Abstract
Ovarian cancer (OCa) is characterized as one of the common reasons for cancer-associated death in women globally. This gynecological disorder is chiefly named the “silent killer” due to lacking an association between disease manifestations in the early stages and OCa. Because of the disease recurrence and resistance to common therapies, discovering an effective therapeutic way against the disease is a challenge. According to documents, some popular herbal formulations, such as curcumin, quercetin, and resveratrol, can serve as an anti-cancer agent through different mechanisms. However, these herbal products may be accompanied by some pharmacological limitations, such as poor bioavailability, instability, and weak water solubility. On the contrary, using nano-based material, e.g., nanoparticles (NPs), micelles, liposomes, can significantly solve these limitations. Therefore, in the present study, we will summarize the anti-cancer aspects of these herbal and-nano-based herbal formulations with a focus on their mechanisms against OCa.
1. Introduction
Ovarian cancer (OCa) is characterized as the fifth most prevalent reason for death in women around the world because of its insidious initiation, weak prognosis, and rapid development. Based on estimations, annually, more than 100,000 females die due to OCa globally [1]. Mainly, OCa is categorized into three types: germ cell, sex-cord-stromal, and epithelia [2]. The most common form of OCa is epithelial OC (EOC), which is a heterogenic disorder, and histologically, EOC can be divided into four main subgroups, endometrioid, serous, mucinous, and clear cell carcinomas [3]. The etiology of OCa has not been completely illustrated yet, but it is shown that obesity, hereditary, aging, alcohol consumption, smoking, and diabetes mellitus are risk factors of OCa [4,5]. Plus, it is expressed that environmental, hormonal, and ovulation factors may have a role in the pathogenesis of OCa [6]. According to reports, OCa progression is linked with various pathways that interfered with the metabolism of energy, like galactose metabolism, which is related to the risk of OCa development [7]. OCa is mostly named the “silent killer” since the observed manifestations in the early stages of the disease are not clearly related to OCa [8]. The signs and symptoms of OCa can be general and ambiguous, such as abdominal pain, abnormal bowel habits, and early satiety [9]. There is a substantial need for using novel therapies for OCa due to disease recurrence and resistance to common therapies, such as chemotherapy and surgery [6,10,11]. In addition, these approaches can significantly along to cytotoxic impacts and severe complications [8]. Therefore, finding and using efficient curative methods against this gynecological tumor is indispensable. Among these, herbal remedies have obtained great attention from thousand years ago owing to their effectiveness against different ailments, such as cancer [12]. It is stated that some herbal compounds, like phenols, alkaloids, and lectins, can exert anti-cancer effects by apoptosis induction [13]. On the other hand, nano-based drug delivery systems, such as nanoparticles (NPs), nano micelles, liposomes, branched dendrimers, nanostructured lipid formulations, and nanocapsules, have been developed recently for the treatment of OCa [14]. Thus, the combination of herbal therapy and nano-based therapy may provide a new horizon in the improvement of OCa. For this reason, in this literature review, the therapeutic potential of some popular nano-based herbal formulations, namely curcumin, quercetin, and resveratrol [15,16,17], will be discussed.
2. Methods
In this literature review, we investigated accessible information from Google Scholar, PubMed, Scopus, Web of Science, Science direct, and Scientific Information Database until 2021. The MeSH terms used were: periodontitis, herbal medicine, herbal remedies, and natural products. According to the search strategy, 218 articles were discovered. After checking the titles, abstracts and manuscripts entirely cited, a collection of 126 papers were received and chosen according to the suitability indexes. The papers were performed around herbal medicine of different diseases especially ovarian cancer.
3. Ovarian Cancer and Its Pathogenesis
Presently, the pathogenesis and clinicopathological properties of OCa have not clearly been expressed [18]. However, some theories have been proposed concerning OCa origination (Figure 1) which includes (1) the gonadotropin theory characterizes over the induction of the epithelium of ovarian surface by hormonal receptors resulting in malignancy, (2) the continuous ovulation theory, in the location of which the cells of ovarian surface epithelium are damaged because of constant ovulation, (3) the origin cells for the majority of epithelial ovarian cancers are not derived from the ovary but mostly originated from the fallopian tube and develop to the ovary and more than it [19]. Regarding hormonal conditions, there is evidence that progesterone and androgens can elevate ovarian epithelium proliferation and subsequently lead to the formation of OCa. Indeed, the increment of androgens and estrogens taggers multiple pro-inflammatory agents resulting in immune activation. During ovulatory occurrences, a great number of molecules are produced, such as chemokines and cytokines, plasminogen activators, prostaglandins, interleukins, bioactive eicosanoids, tumor necrosis factors, collagenases, and a large number of growth factors and immune cells, which all trigger a pro-inflammatory occurrence. Such pro-inflammatory agents, like IL-8, CCL2/MCP-1, and CCL5/RANTES, are induced during each cycle of ovulation; therefore, the continuous ovulation theory recommends that the inflammation accompanied by other physiological situations potentiates OCa progression [20,21]. Plus, it is shown that IL-1β, IL-6, and TNF-α formed by activated immune agents and/or the tumor itself, induce the growth of cancer cells and affect the prognosis and clinical status of the disease via increasing resistance to chemotherapy and stimulating symptoms (e.g., weight loss, anemia, depression, and anorexia) [22]. Oxidative stress, namely reactive nitrogen species (RNS) and reactive oxygen species (ROS), are another factor involved in many pathological conditions, such as OCa, by genetic instability enhancement, angiogenesis promotion, and abnormality in cell proliferation [23,24]. Exogenous agents, like hypoxia, infection, and chronic inflammation, are among the main sources of oxidative stress [25]. Mounting evidence has demonstrated that ROS can modulate the biogenesis and expression of microRNAs by epigenetic alterations, regulating biogenesis course, and transcription factors [26]. MicroRNAs, as noncoding RNAs, have a role in chemoresistance, carcinogenesis, proliferation, apoptosis, cell cycle, invasion, and metastasis. Impairments of microRNAs can lead to the onset and progression of OCa [27]. Possibly, the most important feature of any cancer is genetic changes that mediate the development and progression of tumors [28]. In this line, it is revealed that the presence of mutations in PTEN (Phosphatase and tensin homolog), P53, BRCA(Breast Cancer)1, and BRCA2 genes, and tumor suppressor factors can develop OCa (Figure 2) [29,30,31]. Endometrioid, serous, and mucinous types of OCa have shown mutations in KRAS (Kirsten rat sarcoma), β-catenin, TFG-βRII, and BRAF genes, which all are associated with the proliferation and cell growth processes [28].
