The Antibacterial Synthetic Flavonoid BrCl-Flav Exhibits Important Anti-Candida Activity by Damaging Cell Membrane Integrity
Abstract
:1. Introduction
2. Results
2.1. Antifungal Activity of Synthetic Flavonoid
2.1.1. BrCl-Flav Inhibited Candida spp. Growth in a Dose and Time Dependent Manner
2.1.2. BrCl-Flav Has a Potent Fungicidal Activity against Candida spp.
2.2. BrCl-Flav Mode of Action
2.2.1. Complexation of Sorbitol
2.2.2. Exposure to BrCl-Flav Caused Cellular Membrane Damage
2.2.3. BrCl-Flav Induced Irreversible Morphological Damage
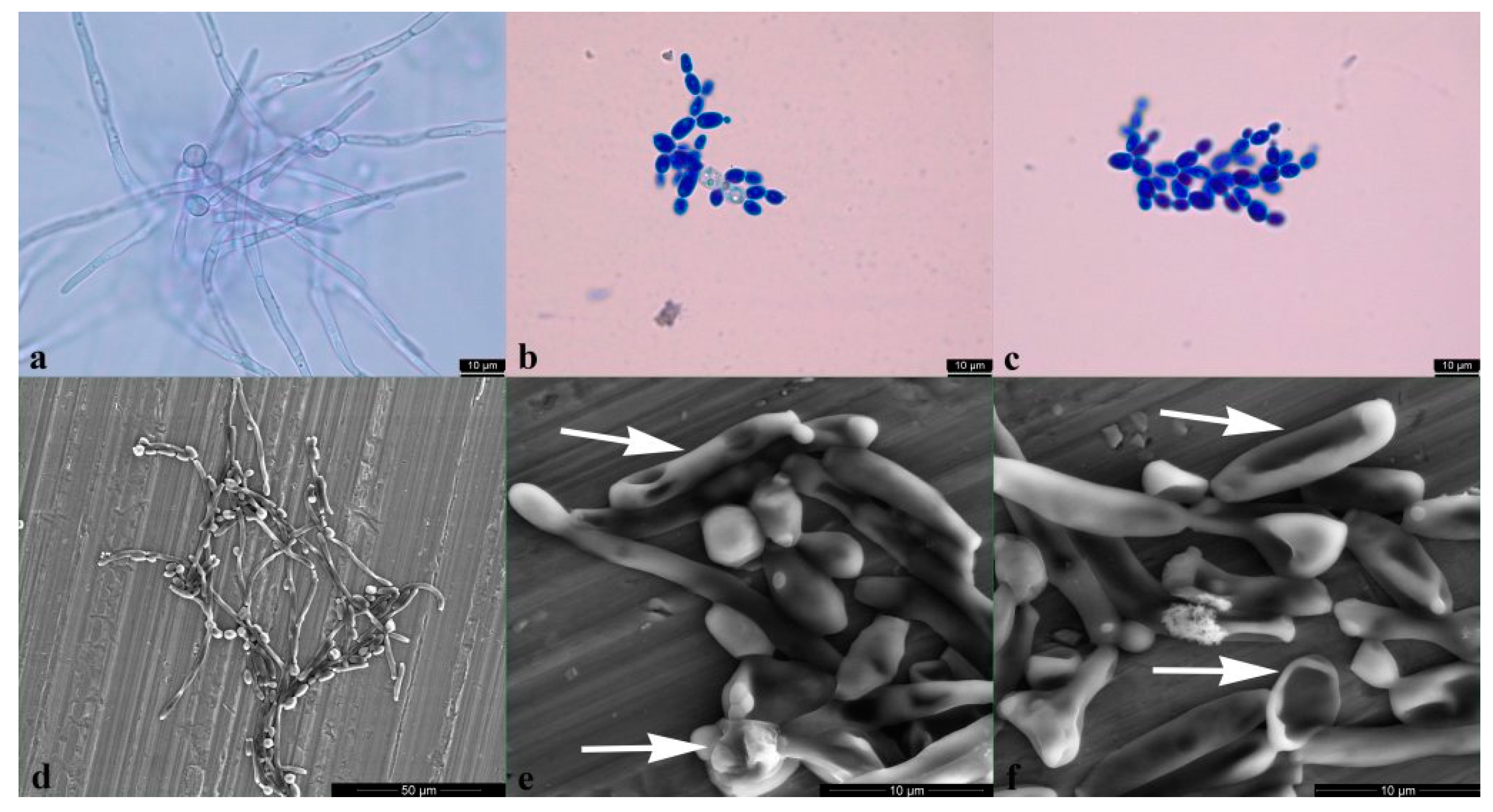
2.2.4. Candida albicans Yeast to Hyphal Transition Was Prevented by BrCl-Flav
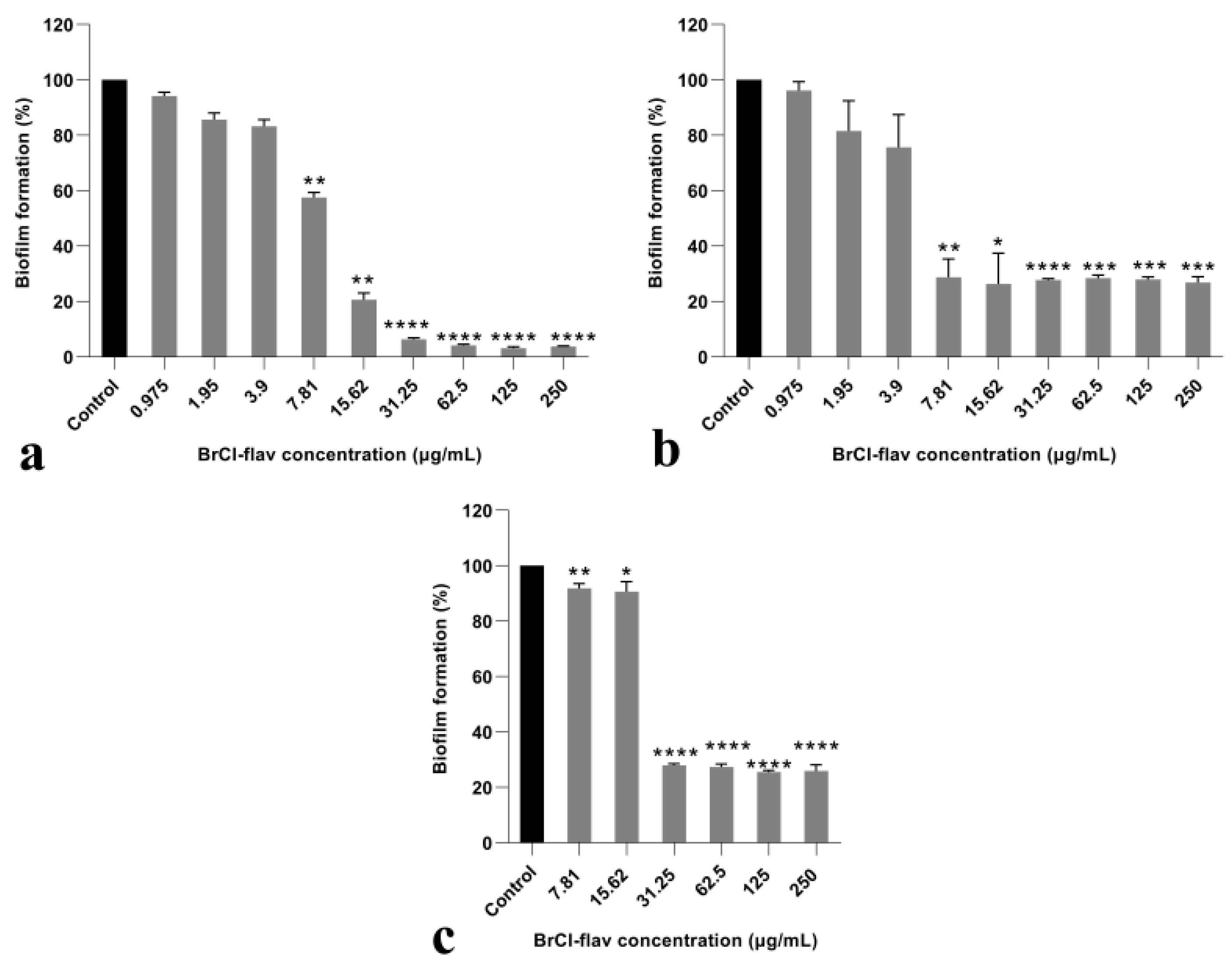
2.2.5. BrCl-Flav Impedes Candida spp. Biofilm Formation
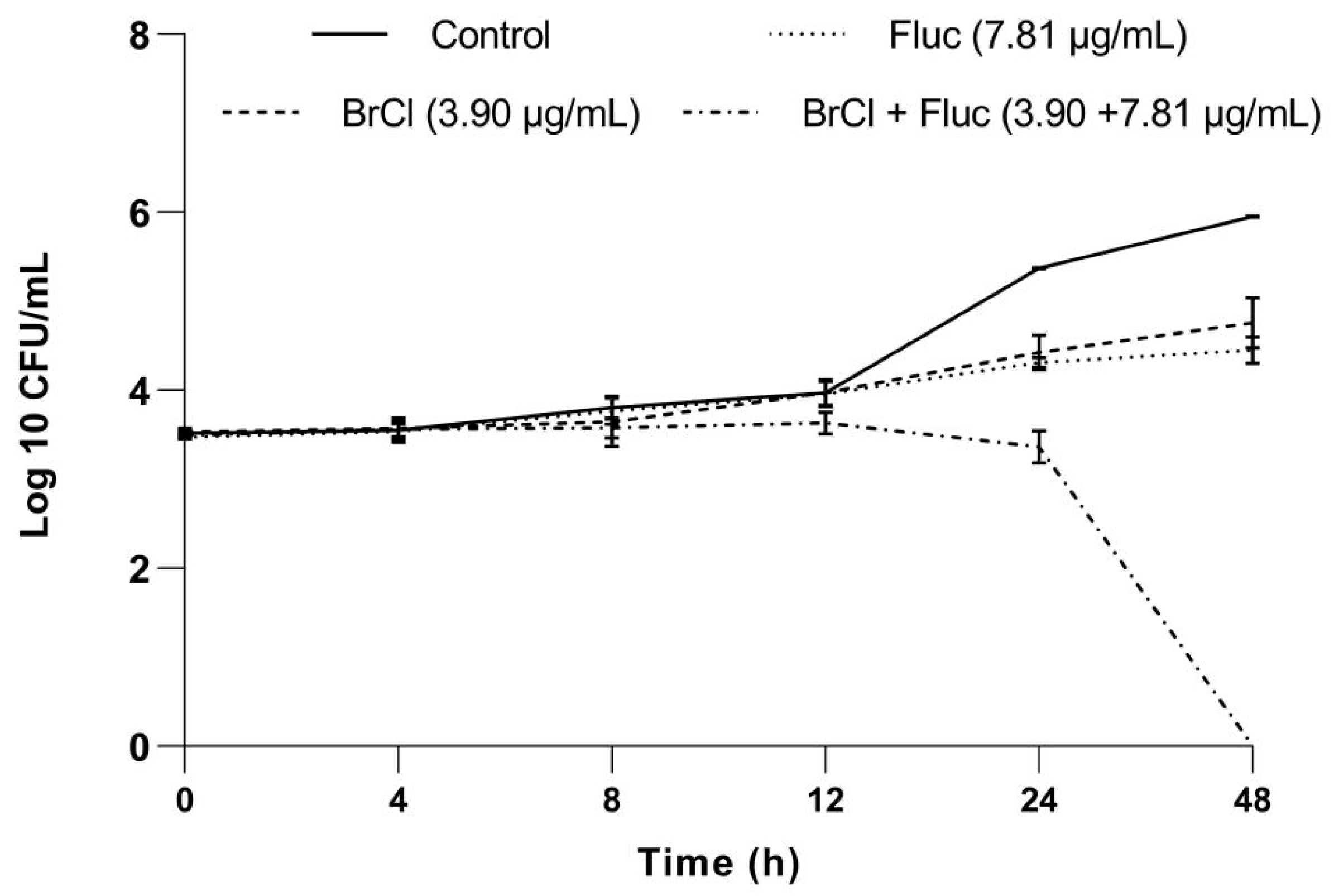
2.3. Effect of BrCl-Flav in Combination with Fluconazole against Candida Strains
3. Discussion
4. Materials and Methods
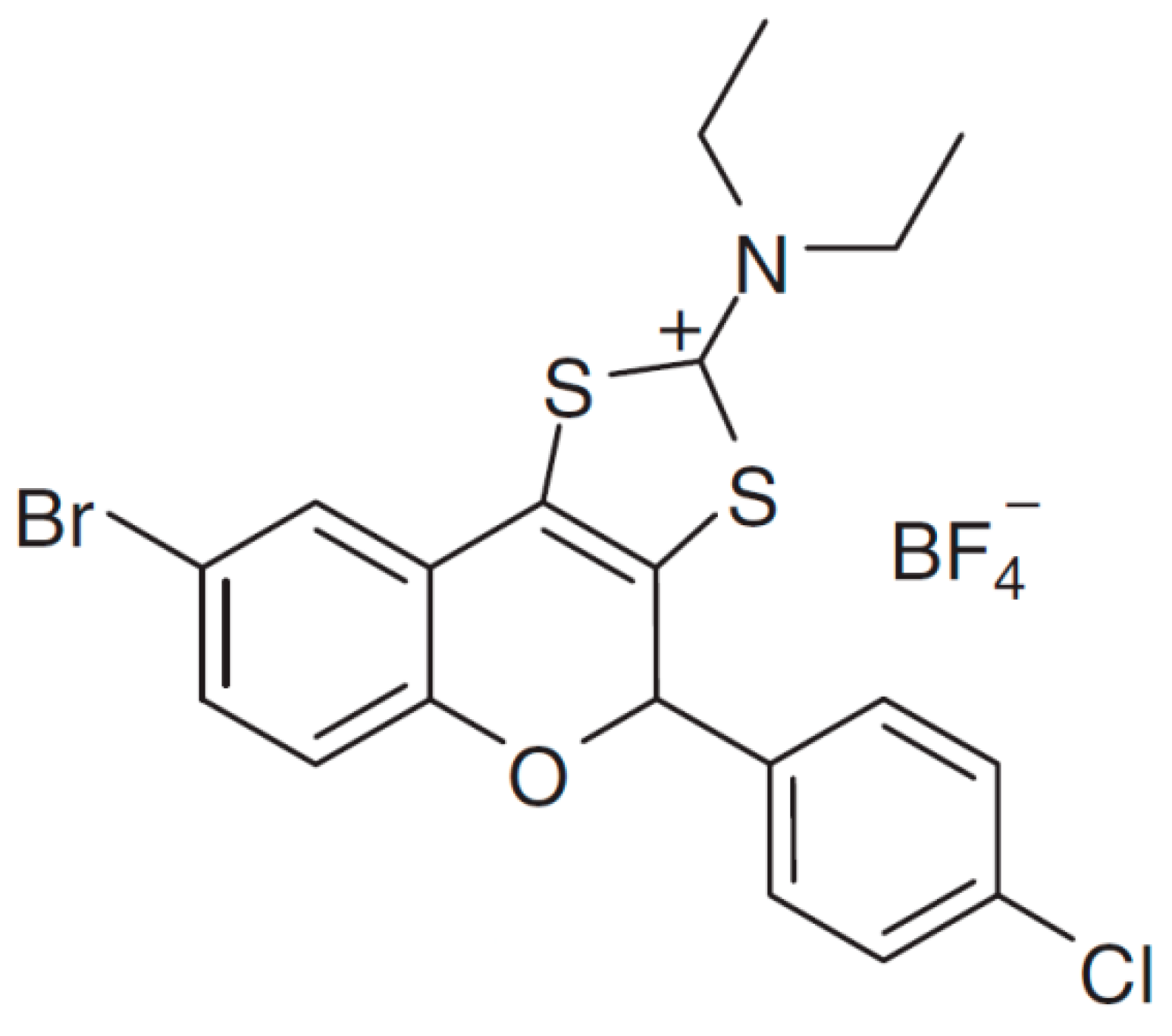
4.1. Chemicals and Fungal Strains
4.2. Assessment of Antifungal Activity
4.2.1. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
4.2.2. Fungal Growth Analysis
4.2.3. Time-Kill Kinetic Assay
4.3. Sorbitol Binding Affinity Assay
4.4. Cell Membrane Permeability Test
4.5. Hyphal Growth Test
4.6. Scanning Electron Microscopy (SEM)
4.7. In Vitro Anti-Biofilm Activity Assay
4.8. Checkerboard Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.O.; de Sousa Cartágenes, M.d.S.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida Infections and therapeutic strategies: Mechanisms of action for traditional and alternative agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef]
