The Effect of Acknowledged and Novel Anti-Rheumatic Therapies on Periodontal Tissues—A Narrative Review
Abstract
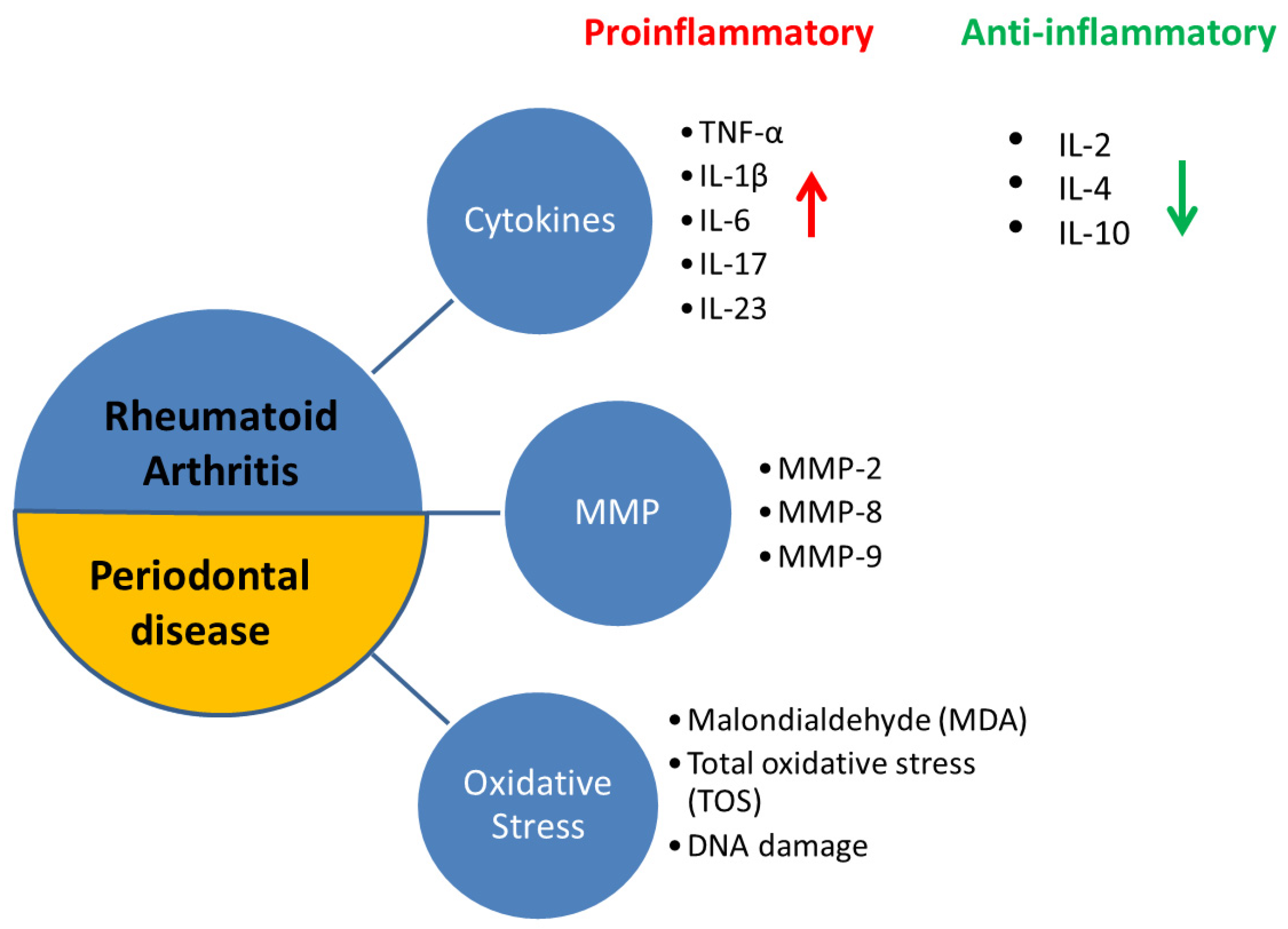
:1. Introduction
2. Ethiopathogenetic Common Ground between Rheumatoid Arthritis and Periodontal Disease
3. Current Treatment Options in Rheumatoid Arthritis and Periodontal Disease
3.1. Corticosteroids
3.2. NSAIDs (Non-Steroidal Anti-Inflammatory Drugs)
3.3. MMP Inhibitors (Matrix Metalloproteinases)
3.4. DMARDs
3.5. Lipid Mediators of Inflammation Resolution
3.6. Small-Molecule Compounds
3.7. RANKL Inhibitors
3.8. Bisphosphonates
4. Future Research Directions and Potential Risks
Lessons Learned from Rheumatoid Arthritis Treatment-Combined Approaches for Periodontitis Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, Regional, and National Burden of Severe Periodontitis, 1990–2019: An Analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef] [PubMed]
- Monsarrat, P.; Blaizot, A.; Kemoun, P. Clinical research activity in periodontal medicine: A systematic mapping of trial reg-isters. J. Clin. Periodontol. 2016, 43, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Hassan, N.; Khatoon, K.; Mirza, M.A.; Naseef, P.P.; Kuruniyan, M.S.; Iqbal, Z. Periodontitis and Systemic Disorder—An Overview of Relation and Novel Treatment Modalities. Pharmaceutics 2021, 13, 1175. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Papapanou, P.N.; Philips, K.H.; Offenbacher, S. Periodontal Medicine: 100 Years of Progress. J. Dent. Res. 2019, 98, 1053–1062. [Google Scholar] [CrossRef]
- Hussain, S.B.; Botelho, J.; Machado, V.; Zehra, S.A.; Mendes, J.J.; Ciurtin, C.; Orlandi, M.; D’Aiuto, F. Is There a Bidirectional Association between Rheumatoid Arthritis and Periodontitis? A Systematic Review and Meta-Analysis. Semin. Arthritis Rheum. 2020, 50, 414–422. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, Y.; Bian, X.; Ge, H.; Wang, J.; Zhang, Z. Non-Surgical Periodontal Treatment Improves Rheumatoid Arthritis Disease Activity: A Meta-Analysis. Clin. Oral Investig. 2021, 25, 4975–4985. [Google Scholar] [CrossRef]
- Drosos, A.A.; Pelechas, E.; Voulgari, P.V. Rheumatoid Arthritis Treatment. A Back to the Drawing Board Project or High Expectations for Low Unmet Needs? J. Clin. Med. 2019, 8, 1237. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, M.; Mousavi, M.J.; Jamalzehi, S.; Alimohammadi, R.; Bezvan, M.H.; Mohammadi, H.; Aslani, S. Strategies toward Rheumatoid Arthritis Therapy; the Old and the New. J. Cell. Physiol. 2019, 234, 10018–10031. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Koeller, M.; Weisman, M.H.; Emery, P. New Therapies for Treatment of Rheumatoid Arthritis. Lancet 2007, 370, 1861–1874. [Google Scholar] [CrossRef]
- Hazlewood, G.S.; Whittle, S.L.; Kamso, M.M.; Akl, E.A.; Wells, G.A.; Tugwell, P.; Thomas, M.; Lee, C.; Ejaredar, M.; Choudhary, D.; et al. Disease-modifying Anti-rheumatic Drugs for Rheumatoid Arthritis: A Systematic Review and Network Meta-analysis. Cochrane Database Syst. Rev. 2020, 2020, CD013562. [Google Scholar] [CrossRef]
- Li, R.; Tian, C.; Postlethwaite, A.; Jiao, Y.; Garcia-Godoy, F.; Pattanaik, D.; Wei, D.; Gu, W.; Li, J. Rheumatoid arthritis and periodontal disease: What are the similarities and differences? Int. J. Rheum. Dis. 2017, 20, 1887–1901. [Google Scholar] [CrossRef] [PubMed]
- Bunte, K.; Beikler, T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef] [Green Version]
- Kozak, M.; Dabrowska-Zamojcin, E.; Mazurek-Mochol, M.; Pawlik, A. Cytokines and Their Genetic Polymorphisms Related to Periodontal Disease. J. Clin. Med. 2020, 9, 4045. [Google Scholar] [CrossRef]
- Franco, C.; Patricia, H.-R.; Timo, S.; Claudia, B.; Marcela, H. Matrix Metalloproteinases as Regulators of Periodontal Inflamma-tion. Int. J. Mol. Sci. 2017, 18, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkataraman, A.; Almas, K. Rheumatoid Arthritis and Periodontal Disease. An Update. N. Y. State Dent. J. 2015, 81, 30–36. [Google Scholar]
- Alam, J.; Jantan, I.; Bukhari, S.N. Rheumatoid Arthritis: Recent Advances on Its Etiology, Role of Cytokines and Pharmacotherapy. Biomed. Pharmacother. 2017, 92, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Dionigi, C.; Larsson, L.; Carcuac, O.; Berglundh, T. Cellular Expression of DNA Damage/Repair and Reactive Oxygen/Nitrogen Species in Human Periodontitis and Peri-implantitis Lesions. J. Clin. Periodontol. 2020, 47, 1466–1475. [Google Scholar] [CrossRef]