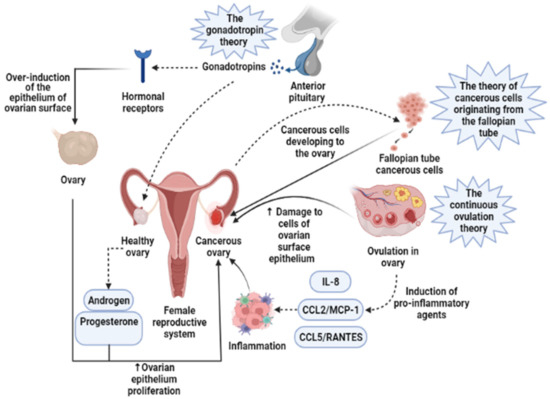
Figure 1.
Three main theories regarding the development of ovarian cancer are based on induction of the epithelium of ovarian surface by hormonal receptors, increased induction of pro-inflammatory agents during continuous ovulation, and cancerous cells originating from the fallopian tube. IL-8, Interleukin-8; CCL2/MCP-1, Monocyte chemoattractant protein-1; CCL5/RANTES, CC Chemokine Ligand-5.
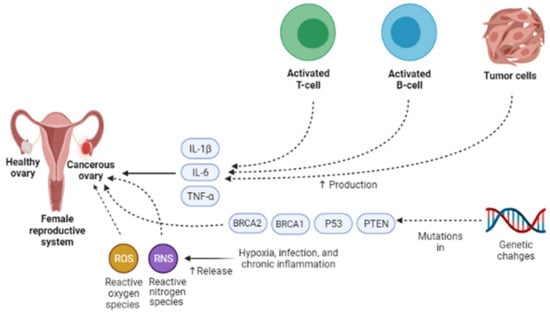
Figure 2.
Different endogenous and exogenous factors modify the development and prognosis of ovarian cancer. IL-1β, IL-6, and TNF-α accelerate the growth of cancer cells and affect the prognosis and clinical status of the disease via increasing resistance to chemotherapy and stimulating symptoms. Exogenous factors, such as hypoxia, infection, and chronic inflammation, are the main sources of oxidative stress, namely reactive nitrogen species (RNS) and reactive oxygen species (ROS). They can contribute to ovarian cancer development via genetic instability enhancement, angiogenesis promotion, and abnormality in cell proliferation. One of the most important features of ovarian cancer is genetic changes that mediate the development and progression of tumors. In ovarian cancer, the presence of mutations in PTEN, P53, BRCA1, and BRCA2 genes, tumor suppressor factors, can lead to ovarian cancer development. IL-1β, Interleukin-1β; IL-6, Interleukin-6; TNF-α, Tumor necrosis factor α; PTEN, Phosphatase and tensin homolog; BRCA1, Breast cancer type 1; BRCA2, Breast cancer type 1.
4. Use of Curcumin and Its Nanoformulations against Ovarian Cancer
Curcumin (CUR) is characterized as a yellow and hydrophobic herbal component that is originated from the turmeric plant (Curcuma longa L. Zingiberaceae) [32]. Growing evidence has shown some positive effects of CUR in medicine, such as anti-tumor, anti-inflammatory, anti-oxidative, immunoregulatory, anti-fungus, and anti-bacterial features [33,34], such as breast, ovarian, prostate, gastric, colorectal, pancreatic, and cervical cancers [35,36,37,38]. Regarding anti-cancer mechanisms of CUR, it has to be said that several signaling pathways are affected by that, for example, JAK (Janus Activated Kinase)/STAT (signal transducer and activator), PI3K (Phosphoinositide 3-kinases)/Akt, MAPK (mitogen-activated protein kinase), NF-ĸB, p53, Wnt/β-catenin, and apoptosis-related signaling (Figure 3). Furthermore, CUR can suppress epithelial-mesenchymal transition (EMT), angiogenesis, proliferation, metastasis, and invasion of the tumor by modulating the expression of non-coding RNA (ncRNA) associated with the tumor [39,40,41,42]. In the study of Shi et al., it was revealed that CUR can considerably suppress the growth and stimulate apoptosis in human OCa cell line Ho-8910. In their research, the use of 40 μM CUR caused a reduction in pro-caspase-3, Bcl-XL, and Bcl-2, whereas Bax and p53 levels were elevated in the treated cells with CUR [38]. Triggering AMP-activated protein kinase (AMPK), which stimulates cell apoptosis and inhibits cell proliferation in several cancers, in a p38-dependent way is another mechanism of CUR action in ovarian cancer cells CaOV3 [43]. In an animal investigation on OCa, it was manifested that CUR can dramatically suppresses STAT3 and NF-ĸB signaling pathways [44]. Liu et al. have pointed out that CUR can stimulate human OCa cell autophagy through AKT/mTOR (mammalian target of rapamycin)/p70S6K pathway suppression [45]. Despite these, the clinical application of CUR has been limited owing to its instability and low water solubility, which in turn give rise to poor bioavailability of CUR in cancerous cells. Attempts toward elevating the therapeutic effectiveness of Cur have been carried out through various techniques [46]. For instance, drug delivery systems based on NP have attracted much attention. CUR can be encapsulated inside NPs to promote its water solubility, biocompatibility, and protection from breaking down, and enhance CUR accumulation in cancerous regions because of increased permeability and retention impact [47,48]. In the investigation of Xu et al., CUR was encapsulated with niosome and its therapeutic effects against OCa cells were assessed [49]. In the field of nanotechnology, niosomes are known as nonionic vesicles with a bilayer construction which have a high bioavailability and are a good candidate for drug delivery systems [49,50]. Xu and colleagues concluded that CUR-niosomes increase cytotoxic influences and induce apoptosis against OCa cells A2780 in comparison with free CUR [49]. An in vivo and in vitro investigation showed that nanocurcumin in combination with cisplatin, a common treatment for OCa, could lead to a remarked reduction of the weight and volume of ovarian tumors. In addition, this treatment decreased PI3K, JAK, TGF-β, Ki67 expression, and Akt phosphorylation [51]. Hu et al. (2020) demonstrated that the use of Docetaxel curcumin/methoxy poly (ethylene glycol)-poly (L-lactic acid) (MPEG-PLA) copolymers nanomicelles cause the Suppression of tumor proliferation and angiogenesis (Table 1). The study of Bondi et al. (2017) concluded that biocompatible Lipid nanoparticles as carriers improved curcumin efficacy in ovarian cancer treatment and caused the Reduction of cell colony survival, inhibition of tumor growth, and apoptosis induction (Table 1). In the study of Ghaderi et al. (2021) OCa cells were treated with free curcumin and Gemni-Cur the anticancer activity was investigated by uptake kinetics, cellular viability, and apoptotic assays. The results illustrate that Gemini-Cur nanoparticles have a great potential for developing novel therapeutics against ovarian cancer (Table 1). Previous studies also demonstrated that the use of curcumin and paclitaxel co-delivery by hyaluronic acid-modified drug-loaded polyethyleneimine and stearic acid caused Downregulation of P-glycoprotein, and suppression of tumor cell migration (Table 1). Dendrosomal nano-curcumin caused the reduction of cancer cell viability, a decrease of LncRNAs expression of H19 and HOTAIR, and an increase in the expression of MEG3 LncRNA and Bcl2 protein (Table 1). The study of Sandhiutami et al. (2021) showed that co-use of curcumin nanoparticles and Cisplatin caused the Decrease of ovarian tumor weight and volume, reduction of PI3K, TGF-β, JAK, and Ki67 expression, Akt and STAT3 phosphorylation, and decrease of IL-6 level (Table 1). In general, Curcumin is an efficient agent with anti-tumor, antioxidant, and anti-inflammatory activities. The main mechanisms of action by which curcumin exhibits its unique anticancer activity include inducing apoptosis and inhibiting proliferation and invasion of tumors by suppressing a variety of cellular signaling pathways.