- Tobudic, S.; Kratzer, C.; Lassnigg, A.; Presterl, E. Antifungal susceptibility of Candida albicans in biofilms. Mycoses 2012, 55, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Barchiesi, F.; Orsetti, E.; Osimani, P.; Catassi, C.; Santelli, F.; Manso, E. Factors Related to outcome of bloodstream infections due to Candida parapsilosis complex. BMC Infect. Dis. 2016, 16, 387. [Google Scholar] [CrossRef] [Green Version]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Douglas, L.J. Candida biofilms and their role in infection. Trends. Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Ngo-Mback, M.N.L.; Babii, C.; Jazet Dongmo, P.M.; Kouipou Toghueo, M.R.; Stefan, M.; Fekam Boyom, F. Anticandidal and synergistic effect of essential oil fractions from three aromatic plants used in Cameroon. J. Mycol. Med. 2020, 30, 100940. [Google Scholar] [CrossRef]
- Jin, Y.-S. Recent advances in natural antifungal flavonoids and their derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 126589. [Google Scholar] [CrossRef]
- Sarbu, L.G.; Bahrin, L.G.; Babii, C.; Stefan, M.; Birsa, M.L. Synthetic flavonoids with antimicrobial activity: A review. J. Appl. Microbiol. 2019, 127, 1282–1290. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Sagrera, G.; Bertucci, A.; Vazquez, A.; Seoane, G. Synthesis and antifungal activities of natural and synthetic biflavonoids. Bioorg. Med. Chem. 2011, 19, 3060–3073. [Google Scholar] [CrossRef]
