Abstract
This manuscript deals with the synthesis and computational and experimental evaluation of the antimicrobial activity of twenty-nine 4-(indol-3-yl)thiazole-2-amines and 4-ιndol-3-yl)thiazole acylamines. An evaluation of antibacterial activity against Gram (+) and Gram (−) bacteria revealed that the MIC of indole derivatives is in the range of 0.06–1.88 mg/mL, while among fourteen methylindole derivatives, only six were active, with an MIC in the range of of 0.47–1.88 mg/mL. S. aureus appeared to be the most resistant strain, while S. Typhimurium was the most sensitive. Compound 5x was the most promising, with an MIC in the range of 0.06–0.12 mg/mL, followed by 5d and 5m. An evaluation of these three compounds against resistant strains, namely MRSA P. aeruginosa and E. coli, revealed that they were more potent against MRSA than ampicillin. Furthermore, compounds 5m and 5x were superior inhibitors of biofilm formation, compared to ampicillin and streptomycin, in terms Compounds 5d, 5m, and 5x interact with streptomycin in additive manner. The antifungal activity of some compounds exceeded or was equipotent to those of the reference antifungal agents bifonazole and ketoconazole. The most potent antifungal agent was found to be compound 5g. Drug likeness scores of compounds was in a range of −0.63 to 0.29, which is moderate to good. According to docking studies, E. coli MurB inhibition is probably responsible for the antibacterial activity of compounds, whereas CYP51 inhibition was implicated in antifungal activity. Compounds appeared to be non-toxic, according to the cytotoxicity assessment in MRC-5 cells.
1. Introduction
There is a universal current interest in the development of new antimicrobial agents due to the growing emergence of bacterial resistance to antibiotic therapy and to newly emerging pathogens. Antimicrobial resistance (AMR) poses a severe worldwide risk of growing concern to human, animal, and environment health. This is due to the appearance, spread, and persistence of multidrug-resistant (MDR) bacteria, or “superbugs” [1]. AMR is probably due to the unnecessary use of antibiotics in animals (food, pets, marine) and humans, antibiotics sold over the counter, increased global travel, and poor hygiene, especially in developing countries. Another reason could be the release of non-metabolized antibiotics or their residues into the environment through manure/feces. In the absence of the effective treatment, this leads to human losses and the prolonged stay of patients in hospital. Thus, there is an increasing demand for the introduction of new antimicrobial agents due to the developing resistance to conventional antibiotics [2].
Indole, an aromatic heterocyclic organic compound, has attracted the interest of scientists since it possesses a wide variety of pharmacological properties, such as analgesic [3,4], anti-inflammatory [3,5,6], antimicrobial [7,8,9,10], anticancer [11,12,13], anticonvulsant [14,15] and antiviral [16,17], COX-inhibitory [18,19], antidiabetic [20,21], and antitubercular [22,23] activities. Furthermore, indole moiety is present in some drug molecules, such as: indomethacin; yohimbine, an approved drug for the treatment of sexual disorders [24,25]; delavirdine, anti-HIV drug [26]; reserpine (Figure 1), and many others. Indole-based pharmaceuticals represent a very important class of therapeutic molecules and will probably replace many existing pharmaceuticals in the future [27].
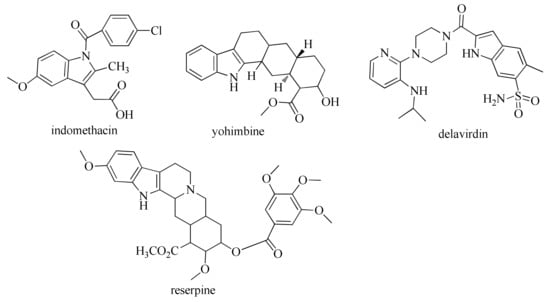
Figure 1.
Approved drugs with indole moiety.
On the other hand, it is well known that natural products have served as a powerful source for drug discovery. There have been many important drugs developed from plants and microorganisms [28]. One of them is streptochlorin, a bioactive compound first isolated from marine Streptomyces sp. by Japanese scientist H. Watanabe in 1988 [29]. Since streptochlorin lacks antifungal potency in low concentrations, Zhang et al. [30] and Gao et al. [31] made different structural modifications to it and showed the importance of an indole ring in the structure of streptochlorin, replacing the oxazole ring with imidazole as a bioisostere to discover novel streptochlorin analogues (Figure 2).
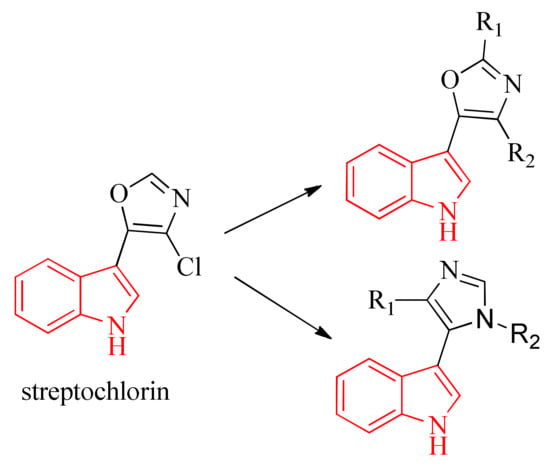
Figure 2.
Oxazole and Imidazole analogues of streptochlorin.
Another interesting core is thiazole. Its derivatives have been reported to possess a wide range of biological properties, such as antimicrobial [32,33,34], anticancer [35,36,37], anti-inflammatory [38,39,40], antiviral [41,42], anti-HIV [43,44], antidiabetic [45,46], carbonic anhydrase inhibitory [47,48,49], antitubercular [50,51], and many other [52,53,54] activities. Furthermore, penicillin derivatives [55]; bleomycin [56]; tiazofurin [57], an antineoplastic drug; vitamin B1 [58]; ritonavir, an approved drug for the treatment of HIV [59]; and meloxicam [60] (Figure 3) are also very important thiazole-containing agents approved for human use.
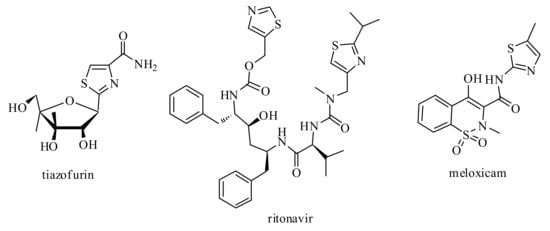
Figure 3.
Approved drugs with thiazole moiety.
One approach in drug design is molecular hybridization based on the combination of two or more pharmacophores of different biologically active molecules in the frame of one single molecule [61]. The aim of this approach is mainly to improve the activity profile and to reduce undesired side effects [62].
Prompted by the factors mentioned above, as well as based on our previous results [63,64], we designed and synthesized new derivatives incorporating two pharmacophores, indole and thiazole moieties, in the frame of one molecule.
The established human fibroblast-derived MRC-5 normal cell line was chosen to be applied in this work in order to test the cytotoxicity of the new synthesized chemical molecules since its application has been licensed by regulatory bodies for human vaccine production and new drug development [65,66]. To this end, it is reasonable for MRC-5 to be used to develop new antimicrobial and antiviral agents, as well as anticancer therapeutics, in order to evaluate its selectivity in cytotoxicity for bacteria, virus, and malignant cells opposite to normal human cells, as previously published [44,67,68].
2. Results and Discussion
2.1. Chemistry
The title compounds were synthesized according to Scheme 1. Substituted 3-(α-chlorouracil) indoles (3a–l) obtained by acylation of the corresponding indoles (1a–k) with chloroacetic (2a) and α-chloropropionic acid chlorides (2b) were used as starting compounds for their synthesis. 3-(α-chlorouracil) indoles (3a–l), upon heating in methanol with thiourea (4a) and its derivatives (4b), in the presence of a base, were converted into the target 4- (indol-3-yl)thiazole-2-amines (5a–o) (Scheme 1). Compounds of both groups were obtained good yields in a range of 49–71% for indole-based thiazole derivatives and 49–77% for methylindole thiazole derivatives.
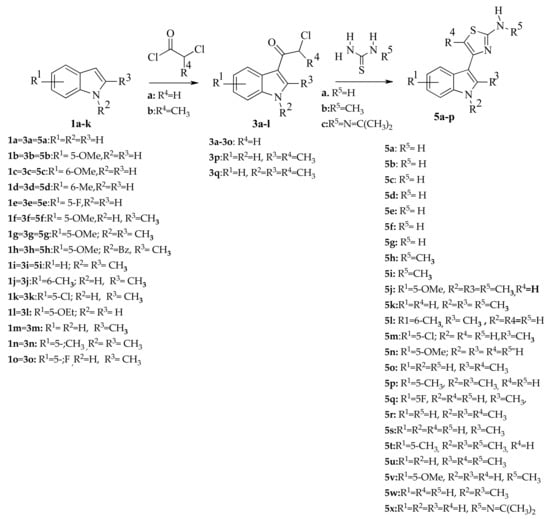
Scheme 1.
Synthesis of indole-based thiazoles 5a-5x. Reagents and conditions. (a) pyridine, toluene, 55–60 °C, 1h C; (b) Ki, Na2CO3, abs. MeOH, reflux.
The structure of all of the obtained compounds was confirmed by 1H-NMR and 13C-NMR spectroscopy. In the 1H-NMR spectra of 3- (α-chlorouracil) indoles (3a–l), the signals of the protons of the –CO–CH2–Cl group are in the range of 4.5–4.7 ppm, while the signals of the NH protons of the indole ring were observed at 11.59–12.01 ppm. In the 1H-NMR spectra of 4- (indol-3-yl)thiazole-2-amines (5a-n, 5p, 5q, 5s) obtained on the basis of 3- (α-chloroacetyl) indoles (3a–o), there is a proton singlet in position 5 of the thiazole fragment in the range of 6.2–6.5 ppm. In the 1H-NMR spectra of compounds (5o, 5r, 5u) synthesized using 3- (α-chloropropionyl) indoles (3p–3q), the signal of the protons of the methyl group is in the range of 2.12–2.22 ppm. The signal of the protons of the NH2 group in 4-(indol-3-yl) thiazol-2-amines (5a–5f, 5h–m), formed upon condensation of 3-(α-chlorouracil) indoles with unsubstituted thiourea (4a), appears in the region of 6.6–6.8 ppm, while in the spectra of compounds (5g, 5n, 5o) obtained using N-methylthiourea (4b), the signal of the protons of the N-methyl group is in the range of 2.8–2.9 ppm. In the spectrum of compound 5p, the protons of the methyl groups of the isopropenyl fragment are represented by a doublet at 2.12 ppm.
For the synthesis of 4-(indol-3-yl)thiazol-2-amines (6a–f) containing an acyl residue in the amino group, 4- (indol-3-yl) thiazol-2-amines (5b, 5c, 5w) were treated with acid chlorides of the corresponding acids (7a–d) in pyridine (Scheme 2).
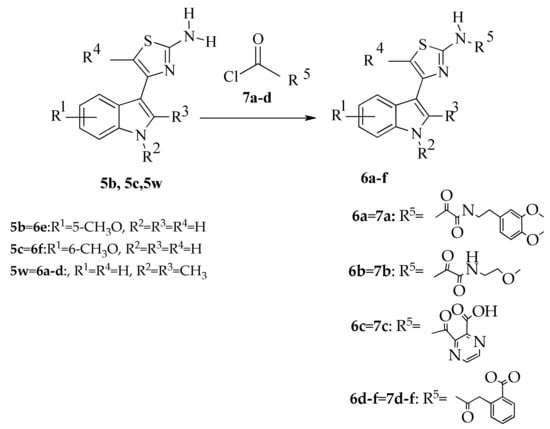
Scheme 2.
Synthesis of indole-based thiazoles 6a-f by acylation of aminothiazoles 5b, 5c, 5w. Reagents and conditions: pyridine, 5h, stiurring stiurring.
In the 13C-NMR spectra, the signal of the O–CH3 group was observed in the range of 55.84–55.97 ppm, with that of the methyl group in the range of 14.22–14.78 ppm. C–NH2 signals appeared at 167.11–167.34ppm, while C=O appeared at 158.27–170.02 ppm. The signals of C–OCH3 group are represented at 154.02–154.21 ppm, while those of COOH are represented at 172.12 ppm. Finally, the signals of the C–NHCH3 group were observed at 163.29 ppm.
In the 1H-NMR spectra of 4- (indol-3-yl) thiazol-2-amines (6a–f) formed as a result of acylation of compounds (5b, 5c, 5w), the signals of the acyl group protons were observed.
2.2. Biological Evaluation
All compounds (29) were evaluated for their possible antibacterial activity using the microdilution method and were divided in two subgroups. The first group consists of indole-based thiazole derivatives (5a–f, 5i, 5l–o, 5q, 5s, 5u, 5v,5x), and the second group is methylindole-based thiazole derivatives (5g-5k, 5p,5r, 5t, 5w, 6a–6f). Ampicillin and streptomycin were used as reference drugs. All compounds of the first subgroup of indole-based thiazoles showed antibacterial activity, with MIC in the range of 0.06–1.88 mg/mL and MBC of 0.12–3.75 mg/mL. The antibacterial potency of the tested compounds is shown in Table 1, and activity order can be presented as follows: 5x > 5m > 5d > 5e > 5a > 5q > 5s > 5b > 5v > 5f > 5l > 5c = 5n = 5o > 5i > 5u. Thus, the best activity was achieved for compound 5x, with MIC and MBC of 0.06–0.12 mg/mL and 0.12–0.23 mg/mL, respectively, whereas compound 5u showed the lowest activity, with MIC/MBC in the range of 0.47–3.75/0.94–3.75 mg/mL.

Table 1.
Antibacterial activity of indole derivatives. (MIC and MBC in mg/mL).
The most sensitive bacterium was found to be S. Typhimurium (ATCC 13311), with the lowest MIC of 0.06 mg/mL (5x) and 0.12 mg/mL (5a) and the highest at 1.88 mg/mL (5o and 5u). S. aureus (ATCC 6538) was the most resistant strain, with the lowest MIC of 0.12 mg/mL (5m and 5x), and the highest at 3.75 mg/mL (5i). In general, all strains were moderately sensitive to the compounds tested.
Compound 5e showed promising activity against B. cereus and L. monocytogenes, with MIC/MBC of 0.12/0.23 mg/mL. Nevertheless, none of compounds exceeded the activity of the reference drugs.
Compound 5x exhibited the highest activity among the tested compounds against S. Typhimurium (ATCC 13311), while compound 5m exhibited the highest activity against B. cereus and the most resistant bacterium, S. aureus, (ATCC 6538) with MIC of 0.06 mg/mL and MBC of 0.12 mg/mL, exceeding the activity of ampicillin. Good activity against S. Typhimurium (ATCC 13311) was observed for compound 5a, whereas compound 5e showed good activity against B. cereus and L. monocytogenes, with MIC/MBC of 0.12/0.23 mg/mL. Nevertheless, none of other compounds exceeded the activity of the reference drugs.
