, G.; Finazzi-Agrò, A.; et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat. Neurosci. 2008, 11, 152–159. [Google Scholar] [CrossRef]
- Behl, T.; Chadha, S.; Sachdeva, M.; Sehgal, A.; Kumar, A.; Venkatachalam, T.; Hafeez, A.; Aleya, L.; Arora, S.; Batiha, G.E.S.; et al. Understanding the possible role of endocannabinoid system in obesity. Prostaglandins Other Lipid Mediat. 2021, 152, 106520. [Google Scholar] [CrossRef] [PubMed]
- de Moura, E.D.M.; Dos Reis, S.A.; da Conceição, L.L.; Sediyama, C.; Pereira, S.S.; de Oliveira, L.L.; Gouveia Pelúzio, M.D.C.; Martinez, J.A.; Milagro, F.I. Diet-induced obesity in animal models: Points to consider and influence on metabolic markers. Diabetol. Metab. Syndr. 2021, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Balsevich, G.; Sticht, M.; Bowles, N.P.; Singh, A.; Lee, T.T.Y.; Li, Z.; Chelikani, P.K.; Lee, F.S.; Borgland, S.L.; Hillard, C.J.; et al. Role for fatty acid amide hydrolase (FAAH) in the leptin-mediated effects on feeding and energy balance. Proc. Natl. Acad. Sci. USA 2018, 115, 7605–7610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cifani, C.; Avagliano, C.; Micioni Di Bonaventura, E.; Giusepponi, M.E.; De Caro, C.; Cristiano, C.; La Rana, G.; Botticelli, L.; Romano, A.; Calignano, A.; et al. Modulation of Pain Sensitivity by Chronic Consumption of Highly Palatable Food Followed by Abstinence: Emerging Role of Fatty Acid Amide Hydrolase. Front. Pharmacol. 2020, 11, 266. [Google Scholar] [CrossRef]
- Liu, J.; Cinar, R.; Xiong, K.; Godlewski, G.; Jourdan, T.; Lin, Y.; Ntambi, J.M.; Kunos, G. Monounsaturated fatty acids generated via stearoyl CoA desaturase-1 are endogenous inhibitors of fatty acid amide hydrolase. Proc. Natl. Acad. Sci. USA 2013, 110, 18832–18837. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, T.; Mahadevan, A.; Coccurello, R.; Chang, J.W.; Allarà, M.; Chen, Y.; Giacovazzo, G.; Lichtman, A.; Cravatt, B.; Moles, A.; et al. A novel fluorophosphonate inhibitor of the biosynthesis of the endocannabinoid 2-arachidonoylglycerol with potential anti-obesity effects. Br. J. Pharmacol. 2013, 169, 784–793. [Google Scholar] [CrossRef] [Green Version]
- Palma-Chavez, A.; Konar-Nié, M.; Órdenes, P.; Maurelia, F.; Elizondo-Vega, R.; Oyarce, K.; López, S.; Rojas, J.; Steinberg, X.; García-Robles, M.A.; et al. Glucose Increase DAGLα Levels in Tanycytes and Its Inhibition Alters Orexigenic and Anorexigenic Neuropeptides Expression in Response to Glucose. Front. Endocrinol. 2019, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Touriño, C.; Oveisi, F.; Lockney, J.; Piomelli, D.; Maldonado, R. FAAH deficiency promotes energy storage and enhances the motivation for food. Int. J. Obes. 2010, 34, 557–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaitheesvaran, B.; Yang, L.; Hartil, K.; Glaser, S.; Yazulla, S.; Bruce, J.E.; Kurland, I.J. Peripheral Effects of FAAH Deficiency on Fuel and Energy Homeostasis: Role of Dysregulated Lysine Acetylation. PLoS ONE 2012, 7, e33717. [Google Scholar] [CrossRef] [Green Version]
- Brown, W.H.; Gillum, M.P.; Lee, H.-Y.; Camporez, J.P.G.; Zhang, X.-M.; Jeong, J.K. Fatty acid amide hydrolase ablation promotes ectopic lipid storage and insulin resistance due to centrally mediated hypothyroidism. Proc. Natl. Acad. Sci. USA 2012, 109, 14966–14971. [Google Scholar] [CrossRef] [Green Version]
- Grevengoed, T.J.; Trammell, S.A.J.; McKinney, M.K.; Petersen, N.; Cardone, R.L.; Svenningsen, J.S.; Ogasawara, D.; Nexøe-Larsen, C.C.; Knop, F.K.; Schwartz, T.W.; et al. N-acyl taurines are endogenous lipid messengers that improve glucose homeostasis. Proc. Natl. Acad. Sci. USA 2019, 116, 24770–24778. [Google Scholar] [CrossRef]
- Jung, K.-M.; Clapper, J.R.; Fu, J.; D’Agostino, G.; Guijarro, A.; Thongkham, D.; Avanesian, A.; Astarita, G.; DiPatrizio, N.V.; Frontini, A.; et al. 2-Arachidonoylglycerol Signaling in Forebrain Regulates Systemic Energy Metabolism. Cell Metab. 2012, 15, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Chon, S.-H.; Douglass, J.D.; Zhou, Y.X.; Malik, N.; Dixon, J.L.; Brinker, A.; Quadro, L.; Storch, J. Over-Expression of Monoacylglycerol Lipase (MGL) in Small Intestine Alters Endocannabinoid Levels and Whole Body Energy Balance, Resulting in Obesity. PLoS ONE 2012, 7, e43962. [Google Scholar] [CrossRef] [Green Version]
- Tardelli, M.; Bruschi, F.V.; Claudel, T.; Fuchs, C.D.; Auer, N.; Kunczer, V.; Stojakovic, T.; Scharnagl, H.; Habib, A.; Grabner, G.F.; et al. Lack of monoacylglycerol lipase prevents hepatic steatosis by favoring lipid storage in adipose tissue and intestinal malabsorption. J. Lipid Res. 2019, 60, 1284–1292. [Google Scholar] [CrossRef]