- Babii, C.; Bahrin, L.G.; Neagu, A.N.; Gostin, I.; Mihasan, M.; Birsa, L.M.; Stefan, M. Antibacterial activity and proposed action mechanism of a new class of synthetic tricyclic flavonoids. J. Appl. Microbiol. 2016, 120, 630–637. [Google Scholar] [CrossRef]
- Alexander, B.D.; Johnson, M.D.; Pfeiffer, C.D.; Jiménez-Ortigosa, C.; Catania, J.; Booker, R.; Castanheira, M.; Messer, S.A.; Perlin, D.S.; Pfaller, M.A. Increasing echinocandin resistance in Candida glabrata: Clinical failure correlates with presence of fks mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 2013, 56, 1724–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baddley, J.W.; Patel, M.; Bhavnani, S.M.; Moser, S.A.; Andes, D.R. Association of fluconazole pharmacodynamics with mortality in patients with candidemia. Antimicrob. Agents Chemother. 2008, 52, 3022–3028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seleem, D.; Pardi, V.; Murata, R.M. Review of flavonoids: A diverse group of natural compounds with anti-Candida albicans activity in vitro. Arch. Oral. Biol. 2017, 76, 76–83. [Google Scholar] [CrossRef]
- Abdelrahmman, K.; El-Behairy, M.F.; Alsherbiny, M.A.; Mazeed, T.E. In vitro activity of dihydropyrazole derivatives against Candida species. Bull. Fac. Pharm. Cairo Univ. 2018, 56, 80–82. [Google Scholar] [CrossRef]