According to structure-activity relationships, the presence of propan-2-ylidenhydrazine substituent at position 2 of the thiazole ring (5x) appeared to be most beneficial for antibacterial activity. The introduction of an Me group at position 2 and a 5-Cl substituent to the indole ring, as well as the replacement of propan-2-ylidenhydrazine by an amino group (5m) slightly decreased the activity. The presence of an amino group in position 2 of thiazole, as well as a 6-Me-group in the indole ring led to compound, 5d less active than previous. The replacement of the 5-Cl of compound 5m by a 5-OMe group and the introduction a methylamino group in position 2 of the thiazole ring (5i) appeared to be detrimental to antibacterial activity. The presence of 2-methylamino, as well as a methyl group, in position 5 of the thiazole ring (5u) had the most negative effect. It should be mentioned that derivatives with a 2-NH2 group in the thiazole ring, independent of substituents in the indole ring (5a, 5d, 5e, 5m, 5q and 5s), were among the most potent.
Thus, it can be concluded that antibacterial activity depends not only on substituents and their position in the indole ring but also on substituents in position 2 of the thiazole moiety.
The three most active compounds (5x, 5m and 5d) were also studied for their activity against resistant strains, including methicillin-resistant S. aureus, P. aeruginosa, and E. coli. From the results, presented in Table 2, it is obvious that all compounds appeared to be more potent against MRSA than ampicillin, whereas streptomycin did not exhibit bactericidal activity. As far as the other two resistant strains are concerned, these compounds were less active than both reference compounds, even though ampicillin did not show bactericidal activity.

Table 2.
FICI indexes of combinations of selected compounds with streptomycin.
The compounds were evaluated then for their ability to stop biofilm formation. The obtained results are promising. Both compounds (5m and 5x) showed stronger inhibition of biofilm formation than both reference compounds in terms of concentration of MIC. Compound 5x was active at lower concentration compared to 5m (0.47 mg/mL and 0.84 mg/mL, respectively). However, it should also be mentioned that the second, in order of activity, compound 5m, was more potent against biofilm formation than both reference drugs, even at a concentration of 0.5 MIC, while the ability of compound 5d was less not only than that of both reference drugs but also than that of the other two compounds. Both compounds 5m and 5x displayed strong antimicrobial potential, represented by both low MICs towards non-resistant (Table 1) and resistant strains (Table 3) and by strong antibiofilm potential towards P. aeruginosa. Since the majority of infections are associated with biofilm-forming microorganisms, these compounds have promising potential for the development of novel antibiofilm therapeutics since they can reduce growth of both planktonic and biofilm-associated microbial cells.

Table 3.
Antibacterial activity against resistant strains (MIC/MBC in mg/mL) and inhibition of biofilm formation (%).
As far as the second subgroup of compounds is concerned (methylindols), they did not show remarkable antibacterial activity (Table S1, Antibacterial activity of methylindole derivatives. (MIC and MBC in mg/mL, Supplementary Files)). More than half of the compounds were of very low activity (MIC/MBC > 3.75 mg/mL), and only compounds 5g, 5h, 5i, 5j, 5k, and 5w showed moderate activity, with MIC of 0.47–1.88 mg/mL and MBC of 0.94–3.75 mg/mL against bacteria tested, except S. aureus. As in case of indole derivatives, S. aureus was the most resistant bacteria, followed by L.monocytogenes, while B. cereus was the most sensitive strain.
According to structure-activity relationships, the presence of 2-Me, 6-OMe substitution in the methylindole ring and 2-NH2 substitution in the thiazole ring (5g) appeared to be the most beneficial.
2.3. Additive Effect of Selected Indole Derivatives in Combination with Streptomycin
The three selected compounds were determined for the interactions with antibiotic streptomycin using checkboard assay. All of the examined compounds were additives with streptomycin (FICI 1.5, Table 2), suggesting, based on the in vitro data, that the combination of compounds with this antibiotic can reduce its MIC and subsequently increase its efficiency.
2.4. P. aeruginosa Time-Kill Curve Assay Efficient of P. aeruginosa Bactericidal Effect after 1 h
The bactericidal nature of three more active compounds, 5d, 5m, and 5x against P. aeruginosa was determined by a time-kill curve study. The treatment with the MBC of all selected compounds drastically reduced the number of P. aeruginosa CFU (Figure 4). Even after 1 h of treatment with compounds 5d, 5m, and 5x, the number of bacterial CFU was reduced by more than 90%, while the 2-h treatment induced a reduction of more than 94%. After 6h, none of the P. aeruginosa colonies treated with the selected compounds (5d, 5m, and 5x) remained viable.
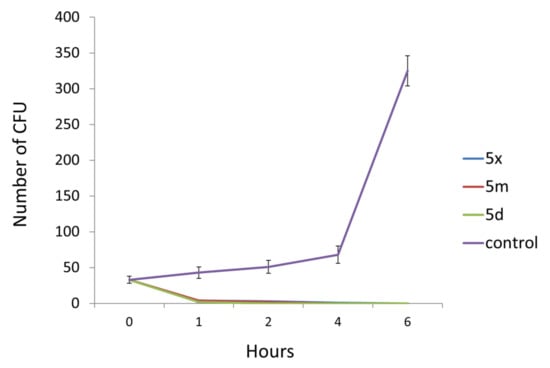
Figure 4.
Number of P. aeruginosa CFU after different time intervals of antimicrobial treatment with MBC of tested compounds.
2.5. Antifungal Activity
Compounds of both groups were then evaluated for antifungal activity. All compounds (indole-based thiazole derivatives) (Table 4) showed antifungal activity, with MIC in the range of 0.06–1.88 mg/mL and MBC of 0.12–3.75 mg/mL. The order of their activity can be presented as follows: 5x > 5l > 5d > 5b > 5m > 5v > 5n = 5o > 5e > 5a = 5q > 5u > 5s > 5i > 5f > 5c. Thus, the most active compound appeared, again, to be compound 5x, with MIC and MBC of 0.06–0.12 mg/mL and 0.12–0.23 mg/mL, respectively, while compound 5c showed the lowest activity, with MIC in the range of 0.12–1.88 mg/mL and MBC of 0.23–3.75 mg/mL.

Table 4.
Antifungal activity of indole derivatives. (MIC and MBC in mg/mL).
It is worth noting that 7 (5d, 5e, 5l, 5o, 5u, 5v, and 5x) out of 16 compounds appeared to be more potent than ketoconazole against some fungal species. Thus, compound 5x was more active against all fungal species than bifonazole and ketoconazole, while compounds 5d was almost equipotent to ketoconazole. Compounds 5l and 5v exhibited higher activity than both reference drugs against A. niger and A. versicolor, while 5l, 5q, and 5v appeared to be equipotent to ketoconazole against the most resistant strain, A. fumigatus. Additionally, compound 5q was equipotent to ketoconazole against A. niger, while 5l was almost equipotent to bifonazole against T viride and more potent than ketoconazole (MIC/MBC of 0.12/0.23 mg/mL) against P.v.c. On the other hand, compounds 5a, 5d, 5e, 5m, 5o and 5u were found to be equipotent to ketoconazole against A.versicolor. It is worth noting that all compounds exhibited superior activity to that of ketoconazole against T. viride. Furthermore, compound 5x demonstrated higher activity than bifonazole against five species, being almost equipotent against A. fumigatus. It is known that clinically, aspergillosis, due to A. fumigatus, represents 90% of systemic fungal infections in immunocompromised patients.
The structure-activity relationships revealed that, like in case of antibacterial activity, the presence of a propan-2-ylidenhydrazine substituent in position 2 of the thiazole ring (5x) is very beneficial for antifungal activity. Additionally, the presence of a methyl group in positions 2 and 6 of the indole ring and an amino group in position 2 of the thiazole ring (5l) appeared to be beneficial. The removal of an Me group from position 2 of the indole ring led to compound 5d with decreased activity. The replacement of a 6-Me group by a 5-OMe substituent in the indole ring led to a less active compound 5b compared to compound 5d, while the presence of a 6-Cl substitution in the indole ring and a 2-NH2 in the thiazole ring (5m) further decreased the activity. A negative influence on antifungal activity was observed with the presence of a methylamino group instead of an amino group in position 2 of the thiazole ring, the replacement of a 6-Cl substituent with 5-OMe, as well as the removal of a 2-Me group in the indole ring, leading to a less potent compound 5v. Furthermore, the combination of a 2-NH2 substitution in the thiazole ring with 6-OMe in the indole ring (5c) appeared to be detrimental to antifungal activity. In general, it was observed that the presence of a methoxy group in either position 5 or 6 of the indole ring, in combination with 2-NH2 substitution in thiazole ring, has a negative effect on the antifungal activity of the compounds.
Regarding the second group, methylindole derivatives, all compounds showed promising antifungal activity, (Table 5) with MIC of 0.06–1.88 mg/mL and MFC in the range of 0.12–3.75 mg/mL. The order of their activity is: 5r > 5t = 6c = 6f > 5g > 6d > 5k > 6e > 5p = 6b > 5h > 5w. Thus, the best activity in the group of methylindole derivatives was achieved for compound 5r, with MIC in the range of 0.12–0.47 mg/mol and MFC of 0.23–0.94 mg/mL, whereas the less active compound appeared to be 5w (MIC and MFC of 0.12–1.88 mg/mL and 0.23–3.75 mg/mL, respectively). The most sensitive fungal was found to be T. viride, followed by A. niger, while P.v.c was the most resistant one, followed by P. funiculosum. Bifonazole exhibited activity, with MIC in the range of 0.10–0.20 mg/mL and MFC of 0.20–0.25 mg/mL, while ketoconazole had MIC of 0.20–1.00 mg/mL and MFC of 0.30–1.50 mg/mL. Most of compounds displayed activity almost equipotent to ketoconazole against almost all fungi tested. Thus, compound 5g showed promising activity against A. niger, with MIC/MFC of 0.06/0.12 mg/mL, better than bifonazole; compounds 5r and 5w appeared to be (MIC/MFC 0.12/0.23 mg/mL) almost equipotent to bifonazole (MIC/MFC 0.15/0.2 mg/mL); while compounds 5g, 6b–6e, and 6g, with MIC/MFC of 0.23/0.47 mg/mL, were equipotent to ketoconazole. On the other hand, compounds 5g, 5r, 5w, as well as 6b and 6d, with MIC and MFC of 0.23 mg/mL and 0.47 mg/mL, respectively, appeared to be equipotent to ketoconazole against A.versicolor, whereas compounds 5h, 5p, 5r and 6e appeared to be equipotent to ketoconazole against the second most resistant fungal, P. funiculosum. Finally, all compounds appeared to be more potent than ketoconazole against T viride, except 6b.

Table 5.
Antifungal activity of methylindole derivatives (MIC and MBC in mg/mL).
The study of the structure-activity relationships of methylindole derivatives revealed that the presence of a 2-Me substituent in the methylindole ring, as well as 2-NH2 and 5-Me groups in the thiazole ring (5r) is very beneficial for antifungal activity. The removal of a methyl group from position 5 of the thiazole moiety of compound 5r was detrimental to antifungal activity, leading to a less active compound (5w). The introduction of a methyl group in position 5 of the methylindole ring, as well as the replacement of a 2-NH2 group with a methylamino and removal of a 5-Me group of thiazole ring (5t) slightly decreased the activity. It should be mentioned that an N-(2-amino-3-acetylpyrazine-2-carboxylic acid substituent in position 2 of the thiazole ring (6c), as well as 2-(2-amino-2-oxopropyl-benzoic acid (6f), had the same influence on antifungal activity as previous substituents. In general, the presence of 2,5-di-Me groups in the methylindole ring, in combination with 2-NH2 substitution in the thiazole ring (5p), an N-(2-methoxyethyl)-2-oxopropanamide (6b) substituent, as well as 2-Me or 5-OMe substitution in the benzylindole ring and 2-NMe substitution in the thiazole ring (5h), had a negative effect on antifungal activity.
Thus, from all mentioned above, it is clear that antifungal activity of these compounds depends on substituents and their position in the methylindole ring, as well as on the nature of the substituents in the thiazole ring.
Finally, it worth noting that methylindole derivatives displayed better antifungal activity than antibacterial but not better than indole derivatives.
2.6. Docking Studies
2.6.1. Docking Studies to Antibacterial Targets
In order to estimate the probable mechanism of antibacterial action of the tested compounds, molecular docking studies on different antibacterial targets (DNA topo IV, E coli primase, Gyrase, Thymidyl kinase, E. coli MurB) were performed. The results are presented in Table 6 and reveal that the calculated free energy of binding to E. coli MurB enzyme (PDB:2Q85) of indole-based thiazolidinones was lower than that of the other enzymes for each compound. Therefore, it may be concluded that the inhibition of E. coli MurB enzyme is probably the most suitable antibacterial mechanism in the tested compounds.

Table 6.
Molecular docking free binding energies (kcal/mol) with antibacterial targets of indole-based thiazole derivatives.
The most active compound, 5x, exhibited the lowest free binding energy (−10.74 Kcal/mol) ) forming three hydrogen bonds: between the hydrogen of indole moiety and the oxygen atom of the side chain of the amino acid Ser228 (distance 2.79 Å), and another two hydrogen bonds between the hydrogen of NH group of the side chain of the compound and the oxygen atoms of the residues Asn50 and Glu324 respectively (2.92 and 2.47 Å respectively). The compound is directed into the E. coli MurB enzyme-active site, into a “channel” composed of amino acids Ile121, Pro110, Ile109, Arg158, Gly122, Gln119, and Arg326, with which it interacts hydrophobically. All these interactions stabilize the complex compound enzyme (Figure 5). It is important to highlight that the hydrogen bond with the residue, Ser228, is crucial for the inhibitory action of this compound because this residue takes part in the proton transfer at the second stage of peptidoglycan synthesis. Hydrogen bond interactions with this residue were also observed for most of the compounds (Table 6).
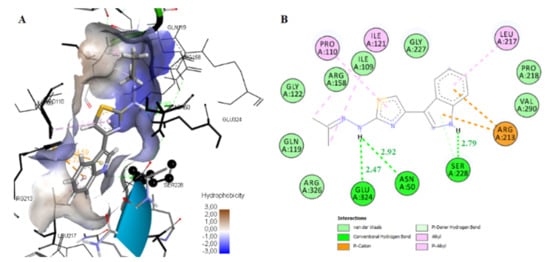
Figure 5.
Binding mode in 3D (A) and 2D (B) of compound 5x into the enzyme-active side of E. coli MurB (PBD: 2Q85). Green dotted lines represent the hydrogen bonds; pink-colored dotted lines and light green spheres represent pi-pi and hydrophobic interactions, respectively.