- Martu, M.-A.; Surlin, P.; Lazar, L.; Maftei, G.A.; Luchian, I.; Gheorghe, D.-N.; Rezus, E.; Toma, V.; Foia, L.-G. Evaluation of Oxidative Stress before and after Using Laser and Photoactivation Therapy as Adjuvant of Non-Surgical Periodontal Treatment in Patients with Rheumatoid Arthritis. Antioxidants 2021, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Goronzy, J.J.; Weyand, C.M. DNA damage, metabolism and aging in pro-inflammatory T cells: Rheumatoid arthritis as a model system. Exp. Gerontol. 2018, 105, 118–127. [Google Scholar] [CrossRef]
- de Molon, R.S.; Rossa, C., Jr.; Thurlings, R.M.; Cirelli, J.A.; Koenders, M.I. Linkage of periodontitis and rheumatoid arthritis: Current evidence and potential biological interactions. Int. J. Mol. Sci. 2019, 20, 4541. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-Inflammatory and Immune-Regulatory Cytokines in Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Romano, F.; Del Buono, W.; Bianco, L.; Arena, M.; Mariani, G.M.; Di Scipio, F.; Berta, G.N.; Aimetti, M. Gingival Crevicular Fluid Cytokines in Moderate and Deep Sites of Stage III Periodontitis Patients in Different Rates of Clinical Progression. Biomedicines 2020, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Jajoo, N.S.; Shelke, A.U.; Bajaj, R.S. Periodontitis and Rheumatoid Arthritis: The Common Thread. Clin. Rev. Bone Miner. Metab. 2020, 18, 18–30. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, Z.; Li, Y.; Han, Y.; Zhou, Y.; Cao, X. Rheumatoid Arthritis Risk in Periodontitis Patients: A Systematic Review and Me-Ta-Analysis. Jt. Bone Spine 2020, 87, 556–564. [Google Scholar] [CrossRef]
- Calderaro, D.C.; Corrêa, J.D.; Ferreira, G.A.; Barbosa, I.G.; Martins, C.C.; Silva, T.A.; Teixeira, A.L. Influence of periodontal treatment on rheumatoid arthritis: A systematic review and meta-analysis. Rev. Bras. Reumatol. 2017, 57, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Mercado, F.B.; Marshall, R.I.; Klestov, A.C.; Bartold, P.M. Relationship between Rheumatoid Arthritis and Periodontitis. J. Periodontol. 2001, 72, 779–787. [Google Scholar] [CrossRef]
- Schmickler, J.; Rupprecht, A.; Patschan, S.; Patschan, D.; Müller, G.A.; Haak, R.; Mausberg, R.F.; Schmalz, G.; Kottmann, T.; Ziebolz, D. Cross-sectional Evaluation of Periodontal Status and Microbiologic and Rheumatoid Parameters in a Large Cohort of Patients with Rheu-Matoid Arthritis. J. Periodontol. 2017, 88, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Arkema, E.V.; Karlson, E.W.; Costenbader, K.H. A Prospective Study of Periodontal Disease and Risk of Rheumatoid Arthritis. J. Rheumatol. 2010, 37, 1800–1804. [Google Scholar] [CrossRef] [Green Version]
- Bartold, P.M. Lifestyle and Periodontitis: The Emergence of Personalized Periodontics. Periodontol. 2000 2018, 78, 7–11. [Google Scholar] [CrossRef]
- Burmester, G.R.; Pope, J.E. Novel Treatment Strategies in Rheumatoid Arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef]
- Drosos, A.A.; Pelechas, E.; Voulgari, P.V. Treatment Strategies Are More Important than Drugs in the Management of Rheumatoid Arthritis. Clin. Rheumatol. 2020, 39, 1363–1368. [Google Scholar] [CrossRef]
- Baschant, U.; Lane, N.E.; Tuckermann, J. The Multiple Facets of Glucocorticoid Action in Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2012, 8, 645–655. [Google Scholar] [CrossRef]
- Ciccarelli, F.; De Martinis, M.; Ginaldi, L. Glucocorticoids in Patients with Rheumatic Diseases: Friends or Enemies of Bone? Curr. Med. Chem. 2015, 22, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Komerik, N.; Akkaya, A.; Yıldız, M.; Buyukkaplan, U.S.; Kuru, L. Oral health in patients on inhaled corticosteroid treatment. Oral Dis. 2005, 11, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Brasil-Oliveira, R.; Cruz, Á.A.; Sarmento, V.A.; Souza-Machado, A.; Lins-Kusterer, L. Corticosteroid Use and Periodontal Disease: A Systematic Review. Eur. J. Dent. 2020, 14, 496–501. [Google Scholar] [CrossRef]
- Sfetcu, L.; Didilescu, A.C.; Vlădan, C.; Dincă, O.; Miricescu, D.; Băncescu, G.; Bucur, A.; Tribus, L.C. The Effects of Predni-Sone/Ketoprofen Administration in Association with Amoxicillin Clavulanate Following Periodontal Surgical Therapy in Patients with Severe Chronic Periodontitis. Medicina 2021, 57, 447. [Google Scholar] [CrossRef]
- Crofford, L.J. Use of NSAIDs in Treating Patients with Arthritis. Arthritis Res. Ther. 2013, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Agossa, K.; Morand, D.N.; Tenenbaum, H.; Davideau, J.L.; Huck, O. Systemic Application of Anti-Inflammatory Agents in Periodontal Treatment. Clin. Anti-Inflamm. Anti-Allergy Drugs 2015, 2, 3–13. [Google Scholar] [CrossRef]
