MicroRNA-100 Mediates Hydrogen Peroxide-Induced Apoptosis of Human Retinal Pigment Epithelium ARPE-19 Cells
Abstract
1. Introduction
2. Results
2.1. Oxidative Stress Increased miR-100 Biosynthesis in Human ARPE-19 Cells
2.2. Signaling Profiles of Oxidative Stress-Driven Cytotoxicity of ARPE-19 Cells
2.3. Signal Pathways Involved in Oxidative-Stress-Induced miR-100 Biosynthesis and Cytotoxicity in ARPE-19 Cells
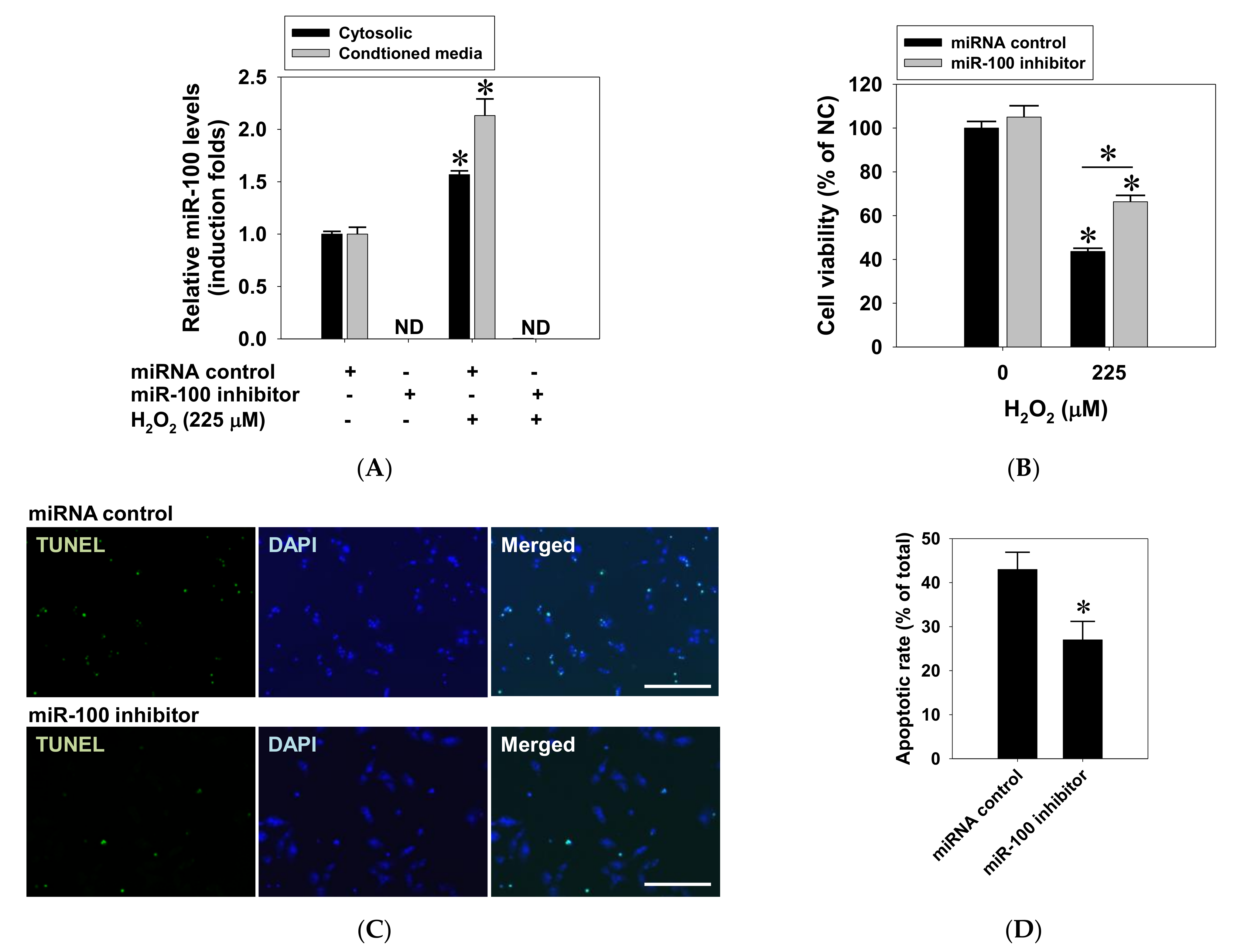
2.4. Suppressive Effect of miR-100 Antagomir on Oxidative Cell Death in ARPE-19 Cells
2.5. Effects of miR-100 Antagomir on AMPK/mTOR and Antioxidant Expression in ARPE-19 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Treatment
4.3. Cytotoxicity Assay
4.4. MiRNA Semi-Quantification
4.5. Western Blotting
4.6. TUNEL Staining
4.7. Transfection of miR-100 Inhibitor
4.8. Immunofluorescent Staining
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedman, D.S.; O’Colmain, B.J.; Munoz, B.; Tomany, S.C.; McCarty, C.; de Jong, P.T.; Nemesure, B.; Mitchell, P.; Kempen, J. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar] [PubMed]
- Yang, K.; Liang, Y.B.; Gao, L.Q.; Peng, Y.; Shen, R.; Duan, X.R.; Friedman, D.S.; Sun, L.P.; Mitchell, P.; Wang, N.L.; et al. Prevalence of age-related macular degeneration in a rural Chinese population: The Handan Eye Study. Ophthalmology 2011, 118, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R.; Wang, J.J.; Ji, G.J.; Taylor, B.; Oizumi, T.; Daimon, M.; Kato, T.; Kawata, S.; Kayama, T.; Tano, Y.; et al. Prevalence and risk factors for age-related macular degeneration in an adult Japanese population: The Funagata study. Ophthalmology 2008, 115, 1376–1381. [Google Scholar] [CrossRef]
- Chen, S.J.; Cheng, C.Y.; Peng, K.L.; Li, A.F.; Hsu, W.M.; Liu, J.H.; Chou, P. Prevalence and associated risk factors of age-related macular degeneration in an elderly Chinese population in Taiwan: The Shihpai Eye Study. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3126–3133. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R.; Wang, J.J.; Aung, T.; Tan, D.T.; Mitchell, P.; Sandar, M.; Saw, S.M.; Wong, T.Y. Prevalence of age-related macular degeneration in a Malay population: The Singapore Malay Eye Study. Ophthalmology 2008, 115, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Feigl, B. Age-related maculopathy—Linking aetiology and pathophysiological changes to the ischaemia hypothesis. Prog. Retin. Eye Res. 2009, 28, 63–86. [Google Scholar] [CrossRef] [PubMed]
- Van Lookeren Campagne, M.; LeCouter, J.; Yaspan, B.L.; Ye, W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J. Pathol. 2014, 232, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A. The pathogenesis of age-related macular degeneration. Mol. Vis. 1999, 5, 24. [Google Scholar]
- Algvere, P.V.; Seregard, S. Age-related maculopathy: Pathogenetic features and new treatment modalities. Acta Ophthalmol. Scand. 2002, 80, 136–143. [Google Scholar] [CrossRef]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Khandhadia, S.; Lotery, A. Oxidation and age-related macular degeneration: Insights from molecular biology. Expert Rev. Mol. Med. 2010, 12, e34. [Google Scholar] [CrossRef] [PubMed]