- Yoshida, K.; Kita, Y.; Tokuoka, S.M.; Hamano, F.; Yamazaki, M.; Sakimura, K.; Kano, M.; Shimizu, T. Monoacylglycerol lipase deficiency affects diet-induced obesity, fat absorption, and feeding behavior in CB1 cannabinoid receptor-deficient mice. FASEB J. 2019, 33, 2484–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglass, J.D.; Zhou, Y.X.; Wu, A.; Zadrogra, J.A.; Gajda, A.M.; Lackey, A.I.; Lang, W.; Chevalier, K.M.; Sutton, S.W.; Zhang, S.P.; et al. Global deletion of MGL in mice delays lipid absorption and alters energy homeostasis and diet-induced obesity. J. Lipid Res. 2015, 56, 1153–1171. [Google Scholar] [CrossRef] [Green Version]
- Powell, D.R.; Gay, J.P.; Wilganowski, N.; Doree, D.; Savelieva, K.V.; Lanthorn, T.H.; Read, R.; Vogel, P.; Hansen, G.M.; Brommage, R.; et al. Diacylglycerol Lipase α Knockout Mice Demonstrate Metabolic and Behavioral Phenotypes Similar to Those of Cannabinoid Receptor 1 Knockout Mice. Front. Endocrinol. 2015, 6, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sipe, J.C.; Chiang, K.; Gerber, A.L.; Beutler, E.; Cravatt, B.F. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc. Natl. Acad. Sci. USA 2002, 99, 8394–8399. [Google Scholar] [CrossRef] [Green Version]
- Chiang, K.P.; Gerber, A.L.; Sipe, J.C.; Cravatt, B.F. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: Evidence for a link between defects in the endocannabinoid system and problem drug use. Hum. Mol. Genet. 2004, 13, 2113–2119. [Google Scholar] [CrossRef]
- Aberle, J.; Fedderwitz, I.; Klages, N.; George, E.; Beil, F.U. Genetic Variation in Two Proteins of the Endocannabinoid System and their Influence on Body Mass Index and Metabolism under Low Fat Diet. Horm. Metab. Res. 2007, 39, 395–397. [Google Scholar] [CrossRef]
- de Luis, D.; Aller, R.; Izaola, O.; Conde, R.; de la Fuente, B.; Sagrado, M.G. Genetic variation in the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) and their influence on weight loss and insulin resistance under a high monounsaturated fat hypocaloric diet. J. Diabetes Complicat. 2013, 27, 235–239. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Aller, R.; Izaola, O.; Conde, R.; Sagrado, M.G.; Primo, D.; Castro, M.J. Relationship among metabolic syndrome, C358A polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) and insulin resistance. J. Diabetes Complicat. 2012, 26, 328–332. [Google Scholar] [CrossRef]
- de Luis, D.A.; González Sagrado, M.; Aller, R.; Izaola, O.; Conde, R. Relation of C358A polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) with obesity and insulin resistance. Nutr. Hosp. 2010, 25, 993–998. [Google Scholar] [PubMed]
- de Luis, D.A.; Gonzalez Sagrado, M.; Aller, R.; Izaola, O.; Conde, R. Effects of C358A missense polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase on weight loss after a hypocaloric diet. Metab. Clin. Exp. 2011, 60, 730–734. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Gonzalez Sagrado, M.; Aller, R.; Izaola, O.; Conde, R.; Romero, E. C358A missense polymorphism of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) and visfatin levels in obese females. Int. J. Obes. 2010, 34, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Luis, D.A.; Izaola, O.; Aller, R.; de La Fuente, B.; Pacheco, D. Effects of C358A polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) on weight loss, adipocytokines levels, and insulin resistance after a high polyunsaturated fat diet in obese patients. J. Endocrinol. Investig. 2013, 36, 965–969. [Google Scholar] [CrossRef]
- de Luis, D.A.; Sagrado, M.G.; Aller, R.; Izaola, O.; Conde, R.; Romero, E. C358A missense polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) and insulin resistance in patients with diabetes mellitus type 2. Diabetes Res. Clin. Pract. 2010, 88, 76–80. [Google Scholar] [CrossRef]
- de Luis, D.A.; Sagrado, M.G.; Pacheco, D.; Terroba, M.C.; Martin, T.; Cuellar, L.; Ventosa, M. Effects of C358A missense polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase on weight loss and cardiovascular risk factors 1 year after biliopancreatic diversion surgery. Surg. Obes. Relat. Dis. 2010, 6, 516–520. [Google Scholar] [CrossRef]
- de Luis, D.A.; Sagrado, M.G.; Aller, R.; Izaola, O.; Conde, R. Effects of C358A missense polymorphism of the degrading enzyme fatty acid amide hydrolase on weight loss, adipocytokines, and insulin resistance after 2 hypocaloric diets. Metab. Clin. Exp. 2010, 59, 1387–1392. [Google Scholar] [CrossRef]
- Durand, E.; Lecoeur, C.; Delplanque, J.; Benzinou, M.; Degraeve, F.; Boutin, P.; Marre, M.; Balkau, B.; Charpentier, G.; Froguel, P.; et al. Evaluating the Association of FAAH Common Gene Variation with Childhood, Adult Severe Obesity and Type 2 Diabetes in the French Population. Obes. Facts 2008, 1, 305–309. [Google Scholar] [CrossRef]