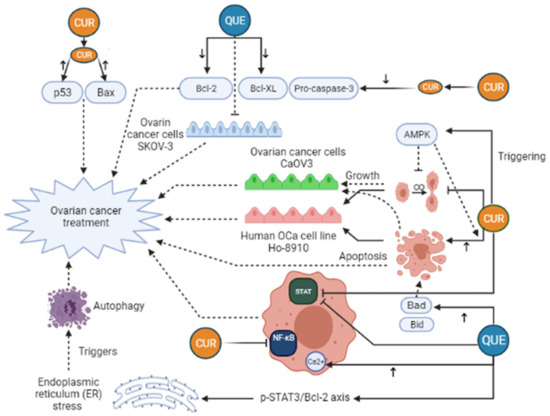
Figure 3.
Curcumin (CUR) and Quercetin (Que) can exert an anti-cancerous effect on ovarian cancer in many different pathways. CUR triggers AMP-activated protein kinase (AMPK) that leads to stimulation of cell apoptosis and inhibition of cell proliferation. Moreover, CUR can decrease pro-caspase-3, Bcl-XL, and Bcl-2 levels, whereas Bax and p53 levels rise in the treated cells with CUR. These changes lead to ovarian cancer treatment. Furthermore, CUR can exert a significant inhibitory effect on STAT3 and NF-ĸB signaling pathways. Quercetin (Que) can modify many pathways and play a role in ovarian cancer treatment. Que decreases the anti-apoptotic agents, like Bcl-2, Bcl-xL, while it elevates the expression of pro-apoptotic agents, such as Bad and Bid, leading to increased apoptosis and ovarian cancer treatment. In addition, the elevation of cytosolic Ca2+ levels due to Que consumption can take part in ovarian cancer treatment. Que triggers autophagy by endoplasmic reticulum (ER) stress by the p-STAT3/Bcl-2 axis as well. Bcl-XL, B-cell lymphoma-extra-large; BAX, BCL2-associated X protein; Bcl-2, B-cell lymphoma 2; Bad, BCL2 associated agonist of cell death; Bid, BH3-interacting domain death agonist; STAT, Signal transducer and activator of transcription; NF-ĸB, Nuclear factor-kappaB.

Table 1.
Nano-based formulations of curcumin, quercetin, and resveratrol through various mechanisms affect ovarian cancer.
5. Use of Quercetin and Its Nanoformulations against Ovarian Cancer
Quercetin (Que) is a polyphenolic compound present in various vegetables and fruits [64]. Several pharmacologic effects have been demonstrated for Que, such as anti-cancer, anti-proliferation, anti-inflammation, anti-oxidant, as well as anti-diabetes influences [65,66,67]. The growth of numerous cancers, like ovarian, colon, prostate, breast, cervical, gastric, and lung cancer, has been manifested to be diminished by Que [68,69,70,71,72,73]. Predominantly, the anti-cancer effects of Que are linked with the modulation of PI3K/Akt/ mTOR, STAT signaling pathway, expression of heat shock protein (HSP), intracellular pH modification, regulation of apoptosis-associated proteins, and the regulation of matrix metalloproteinases (MMPs), fibronectin, and vascular endothelial growth factor (VEGF) [74,75,76]. Apoptosis induction mechanisms have a key role in exerting anti-cancer impacts of Que possibly through the elevation of cytosolic Ca2+ levels, ROS generation, reduction of mitochondrial membrane potential, and surviving modulation [77,78,79,80]. Que also decreases the anti-apoptotic agents, for example, Bcl-2, Bcl-xL, whereas elevates the expression of pro-apoptotic agents, for instance, Bax, Bad, Bid, cyto-c, caspase-3, and caspase-9 (Figure 3) [81]. In vivo and in vitro studies have indicated that Que has a cytotoxic effect on OCa cells [67]. It is stated that Que can suppress the proliferation of OCa cells SKOV-3 in a dose-and time-dependent way. In addition, it can potentiate the apoptosis of these cell lines and attenuate the expression of survivin protein [82]. Regarding this disease, Liu and colleagues expressed that Que triggers autophagy by endoplasmic reticulum (ER) stress by the p-STAT3/Bcl-2 axis [83]. In this line, Yi et al. also highlighted that Que sensitizes human OCa cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), one of the strong anti-tumor agents in various cancer types [84,85,86]. They also observed that Que stimulated the expression of death receptor 5 (DR5) by JNK activation and CCAAT enhancer-binding protein homologous protein (CHOP) upregulation, while death receptor 4 (DR4) expression did not change by this phenol [84]. DR4 and DR5 belong to the TNF family and are induced through TRAIL, and CHOP is defined as a transcriptional factor that enhances apoptosis by the mediation of proportion of prosurvival Bcl-2 and the proapoptotic Bax [87,88,89]. Based on another work, Que stimulates radiosensitization via ATM phosphorylation induction and increases p53 protein expression [90]. However, the pharmacological applications of quercetin are limited by its insolubility in water. Several approaches have been investigated to overcome these obstacles, such as the use of micelle, polymeric NPs, microemulsions, solid lipid NPs, liposomes, as well as liquid crystal systems [91,92,93]. It is stated that one of the ways to promote water solubility of hydrophobic medicinal compounds is encapsulation by polymer micelles [58]. Micelles are nanoscale colloidal aggregates obtained from amphiphilic surfactants, which their core and shell are hydrophobic and hydrophilic, respectively. These features make them a suitable carrier for hydrophobic drug delivery [94]. The line with this notion, Gao et al. encapsulated Que into the micelles of monomethoxy poly (ethylene glycol)-poly(3-caprolactone) (MPEG-PCL), these QU loaded MPEG-PCL (QU/MPEG-PCL) micelles with a drug loading of 6.9% had a mean particle size of 36 nm, rendering the complete dispersion of quercetin in water and they illustrated that intravenous injection of these micelles can significantly repress the growth of ovarian tumors by inducing the apoptosis of cancer cells and suppressing angiogenesis in vivo [58]. In the drug delivery system, also poly (3-caprolactone) and poly (ethylene glycol) (PCL/PEG) are block copolymers that are amphiphilic, biodegradable, and can easily be produced. [95,96]. Another work assessed the potential of PEGylated liposomal quercetin (Lipo-Que) in OCa cells in vivo and in vitro. Lipo-Que was prepared using a solid dispersion method, and the obtained Lipo-Que is monodisperse with a mean diameter of 163 ± 10 nm. They implicated that Lipo-Que suppresses the proliferation and growth, stimulates cell cycle arrest and apoptosis of ovarian tumors [60]. Liposomes are a drug delivery system that provides the possibility of administration of the lipophilic and hydrophilic drugs in a united formulation, and their outer membrane can be modified by the surface attachment with the PEG and/or other targeting molecules to boost their specificity [97]. Generally, quercetin is a desirable anticancer agent because of its natural origin, safety, and low cost relative to synthetic cancer drugs. Que decreases the expression of survivin protein, induces the expression DR5 and ATM phosphorylation, and increases p53 protein expression.
