Choroidal Volume Evaluation after Photodynamic Therapy Using New Optical Coherence Tomography Imaging Algorithm
Abstract
:1. Introduction
2. Results
2.1. Patient Population
2.2. Inter-Test Repeatability
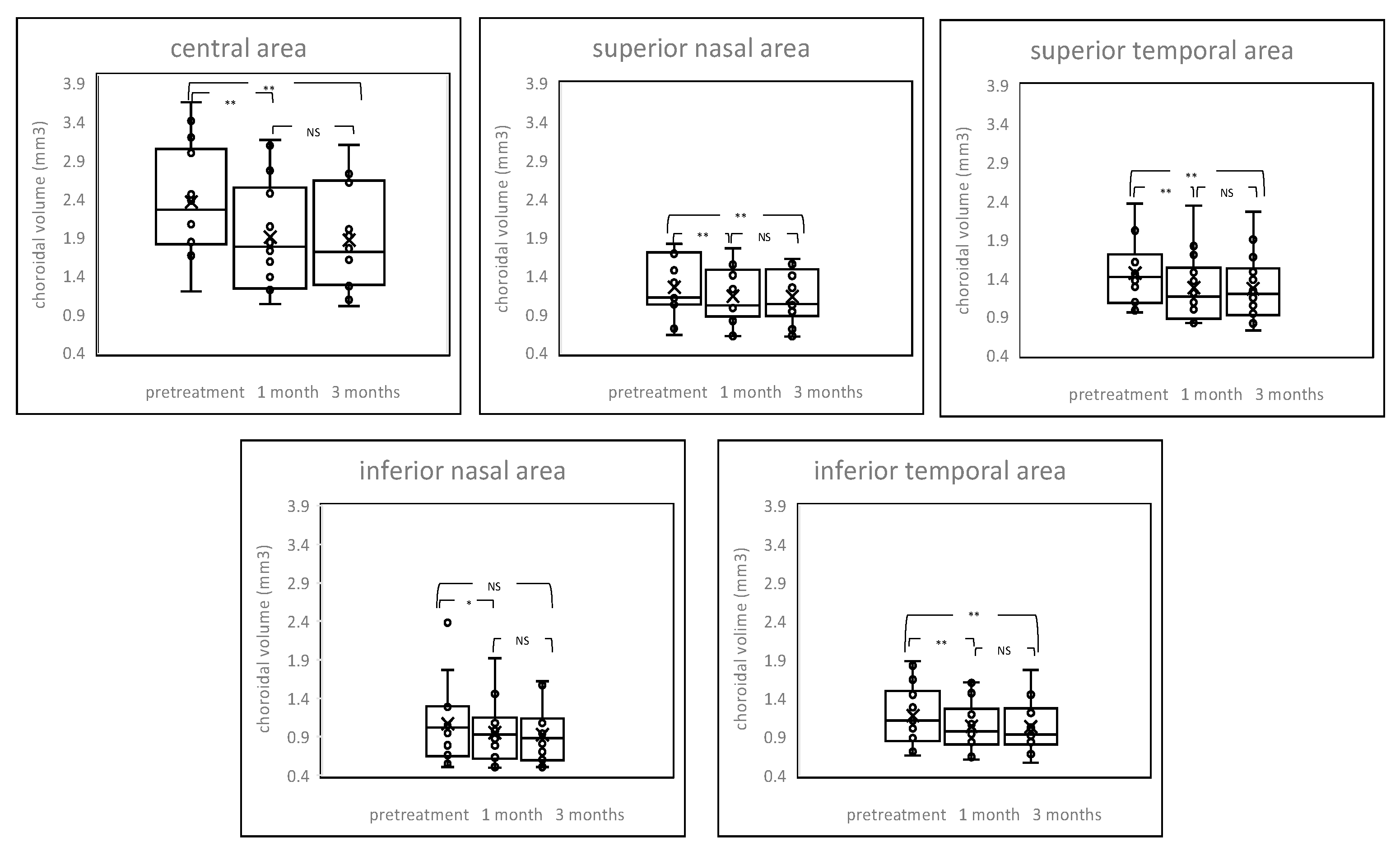
2.3. Changes of Choroidal Volume
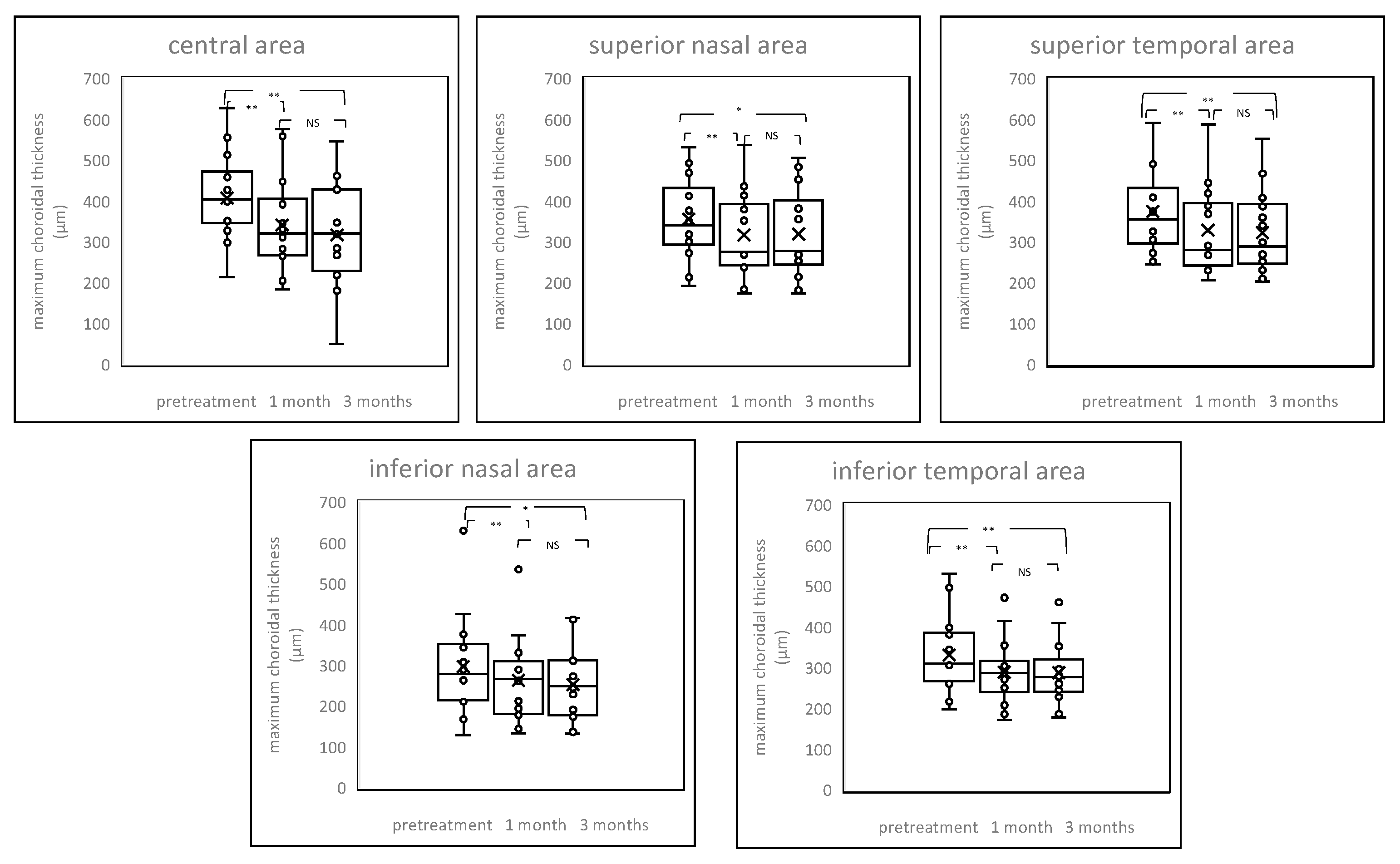
2.4. Chenges of Choroildal Thickness
2.5. Case Presentations
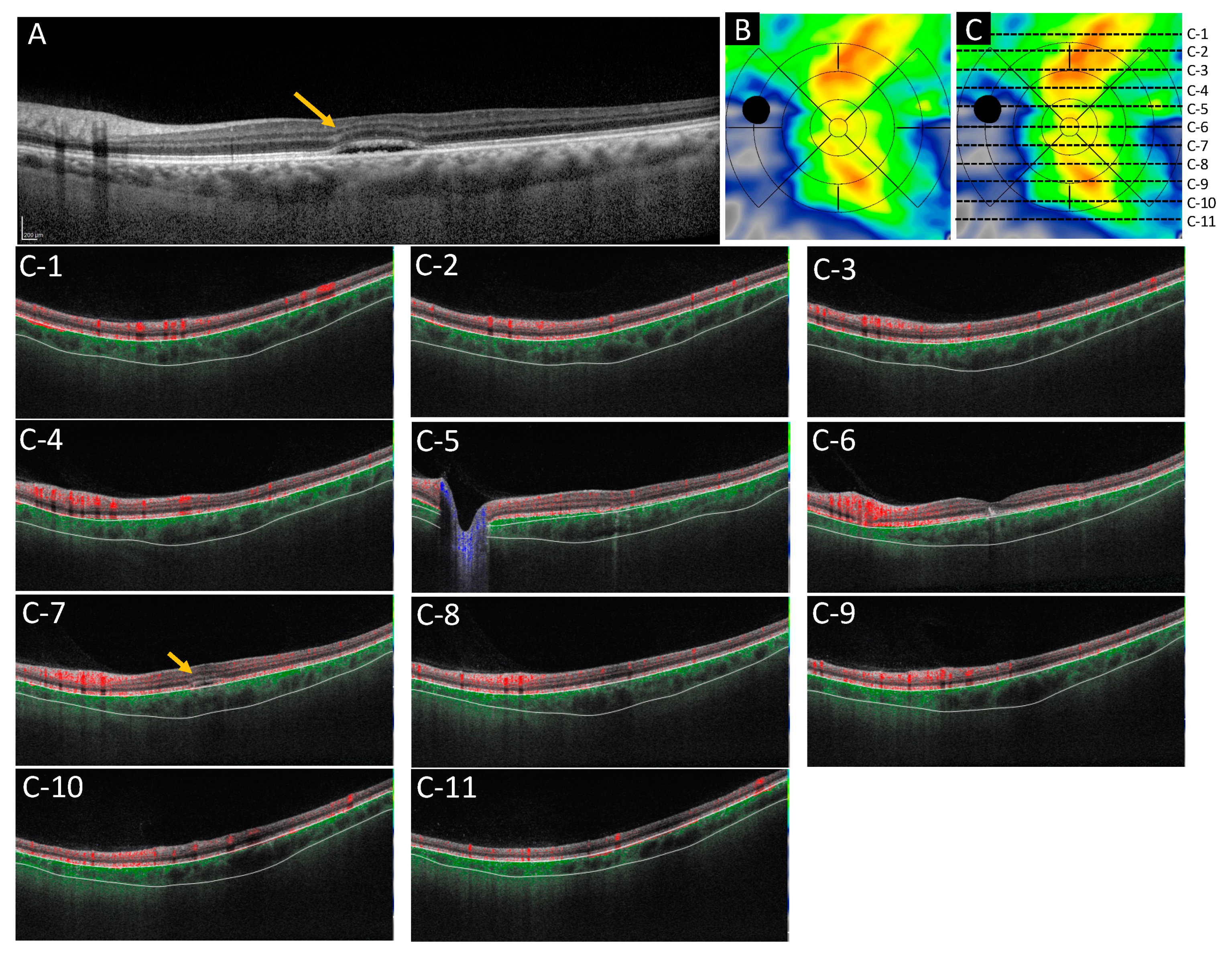
2.5.1. Case 1: A 46 Year-Old-Male
2.5.2. Case 2: A 53-Year-Old Male
3. Discussion
4. Materials and Methods
4.1. Population
4.2. Treatment Conditions
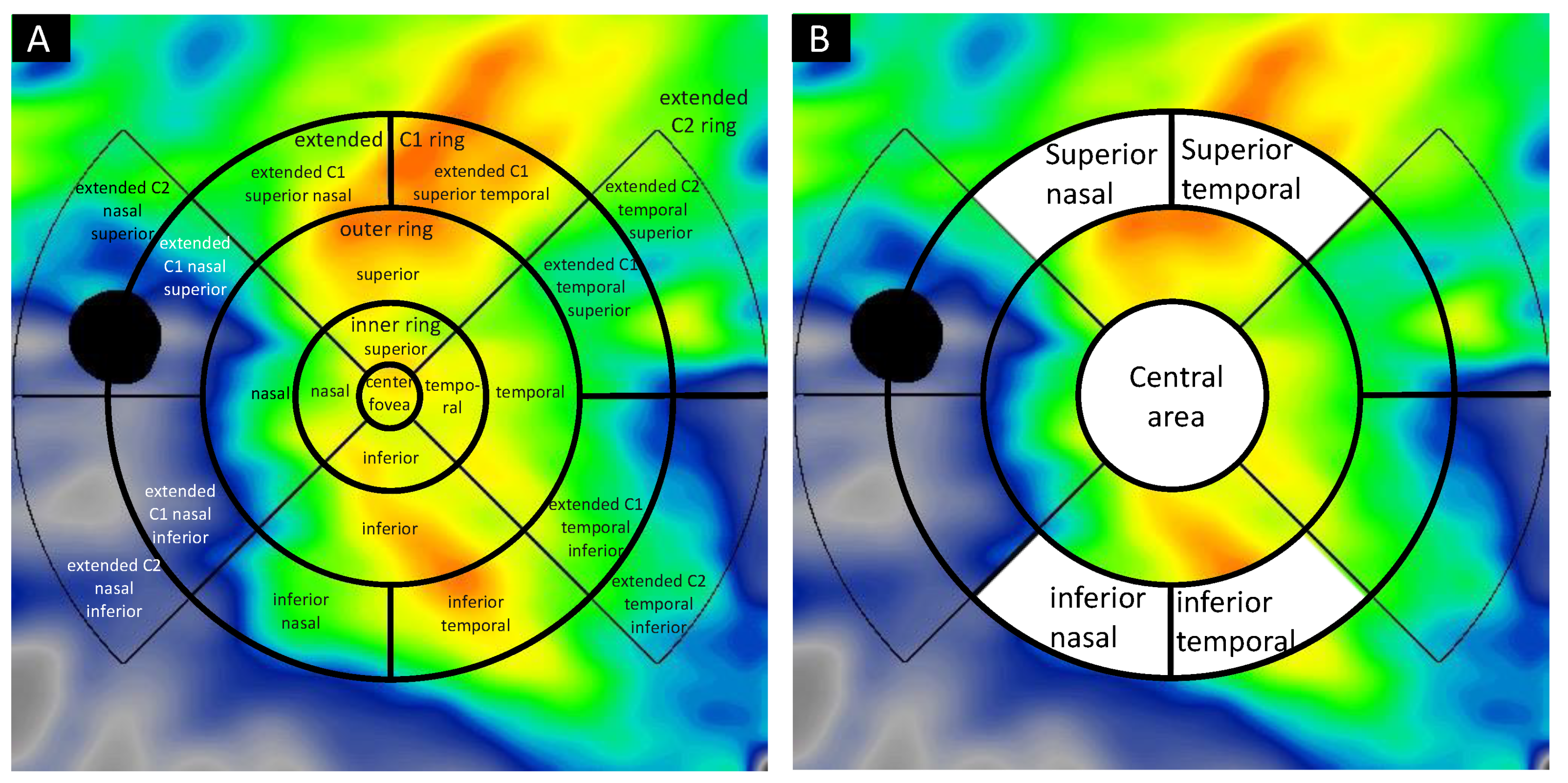
4.3. Image Acquisition and Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TAP | Treatment of Age-Related Macular Degeneration with Photodynamic Therapy |
| VIP | Verteporfin in Photodynamic Therapy |
| PDT | Photodynamic therapy |
| CSC | Central serous chorioretinopathy |
| RPE | Retinal pigment epithelium |
| ICGA | Indocyanine green angiography |
| OCT | Optical coherence tomography |
| SS | Swept-source |
| ARI | Advanced Retina Imaging |
| ICC | Intraclass correlation coefficient |
| CI | Confidence interval |
| LogMAR | Logarithm of the minimum angle of resolution |
| FA | Fluorescein angiography |
| BCVA | Best-corrected visual acuity |
| VEGF | Vascular endothelial growth factor |
| ETDRS | Early Treatment Diabetic Retinopathy Study |
References
- Chan, W.M.; Lai, T.Y.; Tano, Y.; Liu, D.T.; Li, K.K.; Lam, D.S. Photodynamic therapy in macular diseases of asian populations: When East meets West. Jpn. J. Ophthalmol. 