2.6.2. Docking to Antifungal Targets
All compounds and the reference drug ketoconazole were docked to lanosterol 14α-demethylase from pathogenic yeast C. albicans (CYP51Ca) and dihydrofolate reductase in order to predict the possible mechanism of antifungal action. According to the docking score, it seems that CYP51Ca was the most suitable enzyme for antifungal potency (Table S2).
Docking studies revealed that all the tested compounds may bind CYP51Ca enzymes in an analogous mode to that of reference drug ketoconazole (Figure 6 and Figure 7). Compound 5x showed the lowest free binding energy of −12.55 kcal/mol. This value is particularly due to the hydrogen bond formation between the nitrogen atom of the indole ring and the heme group of the enzyme (distance 1.98 A, Figure 6A). Hydrophobic interactions with the residues, Tyr118, Thr122, Phe126, Ile131, Tyr132, Phe288, Phe233, Thr311, Leu376, Phe380, Met508, and Val509 also contribute to the stability of the complex enzyme compound.
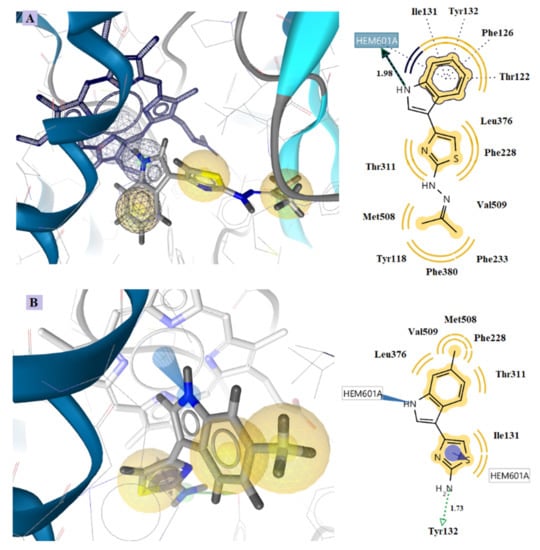
Figure 6.
Docking of the most active compounds 5x (A) and 5d (B) to CYP51Ca receptor. Red and green dotted arrows indicate H-bond, blue arrows aromatic interactions and yellow spheres hydrophobic interactions.
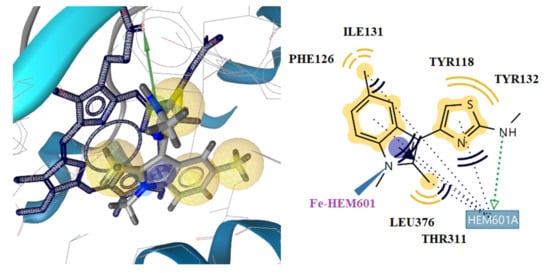
Figure 7.
Docking of compound 5t to CYP51Ca receptor. Red and green dotted arrows indicate H-bond, blue arrows aromatic interactions and yellow spheres hydrophobic interactions.
Furthermore, compound 5d is placed inside the enzyme on the side of the heme group, binding by hydrophobic and aromatic interactions to the Fe of the heme group. This interaction with the Fe is stronger than hydrogen bonds, indicating the high inhibitory activity of this compound (Figure 6B). The benzene ring of ketoconazole also forms aromatic and hydrophobic interactions with the heme (Figure 7). However, compounds 5x and 5d form more stable complexes with the enzyme due to their stronger hydrogen bond formation and the interaction with the Fe of heme, respectively. This is probably the reason why these compounds have better antifungal activity than ketoconazole.
The results of docking of methyl indole derivatives are shown in Table S3.
According to the docking studies, all the tested methylindole-based thiazolidinone derivatives may bind to CYP51Ca enzymes in an analogous mode to that of reference drug ketoconazole (Figure 8). Compound 5t showed the lowest free binding energy, −11.28 kcal/mol. This value is particularly due to the hydrogen bond formation between the nitrogen atom of the methylindole ring of the compound and the hem group of the enzyme (distance 2.53 A, Figure 7). Hydrophobic interactions with the residues, Tyr118, Phe126, Ile131, Tyr132, Thr311, Leu376, and hem also contribute to the stability of the complex enzyme compound.
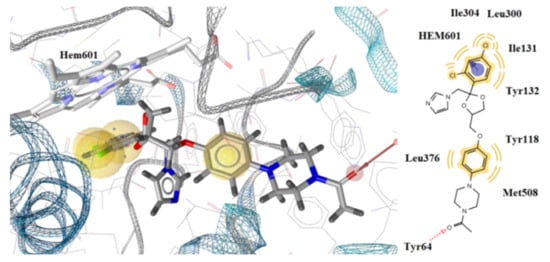
Figure 8.
Docking of ketoconazole to CYP51Ca receptor. Red and green dotted arrows indicate H-bond, blue arrows aromatic interactions and yellow spheres hydrophobic interactions.
Furthermore, compounds 5t and 5r are placed inside the enzyme on the side of the hem group, binding to the Fe of hem through hydrophobic and aromatic interactions. (Figure 7 and Figure 9). However, compounds 5t and 5r form more stable complexes with the enzyme due to their stronger interaction with the Fe of hem than the interaction observed between the benzene of ketoconazole and hem (Figure 8). This is probably the reason why these compounds have better antifungal activity than ketoconazole.
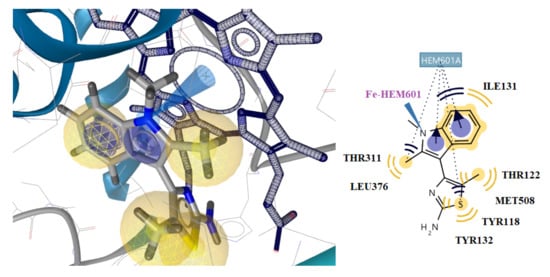
Figure 9.
Docking of the most active compound 5r to CYP51Ca receptor. Red and green dotted arrows indicate H-bond, blue arrows aromatic interactions and yellow spheres hydrophobic interactions.
2.7. Drug-Likeness
In order to see whether our compounds can be bioactive oral drug candidates, the prediction of drug-likeness was performed based on several rules [69,70,71,72,73].
The bioavailability and drug-likeness scores of all compounds are shown in Table S4. According to Table S4 none of the compounds violated any rule, and their bioavailability score was about 0.55. Moreover, all compounds displayed moderate-to-good drug-likeness scores (−0.63 to 0.29). Figure 10 presents the bioavailability radar of some of the compounds. The best of the in-silico predictions results was achieved for compounds with a drug-likeness score of 0.29.
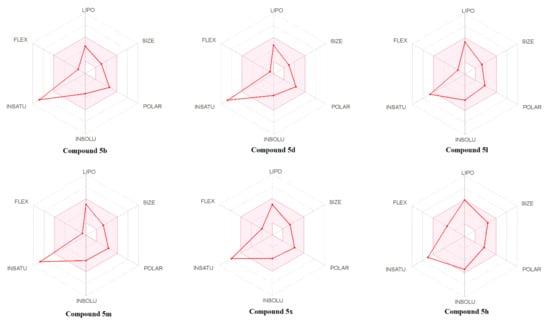
Figure 10.
Bioavailability radar of the tested compounds. The pink area represents the optimal range for each property for oral bioavailability, (lipophilicity (LIPO): XLOGP3 between −0.7 and +5.0, molecular weight (SIZE): MW between 150 and 500 g/mol, polarity (POLAR): TPSA between 20 and 130 Å2, solubility (INSOLU): log S not higher than 6, saturation (INSATU): fraction of carbons in the sp3 hybridization not less than 0.25, and flexibility (FLEX): no more than 9 rotatable bonds).
2.8. Cytotoxicity Assessment
The assessment of cellular cytotoxicity of the compounds in normal human MRC-5 cells was evaluated at two concentrations in culture, i.e., 1 × 10−5 M (Figure 11A,B) and 1 × 10−6 M (Figure 11A,B). No substantial effect on cell proliferation after 48 h exposure was observed in cultures, since the growth was ≥80% for all the tested agents compared to control untreated cultures (Figure 11A, B). Moreover, the percentage of dead cells accumulated in the cultures was very low, since the maximum number did not exceed that of 2–2.5% (Data not shown).
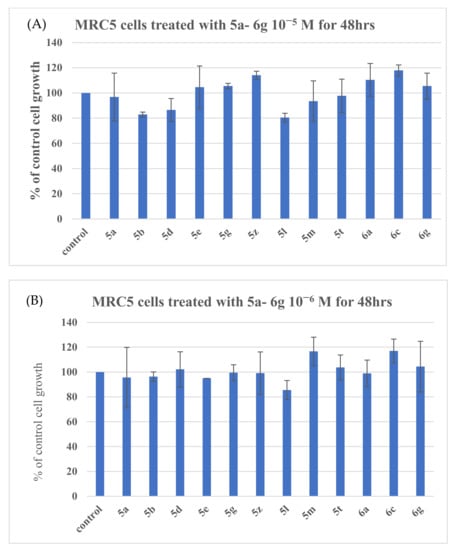
Figure 11.
Assessment of cell proliferation of MRC-5 cells exposed to different compounds in culture. MRC-5 cells grown in culture were separately incubated with each of the compounds at concentrations 1 × 10−5 M (10 μΜ) (panel A) and 1 × 10−6 M (1 μM) (panel B) for 48 h [44].
3. Materials and Methods
3.1. General Procedure for the Synthesis of 3- (α-chlorouracil) -Indoles 2a–q
Starting indoles 1a, 1d, 1j, and 1f were commercially available, while starting indoles 1b, 1c, 1e, and 1i were obtained by decarboxylation of the corresponding indol-2-yl-carboxylic acids [74], and indole 1g was obtained according to [75]. Indoles 1h and 1i were prepared by alkylation of indoles 1f and 1m with benzyl chloride and methyl iodide, respectively, according to [76]. Finally, indoles 1k, 1j и, and 1o were obtained following Chapman et al. [77].
3.2. General Method for Synthesis of 3- (α-chlorouracil) Indoles
To a stirred solution of the corresponding indoles, 1a–o (0.1 mol), and pyridine (8.1 mL, 0.1 mol) in toluene (250 mL) at 55–60 °C, the corresponding acyl chlorides, 2a–b, (0.1 mol) were added dropwise over 1 h. The mixture was stirred at the same temperature for another 1 h, cooled to room temperature, poured into water (1 L), and kept for 3 h at a temperature of 5–8 °C. The precipitate that formed was filtered out, washed on a filter with water (0.5 L), dried, and recrystallized from an appropriate solvent.
3-(α-Chloroacetyl) indole (3a). Yield 49%; mp. 231–232 °C (from ethanol), according to [63] mp. is 230–232 °C.
5-Methoxy-3- (α-chloroacetyl) indole (3b). Yield 56%; mp.193–194 Co (from aqueous acetone), 1H-NMR (300 MHz, DMSO-d6, ppm) 11.73 (s, 1H NH), 8.15 (s, 1H, C2-H), 7.68 (s, 1H, C4-H), 7.32 (d, J = 9.0 Hz, 1H, C6), 6.75 (d, J = 6.0 Hz, 1H, C7-H) 4.63 (s, 2H, –CH2–Cl), 3.81 (s, 3H, 5-CH3O). Anal.Calcd.For C11H10ClNO2 (%):C, 59.07; H, 4.51; N, 6.26. Found (%): C, 59.03; H, 4.55; N. 6.30.
6-Methoxy-3-(α-chloroacetyl) indole (3c). Yield 52%; mp.175–177 °C (from aqueous acetone), 1H-NMR (300 MHz, DMSO-d6, ppm) 11.61 (s, 1H NH), 8.10 (s, 1H, C2-H), 8.04 (d, J = 3.0 Hz, 1H, C4-H), 6.92 (d, J = 3.0 Hz, 1H C7-H), 6.80 (dd, J = 9.0Hz, J = 6.0Hz, 1H, C5-H), 4.60 (s, 2H, –CH2–Cl), 3.83 (s, 3H, 6-CH3O). Anal.Calcd.For C11H10ClNO2 (%):C, 59.07; H, 4.51; N, 6.26. Found (%): C, 59.04; H, 4.56; N. 6.29.
6-Methyl-3-(α-chloroacetyl) indole (3d). Yield 56%, mp.163–164 °C (from ethanol), 1H-NMR (300 MHz, DMSO-d6, ppm) 11.59 (s, 1H NH), 7.93 (s, 1H, C2-H), 7.74 (d, J = 6.0 Hz, 1H, C4-H), 7.13 (s, 1H, C7-H), 6.95 (d, J = 9.0 Hz, 1H, C7-H), 4.55 (s, 2H, –CH2–Cl), 2, 48 (s, 3H, 6-CH3). Anal.Calcd.For C11H10ClNO (%):C, 63.62; H, 4.85; N, 6.75. Found (%): C, 63.58; H, 4.90; N. 6.70.
5-Fluoro-3- (α-chloroacetyl) indole (3e). Yield 64%; mp.165–166 °C (from ethanol), 1H-NMR (300 MHz, DMSO-d6, ppm) 12.01 (s, 1H NH), 8.34 (d, J = 3.0 Hz, 1H, C2-H), 7.84 (dd, J = 12.0 Hz, J = 3.0Hz, 1H, C4-H), 7.43 (dd, J = 9.0 Hz, J = 3.0 Hz, 1H, C7-H), 6.96 (m, 1H, C6-H), 4.66 (s, 2H, –CH2–Cl). Anal.Calcd.For C10H7ClFNO (%):C, 56.76; H, 3.33; N, 6.62. Found (%): C, 56.72; H, 3.35; N. 6.65.
2-Methyl-5-methoxy-3- (α-chloroacetyl) indole (3f). Yield 66%; mp.151–152 °C (from iso-propanol). 1H-NMR (300 MHz, DMSO-d6, ppm) 11.60 (s, 1H NH), 7.46 (s, 1H, C4-H), 7.21 (d, J = 6.0 Hz, 1H, C6-H), 6.74 (d, J = 4.0Hz, 1H, C7-H), 4.65 (s, 2H, –CH2–Cl), 3.83 (s, 3H, 5-CH3O), 2.69 (s, 3H, 2-CH3). Anal.Calcd.For C12H12ClNO2 (%):C, 60.64; H, 5.09; N, 5.89. Found (%): C, 60.66; H, 5.12; N, 5.85.
2,6-Dimethyl-3-(α-chloroacetyl) indole (3g). Yield 59%; mp. 163–165 °C (from ethanol), 1H-NMR (400 MHz, DMSO-d6, ppm) 11.61 (s, 1H NH), 7.74 (d, J = 6.0 Hz, 1H, C4-H), 7.13 (s, 1H, C7-H), 6.95 (d, J = 9.0Hz, 1H, C7-H), 4.65 (s, 2H, –CH2–Cl), 2.71 (s, 3H, 2-CH3). 2.43 (s, 3H, 6-CH3). Anal.Calcd.For C12H12ClNO (%):C, 65.02; H, 5.46; N, 6.32. Found (%): C, 65.06; H, 5.49; N, 6.35.