- Hollyfield, J.G.; Bonilha, V.L.; Rayborn, M.E.; Yang, X.; Shadrach, K.G.; Lu, L.; Ufret, R.L.; Salomon, R.G.; Perez, V.L. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 2008, 14, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Zarbin, M.A. Current concepts in the pathogenesis of age-related macular degeneration. Arch. Ophthalmol. 2004, 122, 598–614. [Google Scholar] [CrossRef]
- Moriarty-Craige, S.E.; Adkison, J.; Lynn, M.; Gensler, G.; Bressler, S.; Jones, D.P.; Sternberg, P., Jr. Antioxidant supplements prevent oxidation of cysteine/cystine redox in patients with age-related macular degeneration. Am. J. Ophthalmol. 2005, 140, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.S. MicroRNA function: Multiple mechanisms for a tiny RNA? RNA 2005, 11, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Loscher, C.J.; Hokamp, K.; Wilson, J.H.; Li, T.; Humphries, P.; Farrar, G.J.; Palfi, A. A common microRNA signature in mouse models of retinal degeneration. Exp. Eye Res. 2008, 87, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Loscher, C.J.; Hokamp, K.; Kenna, P.F.; Ivens, A.C.; Humphries, P.; Palfi, A.; Farrar, G.J. Altered retinal microRNA expression profile in a mouse model of retinitis pigmentosa. Genome Biol. 2007, 8, R248. [Google Scholar] [CrossRef]
- Hackler, L., Jr.; Wan, J.; Swaroop, A.; Qian, J.; Zack, D.J. MicroRNA profile of the developing mouse retina. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1823–1831. [Google Scholar] [CrossRef]
- Ren, C.; Liu, Q.; Wei, Q.; Cai, W.; He, M.; Du, Y.; Xu, D.; Wu, Y.; Yu, J. Circulating miRNAs as potential biomarkers of age-related macular degeneration. Cell. Physiol. Biochem. 2017, 41, 1413–1423. [Google Scholar] [CrossRef]
- Romano, G.L.; Platania, C.B.M.; Drago, F.; Salomone, S.; Ragusa, M.; Barbagallo, C.; Di Pietro, C.; Purrello, M.; Reibaldi, M.; Avitabile, T.; et al. Retinal and circulating miRNAs in age-related macular degeneration: An in vivo animal and human study. Front. Pharmacol. 2017, 8, 168. [Google Scholar] [CrossRef]
- Askou, A.L.; Alsing, S.; Holmgaard, A.; Bek, T.; Corydon, T.J. Dissecting microRNA dysregulation in age-related macular degeneration: New targets for eye gene therapy. Acta Ophthalmol. 2017, 96, 9–23. [Google Scholar] [CrossRef]
- Menard, C.; Rezende, F.A.; Miloudi, K.; Wilson, A.; Tetreault, N.; Hardy, P.; SanGiovanni, J.P.; De Guire, V.; Sapieha, P. MicroRNA signatures in vitreous humour and plasma of patients with exudative AMD. Oncotarget 2016, 7, 19171–19184. [Google Scholar] [CrossRef][Green Version]
- Nagaraja, A.K.; Creighton, C.J.; Yu, Z.; Zhu, H.; Gunaratne, P.H.; Reid, J.G.; Olokpa, E.; Itamochi, H.; Ueno, N.T.; Hawkins, S.M.; et al. A link between mir-100 and FRAP1/mTOR in clear cell ovarian cancer. Mol. Endocrinol. 2010, 24, 447–463. [Google Scholar] [CrossRef]