2006, 50, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.M.; Lim, T.H.; Pece, A.; Silva, R.; Yoshimura, N. Verteporfin PDT for non-standard indications—A review of current literature. Graefes. Arch. Clin. Exp. Ophthalmol. 2010, 248, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.M.; Ruiz-Moreno, J.M.; Gomez-Ulla, F.; Montero, J.A.; Gregório, T.; Cachulo, M.L.; Pires, I.A.; Cunha-Vaz, J.G.; Murta, J.N. Photodynamic therapy for chronic central serous chorioretinopathy: A 4-year follow-up study. Retina 2013, 33, 309–315. [Google Scholar] [CrossRef]
- Chan, W.M.; Lam, D.S.; Lai, T.Y.; Tam, B.S.; Liu, D.T.; Chan, C.K.M. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: A novel treatment at the primary disease level. Br. J. Ophthalmol. 2003, 87, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Cardillo Piccolino, F.; Eandi, C.M.; Ventre, L.; Rigault de la Longrais, R.C.; Grignolo, F.M. Photodynamic therapy for chronic central serous chorioretinopathy. Retina 2003, 23, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.Y.; Chan, W.M.; Lam, D.S. Transient reduction in retinal function revealed by multifocal electroretinogram after photodynamic therapy. Am. J. Ophthalmol. 2004, 137, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Gomi, F.; Ueno, C.; Nishida, K. Reduced-fluence photodynamic therapy for subfoveal serous pigment epithelial detachment with choroidal vascular hyperpermeability. Am. J. Ophthalmol. 2012, 154, 865–871. [Google Scholar] [CrossRef]
- van Dijk, E.H.C.; Fauser, S.; Breukink, M.B.; Blanco-Garavito, R.; Groenewoud, J.M.M.; Keunen, J.E.E.; Peters, P.J.H.; Dijkman, G.; Souied, E.H.; MacLaren, R.E.; et al. Half-Dose Photodynamic Therapy versus High-Density Subthreshold Micropulse Laser Treatment in Patients with Chronic Central Serous Chorioretinopathy: The PLACE Trial. Ophthalmology 2018, 125, 1547–1555. [Google Scholar] [CrossRef]