- Grolmusz, V.K.; Stenczer, B.; Fekete, T.; Szendei, G.; Patócs, A.; Rácz, K.; Reismann, P. Lack of Association between C385A Functional Polymorphism of the Fatty Acid Amide Hydrolase Gene and Polycystic Ovary Syndrome. Exp. Clin. Endocrinol. Diabetes 2013, 121, 338–342. [Google Scholar] [CrossRef]
- Monteleone, P.; Tortorella, A.; Martiadis, V.; Di Filippo, C.; Canestrelli, B.; Maj, M. The cDNA 385C to A missense polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) is associated with overweight/obesity but not with binge eating disorder in overweight/obese women. Psychoneuroendocrinology 2008, 33, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Brönner, G.; Wandolski, M.; Carrie, J.; Nguyen, T.T.; Greene, B.H.; Scherag, A.; Grallert, H.; Vogel, C.I.; Scherag, S.; et al. Mutation screen and association studies for the fatty acid amide hydrolase (FAAH) gene and early onset and adult obesity. BMC Med. Genet. 2010, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Sipe, J.C.; Waalen, J.; Gerber, A.; Beutler, E. Overweight and obesity associated with a missense polymorphism in fatty acid amide hydrolase (FAAH). Int. J. Obes. 2005, 29, 755–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thethi, T.K.; Sigel, A.; Japa, S.; Katalenich, B.; Liu, S.; Nguyen, T.; Larrazolo, J.; Syu, S.; Carefoot, E.; McDuffie, R.; et al. Racial and sex differences in the polymorphisms of the endocannabinoid receptor genes in obesity. J. Diabetes Complicat. 2020, 34, 107682. [Google Scholar] [CrossRef]
- Vazquez-Roque, M.I.; Camilleri, M.; Vella, A.; Carlson, P.; Laugen, J.; Zinsmeister, A.R. Association of genetic variation in cannabinoid mechanisms and gastric motor functions and satiation in overweight and obesity. Neurogastroenterol. Motil. 2011, 23, 637-e257. [Google Scholar] [CrossRef] [Green Version]
- Yagin, N.L.; Aliasgari, F.; Aliasgharzadeh, S.; Mahdavi, R.; Akbarzadeh, M. The influence of the fatty acid amide hydrolase 385C>A single nucleotide polymorphisms on obesity susceptibility. Mol. Biol. Rep. 2019, 46, 5049–5055. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sonnenberg, G.E.; Baye, T.M.; Littrell, J.; Gunnell, J.; DeLaForest, A.; MacKinney, E.; Hillard, C.J.; Kissebah, A.H.; Olivier, M.; et al. Obesity-related dyslipidemia associated with FAAH, independent of insulin response, in multigenerational families of Northern European descent. Pharmacogenomics 2009, 10, 1929–1939. [Google Scholar] [CrossRef] [Green Version]
- Jensen, D.P.; Andreasen, C.H.; Andersen, M.K.; Hansen, L.; Eiberg, H.; Borch-Johnsen, K.; Jørgensen, T.; Hansen, T.; Pedersen, O. The functional Pro129Thr variant of the FAAH gene is not associated with various fat accumulation phenotypes in a population-based cohort of 5801 whites. J. Mol. Med. 2007, 85, 445–449. [Google Scholar] [CrossRef]
- Knoll, N.; Volckmar, A.L.; Pütter, C.; Scherag, A.; Kleber, M.; Hebebrand, J.; Hinney, A.; Reinehr, T. The Fatty Acid Amide Hydrolase (FAAH) Gene Variant rs324420 AA/AC is not Associated with Weight Loss in a 1-Year Lifestyle Intervention for Obese Children and Adolescents. Horm. Metab. Res. 2012, 44, 75–77. [Google Scholar] [CrossRef]
- Lieb, W.; Manning, A.K.; Florez, J.C.; Dupuis, J.; Cupples, L.A.; McAteer, J.B.; Vasan, R.S.; Hoffmann, U.; O’Donnell, C.J.; Meigs, J.B.; et al. Variants in the CNR1 and the FAAH Genes and Adiposity Traits in the Community. Obesity 2009, 17, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, E.; Nobrega, J.N.; Hill, M.N.; Tyndale, R.F.; Lee, F.S.; Hendershot, C.S.; Best, L.M.; Di Ciano, P.; Balsevich, G.; Sloan, M.E.; et al. D3 dopamine receptors and a missense mutation of fatty acid amide hydrolase linked in mouse and men: Implication for addiction. Neuropsychopharmacology 2020, 45, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.J.D.M.; Genelhu, V.; Pimentel, M.M.G.; Celoria, B.M.J.; Mangia, R.F.; Aveta, T.; Silvestri, C.; Di Marzo, V.; Francischetti, E.A. Circulating Endocannabinoids and the Polymorphism 385C>A in Fatty Acid Amide Hydrolase (FAAH) Gene May Identify the Obesity Phenotype Related to Cardiometabolic Risk: A Study Conducted in a Brazilian Population of Complex Interethnic Admixture. PLoS ONE 2015, 10, e0142728. [Google Scholar] [CrossRef]
- Papazoglou, D.; Panagopoulos, I.; Papanas, N.; Gioka, T.; Papadopoulos, T.; Papathanasiou, P.; Kaitozis, O.; Papatheodorou, K.; Maltezos, E. The Fatty Acid Amide Hydrolase (FAAH) Pro129Thr Polymorphism is not Associated with Severe Obesity in Greek Subjects. Horm. Metab. Res. 2008, 40, 907–910. [Google Scholar] [CrossRef]
