A Large Sample Retrospective Study on the Distinction of Voriconazole Concentration in Asian Patients from Different Clinical Departments
Abstract
:1. Introduction
2. Results
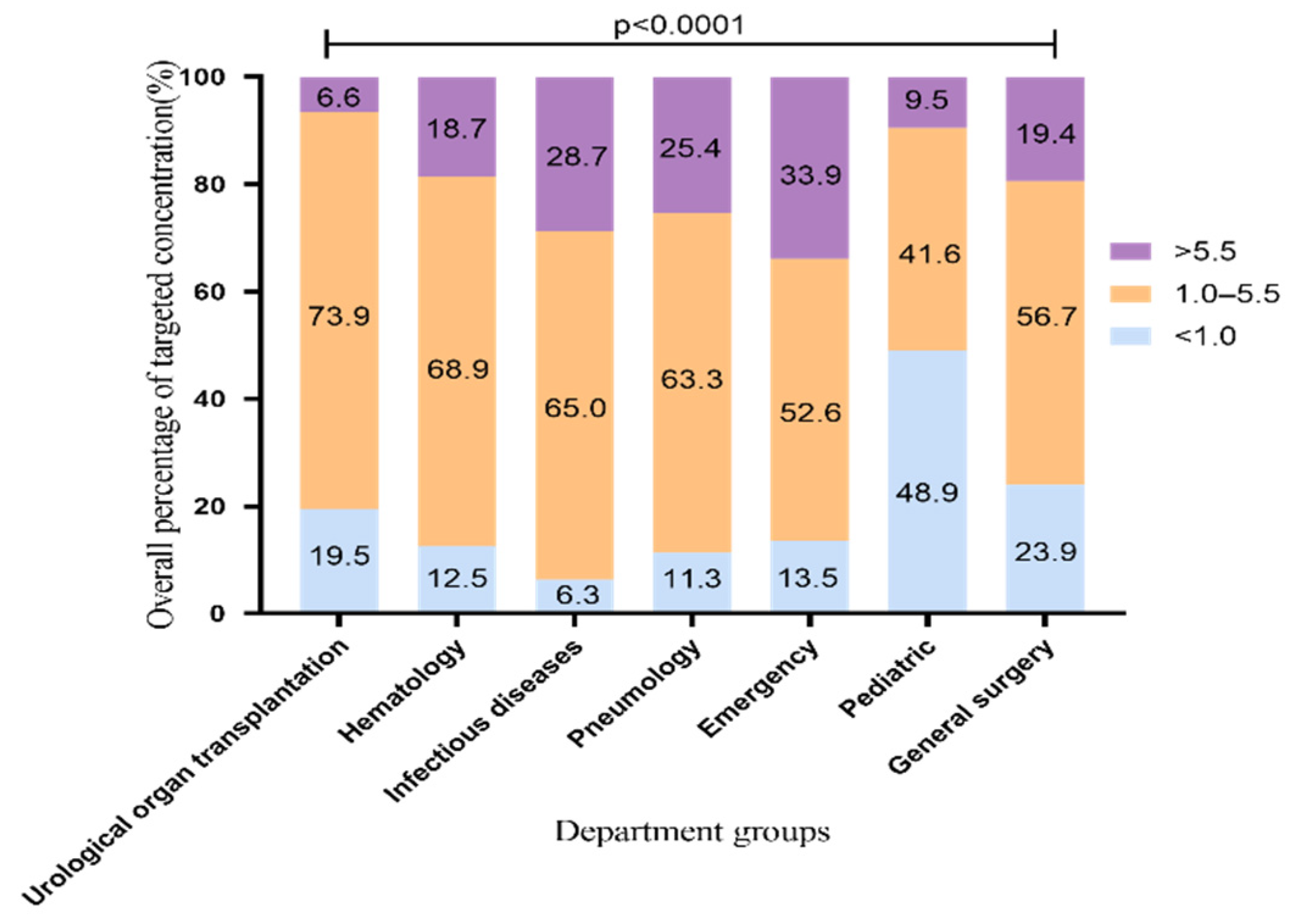
2.1. The Overall Standard Rate of VRZ Trough Concentration
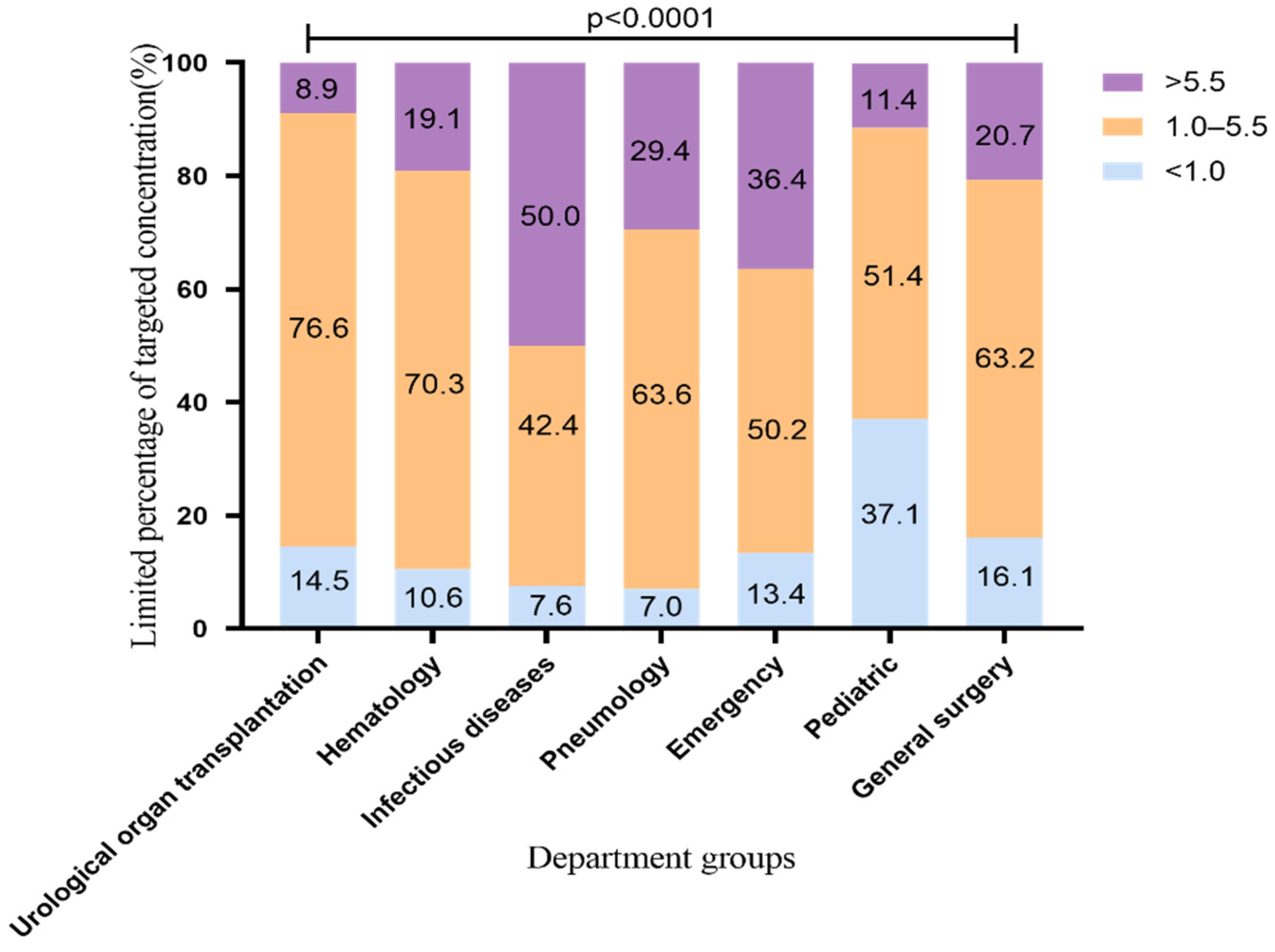
2.2. The Standard Rate of VRZ Trough Concentration Using 200 mg q12h
2.3. Patient Characteristics
2.4. Difference of VRZ Concentration among Seven Departments
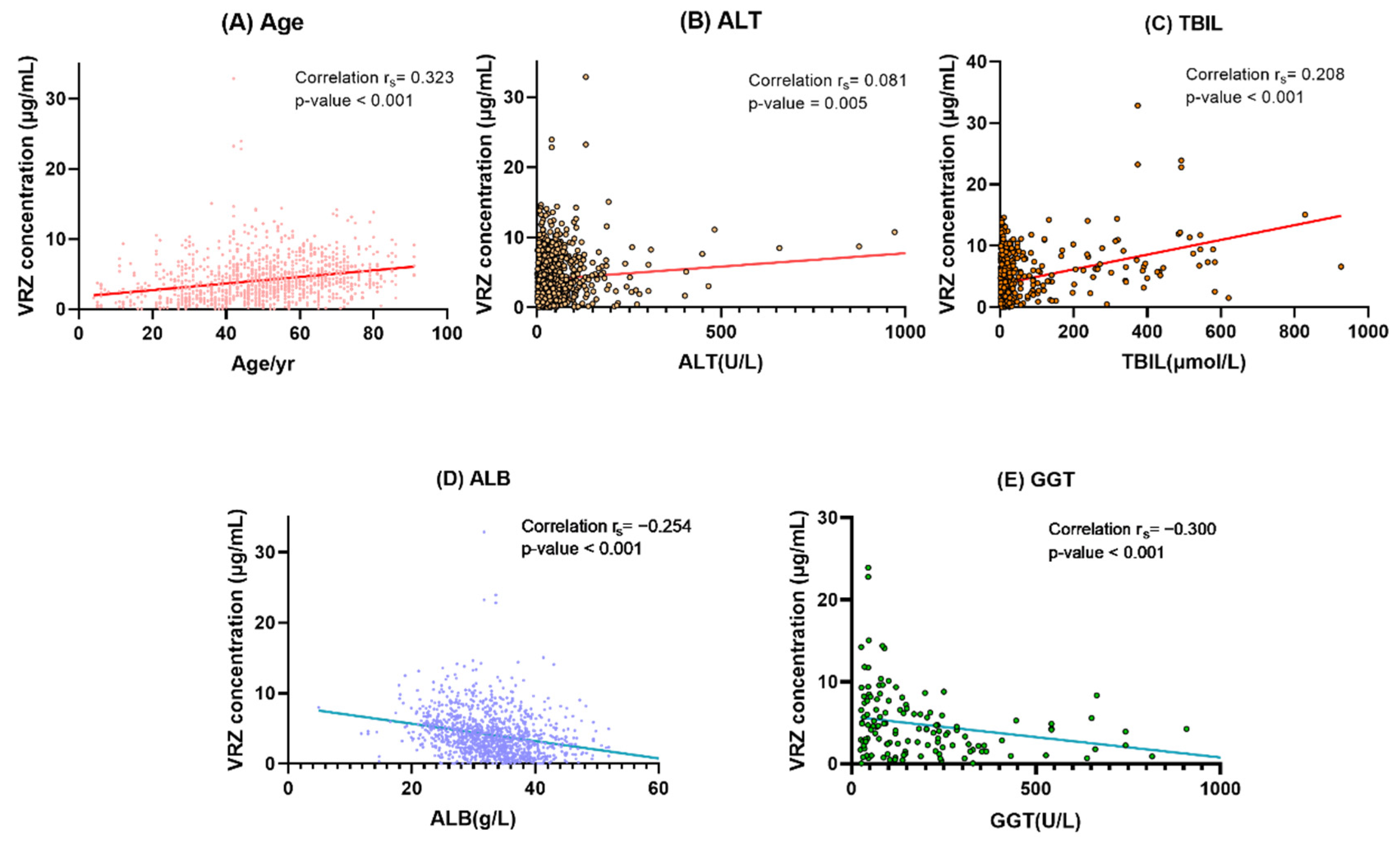
2.5. Determinants of VRZ Trough Concentration
+ 0.010 × total bilirubin − 0.100 × albumin − 0.004 × gamma-glutamyl transferase
2.6. Diagnosis of the Multiple Linear Model
3. Discussion
4. Materials and Methods
4.1. The Patients and Inclusion Criteria
4.2. VRZ Administration and Data Collection
4.3. Blood Sampling and Analytical Assays
4.4. CPY2C19 Genotyping and Phenotype Assignment
4.5. The Standard Rate of VRZ Trough Concentration
4.6. Differences of VRZ Concentration among Different Departments and the Influencing Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosseini-Moghaddam, S.M.; Ouédraogo, A.; Naylor, K.L.; Bota, S.E.; Husain, S.; Nash, D.M.; Paterson, J.M. Incidence and outcomes of invasive fungal infection among solid organ transplant recipients: A population-based cohort study. Transpl. Infect. Dis. 2020, 22, e13250. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, H.; Chen, J.; Li, J.; Ma, J.; Li, J.; Liang, Y.; Wang, J.; Li, Y.; Yu, K. Invasive fungal infection in patients receiving chemotherapy for hematological malignancy: A multicenter, prospective, observational study in China. Tumour Biol. 2015, 36, 757–767. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Pappas, P.G.; Wingard, R.J. Invasive Fungal Pathogens: Current Epidemiological Trends. Clin. Infect. Dis. 2006, 43, S3–S14. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Rosanova, M.T.; Bes, D.; Serrano Aguilar, P.; Sberna, N.; Lede, R. Efficacy and safety of voriconazole in immunocompromised patients: Systematic review and meta-analysis. Infect. Dis. 2018, 50, 489–494. [Google Scholar] [CrossRef]
- Pearson, M.M.; Rogers, P.D.; Cleary, J.D.; Chapman, S.W. Voriconazole: A New Triazole Antifungal Agent. Ann. Pharmacother. 2003, 37, 420–432. [Google Scholar] [CrossRef]