- Nicolo, M.; Eandi, C.M.; Alovisi, C.; Grignolo, F.M.; Traverso, C.E.; Musetti, D.; Cardillo Piccolino, F. Half-fluence versus half-dose photodynamic therapy in chronic central serous chorioretinopathy. Am. J. Ophthalmol. 2014, 157, 1033–1037. [Google Scholar] [CrossRef]
- van Rijssen, T.J.; van Dijk, E.H.C.; Yzer, S.; Ohno-Matsui, K.; Keunen, J.E.E.; Schlingemann, R.O.; Sivaprasad, S.; Querques, G.; Downes, S.M.; Fauser, S.; et al. Central serous chorioretinopathy: Towards an evidence-based treatment guideline. Prog. Retin. Eye. Res. 2019, 73, 100770. [Google Scholar] [CrossRef]
- Schlotzer-Schrehardt, U.; Viestenz, A.; Naumann, G.O.; Laqua, H.; Michels, S.; Schmidt-Erfurth, U. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes. Arch. Clin. Exp. Ophthalmol. 2002, 240, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Laqua, H.; Schlötzer-Schrehard, U.; Viestenz, A.; Naumann, G.O.H. Histopathological changes following photodynamic therapy in human eyes. Arch. Ophthalmol. 2002, 120, 835–844. [Google Scholar]
- Schmidt-Erfurth, U.; Michels, S.; Barbazetto, I.; Laqua, H. Photodynamic effects on choroidal neovascularization and physiological choroid. Investig. Ophthalmol. Vis. Sci. 2002, 43, 830–841. [Google Scholar]