- Golub, L.M.; Lee, H.M. Periodontal therapeutics: Current host-modulation agents and future directions. Periodontol. 2000 2020, 82, 186–204. [Google Scholar] [CrossRef] [Green Version]
- Shiloah, J.; Bland, P.S.; Scarbecz, M.; Patters, M.R.; Stein, S.H.; Tipton, D.A. The Effect of Long-term Aspirin Intake on the Outcome of Non-surgical Periodontal Therapy in Smokers: A Double-blind, Randomized Pilot Study. J. Periodontal. Res. 2014, 49, 102–109. [Google Scholar] [CrossRef]
- Fields, G.B. The Rebirth of Matrix Metalloproteinase Inhibitors: Moving Beyond the Dogma. Cells 2019, 8, 984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, M.J.; Karami, J.; Aslani, S.; Tahmasebi, M.N.; Vaziri, A.S.; Jamshidi, A.; Farhadi, E.; Mahmoudi, M. Transformation of Fibroblast-like Synoviocytes in Rheumatoid Arthritis; from a Friend to Foe. Autoimmun. Highlights 2021, 12, 1–3. [Google Scholar] [CrossRef]
- Wada, T.T.; Araki, Y.; Sato, K.; Aizaki, Y.; Yokota, K.; Kim, Y.T.; Oda, H.; Kurokawa, R.; Mimura, T. Aberrant Histone Acetylation Contributes to Elevated Interleukin-6 Production in Rheumatoid Arthritis Synovial Fibroblasts. Biochem. Biophys. Res. Commun. 2014, 444, 682–686. [Google Scholar] [CrossRef]
- Araki, Y.; Mimura, T. Matrix Metalloproteinase Gene Activation Resulting from Disordred Epigenetic Mechanisms in Rheumatoid Arthritis. Int. J. Mol. Sci. 2017, 18, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, D.A.; Barter, M.J.; Wilkinson, D.J. Recent Advances in Understanding the Regulation of Metalloproteinases. F1000Research 2019, 8, PMC6381797. [Google Scholar] [CrossRef] [Green Version]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Zhou, C.; Nandakumar, K.S. Molecular and Cellular Pathways Contributing to Joint Damage in Rheumatoid Arthritis. Mediat. Inflamm. 2020, 2020, 3830212. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, T.; Takahashi, N.; Kida, D.; Kaneko, A.; Hirano, Y.; Fujibayashi, T.; Kanayama, Y.; Hanabayashi, M.; Yabe, Y.; Takagi, H.; et al. Improvement in Matrix Metalloproteinase-3 Independently Predicts Low Disease Activity at 52 Weeks in Bio-Switch Rheumatoid Arthritis Patients Treated with Abatacept. Clin. Exp. Rheumatol. 2020, 38, 933–939. [Google Scholar]
- Abdelrahman, M.A.; Sakr, H.M.; Shaaban, M.A.; Afifi, N. Serum and Synovial Matrix Metalloproteinases 1 and 3 in Patients with Early Rheumatoid Arthritis: Potentially Prospective Biomarkers of Ultrasonographic Joint Damage and Disease Activity. Egypt. J. Intern. Med. 2019, 31, 965–971. [Google Scholar]
- Kaneko, K.; Williams, R.O.; Dransfield, D.T.; Nixon, A.E.; Sandison, A.; Itoh, Y. Selective Inhibition of Membrane Type 1 Matrix Metalloproteinase Abrogates Progression of Experimental Inflammatory Arthritis: Synergy with Tumor Necrosis Factor Blockade. Arthritis Rheumatol. 2016, 68, 521–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, J.H.; Starr, A.E.; Kappelhoff, R.; Yan, R.; Roberts, C.R.; Overall, C.M. Matrix Metalloproteinase 8 Deficiency in Mice Exac-Erbates Inflammatory Arthritis through Delayed Neutrophil Apoptosis and Reduced Caspase 11 Expression. Arthritis Rheum 2010, 62, 3645–3655. [Google Scholar] [CrossRef]
- Li, K.; Tay, F.R.; Yiu, C.K. The past, present and future perspectives of matrix metalloproteinase inhibitors. Pharmacol. Ther. 2020, 207, 107465. [Google Scholar] [CrossRef]
- Hasan, U.H.; Uttra, A.M.; Qasim, S.; Ikram, J.; Saleem, M.; Niazi, Z.R. Phytochemicals Targeting Matrix Metalloproteinases Regulating Tissue Degradation in Inflammation and Rheumatoid Arthritis. Phytomedicine 2020, 66, 153134. [Google Scholar]
- Hemmings, F.J.; Farhan, M.; Rowland, J.; Banken, L.; Jain, R. Tolerability and Pharmacokinetics of the Collagenase-Selective Inhibitor Trocade in Patients with Rheumatoid Arthritis. Rheumatology 2001, 40, 537–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; McKelvey, K.; Shen, K.; Minhas, N.; March, L.; Park, S.Y.; Jackson, C.J. Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatology 2014, 53, 2270–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, S.; Forteza, J.; López-Otin, C.; Gómez-Reino, J.J.; González, A.; Conde, C. Matrix Metalloproteinase-8 Deficiency Increases Joint Inflammation and Bone Erosion in the K/BxN Serum-Transfer Arthritis Model. Arthritis Res. Ther. 2010, 12, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Q.; Kou, L.; Tu, Y.; Zhu, L. MMP-Responsive ‘Smart’ Drug Delivery and Tumor Targeting. Trends Pharmacol. Sci. 2018, 39, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Golub, L.M.; Sorsa, T.; Lee, H.M.; Ciancio, S.; Sorbi, D.; Ramamurthy, N.S.; Gruber, B.; Salo, T.; Konttinen, Y.T. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis gingiva. J. Clin. Periodontol. 1995, 22, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Checchi, V.; Maravic, T.; Bellini, P.; Generali, L.; Consolo, U.; Breschi, L.; Mazzoni, A. The Role of Matrix Metalloproteinases in Periodontal Disease. Int. J. Environ. Res. Public Health 2020, 17, 4923. [Google Scholar] [CrossRef]