- Lobo, C.I.V.; Lopes, A.C.U.d.A.; Klein, M.I. Compounds with distinct targets present diverse antimicrobial and antibiofilm efficacy against Candida albicans and Streptococcus mutans, and combinations of compounds potentiate their effect. J. Fungi 2021, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Srivastava, V.; Ahmad, A. Dodonaea viscosa var angustifolia derived 5,6,8-trihydroxy-7,4’ dimethoxy flavone inhibits ergosterol synthesis and the production of hyphae and biofilm in Candida albicans. J. Ethnopharmacol. 2020, 259, 112965. [Google Scholar] [CrossRef]
- Lin, M.Y.; Yuan, Z.L.; Hu, D.D.; Hu, G.H.; Zhang, R.L.; Zhong, H.; Yan, L.; Jiang, Y.Y.; Su, J.; Wang, Y. Effect of loureirin a against Candida albicans biofilms. Chin. J. Nat. Med. 2019, 17, 616–623. [Google Scholar] [CrossRef]
- Pricopie, A.I.; Ionuț, I.; Marc, G.; Arseniu, A.M.; Vlase, L.; Grozav, A.; Găină, L.I.; Vodnar, D.C.; Pîrnău, A.; Tiperciuc, B.; et al. Design and synthesis of novel 1,3-thiazole and 2-hydrazinyl-1,3-thiazole derivatives as anti-Candida agents: in vitro antifungal screening, molecular docking study, and spectroscopic investigation of their binding interaction with bovine serum albumin. Molecules 2019, 24, 3435. [Google Scholar] [CrossRef] [Green Version]
- Illicachi, L.A.; Montalvo-Acosta, J.J.; Insuasty, A.; Quiroga, J.; Abonia, R.; Sortino, M.; Zacchino, S.; Insuasty, B. Synthesis and dft calculations of novel vanillin-chalcones and their 3-aryl-5-(4-(2-(dimethylamino)-ethoxy)-3-methoxyphenyl)-4,5-dihydro-1h-pyrazole-1-carbaldehyde derivatives as antifungal agents. Molecules 2017, 22, 1476. [Google Scholar] [CrossRef] [Green Version]
