In Vitro and Clinical Compassionate Use Experiences with the Drug-Repurposing Approach CUSP9v3 in Glioblastoma
Abstract
:1. Introduction
2. Results
2.1. CUSP9v3 Reduces the Viability of Glioblastoma Cells
2.2. CUSP9v3 Abolishes Anchorage-Independent Growth of Glioblastoma Cells
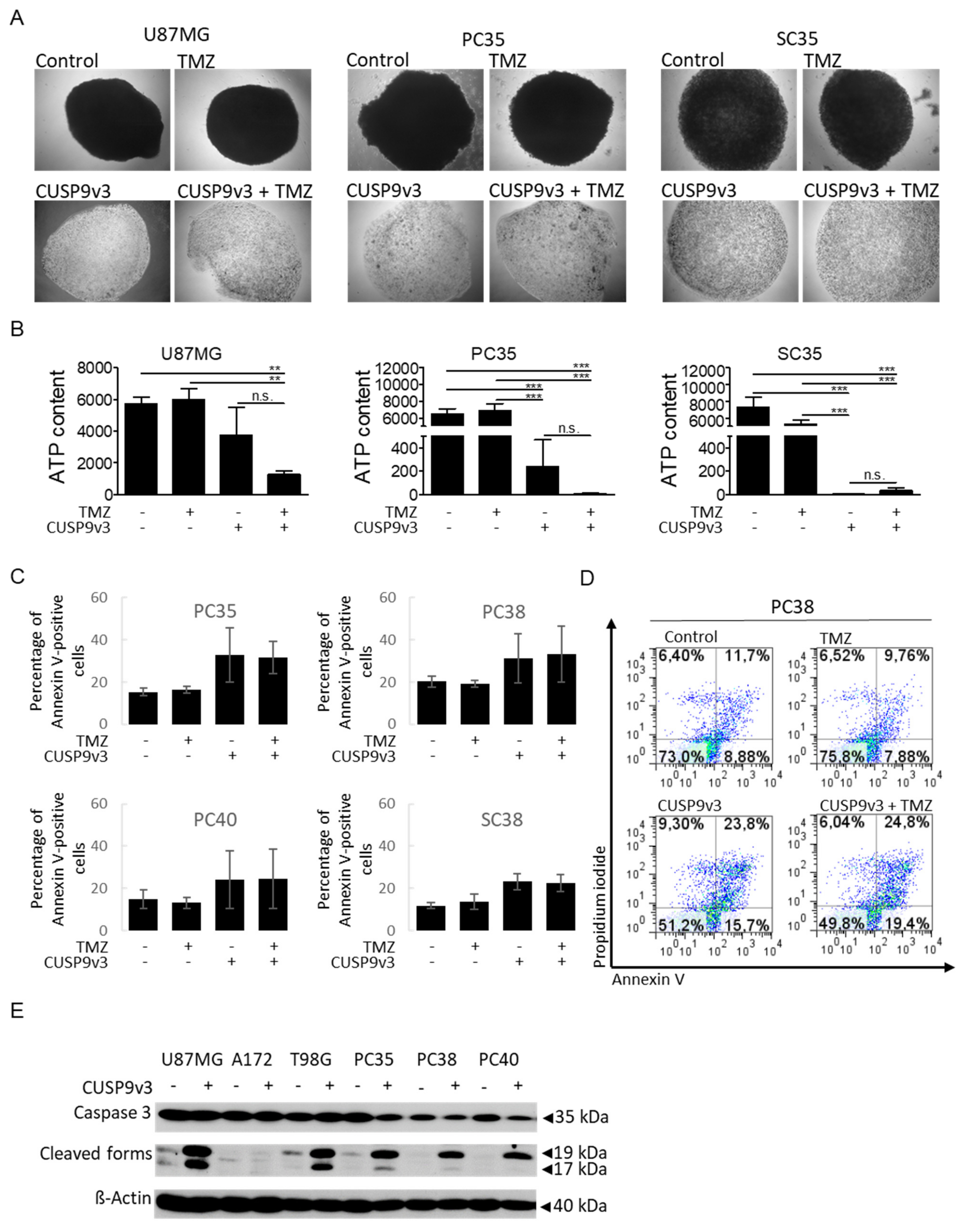
2.3. CUSP9v3 Inhibits 3-Dimensional Tumor Growth
2.4. CUSP9v3 Leads to Enhanced Apoptosis
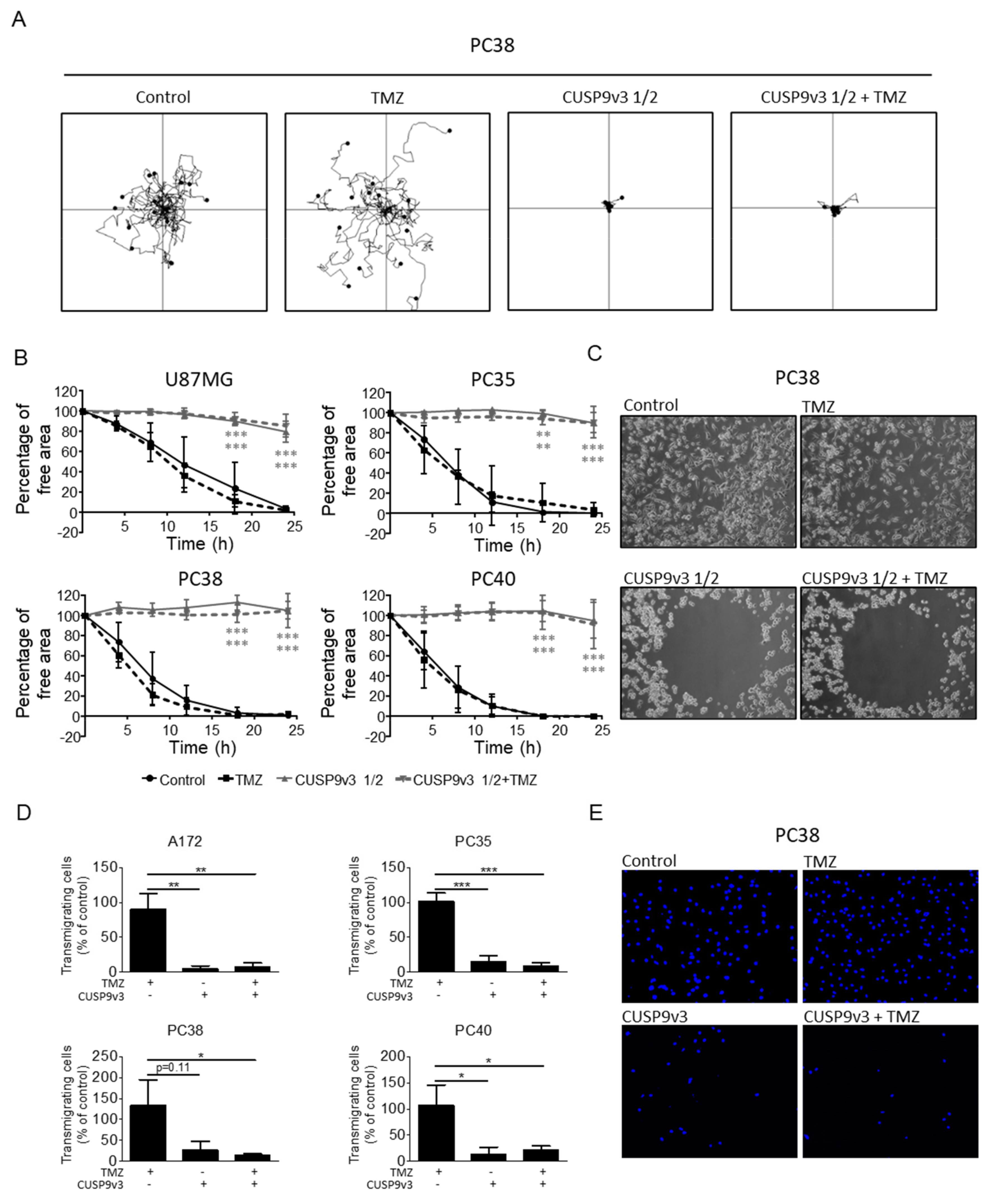
2.5. CUSP9v3 Impairs Non-Directed and Directed Movement of Glioblastoma Cells
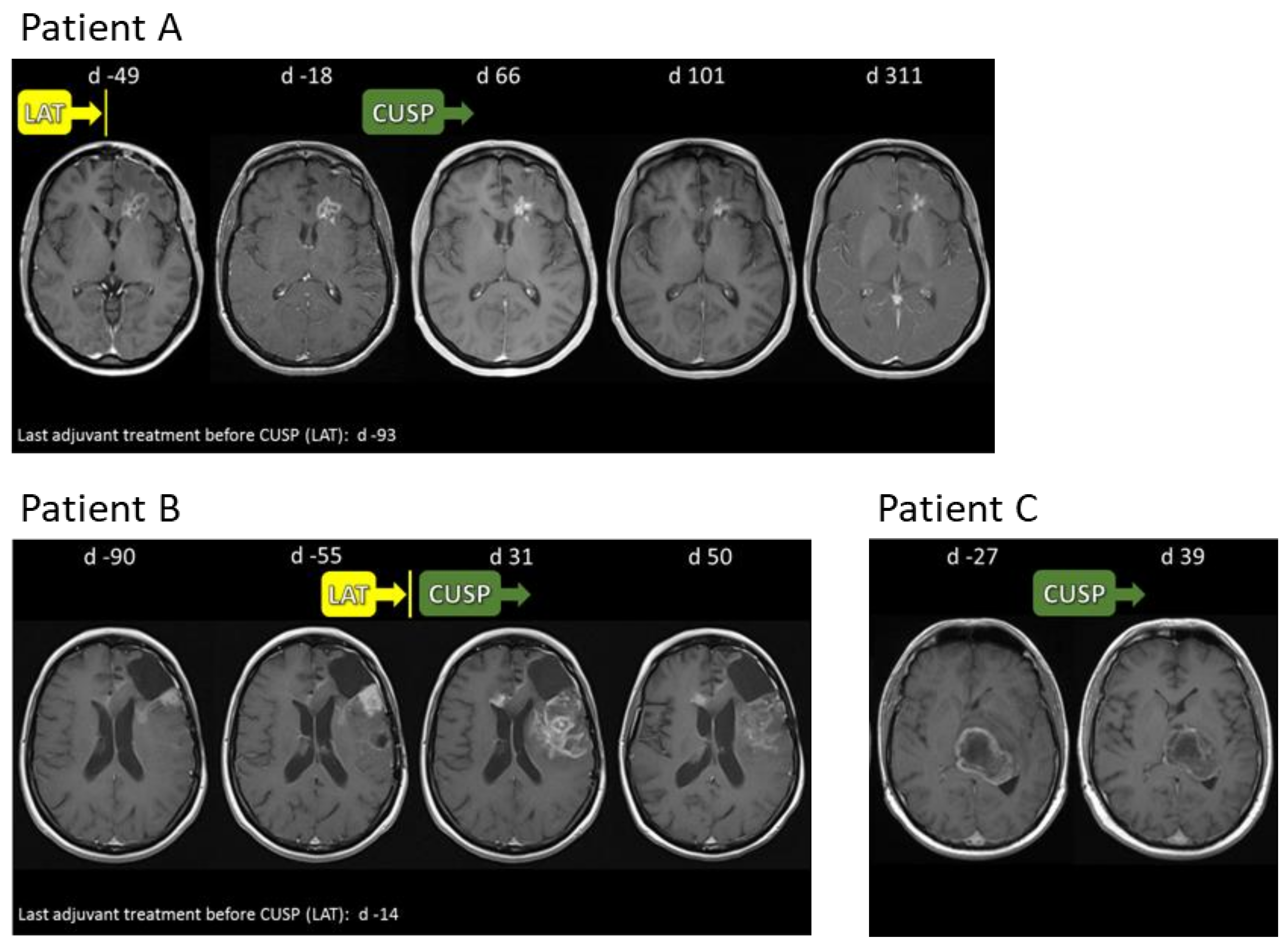
2.6. Compassionate Use Experience
2.7. Safety and Tolerability of CUSP9v3 or CUSP9v3r/q
2.8. CUSP9v3 Affects Contrast Enhancement on Magnetic Resonance Imaging (MRI)
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Cultures and Growth Conditions
4.3. Cell Viability Assays
4.4. Soft Agar Assay
4.5. Spheroid Assay
4.6. Cell Migration Assays
4.7. Western Blot Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro-Oncology 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Cazzato, E.; Ladewig, E.; Frattini, V.; Rosenbloom, D.I.S.; Zairis, S.; Abate, F.; Liu, Z.; Elliott, O.; Shin, Y.-J.; et al. Clonal evolution of glioblastoma under therapy. Nat. Genet. 2016, 48, 768–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kast, R.E.; Boockvar, J.A.; Brüning, A.; Cappello, F.; Chang, W.-W.; Cvek, B.; Dou, Q.P.; Duenas-Gonzalez, A.; Efferth, T.; Focosi, D.; et al. A conceptually new treatment approach for relapsed glioblastoma: Coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the international initiative for accelerated improvement of glioblastoma care. Oncotarget 2013, 4, 502–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kast, R.E.; Karpel-Massler, G.; Halatsch, M.