- Moriyama, B.; Obeng, A.O.; Barbarino, J.; Penzak, S.R.; Henning, S.A.; Scott, S.A.; Agúndez, J.; Wingard, J.R.; McLeod, H.L.; Klein, T.E. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP2C19 and Voriconazole Therapy. Clin. Pharm. 2017, 102, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Chen, S.; Sun, J.; Cai, J.; Cheng, X.; Dong, H.; Wang, X.; Xing, J.; Dong, W.; Yao, H. Identification of factors influencing the pharmacokinetics of voriconazole and the optimization of dosage regimens based on Monte Carlo simulation in patients with invasive fungal infections. J. Antimicrob. Chemother. 2014, 69, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, B.H.; Nam, W.S.; Yoon, S.H.; Cho, J.Y.; Shin, S.G.; Jang, I.J.; Yu, K.S. Effect of CYP2C19 polymorphism on the pharmacokinetics of voriconazole after single and multiple doses in healthy volunteers. J. Clin. Pharm. 2012, 52, 195–203. [Google Scholar] [CrossRef]
- Pascual, A.; Calandra, T.; Bolay, S.; Buclin, T.; Bille, J.; Marchetti, O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis 2008, 46, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Boyd, A.E.; Simon, M.; Howard, S.J.; Moore, C.B.; Keevil, B.G.; Denning, D.W. Adverse reactions to voriconazole. Clin. Infect. Dis. 2004, 39, 1241–1244. [Google Scholar] [CrossRef]
- Friberg, L.E.; Ravva, P.; Karlsson, M.O.; Liu, P. Integrated population pharmacokinetic analysis of voriconazole in children, adolescents, and adults. Antimicrob. Agents Chemother. 2012, 56, 3032–3042. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zhu, H.; Sun, J.; Cheng, X.; Xie, J.; Dong, H.; Chen, L.; Wang, X.; Xing, J.; Dong, Y. Efficacy and safety of voriconazole and CYP2C19 polymorphism for optimised dosage regimens in patients with invasive fungal infections. Int. J. Antimicrob. Agents 2014, 44, 436–442. [Google Scholar] [CrossRef]
- Levine, M.T.; Chandrasekar, P.H. Adverse effects of voriconazole: Over a decade of use. Clin. Transplant. 2016, 30, 1377–1386. [Google Scholar] [CrossRef]
- Luong, M.L.; Hosseini-Moghaddam, S.M.; Singer, L.G.; Chaparro, C.; Azad, S.; Lazar, N.; Boutros, P.C.; Keshavjee, S.; Rotstein, C.; Husain, S. Risk factors for voriconazole hepatotoxicity at 12 weeks in lung transplant recipients. Am. J. Transplant. 2012, 12, 1929–1935. [Google Scholar] [CrossRef]
- Pascual, A.; Csajka, C.; Buclin, T.; Bolay, S.; Bille, J.; Calandra, T.; Marchetti, O. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: Population pharmacokinetics-based analysis of adult patients with invasive fungal infections. Clin. Infect. Dis. 2012, 55, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Zwald, F.O.; Spratt, M.; Lemos, B.D.; Veledar, E.; Lawrence, C.; Marshall Lyon, G.; Chen, S.C. Duration of voriconazole exposure: An independent risk factor for skin cancer after lung transplantation. Derm. Surg 2012, 38, 1369–1374. [Google Scholar] [CrossRef]