- Andrade, J.T.; Santos, F.R.S.; Lima, W.G.; Sousa, C.D.F.; Oliveira, L.; Ribeiro, R.; Gomes, A.; Araújo, M.G.F.; Villar, J.; Ferreira, J.M.S. Design, synthesis, biological activity and structure-activity relationship studies of chalcone derivatives as potential anti-Candida agents. J. Antibiot. 2018, 71, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Seleem, D.; Benso, B.; Noguti, J.; Pardi, V.; Murata, R.M. In vitro and in vivo antifungal activity of lichochalcone-a against Candida albicans biofilms. PLoS ONE 2016, 11, e0157188. [Google Scholar] [CrossRef]
- Pereira Fde, O.; Mendes, J.M.; Lima, I.O.; Mota, K.S.; Oliveira, W.A.; Lima Ede, O. Antifungal Activity of geraniol and citronellol, two monoterpenes alcohols, against Trichophyton rubrum involves inhibition of ergosterol biosynthesis. Pharm. Biol. 2015, 53, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Zhao, X.; Yang, L.; Su, P.; Fu, P.; Peng, J.; Yang, N.; Guo, G. Antimicrobial peptide amp-17 affects Candida albicans by disrupting its cell wall and cell membrane integrity. Infect. Drug Resist. 2020, 13, 2509–2520. [Google Scholar] [CrossRef]
- Douglas, L.M.; Konopka, J.B. Plasma membrane organization promotes virulence of the human fungal pathogen Candida albicans. J. Microbiol. 2016, 54, 178–191. [Google Scholar] [CrossRef] [Green Version]