2-Methyl-5-chloro-3-(α-chloroacetyl) indole (3h). Yield 61%; mp. 134–135 °C (from ethanol), 1H-NMR (300 MHz, DMSO-d6, ppm) 11.99 (s, 1H NH), 7.94 (s, 1H, C4-H), 7.32 (d, J = 9.0 Hz, 1H, C7-H), 7.08 (dd, J = 9.0 Hz, J = 3.0 Hz, 1H, C6-H), 4.68 (s, 2H, –CH2–Cl), 2.73 (s, 3H, 2- CH3). Anal.Calcd.For C11H9Cl2NO (%):C, 54.57; H, 3.75; N, 5.79. Found (%): C, 54.60; H, 3.80; N, 6.75.
5-Ethoxy-3-(α-chloroacetyl) indole (3i). Yield 59%; mp. 149–151 °C (from ethanol), 1H-NMR (300 MHz, DMSO-d6, ppm) 11.73 (s, 1H NH), 8.25 (s, 1H, C2-H), 7.58 (s, 1H, C4-H), 7.52 (d, J = 9.0 Hz, 1H, C6), 6.81 (d, J = 6.0 Hz, 1H, C7-H) 4.59 (s, 2H, –CH2–Cl),), 4.12 (q, J = 20.0 Hz, 2H, -O-CH2 -), 1.41 (t, J = 6.0 Hz, 3H, CH3–CH2–O). Anal.Calcd.For C12H12ClNO2 (%):C, 60.64; H, 5.09; N, 5.89. Found (%): C, 60.62; H, 5.11; N, 5.86.
2-methyl-3-(α-chloroacetyl) indole (3j). Yield 74%; mp.221–222 °C (from acetonitrile), according to [6] so pl. 220–221 Co. 1H-NMR (300 MHz, DMSO-d6, ppm) 11.84 (s, 1H NH), 7.89 (d, J = 3Hz, 1H, C4-H), 7.34 (m, 1H, C7-H), 7.11 (m, 2H, C5-H, C6-H), 4.69 (s, 2H, –CH2–Cl), 2.74 (d, J = 3.0Hz, 3H, 2-CH3). Anal.Calcd.For C11H10ClNO (%):C, 63.62; H, 4.85; N, 6.75. Found (%): C, 63.64; H, 4.89; N, 6.73.
2-Methyl-5-fluoro-3-(α-chloroacetyl) indole (3k). Yield 72%; mp. 168–170 °C (from ethanol). 1H-NMR (400 MHz, DMSO-d6, ppm) 11.90 (s, 1H NH), 7.65 (dd, J = 12.0 Hz, J = 3.0 Hz, 1H, C4-H), 7.31 (dd, J = 9.0 Hz, J = 3.0 Hz, 1H, C7-H), 6.88 (m, 1H, C6-H), 4.67 (s, 2H, –CH2–Cl), 2.72 (s, 3H, 2-CH3). Anal.Calcd.For C11H9FClNO (%):C, 58.55; H, 4.02; N, 6.21. Found (%): C, 58.52; H, 4.05; N, 6.24.
2-Methyl-3-(α-chloropropionyl) indole (3l). Output 65%; mp. 211–213 °C (from ethanol). 1H-NMR (400 MHz, DMSO-d6, ppm) 11.63 (s, 1H NH), 7.79 (d, J = 3Hz, 1H, C4-H), 7.24 (m, 1H, C7-H), 6.99 (m, 2H, C5-H, C6-H), 4.69 (s, 1H, CH3–CH–Cl), 2.74 (d, J = 3.0Hz, 3H, 2-CH3), 2.45 (d, J = 4.0Hz, 3H, CH3–CH–Cl). Anal.Calcd.For C11H11NO (%):C, 76.28; H, 6.40; N, 8.09. Found (%): C, 76.31; H, 6.4.5; N, 8.05.
3.3. General Procedure for the Synthesis of Indol-2-ylthiazoles 5a–x
A mixture of the corresponding 3-(α-chlorouracil)-indole, 3a-q (0.1 mol), thiourea 4a–c (0.125 mol), potassium iodide (0.01 mol), and sodium carbonate (0.3 mol) in absolute methanol (250 mL) was refluxed with stirring until completion of the reaction (monitoring by TLC), cooled to room temperature, poured into water (1L), and an aqueous ammonia solution was added to pH 10. The precipitate formed was filtered off, washed on a filter with water (0.5 L), dried, and recrystallized from an appropriate solvent.
4-(1H-indol-3-yl) thiazol-2-amine (5a). Yield 56%, mp.148–150 °C (from isopropanol), 1H-NMR (300 MHz, DMSO-d6, ppm) 10.95 (s, 1H NH), 7.90 (d, J = 3.0 Hz, 1H, C4-H), 7.57 (s, 1H, CH tiazol), 7.37 (d, J = 3.0 Hz, 1H, C2-H), 6.89 (m, 3H, C6-H, C7-H), 6.43 (m, 1H, C5-H), 6.38 (m, 2H, NH2). 13C-NMR (CDCl3; 125 MHz) δ ppm: 109.25, 111.23, 111.76, 119.52, 121.03, 121.85, 125.34, 125.44, 135.14, 135.98, 167.34 (C-NH2). Anal.Calcd.For C11H9N3S (%): C, 61.37; H, 4.21; N, 19.52. Found (%): C, 61.35; H, 4.22; N, 19.54.
4-(5-Methoxy-1H-indol-3-yl) thiazol-2-amine (5b). Yield 66%; mp. 182–183 °C (from isopropanol), 1H-NMR (300 MHz, DMSO-d6, ppm) 10.80 (s, 1H NH), 7.53 (d, J = 3.0 Hz, 1H, C2-H), 7.40 (s, 1H, C4-H), 7.26 (d, J = 3.0 Hz, 1H, C7-H), 6.74 (dd, J = 9.0 Hz, J = 3.0 Hz, 1H, C6-H), 6.61 (s, 2H, NH2), 6.49 (s, 1H, CH thiazol), 3.83 (s, 3H, 5-CH3O). 13C-NMR (CDCl3; 125 MHz) δ ppm: 55.84 (O-CH3), 108.16, 109.16, 109.87, 112.16, 112.97, 124.05, 128.11, 129.13, 135.26, 153.79 (C-O-CH3), 167.34 (C-NH2). Anal.Calcd.For C12H11N3OS (%): C, 58.76; H, 4.52; N, 17.13. Found (%): C, 58.78; H, 4.50; N, 17.11.
4-(6-Methoxy-1H-indol-3-yl) thiazol-2-amine (5c). Yield 54%; m.p.266–267 °C (from aqueous acetone), 1H-NMR (200 MHz, DMSO-d6, ppm) 10.70 (s, 1H NH), 7.76 (d, J = 10.0 Hz, 1H, C2-H), 7.43 (d, J = 2.0 Hz, 1H, C5-H), 6.87 (d, J = 4.0 Hz, 1H, C5-H), 6.85 (d, J = 2.0 Hz, 1H), 6.69 (d, J = 2.0 Hz, 2H, NH2) 6.43 (s, 1H, CH tiazole), 3.81 (s, 3H, 6-CH3O). 13C-NMR (CDCl3; 125 MHz) δ ppm: 55.69 (O-CH3), 96.24, 108.11, 108.23, 111.87, 117.58, 121.03, 124.17, 135.88, 135.94, 156.17 (C–O–CH3), 167.32 (C-NH2). Anal.Calcd.For C12H11N3OS (%): C, 58.76; H, 4.52; N, 17.13. Found (%): C, 58.74; H, 4.58; N, 17.08.
4-(6-Methyl-1H-indol-3-yl) thiazol-2-amine (5d). Yield 54%; m.p.166–168 °C (from isopropanol), 1H-NMR (200 MHz, DMSO-d6, ppm) 10.79 (s, 1H NH), 7.74 (d, J = 8.0 Hz, 1H, C2-H), 7.52 (d, J = 2.0Hz, 1H, C4-H), 7.16 (s, 1H, C5-H), 6.87 (d, J = 8.0 Hz, 2H, NH2), 6.80 (s, 1H, C7-H), 6.47 (s, 1H, CH tiazole), 2.60 (s, 3H, 6-CH3). 13C-NMR (CDCl3; 125 MHz) δ ppm: 21.68 (CH3), 110.13, 111.25, 111.97, 120.63, 121.77, 121.93, 124.37, 131.59, 135.40, 135.92, 167.11 (C–NH2). Anal.Calcd.For C12H11N3S (%): C, 62.86; H, 4.84; N, 18.33. Found (%): C, 62.81; H, 4.87; N, 18.36.
4-(5-Fluoro-1H-indol-3-yl) thiazol-2-amine (5e). Yield 56%; m.p.142–143 °C (from isopropanol), 1H-NMR (300 MHz, DMSO-d6, ppm) 11.06 (s, 1H NH), 7.64 (d, J = 6.0 Hz, 2H, C2-H, C4- H), 7.33 (dd, J = 9.0 Hz, J = 3.0 Hz, 1H, C6-H), 6.85 (t, J = 15.0 Hz, 1H, C7-H), 6.68 (s, 2H, NH2) 6.45 (s, 1H, CH tiazole). 13C-NMR (CDCl3; 125 MHz) δ ppm: 109.63, 109.82, 111.00, 112.46, 115.69, 124.58, 130.12, 132.22, 135.73, 157.60 (C-F), 167.24 (C-NH2). Anal.Calcd.For C11H8FN3S (%):C, 56.64; H, 3.46; F, 8.14; N, 18.01. Found (%): C, 56.62; H, 3.48; N, 18.03.
4-(5-Methoxy-2-methyl-1H-indol-3-yl) thiazol-2-amine (5f). Yield 63%; m.p.177–179 °C (from aqueous acetone), 1H-NMR (200 MHz, DMSO-d6, ppm) 10.59 (s, 1H, NH), 7.36 (d, J = 2.0 Hz, 1H, C4-H), 7, 15 (d, J = 4.0 Hz, 1H, C6-H), 6.62 (d, J = 6.0 Hz, 2H, -NH2), 6.56 (d, J = 4.0 Hz, 1H, C7-H), 6.25 (s, 1H, CH tiazole), 3.81 (s, 3H, 5-CH3O), 2.55 (s, 3H, 2-CH3). 13C-NMR (CDCl3; 125 MHz) δ ppm: 14.22 (CH3), 55.82 (O–CH3), 99.71, 108.12, 109.14, 112.11, 112.15, 127.61, 129.43, 135.78, 136.55, 154.21 (C–O–CH3), 167.33 (C–NH2). Anal.Calcd.For C13H13N3OS (%): C, 60.21; H, 5.05; N, 16.20. Found (%): C, 60.14; H, 5.03; N, 16.23.
4-(5-Methoxy-1,2-dimethyl-1H-indol-3-yl) thiazol-2-amine (5g). Yield 61%; m.p.130–131 °C (from acetone), 1H-NMR (300 MHz, DMSO-d6, ppm) 10.61 (s, 1H, NH indole), 7.43 (s, 1H, C4-H), 7.14 (s, 1H, NH-CH3) 7.13 (d, J = 3.0 Hz, 1H, C7-H), 6.62 (d, J = 9.0 Hz, 1H, C6), 6.29 (s, 1H, CH tiazole), 3.80 (s, 3H, 5-CH3O), 2.64 (s, 3H, 2-CH3). 13C-NMR (CDCl3; 125 MHz) δ ppm: 14.25 (CH3), 55.97 (O–CH3), 99.79, 109.63, 109.72, 112.01, 112.13, 127.45, 129.41, 135.44, 136.56, 154.22 (C–O–CH3), 167.31 (C–NH2). Anal.Calcd.For C14H15N3OS (%): C, 61.51; H, 5.53; N, 15.37. Found (%): C, 61.52; H, 5.42; N, 15.33.
4-(1-Benzyl-5-methoxy-2-methyl-1H-indol-3-yl)-N-methylthiazol-2-amine (5h). Yield 56%; mp. 194–196 °C (from isopropanol), 1H-NMR (300 MHz, DMSO-d6, ppm) 10.47 (s, 1H, NH), 7.71 (d, J = 6.0 Hz, 1H, C4-H), 7.03 (s, 1H, C7-H), 6.78 (d, J = 9.0 Hz, 1H, C5-H), 6.43 (s, 2H, NH2), 6.27 (s, 1H, CH tiazole), 2.91 (s, 3H, 2-CH3) 2.42 (s, 3H, 6-CH3. 13C-NMR (CDCl3; 125 MHz) δ ppm: 11.12 (CH3), 32.14 (N–CH3), 55.86 (O–CH3), 52.03, 99.12, 108.42, 109.26, 112.33, 125.76, 127.62, 127.64, 128.11, 128.12, 128.55, 129.69, 135.67, 137.12, 138.01, 154.21(C–O–CH3), 163.22 (C–N–CH3). Anal.Calcd.For C21H21N3OS (%): C, 69.39; H, 5.82; N, 11.56. Found (%): C, 69.42; H, 5.80; N, 11.59.
4-(5-Chloro-2-methyl-1H-indol-3-yl) thiazol-2-amine (5i). Yield 56%; m.p.127–128 °C (from isopropanol), 1H-NMR (200 MHz, DMSO-d6, ppm) 10.98 (s, 1H NH), 7.89 (d, J = 2.0 Hz, 1H, C4-H), 7.22 (d, J = 10.0 Hz, 1H, C7-H), 6.93 (d, J = 4.0 Hz, 1H, C6-H), 6.68 (s, 2H, NH2), 6.25 (s, 1H, CH tiazole), 2.51 (s, 3H, 2-CH3). 13C-NMR (CDCl3; 125 MHz) δ ppm: 14.28 (CH3), 32.05 (N–CH3), 55.83 (O–CH3), 99.15, 108.17, 109.66, 112.03, 112.07, 127.51, 129.44, 134.42, 135.73, 154.01 (C–O–CH3), 164.42 (C–NH2). Anal.Calcd.For C14H15N3OS (%): C, 61.51; H, 5.53; N, 15.37;. Found (%): C, 61.47; H, 5.56; N, 15.32.
4-(5-Methoxy-1,2-dimethyl-1H-indol-3-yl)-N-methylthiazol-2-amine (5j). Yield 59%; mp.203–204 °C (from isopropanol), 1H-NMR (200 MHz, DMSO-d6, ppm) 10.77 (s, 1H NH), 7.53 (d, J = 4.0 Hz, 1H, C2-H), 7.30 (dd, J = 14.0 Hz, J = 10.0 Hz, 2H, C4-H, C7-H), 6.69 (s, 2H, NH2), 6.63 (s, 1H, C6-H), 6.43 (s, 1H, CH tiazole), 4.07 (q, J = 20.0 Hz, 2H, –O–CH2–), 1.42 (t, J = 6.0 Hz, 3H, CH3-CH2-O). 13C-NMR (CDCl3; 125 MHz) δ ppm: 10.15 (CH3), 30.26 (N–CH3), 32.02 (N–CH3), 55.81 (O–CH3), 99.62, 108.51, 109.41, 106.78, 112.33, 128.34, 129.75, 135.72, 138.02, 154.11 (C–O–CH3), 164.40 (C-NH2). Anal.Calcd.For C15H17N3OS (%): C, 62.69; H, 5.96; N, 14.62. Found (%): C, 62.75; H, 5.91; N, 14.58.