- Preshaw, P.M. Host modulation therapy with anti-inflammatory agents. Periodontol. 2000 2018, 76, 131–149. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Hefti, A.F.; Novak, M.J.; Michalowicz, B.S.; Pihlstrom, B.L.; Schoor, R.; Trummel, C.L.; Dean, J.; Van Dyke, T.E.; Walker, C.B.; et al. Subantimicrobial Dose Doxycycline Enhances the Efficacy of Scaling and Root Planing in Chronic Periodontitis: A Multicenter Trial. J. Periodontol. 2004, 75, 1068–1076. [Google Scholar] [CrossRef]
- Elashiry, M.; Morandini, A.C.; Cornelius Timothius, C.J.; Ghaly, M.; Cutler, C.W. Selective Antimicrobial Therapies for Periodontitis: Win the “Battle and the War”. Int. J. Mol. Sci. 2021, 22, 6459. [Google Scholar] [CrossRef] [PubMed]
- Golub, L.M.; Elburki, M.S.; Walker, C.; Ryan, M.; Sorsa, T.; Tenenbaum, H.; Goldberg, M.; Wolff, M.; Gu, Y. Non-Antibacterial Tetracycline Formulations: Host-Modulators in the Treatment of Periodontitis and Relevant Systemic Diseases. Int. Dent. J. 2016, 66, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Cazalis, J.; Tanabe, S.I.; Gagnon, G.; Sorsa, T.; Grenier, D. Tetracyclines and Chemically Modified Tetracycline-3 (CMT-3) Modulate Cyto-Kine Secretion by Lipopolysaccharide-Stimulated Whole Blood. Inflammation 2009, 32, 130–137. [Google Scholar] [CrossRef]
- Piperigkou, Z.; Manou, D.; Karamanou, K.; Theocharis, A.D. Strategies to Target Matrix Metalloproteinases as Therapeutic Approach in Cancer. Methods Mol. Biol. 2018, 1731, 325–348. [Google Scholar] [PubMed]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 329, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.F.; Isaacs, J.D. Novel Therapies for Immune-Mediated Inflammatory Diseases: What Can We Learn from Their Use in Rheu-Matoid Arthritis, Spondyloarthritis, Systemic Lupus Erythematosus, Psoriasis, Crohn’s Disease and Ulcerative Colitis? Ann. Rheum. Dis. 2018, 77, 175–187. [Google Scholar] [CrossRef]
- Kerschbaumer, A.; Sepriano, A.; Smolen, J.S.; Heijde, D.; Dougados, M.; Vollenhoven, R.; McInnes, I.B.; Bijlsma, J.W.; Burmester, G.R.; Wit, M.; et al. Efficacy of pharmacological treatment in rheumatoid arthritis: A systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, 744–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, R.; Al Nokhatha, S.A.; Conway, R. JAK Inhibitors in Rheumatoid Arthritis: An Evidence-Based Review on the Emerging Clinical Data. J. Inflamm. Res. 2020, 13, 519. [Google Scholar] [CrossRef]
- Apolinário Vieira, G.H.; Aparecida Rivas, A.C.; Figueiredo Costa, K.; Ferreira Oliveira, L.F.; Tanaka Suzuki, K.; Reis Messora, M.; Sprone Ricoldi, M.; Gonçalves de Almeida, A.L.; Taba, M., Jr. Specific inhibition of IL-6 receptor attenuates inflammatory bone loss in experimental periodontitis. J. Periodontol. 2021, 92, 1460–1469. [Google Scholar] [CrossRef]
- Garlet, G.P.; Cardoso, C.R.; Campanelli, A.P.; Ferreira, B.R.; Avila-Campos, M.J.; Cunha, F.Q.; Silva, J.S. The Dual Role of P55 Tumour Necrosis Factor-α Receptor in Actinobacillus Actinomycetemcomitans-induced Experimental Periodontitis: Host Protection and Tissue Destruction. Clin. Exp. Immunol. 2007, 147, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Pers, J.O.; Saraux, A.; Pierre, R.; Youinou, P. Anti–TNF-α Immunotherapy Is Associated with Increased Gingival Inflammation without Clinical Attachment Loss in Subjects with Rheumatoid Arthritis. J. Periodontol. 2008, 79, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Mayer, Y.; Balbir-Gurman, A.; Machtei, E.E. Anti-tumor Necrosis Factor-alpha Therapy and Periodontal Parameters in Patients with Rheumatoid Arthritis. J. Periodontol. 2009, 80, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Mayer, Y.; Elimelech, R.; Balbir-Gurman, A.; Braun-Moscovici, Y.; Machtei, E.E. Periodontal condition of patients with autoimmune diseases and the effect of anti-tumor necrosis factor-α therapy. J. Periodontol. 2013, 84, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Mikuls, T.R.; Payne, J.B.; Yu, F.; Thiele, G.M.; Reynolds, R.J.; Cannon, G.W.; Markt, J.; McGowan, D.; Kerr, G.S.; Redman, R.S.; et al. Perio-Dontitis and Porphyromonas Gingivalis in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2014, 66, 1090–1100. [Google Scholar] [CrossRef] [Green Version]