-E. CUSP9* treatment protocol for recurrent glioblastoma: Aprepitant, artesunate, auranofin, captopril, celecoxib, disulfiram, itraconazole, ritonavir, sertraline augmenting continuous low dose temozolomide. Oncotarget 2014, 5, 8052–8082. [Google Scholar] [CrossRef] [Green Version]
- Abbruzzese, C.; Matteoni, S.; Signore, M.; Cardone, L.; Nath, K.; Glickson, J.D.; Paggi, M.G. Drug repurposing for the treatment of glioblastoma multiforme. J. Exp. Clin. Cancer Res. 2017, 36, 1–11. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Siegelin, M.D.; Schneider, E.; Westhoff, M.-A.; Wirtz, C.R.; Karpel-Massler, G. Current state and future perspective of drug repurposing in malignant glioma. Semin. Cancer Biol. 2019, 68, 92–104. [Google Scholar] [CrossRef]
- Halatsch, M.-E.; Salacz, M.; Schmitz, B.; Karpel-Massler, G.; Kast, R.E. EXTH-79. Initial experiences with compassionate-use CUSP9v3/v4 for recurrent glioblastoma. Neuro-Oncology 2017, 19, vi90. [Google Scholar] [CrossRef]
- Skaga, E.; Skaga, I.; Grieg, Z.; Sandberg, C.J.; Langmoen, I.A.; Vik-Mo, E.O. The efficacy of a coordinated pharmacological blockade in glioblastoma stem cells with nine repurposed drugs using the CUSP9 strategy. J. Cancer Res. Clin. Oncol. 2019, 145, 1495–1507. [Google Scholar] [CrossRef] [Green Version]