4-(1,2-Dimethyl-1H-indol-3-yl)-N-methylthiazol-2-amine (5k). Yield 62%; mp.184–185 °C (from isopropanol), 1H-NMR (200 MHz, DMSO-d6, ppm) 10.71 (s, 1H, NH) 7.35 (d, J = 8.0 Hz, 1H, C4-H), 7.28 (d, J = 8.0 Hz, 1H, C5-H), 6.92 (m, 2H, C6-H, C7-H), 6.32 (s, 2H, NH2), 2.48 (s, 3H, 2-CH3), 2.12 (s, 3H, CH3-tiazole). 13C-NMR (CDCl3; 125 MHz) δ ppm: 11.06 (CH3), 14.95 (CH3), 99.35, 113.23, 119.35, 121.03, 121.05, 128.65, 132.57, 134.45, 135.26, 140.38, 167.85 (C-NH2). Anal.Calcd.For C14H15N3S (%): C, 65.34; H, 5.87; N, 16.33 Found (%): C, 65.32; H, 5.88; N, 16.31.
4-(2,6-Dimethyl-1H-indol-3-yl)thiazol-2-amine (5l). Yield 49%; m.p.155–156 °C (from acetone), 1H-NMR (300 MHz, DMSO-d6, ppm) 10.89 (s, 1H, NH), 7.61 (d, J = 3.0 Hz, 1H, C4-H), 7.182 (d, J = 3.0 Hz, 1H, C7-H), 6.80 (m, 3H, C6-H indole + NH2), 6.26 (s, 1H CH tiazole) 2.62 (s, 3H, 2-CH3). 13C-NMR (CDCl3; 125 MHz) δ ppm: 14.24 (CH3), 21.13 (CH3), 99.13, 109.36, 111.04, 120.02, 121.67, 125.79, 131.42, 134.44, 135.12, 135.16, 167.38 (C–NH2). Anal.Calcd.For C13H13N3S (%): C, 64.17; H, 5.39; N, 17.27. Found (%): C, 64.15; H, 5.43; N, 17.30.
4-(5-Chloro-2-methyl-1H-indol-3-yl)thiazol-2-amine (5m). Yield 67%; m. 143–145 °C (from isopropanol), 1H-NMR (300 MHz, DMSO-d6, ppm) 10.79 (s, 1H, NH), 7.84 (d, J = 6.0 Hz, 1H, C4-H), 7.24 (d, J = 6.0 Hz, 1H, C7-H), 6.94 (dd, J = 12.0 Hz, J = 6.0 Hz, 2H, C5-H, C6-H), 6.61 (s, 2H, NH2), 6.28 (s, 1H, CH tiazole), 2.62 (s, 3H, 2-CH3). 13C-NMR (CDCl3; 125 MHz) δ ppm: 14.00 (CH3), 99.75, 109.36, 114.75, 121.87, 122.13, 125.43, 129.14, 134.55, 135.12, 135.67, 167.45 (C–NH2). Anal.Calcd.For C12H10ClN3S (%): C, 54.65; H, 3.82; Cl, 13.44; N, 15.93. Found (%): C, 54.62; H, 3.84; N, 15.96
4-(5-Ethoxy-1H-indol-3-yl)thiazol-2-amine (5n). Yield 56%; mp 210–212 °C (from aqueous acetone), 1H-NMR (300 MHz, DMSO-d6, ppm) 10.72 (s, 1H, NH), 7.31 (d, J = 6.0 Hz, 1H, C4-H), 7.22 (d, J = 6.0 Hz, 1H, C7-H), 6.96 (s, 1H, NH-CH3), 6.94 (dd, J = 12.0 Hz, J = 6.0 Hz, 2H, C5-H, C6-H), 2.84 (d, 3H, J = 6.0Hz, NH-CH3 tiazole), 2.48 (s, 3H, 2-CH3) 2.22 (s, 3H, C–CH3-ttiazole). 13C-NMR (CDCl3; 125 MHz) δ ppm: 14.05 (CH3), 64.00, 107.98, 108.01, 110.56, 110.58, 117.21, 122.43, 127.92, 128.67, 135.74, 153.11, 167.30 (C–NH2). Anal.Calcd.For C13H13N3OS (%): C, 60.21; H, 5.05; N, 16.20. Found (%): C, 60.18; H, 5.07; N, 16.15.
5-Methyl-4-(2-methyl-1H-indol-3-yl)thiazol-2-amine (5o). Yield 59%; mp.132–133 °C (from acetone), 1H-NMR (200 MHz, DMSO-d6, ppm) 10.82 (s, 1H, NH-indole), 7.57 (s, 1H, C2-H), 7.44 (s, 1H, C4-H), 7.21 (d, J = 6.0 Hz, 1H, C7-H), 7.19 (s, 1H, NH–CH3), 6.72 (d, J = 4.0 Hz, 1H, C6-H), 6, 50 (s, 1H, CH tiazole), 3.84 (s, 3H, 5-OCH3), 2.98 (s, 3H, NH–CH3). 13C-NMR (CDCl3; 125 MHz) δ ppm: 11.06 (CH3), 14.95 (CH3), 99.35, 113.23, 119.35, 121.03, 121.05, 128.65, 132.57, 134.45, 135.26, 140.38, 167.85 (C–NH2). Anal.Calcd.For C13H13N3S (%): C, 64.17; H, 5.39; N, 17.27. Found (%): C, 64.18; H, 5.34; N, 17.32.
4-(1,2,5-Trimethyl-1H-indol-3-yl)thiazol-2-amine (5p). Yield 71%; mp 196–197 °C (from ethanol), 1H-NMR (400 MHz, DMSO-d6, ppm) 11.44 (s, 1H, NH-indole), 7.85 (s, 1H, C2-H), 7.81 (m, 1H, C4-H), 7.45 (m, 1H, C5-H), 7.14 (m, 2H, C6-H, C7-H), 7.01 (s, 1H, CH thiazole), 4.99 (s, 1H, –NH–N = C), 2.12 (d, 6H, –C–(CH3) 2). 13C-NMR (CDCl3; 125 MHz) δ ppm: 10.56 (CH3), 21.73 (CH3), 30.64 (N-CH3), 99.63, 108.02, 109.64, 123.75, 128.11, 128.34, 128.72, 133.62, 135.71, 138.02, 167.32 (C–NH2. Anal.Calcd.For C14H15N3S (%): C, 65.34; H, 5.87; N, 16.33. Found (%): C, 65.38; H, 5.83; N, 16.35.
4-(5-Fluoro-2-methyl-1H-indol-3-yl)thiazol-2-amine (5q). Yield 49%; mp. 155–156 °C (from acetone), 1H-NMR (300 MHz, DMSO-d6, ppm) 10,89 (s, 1H, NH), 7.61 (d, J = 3.0 Hz, 1H, C4-H), 7.182 (d, J = 3.0 Hz, 1H, C7-H), 6,80 (m, 3H, C6-H indole +NH2), 6.26 (s, 1H C–H tiazole) 2.62(s, 3H, 2-CH3). 13C-NMR (CDCl3; 125 MHz) δ ppm: 14.36 (CH3), 99.65, 109.52, 110.25, 112.49, 116.02, 130.00, 130.85, 134.12, 135.97, 158.11 (C–F), 167.21 (C–NH2). Anal.Calcd.For C12H10FN3S (%): C, 58.28; H, 4.08; F, 7.68; N, 16.99. Found (%): C, 58.30; H, 4.05; N, 16.96.
4-(1,2-Dimethyl-1H-indol-3-yl)-5-methylthiazol-2-amine (5r). Yield 49%; mp.210–212 °C (from acetone), 1H-NMR (200 MHz, DMSO-d6, ppm) 7.37 (d, J = 4.5 Hz, 2H, C4-H, C5-H,), 7.27 (d, J = 3.0 Hz, 1H), 6.99 (dt, J = 4.0 Hz, 18.0 Hz, 2H, C7-H, C6-H), 6.39 (s, 2H, NH2), 3.74(s, 3H, N–CH3) 2.40 (s, 3H, 2-CH3), 2,12 (s, 3H, CH3 tiazole). 13C-NMR (CDCl3; 125 MHz) δ ppm: 10.52 (CH3), 10.78 (CH3), 30.34 (N–CH3), 99.65, 109.85, 120.12, 121.14, 121.48, 128.24, 132.67, 136.24, 138.07, 140.53, 167.40 (C–NH2). Anal.Calcd.For C14H15N3S (%): C, 65.34; H, 5.87; N, 16.33. Found (%): C, 65.32; H, 5.86; N, 16.38. Anal.Calcd.For C14H15N3S (%): C, 65.34; H, 5.87; N, 16.33. Found (%): C, 65.32; H, 5.86; N, 16.38.
4-(2-Methyl-1H-indol-3-yl)thiazol-2-amine (5s). Yield 67%; mp.153–154 °C (from i-propanol), 1H-NMR (300 MHz, DMSO-d6, ppm) 10.79 (s, 1H, N-H), 7.84 (d, J = 6.0 Hz, 1H, C4-H), 7.24 (d, J = 6.0 Hz, 1H, C7-H), 6.94 (dd, J = 12.0 Hz, J = 6.0 Hz, 2H, C5-H, C6-H), 6.61(s, 2H, NH2), 6.28(s, 1H, C-H tiazole), 2.62(s, 3H, 2-CH3). 13C-NMR (CDCl3; 125 MHz) δ ppm: 14.29 (CH3), 99.62, 109.42, 110.99, 119.68, 121.00, 121.83, 129.03, 134.44, 135.76, 167.30 (C–NH2). Anal.Calcd.For C12H11N3S (%): C, 62.86; H, 4.84; N, 18.33. Found (%): C, 62.84; H, 4.82; N, 18.36.
N-Methyl-4-(1,2,5-trimethyl-1H-indol-3-yl)thiazol-2-amine (5t). Yield 64%; mp 169–171 °C (i-propanol), 1H-NMR (200 MHz, DMSO-d6, ppm) 7.60 (s, 1H, C4-H), 7.25 (s, 1H, NH–CH3), 7.15(d, J = 6.0 Hz, 1H, C7-H), 6.89 (d, J = 6.0 Hz, 1H, C6-H), 6.33(s,1H, C-H tiazole), 3.70(s, 3H, N–CH3), 2.97(s, 3H, NH–CH3 tiazole), 2.68(s, 3H, 2-CH3), 2.43(s, 3H, 5-CH3). 13C-NMR (CDCl3; 125 MHz) δ ppm: 10.61 (CH3), 20.74 (CH3), 30.74 (CH3), 31.32 (N–CH3), 99.74, 108.03, 109.61, 123.71, 127.89, 128.31, 128.93, 133.22, 138.21, 135.72, 163.92 (C–NH2). Anal.Calcd.For C15H17N3S (%): C, 66.39; H, 6.31; N, 15.48. Found (%): C, 66.35; H, 6.28; N, 15.52.
4-(2-Methyl-1H-indol-3-yl)- N,5-dimethyl-thiazol-2-amine (5u). Yield 5%6; mp. 184–185 °C (agues acetone), 1H-NMR (300 MHz, DMSO-d6, ppm)10.72 (s, 1H, N-H), 7.31 (d, J = 6.0 Hz, 1H, C4-H), 7.22 (d, J = 6.0 Hz, 1H, C7-H), 6.96 (s, 1H, NH–CH3), 6.94 (dd, J = 12.0 Hz, J = 6.0 Hz, 2H, C5-H, C6-H), 2.84(d, 3H, J = 6.0 Hz, NH–CH3 tiazole),2.48(s, 3H, 2-CH3) 2,22 (s, 3H,C–CH3- tiazole). 13C-NMR (CDCl3; 125 MHz) δ ppm: 14.78 (CH3), 31.56 (NH-CH3), 55.82 (O–CH3), 99.26, 108.37, 109.82, 112.20, 112.31, 126.43, 126.51, 134.88, 135.22, 154.02 (C–O–CH3), 163.43 (C–NHCH3). Anal.Calcd.For C14H15N3S (%): C, 65.34; H, 5.87; N, 16.33. Found (%): C, 65.36; H, 5.84; N, 16.32.
4-(5-Methoxy-1H-indol-3-yl)-N-methylthiazol-2-amine (5v). Yield 59%; mp.132–133 °C (acetone), 1H-NMR (200 MHz, DMSO-d6, ppm) 10.82 (s, 1H, NH-indole), 7.57 (s, 1H, C2-H), 7.44 (s, 1H, C4-H), 7.21 (d, J = 6.0 Hz, 1H, C7-H), 7.19 (s, 1H, NH–CH3), 6.72 (d, J = 4.0 Hz, 1H, C6-H), 6,50 (s, 1H,C–H tiazole), 3,84 (s, 3H, 5-OCH3), 2,98 (s, 3H, NH–CH3). 13C-NMR (CDCl3; 125 MHz) δ ppm: 31.25 (NH-CH3), 55.88 (O–CH3), 108.26, 109.57, 111.13, 112.06, 112.54, 124.63, 128.96, 129.71, 135.74, 154.12 (C–O–CH3), 163.29 (C–NHCH3). Anal.Calcd.For C13H13N3OS (%): C, 60.21; H, 5.05; N, 16.20. Found (%): C, 60.25; H, 5.04; N, 16.23.
4-(1,2-Dimethyl-1H-indol-3-yl)thiazol-2-amine (5w). Yield 63%; mp.162–164 °C (i-propanol), 1H-NMR (400 MHz, DMSO-d6, ppm) 7.83 (d, J = 6.0 Hz, 1H, C4-H), 7.28 (d, J = 6.0 Hz, 1H, C7-H), 7.03(m, 2H, C5-H, C6-H), 6.48(s, 2H, NH2), 6.32(s,1H, C–H tiazole), 3.73 (s, 3H, N–CH3), 2.67(s, 3H, 2-CH3). 13C-NMR (CDCl3; 125 MHz) δ ppm: 10.23 (CH3), 30.32 (N-CH3), 99.15, 109.62, 109.66, 120.04, 121.41, 121.82, 128.25, 136.21, 138.11, 167.32 (C–NH2). Anal.Calcd.For C13H13N3S(%): C,,64.17; H,5.39: N, 17.27. Found (%): C, 64.14; H, 5.41; N, 17.30.