- Manabe, S.; Shiragami, C.; Hirooka, K.; Izumibata, S.; Tsujikawa, A.; Shiraga, F. Change of regional choroid thickness after reduced-fluence photodynamic therapy for chronic central serous chorioretinopathy. Am. J. Ophthalmol. 2015, 159, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Agawa, T.; Miura, M.; Ikuno, Y.; Makita, S.; Fabritius, T.; Iwasaki, T.; Goto, H.; Nishida, K.; Yasuno, Y. Choroidal thickness measurement in healthy Japanese subjects by three-dimensional high-penetration optical coherence tomography. Graefes. Arch. Clin. Exp. Ophthalmol. 2011, 249, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Lee, J.Y.; Lee, E.K.; Kim, J.Y. Comparison of visula/anatomical outcomes and recurrence rate between oral spironolactone and photodynamic therapy for nonresolving central serous chorioretinopathy. Retina 2020, 40, 1191–1199. [Google Scholar] [CrossRef]
- Kim, Y.K.; Ryoo, N.K.; Woo, S.J.; Park, K.H. Comparison of visual and anatomical outcomes of half-fluence and half-dose photodynamic therapy in eyes with chronic central serous chorioretinopathy. Graefes. Arch. Clin. Exp. Ophthalmol. 2015, 253, 2063–2073. [Google Scholar] [CrossRef]
- Zhao, M.; Alonso-Caneiro, D.; Lee, R.; Cheong, A.M.Y.; Yu, W.Y.; Wong, H.Y.; Lam, A.K.C. Comparison of Choroidal Thickness Measurements Using Semiautomated and Manual Segmentation Methods. Optom. Vis. Sci. 2020, 97, 121–127. [Google Scholar] [CrossRef]
- Huynh, E.; Chandrasekera, E.; Bukowska, D.; McLenachan, S.; Mackey, D.A.; Chen, F.K. Past, Present, and Future Concepts of the Choroidal Scleral Interface Morphology on Optical Coherence Tomography. Asia Pac. J. Ophthalmol. 2017, 6, 94–103. [Google Scholar]
- Cogliati, A.; Canavesi, C.; Hayes, A.; Tankam, P.; Duma, V.F.; Santhanam, A.; Thompson, K.P.; Rolland, J.P. MEMS-based handheld scanning probe with pre-shaped input signals for distortion-free images in Gabor-domain optical coherence microscopy. Opt. Express 2016, 24, 13365–13374. [Google Scholar] [CrossRef]