- Shi, W.; Alajez, N.M.; Bastianutto, C.; Hui, A.B.; Mocanu, J.D.; Ito, E.; Busson, P.; Lo, K.W.; Ng, R.; Waldron, J.; et al. Significance of Plk1 regulation by miR-100 in human nasopharyngeal cancer. Int. J. Cancer 2010, 126, 2036–2048. [Google Scholar] [CrossRef]
- Xu, C.; Zeng, Q.; Xu, W.; Jiao, L.; Chen, Y.; Zhang, Z.; Wu, C.; Jin, T.; Pan, A.; Wei, R.; et al. miRNA-100 inhibits human bladder urothelial carcinogenesis by directly targeting mTOR. Mol. Cancer Ther. 2012, 12, 207–219. [Google Scholar] [CrossRef]
- Gong, Y.; He, T.; Yang, L.; Yang, G.; Chen, Y.; Zhang, X. The role of miR-100 in regulating apoptosis of breast cancer cells. Sci. Rep. 2015, 5, 11650. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, R.; He, Y.; Fu, X.; Fu, L.; Zhu, Z.; Dong, J.T. MicroRNA 100 sensitizes luminal A breast cancer cells to paclitaxel treatment in part by targeting mTOR. Oncotarget 2015, 7, 5702–5714. [Google Scholar] [CrossRef]
- Fahim Golestaneh, A.; Lecker, L.S.M.; Schlegel, J.; Nowrouzi, A.; Schwager, C.; Meister, S.; Weichert, W.; Debus, J.; Abdollahi, A. Large scale in vivo micro-RNA loss of function screen identified miR-29a, miR-100 and miR-155 as modulators of radioresistance and tumor-stroma communication. Int. J. Cancer 2019, 144, 2774–2781. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Guo, J.; He, H.; Mi, X.; Chen, C.; Xie, J.; Wang, S.; Wu, P.; Cao, F.; et al. miR-100 maintains phenotype of tumor-associated macrophages by targeting mTOR to promote tumor metastasis via Stat5a/IL-1ra pathway in mouse breast cancer. Oncogenesis 2018, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.Y.; Shi, Q.; Zheng, Z.Y.; Gong, J.; Zeng, C.; Yang, J.; Zhuang, S.M. MicroRNA-100 promotes the autophagy of hepatocellular carcinoma cells by inhibiting the expression of mTOR and IGF-1R. Oncotarget 2014, 5, 6218–6228. [Google Scholar] [CrossRef]
- Sucharov, C.; Bristow, M.R.; Port, J.D. miRNA expression in the failing human heart: Functional correlates. J. Mol. Cell. Cardiol. 2008, 45, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Li, G.; Chen, L.; Guo, J.; Liu, Y. Downregulation of microRNA-100 protects H2O2-induced apoptosis in neonatal cardiomyocytes. Int. J. Clin. Exp. Pathol. 2015, 8, 5491–5496. [Google Scholar] [PubMed]
- Kong, N.; Lu, X.; Li, B. Downregulation of microRNA-100 protects apoptosis and promotes neuronal growth in retinal ganglion cells. BMC Mol. Biol. 2014, 15, 25. [Google Scholar] [CrossRef]
- Walz, J.M.; Wecker, T.; Zhang, P.P.; Cakir, B.; Gruening, B.; Agostini, H.; Reuer, T.; Ludwig, F.; Boneva, S.; Faerber, L.; et al. Impact of angiogenic activation and inhibition on miRNA profiles of human retinal endothelial cells. Exp. Eye Res. 2019, 181, 98–104. [Google Scholar] [CrossRef]