4-(1H-Indol-3-yl)-2-(2-(propan-2-ylidene)hydrazinyl)thiazole (5x). Yield 71%; mp. 233–35 °C (ethanol), 1H-NMR (400 MHz, DMSO-d6, ppm) 11.44 (s, 1H, NH-indole), 7.85 (s, 1H, C2-H), 7.81 (m, 1H, C4-H), 7.45 (m, 1H, C5-H), 7.14 (m, 2H, C6-H, C7-H), 7.01(s, 1H, C–H tiazole), 4,99 (s, 1H, -NH–N=C), 2.12 (d, 6H, –C–(CH3)2). 13C-NMR (CDCl3; 125 MHz) δ ppm: 19.35 (CH3), 25.47 (CH3), 109.63, 111.00, 111.13, 119.88, 121.83, 122.03, 124.35, 124.91, 135.18, 135.45, 147.50, 170.28 (N–C–(CH3)2).Anal.Calcd.For C14H14N4S (%): C, 62.20; H, 5.22; N, 20.72. Found (%): C, 62.17; H, 5.24; N, 20.74.
3.4. General Procedure for the Synthesis of indol-2-ylthiazoles 6a–f
To a solution of the corresponding aminothiazole (5b, 5c, 5w) (10 mmol) in pyridine (50 mL), acid chloride was added (7a-b) (10 mmol). The reaction mass was stirred for 5 h and poured into water (200mL). The precipitate that formed was filtered off, washed thoroughly with water, dried in air, and recrystallized from ethanol.
N1-(3,4-Dimethoxyphenethyl)-N2-(4-(1,2-dimethyl-1H-indol-3-yl)thiazol-2-yl)oxalamide (6a). Yield 69%;mp.155–156 °C (from ethanol), 1H-NMR (200 MHz, DMSO-d6, ppm) 9.14 (t, 1H, C2-H Ar), 8.01 (d, J = 6.0 Hz, 1H, C5-H Ar), 7.45 (d, J = 6.0 Hz, 1H, C6-H Ar), 7.26 (s, 1H, C-H tiazole), 7.10 (m, 2H, C4-H, C7-H indole), 6.83 (s, 2H, NH–CO), 6,79 (m, 2H, C5-H, C6-H indole), 3.73 (s, 6H, OCH3), 3.45 (m, 2H, –CH2–), 3.33 (s, 3H, N-CH3), 2.75 (m, 2H, –CH2–), 2,69 (s, 3H, 2-CH3). 13C-NMR (CDCl3; 125 MHz) δ ppm: 19.35 (CH3), 25.47 (CH3), 109.63, 111.00, 111.13, 119.88, 121.83, 122.03, 124.35, 124.91, 135.18, 135.45, 147.50, 170.28 (N–C–(CH3)2). Anal.Calcd.For C25H26N4O4S (%):C, 62.74; H, 5.48; N, 11.71. Found (%): C, 62.72; H, 5.49; N, 11.70.
N1-(4-(1,2-Dimethyl-1H-indol-3-yl)thiazol-2-yl)2-N2-(2-methoxyethyl)oxalamide (6b). Yield 76%; mp.172–173 °C (ethanol), 1H-NMR (200 MHz, DMSO-d6, ppm) 12.47 (s, 1H, -NH-C=O), 9.07 (d, J = 2.0 Hz, 1H, O=C–NH–CH2–), 8.01 d,d, J = 6.0 Hz, 1H, C4-H), 7.45 (d, J = 6.0 Hz, 1H, C7-H), 7.27 (s, 1H, C–H tiazole), 7.10 (m, 2H, C5-H, C6-H), 3.73 (s, 3H, N–CH3), 3.38 (m, 4H, –CH2–CH2–), 3.33 (s, 3H, CH3–O), 2.69 (s, 3H, 2- CH3). 13C-NMR (CDCl3; 125 MHz) δ ppm: 10.72 (CH3), 30.23 (N–CH3), 35.41, 40.63, 56.81 (O–CH3), 56.83 (O-CH3), 99.26, 108.53, 109.11, 112.31, 112.84, 120.03, 121.45, 121.67, 122.13, 128.73, 130.24, 135.11, 136.74, 138.06, 148.15, 150.32 (C–O–CH3), 161.25 (C=O), 162.47(C=O), 163.18. Anal.Calcd.For C18H20N4O3S (%): C, 58.05; H, 5.41; N, 15.04. Found (%): C, 58.02; H, 5.44; N, 15.10.
3-((4-(1,2-Dimethyl-1H-indol-3-yl)thiazol-2-yl)carbamoyl) pyrazine-2-carboxylic acid (6c). Yield 75%; mp.304–305 °C (ethanol), 1H-NMR (DMSO-d6; 125 MHz) δ ppm: 2.49 (s, 3H, CH3), 3.70 (Ss, 3H, N–CH3), 4.20 (s, 2H, –CH2–), 7.11 (s, 2H, Ar, benz), 7.18–7.50 (m, 2H, Ar), 7.51–7.69 (m, 1H, Ar), 7.76–7.82 (m, 2H, Ar), 8.98 (s, br, 1H, NH–C=O), 11.02 (s, br, 1H, –OH). 13C-NMR (CDCl3; 125 MHz) δ ppm: 11.61 (CH3), 29.59 (N–CH3), 39.81, 58.91 (O–CH3), 70.36, 107.34, 106,35, 106,84, 119.18, 120.10, 121.30, 126.42, 135.64, 136.66, 146.84, 155.06, 157.14(C=O), 158.27 (C=O). Anal.Calcd.For C19H15N5O3S (%): C, 58.01; H, 3.84; N, 17.80. Found (%): C, 58.05; H, 3.82; N, 17.78.
2-(2-((4-(1,2-Dimethyl-1H-indol-3-yl)thiazol-2-yl)amino)-2-oxoethyl)benzoic acid (6d). Yield 77%; mp.205–206 °C (ethanol), 1H-NMR (200 MHz, DMSO-d6, ppm) 12.77 (s, 1H, OH), 12.19 (s, 1H, –NH–C=O), 7,93 (d, J = 4.0 Hz, 2H, C4-H, C-H Ar), 7.54 (t, J = 10.0 Hz, 1H, C6-H), 7.42 (dd, J = 10.0 Hz, J = 6.0 Hz, 3H, C5-H, C6-H, C-H Ar), 7.10 (m, 2H, C7-H, C-H Ar), 7.04 (s, 1H, C-H tiazole), 4.23 (s, 2H, –CH2–Ar), 3.73 (s, 3H, N–CH3), 2.67 (s, 3H, 2-CH3). 17 13C-NMR (CDCl3; 125 MHz) δ ppm: 10.42 (CH3), 29.88 (N–CH3), 99.65, 108.93, 109.25, 109.31, 120.12, 121.43, 121.82, 128.74, 135.14, 136.25, 138.03, 142.63, 14377, 143.81, 161.76 (C=O), 162.64, 163.48 (COOH). Anal.Calcd.For C22H19N3O3S (%): C, 65.17; H, 4.72; N, 10.36. Found (%): C, 65.15; H, 4.74; N, 10.38.
2-(2-((4-(5-Methoxy-1H-indol-3-yl)thiazol-2-yl)amino)-2-oxoethyl)benzoic acid (6e). Yield 71%; mp. 245–247 °C (ethanol), 1H-NMR (DMSO-d6; 125 MHz) δ ppm: 3.83 (s, 5H, O-CH3, CH2), 6.65 (d, 1H, Ar), 6.88 (s, 1H, Ar), 7.22–7.31 (m, 3H, Ar), 7.38–7.46 (m, 2H, Ar), 7.71–7.82 (m, 1H, Ar), 8.67 (s, 1H, =CH–NH), 9.25 (s, br, 1H, NH–C=O), 10.18 (s, br, 1H, NH), 11.09 (s, br, 1H, OH.) 13C-NMR (CDCl3; 125 MHz) δ ppm: 10.71 (CH3), 30.62 (N-CH3), 95.22, 109.32, 109.55, 119.02, 121.31, 121.52, 123.82, 128.66, 128.70, 130.11, 131.10, 132.05, 134.82, 135.24, 136.83, 138.02, 162.77, 164.73 (C=O), 167.64 (COOH). Anal.Calcd.For C21H17N3O4S (%):C, 61.90; H, 4.21; N, 10.31. Found (%):C, 61.88; H, 4.22; N, 10.34.
2-(2-((4-(6-Methoxy-1H-indol-3-yl)thiazol-2-yl)amino)-2-oxoethyl)benzoic acid (6f). Yield 69%; 229–230 °C (ethanol), 1H-NMR (DMSO-d6; 125 MHz) δ ppm: 3.84 (s, 5H, O–CH3, CH2), 6.68 (d, 1H, Ar), 6.90 (s, 1H, Ar), 7.11 (s, 1H, thiaz), 7.18–7.25 (m, 1H, Ar), 7.35–7.40 (m, 2H, Ar), 7.61–7.68 (m, 3H, Ar), 8.62 (s, 1H, =CH-NH), 9.21 (s, br, 1H, NH–C=O), 10.05 (s, br, 1H, NH), 11.08 (s, br, 1H, –OH). 13C-NMR (CDCl3; 125 MHz) δ ppm: 37.13, 55.82 (O–CH3), 95.22, 109.31, 109.66, 111.21, 117.41, 121.34, 124.35, 125.96, 127.54, 130.02, 132.33, 134.42, 135.11, 136.76, 138.39, 156.52 (C–OCH3), 162.63, 170.02 (C=O), 172.12 (COOH). Anal.Calcd.For C21H17N3O4S (%): C, 61.90; H, 4.21; N, 10.31. Found (%): C, 61.88; H, 4.19; N, 10.37.
3.5. Biological Evaluation
3.5.1. Antibacterial Action
The following Gram-negative bacteria: Escherichia coli (ATCC 35210), Enterobacter cloacae (clinical isolate), Salmonella typhimurium (ATCC 13311), as well as Gram-positive bacteria: Listeria monocytogenes (NCTC 7973), Bacillus cereus (clinical isolate), and Staphylococcus aureus (ATCC 6538) were used. The bacterial strains were supplied by the Mycological Laboratory, Department of Plant Physiology, Institute for Biological Research” Siniša Stankovic”, Belgrade, Serbia.
The minimum inhibitory and bactericidal (MIC/MBC) concentrations were defined, as described previously [78,79]. Resistant strains used were isolates of S. aureus, E. coli, and P. obtained as reported by Kartsev et al. [78]
3.5.2. Biofilm Formation Inhibition
Evaluation was performed as described previously [80], with some modifications. The calculation of % inhibition was performed using the following equation:
[(A620 control − A620 sample)/A620 control] × 100
3.5.3. Checkboard Assay
A checkboard assay was used for the determination of interactions among the selected compounds and antibiotic and streptomycin. The assay was carried out with 96-well microplates containing TSB medium for the resistant P.aeruginosa strain, supplemented with examined compounds in concentrations ranging from 1/16 to 4 × MIC, as described previously, [81] in the checkboard manner. The microplates were incubated for 24 h at 37 °C. The MIC of the combinations of examined compounds with streptomycin was determined as for the antimicrobial assay. The fractional inhibitory concentration index (FICI) was calculated by following equation:
FICI = FIC10/MIC10 + FIC20/MIC20
FIC10 and FIC20 are the MIC values of the combination of tested compounds and antibiotics, and MIC10 and MIC20 represent the MIC values of individual agents. The following cut-offs: FIC ≤ 0.5 synergistic, >0.5 < 2 additive, ≥2 < 4 indifferent, and FIC > 4 antagonistic effects were used for the discussion of obtained results.
3.5.4. Time-Kill Curve Assay
The impact of time on the bactericidal effects of selected compounds was evaluated as described in [82], with some modifications. P. aeruginosa cells were incubated with the MBC of compounds with a total volume of 100 µL, which was rubbed into plate-count agar plates with a sterile spreader after 1, 2, 4, and 6 h of treatment. Plates were incubated at 37 °C, and the number of colonies was counted after 24 h.
3.5.5. Antifungal Activity
The strains supplied by Institute for Biological Research ‘‘Siniša Stankovic were: Aspergillus niger (ATCC 6275), Aspergillus fumigatus (ATCC 1022), Aspergillus versicolor (ATCC 11730), Penicillium funiculosum (ATCC 36839), Trichoderma viride (IAM 5061), and Penicillium verrucosum var. cyclopium (food isolate). All experiments were performed in duplicate and repeated three times [83,84].
3.6. Docking Studies
Docking simulation was performed using AutoDock 4.2®so software, according to our previous paper [78].
3.6.1. Docking Studies for Prediction of the Mechanism of Antibacterial Activity
In order to predict the possible mechanism of antibacterial activity of the tested compounds, the enzymes, E. coli DNA gyrB, thymidylate kinase, E. coli primase, E. coli MurB, and DNA topo IV were chosen for docking studies. As the first step, all the co-crystalized original ligands were redocked in the active sites of all enzymes in order to validate the protocol. The RMSD values were in the range of 0.86 to 1.63 Å.
3.6.2. Docking Studies for Prediction of the Mechanism of Antifungal Activity
In order to predict the possible mechanism of antifungal activity of the tested compounds, enzymes CYP51 14α-lanosterol demethylase and dihydrofolate reductase were used. The X-ray crystal structures 5V5Z and 4HOF respectively for each enzyme were obtained for the Protein Data Bank. The docking box was centered on the heme molecule, at the active center of the CYP51 14α-lanosterol demethylase enzyme, both with a target box of 50 × 50 × 50 Å. All selected X-ray crystal structures were in complex with inhibitors. Docking of these inhibitors to their enzyme structures was performed for verification of the method with RMSD values 0.85 and 1.36 Å for CYP51 14α-lanosterol demethylase and dihydrofolate reductase, respectively (Figure S1). Furthermore, the reference drug, ketoconazole, was docked to the active site of 5V5Z structure.
3.7. In-Silico Predictive Studies
Drug-likeness prediction of all compounds was performed as described in our previous paper [85].
3.8. Assessment of Cytotoxicity
The growth of MRC-5 cells was previously described [44]. For the assessment of cytotoxicity, the cells were seeded in a 96-well plate at an initial concentration of 5 × 104 cells/mL and allowed to attach for at least 3h before the addition of the compounds at two different concentrations: 1 × 10−5 M (10 μΜ) and 1 × 10−6 M (1 μΜ). Note that the concentration of DMSO in culture was ≤ 0.2% v/v, in which no detectable effect on cell proliferation was observed (1). The evaluation of cytotoxicity of each compound and the measure of the number of dead cells was described previously [44,67,68].
4. Conclusions
This manuscript reported on the design, synthesis, and in silico and biological evaluation of twenty-nine 4-(indol-3-yl)thiazole-2-amines (5a–5x) and 4-indol-3-yl)thiazole acylamines (6a–6f) as antimicrobial agents.