6. Use of Resveratrol and Its Nanoformulations against Ovarian Cancer
Resveratrol (Res) is defined as a non-flavonoid polyphenol compound possessing stilbene structural components, which are extensively found in lilies, grapes, and other herbs [63]. Res has been illustrated to have anti-tumor, anti-inflammatory, anti-oxidation, immunoregulatory, anti-virus, anti-microbial, neuroprotective, and anti-atherosclerosis influences [63,98,99]. Some documents revealed the positive effects of Res in different cancers, such as skin, ovarian, breast, colorectal, lung, and uterine cancer [100,101,102,103,104,105]. Several mechanisms are involved in exerting anti-tumor action of Res, for example, inflammation suppression through NLRP3 inflammasome inhibition, cyclooxygenase (COX) curbing, nuclear factor erythroid 2-related factor 2 (Nrf2) induction, and mitogen-activated protein (MAP) kinase phosphatase-1 (MKP-1) stimulation, which inhibits NF-ĸB pathway [99,106,107,108]. In OCa, the administration of Res curbs growth and stimulates cell death through apoptosome complex formation, caspase activation, and mitochondrial secretion of cytochrome c [109]. In the study of Kueck et al., Res suppressed glucose metabolism in OCa cells [110]. It seems that glycolysis, conversion of glucose into 3-carbon carbohydrates, and subsequently ATP formation, are needed for the enhancement of tumor growth [111]. Another study by Zhong and colleagues exhibited accumulated G1 phase, elevated apoptosis fraction, and simultaneous inhibition of STAT3, Notch, and Wnt signaling pathway (Figure 4) [112]. Some other investigations have manifested the anti-cancer effect of Res against OCa through AMPK activation, downregulation of the protein cyclin D1, EMT inhibition [113,114,115]. Regardless of the favorable results of Res in cancer treatment, its wide utilization has been limited because of its poor bioavailability, low solubility in water, instability, and unfavorable systemic delivery [116,117,118]. In contrast, nanotechnology-based strategies have been widely used to acquire promoted oral bioavailability, higher solubility, promoted solubility, and targeted release of Res [61]. In this line, Khatun et al. in their in vitro study used Res—(Zinc oxide) ZnO nanohybrid against OCa cell lines and demonstrated that this nanoformulation exerts anti-cancer effects by the generation of ROS [119]. ZnO NPs have attracted much attention due to their utility in cancer therapy and targeted drug delivery. In human cancer cells, these NPs can stimulate apoptosis by ROS generation, which in turn is associated with cellular apoptosis and DNA damage [119]. An in vivo investigation indicated that harnessing Res–BSA NPs attenuates tumor growth in nude mice with OCa through the induction of ovarian cancer cell necrosis and cellular apoptosis induction (Figure 4) [62]. BSA (bovine serum albumin) is a natural protein that is capable of the formation of complexes in different shapes. In addition to other beneficial features, BSA is nonimmunogenic, non-toxic, biodegradable, biocompatible. Thus, albumin particles can be a good candidate for drug delivery system [120]. In summary, resveratrol is a desirable substance with anti-cancer and anti-inflammatory activities. The anti-cancer effect of resveratrol is correlated with the damage of mitochondrial function that leads to increased ROS, apoptosis, downregulation of the protein cyclin D1 can fight against OCa.
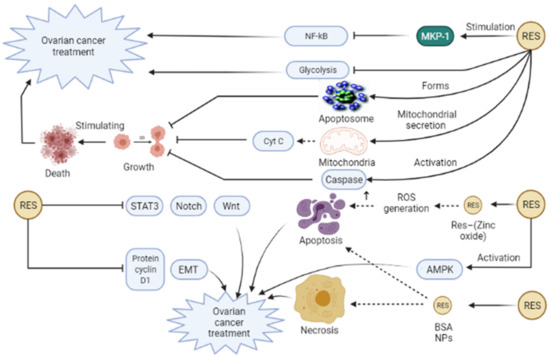
Figure 4.
Resveratrol (Res) can trigger various mechanisms involved in ovarian cancer treatment. Res can stimulate mitogen-activated protein (MAP) kinase phosphatase-1 (MKP-1) that leads to inhibition of NF-ĸB pathway and subsequently contributes to inflammation suppression and ovarian cancer improvement. Furthermore, the administration of Res can stimulate apoptosome complex formation, caspase activation, and mitochondrial secretion of cytochrome c, which all end in inhibition of growth and stimulation of cell death. In addition, Res suppresses glycolysis in ovarian cancer cells that can be effective in ovarian cancer treatment. Res can act against ovarian cancer through AMPK activation, downregulation of the protein cyclin D1, and inhibition of EMT, STAT3, Notch, and Wnt signaling pathways, leading to ovarian cancer treatment. Moreover, consumption of Res—(Zinc oxide) ZnO nanohybrid can lead to the generation of ROS in ovarian cancer cell lines and exert anti-cancer effects on ovarian cancer. In addition, Res–bovine serum albumin (BSA) NPs induce ovarian cancer cell necrosis and cellular apoptosis, attenuating tumor growth in ovarian cancer. EMT, Epithelial–mesenchymal transition; Cyst C, Cytochrome c; STAT3, Signal transducer, and activator of transcription 3; NF-ĸB, Nuclear factor-kappaB.
7. Conclusions
Recently, herbal remedy using some popular herbal spices, including CUR, Que, and Res has acquired much attention in the treatment of OCa, as one of the common gynecologic cancers, through different mechanisms. For example, CUR through suppression of EMT, angiogenesis, and STAT3 and NF-ĸB signaling, modulation of the expression of tumor-related-ncRNA, apoptosis stimulation, AMPK activation, inhibition of STAT3 and NF-ĸB signaling, and induction of autophagy can affect OCa. Que decreases the expression of survivin protein, induces the expression DR5 and ATM phosphorylation, and increases p53 protein expression. Res through mitochondrial secretion of cytochrome c, inhibition of glucose metabolism and STAT3, Notch, and Wnt signaling, and downregulation of the protein cyclin D1 can fight against OCa. However, these herbal products can have some negative aspects in terms of pharmacology, such as instability, poor bioavailability, and poor water solubility. Based on the evidence, using nano-based formulations from these herbal therapeutic candidates, for instance, gemini, ZnO nanohybrids, PEGylated liposome, NPs, micelles, niosome, not only can overcome these obstacles but also can improve the therapeutic potential of herbal medicine against OCa. However, more and larger researches are needed to show their therapeutic effects and mechanisms.