- Mehta, N.; Cheng, Y.; Alibhai, A.Y.; Duker, J.S.; Wang, R.K.; Waheed, N.K. Optical coherence tomography angiography distortion correction in widefield montage images. Quant. Imaging. Med. Surg. 2021, 11, 928–938. [Google Scholar] [CrossRef]
- Drexler, W.; Liu, M.; Kumar, A.; Kamali, T.; Unterhuber, A.; Leitgeb, R.A. Optical coherence tomography today: Speed, contrast, and multimodality. J. Biomed. Opt. 2014, 19, 071412. [Google Scholar] [CrossRef]
- Twa, M.D.; Schulle, K.L.; Chiu, S.J.; Farsiu, S.; Berntsen, D.A. Validation of Macular Choroidal Thickness Measurements from Automated SD-OCT Image Segmentation. Optom. Vis. Sci. 2016, 93, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.L.; Yu, H.G. Choroidal thickness after full-fluence and half-fluence photodynamic therapy in chronic central serus chorioretinopathy. Retina 2015, 35, 1555–1560. [Google Scholar] [CrossRef]
- Barteselli, G.; Chhablani, J.; El-Emam, S.; Wang, H.; Chuang, J.; Kozak, I.; Cheng, L.; Bartsch, D.U.; Freeman, W.R. Choroidal volume variations with age, axial length, and sex in healthy subjects: A three-dimensional analysis. Ophthalmology 2012, 119, 2572–2578. [Google Scholar] [CrossRef]
- Kur, J.; Newman, E.A.; Chan-Ling, T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res. 2012, 31, 377–406. [Google Scholar] [CrossRef]
- Bill, A.; Törnquist, P.; Alm, A. Permeability of the intraocular blood vessels. Trans. Ophthalmol. Soc. U. K. 1980, 100, 332–336. [Google Scholar] [PubMed]