- Li, Z.W.; Peng, F.H.; Yan, M.; Liang, W.; Liu, X.L.; Wu, Y.Q.; Lin, X.B.; Tan, S.L.; Wang, F.; Xu, P. Impact of CYP2C19 Genotype and Liver Function on Voriconazole Pharmacokinetics in Renal Transplant Recipients. Drug Monit. 2017, 39, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Mafuru, M.; Wu, S.; He, S.; Lu, X.; Huang, J.; Jiang, H. The Influence of Proinflammatory Cytokines on Voriconazole Trough Concentration in Patients with Different Forms of Hematologic Disorders. J. Clin. Pharm. 2019, 59, 1340–1350. [Google Scholar] [CrossRef]
- Hu, L.; Dai, T.T.; Zou, L.; Li, T.M.; Ding, X.S.; Yin, T. Therapeutic Drug Monitoring of Voriconazole in Children from a Tertiary Care Center in China. Antimicrob. Agents Chemother. 2018, 62, e00955-18. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Zhang, C.; Qin, Z.; Wang, D.; Yang, J.; Zhang, X. Impact of CYP2C19 Phenotype and Drug-Drug Interactions on Voriconazole Concentration in Pediatric Patients. Antimicrob. Agents Chemother. 2021, 65, Aac0020721. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Yan, J.; Gao, L.; Zhou, L.; Wang, Y.; Li, Q.; Wang, J.; Chen, T.; Wang, T. Voriconazole therapeutic drug monitoring in critically ill patients improves efficacy and safety of antifungal therapy. Basic Clin. Pharm. Toxicol. 2020, 127, 495–504. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, M.; Fu, P.; Xiao, X. Risk factors associated with insufficient and potentially toxic voriconazole plasma concentrations: An observational study. J. Chemother. 2019, 31, 401–407. [Google Scholar] [CrossRef]
- You, H.; Dong, Y.; Zou, Y.; Zhang, T.; Lei, J.; Chen, L.; Wang, X.; Dong, Y.; Wang, T. Voriconazole therapeutic drug monitoring: Factors associated with supratherapeutic and subtherapeutic voriconazole concentrations. Int. J. Clin. Pharm. 2018, 56, 239–246. [Google Scholar] [CrossRef]
- Allegra, S.; Fatiguso, G.; De Francia, S.; Favata, F.; Pirro, E.; Carcieri, C.; De Nicolò, A.; Cusato, J.; Di Perri, G.; D’Avolio, A. Therapeutic drug monitoring of voriconazole for treatment and prophylaxis of invasive fungal infection in children. Br. J. Clin. Pharm. 2018, 84, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Shao, B.; Ma, Y.; Li, Q.; Wang, Y.; Zhu, Z.; Zhao, H.; Sun, J.; Dong, L.; Zhu, Y.; Zhao, N. Effects of cytochrome P450 3A4 and non-genetic factors on initial voriconazole serum trough concentrations in hematological patients with different cytochrome P450 2C19 genotypes. Xenobiotica 2017, 47, 1121–1129. [Google Scholar] [CrossRef]