Author Contributions
F.R.-T. contributed to the acquisition, analysis, and interpretation of data for the work. R.A. and H.M. designed the framework of the manuscript. H.R.-S., M.K.G., F.A.Z., and A.B. contributed to the write-up of the review article. The final version of the draft manuscript was critically reviewed and proofread by R.A. and H.R.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was not financially supported.
Acknowledgments
This work was not financially supported and was carried out in a personal capacity. The figures were created by the web-based software BioRender.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xia, L.; Wang, Y.; Cai, S.; Xu, M. DGAT1 Expression Promotes Ovarian Cancer Progression and Is Associated with Poor Prognosis. J. Immunol. Res. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 2, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.V.; Prahm, K.P.; Christensen, I.J.; Hansen, A.; Høgdall, C.K.; Høgdall, E.V. Gene expression profile association with poor prognosis in epithelial ovarian cancer patients. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Henley, S.J.; Peipins, L.A.; Rim, S.H.; Larson, T.C.; Miller, J.W. Geographic Co-Occurrence of Mesothelioma and Ovarian Cancer Incidence. J. Women’s Health 2020, 29, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–25. [Google Scholar] [PubMed]
- Gong, G.; Lin, T.; Yuan, Y. Integrated analysis of gene expression and DNA methylation profiles in ovarian cancer. J. Ovarian Res. 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Li, X. Identification of an energy metabolism-related gene signature in ovarian cancer prognosis. Oncol. Rep. 2020, 43, 1755–1770. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.R.; Bhattacharya, R.; Mukherjee, P. Fabrication and functional characterization of goldnanoconjugates for potential application in ovarian cancer. J. Mater. Chem. 2010, 20, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Gatti, A.; Plotti, F.; Montera, R.; Terranova, C.; Nardone, C.D.C. Paraneoplastic Arthritis in Advanced Ovarian Cancer and its Correlation with CA125 and HE4 Levels: A Case Report. Ann. Case Rep. 2021, 6, 608. [Google Scholar]
- Chava, S.; Bugide, S.; Edwards, Y.J.; Gupta, R. Disruptor of telomeric silencing 1-like promotes ovarian cancer tumor growth by stimulating pro-tumorigenic metabolic pathways and blocking apoptosis. Oncogenesis 2021, 10, 1–14. [Google Scholar] [CrossRef]
- Zahradnikova, M.; Ihnatova, I.; Lattova, E.; Uhrik, L.; Stuchlikova, E.; Nenutil, R.; Valik, D.; Nalezinska, M.; Chovanec, J.; Zdrahal, Z.; et al. N-Glycome changes reflecting resistance to platinum-based chemotherapy in ovarian cancer. J. Proteom. 2021, 230, 103964. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Hazra, J.; Pal, K.; Nelson, V.K.; Pal, M. Prostate cancer: Therapeutic prospect with herbal medicine. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100034. [Google Scholar] [CrossRef]
- Safarzadeh, E.; Shotorbani, S.S.; Baradaran, B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv. Pharm. Bull. 2014, 4, 421–427. [Google Scholar] [PubMed]
- Barani, M.; Bilal, M.; Sabir, F.; Rahdar, A.; Kyzas, G.Z. Nanotechnology in ovarian cancer: Diagnosis and treatment. Life Sci. 2021, 266, 118914. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Wang, Y.; Li, J.I.; Huang, Q. Preparation of Curcumin Sub-micrometer Dispersions by High-Pressure Homogenization. J. Agric. Food Chem. 2010, 58, 2848–2853. [Google Scholar] [CrossRef] [PubMed]
- Di Bari, L.; Ripoli, S.; Pradhan, S.; Salvadori, P. Interactions between quercetin and warfarin for albumin binding: A new eye on food/drug interference. Chirality Pharmacol. Biol. Chem. Conseq. Mol. Asymmetry 2010, 22, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Aggarwal, B.B.; Orhan, I.E.; Barreca, D.; Battino, M.; Belwal, T.; Bishayee, A.; Daglia, M.; Devkota, H.P.; Echeverria, J.; et al. Resveratrol, a popular dietary supplement for human and animal health: Quantitative research literature analysis-A review. Anim. Sci. Pap. Rep. 2019, 37, 103–118. [Google Scholar]
- Chuang, L.; Lyu, Y.; Liu, C. Identification of Molecular Markers Associated with Ovarian Cancer Prognosis Using Bioinformatics Analysis. Int. J. Cogn. Comput. Eng. 2020, 7, 11023–11036. [Google Scholar]
- Saed, G.M.; Diamond, M.P.; Fletcher, N.M. Updates of the role of oxidative stress in the pathogenesis of ovarian cancer. Gynecol. Oncol. 2017, 145, 595–602. [Google Scholar] [CrossRef]
- Zare, H.; Shafabakhsh, R.; Reiter, R.J.; Asemi, Z. Melatonin is a potential inhibitor of ovarian cancer: Molecular aspects. J. Ovarian Res. 2019, 12, 1–8. [Google Scholar] [CrossRef]
- Freedman, R.S.; Deavers, M.; Liu, J.; Wang, E. Peritoneal inflammation–A microenvironment for epithelial ovarian cancer (EOC). J. Transl. Med. 2004, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Macciò, A.; Madeddu, C. Inflammation and ovarian cancer. Cytokine 2012, 58, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Belotte, J. The role of oxidative stress in the development of cisplatin resistance in epithelial ovarian cancer. Reprod. Sci. 2014, 21, 503–508. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Cohen, J.; Hummel, C.; Burky, R.; Cruz, A.; Farias-Eisner, R. The Role of Oxidative Stress in Ovarian Cancer: Implications for the Treatment of Patients. Cancer 2014, 41–50. [Google Scholar] [CrossRef]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhou, D.; Wang, Q.; Liu, W.; Yu, F.; Wu, F.; Chen, C. Crosstalk of microRNAs and oxidative stress in the pathogenesis of cancer. Oxidative Med. Cell. Longev. 2020, 2020, 2415324. [Google Scholar] [CrossRef] [PubMed]
- Kinose, Y.; Sawada, K.; Nakamura, K.; Kimura, T. The Role of MicroRNAs in Ovarian Cancer. BioMed Res. Int. 2014, 2014, 249393. [Google Scholar] [CrossRef] [PubMed]
- Karst, M.A.; Drapkin, R. Ovarian cancer pathogenesis: A model in evolution. J. Oncol. 2010, 2010, 932371. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q. Effects of BRCA1-and BRCA2-related mutations on ovarian and breast cancer survival: A meta-analysis. Clin. Cancer Res. 2015, 21, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.K.; Sorosky, J.I.; Dolan, M.; Anderson, B.; Buller, R.E. Distant metastases in ovarian cancer: Association with p53 mutations. Clin. Cancer Res. 1999, 5, 2485–2490. [Google Scholar] [PubMed]