- Yagin, N.L.; Aliasgari, F.; Alizadeh, M.; Aliasgharzadeh, S.; Mahdavi, R. Comparison of endocannabinoids levels, FAAH gene polymorphisms, and appetite regulatory substances in women with and without binge eating disorder: A cross—Sectional study. Nutr. Res. 2020, 83, 86–93. [Google Scholar] [CrossRef]
- Bhatia, G.; Bansal, V.; Harismendy, O.; Schork, N.J.; Topol, E.J.; Frazer, K.; Bafna, V. A Covering Method for Detecting Genetic Associations between Rare Variants and Common Phenotypes. PLOS Comput. Biol. 2010, 6, e1000954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harismendy, O.; Bansal, V.; Bhatia, G.; Nakano, M.; Scott, M.; Wang, X.; Dib, C.; Turlotte, E.; Sipe, J.C.; Murray, S.S.; et al. Population sequencing of two endocannabinoid metabolic genes identifies rare and common regulatory variants associated with extreme obesity and metabolite level. Genome Biol. 2010, 11, R118. [Google Scholar] [CrossRef] [Green Version]
- Kuk, A.Y.C.; Li, X.; Xu, J. A fast collapsed data method for estimating haplotype frequencies from pooled genotype data with applications to the study of rare variants. Stat. Med. 2013, 32, 1343–1360. [Google Scholar] [CrossRef]
- Ning, T.; Zou, Y.; Yang, M.; Lu, Q.; Chen, M.; Liu, W.; Zhao, S.; Sun, Y.; Shi, J.; Ma, Q.; et al. Genetic interaction of DGAT2 and FAAH in the development of human obesity. Endocrine 2017, 56, 366–378. [Google Scholar] [CrossRef]
- Fredrikson, G.; Tornqvist, H.; Belfrage, P. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim. Biophys. Acta 1986, 876, 288–293. [Google Scholar] [CrossRef]
- Geurts, L.; Everard, A.; Van Hul, M.; Essaghir, A.; Duparc, T.; Matamoros, S.; Plovier, H.; Castel, J.; Denis, R.G.; Bergiers, M. Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nat. Commun. 2015, 6, 6495. [Google Scholar] [CrossRef] [Green Version]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2021, 1–14. [Google Scholar] [CrossRef]
- Benzinou, M.; Chèvre, J.C.; Ward, K.J.; Lecoeur, C.; Dina, C.; Lobbens, S.; Durand, E.; Delplanque, J.; Horber, F.F.; Heude, B.; et al. Endocannabinoid receptor 1 gene variations increase risk for obesity and modulate body mass index in European populations. Hum. Mol. Genet. 2008, 17, 1916–1921. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.E.; Liebowitz, M.R.; Stein, M.B.; Grunfeld, J.; Van Hove, I.; Simmons, W.K.; Van Der Ark, P.; Palmer, J.A.; Saad, Z.S.; Pemberton, D.J.; et al. The effects of inhibition of fatty acid amide hydrolase (FAAH) by JNJ-42165279 in social anxiety disorder: A double-blind, randomized, placebo-controlled proof-of-concept study. Neuropsychopharmacology 2021, 46, 1004–1010. [Google Scholar] [CrossRef]
- D’Souza, D.C.; Cortes-Briones, J.; Creatura, G.; Bluez, G.; Thurnauer, H.; Deaso, E.; Bielen, K.; Surti, T.; Radhakrishnan, R.; Gupta, A.; et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: A double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry 2019, 6, 35–45. [Google Scholar] [CrossRef]
- Cooper, Z.D.; Craft, R.M. Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacology 2018, 43, 34–51. [Google Scholar] [CrossRef] [Green Version]
- Wagner, E.J. Sex differences in cannabinoid-regulated biology: A focus on energy homeostasis. Front. Neuroendocrinol. 2016, 40, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Reference | Species/Strain and Sex | Drug, Dose, and Route of Administration | Key Findings |
|---|---|---|---|
| FAAH Inhibitors | |||
| Balsevich et al. (2018) [26] | C57BL/6 mice, male | 0.3 mg/kg of URB597 i.p. | After a 12-h overnight fast followed by treatment with URB597, leptin administration was unable to significantly reduce body weight gain or food intake |
| Cifani et al. (2020) [27] | Sprague-Dawley rats, male | 10 mg/kg of PF-3845 i.p. | No significant impact on body weight |
| Liu et al. (2013) [28] | C57BL/6 and SV/129 mice, male | 5 mg/kg of URB597 i.p | SCD1−/− mice fed a HFD demonstrated glucose intolerance and insulin resistance after treatment with URB597 SCD1−/− mice fed a HFD had significantly higher levels of plasma insulin after URB597 administration |
| DAGL Inhibitors | |||
| Bisogno et al. (2013) [29] | C57BL/6 mice, male | O-7460 i.p.; 0 mg/kg, 6 mg/kg, and 12 mg/kg | O-7460 dose-dependently decreased intake of a HFD over 14 h O-7460 (12 mg/kg) significantly decreased body weight at 14 h |
| Palma-Chavez et al. (2019) [30] | Sprague-Dawley rats, female | 30 μL (at 5 × 107 IFU/mL) of shRNA DAGLα-inhibiting adenovirus; administered into third ventricle | In fasting conditions, inhibition of DAGLα reduced NPY and increased POMC expression; in response to glucose, DAGLα inhibition increased NPY expression but decreased POMC (opposite in control rats) |