- Savioli, C.; Ribeiro, A.C.; Fabri, G.M.; Calich, A.L.; Carvalho, J.; Silva, C.A.; Viana, V.S.; Bonfá, E.; Siqueira, J.T. Persistent Periodontal Disease Hampers Anti–Tumor Necrosis Factor Treatment Response in Rheumatoid Arthritis. JCR: J. Clin. Rheumatol. 2012, 18, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sanchez, C.; Rodriguez, C.; Santos-Moreno, P.; Mesa, A.M.; Lafaurie, G.I.; Giraldo, S.Q.; De Avila, J.; Castillo, D.M.; Duran, M.; Chalem, C.P.; et al. Is the Treatment with Biological or Non-Biological DMARDS a Modifier of Periodontal Condition in Patients with Rheumatoid Arthritis? Curr. Rheumatol. Rev. 2017, 13, 139–151. [Google Scholar] [CrossRef]
- Ziebolz, D.; Rupprecht, A.; Schmickler, J.; Bothmann, L.; Krämer, J.; Patschan, D.; Müller, G.A.; Mausberg, R.F.; Schmidt, J.; Schmalz, G.; et al. Association of Different Immunosuppressive Medications with Periodontal Condition in Patients with Rheumatoid Arthritis: Results from a Cross-sectional Study. J. Periodontol. 2018, 89, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Kussmann, M.; Obermueller, M.; Spettel, K.; Winkler, S.; Aletaha, D. In Vitro Evaluation of Disease-Modifying Antirheumatic Drugs against Rheumatoid Arthritis Associated Pathogens of the Oral Microflora. RMD Open 2021, 7, e001737. [Google Scholar] [CrossRef] [PubMed]
- de Smit, M.J.; Westra, J.; Posthumus, M.D.; Springer, G.; Winkelhoff, A.J.; Vissink, A.; Brouwer, E.; Bijl, M. Effect of An-Ti-Rheumatic Treatment on the Periodontal Condition of Rheumatoid Arthritis Patients. Int. J. Environ. Res. Public Health 2021, 18, 2529. [Google Scholar] [CrossRef]
- Angelini, J.; Talotta, R.; Roncato, R.; Fornasier, G.; Barbiero, G.; Dal Cin, L.; Brancati, S.; Scaglione, F. JAK-Inhibitors for the Treatment of Rheumatoid Arthritis: A Focus on the Present and an Outlook on the Future. Biomolecules 2020, 10, 1002. [Google Scholar] [CrossRef]
- Reddig, A.; Voss, L.; Guttek, K.; Roggenbuck, D.; Feist, E.; Reinhold, D. Impact of Different JAK Inhibitors and Methotrexate on Lymphocyte Proliferation and DNA Damage. J. Clin. Med. 2021, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.K.; Lee, I.S. JAK/STAT Pathway Modulates on Porphyromonas Gingivalis Lipopolysaccharide-and Nicotine-Induced Inflammation in Osteoblasts. J. Dent. Hyg. Sci. 2017, 17, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Ito, S.; Murasawa, A.; Ishikawa, H.; Yoshie, H. Effects of Tofacitinib on the Clinical Features of Periodontitis in Patients with Rheumatoid Arthritis: Two Case Reports. BMC Rheumatol. 2019, 3, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Punceviciene, E.; Rovas, A.; Puriene, A.; Stuopelyte, K.; Vitkus, D.; Jarmalaite, S.; Butrimiene, I. Investigating the Relationship between the Severity of Periodontitis and Rheumatoid Arthritis: A Cross-Sectional Study. Clin. Rheumatol. 2021, 40, 3153–3160. [Google Scholar] [CrossRef]
- Serhan, C.N. Treating Inflammation and Infection in the 21st Century: New Hints from Decoding Resolution Mediators and Mecha-Nisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barden, A.E.; Moghaddami, M.; Mas, E.; Phillips, M.; Cleland, L.G.; Mori, T.A. Specialised Pro-Resolving Mediators of Inflammation in Inflammatory Arthritis. Prostaglandins Leukot. Essent. Fat. Acids 2016, 107, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, F.; Kovanen, P.T.; Mardani, R.; Gheibi-Hayat, S.M.; Bo, S.; Sahebkar, A. Resolvins: Emerging Players in Autoimmune and Inflammatory Diseases. Clin. Rev. Allergy Immunol. 2020, 58, 82–91. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in Inflammation: Emergence of the pro-Resolving Superfamily of Mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Doonan, J.; Lumb, F.E.; Pineda, M.A.; Tarafdar, A.; Crowe, J.; Khan, A.M.; Suckling, C.J.; Harnett, M.M.; Harnett, W. Protection against Arthritis by the Parasitic Worm Product ES-62, and Its Drug-like Small Molecule Analogues, Is Associated with Inhibition of Os-Teoclastogenesis. Front. Immunol. 2018, 9, 1016. [Google Scholar] [CrossRef]
- Corbet, M.; Pineda, M.A.; Yang, K.; Tarafdar, A.; McGrath, S.; Nakagawa, R.; Lumb, F.E.; Harnett, W.; Harnett, M.M. ES-62 Suppression of Arthritis Reflects Epigenetic Rewiring of Synovial Fibroblasts to a Joint-Protective Phenotype. BioRxiv 2020. [Google Scholar] [CrossRef]