- Niioka, T.; Fujishima, N.; Abumiya, M.; Yamashita, T.; Ubukawa, K.; Nara, M.; Fujishima, M.; Takahashi, N.; Miura, M. Relationship Between the CYP2C19 Phenotype Using the Voriconazole-to-Voriconazole N-Oxide Plasma Concentration Ratio and Demographic and Clinical Characteristics of Japanese Patients With Different CYP2C19 Genotypes. Drug Monit. 2017, 39, 514–521. [Google Scholar] [CrossRef]
- Hoenigl, M.; Duettmann, W.; Raggam, R.B.; Seeber, K.; Troppan, K.; Fruhwald, S.; Prueller, F.; Wagner, J.; Valentin, T.; Zollner-Schwetz, I. Potential factors for inadequate voriconazole plasma concentrations in intensive care unit patients and patients with hematological malignancies. Antimicrob. Agents Chemother. 2013, 57, 3262–3267. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Lee, S.Y.; Hwang, J.Y.; Lee, S.H.; Yoo, K.H.; Sung, K.W.; Koo, H.H.; Kim, Y.J. Importance of voriconazole therapeutic drug monitoring in pediatric cancer patients with invasive aspergillosis. Pediatr. Blood Cancer 2013, 60, 82–87. [Google Scholar] [CrossRef]
- Lombardi, L.R.; Miano, T.A.; Davis, J.L.; Morgan, S.C.; Goldstein, S.C.; Vardhanabhuti, S.; Mitra, N.; Vozniak, J.M. A retrospective analysis of the effect of patient-specific factors on voriconazole concentrations in oncology patients. J. Oncol. Pharm. Pract. 2012, 18, 3–9. [Google Scholar] [CrossRef]
- Dolton, M.J.; Ray, J.E.; Chen, S.C.; Ng, K.; Pont, L.G.; McLachlan, A.J. Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrob. Agents Chemother. 2012, 56, 4793–4799. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Yee, J.; Kim, J.Y.; Han, H.W.; Kang, S.O.; Lee, K.E.; Gwak, H.S. Factors Associated With Voriconazole Concentration in Pediatric Patients. Drug Monit. 2020, 42, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Q.; Qiao, C.; Yang, Z.C.; Yu, L.; Sun, L.N.; Qian, Y.; Zhang, X.H.; Meng, L.; Zhang, X.Y.; Wang, Y.Q. The Impact of Plasma Protein Binding Characteristics and Unbound. Concentration of Voriconazole on Its Adverse Drug Reactions. Front. Pharm. 2020, 11, 505. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Noto, K.; Ota, R.; Yokoyama, S.; Hosomi, K.; Takada, M.; Matsuoka, H. Voriconazole trough concentration and hepatotoxicity in patients with low serum albumin. Int. J. Clin. Pharm. 2019, 57, 135–143. [Google Scholar] [CrossRef]