| Reference | Species/Strain and Sex | Gene(s) of Interest and Genetic Manipulation * | Key Findings |
|---|---|---|---|
| Balsevich et al. (2018) [26] | C57BL/6 mice, male | Mouse line with Faah C385A knock-in | After a 16-h overnight fast, C385A knock-in mice did not demonstrate an expected decrease in cumulative food intake or body weight after leptin administration |
| Brown et al. (2012) [33] | 129SvJ-C57BL/6 mice, both male and female | Faah −/− mice | Faah−/− mice had significantly high levels of liver triglycerides and liver diacylglycerols compared to WT mice Deletion of Faah resulted in ectopic lipid accumulation at the liver |
| Chon et al. (2012) [36] | C57/BL6J-SJL mice, male | Small intestine Mgll was overexpressed in mice (iMGL mice) | After three weeks of a HFD, iMGL mice (vs. WT) had significantly greater weight gain, increased percent body fat, increased adipose tissue mass, greater ectopic fat depositions in the liver and small intestine, and increased foot intake with reduced energy expenditure |
| Douglass et al. (2015) [39] | 129/SvEv-C57BL/6 mice, both male and female | Mgll−/− mice | At baseline, Mgll −/− and WT mice did not differ in body weight Throughout 12 weeks of feeding, Mgll −/− mice had lower body weight compared to WT mice, in both males and females, and under both HFD and low-fat diets LFD At baseline and after 12 weeks of low-fat dieting, Mgll −/− mice had a significantly lower fat mass than WT mice Throughout the 12-week feeding period, there were no significant differences in cumulative or average daily food intake; after the feeding period, LFD-fed male Mgll −/− mice had a significantly higher cumulative food intake Male Mgll −/− mice had significantly lower triglyceride and cholesterol levels under both diets; when considering both sexes, only Mgll −/− fed a HFD demonstrated significantly lower triglyceride and cholesterol levels |
| Grevengoed et al. (2019) [34] | C57BL/6N mice, male | Single amino-acid substitution in Faah (S268D) that selectively disrupts N-acyl taurine (NAT) but not N-acylethanolamine (NAE) hydrolytic activity | Faah-S268D mice had improved insulin sensitivity, increased glucagon secretion after insulin, and lower food intake in response to leptin treatment compared to WT mice |
| Jung et al. (2012) [35] | C57BL/6J mice, male | Mgll gene; generation of mice with forebrain neurons overexpressing Mgll (using CaMKIIα promoter) | Mgll-overexpressing mice showed reduced weight gain, decreased adiposity, and increased lean mass compared to WT mice; this was accompanied by lower plasma triglyceride levels, decreased serum glucose levels, and increased glucose uptake despite increased frequency of feeding and greater food intake Mgll-overexpressing mice were resistant to diet-induced obesity compared to WT |
| Powell et al. (2015) [40] | 129S5/SvEvBrd x C57BL/6-Tyrc−Brd mice, both male and female | Dagla and Daglb genes; generation of Dagla KO, Daglb KO, and Dagla/Daglb double KO mice | Dagla KO mice had significantly lower body weight and body fat mass compared to WT mice (under both chow-fed and HFD) Dagla KO mice had significantly lower total food intake compared to WT mice Dagla KO mice had significantly lower fasting insulin, total triglyceride, and total cholesterol compared to WT mice |
| Tardelli et al. (2019) [37] | C57BL/6 mice, male | Mgll −/− mice | Under either chow or Western diet, Mgll −/− mice experienced significantly less weight gain compared to WT mice, but Mgll −/− mice had a significantly greater gonadal white adipose tissue to body weight ratio; no significant differences in food intake Mgll −/− fed under a Western diet had significantly lower levels of plasma and hepatic triglycerides and plasma insulin compared to WT mice; no significant differences in plasma cholesterol |
| Touriño et al. (2010) [31] | 129SvJ-C57BL/6 mice, male | Faah −/− mice | Faah−/− mice had significantly greater body weight (standard diet and high-fat diet) compared to WT mice; this difference increased over time in Faah−/−mice fed with the HFD No significant effect on total food intake; however, Faah−/− mice demonstrated greater reinforcement and motivation effects from food When fed a HFD, Faah−/− mice had significantly higher fat mass, triglyceride levels, glucose, and insulin levels compared to WT mice |
| Vaitheesvaran et al. (2012) [32] | C57BL/6 mice, male | Faah −/− mice | Faah −/− mice had significantly higher body weight, food intake (regular chow diet), and fat mass compared to WT mice Faah −/− mice had significantly higher levels of plasma insulin and plasma triglycerides compared to WT mice |