- Mori, K.; Gehlbach, P.L.; Yoneya, S.; Shimizu, K. Asymmetry of choroidal venous vascular patterns in the human eye. Ophthalmology 2004, 111, 507–512. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hoshino, J.; Mukai, R.; Nakamura, K.; Kikuchi, Y.; Kishi, S.; Akiyama, H. Vortex Vein Anastomosis at the Watershed in Pachychoroid Spectrum Diseases. Ophthalmol. Retina 2020, 4, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Ledesma-Gil, G.; Gemmy Cheung, C.M. Intervortex venous anastomosis in pachychoroid -related disorders. Retina 2021, 41, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Matsumoto, H.; Miyakubo, T.; Akiyama, H. Reduced vascular density in the choroid after treatment with photodynamic theraphy combined with aflibercept in patients with polypoidal choroidal vasculopathy. Retina 2021, 41, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Dai, Y.; Shi, Y.; Russell, J.F.; Lyu, C.; Noorikolouri, J.; Feuer, W.J.; Chu, Z.; Zhang, Q.; de Sisternes, L.; et al. Age-Related Changes in Choroidal Thickness and the Volume of Vessels and Stroma Using Swept-Source OCT and Fully Automated Algorithms. Ophthalmol. Retina 2020, 4, 204–215. [Google Scholar] [CrossRef]
- Agrawal, R.; Gupta, P.; Tan, K.A.; Cheung, C.M.; Wong, T.Y.; Cheng, C.Y. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci. Rep. 2016, 6, 21090. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Gregori, G.; Schaal, K.B.; Legarreta, A.D.; Miller, A.R.; Roisman, L.; Feuer, W.J.; Rosenfeld, P.J. Choroidal Thickness and Choroidal Vessel Density in Nonexudative Age-Related Macular Degeneration Using Swept-Source Optical Coherence Tomography Imaging. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6256–6264. [Google Scholar] [CrossRef] [PubMed]