- Saito, M.; Okamoto, A.; Kohno, T.; Takakura, S.; Shinozaki, H.; Isonishi, S.; Yasuhara, T.; Yoshimura, T.; Ohtake, Y.; Ochiai, K.; et al. Allelic imbalance and mutations of the PTEN gene in ovarian cancer. Int. J. Cancer 2000, 85, 160–165. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Dawood, M.A.; Elnesr, S.S.; Dhama, K. Curcumin and its different forms: A review on fish nutrition. Aquaculture 2020, 532, 736030. [Google Scholar] [CrossRef]
- Tang, W.; Du, M.; Zhang, S.; Jiang, H. Therapeutic effect of curcumin on oral diseases: A literature review. Phytother. Res. 2021, 35, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, F.; Majeed, M.; Jamialahmadi, T.; Sahebkar, A. Curcumin: A Review of Its Effects on Epilepsy. Stud. Biomark. New Targets Aging Res. Iran 2021, 1291, 363–373. [Google Scholar]
- Kuttan, R.; Sudheeran, P.; Josph, C. Turmeric and curcumin as topical agents in cancer therapy. Tumori J. 1987, 73, 29–31. [Google Scholar] [CrossRef]
- Shishodia, S.; Chaturvedi, M.M.; Aggarwal, B.B. Role of curcumin in cancer therapy. Curr. Probl. Cancer 2007, 31, 243–305. [Google Scholar] [CrossRef] [PubMed]
- Thangapazham, R.L.; Puri, A.; Tele, S.; Blumenthal, R.; Maheshwari, R.K. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int. J. Oncol. 2008, 32, 1119–1123. [Google Scholar] [CrossRef]
- Shi, M.; Cai, Q.; Yao, L.; Mao, Y.; Ming, Y.; Ouyang, G. Antiproliferation and apoptosis induced by curcumin in human ovarian cancer cells. Cell Biol. Int. 2006, 30, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, W.; Han, N.; Zou, Y.; Yin, D. Curcumin inhibits proliferation, migration, invasion and promotes apoptosis of retinoblastoma cell lines through modulation of miR-99a and JAK/STAT pathway. BMC Cancer 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Ghasemi, F.; Shafiee, M.; Banikazemi, Z.; Pourhanifeh, M.H.; Khanbabaei, H.; Shamshirian, A.; Moghadam, S.A.; ArefNezhad, R.; Sahebkar, A.; Avan, A.; et al. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathol. Res. Pract. 2019, 215, 152556. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, W.; Xie, C.; Huang, C.; Zhu, J.; Liang, Z.; Deng, F.; Zhu, M.; Zhu, W.; Wu, R.; et al. Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phytother. Res. 2014, 28, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, K.; Liu, J.; Yang, J.; Tian, Y.; Yang, C.; Li, Y.; Shao, M.; Su, W.; Song, N. Curcumin regulates cancer progression: Focus on ncRNAs and molecular signaling pathways. Front. Oncol. 2021, 11, 1202. [Google Scholar] [CrossRef]
- Pan, W. AMPK mediates curcumin-induced cell death in CaOV3 ovarian cancer cells. Oncol. Rep. 2008, 20, 1553–1559. [Google Scholar] [PubMed]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Sahin, N.; Tastan, H.; Özercan, İ.H.; Güler, O.; Kahraman, N.; Kucuk, O.; Ozpolat, B. Chemopreventive and antitumor efficacy of curcumin in a spontaneously developing hen ovarian cancer model. Cancer Prev. Res. 2018, 11, 59–67. [Google Scholar] [CrossRef]
- Liu, L.-D.; Pang, Y.X.; Zhao, X.R.; Li, R.; Jin, C.J.; Xue, J.; Dong, R.Y.; Liu, P.S. Curcumin induces apoptotic cell death and protective autophagy by inhibiting AKT/mTOR/p70S6K pathway in human ovarian cancer cells. Arch. Gynecol. Obstet. 2019, 299, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.; Dehghan, G.; Baradaran, B.; Zarebkohan, A.; Mansoori, B.; Soleymani, J.; Dolatabadi, J.E.N.; Hamblin, M.R. Co-delivery of curcumin and Bcl-2 siRNA by PAMAM dendrimers for enhancement of the therapeutic efficacy in HeLa cancer cells. Colloids Surf. B Biointerfaces 2020, 188, 110762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Chen, Y.; Zhang, Y.; Wang, Y.; Zhang, Y.; Song, L.; Jiang, B.; Su, G.; Li, Y.; et al. Glutathione-responsive self-delivery nanoparticles assembled by curcumin dimer for enhanced intracellular drug delivery. Int. J. Pharm. 2018, 549, 230–238. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Zhang, Y.; Zhang, Y.; Zhu, Y.; Shi, J.; Sun, Y.; Huang, Q. Encapsulation of curcumin within poly (amidoamine) dendrimers for delivery to cancer cells. J. Mater. Sci. Mater. Med. 2013, 24, 2137–2144. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Chen, W.R.; Tsosie, J.K.; Xie, X.; Li, P.; Wan, J.B.; He, C.W.; Chen, M.W. Niosome Encapsulation of Curcumin: Characterization and Cytotoxic Effect on Ovarian Cancer Cells. J. Nanomater. 2016, 2016, 6365295. [Google Scholar] [CrossRef]
- Puras, G.; Mashal, M.; Zárate, J.; Agirre, M.; Ojeda, E.; Grijalvo, S.; Eritja, R.; Diaz-Tahoces, A.; Navarrete, G.M.; Avilés-Trigueros, M.; et al. A novel cationic niosome formulation for gene delivery to the retina. J. Control. Release 2014, 174, 27–36. [Google Scholar] [CrossRef]
- Sandhiutami, N.M.D.; Arozal, W.; Louisa, M.; Rahmat, D.; Wuyung, P.E. Curcumin Nanoparticle Enhances the Anticancer Effect of Cisplatin by Inhibiting PI3K/AKT and JAK/STAT3 Pathway in Rat Ovarian Carcinoma Induced by DMBA. Front. Pharmacol. 2021, 11, 2199–2210. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.-Y.; Li, Z.-Y.; Ning, S.-Q. Evaluation of the efficacy of paclitaxel with curcumin combination in ovarian cancer cells. Oncol. Lett. 2016, 12, 3944–3948. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, Y.; Ran, M.; Wang, B.; Lin, Y.; Cheng, Y.; Zheng, S. Co-Delivery of Docetaxel and Curcumin via Nanomicelles for Enhancing Anti-Ovarian Cancer Treatment. Int. J. Nanomed. 2020, 15, 9703–9715. [Google Scholar] [CrossRef] [PubMed]
- Bondì, M.L.; Emma, M.R.; Botto, C. Biocompatible lipid nanoparticles as carriers to improve curcumin efficacy in ovarian cancer treatment. J. Agric. Food Chem. 2017, 65, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, S.; Babaei, E.; Hussen, B.M.; Mahdavi, M.; Azeez, H.J. Gemini Curcumin Suppresses Proliferation of Ovarian Cancer OVCAR-3 Cells via Induction of Apoptosis. Anti-Cancer Agents Med. Chem. 2021, 21, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-D.; Li, J.-Q.; Chen, F.-Y. Co-Delivery of Curcumin and Paclitaxel by “Core-Shell” Targeting Amphiphilic Copolymer to Reverse Resistance in the Treatment of Ovarian Cancer. Int. J. Nanomed. 2019, 14, 9453–9467. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, E.S.; Zarei, M.A.; Kashani, H.H.; Salimian, M.; Kashani, N.R.; Nikzad, H. Altered Long Non-coding RNAs Expression and Cytotoxic and Anti-proliferative Activity of Dendrosomal Nano-curcumin in Ovarian Cancer Cells. Indian J. Gynecol. Oncol. 2021, 19, 1–9. [Google Scholar] [CrossRef]