The subgroup of indole-based thiazolidinone derivatives (5a–f, 5i, 5l–o, 5q, 5s, 5u, 5v, 5x) showed antibacterial activity, with MIC in the range of 0.06–1.88 mg/mL and MBC of 0.12–3.75 mg/mL. Nevertheless, only one compound, 5x, exceeded the activity of ampicillin against S. typhimurium. The most sensitive bacteria was found to be S. typhimurium, while S. aureus was the most resistant one The three most active compounds, 5d, 5m, and 5x, appeared to be active against three resistant strains MRSA, E. coli, and P. aeruginosa, showing better activity against MRSA than both reference drugs. An evaluation of their ability to stop biofilm formation revealed that two compounds (5m and 5x) exhibited stronger inhibition of biofilm formation than both reference drugs in concentration of MIC. Additionally, compound 5m was more potent against biofilm formation than both reference drugs, even in concentrations of 0.5 MIC.
The determination of the interactions of these selected compounds with antibiotic streptomycin using checkboard assay demonstrated that all compounds were additive with streptomycin, suggesting, based on the in vitro data, that a combination of compounds with this antibiotic can reduce its MIC and subsequently increase its efficiency. Furthermore, the bactericidal nature of three more active compounds, 5d, 5m, and 5x, against P. aeruginosa was determined by a time-kill curve study. It was found that after 1 h of treatment with compounds 5d, 5m, and 5x, the number of bacterial CFU was reduced by more than 90%, while after 6h, none of the P. aeruginosa colonies treated with the selected compounds (5d, 5m and 5x) remained viable.
In the case of methylindole-based thiazolidinones, only 6 out of 14 compounds showed moderate activity; the rest were of very low activity.
According to results of an antifungal activity evaluation, the tested compounds displayed promising potency against all the fungi. In the subgroup of indole-based thiazolidinone derivatives, 7 (5d, 5e, 5l, 5o, 5u, 5v and 5x) out of 16 compounds appeared to be more potent than ketoconazole against some fungal species. Compound 5x demonstrated higher activity than bifonazole against five species, being almost equipotent against A. fumigatus, the most resistant strain.
Regarding the second group, methylindole-based thiazolidinone derivatives, all compounds showed promising antifungal activity, with most of them displaying activity almost equipotent to ketoconazole against almost all fungi tested. It should be mentioned that compound 5g (MIc = 0.06 mg/mL) was more potent than bifonazole (MIC of 0.15 mg/mL), while two compounds (5r and 5w) were equipotent. The most sensitive fungal strain appeared to be T. viride, followed by A. niger, while P.v.c was the most resistant one, followed by P. funiculosum.
The diverse sensitivity of bacteria and fungi to the tested compounds demonstrates the dependence of activity on substituents and their position in indole/methylindole rings, as well as on the nature of substituent of the thiazole ring.
According to docking studies, E. coli MurB inhibition is probably responsible for the antibacterial activity of compounds, whereas CYP51 inhibition is implicated in antifungal activity.
All compounds exhibited moderate-to-good drug-likeness scores, ranging from −0.63 to 0.29.
An evaluation of the cytotoxicity of the compounds in normal human MRC-5 cells revealed that the compounds are not toxic.
Finally, compound 5x, 4-(1H-indol-3-yl)-2-(2-(propan-2-ylidene)hydrazinyl)thiazole, can be considered as lead compound for further development of more potent and safe antibacterial and antifungal agents.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ph14111096/s1. Copies of 1H and 13C NMR spectra for all new synthesized compounds have been submitted along with the manuscript, Table S1, and Figure S1.
Author Contributions
Conceptualization, A.G. and V.K.; methodology, S.S. and M.S.; software, A.P.; formal analysis, S.S.; investigation, I.N., M.I., M.K., J.G., D.T. and I.S.V.; data curation, A.G., S.S. and M.S.; original draft preparation, A.G. and M.I.; review and editing, A.G. and M.I.; supervision, A.G. and V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Serbian Ministry of Education, Science and Technological Development [Contract No. 451-03-9/2021-14/200007].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing contains in this article.
Acknowledgments
This research is funded by the Serbian Ministry of Education, Science and Technological Development [Contract No. 451-03-9/2021-14/200007].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Tehranchian, S.; Akbarzadeh, T.; Fazeli, M.R.; Jamalifar, H.; Shafiee, A. Synthesis and antibacterial activity of 1-[1,2,4-triazol-3-yl] and 1-[1,3,4-thiadiazol-2-yl]-3-methylthio-6,7-dihydrobenzo[c]thiophen-4(5H)ones. Bioorganic Med. Chem. Lett. 2005, 15, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, K.R.; Lamie, R.F.; Omar, H.A. 3-methyl-2-phenyl-1-substituted-indole derivatives as indomethacin analogs: Design, synthesis and biological evaluation as potential anti-inflammatory and analgesic agents. J. Enzyme Inhib. Med. Chem. 2016, 31, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tang, L.; Zhu, H.; Xu, T.; Qiu, C.; Zheng, S.; Gu, Y.; Feng, J.; Zhang, Y. Design, Synthesis, and Structure–Activity Relationship Study of Novel Indole-2-carboxamide Derivatives as Anti-inflammatory Agents for the Treatment of Sepsis. J. Med. Chem. 2016, 59, 4637–4650. [Google Scholar] [CrossRef]
- Shaker, A.M.M.; Abdelall, E.K.A.; Abdellatif, K.R.A.; Abdel-Rahman, H.M. Synthesis and biological evaluation of 2-(4-methylsulfonyl phenyl) indole derivatives: Multi-target compounds with dual antimicrobial and anti-inflammatory activities. BMC Chem. 2020, 14, 23–37. [Google Scholar] [CrossRef]
- Fatahala, S.S.; Khedr, M.A.; Mohamed, M.S. Synthesis and Structure Activity Relationship of Some Indole Derivatives as Potential Anti-inflammatory Agents. Acta Chim. Slov. 2017, 64, 865–876. [Google Scholar] [CrossRef]
- Kaur, H.; Sing, J.; Narasimhan, B. Indole hybridized diazenyl derivatives: Synthesis, antimicrobial activity, cytotoxicity evaluation and docking studies. BMC Chem. 2019, 13, 65–82. [Google Scholar] [CrossRef]
- Kaur, J.; Utreja, D.; Jain, N.; Sharma, S. Recent Developments in the Synthesis and Antimicrobial Activity of Indole and its Derivatives. Curr. Org Synth. 2019, 16, 17–37. [Google Scholar] [CrossRef]
- Tiwari, S.; Kirar, S.; Banerjee, U.C.; Neerupudi, K.B.; Singh, S.; Wani, A.A.; Bharatam, P.V.; Singh, I.P. Synthesis of N-substituted indole derivatives as potential antimicrobial and antileishmanial agents. Bioorganic Chem. 2020, 99, 103787–103798. [Google Scholar] [CrossRef]
- Dixit, A.; Pathak, D.; Sharma, G.K. Synthesis, antibacterial and free radical scavenging activity of some newer N-((10-nitro-1H-indolo [1, 2-c]quinazolin-12-yl)methylene)benzenamines. Eur. Pharm. J. 2019, 67, 7–16. [Google Scholar] [CrossRef]
- Kaur, K.; Jaitak, V. Recent Development in Indole Derivatives as Anticancer Agents for Breast Cancer. Anticancer Agents Med. Chem. 2019, 19, 962–983. [Google Scholar] [CrossRef]
- Prakash, B.; Amuthavalli, A.; Edison, D.; Sivaramkumar, M.; Velmurugan, S. Novel indole derivatives as potential anticancer agents:Design, synthesis and biological screening. Med. Chem. Res. 2018, 27, 321–331. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, A.; Di Vita, G.; Ciancimino, C.; Attanzio, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Tesoriere, V.; Livrea, M.A.; et al. 3-[4-(1H-Indol-3-yl)-1,3-thiazol-2-yl]-1H-pyrrolo [2,3-b]pyridines, Nortopsentin Analogues with Antiproliferative Activity. Mar. Drugs 2015, 13, 1901–1924. [Google Scholar] [CrossRef]
- Swathi, K.; Sarangapani, M. Evaluation of Anti-Epileptic Effect of New Indole Derivatives by Estimation of Biogenic Amines Concentrations in Rat Brain. Adv. Exp. Med. Biol. 2017, 988, 39–48. [Google Scholar] [CrossRef]
- Saini, S. Synthesis and Anticonvulsant Studies of Thiazolidinone and Azetidinone Derivatives from Indole Moiety. Drug Res. 2019, 69, 445–450. [Google Scholar] [CrossRef]
- Zhang, M.-Z.; Chen, Q.; Yang, G.-F. A review on recent developments of indole-containing antiviral agents. Eur. J. Med. Chem. 2015, 89, 421–441. [Google Scholar] [CrossRef]
- Bardiot, D.; Koukni, M.; Smets, W.; Carlens, G.; McNaughton, M.; Kaptein, S.; Dallmeier, K.; Chaltin, P.; Neyts, J.; Marchand, A. Discovery of Indole Derivatives as Novel and Potent Dengue Virus Inhibitors. J. Med. Chem. 2018, 61, 8390–8401. [Google Scholar] [CrossRef]
- Bhat, M.A.; Al-Omar, M.A.; Raish, M.; Ansari, M.A.; Hatem, A.; Abuelizz, H.A.; Bakheit, A.H.; Naglah, A.N. Indole Derivatives as Cyclooxygenase Inhibitors: Synthesis, Biological Evaluation and Docking Studies. Molecules 2018, 23, 1250. [Google Scholar] [CrossRef]
- Atta-Allah, S.R.; Nassar, I.F.; El-Sayed, W.A. Design, synthesis and anti-inflammatory vel 5-(Indol-3-yl)thiazolidinone derivatives as COX-2 inhibitors. J. Pharm. Therap. Res. 2020, 4, 23–25. [Google Scholar] [CrossRef]
- Gani, R.S.; Timanagouda, K.; Joshic, S.D.; Hiremath, M.B.; Mujawar, S.B.H.; Kudva, A.K. Synthesis of novel indole, 1,2,4-triazole derivatives as potential glucosidase inhibitors. J. King Saud Univ. Sci. 2020, 32, 3388–3399. [Google Scholar] [CrossRef]
- Ramya, V.; Vembu, S.; Ariharasivakumar, G.; Gopalakrishnan, M. Synthesis, Characterisation, Molecular Docking, Anti-microbial and Anti-diabetic Screening of Substituted 4-indolylphenyl-6-arylpyrimidine-2-imine Derivatives. Drug Res. 2017, 67, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.S.; Pal, M. Indole derivatives as anti-tubercular agents: An overview on their synthesis and biological activities. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cihan-Üstündağ, G.; Şatana, D.; Özhan, G.; Çapan, G. Indole-based hydrazide-hydrazones and 4-thiazolidinones: Synthesis and evaluation as antitubercular and anticancer agents. J. Enzyme Inhib. Med. Chem. 2016, 31, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Herlé, B.; Wanner, M.J.; van Maarseveen, J.H.; Hiemstra, H. Total synthesis of (+)-yohimbine via an enantioselective organocatalytic Pictet-Spengler reaction. J. Org. Chem. 2011, 76, 8907–8912. [Google Scholar] [CrossRef]
- Morales, A. Yohimbine in erectile dysfunction: The facts. Int. J. Impot. Res. 2000, 12 (Suppl 1), S70–S74. [Google Scholar] [CrossRef]
- Scott, L.J.; Perry, C.M. Delavirdine: A review of its use in HIV infection. Drugs 2000, 60, 1411–1444. [Google Scholar] [CrossRef]
- Biswal, S.; Sahoo, U.; Sethy, S.; Kumar, H.K.S.; Banerjee, M. Indole: The molecule of diverse biological activities. Asian J. Pharm. Clin. Res. 2012, 5, 1. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Watanabe, H.; Okuyama, K.; Ozawa, H.; Hirabayashi, K.; Shimomura, M.; Kunitake, T.; Yasuoka, N. A new antibiotic SF2583A, 4-chloro-5-(3′-indolyl) oxazole, produced by Streptomyces. Meiji Seika Kenkyu Nenpo 1988, 27, 55–62. [Google Scholar]
- Zhang, M.Z.; Jia, C.Y.; Gu, Y.C.; Mulholland, N.; Turner, S.; Beattie, D.; Zhang, W.H.; Yang, G.F.; Clough, J. Synthesis and antifungal activity of novel indole-replaced streptochlorin analogues. Eur. J. Med. Chem. 2017, 126, 669–674. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, D.-C.; Liu, C.; Song, Z.-L.; Zhang, M.-Z. Streptochlorin analogues as potential antifungal agents: Design, synthesis, antifungal activity and molecular docking study. Bioorg. Med. Chem. 2021, 35, 116073. [Google Scholar] [CrossRef]
- Althagafi, I.; El-Metwaly, N.; Farghaly, T.A. New Series of Thiazole Derivatives: Synthesis, Structural Elucidation, Antimicrobial Activity, Molecular Modeling and MOE Docking. Molecules 2019, 24, 1741. [Google Scholar] [CrossRef]
- Demirci, S. Synthesis of Thiazole Derivatives as Antimicrobial Agents by Green Chemistry Techniques. JOTCSA 2018, 5, 393–414. [Google Scholar] [CrossRef][Green Version]
- Kamat, V.; Santosh, R.; Poojary, B.; Nayak, S.P.; Kumar, B.K.; Sankaranarayanan, M.; Khanapure, S.; Barretto, D.A.; Vootla, S.K. Pyridine- and Thiazole-Based hydrazides with promising anti-inflammatory and antimicrobial activities along with their in-silico studies. ACS Omega 2020, 5, 25228–25239. [Google Scholar] [CrossRef]