- Halatsch, M.-E.; Kast, R.E.; Dwucet, A.; Hlavac, M.; Heiland, T.; Westhoff, M.-A.; Debatin, K.-M.; Wirtz, C.R.; Siegelin, M.D.; Karpel-Massler, G. Bcl-2/Bcl-xL inhibition predominantly synergistically enhances the anti-neoplastic activity of a low-dose CUSP9 repurposed drug regime against glioblastoma. Br. J. Pharmacol. 2019, 176, 3681–3694. [Google Scholar] [CrossRef]
- Furuta, T.; Sabit, H.; Dong, Y.; Miyashita, K.; Kinoshita, M.; Uchiyama, N.; Hayashi, Y.; Hayashi, Y.; Minamoto, T.; Nakada, M. Biological basis and clinical study of glycogen synthase kinase- 3β-targeted therapy by drug repositioning for glioblastoma. Oncotarget 2017, 8, 22811–22824. [Google Scholar] [CrossRef] [Green Version]
- Zapletalova, D.; André, N.; Deak, L.; Kyr, M.; Bajciova, V.; Mudry, P.; Zdrazilova-Dubska, L.; Demlova, R.; Pavelka, Z.; Zitterbart, K.; et al. Metronomic chemotherapy with the COMBAT regimen in advanced pediatric malignancies: A multicenter experience. Oncology 2012, 82, 249–260. [Google Scholar] [CrossRef]
- Halatsch, M.-E.; Kast, R.E.; Karpel-Massler, G.; Mayer, B.; Zolk, O.; Schmitz, B.; Scheuerle, A.; Maier, L.; Bullinger, L.; Mayer-Steinacker, R.; et al. A phase Ib/IIa trial of 9 repurposed drugs combined with temozolomide for the treatment of recurrent glioblastoma: CUSP9v3. Neuro-Oncology Adv. 2021, 3, 75. [Google Scholar] [CrossRef]
- Karpel-Massler, G.; Kast, R.E.; Westhoff, M.-A.; Dwucet, A.; Welscher, N.; Nonnenmacher, L.; Hlavac, M.; Siegelin, M.D.; Wirtz, C.R.; Debatin, K.-M.; et al. Olanzapine inhibits proliferation, migration and anchorage-independent growth in human glioblastoma cell lines and enhances temozolomide’s antiproliferative effect. J. Neurooncol. 2015, 122, 21–33. [Google Scholar] [CrossRef]