- Bahrin, L.G.; Sarbu, L.G.; Hopf, H.; Jones, P.G.; Babii, C.; Stefan, M.; Birsa, M.L. The influence of halogen substituents on the biological properties of sulfur-containing flavonoids. Bioorg. Med. Chem. 2016, 24, 3166–3173. [Google Scholar] [CrossRef]
- Leite, M.C.A.; Bezerra, A.P.d.B.; Sousa, J.P.D.; Guerra, F.Q.S.; Lima, E.d.O. Evaluation of antifungal activity and mechanism of action of citral against Candida albicans. Evid.-Based Complement Altern. Med. 2014, 2014, 378280. [Google Scholar] [CrossRef] [Green Version]
- Babii, C.; Mihalache, G.; Bahrin, L.G.; Neagu, A.N.; Gostin, I.; Mihai, C.T.; Sarbu, L.G.; Birsa, L.M.; Stefan, M. A novel synthetic flavonoid with potent antibacterial properties: In vitro activity and proposed mode of action. PLoS ONE 2018, 13, e0194898. [Google Scholar] [CrossRef] [Green Version]
- Tempesti, T.C.; Alvarez, M.G.; de Araújo, M.F.; Catunda Júnior, F.E.A.; de Carvalho, M.G.; Durantini, E.N. Antifungal Activity of a novel quercetin derivative bearing a trifluoromethyl group on Candida albicans. Med. Chem. Res. 2012, 21, 2217–2222. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Yang, E.; Yan, L.; Li, T.; Zhuang, H. Antimicrobial compounds produced by vaginal Lactobacillus crispatus are able to strongly inhibit Candida albicans growth, hyphal formation and regulate virulence-related gene expressions. Front. Microbiol. 2017, 8, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevors, J.T.; Merrick, R.L.; Russell, I.; Stewart, G.G. A comparison of methods for assessing yeast viability. Biotechnol. Lett. 1983, 5, 131–134. [Google Scholar] [CrossRef]