- Gao, X.; Wang, B.; Wei, X.; Men, K.; Zheng, F.; Zhou, Y.; Zheng, Y.; Gou, M.; Huang, M.; Guo, G.; et al. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale 2012, 4, 7021–7030. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, B.; Wang, T. Enhancing the anti-ovarian cancer activity of quercetin using a self-assembling micelle and thermosensitive hydrogel drug delivery system. RSC Adv. 2018, 8, 21229–21242. [Google Scholar] [CrossRef]
- Long, Q.; Xie, Y.; Huang, Y.; Wu, Q.; Zhang, H.; Xiong, S.; Liu, Y.; Chen, L.; Wei, Y.; Zhao, X.; et al. Induction of apoptosis and inhibition of angiogenesis by PEGylated liposomal quercetin in both cisplatin-sensitive and cisplatin-resistant ovarian cancers. J. Biomed. Nanotechnol. 2013, 9, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Annaji, M.; Poudel, I.; Boddu, S.H.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Resveratrol-loaded nanomedicines for cancer applications. Cancer Report 2021, 13, e1353. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Peng, Y.; Yao, J.; Sui, L.; Gu, A.; Wang, J. Anticancer activity and molecular mechanism of resveratrol–Bovine serum albumin nanoparticles on subcutaneously implanted human primary ovarian carcinoma cells in Nude mice. Cancer Biother. Radiopharm. 2010, 25, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Zhu, W.; Guo, L.; Pu, L. RGD-Conjugated Resveratrol HSA Nanoparticles as a Novel Delivery System in Ovarian Cancer Therapy. Drug Des. Dev. Ther. 2020, 14, 5747–5756. [Google Scholar] [CrossRef] [PubMed]
- Tavana, E.; Mollazadeh, H.; Mohtashami, E.; Modaresi, S.M.S.; Hosseini, A.; Sabri, H.; Soltani, A.; Javid, H.; Afshari, A.R.; Sahebkar, A. Quercetin: A promising phytochemical for the treatment of glioblastoma multiforme. BioFactors 2020, 46, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Hirpara, K.V.; Aggarwal, P.; Mukherjee, A.J.; Joshi, N.; Burman, A.C. Quercetin and its derivatives: Synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anti-Cancer Agents Med. Chem. 2009, 9, 138–161. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Asemi, Z. Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 2019, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Effects of quercetin on proliferation and migration of human glioblastoma U251 cells. Biomed. Pharmacother. 2017, 92, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.-S.; Hou, Y.C.; Pai, M.H.; Lin, M.T.; Yeh, S.L. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: In vitro and in vivo studies. J. Nutr. Biochem. 2018, 51, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.B.; Mir, H.; Kapur, N.; Gales, D.N.; Carriere, P.P.; Singh, S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, P.; Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater. Sci. Eng. C 2019, 100, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.N.; Srivastava, R.A.K. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine 2019, 52, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Khan, I.A.; ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Niaz, K.; Maqbool, F.; Ismail Hassan, F.; Abdollahi, M.; Nagulapalli Venkata, K.C.; Nabavi, S.M.; Bishayee, A. Molecular targets underlying the anticancer effects of quercetin: An update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The anti-cancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic potential of quercetin: New insights and perspectives for human health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Hwang, J.T.; Kwon, D.Y.; Surh, Y.J.; Park, O.J. Induction of apoptosis by quercetin is mediated through AMPKα1/ASK1/p38 pathway. Cancer Lett. 2010, 292, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.-Y.; Wu, Y.C.; Chung, J.G.; Yang, J.S.; Lu, H.F.; Tsou, M.F.; Wood, W.G.; Kuo, S.J.; Chen, D.R. Quercetin-induced apoptosis acts through mitochondrial-and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum. Exp. Toxicol. 2009, 28, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and Cancer Chemoprevention. Evid. Based Compl. Altern. Med. 2011, 2011, 591356. [Google Scholar] [CrossRef]
- Teekaraman, D.; Elayapillai, S.P.; Viswanathan, M.P.; Jagadeesan, A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chem. Biol. Interact. 2019, 300, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.X.; Deng, X.H.; Ai, F.; Yuan, G.Y.; Song, H.Y. Effect of quercetin on the proliferation of the human ovarian cancer cell line SKOV-3 in vitro. Exp. Ther. Med. 2015, 10, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, W.; Yang, Z.Y.; Zhou, X.S.; Gong, C.; Zhang, T.R.; Wei, X.; Ma, D.; Ye, F.; Gao, Q.L. Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis 2017, 22, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Zongyuan, Y.; Cheng, G.; Lingyun, Z.; GuiLian, Y.; Wei, G. Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer-binding protein homologous protein (CHOP)-death receptor 5 pathway. Cancer Sci. 2014, 105, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Aggarwal, B.B. Receptor-mediated choreography of life and death. J. Clin. Immunol. 2003, 23, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; O’Rourke, K.; Chinnaiyan, A.M.; Gentz, R.; Ebner, R.; Ni, J.; Dixit, V.M. The receptor for the cytotoxic ligand TRAIL. Science 1997, 276, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Kurbanov, B.M.; Fecker, L.F.; Geilen, C.C.; Sterry, W.; Eberle, J. Resistance of melanoma cells to TRAIL does not result from upregulation of antiapoptotic proteins by NF-κ B but is related to downregulation of initiator caspases and DR4. Oncogene 2007, 26, 3364–3377. [Google Scholar] [CrossRef][Green Version]