- Alovisi, C.; Piccolino, F.C.; Nassisi, M.; Eandi, C.M. Choroidal Structure after Half-Dose Photodynamic Therapy in Chronic Central Serous Chorioretinopathy. J. Clin. Med. 2020, 9, 2734. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]




| CSC (n = 14) | Normal Control (n = 9) | p-Value | |
|---|---|---|---|
| Age | 59.5 (15.85) | 70.4 (7.55) | 0.147 |
| logMAR | 0.15 (0.21) | −0.004 (0.10) | 0.154 |
| Mean refractive error | −0.29 (2.14) | 1.22 (1.23) | 0.086 |
| Spot size (μm) | 4521 (1201) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato-Akushichi, M.; Ono, S.; Klose, G.; Song, Y. Choroidal Volume Evaluation after Photodynamic Therapy Using New Optical Coherence Tomography Imaging Algorithm. Pharmaceuticals 2021, 14, 1140. https://doi.org/10.3390/ph14111140
Sato-Akushichi M, Ono S, Klose G, Song Y. Choroidal Volume Evaluation after Photodynamic Therapy Using New Optical Coherence Tomography Imaging Algorithm. Pharmaceuticals. 2021; 14(11):1140. https://doi.org/10.3390/ph14111140
Chicago/Turabian StyleSato-Akushichi, Miki, Shinji Ono, Gerd Klose, and Youngseok Song. 2021. "Choroidal Volume Evaluation after Photodynamic Therapy Using New Optical Coherence Tomography Imaging Algorithm" Pharmaceuticals 14, no. 11: 1140. https://doi.org/10.3390/ph14111140
APA StyleSato-Akushichi, M., Ono, S., Klose, G., & Song, Y. (2021). Choroidal Volume Evaluation after Photodynamic Therapy Using New Optical Coherence Tomography Imaging Algorithm. Pharmaceuticals, 14(11), 1140. https://doi.org/10.3390/ph14111140