- Djordjevic, D.; Wiedmann, M.; McLandsborough, L.A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002, 68, 2950–2958. [Google Scholar] [CrossRef] [Green Version]
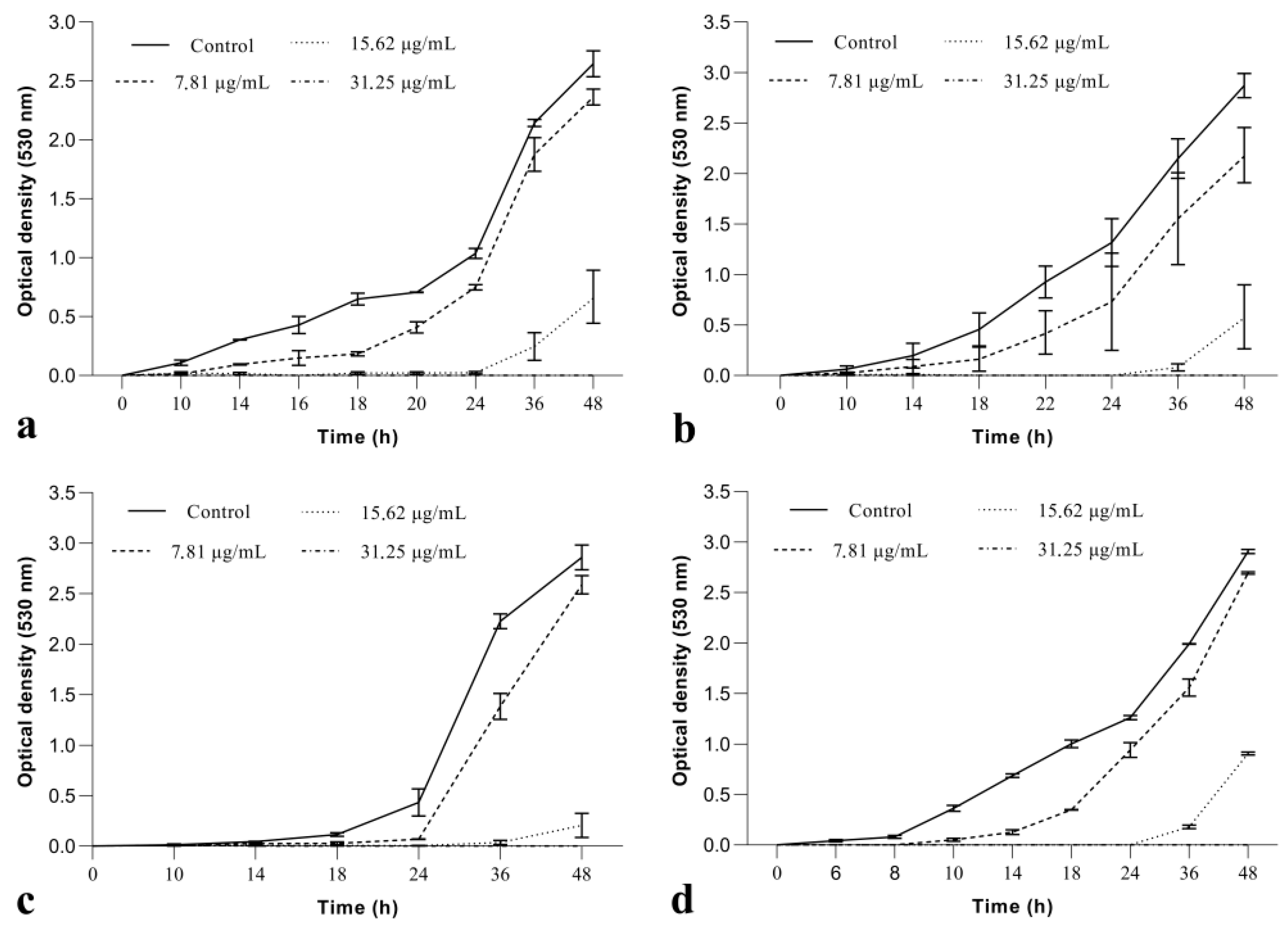
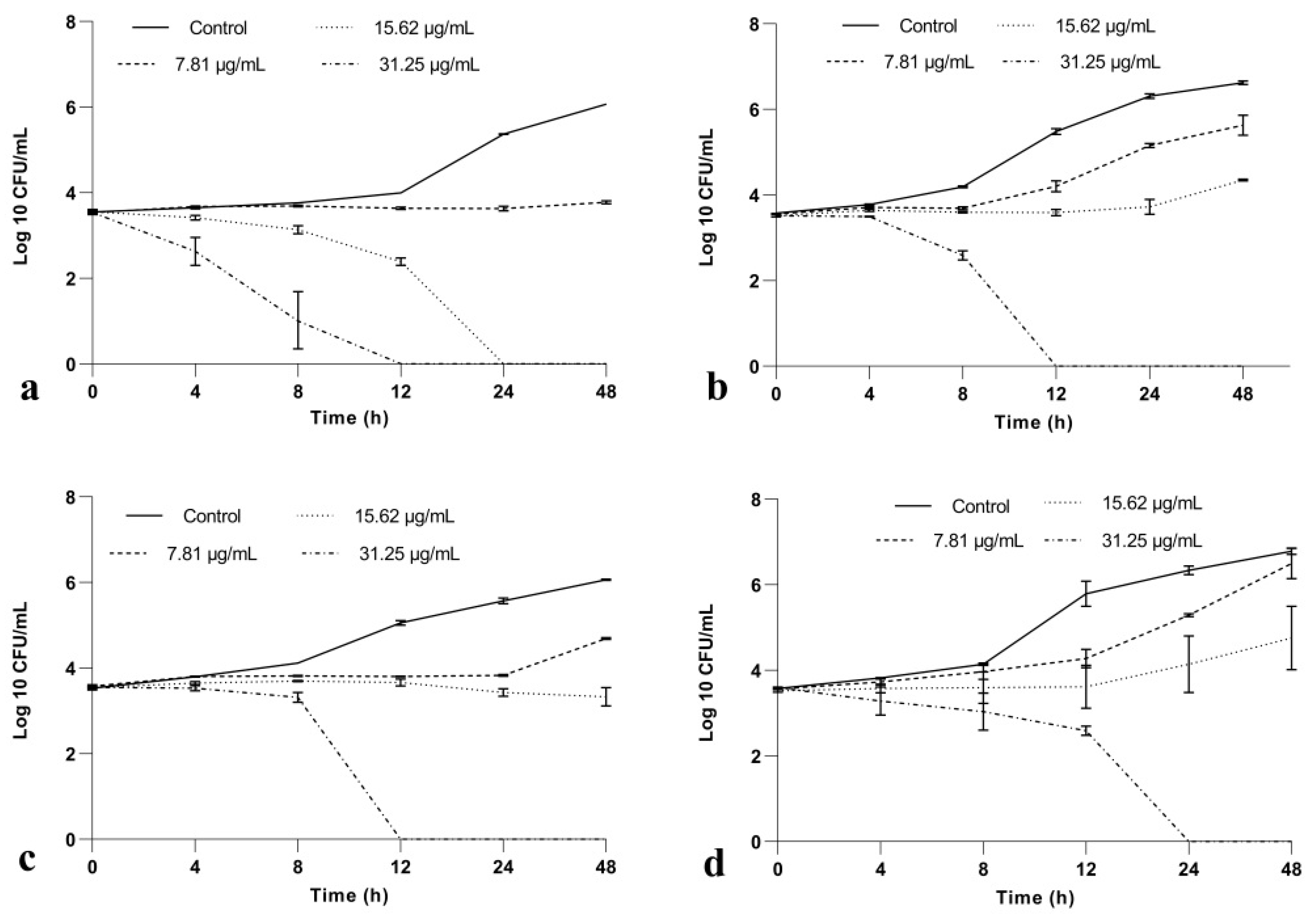

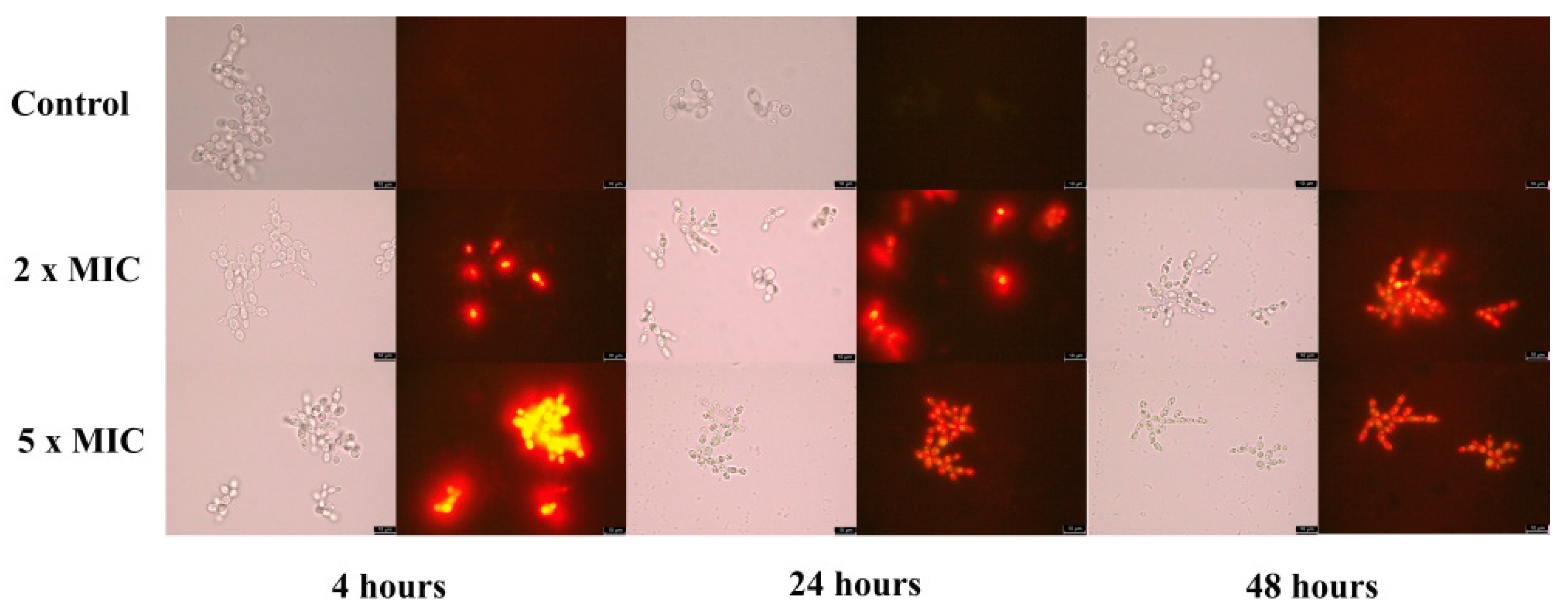
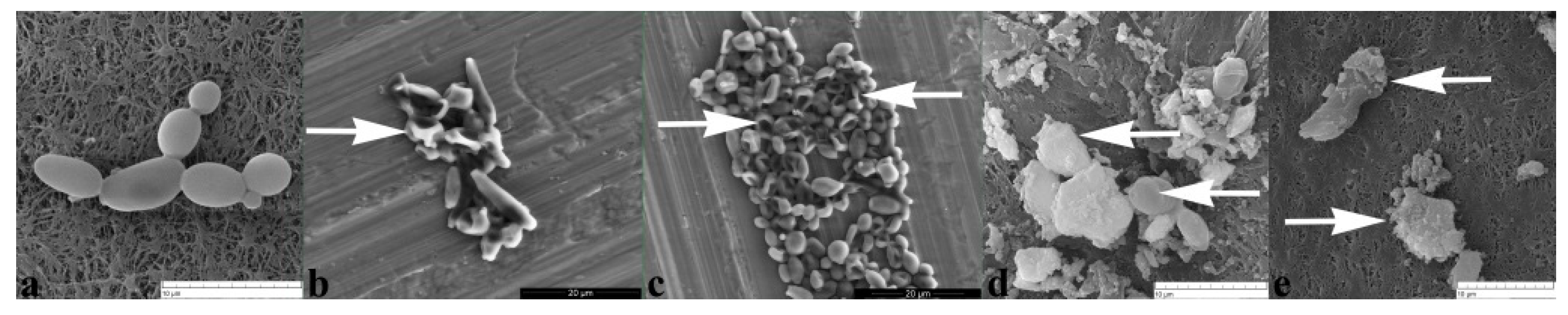
| Candida sp. Strains | BrCl-Flav | DMSO | Fluconazole | |
|---|---|---|---|---|
| MIC (µg/mL) | MFC (µg/mL) | MIC (µg/mL) | MIC (µL/mL) | |
| C. albicans | 15.62 | 31.25 | >250 | 1000 |
| C. parapsilosis | 15.62 | 31.25 | >250 | 3.9 |
| C. krusei | 15.62 | 31.25 | >250 | 62.5 |
| C. glabrata | 15.62 | 31.25 | >250 | 125 |
| Candida krusei | 15.62 | 31.25 | 125 | 62.5 |
| ATCC 6258 | ||||
| Candida sp. Strains | MIC (μg/mL) | |
|---|---|---|
| BrCl-Flav | BrCl-Flav + Sorbitol (0.8 M) | |
| C. albicans | 15.62 | 7.8 |
| C. parapsilosis | 15.62 | 7.8 |
| C. krusei | 15.62 | 15.62 |
| C. glabrata | 15.62 | 7.8 |
| Candida sp. Strains | MIC (µg/mL) | FICI | Interaction | |||
|---|---|---|---|---|---|---|
| Alone | In Combination | |||||
| BrCl-Flav | Fluconazole | BrCl-Flav | Fluconazole | |||
| C. albicans | 15.62 | 1000 | 15.62 | 0.16 | 1 | IND |
| 7.81 | 7.81 | 0.50 | SYN | |||
| 3.9 | 7.81 | 0.25 | SYN | |||
| 1.95 | 7.81 | 0.12 | SYN | |||
| C. krusei | 15.62 | 62.5 | 15.62 | 0.12 | 1 | IND |
| 7.81 | 31.25 | 1 | IND | |||
| 3.9 | 31.25 | 0.75 | ADD | |||
| 1.95 | 31.25 | 0.62 | ADD | |||