- Zaki, I.; Abdelhameid, M.K.; El-Deen, I.M.; Wahab, A.H.; Ashmawy, A.M.; Mohamed, K.O. Design, synthesis and screening of 1, 2, 4-triazinone derivatives as potential antitumor agents with apoptosis inducing activity on MCF-7 breast cancer cell line. Eur. J. Med. Chem. 2018, 156, 563–579. [Google Scholar] [CrossRef]
- Xie, W.; Wu, Y.; Zhang, J.; Mei, Q.; Zhang, Y.; Zhu, N.; Liu, R.; Zhang, H. Design, synthesis and biological evaluations of novel pyridonethiazole hybrid molecules as antitumor agents. Eur. J. Med. Chem. 2018, 145, 35–40. [Google Scholar] [CrossRef]
- de Santana, T.I.; Barbosa, M.O.; Gomes, P.A.T.M.; da Cruz, A.C.N.; da Silva, T.G.; Leite, A.C.L. Synthesis, anticancer activity and mechanism of action of new thiazole derivatives. Eur. J. Med. Chem. 2018, 144, 874–886. [Google Scholar] [CrossRef]
- Kamble, R.D.; Meshram, R.J.; Hese, S.V.; More, R.A.; Kamble, S.S.; Gacche, R.N. Synthesis and in silico investigation of thiazoles bearing pyrazoles derivatives as anti-inflammatory agents. Comp. Biol. Chem. 2016, 61, 86–96. [Google Scholar] [CrossRef]
- Liaras, K.; Fesatidou, M.; Geronikaki, A. Thiazoles and Thiazolidinones asCOX/LOX Inhibitors. Molecules 2018, 23, 685. [Google Scholar] [CrossRef]
- Porwal, P.; Tiwari, T.; Paliwal, S.; Sharma, H.; Kushwah, H.; Pal, K. Synthesis and anti-inflammatory activity of substituted phenyl thiazole derivatives. WJPMR 2021, 7, 152–155. [Google Scholar]
- Galochkina, A.V.; Bollikanda, R.K.; Zarubaev, V.V. Synthesis of novel derivatives of 7,8-dihydro-6H-imidazo [2,1-b][1,3]benzothiazol-5-one and their virus-inhibiting activity against influenza A virus. Arch. Pharm. Chem. Life Sci. 2019, 352, e1800225. [Google Scholar] [CrossRef] [PubMed]
- Gürsoy, E.; Dincel, E.D.; Naesens, L.; Güzeldemirci, N.U. Design and synthesis of novel Imidazo[2,1-b]thiazole derivatives as potent antiviral and antimycobacterial agents. Bioorg. Chem. 2020, 95, 103496–103505. [Google Scholar] [CrossRef]
- Meleddu, R.; Distinto, S.; Corona, A.; Tramontano, E.; Bianco, G.; Melis, C.; Cottiglia, F.; Maccioni, E. Isatin thiazoline hybrids as dual inhibitors of HIV-1reverse transcriptase. J. Enzyme Inhib. Med. Chem. 2017, 32, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Petrou, A.; Eleftheriou, P.; Geronikaki, A.; Akrivou, Μ.; Vizirianakis, Ι. Novel thiazolidin-4-ones as potential non-nucleoside inhibitors of HIV-1 reverse transcriptase. Molecules 2019, 24, 3821. [Google Scholar] [CrossRef] [PubMed]
- Sravanthi, T.V.; Sajitha, L.; Vino, S.; Jayasri, M.A.; Mohanapriya, A.L.; Manju, S.L. Synthesis, docking, and evaluation of novel thiazoles for potent antidiabetic activity. Med. Chem. Res. 2017, 26, 1306–1315. [Google Scholar] [CrossRef]
- Khatik, G.L.; Datusalia, A.K.; Ahsan, W.; Kaur, P.; Vyas, M.; Mittal, A.; Nayak, S.K. A Retrospect Study on Thiazole Derivatives as the Potential Antidiabetic Agents in Drug Discovery and Developments. Curr. Drug Discov. Technol. 2018, 15, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Distinto, S.; Meleddu, R.; Ortuso, F.; Cottiglia, F.; Deplano, S.; Sequeira, L.; Melis, C.; Fois, B.; Angeli, A.; Capasso, C.; et al. Exploring new structural features of the 4-[(3-methyl-4-aryl-2,3-dihydro-1,3-thiazol-2-ylidene)amino]benzenesulphonamide scaffold for the inhibition of human carbonic anhydrases. J. Enzym. Inhib. Med. Chem. 2019, 34, 1526–1533. [Google Scholar] [CrossRef]
- Manasa, K.L.; Pujitha, S.; Sethi, A.; Arifuddin, M.; Alvala, M.; Angeli, A.; Supuran, C.T. Synthesis and Biological Evaluation ofImidazo[2,1-b]Thiazole based Sulfonyl Piperazines as Novel Carbonic Anhydrase II Inhibitor. Metabolites 2020, 10, 136. [Google Scholar] [CrossRef]
- Al-Jaidi, B.A.; Deb, P.K.; Telfah, S.T.; Dakkah, A.N.; Bataineh, Y.A.; Khames Aga, Q.; Al-Dhoun, M.A.; Ahmad Al-Subeihi, A.A.; Odetallah, H.M.; Bardaweel, S.K.; et al. Synthesis and evaluation of 2,4,5-trisubstitutedthiazoles as carbonic anhydrase-III inhibitors. J. Enzym. Inhib. Med. Chem. 2020, 35, 1483–1490. [Google Scholar] [CrossRef]
- Karale, U.B.; Krishna, V.S.; Krishna, E.V.; Choudhari, A.S.; Shukla, M.; Gaikwad, V.R.; Mahizhaveni, B.; Chopra, S.; Misra, S.; Sarkar, D.; et al. Synthesis and biological evaluation of 2,4,5-trisubstituted thiazoles as antituberculosis agents effective against drug-resistant tuberculosis. Eur. J. Med. Chem. 2019, 178, 315–328. [Google Scholar] [CrossRef]
- SAlegaon, S.; Kashniya, K.; Kuncolienkar, S.; Kavalapure, R.; Salve, P.; Palled, M. Synthesis and biological evaluation of some 4-aminoquinoline derivatives as potential antitubercular agents. Future J. Pharm. Sci. 2020, 6, 1–12. [Google Scholar] [CrossRef]
- Mishchenko, M.; Shtrygol, S.; Kaminskyy, D.; Lesyk, R. Thiazole-Bearing 4-Thiazolidinones as NewAnticonvulsant Agents. Sci. Pharm. 2020, 88, 16. [Google Scholar] [CrossRef]
- Kumawat, M.K. Thiazole Containing Heterocycles with Antimalarial Activity. Curr. Drug Discov. Technol. 2018, 15, 196–200. [Google Scholar] [CrossRef]
- de Siqueira, L.R.P.; de Oliveira, M.; Oliveira, A.R.; de Moraes Gomes, P.A.T.; de Oliveira Filho, G.B.; de Oliveira Cardoso, M.V.; Pereira, V.R.A.; da Silva Santos, A.C.; Júnior, P.A.S.; Romanha, A.J.; et al. Synthesis and anti-Trypanosoma cruzi profile of the novel 4-thiazolidinone and 1,3-thiazole derivatives. Front. Drug Chem. Clin. Res. 2019, 2, 1–12. [Google Scholar]
- Ye, J.; Liu, Q.; Wang, C.; Meng, Q.; Sun, H.; Peng, J.; Ma, X.; Liu, K. Benzylpenicillin inhibits the renal excretion of acyclovir by OAT1 and OAT3. Pharmacol. Rep. 2013, 65, 505–512. [Google Scholar] [CrossRef]
- Rahmutulla, B.; Matsushita, K.; Satoh, M.; Seimiya, M.; Tsuchida, S.; Kubo, S.; Shimada, H.; Ohtsuka, M.; Miyazaki, M.; Nomura, F. Alternative splicing of FBP-interacting repressor coordinates c-Myc, P27Kip1/cyclinE and Ku86/XRCC5 expression as a molecular sensor for bleomycin induced DNA damage pathway. Oncotarget 2014, 15, 2404–2417. [Google Scholar] [CrossRef]
- Popsavin, V. Synthesis and antiproliferative activity of two new tiazofurin analogues with 2′-amido functionalities. Bioorganic Med. Chem. Lett. 2006, 16, 2773–2776. [Google Scholar] [CrossRef]
- Wei, L.; Cheng, J.; Meng, Y.; Ren, Y.; Deng, H.; Guo, Y. A novel formulation of thiamine dilaurylsulphate and its preservative effect on apple juice and sterilised milk. Food Chem. 2014, 1, 415–422. [Google Scholar] [CrossRef]
- Sevrioukova, L.F.; Poulos, T.L. Dissecting cytochrome P450 3A4-ligand interactions using ritonavir analogues. Biochemistry 2013, 52, 4474–4481. [Google Scholar] [CrossRef]
- Karateev, A.E. [Meloxicam: The golden mean of nonsteroidal anti-inflammatory drugs]. Ter Arkh. 2014, 86, 99–105, Russian. PMID: 25026810. [Google Scholar] [PubMed]
- Ivasiv, V.; Albertini, C.; Gonçalves, A.E.; Rossi, M.; Bolognesi, M.L. Molecular hybridization as a tool for designing multitarget drug candidates for complex diseases. Curr. Top. Med. Chem. 2019, 19, 1694–1711. [Google Scholar] [CrossRef] [PubMed]
- Viegas-Junior, C.; Danuello, A.; Bolzani, V.d.S.; Barreiro, E.J.; Fraga, C.A.M. Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Horishny, V.; Kartsev, V.G.; Matiychuk, V.S.; Geronikaki, A.; Petrou, A.; Pogodin, D.; Poroikov, V.; Ivanov, M.; Kostic, M.; Sokovic, M. 3-Amino-5- (indol-3-yl) methylene-4-oxo-2-thioxothiazolidine derivatives as antimicrobial agents: Synthesis, computational and biological evaluation. Pharmaceuticals 2020, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Horishny, V.; Kartsev, V.G.; Matiychuk, V.S.; Geronikaki, A.; Petrou, A.; Glamoclija, J.; Ciric, A.; Sokovic, M. 5-(1H-Indol-3-ylmethylene)-4-oxo-2-thioxothiazolidin-3-yl)alkancarboxylic acids as antimicrobial agents. Synthesis, biological evaluation and molecular docking studies. Molecules 2020, 25, 1964. [Google Scholar] [CrossRef]
- Mirchamsy, H.; Shafyi, A.; Bahrami, S.; Kamali, M.; Nazari, P. Use of human diploid cell MRC-5, for production of measles and rubella virus vaccines. Dev Biol Stand. 1976, 37, 297–300. [Google Scholar]
- Rieske, P.; Krynska, B.; Azizi, S. Human fibroblast-derived cell lines have characteristics of embryonic stem cells and cells of neuro-ectodermal origin. Differentiation 2005, 73, 474–483. [Google Scholar] [CrossRef]
- Akrivou, M.G.; Demertzidou, V.P.; Theodoroula, N.F.; Chatzopoulou, F.M.; Kyritsis, K.A.; Grigoriadis, N.; Zografos, A.L.; Vizirianakis, I.S. Uncovering the pharmacological response of novel sesquiterpene derivatives that differentially alter gene expression and modulate the cell cycle in cancer cells. Int. J. Oncol. 2018, 53, 2167–2179. [Google Scholar] [CrossRef]
- Tseligka, E.D.; Rova, A.; Amanatiadou, E.P.; Calabrese, G.; Tsibouklis, J.; Fatouros, D.G.; Vizirianakis, I.S. Pharmacological Development of Target-Specific Delocalized Lipophilic Cation-Functionalized Carboranes for Cancer Therapy. Pharm. Res. 2016, 33, 1945–1958. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. A qualitative and quantitative characterization of known drug databases. J Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Wars, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today: Technologies 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Rubtsov, M.B.; Baichikov, A.G. Synthetic chemico-pharmaceutical preparates. In Meditsina; Moscow, Russia, 1971; pp. 158–159. [Google Scholar]
- Murakami, Y.; Yokoyama, Y.; Miura, T.; Hirasawa, H.; Kamimura, Y.; Mzaki, M. p-Toluenesulfonik acid and cation exchange resin in aprotonic solvent: Valuable catalysts for Fischer indolization. Heterocycles 1984, 22, 1211–1216. [Google Scholar] [CrossRef]
- Suvorov, N.N.; Smushkvic, J.I.; Velezheva, V.S.; Rozhkov, V.S.; Simakov, S.V. Synthesis of N-substituted indoles by extractive alkylation. Chim. Heterocyc. Soedin. 1976, 2, 191–193. [Google Scholar] [CrossRef]
- Chapman, N.B.; Clarke, K.; Hughes, H. 250. Synthesis of some 5-substituted-2-methyltryptamines and their N-mono- and -di-alkyl derivatives. J. Chem. Soc. 1965, 1424–1428. [Google Scholar] [CrossRef]
- Kartsev, V.; Lichitsky, B.; Geronikaki, A.; Petrou, A.; Smiljkovic, M.; Kostic, M.; Radanovic, O.; Soković, M. Design, synthesis and antimicrobial activity of usnic acid derivatives. Med. Chem. Comm. 2018, 9, 870–882. [Google Scholar] [CrossRef]
- Kostić, M.; Smiljković, M.; Petrović, J.; Glamočilija, J.; Barros, L.; Ferreira, I.C.F.R.; Ćirić, A.; Soković, M. Chemical, nutritive composition and a wide range of bioactive properties of honey mushroom Armillariamellea (Vahl: Fr.) Kummer. Food and Function 2017, 8, 3239–3249. [Google Scholar] [CrossRef]
- Cady, N.C.; McKean, K.A.; Behnke, J.; Kubec, R.; Mosier, A.P.; Kasper, S.H.; Burz, D.S.; Musah, R.A. Inhibition of biofilm formation, quorum sensing and infection in Pseudomonas aeruginosa by natural products-inspired organosulfur compounds. PLoS ONE 2012, 7, e38492. [Google Scholar] [CrossRef]
- Aničić, N.; Gaši, U.; Lu, F.; ĆCiric, A.; Ivanov, M.; Jevtic, B.; Dimitrijevic, M.; Andelkovic, B.; Skoric, M.; Nestorovic Živkovic, I.; et al. Antimicrobial and immunomodulating activities of two ]endemic Nepeta species and their major iridoids isolated from natural sources. Pharmaceuticals 2021, 14, 414. [Google Scholar] [CrossRef]
- Smiljkovic, M.; Dias, M.; Stojkovic, D.; Barros, L.; Bukvicki, D.; Ferreira, I.C.F.R.; Sokovic, M. Characterization of phenolic compounds in tincture of edible Nepeta nuda: Development of antimicrobial mouthwash. Food & Function 2018, 9, 5417–5425. [Google Scholar] [CrossRef]
- Kritsi, E.; Matsoukas, M.T.; Potamitis, C.; Detsi, A.; Ivanov, M.; Sokovic, M.; Zoumpoulakis, P. Novel Hit Compounds as Putative Antifungals: The Case of Aspergillus fumigatus. Molecules 2019, 24, 3853. [Google Scholar] [CrossRef] [PubMed]
- Aleksić, M.; Stanisavljević, D.; Smiljković, M.; Vasiljević, P.; Stevanović, M.; Soković, M.; Stojković, D. Pyrimethanil: Between efficient fungicide against Aspergillus rot on cherry tomato and cytotoxic agent on human cell lines. Ann. Appl. Biol. 2019, 175, 228–235. [Google Scholar] [CrossRef]
- Angeli, A.; Kartsev, V.; Petrou, A.; Pinteala, M.; Vydzhak, R.M.; Panchishin, S.Y.; Brovarets, V.; De Luca, V.; Capasso, C.; Geronikaki, A.; et al. New Sulfanilamide Derivatives Incorporating Heterocyclic Carboxamide Moieties as Carbonic Anhydrase Inhibitors. Pharmaceuticals 2021, 14, 828. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).