- Schneider, M.; Ströbele, S.; Nonnenmacher, L.; Siegelin, M.D.; Tepper, M.; Stroh, S.; Hasslacher, S.; Enzenmüller, S.; Strauss, G.; Baumann, B.; et al. A paired comparison between glioblastoma “stem cells” and differentiated cells. Int. J. Cancer 2015, 138, 1709–1718. [Google Scholar] [CrossRef] [Green Version]
- Ströbele, S.; Schneider, M.; Schneele, L.; Siegelin, M.D.; Nonnenmacher, L.; Zhou, S.; Karpel-Massler, G.; Westhoff, M.-A.; Halatsch, M.-E.; Debatin, K.-M. A potential role for the inhibition of PI3K signaling in glioblastoma therapy. PLoS ONE 2015, 10, e0131670. [Google Scholar] [CrossRef] [Green Version]
- Hlavac, M.; Dwucet, A.; Kast, R.E.; Engelke, J.; Westhoff, M.-A.; Siegelin, M.D.; Debatin, K.-M.; Wirtz, C.R.; Halatsch, M.-E.; Karpel-Massler, G. Combined inhibition of RAC1 and Bcl-2/Bcl-xL synergistically induces glioblastoma cell death through down-regulation of the Usp9X/Mcl-1 axis. Cell Oncol. 2019, 42, 287–301. [Google Scholar] [CrossRef]
- Pruss, M.; Dwucet, A.; Tanriover, M.; Hlavac, M.; Kast, R.E.; Debatin, K.-M.; Wirtz, C.R.; Halatsch, M.-E.; Siegelin, M.D.; Westhoff, M.-A.; et al. Dual metabolic reprogramming by ONC201/TIC10 and 2-Deoxyglucose induces energy depletion and synergistic anti-cancer activity in glioblastoma. Br. J. Cancer 2020, 122, 1146–1157. [Google Scholar] [CrossRef] [Green Version]
- Karpel-Massler, G.; Westhoff, M.-A.; Zhou, S.; Nonnenmacher, L.; Dwucet, A.; Kast, R.E.; Bachem, M.G.; Wirtz, C.R.; Debatin, K.-M.; Halatsch, M.-E. Combined inhibition of HER1/EGFR and RAC1 results in a synergistic antiproliferative effect on established and primary cultured human glioblastoma cells. Mol. Cancer Ther. 2013, 12, 1783–1795. [Google Scholar] [CrossRef] [Green Version]