- Saini, L.; Seki, J.T.; Kumar, D.; Atenafu, E.G.; Cole, D.E.; Wong, B.Y.; Božović, A.; Brandwein, J.M. Serum voriconazole level variability in patients with hematological malignancies receiving voriconazole therapy. Can. J. Infect. Dis. Med. Microbiol. 2014, 25, 271–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.; Song, B.L.; Yan, M.; Zou, J.J.; Zhang, M.; Zhou, H.Y.; Wang, F.; Xiao, Y.W.; Xu, P.; Zhang, B.K. Identifying factors affecting the pharmacokinetics of voriconazole in patients with liver dysfunction: A population pharmacokinetic approach. Basic Clin. Pharm. Toxicol. 2019, 125, 34–43. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, T.; Liu, Y.; Wang, J.; Hu, K.; Bao, F.; Zhang, C. Model-based Voriconazole Dose Optimization in Chinese Adult Patients With Hematologic Malignancies. Clin. Ther. 2019, 41, 1151–1163. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, R.; Liu, F.; Li, Y.; Chen, H.; Yao, P.; Sun, F.; Xia, P. Therapeutic drug monitoring and safety of voriconazole in elderly patients. Int. Immunopharmacol. 2020, 78, 106078. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Lin, X.B.; Zhang, B.K.; Xiao, Y.W.; Xu, P.; Wang, F.; Xiang, D.X.; Xie, X.B.; Peng, F.H.; Yan, M. Predictors of Adverse Events and Determinants of the Voriconazole Trough Concentration in Kidney Transplantation Recipients. Clin. Transl. Sci. 2021, 14, 702–711. [Google Scholar] [CrossRef]
- Zeng, G.; Shi, L.; Li, H.; Wang, L.; Zhu, M.; Luo, J.; Zhang, Z. Effect of cyclosporine a and polymorphisms in CYP2C19 and ABCC2 on the concentration of voriconazole in patients undergoing allogeneic hematopoietic stem cell transplantation. Xenobiotica 2020, 50, 614–619. [Google Scholar] [CrossRef]
- Ruiz, J.; Gordon, M.; Villarreal, E.; Peruccioni, M.; Marqués, M.R.; Poveda-Andrés, J.L.; Castellanos-Ortega, Á.; Ramirez, P. Impact of voriconazole plasma concentrations on treatment response in critically ill patients. J. Clin. Pharm. 2019, 44, 572–578. [Google Scholar] [CrossRef]
- Dote, S.; Sawai, M.; Nozaki, A.; Naruhashi, K.; Kobayashi, Y.; Nakanishi, H. A retrospective analysis of patient-specific factors on voriconazole clearance. J. Pharm. Health Care Sci. 2016, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Dorado, S.; Maroñas, O.; Latorre-Pellicer, A.; Rodríguez Jato, M.T.; López-Vizcaíno, A.; Gómez Márquez, A.; Bardán García, B.; Belles Medall, D.; Barbeito Castiñeiras, G.; Pérez Del Molino Bernal, M.L. Impact of CYP2C19 Genotype and Drug Interactions on Voriconazole Plasma Concentrations: A Spain Pharmacogenetic-Pharmacokinetic Prospective Multicenter Study. Pharmacotherapy 2020, 40, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chayakulkeeree, M.; Poovipirom, N.; Siengwattana, P.; Maneerattanaporn, M. Effect of proton pump inhibitor on plasma voriconazole concentration in Thai patients. J. Med. Assoc. Thai. 2015, 98, 232–237. [Google Scholar] [PubMed]