| C. parapsilosis | 15.62 | 3.9 | 15.62 | 0.12 | 1.03 | IND |
| 7.81 | 1.95 | 1 | IND | |||
| 3.9 | 1.95 | 0.75 | ADD | |||
| 1.95 | 3.9 | 1.12 | IND | |||
| C. glabrata | 15.62 | 125 | 15.62 | 31.25 | 1.25 | IND |
| 7.81 | 31.25 | 0.75 | ADD | |||
| 3.9 | 62.5 | 0.75 | ADD | |||
| 1.95 | 125 | 1.12 | IND | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babii, C.; Savu, M.; Motrescu, I.; Birsa, L.M.; Sarbu, L.G.; Stefan, M. The Antibacterial Synthetic Flavonoid BrCl-Flav Exhibits Important Anti-Candida Activity by Damaging Cell Membrane Integrity. Pharmaceuticals 2021, 14, 1130. https://doi.org/10.3390/ph14111130
Babii C, Savu M, Motrescu I, Birsa LM, Sarbu LG, Stefan M. The Antibacterial Synthetic Flavonoid BrCl-Flav Exhibits Important Anti-Candida Activity by Damaging Cell Membrane Integrity. Pharmaceuticals. 2021; 14(11):1130. https://doi.org/10.3390/ph14111130
Chicago/Turabian StyleBabii, Cornelia, Mihaela Savu, Iuliana Motrescu, Lucian Mihail Birsa, Laura Gabriela Sarbu, and Marius Stefan. 2021. "The Antibacterial Synthetic Flavonoid BrCl-Flav Exhibits Important Anti-Candida Activity by Damaging Cell Membrane Integrity" Pharmaceuticals 14, no. 11: 1130. https://doi.org/10.3390/ph14111130
APA StyleBabii, C., Savu, M., Motrescu, I., Birsa, L. M., Sarbu, L. G., & Stefan, M. (2021). The Antibacterial Synthetic Flavonoid BrCl-Flav Exhibits Important Anti-Candida Activity by Damaging Cell Membrane Integrity. Pharmaceuticals, 14(11), 1130. https://doi.org/10.3390/ph14111130