| CUSP9 | CUSP9* | CUSP9v3 | CUSP9v3r/q 1 |
|---|---|---|---|
| aprepitant -antiemetic | aprepitant | aprepitant 80 mg; 1×/d | aprepitant |
| artesunate -antimalarial | artesunate | minocycline -antibiotic 100 mg; 2×/d | minocycline |
| auranofin -antirheumatic | auranofin | auranofin 3 mg; 2×/d | auranofin |
| captopril -antihypertensive | captopril | captopril 50 mg; 2×/d | captopril |
| disulfiram- alcohol deterrent | disulfiram | disulfiram 250 mg; 2×/d | disulfiram |
| copper gluconate -food supplement 2 | celecoxib -NSAID 3 | celecoxib 400 mg; 2×/d | celecoxib |
| ketoconazole -antifungal | itraconazole -antifungal | itraconazole 200 mg; 2×/d | itraconazole |
| nelfinavir -antiretroviral | ritonavir -antiretroviral | ritonavir 400 mg; 2×/d | quetiapine -atypical antipsychotic 25 mg; 1×/d |
| sertraline -antidepressant | sertraline | sertraline 100 mg; 2×/d | sertraline |
| temozolomide -chemotherapeutic | temozolomide | temozolomide 20 mg/m2; 2×/d | temozolomide |
| Age (Years) | Gender | MGMT-Status | IDH-Status | |
|---|---|---|---|---|
| Patient 1 | 69 | M | Pos. | WT |
| Patient 2 | 56 | M | Neg. | WT |
| Patient 3 | 42 | F | Neg. | Mut |
| Patient 4 | 51 | M | Neg. | WT |
| Patient 5 | 41 | F | Pos. | WT |
| Patient 6 | 43 | M | Neg. | WT |
| Patient 7 | 40 | M | Neg. | WT |
| Patient 8 | 44 | M | Neg. | WT |
| Adverse Event | N (%) |
|---|---|
| ALT increased | 3 (38) |
| γGT increased | 2 (25) |
| AST increased | 1 (13) |