| Yoshida et al. (2019) [38] | C57BL/6 mice, male | Mgll −/− mice | Mgll −/− and WT mice had similar body weight gain and food intake when fed a normal chow diet; however, when fed a HFD, Mgll −/− mice gained less weight compared to WT mice Mgll −/− mice had significantly better glucose tolerance and insulin sensitivity compared to WT mice Mgll −/− mice had significantly lighter liver weights compared to WT mice, indicative of less ectopic fat accumulation in the liver Following oral gavage with olive oil, Mgll −/− mice had significantly lower plasma triglyceride levels compared to WT mice |
| Reference | Sample | Gene(s) and Variant(s) and Study Design | Key Findings |
|---|---|---|---|
| Aberle et al. (2007) [43] | n = 451 obese/overweight (BMI > 25 kg/m2) and dyslipidemic | FAAH—Pro129Thr SNP (rs324420) Genetic association with obesity-related traits at baseline and after a 6-week diet intervention | No baseline association After six weeks of low-fat diet, there were significant decreases in triglyceride and total cholesterol levels in CA/AA vs. CC |
| Bhatia et al. (2010) [69] | n = 147 with BMI < 30 kg/m2 n = 142 with BMI > 40 kg/m2 | A novel algorithm called RareCover used to analyze the contribution of rare genetic variants in FAAH and MGLL to the development of obesity phenotypes (based on BMI) | Two 5 Kbp regions in the upstream regulatory segments of FAAH and MGLL genes identified, rare variants that could hinder FAAH and MGLL gene expression |
| De Luis et al. (2010) [46] | n = 279 obese females | FAAH—Pro129Thr SNP (rs324420) Genetic association with obesity-related traits | No significant association with anthropometric parameters or dietary intake A-allele significantly associated with lower triglycerides, glucose, and HOMA |
| De Luis et al. (2010) [48] | n = 143 obese females | FAAH—Pro129Thr SNP (rs324420) Genetic association with obesity-related traits | No significant differences in anthropometric parameters or food intake Significantly higher levels of glucose, insulin, and HOMA in C/C (wildtype) vs. A-allele carriers |
| De Luis et al. (2010) [50] | n = 70 with Type-II Diabetes Mellitus and obesity (BMI > 30 kg/m2) | FAAH—Pro129Thr SNP (rs324420) Genetic association with obesity-related traits | A-allele carriers had significantly higher BMI, fat mass, waist circumference, insulin, HOMA, and lower adiponectin vs. wildtype (C/C) No significant difference in dietary intake |
| De Luis et al. (2010) [51] | n = 67 with BMI > 40 kg/m2 who had undergone biliopancreatic diversion | FAAH—Pro129Thr SNP (rs324420) Genetic association with obesity-related traits at baseline, and then 3, 9, and 12 months after surgery | No significant baseline differences in anthropometric or biochemical parameters At 9 and 12 months, weight loss was greater in A-allele carriers vs. wildtype (C/C) |
| De Luis et al. (2010) [52] | n = 248 with BMI > 30 kg/m2 | FAAH—Pro129Thr SNP (rs324420) Genetic association with obesity-related traits at baseline and after a 3-month diet intervention (low fat or low carbohydrate) | No significant difference between genotypes at baseline or 3 months in anthropometric outcomes, cardiovascular risk factors, or circulating adipocytokines In both diet intervention groups, A-allele carriers failed to show improvement in glucose, insulin, HOMA, and leptin, while wildtype (C/C) did |
| De Luis et al. (2011) [47] | n = 122 with BMI > 30 kg/m2 | FAAH—Pro129Thr SNP (rs324420) Genetic association with obesity-related traits at baseline and after a 3-month diet intervention (hypocaloric) | At baseline, there were no genotype differences in anthropometric or dietary intake measures, but A-allele carriers had significantly lower insulin, HOMA, and C-reactive protein vs. wildtype (C/C) After 3 months, weight, waist circumference, insulin, C-reactive protein, and triglyceride levels were lower in A-allele carriers vs. wildtype (C/C), with no difference in dietary intake |
| De Luis et al. (2012) [45] | n = 799 with mean BMI of 36.7 kg/m2 | FAAH—Pro129Thr SNP (rs324420) Genetic association with obesity-related traits and metabolic syndrome | No significant association with metabolic syndrome No significant differences in anthropometric measures or circulating adipocytokines by genotype, but insulin and HOMA were significantly higher in A-allele carriers vs. wildtype (C/C) |
| De Luis et al. (2013) [44] | n = 95 with BMI > 30 kg/m2 | FAAH—Pro129Thr SNP (rs324420) Genetic association with obesity-related traits at baseline and after a 3-month diet intervention (enriched monounsaturated fat hypocaloric) | No significant differences at baseline between genotypes After 3 months, the wildtype group (C/C) showed greater improvements in insulin, HOMA-R, weight, fat mass, and waist circumference vs. A-allele carriers |