- Kim, D.Y.; Park, H.J.; Lee, Y.J. Factors affecting voriconazole plasma concentrations in patients with invasive fungal infections. Int. J. Clin. Pharm. 2014, 52, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yan, M.; Tang, D.; Xue, L.; Zhang, T.; Dong, Y.; Zhu, L.; Wang, X.; Dong, Y. Therapeutic drug monitoring and safety of voriconazole therapy in patients with Child-Pugh class B and C cirrhosis: A multicenter study. Int. J. Infect. Dis. 2018, 72, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T.; Smith, A.R.; Jacobson, P.A.; Fisher, J.; Rubin, N.T.; Kirstein, M.N. Impact of Obesity on Voriconazole Pharmacokinetics among Pediatric Hematopoietic Cell Transplant Recipients. Antimicrob. Agents Chemother. 2020, 64, e00653-20. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Usuki, K.; Hanada, K.; Orii, T.; Kato, T. Does Diarrhea Influence Plasma Voriconazole Concentration? Drug Monit. 2020, 42, 341–344. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Wu, T.; Jiang, H.; Yang, S.; Zhang, Y. Dose optimisation of voriconazole with therapeutic drug monitoring in children: A single-centre experience in China. Int. J. Antimicrob. Agents 2017, 49, 483–487. [Google Scholar] [CrossRef]
- Boast, A.; Curtis, N.; Cranswick, N.; Gwee, A. Voriconazole dosing and therapeutic drug monitoring in children: Experience from a paediatric tertiary care centre. J. Antimicrob. Chemother. 2016, 71, 2031–2036. [Google Scholar] [CrossRef] [Green Version]
- Ashbee, H.R.; Barnes, R.A.; Johnson, E.M.; Richardson, M.D.; Gorton, R.; Hope, W.W. Therapeutic drug monitoring (TDM) of antifungal agents: Guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 2014, 69, 1162–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.B.; Li, Z.W.; Yan, M.; Zhang, B.K.; Liang, W.; Wang, F.; Xu, P.; Xiang, D.X.; Xie, X.B.; Yu, S.J. Population pharmacokinetics of voriconazole and CYP2C19 polymorphisms for optimizing dosing regimens in renal transplant recipients. Br. J. Clin. Pharm. 2018, 84, 1587–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristic | Value | Range |
|---|---|---|
| Male, N (%) | 478 (70.7%) | |
| Age (years) | 52.0 [40.0–64.0] | 4–91 |
| VRZ concentration (μg/mL) | 3.54 [1.76–5.63] | 0.05–32.86 |
| CYP2C19 phenotypes | ||
| Poor metabolizers, N (%) | 48 (10.6%) | |
| Immediate metabolizers, N (%) | 214 (47.1%) | |
| Extensive metabolizers, N (%) | 188 (41.4%) | |
| Rapid metabolizers, N (%) | 4 (0.9%) | |
| Physiological and biochemical indexes | ||