- Morris, D.R.; Bounds, S.E.; Liu, H.; Ding, W.Q.; Chen, Y.; Liu, Y.; Cai, J. Exosomal miRNA transfer between retinal microglia and RPE. Int. J. Mol. Sci. 2020, 21, 3541. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Pan, J.; Yang, S.; Yao, H.; Li, M.; Li, H.; Lei, H.; Jin, H.; Wang, F. Exosomes mediate an epithelial-mesenchymal transition cascade in retinal pigment epithelial cells: Implications for proliferative vitreoretinopathy. J. Cell. Mol. Med. 2020, 24, 13324–13335. [Google Scholar] [CrossRef]
- Faghiri, Z.; Bazan, N.G. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp. Eye Res. 2010, 90, 718–725. [Google Scholar] [CrossRef]
- Yao, J.; Bi, H.E.; Sheng, Y.; Cheng, L.B.; Wendu, R.L.; Wang, C.H.; Cao, G.F.; Jiang, Q. Ultraviolet (UV) and hydrogen peroxide activate ceramide-ER stress-AMPK signaling axis to promote retinal pigment epithelium (RPE) cell apoptosis. Int. J. Mol. Sci. 2013, 14, 10355–10368. [Google Scholar] [CrossRef]
- Cheng, L.B.; Cheng, L.; Bi, H.E.; Zhang, Z.Q.; Yao, J.; Zhou, X.Z.; Jiang, Q. Alpha-melanocyte stimulating hormone protects retinal pigment epithelium cells from oxidative stress through activation of melanocortin 1 receptor-Akt-mTOR signaling. Biochem. Biophys. Res. Commun. 2014, 443, 447–452. [Google Scholar] [CrossRef]
- Cao, G.F.; Liu, Y.; Yang, W.; Wan, J.; Yao, J.; Wan, Y.; Jiang, Q. Rapamycin sensitive mTOR activation mediates nerve growth factor (NGF) induced cell migration and pro-survival effects against hydrogen peroxide in retinal pigment epithelial cells. Biochem. Biophys. Res. Commun. 2011, 414, 499–505. [Google Scholar] [CrossRef]
- Wu, W.C.; Hu, D.N.; Gao, H.X.; Chen, M.; Wang, D.; Rosen, R.; McCormick, S.A. Subtoxic levels hydrogen peroxide-induced production of interleukin-6 by retinal pigment epithelial cells. Mol. Vis. 2010, 16, 1864–1873. [Google Scholar] [PubMed]
- Zhang, H.; Liu, Y.Y.; Jiang, Q.; Li, K.R.; Zhao, Y.X.; Cao, C.; Yao, J. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radic. Biol. Med. 2014, 69, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cano, M.; Handa, J.T. p62 provides dual cytoprotection against oxidative stress in the retinal pigment epithelium. Biochim. Biophys. Acta 2014, 1843, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, N.G.; Kozhevnikova, O.S.; Telegina, D.V.; Fursova, A.Z.; Stefanova, N.A.; Muraleva, N.A.; Venanzi, F.; Sherman, M.Y.; Kolesnikov, S.I.; Sufianov, A.A.; et al. p62/SQSTM1 coding plasmid prevents age related macular degeneration in a rat model. Aging 2018, 10, 2136–2147. [Google Scholar] [CrossRef]
- Sanchez, C.A.; Andahur, E.I.; Valenzuela, R.; Castellon, E.A.; Fulla, J.A.; Ramos, C.G.; Trivino, J.C. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget 2016, 7, 3993–4008. [Google Scholar] [CrossRef]
- Jaswal, J.S.; Gandhi, M.; Finegan, B.A.; Dyck, J.R.; Clanachan, A.S. p38 mitogen-activated protein kinase mediates adenosine-induced alterations in myocardial glucose utilization via 5′-AMP-activated protein kinase. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1978–H1985. [Google Scholar] [CrossRef]
- Kim, B.J.; Zack, D.J. The role of c-Jun N-terminal kinase (JNK) in retinal degeneration and vision loss. Adv. Exp. Med. Biol. 2018, 1074, 351–357. [Google Scholar]