- Alvarado, R.; To, J.; Lund, M.E.; Pinar, A.; Mansell, A.; Robinson, M.W.; O’Brien, B.A.; Dalton, J.P.; Donnelly, S. The Immune Modulatory Peptide FhHDM-1 Secreted by the Helminth Fasciola Hepatica Prevents NLRP3 Inflammasome Activation by Inhibiting Endo-Lysosomal Acidification in Macrophages. FASEB J. 2017, 31, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saas, P.; Chagué, C.; Maraux, M.; Cherrier, T. Toward the Characterization of Human Pro-Resolving Macrophages? Front. Immunol. 2020, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, R.B.; Gündüz, Ö.S.; Özdemir, A.T.; Akgül, Ö. Low Levels of Pro-Resolving Lipid Mediators Lipoxin-A4, Resolvin-D1 and Resol-Vin-E1 in Patients with Rheumatoid Arthritis. Immunol. Lett. 2020, 227, 34–40. [Google Scholar] [CrossRef]
- Mustonen, A.M.; Nieminen, P. Fatty Acids and Oxylipins in Osteoarthritis and Rheumatoid Arthritis—A Complex Field with Significant Potential for Future Treatments. Curr. Rheumatol. Rep. 2021, 23, 1–8. [Google Scholar] [CrossRef]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes Derived from MiR-92a-3p-Overexpressing Human Mesenchymal Stem Cells Enhance Chondrogenesis and Suppress Cartilage Degradation via Targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Hasturk, H.; Kantarci, A.; Ohira, T.; Arita, M.; Ebrahimi, N.; Chiang, N.; Petasis, N.A.; Levy, B.D.; Serhan, C.N.; Van Dyke, T.E. RvE1 Protects from Local Inflammation and Osteoclastmediated Bone Destruction in Periodontitis. FASEB J. 2006, 20, 401–403. [Google Scholar] [CrossRef]
- Balta, M.G.; Loos, B.G.; Nicu, E.A. Emerging Concepts in the Resolution of Periodontal Inflammation: A Role for Resolvin E1. Front. Immunol. 2017, 8, 1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sharkawy, H.; Aboelsaad, N.; Eliwa, M.; Darweesh, M.; Alshahat, M.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Adjunctive Treatment of Chronic Periodontitis with Daily Dietary Supplementation with Omega-3 Fatty Acids and Low-dose Aspirin. J. Periodontol. 2010, 81, 1635–1643. [Google Scholar] [CrossRef]
- Ikeda, Y.; Nagase, N.; Tsuji, A.; Taniguchi, K.; Kitagishi, Y.; Matsuda, S. Comprehension of the Relationship between Autophagy and Reactive Oxygen Species for Superior Cancer Therapy with Histone Deacetylase Inhibitors. Oxygen 2021, 1, 4. [Google Scholar] [CrossRef]
- Cantley, M.D.; Zannettino, A.C.; Bartold, P.M.; Fairlie, D.P.; Haynes, D.R. Histone Deacetylases (HDAC) in Physiological and Pathological Bone Remodelling. Bone 2017, 95, 162–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treuter, E.; Fan, R.; Huang, Z.; Jakobsson, T.; Venteclef, N. Transcriptional Repression in Macrophages—Basic Mechanisms and Alterations in Metabolic Inflammatory Diseases. FEBS Lett. 2017, 591, 2959–2977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godoy, L.D.; Lucas, J.E.; Bender, A.J.; Romanick, S.S.; Ferguson, B.S. Targeting the Epigenome: Screening Bioactive Compounds That Regulate Histone Deacetylase Activity. Mol. Nutr. Food Res. 2017, 61, 1600744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabiec, A.M.; Korchynskyi, O.; Tak, P.P.; Reedquist, K.A. Histone Deacetylase Inhibitors Suppress Rheumatoid Arthritis Fibroblast-like Synoviocyte and Macrophage IL-6 Production by Accelerating MRNA Decay. Ann. Rheum. Dis. 2012, 71, 424–431. [Google Scholar] [CrossRef]
- Angiolilli, C.; Kabala, P.A.; Grabiec, A.M.; Rossato, M.; Lai, W.S.; Fossati, G.; Mascagni, P.; Steinkühler, C.; Blackshear, P.J.; Reedquist, K.A.; et al. Control of Cytokine MRNA Degradation by the Histone Deacetylase Inhibitor ITF2357 in Rheumatoid Arthritis Fibro-Blast-like Synoviocytes: Beyond Transcriptional Regulation. Arthritis Res. Ther. 2018, 20, 148. [Google Scholar] [CrossRef] [Green Version]
- Oh, B.R.; Suh, D.H.; Bae, D.; Ha, N.; Choi, Y.I.; Yoo, H.J.; Park, J.K.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Therapeutic Effect of a Novel Histone Deacetylase 6 Inhibitor, CKD-L, on Collagen-Induced Arthritis in Vivo and Regulatory T Cells in Rheumatoid Arthritis In Vitro. Arthritis Res. Ther. 2017, 19, 154. [Google Scholar] [CrossRef] [Green Version]
- Park, J.K.; Jang, Y.J.; Oh, B.R.; Shin, J.; Bae, D.; Ha, N.; Choi, Y.; Youn, G.S.; Park, J.; Lee, E.Y.; et al. Therapeutic Potential of CKD-506, a Novel Selective Histone Deacetylase 6 Inhibitor, in a Murine Model of Rheumatoid Arthritis. Arthritis Res. Ther. 2020, 22, 176. [Google Scholar] [CrossRef] [PubMed]