| WBC (109/L) | 6.05 [3.15–9.47] | 0.01–38.58 |
| HCT (%) | 27.30 [22.10–33.20] | 13.00–60.70 |
| HGB (g/L) | 87.0 [71.0–108.0] | 39.0–199.0 |
| PLT (109/L) | 147.0 [54.0–242.0] | 1.0–692.0 |
| ALT (U/L) | 20.8 [11.6–42.1] | 0.2–1948.2 |
| AST (U/L) | 27.2 [16.4–47.6] | 1.2–5596.3 |
| GGT (U/L) | 123.4 [53.1–246.6] | 24.0–1365.1 |
| ALP (U/L) | 154.7 [106.4–233.7] | 36.1–1231.5 |
| TBIL (μmol/L) | 8.4 [5.8–14.7] | 1.6–926.6 |
| ALB (g/L) | 32.6 ± 6.4 | 4.9–76.0 |
| INR | 1.19 [1.06–1.41] | 0.72–9.12 |
| CREA (μmol/L) | 68.4 [49.5–105.7] | 13.4–1062.4 |
| Hospital Departments | Urological Organ Transplantation | Hematology | Infectious Diseases | Pneumology | Emergency | Pediatric | General Surgery | p-Value |
|---|---|---|---|---|---|---|---|---|
| VRZ concentration (μg/mL, mean ± SD) | 2.57 ± 2.07 | 3.60 ± 2.33 | 6.29 ± 4.92 | 4.58 ± 2.77 | 4.71 ± 2.96 | 2.24 ± 2.25 | 3.78 ± 3.11 | <0.001 |
| Variable | Coefficient Index | p-Value |
|---|---|---|
| Gender | 0.067 * | 0.019 |
| Age | 0.323 ** | <0.001 |
| CYP2C19 phenotypes | −0.114 ** | 0.001 |
| Departments | ||
| Hematology | −0.087 ** | 0.002 |
| Infectious diseases | 0.235 ** | <0.001 |
| Pneumology | 0.078 ** | 0.007 |
| Emergency | 0.105 ** | <0.001 |
| Pediatric | −0.103 ** | <0.001 |
| General surgery | −0.026 | 0.357 |
| Physiological and biochemical indexes | 27.30 [22.10–33.20] | 13.00–60.70 |
| WBC | 0.052 | 0.074 |
| HCT | −0.150 ** | <0.001 |
| HGB | −0.154 ** | <0.001 |
| PLT | −0.165 ** | <0.001 |
| ALT | 0.081 ** | 0.005 |
| AST | 0.255 ** | <0.001 |
| GGT | −0.300 ** | <0.001 |
| ALP | −0.282 ** | 0.001 |
| TBIL | 0.208 ** | <0.001 |
| ALB | −0.254 ** | <0.001 |
| INR | 0.395 ** | <0.001 |
| CREA | 0.071 * | 0.014 |
| Variable | Coefficient | T | p-Value | VIF |
|---|---|---|---|---|
| Age | 0.049 | 2.784 | 0.007 | 1.043 |
| ALT | 0.007 | 1.772 | 0.080 | 1.128 |
| TBIL | 0.010 | 2.990 | 0.004 | 1.045 |
| ALB | −0.100 | −2.155 | 0.034 | 1.032 |
| GGT | −0.004 | −2.821 | 0.006 | 1.150 |
| Constant value | 5.195 | 2.768 | 0.007 | |
| F | 6.982 | |||
| P | <0.001 | |||
| R2 | 0.270 | |||
| Factors | References | Number of Patients |
|---|---|---|
| Age | Tian et al. 2021 [21] | 108 |
| Li et al. 2020 [22] | 216 | |
| Mafuru et al. 2019 [19] | 113 | |
| Wei et al. 2019 [23] | 67 | |
| You et al. 2018 [24] | 64 | |
| Allegra et al. 2018 [25] | 237 | |
| Shao et al. 2017 [26] | 86 | |
| Niioka et al. 2017 [27] | 65 | |
| Wang et al. 2014 [8] | 151 | |
| Hoenigl et al. 2013 [28] | 61 | |
| Choi et al. 2013 [29] | 27 | |
| Lombardi et al. 2012 [30] | 32 | |