| De Luis et al. (2013) [49] | n = 99 with BMI > 30 kg/m2 | FAAH—Pro129Thr SNP (rs324420) Genetic association with obesity-related traits at baseline and after a 3-month diet intervention (enriched polyunsaturated fat hypocaloric) | No significant differences at baseline between genotypes After 3 months, there were no significant genotype differences in anthropometric parameter or adipokine changes, while there was improvement in insulin and HOMA-R in wildtype (C/C) vs. A-allele carriers |
| Durand et al. (2008) [53] | n = 1340 healthy adult controls n = 635 obese children n = 896 adults with Class III obesity, i.e., BMI ≥ 40 kg/m2 n = 2238 adults with Type-II Diabetes Mellitus | FAAH—10 SNPs across the entire FAAH locus, including Pro129Thr SNP (rs324420); rs913168, rs17361950, rs6429600, rs324419, rs324418, rs2295633, rs11576941, rs324425, rs7520850 Case-control genetic association | No significant associations with childhood obesity or Type-II Diabetes Mellitus Nominally significant association between rs324420 (C allele was risk allele) and 5 other FAAH SNPs (rs6429600, rs324419, rs324418, rs2295633, and rs7520850) and Class III (adult) obesity No significant association between rs324420 SNP and metabolic traits |
| Grolmusz et al. (2013) [54] | n = 63 patients with polycystic ovary syndrome (mean BMI 29.6 kg/m2) n = 67 healthy controls (mean BMI 21.2 kg/m2) | FAAH—Pro129Thr SNP (rs324420) Case-control genetic association | In cases, free thyroxine was higher in A-allele carriers than wildtype (C/C) In controls, insulin was significantly lower in A-allele carriers than wildtype (C/C) No other significant associations (including BMI) |
| Harismendy et al. (2010) [70] | n = 147 with BMI < 30 kg/m2 n = 142 with BMI > 40 kg/m2 | Sequence-based case-control genetic association (FAAH and MGLL) | One interval in FAAH promoter and 3 intervals in MGLL (promoter, intron 2, intron 3) were associated with obesity |
| Jensen et al. (2007) [62] | n = 5801 classified as normal weight = BMI 18.5–25 kg/m2, overweight = BMI 25–30 kg/m2, and obese = BMI ≥ 30 kg/m2 | FAAH—Pro129Thr SNP (rs324420) Genetic association | No significant association with BMI or waist circumference groups or with any quantitative trait |
| Knoll et al. (2012) [63] | n = 453 overweight or obese children and adolescents (mean 10.8 years old) | FAAH—Pro129Thr SNP (rs324420) Genetic association with obesity-related traits at baseline and after a 1-year lifestyle intervention | No significant association with change in any outcome |
| Kuk et al. (2013) [71] | n = 148 with BMI > 40 kg/m2 n = 150 with BMI < 30 kg/m2 | Sequence-based case-control genetic association (FAAH and MGLL) | Three potentially causal rare variants were identified around MGLL An interaction between two rare variants around FAAH was identified that may increase the risk of obesity |
| Lieb et al. (2009) [64] | n = 2415 participants from a longitudinal cohort | FAAH—9 SNPs across the entire FAAH locus, including Pro129Thr SNP (rs324420); rs12073998, rs6703669, rs3766246, rs324419, rs2295633, rs12029329, rs324425, rs7520850 Genetic association | No significant associations |
| Mansouri et al. (2020) [65] | n = 79 healthy participants | FAAH—Pro129Thr SNP (rs324420) | No significant difference in mean BMI between genotypes |
| Martins et al. (2015) [66] | n = 100 with BMI ≥ 18.5 kg/m2 and < 25 kg/m2 n = 100 with BMI ≥ 30 kg/m2 | FAAH—Pro129Thr SNP (rs324420) Genetic association with obesity and insulin-resistant phenotype | No significant associations |
| Monteleone et al. (2008) [55] | n = 115 overweight/obese females with binge-eating disorder (BED) n = 74 obese females without BED n = 110 healthy controls | FAAH—Pro129Thr SNP (rs324420) Case-control genetic association | A allele was significantly more frequent in overweight/obese females compared to controls |
| Muller et al. (2010) [56] | n = 521 children and adolescents (mean BMI 31.86 kg/m2) and both biological parents n = 501 German children and adolescents (including one sibling) (mean BMI 32.28 kg/m2) and both biological parents n = 8491 adults (mean BMI 27.12 kg/m2) from a population-based study group n = 985 cases (mean BMI 36.04 kg/m2) and n = 588 controls (mean BMI 19.34 kg/m2) | FAAH—five SNPs, including Pro129Thr SNP (rs324420); rs324419, rs873978, rs2295632 and rs932816 Genetic association first in a sample of trios, then replicated in a second cohort of families, then in combined sample SNPs significantly associated with childhood obesity were then screened in a population-based cohort of adults, and then in a case-control sample | Association of G allele of FAAH rs2295632 with early onset obesity in the initial sample of trios, but this did not replicate in the subsequent sample When the two samples were combined, FAAH rs324420 (C allele) and rs2295632 were significantly associated with childhood obesity No significant association between FAAH rs324420 or rs2295632 and adult obesity or BMI in the population-based sample; no significant association with rs324420 in the case-control analysis |