| Infection | 2 (25) |
| Anemia | 1 (13) |
| Leukocytopenia | 1 (13) |
| Thrombocytopenia | 1 (13) |
| PTT prolongation | 0 (0.0) |
| Creatinine increase | 0 (0.0) |
| Nausea | 0 (0.0) |
| Vomiting | 0 (0.0) |
| Thromboembolus | 0 (0.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halatsch, M.-E.; Dwucet, A.; Schmidt, C.J.; Mühlnickel, J.; Heiland, T.; Zeiler, K.; Siegelin, M.D.; Kast, R.E.; Karpel-Massler, G. In Vitro and Clinical Compassionate Use Experiences with the Drug-Repurposing Approach CUSP9v3 in Glioblastoma. Pharmaceuticals 2021, 14, 1241. https://doi.org/10.3390/ph14121241
Halatsch M-E, Dwucet A, Schmidt CJ, Mühlnickel J, Heiland T, Zeiler K, Siegelin MD, Kast RE, Karpel-Massler G. In Vitro and Clinical Compassionate Use Experiences with the Drug-Repurposing Approach CUSP9v3 in Glioblastoma. Pharmaceuticals. 2021; 14(12):1241. https://doi.org/10.3390/ph14121241
Chicago/Turabian StyleHalatsch, Marc-Eric, Annika Dwucet, Carl Julius Schmidt, Julius Mühlnickel, Tim Heiland, Katharina Zeiler, Markus D. Siegelin, Richard Eric Kast, and Georg Karpel-Massler. 2021. "In Vitro and Clinical Compassionate Use Experiences with the Drug-Repurposing Approach CUSP9v3 in Glioblastoma" Pharmaceuticals 14, no. 12: 1241. https://doi.org/10.3390/ph14121241
APA StyleHalatsch, M.-E., Dwucet, A., Schmidt, C. J., Mühlnickel, J., Heiland, T., Zeiler, K., Siegelin, M. D., Kast, R. E., & Karpel-Massler, G. (2021). In Vitro and Clinical Compassionate Use Experiences with the Drug-Repurposing Approach CUSP9v3 in Glioblastoma. Pharmaceuticals, 14(12), 1241. https://doi.org/10.3390/ph14121241