| Dolton et al. 2012 [31] | 201 | |
| ALT | Kang et al. 2020 [32] | 114 |
| AST | Yuan et al. 2020 [33] | 193 |
| Hirata et al. 2019 [34] | 42 | |
| Saini et al. 2014 [35] | 69 | |
| GGT | Cheng et al. 2019 [38] | 166 |
| Mafuru et al. 2019 [19] | 113 | |
| ALP | Zhao et al. 2021 [39] | 93 |
| Wang et al. 2014 [8] | 151 | |
| Saini et al. 2014 [35] | 69 | |
| Lombardi et al. 2012 [30] | 32 | |
| TBIL | Zeng et al. 2020 [40] | 244 |
| Ruiz et al. 2019 [41] | 33 | |
| Saini et al. 2014 [35] | 69 | |
| ALB | Li et al. 2020 [22] | 216 |
| Wei et al. 2019 [23] | 67 | |
| Dote et al. 2016 [42] | 63 | |
| CYP2C19 genotype | Blanco-Dorado et al. 2020 [43] | 78 |
| Yuan et al. 2020 [33] | 193 | |
| Mafuru et al. 2019 [19] | 113 | |
| You et al. 2018 [24] | 64 | |
| Concomitant use | Mafuru et al. 2019 [19] | 113 |
| Hu et al. 2018 [20] | 42 | |
| Chayakulkeeree et al. 2015 [44] | 54 | |
| Kim et al. 2014 [45] | 64 | |
| INR | Wang et al. 2018 [46] | 78 |
| Lombardi et al. 2012 [30] | 32 | |
| PLT | Zhao et al. 2021 [39] | 93 |
| Tang et al. 2019 [36] | 57 | |
| HGB | Zhao et al. 2021 [39] | 93 |
| CREA | Allegra et al. 2018 [25] | 237 |
| Proinflammatory Cytokines | Mafuru et al. 2019 [19] | 113 |
| Obesity | Takahashi et al. 2020 [47] | 44 |
| Diarrhea | Nakayama et al. 2020 [48] | 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Xiao, C.; Hou, J.; Wu, J.; Xiao, Y.; Zhang, B.; Sandaradura, I.; Yan, M. A Large Sample Retrospective Study on the Distinction of Voriconazole Concentration in Asian Patients from Different Clinical Departments. Pharmaceuticals 2021, 14, 1239. https://doi.org/10.3390/ph14121239
Zhao Y, Xiao C, Hou J, Wu J, Xiao Y, Zhang B, Sandaradura I, Yan M. A Large Sample Retrospective Study on the Distinction of Voriconazole Concentration in Asian Patients from Different Clinical Departments. Pharmaceuticals. 2021; 14(12):1239. https://doi.org/10.3390/ph14121239
Chicago/Turabian StyleZhao, Yichang, Chenlin Xiao, Jingjing Hou, Jiamin Wu, Yiwen Xiao, Bikui Zhang, Indy Sandaradura, and Miao Yan. 2021. "A Large Sample Retrospective Study on the Distinction of Voriconazole Concentration in Asian Patients from Different Clinical Departments" Pharmaceuticals 14, no. 12: 1239. https://doi.org/10.3390/ph14121239
APA StyleZhao, Y., Xiao, C., Hou, J., Wu, J., Xiao, Y., Zhang, B., Sandaradura, I., & Yan, M. (2021). A Large Sample Retrospective Study on the Distinction of Voriconazole Concentration in Asian Patients from Different Clinical Departments. Pharmaceuticals, 14(12), 1239. https://doi.org/10.3390/ph14121239