| Ning et al. (2017) [72] | n = 227 with BMI 35.1–61.7 kg/m2 n = 219 with BMI 17.5–23.0 kg/m2 | FAAH—whole-exome sequencing | The novel FAAH c.G944T (p.R315I) variant co-segregated with obesity in the proband’s pedigree; in vitro characterization of FAAH-R315I suggested that it is a loss-of-function mutation |
| Papazoglou et al. (2008) [67] | n = 158 with BMI > 40 kg/m2 n = 145 with BMI > 40 kg/m2 and metabolic syndrome n = 121 with BMI 18.5–25 kg/m2 | FAAH—Pro129Thr SNP (rs324420) Case-control genetic association | No significant associations |
| Sipe et al. (2005) [57] | n = 2667 (1688 White, 614 Black, 365 Asian) | FAAH—Pro129Thr SNP (rs324420) Case-control genetic association | Significant association of A/A genotype with overweight/obese status in Black and White, but not Asian, participants Median BMI was higher in the A/A genotype group compared to C/A + C/C in the pooled sample |
| Thethi et al. (2020) [58] | n = 465 with BMI ≥ 30 kg/m2 n = 202 with BMI ≤ 27 kg/m2 | FAAH—Pro129Thr SNP (rs324420) Case-control genetic association | Significant association of the A allele with obesity, but not when adjusting for age, race, sex, waist-hip ratio, and LDL No association with any other outcome measure |
| Vazquez-Roque et al. (2011) [59] | n = 62 adults overweight or obese (n = 5, mean BMI 24.0 kg/m2; n = 28, mean BMI 28.1 kg/m2; n = 29, mean BMI 34.9 kg/m2) | FAAH—Pro129Thr SNP (rs324420) Genetic association with gastric emptying (GE) of solids and liquids, gastric volume (GV), and satiation [maximum tolerated volume (MTV) after nutrient drink test] | No significant association with GE of solids or liquids, GV, or aggregate symptom score Significant association with MTV: lower MTV in the CC genotype vs. CA/AA |
| Yagin et al. (2019) [60] | n = 180 healthy overweight/obese women (BMI = 25–40 kg/m2) n = 86 women with BMI = 18.5–24.9 kg/m2 | FAAH—Pro129Thr SNP (rs324420) Case-control genetic association | A/A and C/A genotypes were more frequent in overweight/obese women A-allele carriers had significantly higher BMI, waist circumference, neck circumference, waist-to-height ratio, and body fat mass A-allele significantly predicted risk of obesity after adjusting for age, marital status, and physical activity. (OR: 2.38; 95% CI = 1.37–3.79) |
| Yagin et al. (2020) [68] | n = 180 women (mean BMI 32.54 kg/m2) | FAAH—Pro129Thr SNP (rs324420) Genetic association with binge eating disorder | No significant association, though the frequency of the A allele was numerically higher in women with binge eating disorder (p = 0.08) |
| Zhang et al. (2009) [61] | n = 1644 (from 261 extended families) | FAAH—Pro129Thr SNP (rs324420) and four additional SNPs (rs324418, rs1984490, rs2145408 and rs4141964) Genetic association with obesity-related traits | A-allele of rs324420 was significantly associated with higher BMI and fasting triglyceride levels compared to wildtype (C/C genotype) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matheson, J.; Zhou, X.M.M.; Bourgault, Z.; Le Foll, B. Potential of Fatty Acid Amide Hydrolase (FAAH), Monoacylglycerol Lipase (MAGL), and Diacylglycerol Lipase (DAGL) Enzymes as Targets for Obesity Treatment: A Narrative Review. Pharmaceuticals 2021, 14, 1316. https://doi.org/10.3390/ph14121316
Matheson J, Zhou XMM, Bourgault Z, Le Foll B. Potential of Fatty Acid Amide Hydrolase (FAAH), Monoacylglycerol Lipase (MAGL), and Diacylglycerol Lipase (DAGL) Enzymes as Targets for Obesity Treatment: A Narrative Review. Pharmaceuticals. 2021; 14(12):1316. https://doi.org/10.3390/ph14121316
Chicago/Turabian StyleMatheson, Justin, Xin Ming Matthew Zhou, Zoe Bourgault, and Bernard Le Foll. 2021. "Potential of Fatty Acid Amide Hydrolase (FAAH), Monoacylglycerol Lipase (MAGL), and Diacylglycerol Lipase (DAGL) Enzymes as Targets for Obesity Treatment: A Narrative Review" Pharmaceuticals 14, no. 12: 1316. https://doi.org/10.3390/ph14121316
APA StyleMatheson, J., Zhou, X. M. M., Bourgault, Z., & Le Foll, B. (2021). Potential of Fatty Acid Amide Hydrolase (FAAH), Monoacylglycerol Lipase (MAGL), and Diacylglycerol Lipase (DAGL) Enzymes as Targets for Obesity Treatment: A Narrative Review. Pharmaceuticals, 14(12), 1316. https://doi.org/10.3390/ph14121316