- Carling, D. The AMP-activated protein kinase cascade—A unifying system for energy control. Trends Biochem. Sci. 2004, 29, 18–24. [Google Scholar] [CrossRef]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Overvatn, A.; Bjorkoy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef]
- Cadwell, K.; Philips, J.A. Autophagy meets phagocytosis. Immunity 2013, 39, 425–427. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Bao, X.L.; Cong, Y.Y.; Fan, B.; Li, G.Y. Autophagy in age-related macular degeneration: A regulatory mechanism of oxidative stress. Oxid. Med. Cell. Longev. 2020, 2020, 2896036. [Google Scholar] [CrossRef]
- Pakravan, K.; Babashah, S.; Sadeghizadeh, M.; Mowla, S.J.; Mossahebi-Mohammadi, M.; Ataei, F.; Dana, N.; Javan, M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1alpha/VEGF signaling axis in breast cancer cells. Cell. Oncol. 2017, 40, 457–470. [Google Scholar] [CrossRef]
- Grundmann, S.; Hans, F.P.; Kinniry, S.; Heinke, J.; Helbing, T.; Bluhm, F.; Sluijter, J.P.; Hoefer, I.; Pasterkamp, G.; Bode, C.; et al. MicroRNA-100 regulates neovascularization by suppression of mammalian target of rapamycin in endothelial and vascular smooth muscle cells. Circulation 2011, 123, 999–1009. [Google Scholar] [CrossRef]
- Kovacs, B.; Lumayag, S.; Cowan, C.; Xu, S. MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4402–4409. [Google Scholar] [CrossRef]
- Chang, Y.C.; Kao, Y.H.; Hu, D.N.; Tsai, L.Y.; Wu, W.C. All-trans retinoic acid remodels extracellular matrix and suppresses laminin-enhanced contractility of cultured human retinal pigment epithelial cells. Exp. Eye Res. 2009, 88, 900–909. [Google Scholar] [CrossRef]
- Wu, W.C.; Lai, Y.H.; Hsieh, M.C.; Chang, Y.C.; Wu, M.H.; Wu, H.J.; Chang, C.W.; Wu, K.Y.; Kao, Y.H. Pleiotropic role of atorvastatin in regulation of human retinal pigment epithelial cell behaviors in vitro. Exp. Eye Res. 2011, 93, 842–851. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-S.; Chang, Y.-C.; Chen, P.-H.; Li, C.-Y.; Wu, W.-C.; Kao, Y.-H. MicroRNA-100 Mediates Hydrogen Peroxide-Induced Apoptosis of Human Retinal Pigment Epithelium ARPE-19 Cells. Pharmaceuticals 2021, 14, 314. https://doi.org/10.3390/ph14040314
Chang Y-S, Chang Y-C, Chen P-H, Li C-Y, Wu W-C, Kao Y-H. MicroRNA-100 Mediates Hydrogen Peroxide-Induced Apoptosis of Human Retinal Pigment Epithelium ARPE-19 Cells. Pharmaceuticals. 2021; 14(4):314. https://doi.org/10.3390/ph14040314
Chicago/Turabian StyleChang, Yuh-Shin, Yo-Chen Chang, Po-Han Chen, Chia-Yang Li, Wen-Chuan Wu, and Ying-Hsien Kao. 2021. "MicroRNA-100 Mediates Hydrogen Peroxide-Induced Apoptosis of Human Retinal Pigment Epithelium ARPE-19 Cells" Pharmaceuticals 14, no. 4: 314. https://doi.org/10.3390/ph14040314
APA StyleChang, Y.-S., Chang, Y.-C., Chen, P.-H., Li, C.-Y., Wu, W.-C., & Kao, Y.-H. (2021). MicroRNA-100 Mediates Hydrogen Peroxide-Induced Apoptosis of Human Retinal Pigment Epithelium ARPE-19 Cells. Pharmaceuticals, 14(4), 314. https://doi.org/10.3390/ph14040314