- Van Geelen, C.M.; Pennarun, B.; Le, P.T.; de Vries, E.G.; de Jong, S. Modulation of TRAIL resistance in colon carcinoma cells: Different contributions of DR4 and DR5. BMC Cancer 2011, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.-C.; Liu, H.-F.; Chao, J.-I. Survivin and p53 modulate quercetin-induced cell growth inhibition and apoptosis in human lung carcinoma cells. J. Biol. Chem. 2004, 279, 55875–55885. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Yang, Z.; Zhang, L.; Wang, Y.; Gong, W.; Liu, Y. Quercetin suppresses DNA double-strand break repair and enhances the radiosensitivity of human ovarian cancer cells via p53-dependent endoplasmic reticulum stress pathway. OncoTargets Ther. 2018, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, U.; Ali, A.; Khan, M.M.; Siddiqui, M.A.; Akhtar, J.; Ahmad, F.J. Nanotechnology-Based Strategies for Nutraceuticals: A Review of Current Research Development. Nanosci. Technol. Int. J. 2019, 10, 133–155. [Google Scholar] [CrossRef]
- Alexander, A.; Patel, R.J.; Saraf, S.; Saraf, S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J. Control. Release 2016, 241, 110–124. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Wei, T.; Ma, X.; Cheng, Q.; Huo, S.; Zhang, C.; Zhang, Y.; Duan, X.; Liang, X.J. Quercetin-loaded nanomicelles to circumvent human castration-resistant prostate cancer in vitro and in vivo. Nanoscale 2016, 8, 5126–5138. [Google Scholar] [CrossRef] [PubMed]
- Aqeel, R.; Srivastava, N.; Kushwaha, P. Micelles in Cancer Therapy: An Update on Preclinical and Clinical Status. Recent Pat. Nanotechnol. 2021, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Men, K.; Shi, H.; Xiang, M.; Zhang, J.; Song, J.; Long, J.; Wan, Y.; Luo, F.; Zhao, X.; et al. Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale 2011, 3, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Wei, X.; Men, K.; Wang, B.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. PCL/PEG copolymeric nanoparticles: Potential nanoplatforms for anticancer agent delivery. Curr. Drug Targets 2011, 12, 1131–1150. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.; Gupta, A.; Singh, P.; Gupta, C.; Sadhu, V.; Reddy, K.R. Synthesis and characterization of celecoxib loaded PEGylated liposome nanoparticles for biomedical applications. Nano Struct. Nano Objects 2019, 18, 100288. [Google Scholar] [CrossRef]
- Kozuki, Y.; Miura, Y.; Yagasaki, K. Resveratrol suppresses hepatoma cell invasion independently of its anti-proliferative action. Cancer Lett. 2001, 167, 151–156. [Google Scholar] [CrossRef]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Tan, L.; Wang, W.; He, G.; Kuick, R.D.; Gossner, G.; Kueck, A.S.; Wahl, H.; Opipari, A.W.; Liu, J.R. Resveratrol inhibits ovarian tumor growth in an in vivo mouse model. Cancer 2016, 122, 722–729. [Google Scholar] [CrossRef]
- Junco, J.J.; Mancha, A.; Malik, G.; Wei, S.J.; Kim, D.J.; Liang, H.; Slaga, T.J. Resveratrol and P-glycoprotein inhibitors enhance the anti-skin cancer effects of ursolic acid. Mol. Cancer Res. 2013, 11, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for Breast Cancer Prevention and Therapy: Preclinical Evidence and Molecular Mechanisms. Semin. Cancer Biol. 2014, 40, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Honari, M.; Shafabakhsh, R.; Reiter, R.J.; Mirzaei, H.; Asemi, Z. Resveratrol is a promising agent for colorectal cancer prevention and treatment: Focus on molecular mechanisms. Cancer Cell Int. 2019, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Vlachogiannis, I.A.; Tsiani, E. Effects of resveratrol against lung cancer: In vitro and in vivo studies. Nutrients 2017, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Sexton, É.; Van Themsche, C.; Leblanc, K.; Parent, S.; Lemoine, P.; Asselin, E. Resveratrol interferes with AKT activity and triggers apoptosis in human uterine cancer cells. Mol. Cancer 2006, 5, 1–13. [Google Scholar] [CrossRef]
- Shao, B.-Z.; Xu, Z.Q.; Han, B.Z.; Su, D.F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, Y.; Yu, H.; Shen, B.; Liang, Y.; Jin, R.; Liu, X.; Shi, L.; Cai, X. Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy. Cancer Med. 2016, 5, 2061–2068. [Google Scholar] [CrossRef]
- Manju, S.; Ethiraj, K.; Elias, G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: A structure-based approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar]
- Opipari, A.W.; Tan, L.; Boitano, A.E.; Sorenson, D.R.; Aurora, A.; Liu, J.R. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004, 64, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Kueck, A.; Opipari Jr, A.W.; Griffith, K.A.; Tan, L.; Choi, M.; Huang, J.; Wahl, H.; Liu, J.R. Resveratrol inhibits glucose metabolism in human ovarian cancer cells. Gynecol. Oncol. 2007, 107, 450–457. [Google Scholar] [CrossRef]
- Bui, T.; Thompson, C.B. Cancer’s sweet tooth. Cancer Cell 2006, 9, 419–420. [Google Scholar] [CrossRef]
- Zhong, L.-X.; Li, H.; Wu, M.L.; Liu, X.Y.; Zhong, M.J.; Chen, X.Y.; Liu, J.; Zhang, Y. Inhibition of STAT3 signaling as critical molecular event in resveratrol-suppressed ovarian cancer cells. J. Ovarian Res. 2015, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, L.; Luo, Y.; Li, X.; Chen, G.; Wang, Y. Resveratrol inhibits the proliferation and induces the apoptosis in ovarian cancer cells via inhibiting glycolysis and targeting AMPK/mTOR signaling pathway. J. Cell. Biochem. 2018, 119, 6162–6172. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Simeone, P.; Toraldo, D.; Del Boccio, P.; Vergaro, V.; Leporatti, S.; Pieragostino, D.; Tinelli, A.; De Domenico, S.; Alberti, S.; et al. Resveratrol downregulates Akt/GSK and ERK signalling pathways in OVCAR-3 ovarian cancer cells. Mol. Biosyst. 2012, 8, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Baribeau, S.; Chaudhry, P.; Parent, S.; Asselin, É. Resveratrol inhibits cisplatin-induced epithelial-to-mesenchymal transition in ovarian cancer cell lines. PLoS ONE 2014, 9, e86987. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, P.; Ghidoni, R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J. Nutr. Biochem. 2005, 16, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Emília Juan, M.; Buenafuente, J.; Casals, I.; Planas, J.M. Plasmatic levels of trans-resveratrol in rats. Food Res. Int. 2002, 35, 195–199. [Google Scholar] [CrossRef]
- Khatun, M.; Choudhury, S.; Liu, B.; Lemmens, P.; Pal, S.K.; Mazumder, S. Resveratrol–ZnO nanohybrid enhanced anti-cancerous effect in ovarian cancer cells through ROS. RSC Adv. 2016, 6, 105607–105617. [Google Scholar] [CrossRef]
- Yu, Z.; Yu, M.; Zhang, Z.; Hong, G.; Xiong, Q. Bovine serum albumin nanoparticles as controlled release carrier for local drug delivery to the inner ear. Nanoscale Res. Lett. 2014, 9, 343. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).