- Grabiec, A.M.; Potempa, J. Epigenetic Regulation in Bacterial Infections: Targeting Histone Deacetylases. Crit. Rev. Microbiol. 2018, 71, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.; Sohn, H.M.; Ko, Y.J.; Hyun, H.; Lim, W. Inhibition of RANKL-Induced Osteoclastogenesis by Novel Mutant RANKL. Int. J. Mol. Sci. 2021, 22, 434. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, B.; Chen, X.; Su, J. New Insight into Unexpected Bone Formation by Denosumab. Drug Discov. Today 2020, 25, 1919–1922. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kaneko, Y.; Izumi, K.; Takeuchi, T. Efficacy of Denosumab Combined with BDMARDs on Radiographic Progression in Rheumatoid Arthritis. Jt. Bone Spine 2016, 84, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Tanaka, Y.; Soen, S.; Yamanaka, H.; Yoneda, T.; Tanaka, S.; Nitta, T.; Okubo, N.; Genant, H.K.; Van Der Heijde, D. Effects of the Anti-RANKL Antibody Denosumab on Joint Structural Damage in Patients with Rheumatoid Arthritis Treated with Conventional Synthetic Disease-Modifying Antirheumatic Drugs (DESIRABLE Study): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Ann. Rheum. Dis. 2019, 78, 899–907. [Google Scholar] [PubMed] [Green Version]
- Diniz-Freitas, M.; Fernández-Feijoo, J.; Diz Dios, P.; Pousa, X.; Limeres, J. Denosumab-related Osteonecrosis of the Jaw Following Non-surgical Periodontal Therapy: A Case Report. J. Clin. Periodontol. 2018, 45, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Okuma, S.; Matsuda, Y.; Nariai, Y.; Karino, M.; Suzuki, R.; Kanno, T. A Retrospective Observational Study of Risk Factors for Denosumab-Related Osteonecrosis of the Jaw in Patients with Bone Metastases from Solid Cancers. Cancers 2020, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Kuritani, M.; Sakai, N.; Karakawa, A.; Isawa, M.; Chatani, M.; Negishi-Koga, T.; Funatsu, T.; Takami, M. Anti-Mouse RANKL Antibodies Inhibit Alveolar Bone Destruction in Periodontitis Model Mice. Biol. Pharm. Bull. 2018, 41, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Aljohani, S.; Gaudin, R.; Weiser, J.; Tröltzsch, M.; Ehrenfeld, M.; Kaeppler, G.; Smeets, R.; Otto, S. Osteonecrosis of the Jaw in Patients Treated with Denosumab: A Multicenter Case Series. J. Craniomaxillofac. Surg. 2018, 46, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Ueda, N.; Yamada, S.I.; Kato, S.; Iwata, E.; Hayashida, S.; Kojima, Y.; Shinohara, M.; Tojo, I.; Nakahara, H.; et al. Denosumab-Related Osteonecrosis of the Jaw after Tooth Extraction and the Effects of a Short Drug Holiday in Cancer Patients: A Multicenter Retrospective Study. Osteoporos. Int. 2021, 32, 2323–2333. [Google Scholar] [CrossRef]
- Tada, M.; Inui, K.; Sugioka, Y.; Mamoto, K.; Okano, T.; Anno, S.; Koike, T. Use of Bisphosphonate Might Be Important to Improve Bone Mineral Density in Patients with Rheumatoid Arthritis Even under Tight Control: The TOMORROW Study. Rheumatol. Int. 2017, 37, 999–1005. [Google Scholar] [CrossRef]
- Xie, J.; Li, S.; Xiao, L.; Ouyang, G.; Zheng, L.; Gu, Y.; Gao, C.; Han, X. Zoledronic Acid Ameliorates the Effects of Secondary Osteoporosis in Rheumatoid Arthritis Patients. J. Orthop. Surg. Res. Ther. 2019, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.M.; Shenoy, N. Vitamin D, Osteoporosis, and Periodontal Diseases. Int. J. Oral Health Sci. 2018, 8, 6–12. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Pathogenetic Insights from the Treatment of Rheumatoid Arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, R.M.; Ang, D.C. Biologic therapies for autoimmune and connective tissue diseases. Immunol. Allergy Clin. 2017, 37, 283–299. [Google Scholar] [CrossRef]
- Van Dyke, T.E. Pro-resolving mediators in the regulation of periodontal disease. Mol. Aspects Med. 2017, 58, 21–36. [Google Scholar] [CrossRef]
- Jäger, E.; Murthy, S.; Schmidt, C.; Hahn, M.; Strobel, S.; Peters, A.; Stäubert, C.; Sungur, P.; Venus, T.; Geisler, M.; et al. Calci-Um-Sensing Receptor-Mediated NLRP3 Inflammasome Response to Calciprotein Particles Drives Inflammation in Rheumatoid Arthritis. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Guo, C.; Fu, R.; Wang, S.; Huang, Y.; Li, X.; Zhou, M.; Zhao, J.; Yang, N. NLRP3 Inflammasome Activation Contributes to the Pathogenesis of Rheumatoid Arthritis. Clin. Exp. Immunol. 2018, 194, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Choulaki, C.; Papadaki, G.; Repa, A.; Kampouraki, E.; Kambas, K.; Ritis, K.; Bertsias, G.; Boumpas, D.T.; Sidiropoulos, P. Enhanced Activity of NLRP3 Inflammasome in Peripheral Blood Cells of Patients with Active Rheumatoid Arthritis. Arthritis Res. Ther. 2015, 17, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Zheng, Z.; Lin, P.; Fu, X.; Li, F.; Jiang, J.; Zhu, P. ACPAs Promote IL-1β Production in Rheumatoid Arthritis by Activating the NLRP3 Inflammasome. Cell. Mol. Immunol. 2020, 17, 261–271. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, X.; Su, X.; Liu, X.; Ren, K.; Ning, C.; Zhang, Q.; Zhang, S. Daphnes Cortex and Its Licorice-Processed Products Suppress Inflammation via the TLR4/NF-ΚB/NLRP3 Signaling Pathway and Regulation of the Metabolic Profile in the Treatment of Rheumatoid Arthritis. J. Ethnopharmacol. 2021, 283, 114657. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Gao, Y.; Zhao, H.; Wang, Z.; Wang, J. Galangin Protects Human Rheumatoid Arthritis Fibroblast like Synoviocytes via Suppression of the NF ΚB/NLRP3 Pathway. Mol. Med. Rep. 2018, 18, 3619–3624. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Wang, J.; Wen, W.; Pan, T.; Chen, H.; Fu, Y.; Wang, F.; Huang, J.H.; Xu, S. Cinnamaldehyde Suppresses NLRP3 Derived IL-1β via Activating Succinate/HIF-1 in Rheumatoid Arthritis Rats. Int. Immunopharmacol. 2020, 84, 106570. [Google Scholar] [CrossRef]
- Santos-Sierra, S. Targeting Toll-like Receptor (TLR) Pathways in Inflammatory Arthritis: Two Better Than One? Biomolecules 2021, 11, 1291. [Google Scholar] [CrossRef]
- Han, J.; Li, X.; Luo, X.; He, J.; Huang, X.; Zhou, Q.; Han, Y.; Jie, H.; Zhuang, J.; Li, Y.; et al. The Mechanisms of Hydroxychloroquine in Rheumatoid Arthritis Treatment: Inhibition of Dendritic Cell Functions via Toll like Receptor 9 Signaling. Biomed. Pharmacol. 2020, 132, 110848. [Google Scholar] [CrossRef]
- Samarpita, S.; Kim, J.Y.; Rasool, M.K.; Kim, K.S. Investigation of Toll-like Receptor (TLR) 4 Inhibitor TAK-242 as a New Potential an-Ti-Rheumatoid Arthritis Drug. Arthritis Res. Ther. 2020, 22, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monnet, E.; Choy, E.H.; McInnes, I.; Kobakhidze, T.; Graaf, K.; Jacqmin, P.; Lapeyre, G.; Min, C. Efficacy and Safety of NI-0101, an Anti-Toll-like Receptor 4 Monoclonal Antibody, in Patients with Rheumatoid Arthritis after Inadequate Response to Methotrexate: A Phase II Study. Ann. Rheum. Dis. 2020, 79, 316–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speer, E.M.; Dowling, D.J.; Xu, J.; Ozog, L.S.; Mathew, J.A.; Chander, A.; Yin, D.; Levy, O. Pentoxifylline, Dexamethasone and Azithromycin Demonstrate Distinct Age-Dependent and Synergistic Inhibition of TLR-and Inflammasome-Mediated Cytokine Production in Human Newborn and Adult Blood in Vitro. PLoS ONE 2018, 13, e0196352. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, V.J. Azithromycin as an Adjunct to Non-surgical Periodontal Therapy: A Systematic Review. Aust. Dent. J. 2017, 62, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009, 461, 1287–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue Engineering for Bone Regeneration and Osseointegration in the Oral Cavity. Dent. Mater. 2015, 31, 317–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene Therapy Comes of Age. Science 2018, 359, 6372. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Santalla, M.; Bueren, J.A.; Garin, M.I. Mesenchymal Stem/Stromal Cell-Based Therapy for the Treatment of Rheumatoid Arthritis: An Update on Preclinical Studies. EBioMedicine 2021, 69, 103427. [Google Scholar] [CrossRef]
- Martu, M.A.; Rezus, E.; Popa, C.; Solomon, S.M.; Luchian, I.; Pendefunda, A.C.; Sioustis, I.; Anton, D.; Martu, S.; Foia, L. Correlations between systemic therapy with conventional (synthetic) and biological DMARDS, rheumatoid arthritis and periodontal indices of chronic periodontitis. Rom. J. Oral Rehabil. 2018, 10, 161–169. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martu, M.-A.; Maftei, G.-A.; Luchian, I.; Stefanescu, O.M.; Scutariu, M.M.; Solomon, S.M. The Effect of Acknowledged and Novel Anti-Rheumatic Therapies on Periodontal Tissues—A Narrative Review. Pharmaceuticals 2021, 14, 1209. https://doi.org/10.3390/ph14121209
Martu M-A, Maftei G-A, Luchian I, Stefanescu OM, Scutariu MM, Solomon SM. The Effect of Acknowledged and Novel Anti-Rheumatic Therapies on Periodontal Tissues—A Narrative Review. Pharmaceuticals. 2021; 14(12):1209. https://doi.org/10.3390/ph14121209
Chicago/Turabian StyleMartu, Maria-Alexandra, George-Alexandru Maftei, Ionut Luchian, Ovidiu Mihail Stefanescu, Mihaela Monica Scutariu, and Sorina Mihaela Solomon. 2021. "The Effect of Acknowledged and Novel Anti-Rheumatic Therapies on Periodontal Tissues—A Narrative Review" Pharmaceuticals 14, no. 12: 1209. https://doi.org/10.3390/ph14121209
APA StyleMartu, M.-A., Maftei, G.-A., Luchian, I., Stefanescu, O. M., Scutariu, M. M., & Solomon, S. M. (2021). The Effect of Acknowledged and Novel Anti-Rheumatic Therapies on Periodontal Tissues—A Narrative Review. Pharmaceuticals, 14(12), 1209. https://doi.org/10.3390/ph14121209







